SUMMARY
The BH4 domain of Bcl2 is required for its antiapoptotic function, thus constituting a promising anticancer target. We identified a small molecule Bcl2-BH4 domain-antagonist (BDA-366) that binds BH4 with high affinity and selectivity. BDA-366-Bcl2 binding induces conformational change in Bcl2 that abrogates its antiapoptotic function, converting it from a survival to a cell death inducer. BDA-366 suppresses growth of lung cancer xenografts derived from cell lines and patient without significant normal tissue toxicity at effective doses. mTOR inhibition up-regulates Bcl2 in lung cancer cells and tumor tissues from clinical trial patients. Combined BDA-366 and RAD001 treatment exhibits strong synergy against lung cancer in vivo. Development of this Bcl2-BH4 antagonist may provide a strategy to improve lung cancer outcome.
Keywords: Bcl2, BH4 domain, Small molecule antagonist, Lung cancer, Therapy
INTRODUCTION
Lung cancer is the leading cause of cancer-related mortality in the United States, accounting for more deaths than breast, prostate and pancreatic cancer combined (Jemal et al., 2007). Up-regulation of antiapoptotic Bcl2 family members (i.e. Bcl2, Bcl-XL and Mcl-1) and dysregulation of proapoptotic family members (i.e. Bad, Bim, Bax, Bak, etc.) are involved in the mediation of chemo- or radioresistance in human lung cancers (Sartorius and Krammer, 2002; Song et al., 2005), suggesting that Bcl2 family members have the potential to be critical targets for lung cancer treatment.
The Bcl2 family members have homology clustered within four conserved Bcl2 homology (BH) domains (i.e. BH1, BH2, BH3 and BH4) (Kelekar and Thompson, 1998). The BH1, BH2 and BH3 domains form the surface binding pocket of Bcl2 which mediates protein-protein interactions between Bcl2 family members (Castelli et al., 2004; Wang et al., 2000). This hydrophobic surface binding pocket is required for the antiapoptotic function of Bcl2, as studies have shown that mutations at this site abolish the biological function of Bcl2 (Yin et al., 1994). BH3-mimetic Bcl2 inhibitors ABT-737 and ABT-263 (the oral form of ABT-737) bind to the hydrophobic pocket of Bcl2 or Bcl-XL with a high-affinity and subsequently disrupt the antiapoptotic function of Bcl2 and Bcl-XL with potent anti-tumor effect (Konopleva et al., 2006; Oltersdorf et al., 2005; Tse et al., 2008). However, ABT-737 and ABT-263 can induce thrombocytopenia due to their inhibitory effects on both Bcl2 and Bcl-XL (Schoenwaelder et al., 2011). To generate a Bcl2 selective inhibitor, a tethered indole was incorporated into ABT-263 to fill the P4 hot spot, which specifically interacts with aspartic acid (Asp 103) of Bcl2 but not Glu96 of Bcl-XL, leading to the generation of the Bcl2-selective inhibitor ABT-199 (Souers et al., 2013). Since ABT-199 did not cause thrombocytopenia in vivo (Souers et al., 2013), this suggests that highly selective inhibition of Bcl2 may benefit the development of improved Bcl2 antagonists.
The BH4 region is a survival domain that is required for the antiapoptotic function of Bcl2 as demonstrated by the complete abolition of the antiapoptotic activity of Bcl2 or conversion of Bcl2 from survival protein into a proapoptotic molecule when the BH4 domain is removed (Cheng et al., 1997; de Moissac et al., 1999; Hirotani et al., 1999; Hunter et al., 1996; Reed et al., 1996), indicating that the BH4 is an ideal target for screening of small molecules that may convert Bcl2 into a death molecule in tumor tissues for anti-cancer therapeutics. The major goal of the present study is to identify a small molecule Bcl2 BH4 domain antagonist for lung cancer therapy.
