Abstract
Background
Risk for coronary heart disease (CHD) differs by sex, and accumulating evidence suggests sex differences in the effect of coronary risk factors on vascular risk. To date, the existence of a sex difference in the relationship between body mass index (BMI) with CHD has yet to be systematically examined. As sexual dimorphisms in body composition exist, we hypothesized that the relationship between body mass index (BMI) and CHD would differ in women and men.
Methods
From systematic searches of PubMed and EMBASE up to February 2015, we identified 32 published studies of the longitudinal association between BMI and CHD in women and men from population-based cohorts. We also included individual participant data from four large studies. Study results were pooled using random effect models with inverse variance weighting.
Findings
Data from 95 cohorts, 1,219,187 participants, and 37,488 incident cases of CHD were included. Higher BMI was significantly associated with age-adjusted incident CHD: hazard ratios (95% confidence interval) in women and men were 1.04 (1.03–1.05) and 1.05 (1.04–1.07) for one unit BMI, 1.25(1.05–1.49), 1.09(0.91–1.23) for underweight, 1.20 (1.12–1.29), 1.22 (1.12–1.32) for overweight, 1.61 (1.42–1.82) and 1.60 (1.43–1.79) for obesity, respectively). Overall, there was no sex difference in these associations. The women-to-men ratio of the hazard ratios were 0.99 (0.98–1.00) for one unit BMI; 1.08 (0.89–1.31) for underweight; 1.00(0.92–1.07) for overweight; and, 1.05(0.94–1.17) for obesity. Similar results were obtained after multiple-adjustment and in a range of sensitivity analyses.
Interpretation
Higher BMI, measured continuously and categorically, has the same deleterious effects on risk of incident CHD in women and men across diverse populations.
INTRODUCTION
Excess body weight is considered to be one of the most important modifiable risk factors for chronic disease.1–4 Indeed, a strong and continuous association between body mass index (BMI) and coronary heart disease (CHD) has been reported for values of BMI above 20 kg/m2.1 Reliable estimates of both the prevalence of overweight and obesity and the relative risks associated with the condition have become the cornerstones for epidemiologic modelling of the current and projected burden of obesity-related disease. In 2013, an estimated 36.9% of men and 38.0% of women were overweight (BMI >25 kg/m2) worldwide,5 with attributable fractions for CHD as high as 25% in the United States and 58% in the Asia-Pacific Region.6–7
Such estimates are predicated on the assumption that the relationship between BMI and CHD is similar between the sexes, and as such only a single estimate of the relationship is used in predicting the burden of overweight-related disease. However, this may be incorrect as it is becoming increasingly recognised that there are important and clinically meaningful sex differences in the relationships between risk factors and cardiovascular disease – most often to the detriment of women. For example, type 1 diabetes, type 2 diabetes and cigarette smoking have recently been demonstrated to confer significantly greater vascular hazards in women than in men,8–10 whereas the effect of blood pressure on cardiovascular risk is comparable between the sexes.11 Given that sexual dimorphism in the distribution of underlying fat composition is well established,12–13 and that there is a predominance of subcutaneous fat in women - which confers less cardiometabolic risk relative to visceral fat – this may imply a higher relative risk of CHD for men with the same level of BMI.
Although previous reviews have largely reported no sex difference in the relative risk between BMI and CHD, these studies did not specifically compare women and men from within the same study.1–3–14 These estimates may be confounded due to differences in source population and variation in background risk which may have masked a true sex difference in the association. Therefore, we conducted a systematic review with meta-analysis of only those prospective cohort studies that reported sex-specific estimates of the relationship between BMI and CHD in the general population. We hypothesize that higher BMI will be associated with increased risk of CHD, with a stronger association for men than for women.
METHODS
Search Strategy and Selection Criteria
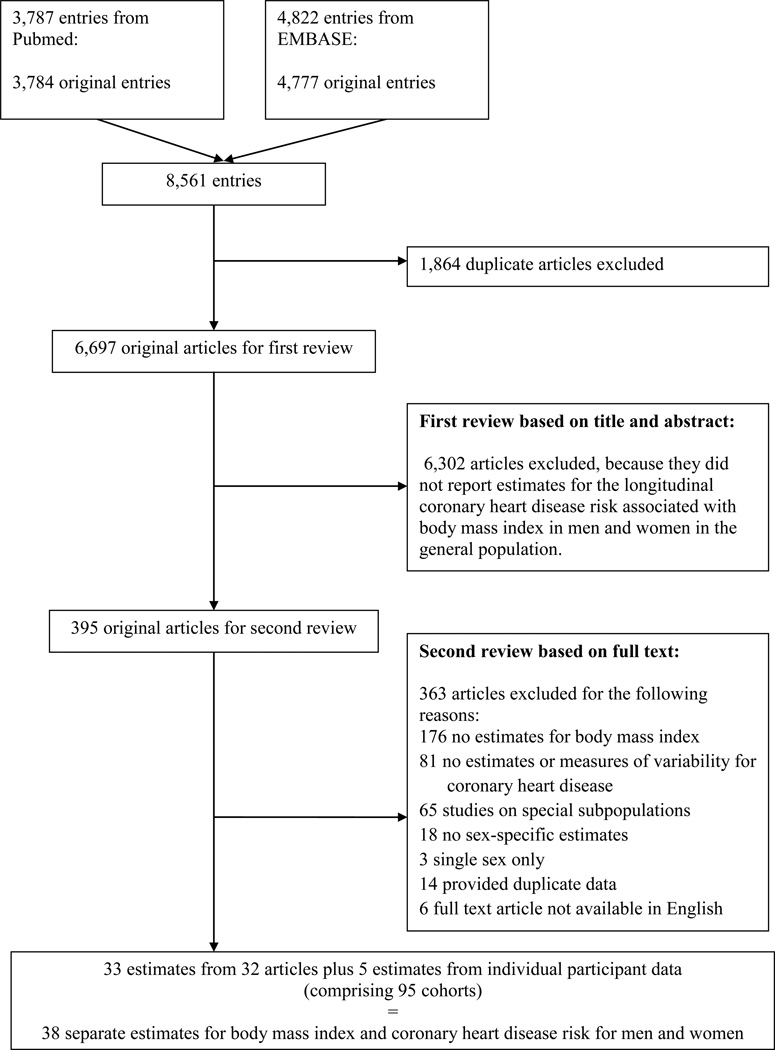
We systematically searched Pubmed and EMBASE for records relating to the longitudinal association between BMI and CHD in women and men in the general population up to February 20th, 2015. The full search criteria used for both sources is available in the Supplemental Methods S1. We excluded studies based on the following criteria: duplicate data from the same study; estimates reported only for z-scores or percentiles of BMI; no report of estimate uncertainty; no report of sex-specific estimates; studies which recruited predominantly from individuals with a prior history of cardiovascular disease or from with selected populations, such as those with kidney disease, diabetes, or hypertension; and articles where the full text was not available in English (Figure 1 and Supplemental Methods S2). All studies included adjustment by age.
Figure 1.
Flowchart of study inclusion/exclusion
A total of 8,561 original entries were reviewed twice for inclusion in the analysis. For the first review, two reviewers (MM-C and SP) split the entries and reviewed titles and abstracts with a conservative view towards including studies until a full text review indicated that estimates for BMI and CHD by sex were not available. For the second review, two reviewers independently graded the full text of all remaining entries. The two reviewers cross-validated their results, and RRH acted as third reviewer when there were discrepancies.
