Abstract
Atopic Dermatitis (AD), the most common chronic inflammatory skin disease, is characterized by an overactive immune response to a host of environmental allergens and dry, itchy skin. Over the past decade important discoveries have demonstrated that AD develops in part from genetic and/or acquired defects in the skin barrier. Histamine is an aminergic neurotransmitter involved in physiologic and pathologic processes such as pruritus, inflammation, and vascular leak. Enhanced histamine release has been observed in the skin of patients with AD and antihistamines are often prescribed for their sedating and anti-itch properties. Recent evidence suggests that histamine also inhibits the terminal differentiation of keratinocytes and impairs the skin barrier, raising the question whether histamine might play a role in AD barrier impairment. This, coupled with the notion that histamine’s effects mediated through the recently identified histamine receptor H4R, may be important in allergic inflammation, has renewed interest in this mediator in allergic diseases. In this paper we summarize the current knowledge on histamine and histamine receptor antagonists in AD and skin barrier function.
Keywords: atopic dermatitis, histamine, histamine receptors, skin barrier, tight junction
1. Atopic Dermatitis (AD) and the Skin Barrier
AD is the most common inflammatory skin disease, affecting up to 15 million Americans (about 17% of children and 6% of adults) [1]. Based on important discoveries over the last decade, it is thought that AD develops as a consequence of an acquired or genetic defect in the skin’s barrier [2,3]. The hypothesis is that these defects promote a more robust immune response to microbes, allergens, antigens, and irritants, and in the case of an atopic subject this response will lead to a largely T helper type 2 (Th2) cell infiltrate characterized by the release of interleukin (IL)-4 and IL-13. The interdependence of the immune and physical barrier systems is now a very active area of investigation and likely to lead to novel approaches to disease prevention and treatment.
It is widely accepted that the stratum corneum (SC) is dysfunctional in AD as a result of abnormal lipids (e.g., reduced ceramides and free fatty acids, an increase in unsaturated chain length) [4], altered expression of epidermal differentiation genes (e.g., loricrin, small proline-rich region proteins (SPRR)), filaggrin null-mutations, imbalance of proteases and protease inhibitors, and trauma from a chronic itch-scratch cycle (reviewed in [5]). We have focused most of our studies on a barrier structure found just below the SC, namely tight junction (TJ). In epithelial cells, TJs function as the “gate” for paracellular (i.e., space between adjacent cells) passage of ions and solutes, which indirectly affects water transport (review in [6]). We have observed a TJ defect in the epidermis of AD subjects, as well as reduced expression of key TJ components, including claudin (cldn)-1 and -23 [7]. More recently, other studies have confirmed and expanded these findings by demonstrating reduced expression of other claudins (e.g., cldn-4 and 8) in the skin of subjects with AD [8,9].
The notion that an impaired skin barrier is a critical feature in the pathogenesis of AD presents an opportunity to develop new therapeutics aimed at repairing the skin barrier as an alternative or complementary approach to the more traditional anti-inflammatory therapies. Importantly, AD often precedes the development of other allergic diseases such as asthma and allergic rhinitis and this pattern of disease progression is often referred to as the Atopic March. This has led many to speculate that effective treatments for AD may diminish the risk for or severity of asthma. In 2002, annual US health care costs for AD were estimated to be as high as $3.8 billion, similar to emphysema and epilepsy [1]. More recently, a US population-based study showed that adult AD subjects have a significantly larger health burden with substantial out-of pocket costs, greater indirect costs (e.g., lost work days) and increased access to the health care system [10]. Despite its high prevalence, effects on quality-of-life and economic burden, there are still few effective treatments for AD and most have focused on inhibiting inflammation. Not surprisingly, this has resulted in high patient (and physician) dissatisfaction with AD management.
To date, AD treatments have exclusively targeted inflammation with the widespread use of topical corticosteroids and frequent use of systemic immunosuppressive drug (reviewed in [11]). As a potentially safer alternative to topical steroids, topical calcineurin antagonists (i.e., tacroliums and pimecrolimus) were developed and received FDA approval in 2000 and 2001, respectively. In the USA, there are no systemic therapies approved by the FDA for treatment of AD. There are very few well-designed trials to support the off-label use of systemic immunosuppressives, with the possible exception of cyclosporine (reviewed in [12]). Cyclosporine is the only systemic treatment approved in some European countries for patients with severe AD [13]. Systemic steroids are often used in clinical practice to control flares. However, clinical studies to support their use in AD are surprisingly absent [14].
Recently a fully human monoclonal antibody directed against the IL-4 receptor α subunit (shared by the Type 1 and Type 2 IL-4 and IL-13 receptors, resulting in the blockage of both IL-4 and IL-13 signaling), called dupilumab (Regeneron Pharmaceuticals), has been shown to be highly effective for the treatment of moderate to severe adult AD subjects [15]. Dupilumab is currently poised to be the first systemic therapy to receive FDA approval for the treatment of adults with moderate to severe AD.
On the other hand, emollient therapy has been recognized for a long time as a critical component of AD patients’ management (reviewed in [11]). Recently it was shown that high-risk neonates who had emollient applied to the entire body on a daily basis since birth halved their risk for AD development [16]. Similarly, a study performed in Japan showed that daily use of emulsion-type moisturizer for the first 32 weeks of life significantly reduced the risk of developing AD [17].
Although the overall hypothesis is that emollients help restore the skin barrier function, mechanistic studies need to be done to test this theory.
Historically, histamine has been recognized as a potent inducer of pruritus. This, coupled with the increased histamine release observed in the skin of patients with AD [18], fostered the widespread use of Histamine Receptor 1 (H1R) antagonists in this disease. However, their clinical efficacy remains controversial. Several authors and clinicians have argued that antihistamines have a role in AD management largely for their soporific effects, essentially as a sleep aid (reviewed in [12]). A recent systematic review reported no high-level evidence to support or refute the efficacy of oral H1 antihistamines as monotherapy in AD patients [19]. Topical antihistamines have also been tried as treatment for AD. Studies evaluating the efficacy of topical doxepin (H1R/H2R and possibly H4R antagonist [20]) in the treatment of AD are conflicting [21,22]. Currently, topical antihistamines are not recommended because of their risk of absorption and contact dermatitis [11]. As we will discuss later in this review, the limited clinical efficacy of antihistamines might reflect their minimal effects on H4R-mediated actions. In addition, it should be highlighted that the vast majority of clinical trials were powered based on itch reduction as the primary endpoint. If one considers that a number of other mediators such as substance P, nerve growth factor, and IL-31 may also be mediating some (or all) of the pruritus observed in AD patients (reviewed in [23]) then it is not surprising that antihistamines have shown limited efficacy in mitigating itch.
In this review we will summarize what is known about histamine’s effects and the role of individual histamine receptors on epidermal skin barrier function. We will discuss how this information helps us better understand AD pathogenesis and the development of new therapeutic strategies.