RESULTS
Screening of small molecules targeting the BH4 domain of Bcl2
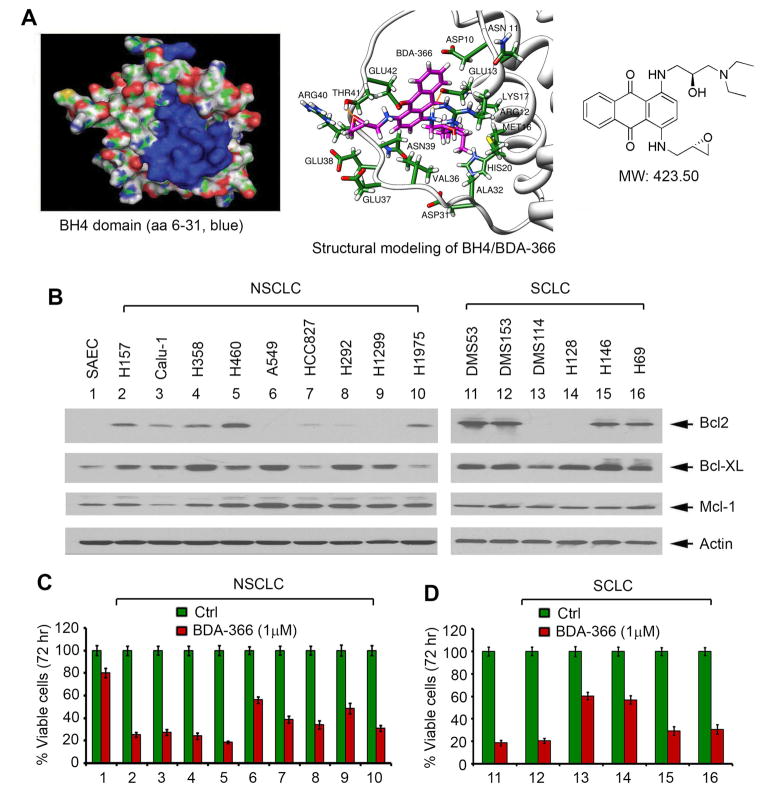
A library containing approximately 300,000 small molecules from the National Cancer Institute (NCI) was used to dock the structural pocket of the BH4 domain (aa6-31; PDB ID codes: 1G5M and 1G5O) using the UCSF DOCK 6.1 program suite (Figure 1A, left panel) as we previously described (Park et al., 2013). The small molecules were ranked according to their energy scores. The top 200 small molecules were selected for screening of cytotoxicity in human lung cancer cells by sulforhodamine B (SRB) assay as described (Liu et al., 2012; Vichai and Kirtikara, 2006). Among these small molecules, one compound (i.e. NSC639366, C24H29N3O4, MW: 423.50) had the most potent activity against human lung cancer cells. We named this lead compound small molecule Bcl2 BH4 domain antagonist (BDA-366). The molecular modeling of this lead in complex with the Bcl2 BH4 domain is shown in Figure 1A, right panel.
Figure 1. BDA-366 targets the BH4 domain of Bcl2 and potently suppresses lung cancer cell growth.
(A) Structural modeling of BDA-366 in the BH4 domain binding pocket of Bcl2 protein. (B) Expression of Bcl2 was analyzed by Western blot in normal small airway epithelial cells (SAEC), small cell lung Cancer (SCLC) and non-small cell lung cancer (NSCLC) cell lines. (C) and (D) A panel of NSCLC and SCLC cell lines and SAEC were treated with BDA-366 (1μM) for 72 hr. Cell viability was determined by analyzing Annexin-V/PI binding by fluorescence-activated cell sorter (FACS). Error bars represent ± S.D. See also Figure S1 and Table S1–2.
The effect of BDA-366 on cell growth and apoptosis was measured by SRB assay and Annexin V/PI binding, respectively, in both small cell lung cancer (SCLC) and non-small cell lung cancer (NSCLC) cell lines that express various levels of endogenous Bcl2. Results indicate that NSCLC cell lines (i.e. H157, Calu-1, H358, H460 and H1975) and SCLC cell lines (i.e. DMS53, DMS153, H146 and H69) that express relatively higher levels of Bcl2 were more sensitive to BDA-366. In contrast, lung cancer cell lines expressing relatively lower or undetectable levels of endogenous Bcl2 (i.e. NSCLC cell lines: A549 and H1299; SCLC cell lines: DMS114 and H128) were less sensitive to BDA-366 (Figures 1B and 1C, Tables S1 and S2). It seems that expression levels of Mcl-1 or Bcl-XL did not significantly affect sensitivity of cells to BDA-366. Based on our findings, we propose that the apoptotic response of BDA-366 may be dependent on the expression levels of Bcl2 in lung cancer cell lines. Importantly, BDA-366 showed less sensitive to normal small airway epithelial cell line (SAEC) (Figure 1C), indicating a relative selectivity against cancer cells compared to normal cells.
BDA-366 selectively binds to Bcl2 at the BH4 domain and induces cell killing
Purified recombinant WT and a panel of Bcl2 BH domain deletion mutant proteins (i.e. ΔBH1, ΔBH2, ΔBH3 and ΔBH4) were commercially obtained from ProteinX Lab (San Diego, CA) as described previously (Wang et al., 2008; Xie et al., 2014). To directly measure BDA-366/Bcl2 binding, a fluorescence polarization assay with a fluorescent Bak peptide (5′-FAM-GQVGRQLAIIGDDINR) was performed as previously described (Bruncko et al., 2007; Enyedy et al., 2001; Wang et al., 2000; Zhang et al., 2002). We found that BDA-366 directly binds to Bcl2 with high binding affinity (Ki =3.3 ± 0.73 nM) (Figure S1A). Deletion of BH1, BH2 or BH3 from Bcl2 protein did not significantly affect its BDA-366 binding. However, the BH4 domain deficient Bcl2 mutant protein (ΔBH4) failed to bind BDA-366 (Figure S1A). These findings indicate that BDA-366 selectively binds to Bcl2 via the BH4 domain. Importantly, BDA-366 did not bind to other Bcl2 family members, including Bcl-XL, Mcl-1, or Bfl-1/A1 (Figure S1B), indicating the specificity of its Bcl2 binding.