Where estimates from multiple reports on the same cohort were available, we prioritized articles in the following order: longest follow-up time; most complete set of estimates; and reporting the most complete set of secondary information . Where studies reported estimates for more than one group and those estimates were heterogeneous, we kept the reported values as separate estimates.15 In contrast, we combined homogeneous estimates from the same study. For instance we combined the estimates for ages 30–59 and 60–79 years in the Bergen study.16
We also included estimates from individual participant data, which were available to us from the Atherosclerosis Risk in Communities study (ARIC), the Asia Pacific Cohort Studies Collaboration (APCSC), the third National Health and Nutrition Examination Survey (NHANES III), and the Scottish Heart Health Extended Cohort Study (SHHEC). We did not solicit raw data from any studies identified in the systematic review. Articles based on studies from which we already had individual participant data were excluded.
Statistical Analysis
Our pre-defined primary endpoint was the pooled women-to-men ratio of the age-adjusted hazard ratios (HRs), or equivalent, relating (continuous and categorical) BMI to incident CHD, with a secondary endpoint as the same ratio after multiple adjustments (allowed to vary across studies). Age-adjusted analyses were used as our primary results because the effect of BMI is chiefly mediated through classical coronary risk factors, including high blood pressure but excluding smoking, leading to over-adjustment.17 Included studies generally reported estimates for CHD risk per unit difference in BMI or by BMI categories. Supplemental Methods S3 describes how estimates were reconciled when studies used different units or cut-points.
For each study, we extracted the HRs for women and men, and their 95% confidence intervals (CI). All studies used HRs, except two studies, which used odds ratios (Table 1). From these, the log HRs and the women-to-men difference in log HRs were computed, together with 95% CIs, assuming a normal distribution. These were pooled using random effects meta-analysis with inverse variance weighting.18 Results were back-transformed to the raw scale, giving pooled HRs and pooled women-to-men ratios of HRs (HRR). We investigated between-study heterogeneity using the I2 statistic, Cochran’s Q test, and meta-regression analyses (on study start date, length of follow-up, total sample size, number of events, the percent female, and study quality), and used funnel plots to assess publication bias.19 The quality of the included studies was assessed using the Newcastle-Ottawa Scale (Supplementary methods S4).20 All analyses were conducted using Stata 11.0.21
Table 1.
Characteristics of included studies
| Cohort | Study Start (year) | Country | Follow-up (years) | Age Range (years) | BMI Measure
(Self- report or Measured) |
Outcome (Fatal
only or All incidence) |
Total N (Percent Women) |
Total Events (Percent Women) |
Prevalent CVD? | Sex-specific Cut-points | BMI Units
(Categories only, One Unit only, Both) |
Normal weight only
as reference |
Overweight and
Obese Combined? |
Maximum adjustment variables |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adventist Health1 | 1976 | USA | 12 | 55-84 | S | F | 20346 (65) | 522 (70) | No | Yes | Cat | Yes | Yes | Age |
| Asia Pacific Cohort
Studies Collaboration (APCSC)-ANZ2* |
1961 | Pool of 9 cohorts |
9 | 20-104 | M | A | 98229 (45) | 3729 (30) | No | No | Both | Yes | No | Age, sbp, tc, hdl, smoking |
| Asia Pacific Cohort
Studies Collaboration (APCSC)--Asia2* |
1961 | Pool of 35 cohorts |
7 | 20-107 | M | A | 331292 (40) | 1391 (29) | No | No | Both | Yes | No | Age, sbp, tc, hdl, smoking |
| Atherosclerosis Risk in
Communities (ARIC)3* |
1987 | USA | 19 | 45-66 | M | A | 14753 (57) | 1616 (42) | No | No | Both | Yes | No | Age, ethnicity, smoking, alcohol, family history |
| Bergen4 | 1963 | Norway | 27 | 30-79 | M | F | 51475 (59) | 3136 (34) | No | No | Unit | Age | ||
| Buffalo Health5 | 1960 | USA | 28 | 20-96 | M | F | 1308 (53) | 190 (43) | No | No | Unit | Age, education, smoking, mean arterial blood pressure |
||
| Consultation Bureau Project6 | 1974 | The Netherlands |
12 | 30-54 | M | F | 49094 (53) | 232 (16) | No | No | Cat | Yes | No | Age |
| Copenhagen City Heart7 | 1976 | Denmark | 14 | 20-93 | M | A | 4417 | 1724 (44) | No | No | Both | No | No | Age, Hypertension, hypercholesterole mia, diabetes |
| Danish Diet, Cancer,and Health8,9 | 1993 | Denmark | 7.7 | 50-64 | M | A | 54783 (53) | 1127 (27) | No | No | Both | No | No | Age, smoking status, fruit and vegetable intake, alcohol consumption, physical activity, total energy intake and educational level. Women also adjusted for hormone replacement therapy and menopausal status. |
| European Prospective Investigation
into Cancer (EPIC-Norfolk)10 |
1993 | UK | 9.1 | 45-79 | M | A | 24508 (55) | 2600 (34) | No | No | Both | No | No | Age, systolic blood pressure, total cholesterol, cigarette smoking, physical activity and alcohol intake |
| Fiji (Indian)11 | 1980 | Fiji | 11 | 30-69 | M | F | 1196 (53) | 31 (32) | No | No | Unit | Age, 2hour glucose, sbp, cholesterol, smoking, physical activity, rural |
||
| Fiji (Melanesian)11 | 1980 | Fiji | 11 | 30-69 | M | F | 1311 (53) | 31 (32) | No | No | Unit | Age, 2hour glucose, sbp, cholesterol, smoking, physical activity, rural |
||
| Finnish Twin12 | 1981 | Finland | 22 | 24-60 | M | F | 15424 (53) | 220 (19) | No | Yes | Unit | Age, leisure time physical activity, smoking, hypertension, binge drinking, life satisfaction, income, schooling, social class |
||
| Framingham Heart13 | 1948 | USA | 44 | 30-62 | M | A | 4255 (56) | 1065 | No | No | Cat | Yes | No | Age,
smoking, hypertension, hypercholesterole mia, diabetes |
| Göteborg±14 | 1971 | Sweden | 15 | 70 | M | A | 1597 (54) | 684 (47) | No | Yes | Both | No | No | Age, Cohort, smoking, SBP, TC |
| Groningen Longitudinal Aging15 | 1993 | The Netherlands |
5 | 57+ | S | A | 5279 | 472 (46) | Yes | No | Unit | Age,
