Abstract
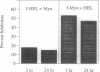
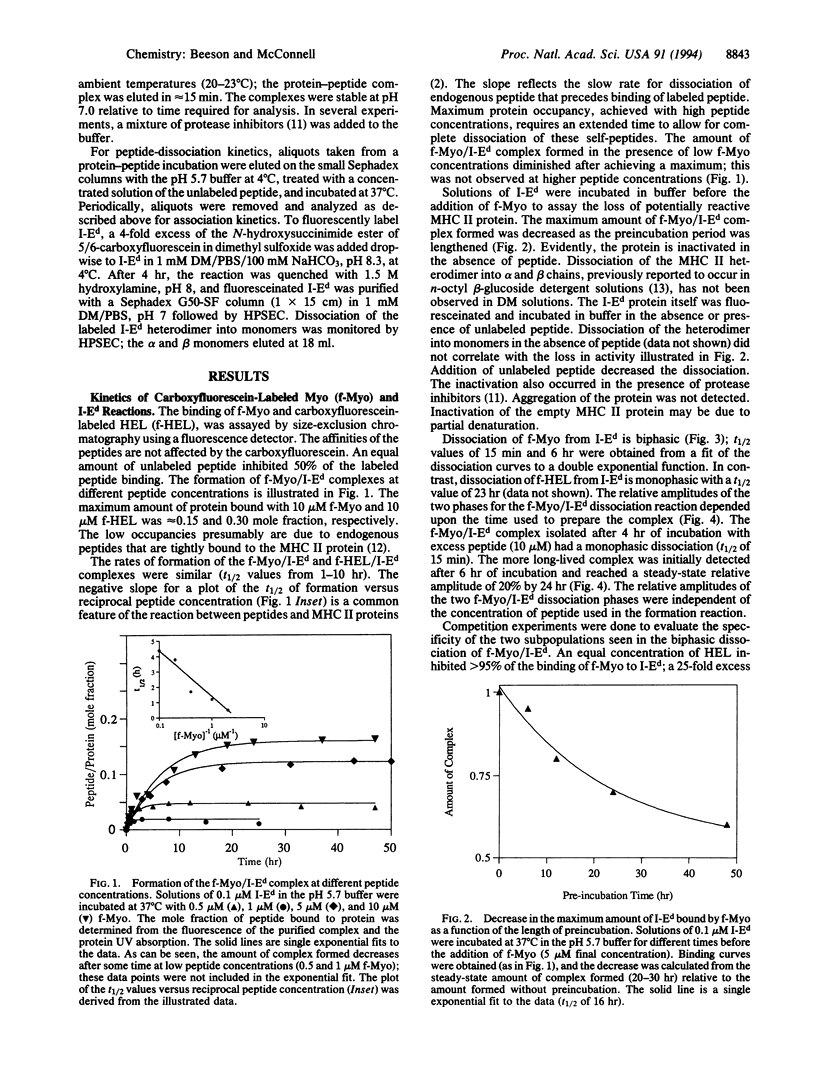
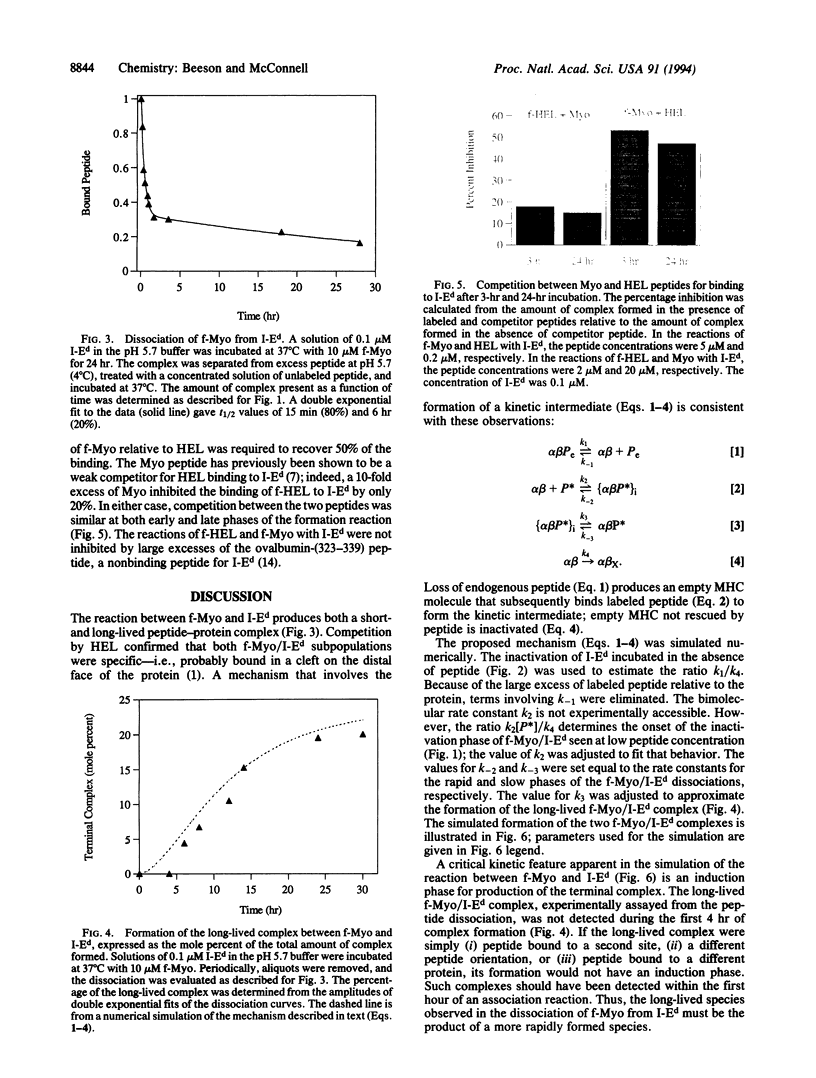
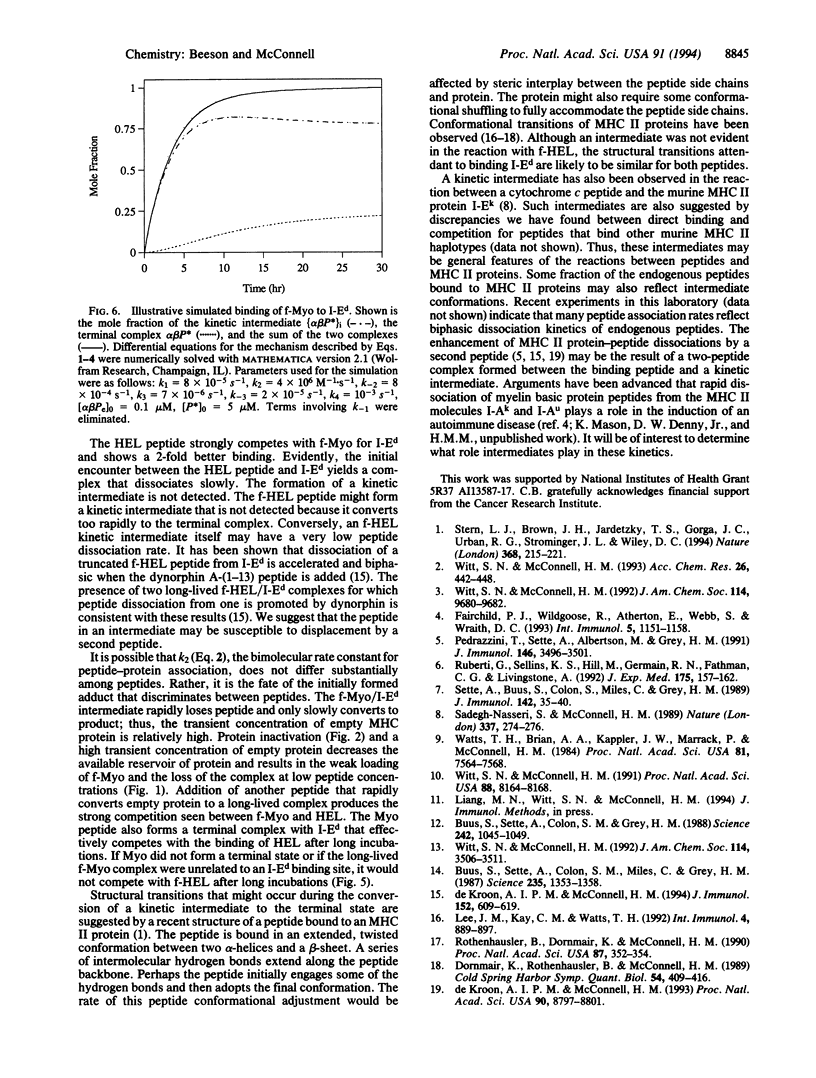
The kinetics of the reactions between fluorescently labeled sperm whale myoglobin-(110-121) peptide and the murine major histocompatibility complex class II protein I-Ed have been analyzed. The presence in solution of both short- and long-lived protein-peptide complexes is demonstrated by the biphasic dissociation of the myoglobin peptide from I-Ed. The formation of the long-lived terminal complex is preceded by a characteristic induction phase. It is shown that the initially formed complex of the myoglobin peptide and I-Ed is a kinetic intermediate that undergoes a unimolecular reaction to form the terminal complex. Reactions between peptides and the class II proteins thus involve an intermediate structurally distinct from the terminal complex. The terminal complex presumably has a structure that is biologically active and similar to the published class II protein-peptide crystal structure.