2. Histamine Overview
Histamine (2-[4-imidazolyl]-ethylamine) is an aminergic neurotransmitter involved in numerous physiologic and pathologic processes, including pruritus, inflammation, and vascular leak. More than a century ago in 1910, Drs. Dale (recipient of the Nobel Prize for Medicine in 1936) and Laidlaw recognized that histamine has biological effects that mimic what is seen in an anaphylactic reaction [24]. A few years later histamine was isolated from lung and liver tissues and named histamine after the Greek word histos (tissue). In 1937, Drs. Bovet (recipient of the Nobel Prize in Physiology and Medicine in 1957) and Staub identified the first compounds capable of blocking histamine-mediated anaphylactic reactions [25]. Ever since, this has been an active and productive field of investigation, with a number of H1R and H2R blockers reaching the lofty blockbuster status defined as annual sales of ≥$1 billion. In fact, cimetidine (H2R-blocker; Tagamet®, GlaxoSmithKline, London, UK) was the first ever blockbuster drug (1985) [26].
Mast cells, basophils, and enterochromaffin cells (found in the gastric mucosa) are widely recognized cellular sources of histamine. However, other cells, including T cells and even keratinocytes, have been shown to produce histamine in response to stimulation [27,28]. The enzyme histidine decarboxylase (HDC) is responsible for histamine synthesis from the amino acid l-histidine. Of note, histamine can be also produced (from l-histidine via HDC) by some fermentative bacteria, including Lactobacilli in the gut [29,30]. This, coupled with recent knowledge about the potential role played by the skin microbiome in AD (reviewed in [31,32,33]), suggests a fascinating mechanism by which cutaneous bacteria might influence skin homeostasis.
In mast cells and basophils, histamine is stored in large quantities and quickly released upon stimulation. In other cell types, such as T cells and dendritic cells, histamine is newly synthesized and released after stimulation. HDC protein expression has recently been reported in cultured human keratinocytes and in the epithelial compartment of skin sections (by immunohistochemistry) [34]. Interestingly, in vitro studies using a human keratinocyte cell line (HaCat) demonstrated that HDC expression could be enhanced by stimulation with mediators present in AD skin lesions (i.e., TNFα, thymic stromal lymphopoietin [TSLP], and house dust mite extract) and it was associated with greater histamine release [34]. These authors also reported greater HDC intensity staining in the epidermis of AD subjects [34].
Histamine concentrations measured in various tissues range from 10−5 to 10−3 M [35]. Unfortunately, the methods for measuring histamine in plasma/serum or tissue samples are not very reliable or reproducible. Gutzmer et al. recently summarized published studies reporting histamine concentrations in different inflammatory skin diseases, including AD (see Table 1 in [36]). Authors highlighted the different methods of detection used and the variability in histamine concentrations measured in healthy and disease states and concluded that there was a need for new detection methods. A new method using liquid chromatography tandem mass spectrometry to measure histamine in plasma and tissues has recently been reported [37].
Histamine can bind to four receptors belonging to the large family of rhodopsin-like G-protein-couples receptors (GPCRs), named in chronological order based on their discovery as H1R, H2R, H3R, and H4R, only described in 2000 [38,39,40,41]. The biological effects of histamine stimulation are determined by the activation of one (or more) of the histamine receptors [42]. Several cell types, including epithelial and endothelial cells, dendritic cells, and neutrophils as well as T and B lymphocytes express both H1R and H2R [36,43]. H3R expression is localized primarily in the central nervous system. H4R is expressed by bone-marrow-derived cells, including T lymphocytes, dendritic cells, mast cells, and eosinophils as well as epithelial cells [44,45,46,47,48]. Interestingly, it has been shown that Langerhans cells, which are a subset of professional antigen-presenting cells that reside in the epidermis, selectively express H4R but not H1R or H2R [49,50]. Human keratinocytes express H1R, H2R, and H4R [51]. This is in contrast with murine keratinocytes where H1R, but not H4R, is expressed constitutively. However, it was shown that H4R expression could be induced upon innate immune stimulation with LPS and peptidoglycan [51]. This difference in H4R expression between human and mouse keratinocytes should be taken into consideration when performing studies investigating histamine biology in murine models of human disease.
The histamine-induced signaling cascade is quite complex. We will superficially summarize this topic and refer readers to expert reviews on this topic for greater details [42,52,53,54]. It is a widely held belief that most of the allergic and inflammatory actions of histamine are mediated by the H1R, a Gαq/11 receptor. Activation of the cellular process after histamine binding to H1R occurs via phospholipase C-mediated calcium mobilization, protein kinase C activation, and nuclear factor-κB-mediated signaling pathways (reviewed in [54]). A hallmark of the H2R signaling pathway is the formation of cAMP [55], while H4R signals through Gi/o receptors, resulting in inhibition of forskolin-induced cAMP production, intracellular calcium mobilization, and actin polymerization [40,55,56]. In addition, H4R has been reported to activate mitogen-activated protein kinases [57] and the JAK/STAT signaling pathway [58,59]. Importantly, histamine receptors can form dimers and oligomers, which allow interaction among histamine receptors as well as other G protein-coupled receptors, and this further increases the complexities of downstream signaling events in response to histamine stimulation.
Several commercially available antihistamines block either H1R or H2R or both, while H3R and H4R are currently being tested in clinical trials [60,61]. Based on their good safety profile, these drugs have been largely used for symptomatic treatment of allergic diseases, pruritic conditions, and gastroesophageal reflux disease (GERD) [62,63]. Antihistamines are recognized for a number of anti-inflammatory effects including inhibitory effects on mast cell and basophil degranulation, inhibition of adhesion molecules and eosinophil or neutrophil chemotaxis, enhancing apoptosis of inflammatory cells, reducing neuroinflammation, and cytokine/chemokine expression [64]. More recently it has become clear that some of the FDA-approved antihistamines are not as selective as we once thought and some have binding affinities for other HR receptors, albeit typically at doses not achieved with standard clinical dosing [64]. Importantly, none of the FDA-approved antihistamines antagonize H3R or H4R at standard dosing regimens. In an epicutaneous allergen challenge murine AD model, treatment with the selective H1R antihistamine, olopatadine, not only suppressed inflammation and scratching by inhibiting cytokine/chemokine production (e.g., IL-31, TSLP, TARC) but also improved the skin barrier function [65,66,67,68,69]. Olopatadine has inhibitory effects on the release of inflammatory mediators (e.g., histamine, leukotriene, thromboxane, and tachykinins), which could explain these broad anti-allergic properties [70]. Olopatadine was approved in 2000 in Japan for the treatment of several conditions including AD, chronic urticaria, and allergic rhinitis. However, in the USA and the European Union it is only available as a topical preparation for ophthalmic or nasal administration.