smoking, depressive symptoms, heart disease, hypertension, diabetes, education, chronic medical conditions |
||
| Hawaii Multiethnic Prospective Cohort16 | 1975 | USA | 20 | 30+ | S | F | 27678 (50) | 1100 (36) | No | Yes | Cat | Yes | No | Age, ethnicity, education, alcohol intake, smoking |
| Health, Aging and Body
Composition (HABC)17 |
1997 | USA | 4.6 | 70-79 | M | A | 2503 (55) | 116 (39) | No | No | Unit | Age,
race, education, smoking, copd, hrt |
||
| Japanese Cardiovascular Risk
Surveys (Japan CVD)18 |
1975 | Japan | 18 | 40-69 | M | A | 9087 (60) | 256 (44) | No | No | Cat | No | Yes | Age, community, total cholesterol, smoking, alcohol intake, time since last meal, menopausal status (women) |
| Japan Collaborative
Cohort (JACC)19 |
1988 | Japan | 19.3 | 40-79 | S | F | 61571 (58) | 640 (38) | No | No | Cat | Yes | No | Age, smoking, alcohol, hours of walking, education, perceived mental stress, fish intake. |
| Japan Arteriosclerosis
Longitudinal (JALS_ECC)20 |
1985 | Pool of 16 cohorts from Japan |
40-90 | M | A | 33128 (53) | 170 (38) | No | No | Cat | No | No | Age, smoking, drinking, sbp, tc |
|
| Japan Public Health
Center (JPHC)21 |
1990 | Japan | 9.7 | 40-69 | S | A | 90679 (52) | 518 (23) | No | No | Cat | Yes | No | Age, smoking, alcohol intake, hypertension, diabetes, physical activity, intake of fish and vegetables, center |
| North Karelia, Kuopio, and
Turku- Loimaa provinces 198722 |
1987 | Finland | 10 | 25-64 | M | A | 11510 | 386 (34) | No | No | Unit | Age, systolic blood pressure, diastolic blood pressure, total cholesterol, HDL cholesterol, diabetes, smoking |
||
| MONICA-KORA23 | 1984 | Germany | 7 | 45-74 | M | A | 6239 (48) | 229 (22) | No | No | Cat | No | No | Age,
survey, cholesterol, smoking, sbp. Education, alcohol, exercise |
| National Health and
Nutrition Examination I (NHANES I)24 |
1982 | USA | 3.9 | 70-86 | M | A | 1581 (61) | 263 (54) | No | Yes | Cat | No | Yes | Age, smoking |
| National Health and
Nutrition Examination III (NHANES III)25* |
1988 | USA | 13 | 17-90 | M | F | 18603 (54) | 973 (51) | No | No | Both | Yes | No | Age, sbp, tc, hdl, smoking |
| Nippon Data 8026 | 1980 | Japan | 24 | 30+ | M | F | 9300 (56) | 175 (50) | Yes | No | Cat | Yes | Yes | Age, SBP, Smoking, Alcohol consumption, Valve HD, history of stroke or angina, total cholesterol, blood glucose, creatinine |
| Nord-Trøndelag Health27 | 1984 | Norway | 10 | 70+ | M | F | 6392 (51) | 607 (40) | No | Yes | Cat | No | No | Age, SBP, smoking |
| Northwick Park Heart28 | 1972 | UK | 30 | 40-64 | M | F | 2202 (31) | 250 (14) | No | Yes | Unit | Age, smoking, cholesterol, sbp |
||
| Populations for Epidemiologic
Studies for the Elderly±29 |
1982 | USA | 6 | 65+ | S | F | 2812 (58) | 144 (50) | No | No | Cat | No | No | Age, chest
pain, diabetes, hypertension, hypertension meds, smoking |
| General Post Office30 | 1966 | UK | 40 | 35-70 | M | F | 1916 (34) | 356 (21) | No | No | Cat | Yes | No | Age |
| Renfrew-Paisley31 | 1972 | Scotland | 20 | 45-64 | M | F | 15424 (53) | 2019 (37) | No | No | Cat | No | No | Age, adjusted FEV1, number of cigarettes smoked per day and social class. |
| Reykjavik32 | 1987 | Iceland | 10 | 34-79 | M | A | 17441 (54) | 181 (24) | Yes | No | Unit | Age, cholesterol, triglycerides, dpb, smoking, prevalent CHD, ECG |
||
| Scottish Heart Health Extended
Cohort (SHHEC)33* |
1984 | Scotland | 20 | 30-75 | M | A | 13343 (51) | 2595 (39) | No | No | Both | Yes | No | Age, sbp, tc, hdl, smoking |
| Swedish Twin Registry34 | 1969 | Sweden | 34 | 16-86 | S | F | 44258 | 3564 (45) | No | No | Cat | Yes | No | Age, smoking |
| Tromsø35 | 1994 | Norway | 15.7 | 25-84 | M | A | 6379 (52) | 925 (39) | No | No | Both | No | No | Age, smoking, SBP, total cholesterol, HDL, triglycerides, HbA1C, self- reported diabetes |
| Province of Zaragoza36 | 1994 | Spain | 5 | 25+ | M | A | 6124 (55) | 155 (43) | No | No | Cat | No | Yes | NR,
only multivariable stated |
| 45 and Up37 | 2006 | Australia | 3.4 | 45-103 | S | A | 151751 (56) | 3096 (43) | No | No | Unit | Yes | No | Age, region of residence, income, education, smoking, alcohol, insurance |
Studies with individual participant data.
Studies reported odds ratios instead of hazard ratios.
Sensitivity Analyses
We ran sensitivity analyses to assess whether the estimates differed by geographical region (Asia vs. Not-Asia), CHD endpoint (fatal only vs. fatal and non-fatal combined), the choice of the reference group (normal weight (18.5–25.0 kg/m2) vs. underweight and normal weight combined (<25 kg/m2), analyses of the overweight and obese groups (two separated groups or one combined group), the use of sex-specific cut-points in BMI (yes/no), study quality, and self-reported or measured BMI.
Individual Participant Data
We used the available individual participant data to further investigate potential heterogeneity in the estimates by assessing the influence of using uniform or sex-specific cut-points, and different adjustment levels (age only, age and smoking, or multivariable adjustment). We also compared estimates from individual participant data with those derived from aggregate published data. Cox proportional hazards regression models were used, separately in each study to provide log HRs and their variances for the overall pooled analyses. We also investigated whether the estimates were sensitive to age, assumptions of linearity, residual confounding by smoking status, and reverse causality by excluding the first 3 years of follow up.
Role of the Funding Source
The funding source played no role in any aspect of this research. The corresponding author had full access to all the study data and had final responsibility for the decision to submit for publication.
RESULTS
Of the 8,561 individual articles that were identified through the systematic search, 395 articles qualified for full-text evaluation (Figure 1). Of these, 32 articles and four studies with individual participant data were included (Table 1), comprising 95 studies, 1,219,187 participants, and 37,488 incident cases of CHD. Study names and abbreviations are found in Table S1.