Structural modeling analysis by computational program reveals that BDA-366 is associated with 8 amino acids (i.e. ASP10, ASN11, ARG12, GLU13, MET16, LYS17, HIS20 and ASP31) in the BH4 domain. We generated a panel of Bcl2 mutants within the BH4 domain at the specific residues that were identified by the initial docking simulations, including D10A, N11A, R12A, E13A, M16A, K17A, H20A and D31A Bcl2 mutants. We also created a compound Bcl2 mutant D10A/N11A/R12A/E13A (AAAA). First, the recombinant proteins of these mutants were generated for BDA-366/Bcl2 binding analysis using a competitive fluorescence polarization as described in “Supplemental Experimental Procedures”. Ki values were: D10A→4.8 ± 0.41nM, N11A→4.1 ± 0.67nM, R12A→4.3 ± 0.54 nM, E13A→4.5 ± 0.71 nM, M16A→ 3.9 ± 0.31nM, K17A→4.2 ± 0.45 nM, H20A→3.8 ± 0.47 nM, D31A→3.7 ± 0.91nM, AAAA→598.64 ± 0.12nM. These findings indicate that single mutation at each individual residue did not significantly reduce Bcl2’s ability to bind BDA-366 but AAAA Bcl2 mutant protein had remarkably decreased BDA-366 binding. Second, WT and all Bcl2 mutants were exogenously overexpressed in H1299 cells that express undetectable levels of endogenous Bcl2. Results indicate that overexpression of exogenous WT or each of the Bcl2 mutants in H1299 cells potently inhibited cisplatin-induced apoptotic cell death (Figures S1C and S1D), indicating that these Bcl2 mutants retain standard anti-apoptotic function. However, overexpression of exogenous WT and each of the Bcl2 single site mutants in H1299 cells failed to prolong cell survival when cells were exposed to BDA-366 and exhibited enhanced sensitivity to BDA-366 (Figure S1E), indicating that BDA-366 not only overcomes Bcl2’s antiapoptotic function but also may convert these Bcl2 proteins into death molecules. In contrast, overexpression of the compound Bcl2 AAAA mutant significantly prolonged cell survival when cells were exposed to BDA-366 (Figure S1E), suggesting that compound mutations (AAAA) at BDA-366 binding residues lead to a phenotype that is resistant to BDA-366. This suggests that BDA-366 binding to these four amino acids (D10, N11, R12 and E13) in the BH4 domain is critical for BDA-366 regulation of Bcl2 and induction of apoptosis.
To determine whether Bcl-2 is the relevant target at the cellular level and whether cell killing is truly dependent on this particular mechanism, Bcl2 was specifically knocked down using three different Bcl2 shRNAs that target different regions in Bcl2 gene in five lung cancer cell lines, including two NSCLC cell lines (H460 and H157) and three SCLC cell lines (DMS53, DMS153 and H146). Stable expression of Bcl2 shRNA1, shRNA2 or shRNA3 efficiently depleted the endogenous Bcl2 in both NSCLC and SCLC cell lines (Figure S1F). This effect of shRNA on Bcl2 expression was highly specific because the control shRNA had no effect. Cells expressing Bcl2 shRNA1, shRNA2, shRNA3 or control shRNA were treated with BDA-366 (1μM) for 72 hr. The level of apoptotic cell death was determined by analysis of Annexin-V/PI binding by FACS as we previously described (Deng et al., 2006; Deng et al., 2001). Importantly, depletion of endogenous Bcl2 from these lung cancer cell lines by Bcl2 shRNA1, shRNA2 or shRNA3 resulted in significantly reduced sensitivity of cells to BDA-366 (Figure S1G).
We further examined whether expression of exogenous Bcl2 can restore the sensitivity of Bcl2-silenced lung cancer cells to BDA-366, and whether the BDA-366/BH4 binding is essential for such effect. WT and BDA-366 binding deficient D10A/N11A/R12A/E13A (AAAA) Bcl2 mutant cDNA in pCIneo or vector-only control were transfected into H460 or DMS53 cells expressing Bcl2 shRNA1. It has been reported that if the shRNA is directed to the 3′ UTR or 5′ UTR of the gene, the effect of the shRNA can be rescued by ectopically expressing the protein using the wild-type or mutant cDNA (Lassus et al., 2002; Lim et al., 2009). Because Bcl2 shRNA1 targets the 5′UTR of endogenous Bcl2, the silencing effect of shRNA1 on Bcl2 expression could be rescued by transfection of exogenous WT or AAAA Bcl2 mutant, as shown in Figure S1H. After transfection, cells were treated with BDA-366 or the BH3 mimetic ABT-199 at the indicated concentrations for 72 hr. Results reveal that expression of exogenous WT Bcl2 restored sensitivity of cells to both BDA-366 and ABT-199 (Figures S1I and S1J), indicating that both BDA-366 and ABT-199 are selective Bcl2 inhibitors. However, expression of AAAA only restored the sensitivity of cells to ABT-199 but not to BDA-366 (Figures S1I and S1J), indicating that compound mutations in the BH4 domain result in a selective resistance to BDA-366 while maintaining sensitivity to the BH3 mimetic (ABT-199). These data suggest that BDA-366, but not ABT-199, acts as a unique BH4 domain inhibitor. The apoptotic effect of BDA-366 on lung cancer cells requires its BH4 binding.