Relatively selective H3R and H4R blockers are currently in various stages of development by many pharma/biotech companies. Since H4R was identified at the beginning of this century, there have been a tremendous number of publications and patent applications. Preclinical data have highlighted the immunomodulatory properties of H4R, including effects on the chemotaxis of eosinophils [48] and mast cells [71], accumulation of FoxP3+ T cells [72] as well as modulation of inflammatory mediators (e.g., downregulation of IL-12 and CCL2) produced by monocytes [73,74]. This has increased enthusiasm that H4R (alone or in association with H1R antagonist) may be an effective new drug class for the treatment of allergic diseases [75,76]. High-throughput drug screening has led to the identification of new selective non-imidazole H4R ligands. As a result, several compounds are currently in preclinical and early clinical development. In Japan, a Phase 2a randomized, double-blind, placebo-controlled, multicenter, parallel-group clinical trial has tested the novel H4R antagonist, JNJ-39758979, in adult subjects with moderate AD. The study was terminated in response to two cases of neutropenia in the treatment group. Although the safety profile of this H4R antagonist remains a real concern, some beneficial effects were observed on disease severity and itch scores [77]. More studies are definitely needed to clarify the safety and efficacy of this compound or other H4R antagonists in the clinical setting.
3. Histamine and the Skin Barrier
In addition to its pro-inflammatory and itch effects, there is a growing body of evidence demonstrating that histamine also plays a role in epidermal terminal differentiation and skin barrier function. Gschwandtner et al. [78] recently demonstrated that histamine dose-dependently suppressed epidermal differentiation as indicated by significant reductions in filaggrin, loricrin, and keratin10 expression using both cultured primary human keratinocytes and an epidermal skin model (e.g., raft culture). In the raft culture system they observed that treatment with histamine induced thinning of the epidermis (50%), which was especially notable for the stratum granulosum, and that these effects were mediated by H1R [78]. These observations are consistent with previous studies showing that treatment with histamine or receptor (H1R and H2R) agonists or antagonists modulates epidermal barrier recovery in murine models [79,80,81]. Topical application of histamine or dimaprit (H2R agonist) delayed skin barrier recovery after tape stripping, as measured by transepidermal water loss (TEWL) in hairless mice [80]. In contrast, treatment with histamine receptor antagonists (e.g., olopatadine/H1R, diphenhydramine/H1R, and cimetidine/H2R) improved epidermal barrier recovery [79,80,81].
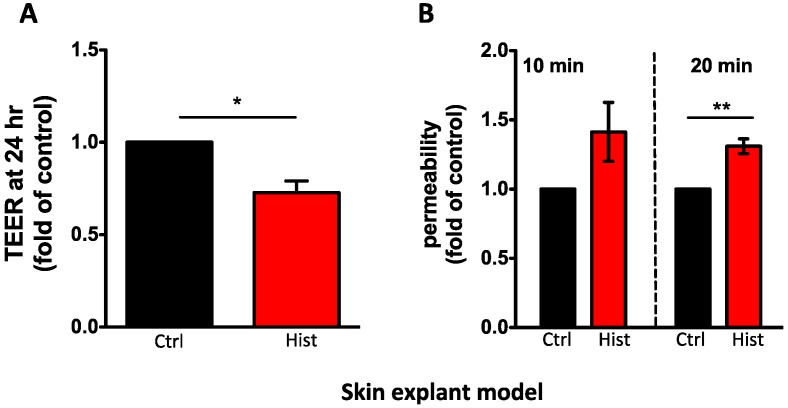
It has been known for some time that histamine, as well other amines such as thrombin, disrupt tight junction (TJ) in endothelial as well as epithelial cells [82,83]. In airway epithelial cells, histamine reduced the expression of a key TJ molecule, ZO-1, and this effect was at least partially abrogated by pretreatment with mepyriamine (an H1R antagonist), but not by ranitidine (an H2R antagonist) [84]. In the human skin equivalent model, Gschwandtner et al. demonstrated a strong suppression of intercellular junction proteins, including TJ components (e.g., cldn-1, -4 and occludin) and desmosomal components (e.g., corneodesmosin and desmoglein-1) after treatment with histamine [78]. These abnormalities were also associated with enhanced penetration of biotin in their skin equivalent model [78]. In our laboratory, we have started to investigate the effect of histamine and selected histamine receptor (H1R, H2R, and H4R) antagonists on epidermal TJ function and composition. We have employed two complementary epidermal models: an in vitro system utilizing cultured primary human keratinocytes (PHK) and an ex vivo system with full-thickness epidermal explants isolated from discarded skin samples from elective surgeries. TJ integrity was quantified by measuring transepithelial electric resistance (TEER) and paracellular flux, as we have previously described [85]. Briefly, TEER was measured using an EVOMX voltohmmeter (World Precision Instruments, Sarasota, FL, USA). To evaluate the paracellular flux of PHK, 0.02% Fluorescein Sodium (Fluka, St. Louis, MO, USA) in PBS (Invitrogen/Gibco, Grand Island, NY, USA) was added to the upper chamber (apical side) and samples were collected from the lower chamber (basal side) after 30 min incubation for PHK culture. PHK, isolated from neonatal foreskin, were cultured in Keratinocyte-SFM (Invitrogen/Gibco, Grand Island, NY, USA) containing 5 ng/mL recombinant EGF, 50 µg/mL BPE, 1% Pen/Strep, and 0.2% Amphotericin B (Invitrogen/Gibco). To induce terminal differentiation, sub-confluent PHK were differentiated in DMEM (Invitrogen/Gibco) containing high calcium (1.8 mM) but no serum or growth factors. In previous studies we determined that PHK grown under these culture conditions are highly differentiated [85]. Histamine dihydrochloride (1–100 µM, Sigma-Aldrich, St. Louis, MO, USA) was added to the culture media from the time of differentiation and replaced every 48 h with the medium change. This model allows us to study the effect of histamine on TJ assembly. De-identified human discarded skin was obtained from the Pathology Department of the University of Rochester Medical Center with the approval of the institutional Research Subject Review Board. As we previously described [85], the epidermis was enzymatically separated from the dermis using dispase and the epidermal sheet was sandwiched between two sterile custom-made Plexiglas discs with an opening of 3-mm diameter and placed in modified Snapwell™ chambers (Corning; Corning, NY, USA). Fresh media (DMEM) with histamine (10 or 100 uM) or media alone were then added to both sides of the transwell. The TEER and paracellular flux of fluorescein of skin explants were measured at 24 h. This model allowed us to investigate the effect of histamine on already formed TJ.