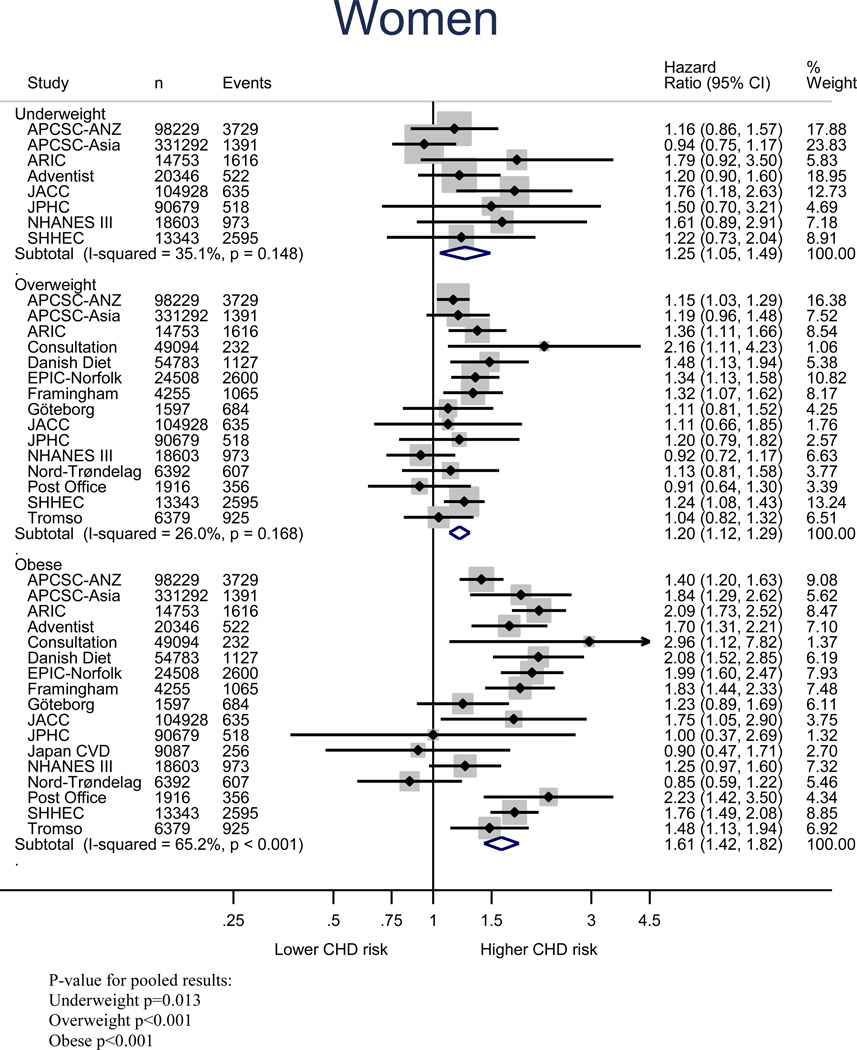
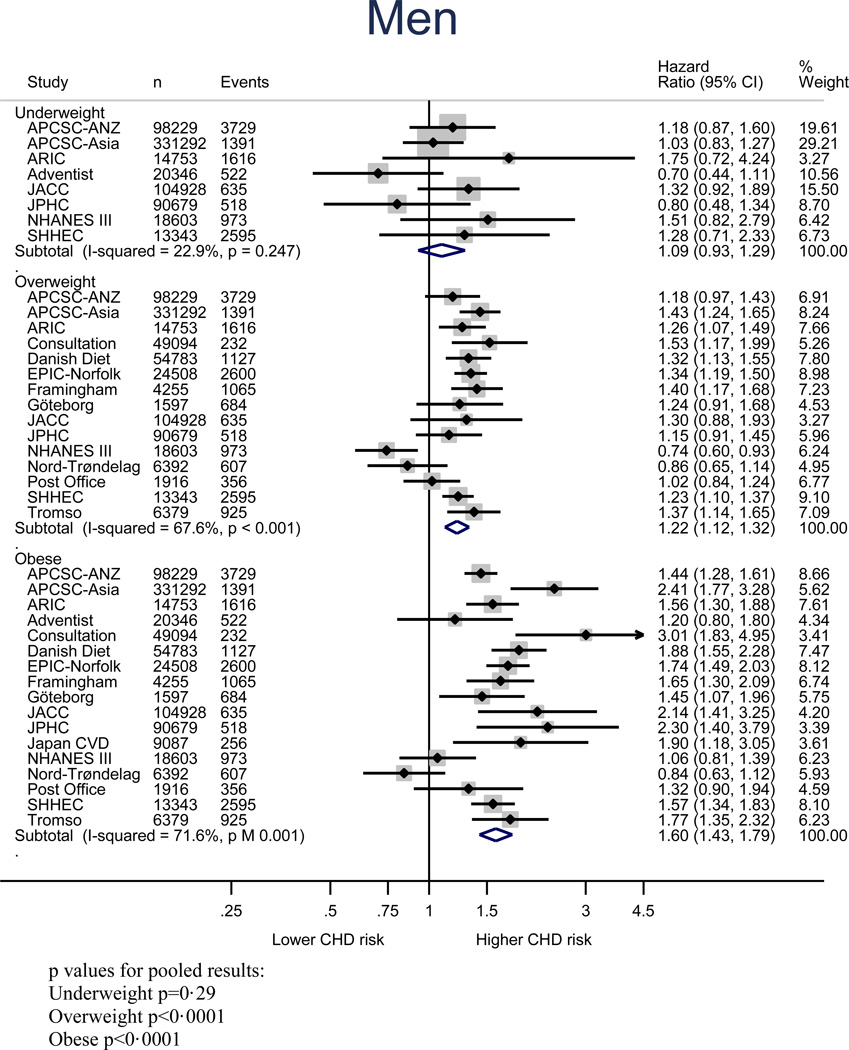
Figure 2 shows the age-adjusted relationship between BMI and risk of CHD in women and men; relative to normal weight, the age-adjusted HR of CHD in underweight, overweight and obese groups were 1.25 (1.05–1.49), 1.20 (1.12–1.29), and 1.61 (1.42–1.82) for women, and 1.09 (0.91–1.23), 1.22 (1.12–1.32), and 1.60 (1.43–1.79) for men, respectively. When measured continuously, a one unit increment in BMI was associated with an age-adjusted increase in risk of CHD of 4% (95% CI: 3–5%) in women and 5% (4–7%) in men (Figure S1). Multiple adjustment had little impact on these estimates (Figure S2 and S3). All pooled estimates showed statistically significant between-study heterogeneity (Table S2), but little evidence of publication bias except for the multiple-adjusted model for the obesity category (Figure S4-S7).
Figure 2.
Age-adjusted coronary heart disease HRs and 95% CIs for BMI categories relative to normal weight in women
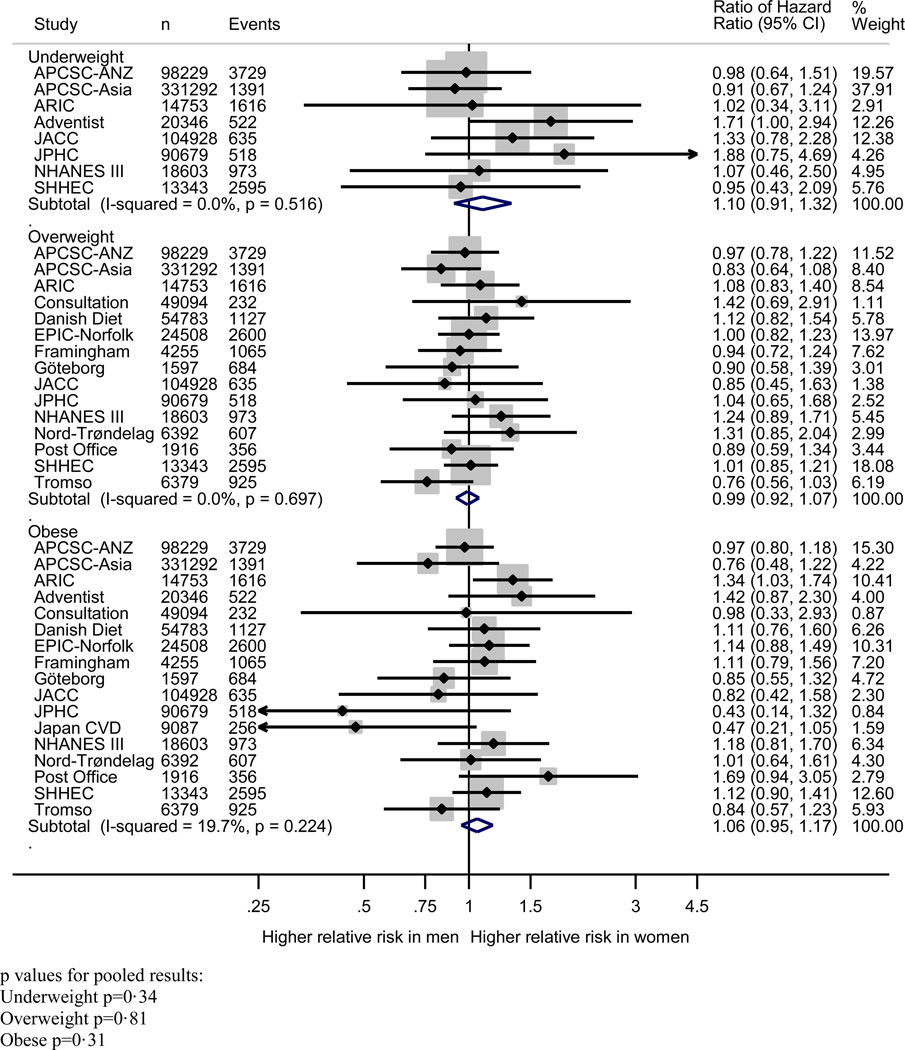
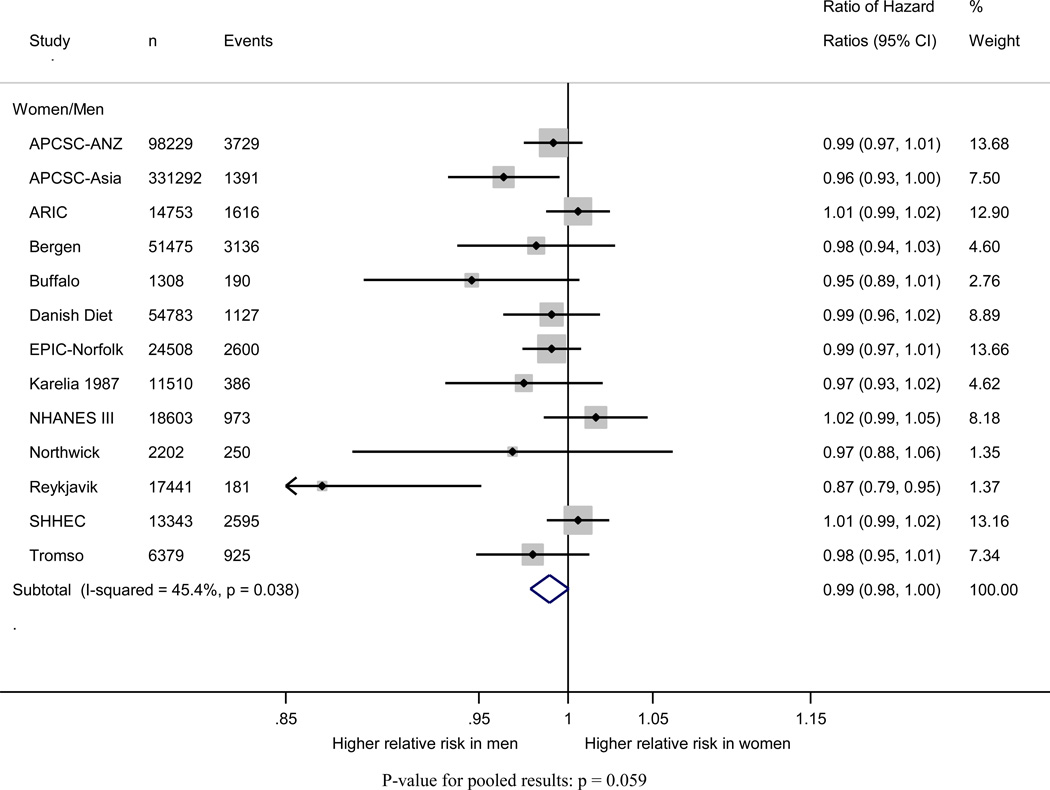
Pooling of the women-to-men ratios of the age-adjusted HRs of incident CHD indicated that, compared to normal weight, the risk of incident CHD did not significantly differ between women and men in any of the weight categories: HRR (95% CI) for underweight 1.08 (0. 89–1.32); overweight 1.00 (0.92–1.07); and obese 1.05 (0.94–1.17) (Figure 3). The pooled HRR with one unit difference in BMI suggested that the excess risk of additional BMI was equivalent in women and men: HRR 0.99 (95% CI: 0.98–1.00) (Figure 4). Multiple adjustment had no material impact on these estimates (Figure S2 and S3).
Figure 3.
Age-adjusted coronary heart disease HRs and 95% CIs for BMI categories relative to normal weight in men
Figure 4.
Women-to-men ratio of age-adjusted coronary heart disease hazard ratios and 95% confidence intervals for body mass index categories
In sensitivity analyses, age-adjusted analyses for one unit difference in BMI were similar to the main results in all subgroups, and little heterogeneity was found by subgroup, with the exception of significant differences between studies from Asia compared to other regions (Table S3). Age-adjusted estimates for BMI categories were more sensitive to subgroup analyses, particularly for women in the underweight group (Table S4); however, estimates for the overweight and obese categories were generally robust to stratification. Subgroup analyses with multiple-adjusted estimates for BMI categories were generally similar to the main results, with the exception of the studies from Asia, which is likely to be a chance finding (Table S4 and S6). Meta-regression did not explain the results for a one unit change in BMI and the small differences for BMI categories are likewise explainable by chance (Table 6 and S7). The included studies were generally of good quality (Table S8) and study quality also did not explain the results (Table S6 and S7).
The individual participant data analyses provided no evidence for any difference between the sex-specific categories versus the WHO categories for overweight or obesity; the difference in HRs between the estimates for WHO categories and the estimates for sex-specific quartiles was 0.038 (−0.025, 0.101) for overweight and 0.15 (−0.060, 0.365) for obesity. The women-to-men ratio of HRs was similar for models adjusted for age and smoking (HRs for one unit BMI 1.00 (0.98–1.02), overweight 1.02 (0.87–1.21), and obesity 1.11 (0.84–1.47)), compared to models adjusted for age alone (corresponding HRs = 0.99 (0.97–1.01), 1.02 (0.85–1.22), 1.07 (0.83–1.37)) (Figure S8).