To test whether BDA-366 reacts with DNA as a crosslinker, electrophoretic mobility shift assay (EMSA) was performed as described (Bartel et al., 2012). Cisplatin, a known DNA binding agent, was used as positive control. Plasmid pUC19 DNA was incubated with BDA-366 or cisplatin. Intriguingly, cisplatin but not BDA-366 can bind to pUC19 DNA to cause mobility shift (Figure S1K). This helps to rule out the possibility of BDA-366 as DNA binding agent.
BDA-366 induces Bcl2 conformational change and abrogates Bcl2 survival function
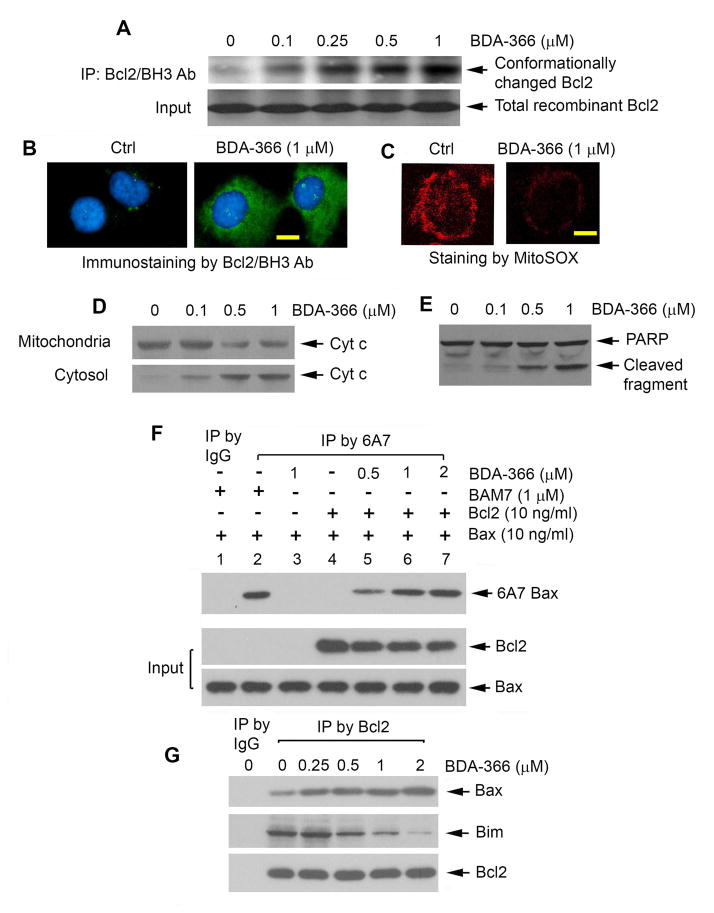
A prior report indicates that the interaction between the nuclear orphan receptor Nur77/TR3 and Bcl2 or the binding of p53 to Bcl2 causes a conformational change in Bcl2 that induces the “exposure” of its own BH3 domain, leading to loss of Bcl2’s antiapoptotic activity (Deng et al., 2006; Lin et al., 2004). Furthermore, removal of the BH4 domain from Bcl2 by caspase 3 results in a conversion of Bcl-2 from a survival protein to a Bax-like death molecule (Cheng et al., 1997). Since BDA-366 is a BH4 domain binding molecule, its binding to BH4 may result in a similar conformational change in Bcl2 to alter its function. It has been reported that the Bcl2/BH3 domain-specific antibody can detect conformational change in the BH3 domain of Bcl2 (Deng et al., 2006; Deng et al., 2009; Lin et al., 2004). To test whether BDA-366 directly induces Bcl2 conformational change, an in vitro cell-free assay was employed. Purified recombinant Bcl2 protein was incubated with increasing concentrations of BDA-366 in lysis buffer, followed by immunoprecipitation (IP) using the anti-Bcl2/BH3 domain antibody. Results indicate that addition of BDA-366 enhanced the ability of the Bcl2/BH3 domain-specific antibody to bind Bcl2, and this occurred in a dose-dependent manner (Figure 2A). These findings appear to provide direct evidence for the notion that binding of BDA-366 with the BH4 domain is able to alter Bcl2’s conformation leading to greater exposure of its own BH3 domain. Since only BDA-366 but not a chemotherapeutic agent (i.e. VP-16 or cisplatin) can directly induce Bcl2 conformational change in a cell-free system (Figure S2A), this shows specificity for BDA-366 induction of such conformational change.
Figure 2. BDA-366 induces Bcl2 conformational change via exposure of its BH3 domain, leading to conversion of Bcl2 from a survival into a killer molecule.