In agreement with published data, we found that histamine reduced TJ integrity. In cultured PHK, we observed a dose-dependent reduction of TEER (10 and 100 μM, p < 0.001, n = 9; Figure 1A) and enhanced permeability (100 μM, p < 0.001, n = 16; Figure 1B). Using the ex vivo model, we confirmed that histamine (100 μM) also reduced TEER (0.7 fold, p < 0.05, n = 3; Figure 2A) and enhanced fluorescein permeability flux (1.3 fold, p < 0.05, n = 3; Figure 2B) in epidermal explants. Based on these findings, we concluded that histamine impaired TJ integrity. Studies are ongoing to establish the signaling pathways mediating the histamine-induced modulation of TJ. Based on published studies in other epithelial and endothelial cell models, we speculate that histamine may act directly on TJ composition; however it is possible that the effect on TJ is indirect and therefore secondary to histamines’ actions on other biological pathways. As mentioned earlier, it has been shown that histamine treatment prevents terminal differentiation, which is mediated by H1R [78]. Impairment of differentiation could potentially affect the development of a mature TJ network, which is typically observed at the level of the stratum granulosum. Additionally, Glatzer et al. [51] demonstrated that histamine induced keratinocyte proliferation that was mediated by H4R. Increased proliferation would likely reduce differentiation and TJ assembly. On the other hand, histamine has been shown to induce the production of a number of inflammatory mediators (e.g., IL-31, human β-defensin 2) [86], which could affect the skin barrier [87,88].
Figure 1.
Histamine reduces TJ integrity in cultured primary human keratinocytes. Histamine dose-dependently reduced (A) transepithelial electric resistance (TEER) and (B) enhanced permeability to fluorescein. TEER is shown as mean area under the curve (AUC) ± SEM on n = 9 experiments; Permeability is shown as mean fold of control ± SEM of n = 16–10. Samples from the same donor were compared and a paired t-test was used for statistical analysis: * p < 0.05; ** p < 0.001, *** p < 0.0001.
Figure 2.
In an ex-vivo model evaluating full-thickness human epidermis placed in a modified micro-snapwell system, histamine (100 μM) (A) reduced TEER (0.7-fold) and (B) enhanced fluorescein permeability flux (20 min time point; 1.3-fold). TEER is shown as mean fold of control ± SEM on n = 3; permeability is shown as mean fold of control ± SEM of n = 3. Samples from the same donor were compared and a paired t-test was used for statistical analysis: * p < 0.05; ** p < 0.001, *** p < 0.0001.
In summary, our preliminary data and published studies demonstrate that histamine disrupts epidermal barriers (TJ and stratum corneum) and that blocking histamine may prevent this unwanted action on the epidermal barriers. Human clinical trials evaluating the effects of specific HR antagonists on skin barrier function and inflammation are needed to better understand the best clinical use of old as well as new, more selective, antihistamines in the management of AD.
4. Conclusions
There is an ongoing debate about the notion that an impaired skin barrier promotes the development of AD. Whether this breach is the consequence of genetic mutations (i.e., FLG null mutations) or something that develops in response to changes in the micro- or macro-environment or both is still unclear. An argument for the acquired pathway is the fact that a number of T-cell-derived cytokines (e.g., IL-4, IL-13, IL-25, IL-22, or IL-17A) found in AD skin can inhibit the epidermal expression of key barrier proteins such as filaggrin, loricrin, and involucrin, which are also markers of terminal differentiation [88,89,90,91]. As discussed in this manuscript, histamine can be added to this list of barrier-modulating agents. Histamine can affect skin barrier integrity by promoting proliferation and inhibiting differentiation of keratinocytes, by disrupting TJ integrity, or by indirectly modulating the parenchymal immune response. This knowledge, along with the observation that H1R or H4R blockade by itself may limit histamine-induced barrier disruption, has led to renewed interest in the role of histamine in allergic inflammation, and may lead to using blockades targeting H1R and H4R as “novel” prevention and/or treatment options for AD patients.
Acknowledgments
The authors are thankful to the Department of Pathology at the University of Rochester Medical Center for providing de-identified discarded skin samples for the study. The present work was supported by the National Institutes of Health, the Dermatology Foundation, a LaRoche Posey North American Foundation 2013 Award, and the National Eczema Association.
Author Contributions
Anna De Benedetto performed the literature review and wrote the paper; Lisa A. Beck and Takeshi Yoshida contributed to the manuscript; Anna De Benedetto and Lisa A. Beck conceived and supervised the experiments; and Sade Fridy, Joo-Eun S. Park, I-Hsin Kuo, and Takeshi Yoshida performed the experiments and analyzed the data.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Bieber T. Atopic dermatitis. N. Engl. J. Med. 2008;358:1483–1494. doi: 10.1056/NEJMra074081. [DOI] [PubMed] [Google Scholar]
- 2.Elias P.M., Wakefield J.S. Mechanisms of abnormal lamellar body secretion and the dysfunctional skin barrier in patients with atopic dermatitis. J. Allergy Clin. Immunol. 2014;134:781–791. doi: 10.1016/j.jaci.2014.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.De Benedetto A., Kubo A., Beck L.A. Skin barrier disruption: A requirement for allergen sensitization? J. Investig. Dermatol. 2012;132:949–963. doi: 10.1038/jid.2011.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Van Smeden J., Janssens M., Kaye E.C., Caspers P.J., Lavrijsen A.P., Vreeken R.J., Bouwstra J.A. The importance of free fatty acid chain length for the skin barrier function in atopic eczema patients. Exp. Dermatol. 2014;23:45–52. doi: 10.1111/exd.12293. [DOI] [PubMed] [Google Scholar]