Figures S8 and S9 suggest that the results did not differ across models using different approaches to account for confounding by smoking status and reverse causality. The association between BMI and CHD is gradually attenuated with increasing age group (Figure S10). Figure S11 shows that when the range of BMI is restricted to 20 kg/m2 or more, the estimated linear association is almost identical to when the entire range of BMI values is used. Finally, sensitivity analyses revealed that the results were generally consistent irrespective of whether individual participant data or published aggregate data were used (Tables S4–S6).
DISCUSSION
This meta-analysis incorporating data on 1,219,187 individuals and 37,488 incident cases of CHD shows definitively that the relationship between BMI and CHD is the same in women and men. Women and men who are overweight or obese experience a similar increase in risk of incident CHD whether measured on a continuous or categorical scale, compared with normal weight individuals. These associations were robust in a wide range of subgroup analyses, and were similar when different definitions and measurements of BMI were used.
That the relationship between BMI and CHD is equivalent in women and men negates our original hypothesis that the effects of increased BMI could be more deleterious in men than in women due to differences in body fat deposition. BMI is widely considered to be an imperfect measure of adiposity, so it remains unknown whether body fat distribution as assessed using more precise measures such as waist circumference or waist to hip ratio would exhibit different patterns with coronary risk in women and men. Nevertheless, we chose BMI – as opposed to other anthropometric indices- as the metric for investigation primarily because BMI remains the most commonly used tool for assessing overweight and obesity at the population level and is routinely measured in clinical practice.22 The relationship between BMI and coronary risk is largely mediated through its adverse effects on other major cardiovascular risk factors, principally type 2 diabetes, lipids and blood pressure,17 and thus, BMI tends not to be a component of commonly used risk prediction scores, such as the Framingham Risk Score. However, in low-income settings where the ability to conduct a plethora of biochemical tests is severely limited, BMI may well be a reasonable proxy for these health indicators. For example, Chiuve and colleagues successfully developed and validated a lifestyle-based cardiovascular prediction model– ‘The Healthy Heart Score’ based on two large US cohorts that included BMI, together with age, smoking, alcohol, activity level and a composite diet score.23
Our results are generally consistent with other large-scale pooling projects on this topic, namely the Prospective Studies Collaboration and the Emerging Risk Factors Collaboration.1–3 In both studies the relative risk estimates for an approximate 5 kg/m2 increment in BMI were fractionally higher in men than in women (1.42 vs. 1.35 in the Prospective Studies Collaboration and 1.26 vs. 1.24 in the Emerging Risk Factors Collaboration), but as neither of the studies specifically compared women and men from within the same study, these data could not definitively address the issue of a sex difference that we have examined here. These studies also caution against the over-adjustment of mediating risk factors and found the same attenuation of risk for CHD death after age 70. While it remains unclear why studies of CHD find an association between overweight and risk, but studies of all-cause mortality do not, this discrepancy does not diminish the important potential contributions of overweight to morbidity and healthcare costs.