(A) Purified recombinant Bcl2 was treated with increasing concentrations of BDA-366 in 1% CHAPS lysis buffer, followed by IP using anti-Bcl2/BH3 domain antibody. BH3 domain-exposed Bcl2 was analyzed by Western blot using Bcl2 antibody. (B) and (C) H460 cells were treated with BDA-366 for 24h, followed by immunostaining using anti-Bcl2/BH3 domain antibody or MitoSOX™ red staining, respectively. Scale bar represents 5 um. (D) and (E) H460 cells were treated with increasing concentrations of BDA-366. Cyt c release and PARP cleavage was analyzed. (F) Purified Bcl2 in the absence or presence of Bax protein was treated with BDA-366 or BAM7, followed by IP using 6A7 Bax antibody. Conformationally changed Bax (i.e. active form of Bax) was analyzed by Western blot using Bax antibody. (G) H460 cells were treated with increasing concentrations of BDA-366. A co-IP was performed using agarose-conjugated Bcl2 antibody. Bcl2-associated Bax or Bim was analyzed by Western blot. See also Figure S2.
BDA-366-induced Bcl2 conformational change was also confirmed by immunofluorescence using a Bcl2/BH3-domain specific antibody as described (Deng et al., 2006; Deng et al., 2009; Lin et al., 2004). Bcl2 immunofluorescence was low or undetectable in untreated H460 cells, and was enhanced significantly in cells treated with BDA-366 (Figure 2B). However, treatment of H460 cells with BDA-366 did not significantly affect Bcl2 expression level (Figure S2B). These findings suggest that the negative regulation of Bcl2 activity by BDA-366 occurs through conformational change and not by a change in its expression.
Because treatment of H460 cells with BDA-366 resulted in mitochondrial dysfunction (i.e. reduced MitoSOX™ red staining and cytochrome c (Cyt c) release) and apoptosis (i.e. PARP cleavage) (Figures 2C, 2D and 2E), the binding of BDA-366 to the BH4 domain may cause exposure of the Bcl2 BH3 domain, which in turn renders Bcl2 eventually able to activate Bax, leading to apoptosis. To test this possibility, a 6A7 Bax antibody that only recognizes the conformationally changed, active, form of Bax was employed (Hsu et al., 1997; Hsu and Youle, 1998). First, purified Bcl2 was treated with BDA-366 in lysis buffer for 1 hr. The mixture of Bcl2/BDA-366 was then incubated with purified recombinant Bax for another 2 hr, followed by IP using 6A7 antibody. Results indicate that BDA-366-treated Bcl2 but not Bcl2 alone enhanced the ability of Bax to bind to 6A7 antibody (Figure 2F, lane 4 vs. lanes 5–7), indicating that BDA-366-treated Bcl2 can induce Bax conformational change leading to Bax activation. BAM7 is a recently identified Bax activator that can directly activate Bax by binding to a Bax trigger site (Gavathiotis et al., 2012). Importantly, BAM7 but not BDA-366 can directly activate Bax (Figure 2F, lane 2 vs. lane 3). These findings indicate that BDA-366 is not a direct Bax activator but can induce Bcl2-dependent Bax activation (Figure 2F, lane 3 vs. lanes 5–7). To test whether BDA-366-induced Bcl2 conformational change increases interaction between Bcl2 and Bax in cells, co-IP experiments were performed following treatment of H460 cells with BDA-366. Results reveal that BDA-366 enhanced Bcl2/Bax interaction in association with decreased Bcl2/Bim binding (Figure 2G), suggesting that the BH3-exposed Bcl2 induced by BDA-366 may have greater ability to interact with Bax than Bim leading to activation of Bax’s cell killing function via a 6A7 conformational change in cancer cells.
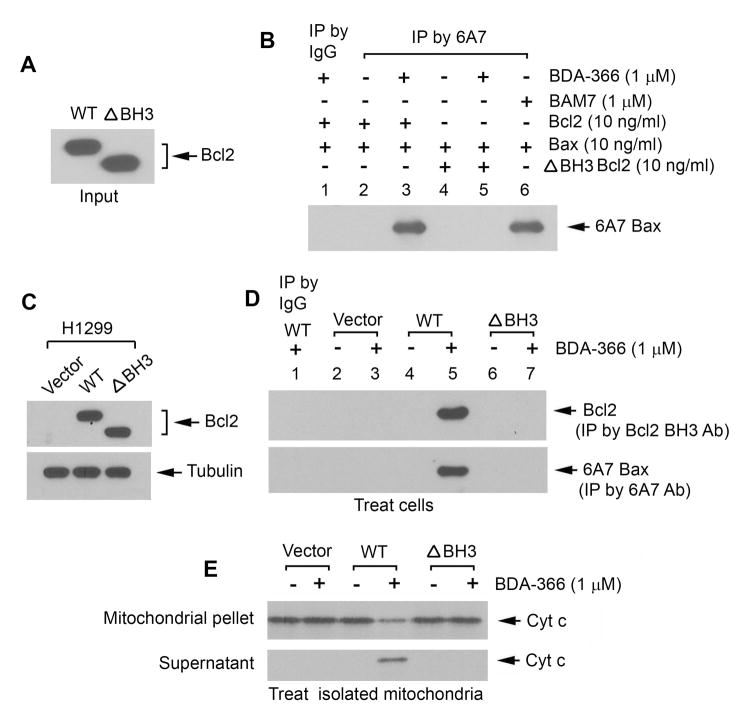