Full text
PDF
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Buus S., Sette A., Colon S. M., Grey H. M. Autologous peptides constitutively occupy the antigen binding site on Ia. Science. 1988 Nov 18;242(4881):1045–1047. doi: 10.1126/science.3194755. [DOI] [PubMed] [Google Scholar]
- Buus S., Sette A., Colon S. M., Miles C., Grey H. M. The relation between major histocompatibility complex (MHC) restriction and the capacity of Ia to bind immunogenic peptides. Science. 1987 Mar 13;235(4794):1353–1358. doi: 10.1126/science.2435001. [DOI] [PubMed] [Google Scholar]
- Dornmair K., Rothenhäusler B., McConnell H. M. Structural intermediates in the reactions of antigenic peptides with MHC molecules. Cold Spring Harb Symp Quant Biol. 1989;54(Pt 1):409–416. doi: 10.1101/sqb.1989.054.01.050. [DOI] [PubMed] [Google Scholar]
- Fairchild P. J., Wildgoose R., Atherton E., Webb S., Wraith D. C. An autoantigenic T cell epitope forms unstable complexes with class II MHC: a novel route for escape from tolerance induction. Int Immunol. 1993 Sep;5(9):1151–1158. doi: 10.1093/intimm/5.9.1151. [DOI] [PubMed] [Google Scholar]
- Lee J. M., Kay C. M., Watts T. H. Conformational changes in mouse MHC class II proteins at acidic pH. Int Immunol. 1992 Aug;4(8):889–897. doi: 10.1093/intimm/4.8.889. [DOI] [PubMed] [Google Scholar]
- Pedrazzini T., Sette A., Albertson M., Grey H. M. Free ligand-induced dissociation of MHC-antigen complexes. J Immunol. 1991 May 15;146(10):3496–3501. [PubMed] [Google Scholar]
- Rothenhäusler B., Dornmair K., McConnell H. M. Specific binding of antigenic peptides to separate alpha and beta chains of class II molecules of the major histocompatibility complex. Proc Natl Acad Sci U S A. 1990 Jan;87(1):352–354. doi: 10.1073/pnas.87.1.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruberti G., Sellins K. S., Hill C. M., Germain R. N., Fathman C. G., Livingstone A. Presentation of antigen by mixed isotype class II molecules in normal H-2d mice. J Exp Med. 1992 Jan 1;175(1):157–162. doi: 10.1084/jem.175.1.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadegh-Nasseri S., McConnell H. M. A kinetic intermediate in the reaction of an antigenic peptide and I-Ek. Nature. 1989 Jan 19;337(6204):274–276. doi: 10.1038/337274a0. [DOI] [PubMed] [Google Scholar]
- Sette A., Buus S., Colon S., Miles C., Grey H. M. Structural analysis of peptides capable of binding to more than one Ia antigen. J Immunol. 1989 Jan 1;142(1):35–40. [PubMed] [Google Scholar]
- Stern L. J., Brown J. H., Jardetzky T. S., Gorga J. C., Urban R. G., Strominger J. L., Wiley D. C. Crystal structure of the human class II MHC protein HLA-DR1 complexed with an influenza virus peptide. Nature. 1994 Mar 17;368(6468):215–221. doi: 10.1038/368215a0. [DOI] [PubMed] [Google Scholar]
- Watts T. H., Brian A. A., Kappler J. W., Marrack P., McConnell H. M. Antigen presentation by supported planar membranes containing affinity-purified I-Ad. Proc Natl Acad Sci U S A. 1984 Dec;81(23):7564–7568. doi: 10.1073/pnas.81.23.7564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witt S. N., McConnell H. M. A first-order reaction controls the binding of antigenic peptides to major histocompatibility complex class II molecules. Proc Natl Acad Sci U S A. 1991 Sep 15;88(18):8164–8168. doi: 10.1073/pnas.88.18.8164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Kroon A. I., McConnell H. M. Enhancement of peptide antigen presentation by a second peptide. Proc Natl Acad Sci U S A. 1993 Oct 1;90(19):8797–8801. doi: 10.1073/pnas.90.19.8797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Kroon A. I., McConnell H. M. Kinetics and specificity of peptide-MHC class II complex displacement reactions. J Immunol. 1994 Jan 15;152(2):609–619. [PubMed] [Google Scholar]