This study has a number of limitations. First, we had to exclude several articles because estimates for women and men were not reported separately (Supplemental Methods S5). Since reporting a combined estimate is probably more likely for those studies with similar estimates for women and men, our analysis may have overestimated heterogeneity by sex. This seems unlikely however, as there were few within-study differences in the estimates for women and men were found. Second, there was significant heterogeneity between studies, which is most likely due to differences in study characteristics or in the background populations from which the cohorts were derived. This does not invalidate the estimates and confidence intervals from our random effects meta-analysis and may merely reflect the precise estimation of the individual studies contributing to the pool, since I2 measures between-study variability as a ratio of between plus within study variability. Third, not all studies provided CHD estimates for BMI as both a continuous and categorical variable, and not all studies provided both age- and multiple-adjusted estimates. We were therefore unable to determine the unique contribution of these changes in definition and analysis to differences in estimates. Fourth, BMI categories and unit cut-points were not consistent across studies and reconciling these differences was complicated. We attempted to standardize these estimates in a systematic way and address this issue using individual participant data, but are limited in our ability to judge how this compromise influenced the pooled estimates. Fifth, there was variation in the set of variables used in adjusted analyses across the studies, which is likely to have impacted the size of the relative risk estimates. But, importantly, differences in the level of adjustment between studies is unlikely to have affected the internal comparisons of the effect of BMI on CHD among women and men from the same study. Finally, our analyses of continuous BMI assumed a log-linear model for the risk of CHD across the full range of BMI. This has previously been demonstrated to be a reasonable assumption for values of BMI above 20 kg/m2,1 within which the values of most people fall. Our sensitivity analyses suggests that the log-linear relationships for both women and men overall are very similar to when those with BMI < 20 kg/m2 are excluded, whilst any deviations from log-linearity are unlikely to greatly affect our sex comparisons.
To our knowledge, this is the first meta-analysis of this topic that included only studies that reported estimates for both women and men. This inclusion criterion facilitated the derivation of ratio estimates from women and men that share the same source population, ensuring better comparability between the sexes. This study also benefits greatly from the availability of individual participant data that enabled a second set of sensitivity analyses to assess the influence of using sex-specific BMI cut-points, differing levels of adjustment for confounding, smoking status, age groups, reverse causality, and assumptions of linearity at the individual level.
In conclusion, increments in BMI confer a similar risk of incident CHD risk for women and men from the same source population. While consensus has yet to be reached about the optimal level of BMI for health,2–24 these results suggest that BMI above the normal range places both women and men at equivalent increased risk for CHD.
Supplementary Material
Figure 5.
Women-to-men ratio of age-adjusted coronary heart disease hazard ratios and 95% confidence intervals for a one unit (kg/m2) increase in body mass index
Acknowledgements
We would like to acknowledge the assistance of Lori Rosman in creating search criteria for this study. The authors thank the investigators, staff, and participants of all studies included in our meta-analysis for their important contributions.
Funding: MLMC was supported by NIH/NLHBI grant T32HL079891.
APCSC was funded by the Australian National Heart and Medical Research Council and Pfizer Inc.
The Atherosclerosis Risk in Communities Study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute contracts (HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HHSN268201100009C, HHSN268201100010C, HHSN268201100011C, and HHSN268201100012C). The authors thank the staff and participants of the ARIC study for their important contributions.
The Third National Health and Nutrition Examination Survey is supported by the Centers for Disease Control and Prevention and provide public access to the data here: http://www.cdc.gov/nchs/nhanes/nh3data.htm
SHHEC was funded by the Scottish Health Department Chief Scientist Organization, British Heart Foundation and FP Fleming Trust.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions: MMC performed the literature search. MMC and SAP acquired, analyzed, and interpreted data for the manuscript, including creating figures and tables. RRH and MW conceived the study and designed and interpreted the analysis for the manuscript. All authors wrote and revised the manuscript, approved the final version for publication, and agree to be accountable for all aspects of the work.
Conflict of Interest: The authors have nothing to declare.
Dedication: This paper is dedicated to the memory of our friend and colleague Dr. Gary Whitlock (1964–2013).
REFERENCES
- 1.Whitlock G, Lewington S, Sherliker P, et al. Body-mass index and cause-specific mortality in 900 000 adults: collaborative analyses of 57 prospective studies. Lancet. 2009;373(9669):1083–1096. doi: 10.1016/S0140-6736(09)60318-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Flegal KM, Kit BK, Orpana H, Graubard BI. Association of All-Cause Mortality With Overweight and Obesity Using Standard Body Mass Index Categories: A Systematic Review and Meta-analysis. Journal of the American Medical Association. 2013;309(1):71–82. doi: 10.1001/jama.2012.113905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wormser D, Kaptoge S, Di Angelantonio E, et al. Separate and combined associations of body-mass index and abdominal adiposity with cardiovascular disease: Collaborative analysis of 58 prospective studies. The Lancet. 2011;377(9771):1085–1095. doi: 10.1016/S0140-6736(11)60105-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berrington De Gonzalez A, Hartge P, Cerhan JR, et al. Body-mass index and mortality among 1.46 million white adults. New England Journal of Medicine. 2010;363(23):2211–2219. doi: 10.1056/NEJMoa1000367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ng M, Fleming T, Robinson M, Thomson B, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: a systematic analysis for the Global Burden of Disease Study 2013. The Lancet. 2014;384(9945):766–781. doi: 10.1016/S0140-6736(14)60460-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hubert HB, Feinleib M, McNamara PM, Castelli WP. Obesity as an independent risk factor for cardiovascular-disease - A 26-year follow-up of participants in the Framingham Heart Study. Circulation. 1983;67(5):968–977. doi: 10.1161/01.cir.67.5.968. [DOI] [PubMed] [Google Scholar]