To further test whether the BH3 domain of Bcl2 is essential for BDA-366 activation of Bax, first, purified recombinant WT and the BH3 deletion (ΔBH3) mutant Bcl2 proteins (Figure 3A) were incubated with purified Bax protein in the absence or presence of BDA-366, followed by IP with 6A7 antibody. Results indicate that, in the presence of BDA-366, WT but not the ΔBH3 mutant Bcl2 can activate Bax via 6A7 conformational change in a cell-free system (Figure 3B). To test this at the cellular or mitochondrial level, WT, ΔBH3 Bcl2 mutant and empty vector (pCIneo) were transfected into H1299 cells that express undetectable levels of endogenous Bcl2 but high levels of endogenous Bax (Figure 3C). Cells or isolated mitochondria from these cells were treated with BDA-366, followed by IP with Bcl2 BH3 or 6A7 antibody, respectively. Results indicate that BDA-366 induced Bcl2 conformational change in association with Bax activation in cells or isolated mitochondria containing WT Bcl2 but not in cells or isolated mitochondria expressing ΔBH3 Bcl2 mutant or vector-only control (Figures 3D and S3A). These results suggest that exposure of Bcl2 BH3 domain induced by BDA-366 is required for BDA-366 activation of Bax in cells or isolated mitochondria. To further test whether BDA-366 induces Cyt c release from mitochondria in a Bcl2 dependent fashion, mitochondria were isolated from H1299 cells expressing WT, ΔBH3 mutant Bcl2 or vector-only control. The isolated mitochondria were treated with BDA-366 (1μM) for 30 min at 30°C. After centrifugation, Cyt c in the supernatant (i.e. Cyt c release) was analyzed by Western blot. Results reveal that BDA-366 induced Cyt c release from the mitochondria isolated from WT Bcl2 but not from ΔBH3 and vector-only control cells (Figure 3E). This indicates that BDA-366-induced Cyt c release occurs in a Bcl2-dependent fashion, which also requires the BH3 domain in Bcl2.
Figure 3. BH3 domain in Bcl2 is essential for BDA-366 regulation of Bcl2 and Bax function and Cytc release.
(A) 10ng of purified WT and BH3 deletion mutant (ΔBH3) Bcl2 protein was analyzed by Western blot. (B) Purified WT or ΔBH3 mutant Bcl2 in the absence or presence of Bax protein was treated with BDA-366 or BAM7, followed by IP using 6A7 Bax antibody. Conformationally changed Bax was analyzed by Western blot using Bax antibody. (C) WT and BH3 deletion mutant (ΔBH3) Bcl2 were stably expressed in H1299 cells. (D) H1299 cells expressing WT, ΔBH3 or vector-only control were treated with BDA-366 for 24h, followed by co-IP using Bcl2 BH3 specific or 6A7 antibody, respectively. (E) Isolated mitochondria from H1299 cells expressing WT, ΔBH3 or vector-only control were treated with BDA-366 in mitochondrial buffer for 30 min at 30°C. After centrifugation, Cyt c in supernatant or mitochondrial pellet was analyzed by Western blot. See also Figure S3.
BDA-366 induces apoptotic cell death in a Bax-dependent manner
Our findings above reveal that the BDA-366-induced conformationally changed Bcl2 (i.e. with exposed BH3 domain) can activate Bax (Figures 2 and 3). To further determine whether Bax is essential for BDA-366 induction of apoptosis, we tested the apoptotic effect of BDA-366 on wild type (WT) and Bax knockout (Bax−/−) MEF cells. Intriguingly, Bax deficient MEF cells were significantly resistant to BDA-366 as compared to WT MEF cells (Figures S3B and S3C), indicating that activation of Bax by conformationally changed Bcl2 may be an essential step for the eventual induction of apoptosis by BDA-366.
BDA-366 induces calcium (Ca2+) release via inhibition of Bcl2/IP3R interaction
Bcl2 has been reported to inhibit Ca2+-driven apoptosis by direct interaction with the inositol 1,4,5-trisphosphate (IP3) receptor via the BH4 domain (Monaco et al., 2012; Rong et al., 2009). Disruption of BH4/IP3R association by synthetic BH4 binding peptide resulted in increased Ca2+ release and apoptosis (Rong et al., 2008; Zhong et al., 2011). To test whether BDA-366/BH4 binding affects the IP3R/Bcl2 interaction and Ca2+ release, H460 cells were treated with increasing concentrations of BDA-366 for 24h. Results reveal that BDA-366 reduced Bcl2/IP3R binding in association with increased Ca2+ release (Figures S3D and S3E). These findings may uncover an additional mechanism by which BDA-366 induces apoptosis in a BH4 binding-dependent manner.