- 5.Kezic S., Novak N., Jakasa I., Jungersted J.M., Simon M., Brandner J.M., Middelkamp-Hup M.A., Weidinger S. Skin barrier in atopic dermatitis. Front. Biosci. (Landmark Ed.) 2014;19:542–556. doi: 10.2741/4225. [DOI] [PubMed] [Google Scholar]
- 6.Brandner J.M.Z.-K.M., Yoshida T., Moll I., Beck L.A., De Benedetto A. Epidermal tight junctions in health and disease. Tissue Barriers. 2015;3 doi: 10.4161/21688370.2014.974451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De Benedetto A., Rafaels N.M., McGirt L.Y., Ivanov A.I., Georas S.N., Cheadle C., Berger A.E., Zhang K., Vidyasagar S., Yoshida T., et al. Tight junction defects in patients with atopic dermatitis. J. Allergy Clin. Immunol. 2011;127:773–786. doi: 10.1016/j.jaci.2010.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Esaki H., Ewald D.A., Ungar B., Rozenblit M., Zheng X., Xu H., Estrada Y.D., Peng X., Mitsui H., Litman T., et al. Identification of novel immune and barrier genes in atopic dermatitis by means of laser capture microdissection. J. Allergy Clin. Immunol. 2015;135:153–163. doi: 10.1016/j.jaci.2014.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Batista D.I., Perez L., Orfali R.L., Zaniboni M.C., Samorano L.P., Pereira N.V., Sotto M.N., Ishizaki A.S., Oliveira L.M., Sato M.N., et al. Profile of skin barrier proteins (filaggrin, claudins 1 and 4) and Th1/Th2/Th17 cytokines in adults with atopic dermatitis. J. Eur. Acad. Dermatol. Venereol. 2014 doi: 10.1111/jdv.12753. [DOI] [PubMed] [Google Scholar]
- 10.Silverberg J.I. Healthcare utilization, patient costs, and access to care in us adults with eczema: A population-based study. JAMA Dermatol. 2015 doi: 10.1001/jamadermatol.2014.5432. [DOI] [PubMed] [Google Scholar]
- 11.Eichenfield L.F., Tom W.L., Berger T.G., Krol A., Paller A.S., Schwarzenberger K., Bergman J.N., Chamlin S.L., Cohen D.E., Cooper K.D., et al. Guidelines of care for the management of atopic dermatitis: Section 2. Management and treatment of atopic dermatitis with topical therapies. J. Am. Acad. Dermatol. 2014;71:116–132. doi: 10.1016/j.jaad.2014.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sidbury R., Davis D.M., Cohen D.E., Cordoro K.M., Berger T.G., Bergman J.N., Chamlin S.L., Cooper K.D., Feldman S.R., Hanifin J.M., et al. Guidelines of care for the management of atopic dermatitis: Section 3. Management and treatment with phototherapy and systemic agents. J. Am. Acad. Dermatol. 2014;71:327–349. doi: 10.1016/j.jaad.2014.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bieber T., Straeter B. Off-label prescriptions for atopic dermatitis in Europe. Allergy. 2015;70:6–11. doi: 10.1111/all.12498. [DOI] [PubMed] [Google Scholar]
- 14.Schmitt J., Schakel K., Schmitt N., Meurer M. Systemic treatment of severe atopic eczema: A systematic review. Acta Derm. Venereol. 2007;87:100–111. doi: 10.2340/00015555-0207. [DOI] [PubMed] [Google Scholar]
- 15.Beck L.A., Thaci D., Hamilton J.D., Graham N.M., Bieber T., Rocklin R., Ming J.E., Ren H., Kao R., Simpson E., et al. Dupilumab treatment in adults with moderate-to-severe atopic dermatitis. N. Engl. J. Med. 2014;371:130–139. doi: 10.1056/NEJMoa1314768. [DOI] [PubMed] [Google Scholar]
- 16.Simpson E.L., Chalmers J.R., Hanifin J.M., Thomas K.S., Cork M.J., McLean W.H., Brown S.J., Chen Z., Chen Y., Williams H.C. Emollient enhancement of the skin barrier from birth offers effective atopic dermatitis prevention. J. Allergy Clin. Immunol. 2014;134:818–823. doi: 10.1016/j.jaci.2014.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Horimukai K., Morita K., Narita M., Kondo M., Kitazawa H., Nozaki M., Shigematsu Y., Yoshida K., Niizeki H., Motomura K., et al. Application of moisturizer to neonates prevents development of atopic dermatitis. J. Allergy Clin. Immunol. 2014;134:824–830. doi: 10.1016/j.jaci.2014.07.060. [DOI] [PubMed] [Google Scholar]
- 18.Sampson H.A., Jolie P.L. Increased plasma histamine concentrations after food challenges in children with atopic dermatitis. N. Engl. J. Med. 1984;311:372–376. doi: 10.1056/NEJM198408093110605. [DOI] [PubMed] [Google Scholar]
- 19.Van Zuuren E.J., Apfelbacher C.J., Fedorowicz Z., Jupiter A., Matterne U., Weisshaar E. No high level evidence to support the use of oral H1 antihistamines as monotherapy for eczema: A summary of a Cochrane systematic review. Syst. Rev. 2014;3:25. doi: 10.1186/2046-4053-3-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nguyen T., Shapiro D.A., George S.R., Setola V., Lee D.K., Cheng R., Rauser L., Lee S.P., Lynch K.R., Roth B.L., et al. Discovery of a novel member of the histamine receptor family. Mol. Pharmacol. 2001;59:427–433. doi: 10.1124/mol.59.3.427. [DOI] [PubMed] [Google Scholar]
- 21.Drake L.A., Fallon J.D., Sober A. Relief of pruritus in patients with atopic dermatitis after treatment with topical doxepin cream. The Doxepin Study Group. J. Am. Acad. Dermatol. 1994;31:613–616. doi: 10.1016/S0190-9622(94)70225-X. [DOI] [PubMed] [Google Scholar]
- 22.Berberian B.J., Breneman D.L., Drake L.A., Gratton D., Raimir S.S., Phillips S., Sulica V.I., Bernstein J.E. The addition of topical doxepin to corticosteroid therapy: An improved treatment regimen for atopic dermatitis. Int. J. Dermatol. 1999;38:145–148. doi: 10.1046/j.1365-4362.1999.00505.x. [DOI] [PubMed] [Google Scholar]
- 23.Kabashima K. New concept of the pathogenesis of atopic dermatitis: Interplay among the barrier, allergy, and pruritus as a trinity. J. Dermatol. Sci. 2013;70:3–11. doi: 10.1016/j.jdermsci.2013.02.001. [DOI] [PubMed] [Google Scholar]
- 24.Dale H.H., Laidlaw P.P. The physiological action of beta-iminazolylethylamine. J. Physiol. 1910;41:318–344. doi: 10.1113/jphysiol.1910.sp001406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bovet D. Introduction to antihistamine agents and antergan derivative. Ann. N. Y. Acad. Sci. 1950;50:1089–1126. doi: 10.1111/j.1749-6632.1950.tb39905.x. [DOI] [PubMed] [Google Scholar]