- 7.Lee C, Colagiuri S, Ezzati M, Woodward M. The burden of cardiovascular disease associated with high body mass index in the Asia-Pacific region. Obesity Reviews. 2011;12(5):e454–e459. doi: 10.1111/j.1467-789X.2010.00849.x. [DOI] [PubMed] [Google Scholar]
- 8.Huxley R, Peters S, Mishra G, Woodward M. Risk of all-cause mortality and vascular events in women versus men with type 1 diabetes: a systematic review and meta-analysis. Lancet Diabetes Endocrinol. 2015;3:198–206. doi: 10.1016/S2213-8587(14)70248-7. [DOI] [PubMed] [Google Scholar]
- 9.Peters S, Huxley R, Woodward M. Diabetes as risk factor for incident coronary heart disease in women compared with men: a systematic review and meta-analysis of 64 cohorts including 858,507 individuals and 28,203 coronary events. Diabetologia. 2014;57:1542–1551. doi: 10.1007/s00125-014-3260-6. [DOI] [PubMed] [Google Scholar]
- 10.Huxley R, Woodward M. Cigarette smoking as a risk factor for coronary heart disease in women compared with men: a systematic review and meta-analysis of prospective cohort studies. Lancet. 2011;8(378):1297–1305. doi: 10.1016/S0140-6736(11)60781-2. [DOI] [PubMed] [Google Scholar]
- 11.Peters S, Huxley R, Woodward M. Comparison of the Sex-Specific Associations Between Systolic Blood Pressure and the Risk of Cardiovascular Disease: A Systematic Review and Meta-Analysis of 124 Cohort Studies, Including 1.2 Million Individuals. Stroke. 2013;44(2394–2401) doi: 10.1161/STROKEAHA.113.001624. [DOI] [PubMed] [Google Scholar]
- 12.Hattori K, Numata N, Ikoma M, Matsuzaka A, Danielson R. Sex differences in the distribution of subcutaneous and internal fat. Human Biology. 1991;63:53–63. [PubMed] [Google Scholar]
- 13.Vague J. The degree of masculine differentiation of obesities: a factor determining predisposition to diabetes, atherosclerosis, gout, and uric calculous disease. American Journal of Clinical Nutrition. 1956;4:20–34. doi: 10.1093/ajcn/4.1.20. [DOI] [PubMed] [Google Scholar]
- 14.Bogers RP, Bemelmans WJE, Hoogenveen RT, et al. Association of overweight with increased risk of coronary heart disease partly independent of blood pressure and cholesterol levels: A meta-analysis of 21 cohort studies including more than 300 000 persons. Archives of Internal Medicine. 2007;167(16):1720–1728. doi: 10.1001/archinte.167.16.1720. [DOI] [PubMed] [Google Scholar]
- 15.Collins VR, Dowse GK, Cabealawa S, Ram P, Zimmet PZ. High mortality from cardiovascular disease and analysis of risk factors in Indian and Melanesian Fijians. Int J Epidemiol. 1996;25(1):59–69. doi: 10.1093/ije/25.1.59. [DOI] [PubMed] [Google Scholar]
- 16.Selmer R, Tverdal A. Body mass index and cardiovascular mortality at different levels of blood pressure: a prospective study of Norwegian men and women. J Epidemiol Community Health. 1995;49(3):265–270. doi: 10.1136/jech.49.3.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lu Y, Hajifathalian K, Ezzati M, Woodward M, Rimm E, Danaei G. Metabolic mediators of the effects of body-mass index, overweight, and obesity on coronary heart disease and stroke: a pooled analysis of 97 prospective cohorts with 1·8 million participants. Lancet. 2014;15(383):970–983. doi: 10.1016/S0140-6736(13)61836-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.DerSimonian R, Laird N. Meta-analysis in clinical trials. Controlled Clinical Trials. 1986;7:177–178. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 19.Woodward M. Epidemiology: Study Design and Data Analysis. 3rd ed. Boca Raton, FL: CRC Press; 2013. [Google Scholar]
- 20.Wells G, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. 2013 Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
- 21.StataCorp. Stata Statistical Software Release 11. College Station, TX: StataCorp LP; 2009. [Google Scholar]
- 22.Huxley R, Mendis S, Zheleznyakov E, Reddy S, Chan J. Body mass index, waist circumference and waist:hip ratio as predictors of cardiovascular risk--a review of the literature. Eur J Clin Nutr. 2010;64(1):16–22. doi: 10.1038/ejcn.2009.68. [DOI] [PubMed] [Google Scholar]
- 23.Chiuve S, Cook N, Shay C, et al. Lifestyle-based prediction model for the prevention of CVD: the Healthy Heart Score. J Am Heart Assoc. 2014;3(4):e000954. doi: 10.1161/JAHA.114.000954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Willett WC, Hu FB, Thun M. Overweight, Obesity, and All-Cause Mortality. Journal of the American Medical Association. 2013;309(16):1681–1682. doi: 10.1001/jama.2013.3075. [DOI] [PubMed] [Google Scholar]
* References of included studies
- 1.Singh PN, Lindsted KD, Fraser GE. Body weight and mortality among adults who never smoked. Am J Epidemiol. 1999;150:1152–1164. doi: 10.1093/oxfordjournals.aje.a009942. [DOI] [PubMed] [Google Scholar]
- 2.Woodward M, Barzi F, Martiniuk A, et al. Cohort profile: the Asia Pacific Cohort Studies Collaboration. International Journal of Epidemiology. 2006;35:1412–1416. doi: 10.1093/ije/dyl222. [DOI] [PubMed] [Google Scholar]
- 3.The Aric Investigators. The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. American Journal of Epidemiology. 1989;129:687–702. [PubMed] [Google Scholar]
- 4.Selmer R, Tverdal A. Body mass index and cardiovascular mortality at different levels of blood pressure: a prospective study of Norwegian men and women. J Epidemiol Community Health. 1995;49:265–270. doi: 10.1136/jech.49.3.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dorn JM, Schisterman EF, Winkelstein W, Jr, Trevisan M. Body mass index and mortality in a general population sample of men and women. The Buffalo Health Study. Am J Epidemiol. 1997;146:919–931. doi: 10.1093/oxfordjournals.aje.a009218. [DOI] [PubMed] [Google Scholar]
- 6.Seidell JC, Verschuren WM, van Leer EM, Kromhout D. Overweight, underweight, and mortality. A prospective study of 48,287 men and women. Arch Intern Med. 1996;156:958–963. doi: 10.1001/archinte.156.9.958. [DOI] [PubMed] [Google Scholar]
- 7.Mogelvang R, Scharling H, Jensen JS. A simple linear model for the effect of changes in metabolic risk factors on coronary heart disease. J Intern Med. 2006;259:561–568. doi: 10.1111/j.1365-2796.2006.01634.x. [DOI] [PubMed] [Google Scholar]
- 8.Stegger JG, Schmidt EB, Obel T, et al. Body composition and body fat distribution in relation to later risk of acute myocardial infarction: a Danish follow-up study. SOURCE International Journal of Obesity. 2011 doi: 10.1038/ijo.2010.278. Date of Publication: 1 Feb 2011 2011. [DOI] [PubMed] [Google Scholar]
- 9.Jensen MK, Chiuve SE, Rimm EB, et al. Obesity, behavioral lifestyle factors, and risk of acute coronary events. Circulation. 2008;117:3062–3069. doi: 10.1161/CIRCULATIONAHA.107.759951. [DOI] [PubMed] [Google Scholar]
- 10.Canoy D, Boekholdt SM, Wareham N, et al. Body fat distribution and risk of coronary heart disease in men and women in the European Prospective Investigation Into Cancer and Nutrition in Norfolk cohort: a population-based prospective study. Circulation. 2007;116:2933–2943. doi: 10.1161/CIRCULATIONAHA.106.673756. [DOI] [PubMed] [Google Scholar]
- 11.Collins VR, Dowse GK, Cabealawa S, Ram P, Zimmet PZ. High mortality from cardiovascular disease and analysis of risk factors in Indian and Melanesian Fijians. Int J Epidemiol. 1996;25:59–69. doi: 10.1093/ije/25.1.59. [DOI] [PubMed] [Google Scholar]