BDA-366 induces autophagic cell death of human lung cancer cells
It is well known that Bcl2 directly interacts with Beclin-1 and suppresses Beclin-1-dependent autophagy (Pattingre et al., 2005). Inhibition of Bcl2 by ABT-737 not only induces apoptosis but also autophagy (Maiuri et al., 2007). Importantly, a recent report indicates that removal of the BH4 domain from Bcl2 protein promotes an autophagic process that impairs tumor growth (Trisciuoglio et al., 2013). To test whether disruption of Bcl2 BH4 by BDA-366 stimulates autophagy, H460 cells were treated with BDA-366 (1 μM) for 24h. Increased levels of LC3-II were observed after treatment with BDA-366 (Figure S3F). To further quantify the level of autophagy, a GFP-LC3 construct was used to indicate autophagosomes as described (Liang et al., 2006; Maiuri et al., 2007). After treatment with BDA-366, GFP-LC3 redistributes from a diffuse staining pattern in the cytoplasm and nucleus to a cytoplasmic punctate structure that specifically labels pre-autophagosomal and autophagosomal membranes (i.e. GFP-LC3 vac cells) (Figure S3G). Treatment of cells with BDA-366 significantly increases the percentage of GFP-LC3vac cells as compared to DMSO control (Figure S3H). These findings reveal that, in addition to apoptosis, BDA-366 may also stimulate autophagic cell death by inhibiting Bcl2 activity in human lung cancer cells.
BDA-366 suppresses lung cancer growth via induction of apoptosis in animal models
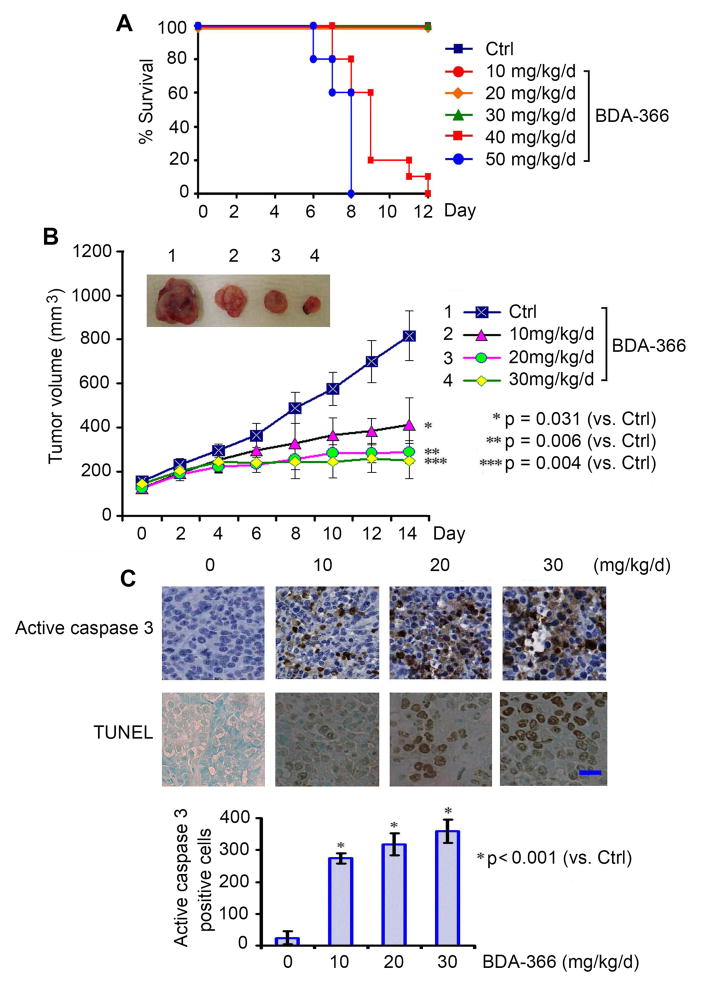
In order to define the appropriate doses for in vivo experimentation, we first determined the maximum tolerated dose (MTD) as previously described (Park et al., 2013). Nu/Nu nude mice were treated in groups of 6 per dose level with increasing doses of BDA-366 (10–50mg/kg/d) intraperitoneally (i.p.) for up to 12 days. Doses of 40 and 50mg/kg/d were uniformly lethal in the 6 mice within 8 or 12 days, respectively (Figure 4A). The dose range between 10 and 30mg/kg/d was tolerable with no death recorded for up to 12 days of daily administration (Figure 4A). To uncover the mechanism of lethality, in addition to 12-day treatment at the MTD as shown in Figure 4A, we also measured standard single-dose MTD treatment in normal C57BL/6 mice as described (Hamblett et al., 2004). Treatment of mice with a single dose of 300mg/kg i.p. did not cause weight loss or other toxicities, including hematologic, liver and kidney functions (Figures S4A, S4B and S4C). However, a single dose of 400mg/kg resulted in death of mice in 4 days. Both ALT and AST were significantly enhanced (Figures S4A and S4B). Pathological analysis for all organs indicated microsteatosis in the liver and atrophic white pulp in the spleen (Figure S4C). Based on these findings, mice might die mainly from liver damage at a single 400mg/kg dose. Thus, the single dose MTD of BDA-366 should range from 300–400 mg/kg. 10% of single dose MTD can usually be considered as the maximum therapeutic dose (30~40mg/kg) for continuous treatment. We therefore considered doses between 10 and 30mg/kg/d to be relatively safe.
Figure 4. BDA-366 potently represses lung cancer in vivo.