- 26.Leurs R., Vischer H.F., Wijtmans M., de Esch I.J. En route to new blockbuster anti-histamines: Surveying the offspring of the expanding histamine receptor family. Trends Pharmacol. Sci. 2011;32:250–257. doi: 10.1016/j.tips.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 27.Kubo Y., Nakano K. Regulation of histamine synthesis in mouse CD4+ and CD8+ T lymphocytes. Inflamm. Res. 1999;48:149–153. doi: 10.1007/s000110050438. [DOI] [PubMed] [Google Scholar]
- 28.Malaviya R., Morrison A.R., Pentland A.P. Histamine in human epidermal cells is induced by ultraviolet light injury. J. Investig. Dermatol. 1996;106:785–789. doi: 10.1111/1523-1747.ep12346356. [DOI] [PubMed] [Google Scholar]
- 29.Thomas C.M., Hong T., van Pijkeren J.P., Hemarajata P., Trinh D.V., Hu W., Britton R.A., Kalkum M., Versalovic J. Histamine derived from probiotic Lactobacillus reuteri suppresses TNF via modulation of PKA and ERK signaling. PLoS ONE. 2012;7:e31951. doi: 10.1371/journal.pone.0031951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ferstl R., Frei R., Schiavi E., Konieczna P., Barcik W., Ziegler M., Lauener R.P., Chassard C., Lacroix C., Akdis C.A., et al. Histamine receptor 2 is a key influence in immune responses to intestinal histamine-secreting microbes. J. Allergy Clin. Immunol. 2014;134:744–746. doi: 10.1016/j.jaci.2014.04.034. [DOI] [PubMed] [Google Scholar]
- 31.Penders J., Stobberingh E.E., van den Brandt P.A., Thijs C. The role of the intestinal microbiota in the development of atopic disorders. Allergy. 2007;62:1223–1236. doi: 10.1111/j.1398-9995.2007.01462.x. [DOI] [PubMed] [Google Scholar]
- 32.Nakamizo S., Egawa G., Honda T., Nakajima S., Belkaid Y., Kabashima K. Commensal bacteria and cutaneous immunity. Semin. Immunopathol. 2015;37:73–80. doi: 10.1007/s00281-014-0452-6. [DOI] [PubMed] [Google Scholar]
- 33.Sanford J.A., Gallo R.L. Functions of the skin microbiota in health and disease. Semin. Immunol. 2013;25:370–377. doi: 10.1016/j.smim.2013.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gutowska-Owsiak D., Greenwald L., Watson C., Selvakumar T.A., Wang X., Ogg G.S. The histamine-synthesizing enzyme histidine decarboxylase is upregulated by keratinocytes in atopic skin. Br. J. Dermatol. 2014;171:771–778. doi: 10.1111/bjd.13199. [DOI] [PubMed] [Google Scholar]
- 35.Simons F.E., Simons K.J. The pharmacology and use of H1-receptor-antagonist drugs. N. Engl. J. Med. 1994;330:1663–1670. doi: 10.1056/NEJM199406093302307. [DOI] [PubMed] [Google Scholar]
- 36.Gutzmer R., Gschwandtner M., Rossbach K., Mommert S., Werfel T., Kietzmann M., Baeumer W. Pathogenetic and therapeutic implications of the histamine H4 receptor in inflammatory skin diseases and pruritus. Front. Biosci. (Schol. Ed.) 2011;3:985–994. doi: 10.2741/203. [DOI] [PubMed] [Google Scholar]
- 37.Chimalakonda K.C., Pang E., Weaver J.L., Howard K.E., Patel V., Boyne M.T., 2nd Development and validation of a liquid-chromatography tandem mass spectrometry method to determine in vitro and in vivo histamine release. J. Pharm. Biomed. Anal. 2014;102:494–499. doi: 10.1016/j.jpba.2014.10.016. [DOI] [PubMed] [Google Scholar]
- 38.Ash A.S., Schild H.O. Receptors mediating some actions of histamine. Br. J. Pharmacol. Chemother. 1966;27:427–439. doi: 10.1111/j.1476-5381.1966.tb01674.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lovenberg T.W., Roland B.L., Wilson S.J., Jiang X., Pyati J., Huvar A., Jackson M.R., Erlander M.G. Cloning and functional expression of the human histamine H3 receptor. Mol. Pharmacol. 1999;55:1101–1107. [PubMed] [Google Scholar]
- 40.Oda T., Morikawa N., Saito Y., Masuho Y., Matsumoto S. Molecular cloning and characterization of a novel type of histamine receptor preferentially expressed in leukocytes. J. Biol. Chem. 2000;275:36781–36786. doi: 10.1074/jbc.M006480200. [DOI] [PubMed] [Google Scholar]
- 41.Arrang J.M., Garbarg M., Schwartz J.C. Auto-inhibition of brain histamine release mediated by a novel class (H3) of histamine receptor. Nature. 1983;302:832–837. doi: 10.1038/302832a0. [DOI] [PubMed] [Google Scholar]
- 42.O’Mahony L., Akdis M., Akdis C.A. Regulation of the immune response and inflammation by histamine and histamine receptors. J. Allergy Clin. Immunol. 2011;128:1153–1162. doi: 10.1016/j.jaci.2011.06.051. [DOI] [PubMed] [Google Scholar]
- 43.Sander L.E., Lorentz A., Sellge G., Coeffier M., Neipp M., Veres T., Frieling T., Meier P.N., Manns M.P., Bischoff S.C. Selective expression of histamine receptors H1R, H2R, and H4R, but not H3R, in the human intestinal tract. Gut. 2006;55:498–504. doi: 10.1136/gut.2004.061762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mommert S., Gschwandtner M., Koether B., Gutzmer R., Werfel T. Human memory Th17 cells express a functional histamine H4 receptor. Am. J. Pathol. 2012;180:177–185. doi: 10.1016/j.ajpath.2011.09.028. [DOI] [PubMed] [Google Scholar]
- 45.Baumer W., Wendorff S., Gutzmer R., Werfel T., Dijkstra D., Chazot P., Stark H., Kietzmann M. Histamine H4 receptors modulate dendritic cell migration through skin—Immunomodulatory role of histamine. Allergy. 2008;63:1387–1394. doi: 10.1111/j.1398-9995.2008.01720.x. [DOI] [PubMed] [Google Scholar]
- 46.Dunford P.J., O’Donnell N., Riley J.P., Williams K.N., Karlsson L., Thurmond R.L. The histamine H4 receptor mediates allergic airway inflammation by regulating the activation of CD4+ T cells. J. Immunol. 2006;176:7062–7070. doi: 10.4049/jimmunol.176.11.7062. [DOI] [PubMed] [Google Scholar]
- 47.Jemima E.A., Prema A., Thangam E.B. Functional characterization of histamine H4 receptor on human mast cells. Mol. Immunol. 2014;62:19–28. doi: 10.1016/j.molimm.2014.05.007. [DOI] [PubMed] [Google Scholar]
- 48.O’Reilly M., Alpert R., Jenkinson S., Gladue R.P., Foo S., Trim S., Peter B., Trevethick M., Fidock M. Identification of a histamine H4 receptor on human eosinophils—Role in eosinophil chemotaxis. J. Recept. Signal Transduct. Res. 2002;22:431–448. doi: 10.1081/RRS-120014612. [DOI] [PubMed] [Google Scholar]