- 12.Korkeila M, Rissanen A, Sorensen TI, Kaprio J. BMI, weight stability and mortality among adults without clinical co-morbidities: a 22-year mortality follow-up in the Finnish twin cohort. Obes Facts. 2009;2:344–351. doi: 10.1159/000261416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wilson PW, D'Agostino RB, Sullivan L, Parise H, Kannel WB. Overweight and obesity as determinants of cardiovascular risk: the Framingham experience. Arch Intern Med. 2002;162:1867–1872. doi: 10.1001/archinte.162.16.1867. [DOI] [PubMed] [Google Scholar]
- 14.Dey DK, Lissner L. Obesity in 70-year-old subjects as a risk factor for 15-year coronary heart disease incidence. Obes Res. 2003;11:817–827. doi: 10.1038/oby.2003.113. [DOI] [PubMed] [Google Scholar]
- 15.Kempen GIJM, Van Jaarsveld CHM, Van Sonderen E, Sanderman R, Ormel J. Risk factors for developing cardiac disease in late middle-aged and older men and women: A prospective study [4] Journal of the American Geriatrics Society. 2001;49:1575–1577. doi: 10.1046/j.1532-5415.2001.4911261.x. [DOI] [PubMed] [Google Scholar]
- 16.Maskarinec G, Meng L, Kolonel LN. Alcohol intake, body weight, and mortality in a multiethnic prospective cohort. Epidemiology. 1998;9:654–661. [PubMed] [Google Scholar]
- 17.Nicklas BJ, Penninx BW, Cesari M, et al. Association of visceral adipose tissue with incident myocardial infarction in older men and women: the Health, Aging and Body Composition Study. American Journal of Epidemiology. 2004;160:741–749. doi: 10.1093/aje/kwh281. [DOI] [PubMed] [Google Scholar]
- 18.Iso H, Sato S, Kitamura A, et al. Metabolic syndrome and the risk of ischemic heart disease and stroke among Japanese men and women. Stroke. 2007;38:1744–1751. doi: 10.1161/STROKEAHA.106.469072. [DOI] [PubMed] [Google Scholar]
- 19.Cui R, Hiroyasu I, Tanabe N, Watanabe Y, Tamakoshi A JACC Study Group. Association between weight change since 20 years of age with mortality from myocardial infarction and chronic heart failure in the Japan Collaborative Cohort (JACC) Study. Circulation Journal. 2014;78:649–655. doi: 10.1253/circj.cj-13-1057. [DOI] [PubMed] [Google Scholar]
- 20.Yatsuya H, Toyoshima H, Yamagishi K, et al. Body mass index and risk of stroke and myocardial infarction in a relatively lean population: meta-analysis of 16 Japanese cohorts using individual data. Circ Cardiovasc Qual Outcomes. 2010;3:498–505. doi: 10.1161/CIRCOUTCOMES.109.908517. [DOI] [PubMed] [Google Scholar]
- 21.Chei CL, Iso H, Yamagishi K, Inoue M, Tsugane S. Body mass index and weight change since 20 years of age and risk of coronary heart disease among Japanese: the Japan Public Health Center-Based Study. Int J Obes (Lond) 2008;32:144–151. doi: 10.1038/sj.ijo.0803686. [DOI] [PubMed] [Google Scholar]
- 22.Silventoinen K, Jousilahti P, Vartiainen E, Tuomilehto J. Appropriateness of anthropometric obesity indicators in assessment of coronary heart disease risk among Finnish men and women. Scand J Public Health. 2003;31:283–290. doi: 10.1080/14034940210165046. [DOI] [PubMed] [Google Scholar]
- 23.Ladwig KH, Marten-Mittag B, Lowel H, Doring A, Wichmann HE. Synergistic effects of depressed mood and obesity on long-term cardiovascular risks in 1510 obese men and women: results from the MONICA-KORA Augsburg Cohort Study 1984–1998. International Journal of Obesity. 2006;30:1408–1414. doi: 10.1038/sj.ijo.0803285. [DOI] [PubMed] [Google Scholar]
- 24.Harris TB, Launer LJ, Madans J, Feldman JJ. Cohort study of effect of being overweight and change in weight on risk of coronary heart disease in old age. Bmj. 1997;314:1791–1794. doi: 10.1136/bmj.314.7097.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ezzati T, Massey J, Waksberg J, Chu A, Maurer K. Sample design: Third National Health and Nutrition Examination Survey. Vital and health statistics Series 2. 1992;113:1–35. [PubMed] [Google Scholar]
- 26.Nakamura Y, Turin TC, Rumana N, et al. Risk factors for heart failure and coronary heart disease mortality over 24-year follow-up period in Japan: NIPPON DATA80. CVD Prevention and Control. 2010;5:97–103. [Google Scholar]
- 27.Ellekjaer H, Holmen J, Vatten L. Blood pressure, smoking and body mass in relation to mortality from stroke and coronary heart disease in the elderly. A 10-year follow-up in Norway. Blood Press. 2001;10:156–163. doi: 10.1080/080370501753182370. [DOI] [PubMed] [Google Scholar]
- 28.Kim J, Meade T, Haines A. Skinfold thickness, body mass index, and fatal coronary heart disease: 30 year follow up of the Northwick Park heart study. J Epidemiol Community Health. 2006;60:275–279. doi: 10.1136/jech.2005.042200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Seeman T, De Leon CM, Berkman L, Ostfeld A. Risk factors for coronary heart disease among older men and women: A prospective study of community-dwelling elderly. American Journal of Epidemiology. 1993;138:1037–1049. doi: 10.1093/oxfordjournals.aje.a116822. [DOI] [PubMed] [Google Scholar]
- 30.Ferrie JE, Singh-Manoux A, Kivimaki M, et al. Cardiorespiratory risk factors as predictors of 40-year mortality in women and men. Heart. 2009;95:1250–1257. doi: 10.1136/hrt.2008.164251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Murphy NF, MacIntyre K, Stewart S, Hart CL, Hole D, McMurray JJ. Long-term cardiovascular consequences of obesity: 20-year follow-up of more than 15 000 middle-aged men and women (the Renfrew-Paisley study) Eur Heart J. 2006;27:96–106. doi: 10.1093/eurheartj/ehi506. [DOI] [PubMed] [Google Scholar]
- 32.Thorgeirsson G, Thorgeirsson G, Sigvaldason H, Witteman J. Risk factors for out-of-hospital cardiac arrest: the Reykjavik Study. Eur Heart J. 2005;26:1499–1505. doi: 10.1093/eurheartj/ehi179. [DOI] [PubMed] [Google Scholar]
- 33.Woodward M, Brindle P, Tunstall-Pedoe H. Adding social deprivation and family history to cardiovascular risk assessment –the ASSIGN score from the Scottish Heart Health Extended Cohort (SHHEC) Heart. 2007;93(2):172–176. doi: 10.1136/hrt.2006.108167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Carlsson S, Andersson T, de Faire U, Lichtenstein P, Michaelsson K, Ahlbom A. Body mass index and mortality: is the association explained by genetic factors? Epidemiology. 2011;22:98–103. doi: 10.1097/EDE.0b013e3181fce2a2. [DOI] [PubMed] [Google Scholar]
- 35.Horvei LD, Brækkan SK, Mathiesen EB, Njølstad I, Wilsgaard T, Hansen JB. Obesity measures and risk of venous thromboembolism and myocardial infarction. European Journal of Epidemiology. 2014;29:821–830. doi: 10.1007/s10654-014-9950-z. [DOI] [PubMed] [Google Scholar]
- 36.Marin A, Medrano MJ, Gonzalez J, et al. Risk of ischaemic heart disease and acute myocardial infarction in a Spanish population: observational prospective study in a primary-care setting. BMC Public Health. 2006;6:38. doi: 10.1186/1471-2458-6-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Joshy G, Korda RJ, Attia J, Bauman AE, Banks E. Body mass index and incident hospitalization for cardiovascular disease in 158 546 participants from the 45 and Up Study. International Journal of Obesity. 2014;38:848–856. doi: 10.1038/ijo.2013.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.