- 49.Gschwandtner M., Rossbach K., Dijkstra D., Baumer W., Kietzmann M., Stark H., Werfel T., Gutzmer R. Murine and human Langerhans cells express a functional histamine H4 receptor: Modulation of cell migration and function. Allergy. 2010;65:840–849. doi: 10.1111/j.1398-9995.2009.02279.x. [DOI] [PubMed] [Google Scholar]
- 50.Ohtani T., Aiba S., Mizuashi M., Mollah Z.U., Nakagawa S., Tagami H. H1 and H2 histamine receptors are absent on Langerhans cells and present on dermal dendritic cells. J. Investig. Dermatol. 2003;121:1073–1079. doi: 10.1046/j.1523-1747.2003.12570.x. [DOI] [PubMed] [Google Scholar]
- 51.Glatzer F., Gschwandtner M., Ehling S., Rossbach K., Janik K., Klos A., Baumer W., Kietzmann M., Werfel T., Gutzmer R. Histamine induces proliferation in keratinocytes from patients with atopic dermatitis through the histamine 4 receptor. J. Allergy Clin. Immunol. 2013;132:1358–1367. doi: 10.1016/j.jaci.2013.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.MacGlashan D., Jr. Histamine: A mediator of inflammation. J. Allergy Clin. Immunol. 2003;112:S53–S59. doi: 10.1016/S0091-6749(03)01877-3. [DOI] [PubMed] [Google Scholar]
- 53.Jutel M., Akdis M., Akdis C.A. Histamine, histamine receptors and their role in immune pathology. Clin. Exp. Allergy. 2009;39:1786–1800. doi: 10.1111/j.1365-2222.2009.03374.x. [DOI] [PubMed] [Google Scholar]
- 54.Akdis C.A., Simons F.E. Histamine receptors are hot in immunopharmacology. Eur. J. Pharmacol. 2006;533:69–76. doi: 10.1016/j.ejphar.2005.12.044. [DOI] [PubMed] [Google Scholar]
- 55.Lim H.D., van Rijn R.M., Ling P., Bakker R.A., Thurmond R.L., Leurs R. Evaluation of histamine H1-, H2-, and H3-receptor ligands at the human histamine H4 receptor: Identification of 4-methylhistamine as the first potent and selective H4 receptor agonist. J. Pharmacol. Exp. Ther. 2005;314:1310–1321. doi: 10.1124/jpet.105.087965. [DOI] [PubMed] [Google Scholar]
- 56.Leurs R., Chazot P.L., Shenton F.C., Lim H.D., de Esch I.J. Molecular and biochemical pharmacology of the histamine H4 receptor. Br. J. Pharmacol. 2009;157:14–23. doi: 10.1111/j.1476-5381.2009.00250.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Morse K.L., Behan J., Laz T.M., West R.E., Jr., Greenfeder S.A., Anthes J.C., Umland S., Wan Y., Hipkin R.W., Gonsiorek W., et al. Cloning and characterization of a novel human histamine receptor. J. Pharmacol. Exp. Ther. 2001;296:1058–1066. [PubMed] [Google Scholar]
- 58.Horr B., Borck H., Thurmond R., Grosch S., Diel F. STAT1 phosphorylation and cleavage is regulated by the histamine (H4) receptor in human atopic and non-atopic lymphocytes. Int. Immunopharmacol. 2006;6:1577–1585. doi: 10.1016/j.intimp.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 59.Michel I., Borck H., McElligott S., Krieg C., Diel F. Histamine receptor H4R-selective ligands influence the STAT6 transcription activation domain (TAD) and the DNA-binding. Inflamm. Res. 2008;57:S47–S48. doi: 10.1007/s00011-007-0623-1. [DOI] [PubMed] [Google Scholar]
- 60.Kuhne S., Wijtmans M., Lim H.D., Leurs R., de Esch I.J. Several down, a few to go: Histamine H3 receptor ligands making the final push towards the market? Expert Opin. Investig. Drugs. 2011;20:1629–1648. doi: 10.1517/13543784.2011.625010. [DOI] [PubMed] [Google Scholar]
- 61.Kiss R., Keseru G.M. Novel histamine H4 receptor ligands and their potential therapeutic applications: An update. Expert Opin. Ther. Pat. 2014;24:1185–1197. doi: 10.1517/13543776.2014.959494. [DOI] [PubMed] [Google Scholar]
- 62.Van der Pol R., Langendam M., Benninga M., van Wijk M., Tabbers M. Efficacy and safety of histamine-2 receptor antagonists. JAMA Pediatri. 2014;168:947–954. doi: 10.1001/jamapediatrics.2014.1273. [DOI] [PubMed] [Google Scholar]
- 63.Simons F.E., Simons K.J. H1 antihistamines: Current status and future directions. World Allergy Organ. J. 2008;1:145–155. doi: 10.1097/WOX.0b013e318186fb3a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bielory L., Ghafoor S. Histamine receptors and the conjunctiva. Curr. Opin. Allergy Clin. Immunol. 2005;5:437–440. doi: 10.1097/01.all.0000183113.63311.11. [DOI] [PubMed] [Google Scholar]
- 65.Amano T., Takeda T., Yano H., Tamura T. Olopatadine hydrochloride accelerates the recovery of skin barrier function in mice. Br. J. Dermatol. 2007;156:906–912. doi: 10.1111/j.1365-2133.2007.07796.x. [DOI] [PubMed] [Google Scholar]
- 66.Higashi M., Ohsawa I., Oda F., Yamada Y., Kawana S., Iida K., Mitsuishi T. Histamine H1-receptor antagonistic drug olopatadine suppresses TSLP in atopic dermatitis model mice. Allergol. Int. 2013;62:137–138. doi: 10.2332/allergolint.12-LE-0466. [DOI] [PubMed] [Google Scholar]
- 67.Ito T., Fujiyama T., Hashizume H., Tokura Y. Antihistaminic drug olopatadine downmodulates T cell chemotaxis toward CXCL10 by reducing CXCR3 expression, F-actin polymerization and calcium influx in patients with alopecia areata. J. Dermatol. Sci. 2013;72:68–71. doi: 10.1016/j.jdermsci.2013.04.029. [DOI] [PubMed] [Google Scholar]
- 68.Murota H., El-latif M.A., Tamura T., Katayama I. Olopatadine hydrochloride decreases tissue interleukin-31 levels in an atopic dermatitis mouse model. Acta Derm. Venereol. 2014;94:78–79. doi: 10.2340/00015555-1648. [DOI] [PubMed] [Google Scholar]
- 69.Tamura T., Matsubara M., Amano T., Chida M. Olopatadine ameliorates rat experimental cutaneous inflammation by improving skin barrier function. Pharmacology. 2008;81:118–126. doi: 10.1159/000110112. [DOI] [PubMed] [Google Scholar]
- 70.Ohmori K., Hayashi K., Kaise T., Ohshima E., Kobayashi S., Yamazaki T., Mukouyama A. Pharmacological, pharmacokinetic and clinical properties of olopatadine hydrochloride, a new antiallergic drug. Jpn. J. Pharmacol. 2002;88:379–397. doi: 10.1254/jjp.88.379. [DOI] [PubMed] [Google Scholar]
- 71.Hofstra C.L., Desai P.J., Thurmond R.L., Fung-Leung W.P. Histamine H4 receptor mediates chemotaxis and calcium mobilization of mast cells. J. Pharmacol. Exp. Ther. 2003;305:1212–1221. doi: 10.1124/jpet.102.046581. [DOI] [PubMed] [Google Scholar]
- 72.Morgan R.K., McAllister B., Cross L., Green D.S., Kornfeld H., Center D.M., Cruikshank W.W. Histamine 4 receptor activation induces recruitment of FOXP3+ T cells and inhibits allergic asthma in a murine model. J. Immunol. 2007;178:8081–8089. doi: 10.4049/jimmunol.178.12.8081. [DOI] [PubMed] [Google Scholar]
- 73.Gutzmer R., Diestel C., Mommert S., Kother B., Stark H., Wittmann M., Werfel T. Histamine H4 receptor stimulation suppresses IL-12p70 production and mediates chemotaxis in human monocyte-derived dendritic cells. J. Immunol. 2005;174:5224–5232. doi: 10.4049/jimmunol.174.9.5224. [DOI] [PubMed] [Google Scholar]
- 74.Dijkstra D., Leurs R., Chazot P., Shenton F.C., Stark H., Werfel T., Gutzmer R. Histamine downregulates monocyte CCL2 production through the histamine H4 receptor. J. Allergy Clin. Immunol. 2007;120:300–307. doi: 10.1016/j.jaci.2007.03.024. [DOI] [PubMed] [Google Scholar]
- 75.Thurmond R.L., Gelfand E.W., Dunford P.J. The role of histamine H1 and H4 receptors in allergic inflammation: The search for new antihistamines. Nat. Rev. Drug Discov. 2008;7:41–53. doi: 10.1038/nrd2465. [DOI] [PubMed] [Google Scholar]
- 76.Ohsawa Y., Hirasawa N. The role of histamine H1 and H4 receptors in atopic dermatitis: From basic research to clinical study. Allergol. Int. 2014;63:533–542. doi: 10.2332/allergolint.13-RA-0675. [DOI] [PubMed] [Google Scholar]
- 77.Murata Y., Song M., Kikuchi H., Hisamichi K., Xu X.L., Greenspan A., Kato M., Chiou C.F., Kato T., Guzzo C., et al. Phase 2a, randomized, double-blind, placebo-controlled, multicenter, parallel-group study of a H4 R-antagonist (JNJ-39758979) in Japanese adults with moderate atopic dermatitis. J. Dermatol. 2014;42:129–139. doi: 10.1111/1346-8138.12726. [DOI] [PubMed] [Google Scholar]
- 78.Gschwandtner M., Mildner M., Mlitz V., Gruber F., Eckhart L., Werfel T., Gutzmer R., Elias P.M., Tschachler E. Histamine suppresses epidermal keratinocyte differentiation and impairs skin barrier function in a human skin model. Allergy. 2013;68:37–47. doi: 10.1111/all.12051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tamura T., Komai M. Effect of olopatadine hydrochloride, an anti-histamine drug, on rhinitis induced by intranasal instillation of toluene-2,4-diisocyanate in rats. Int. Immunopharmacol. 2008;8:916–921. doi: 10.1016/j.intimp.2008.02.009. [DOI] [PubMed] [Google Scholar]
- 80.Ashida Y., Denda M., Hirao T. Histamine H1 and H2 receptor antagonists accelerate skin barrier repair and prevent epidermal hyperplasia induced by barrier disruption in a dry environment. J. Investig. Dermatol. 2001;116:261–265. doi: 10.1046/j.1523-1747.2001.01238.x. [DOI] [PubMed] [Google Scholar]
- 81.Lin T.K., Man M.Q., Santiago J.L., Park K., Roelandt T., Oda Y., Hupe M., Crumrine D., Lee H.J., Gschwandtner M., et al. Topical antihistamines display potent anti-inflammatory activity linked in part to enhanced permeability barrier function. J. Investig. Dermatol. 2013;133:469–478. doi: 10.1038/jid.2012.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zabner J., Winter M., Excoffon K.J., Stoltz D., Ries D., Shasby S., Shasby M. Histamine alters E-cadherin cell adhesion to increase human airway epithelial permeability. J. Appl. Physiol. 2003;95:394–401. doi: 10.1152/japplphysiol.01134.2002. [DOI] [PubMed] [Google Scholar]
- 83.Srinivas S.P., Satpathy M., Guo Y., Anandan V. Histamine-induced phosphorylation of the regulatory light chain of myosin II disrupts the barrier integrity of corneal endothelial cells. Investig. Ophthalmol. Vis. Sci. 2006;47:4011–4018. doi: 10.1167/iovs.05-1127. [DOI] [PubMed] [Google Scholar]
- 84.Takeuchi K., Kishioka C., Ishinaga H., Sakakura Y., Majima Y. Histamine alters gene expression in cultured human nasal epithelial cells. J. Allergy Clin. Immunol. 2001;107:310–314. doi: 10.1067/mai.2001.112127. [DOI] [PubMed] [Google Scholar]
- 85.Kuo I.H., Carpenter-Mendini A., Yoshida T., McGirt L.Y., Ivanov A.I., Barnes K.C., Gallo R.L., Borkowski A.W., Yamasaki K., Leung D.Y., et al. Activation of epidermal toll-like receptor 2 enhances tight junction function: Implications for atopic dermatitis and skin barrier repair. J. Investig. Dermatol. 2013;133:988–998. doi: 10.1038/jid.2012.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kanda N., Watanabe S. Histamine enhances the production of human beta-defensin-2 in human keratinocytes. Am. J. Physiol. Cell Physiol. 2007;293:C1916–C1923. doi: 10.1152/ajpcell.00293.2007. [DOI] [PubMed] [Google Scholar]
- 87.Niyonsaba F., Ushio H., Nakano N., Ng W., Sayama K., Hashimoto K., Nagaoka I., Okumura K., Ogawa H. Antimicrobial peptides human beta-defensins stimulate epidermal keratinocyte migration, proliferation and production of proinflammatory cytokines and chemokines. J. Investig. Dermatol. 2007;127:594–604. doi: 10.1038/sj.jid.5700599. [DOI] [PubMed] [Google Scholar]
- 88.Danso M.O., van Drongelen V., Mulder A., van Esch J., Scott H., van Smeden J., El Ghalbzouri A., Bouwstra J.A. TNF-alpha and Th2 cytokines induce atopic dermatitis-like features on epidermal differentiation proteins and stratum corneum lipids in human skin equivalents. J. Investig. Dermatol. 2014;134:1941–1950. doi: 10.1038/jid.2014.83. [DOI] [PubMed] [Google Scholar]
- 89.Howell M.D., Kim B.E., Gao P., Grant A.V., Boguniewicz M., Debenedetto A., Schneider L., Beck L.A., Barnes K.C., Leung D.Y. Cytokine modulation of atopic dermatitis filaggrin skin expression. J. Allergy Clin. Immunol. 2007;120:150–155. doi: 10.1016/j.jaci.2007.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gutowska-Owsiak D., Schaupp A.L., Salimi M., Taylor S., Ogg G.S. Interleukin-22 downregulates filaggrin expression and affects expression of profilaggrin processing enzymes. Br. J. Dermatol. 2011;165:492–498. doi: 10.1111/j.1365-2133.2011.10400.x. [DOI] [PubMed] [Google Scholar]
- 91.Thyssen J.P., Kezic S. Causes of epidermal filaggrin reduction and their role in the pathogenesis of atopic dermatitis. J. Allergy Clin. Immunol. 2014;134:792–799. doi: 10.1016/j.jaci.2014.06.014. [DOI] [PubMed] [Google Scholar]