(A) Nu/Nu nude mice were treated with increasing doses (10–50mg/kg/d) of BDA-366 by i.p. for 12 days (n = 6 mice per group). The percentage survival of mice was calculated. (B) Nu/Nu nude mice with H460 lung cancer xenografts were treated with increasing doses of BDA-366 (0, 10, 20 and 30mg/kg/d) for 14 days (n = 6 mice per group). Tumor volume was measured once every 2 days. After 14 days, the mice were sacrificed and the tumors were removed and analyzed. All P values are compared with control group. Error bars represent ± S.D. (C) Active caspase 3 by IHC staining or TUNEL assay was performed in tumor tissues at the end of experiments and quantified as described in “Supplemental Experimental Procedures”. Scale bar represents 10 um. See also Figure S4.
To test the potency of BDA-366 in vivo, lung cancer xenografts derived from H460 cells were treated with increasing doses (0, 10, 20, 30mg/kg/d) of BDA-366 via i.p. for 14 days. Results show that treatment of mice with BDA-366 resulted in a dose-dependent repression of lung cancer in vivo (Figure 4B). To assess whether BDA-366 induced suppression of tumor growth via apoptosis in vivo, representative samples from harvested tumor tissues were analyzed by immunohistochemistry (IHC) for active caspase 3 or TUNEL assay as described (Hao et al., 2012; Oltersdorf et al., 2005). A dose-dependent apoptosis induction was observed in tumor tissues after BDA-366 treatment (Figure 4C). Importantly, doses of 10–30mg/kg/d not only potently suppressed tumor growth but were also well tolerated without significant toxicity to mice. Slight weight loss was observed in mice treated with the dose of 30mg/kg but there were no decreases in neutrophils, lymphocytes, red blood cells (RBC) and platelets (PLT) in blood. Tests of kidney (BUN) and liver (ALT and AST) function were in the normal range (Figures S4D and S4E). Histopathology of harvested normal tissues (heart, liver, lung, brain, spleen, kidney, intestine, etc.) revealed no evidence of normal tissue toxicities after treatment with doses of 10–30mg/kg/d (Figure S4F). These findings suggest that doses between 10mg/kg and 30mg/kg provide the optimal therapeutic index for BDA-366 for in vivo experimentation involving lung cancer xenografts.
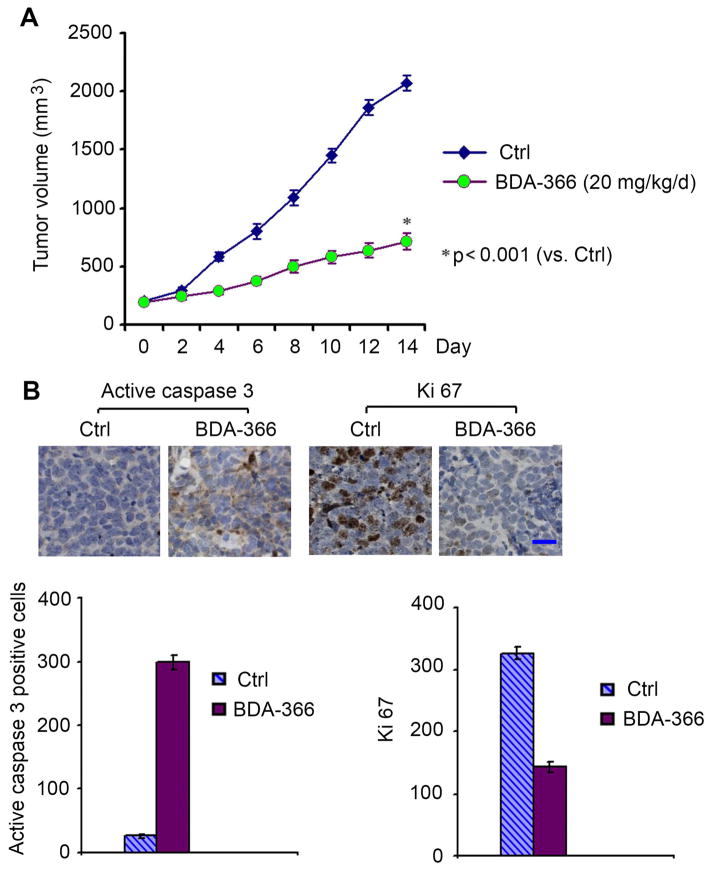
In addition to NSCLC cell lines, BDA-366 also efficiently suppressed growth of SCLC cell lines (Figure 1). To evaluate the anti-tumor activity of BDA-366 against SCLC in vivo, we established patient-derived xenografts (PDX), without intervening in vitro culture, which are expected to better recapitulate the SCLC tumor setting. We tested the potency of BDA-366 (20mg/kg/d) administered for 2 weeks in a PDX obtained from a patient with refractory SCLC. Intriguingly, BDA-366 potently suppressed the growth of the SCLC PDX, which occurred through apoptosis in tumor tissues (Figure 5). These findings indicate that BDA-366 may potentially be effective in patients with SCLC where there are currently limited treatment options.
Figure 5. BDA-366 represses tumor growth in xenografts derived from SCLC patient.
Mice carrying xenografts derived from a patient with refractory SCLC were treated with BDA-366 (20mg/kg/d) via i.p. for 2 weeks (n = 6 mice per group). Tumor volume (A) and active caspase 3 in tumor tissues (B) were analyzed. Error bars represent ± S.D. Scale bar represents 10 um.
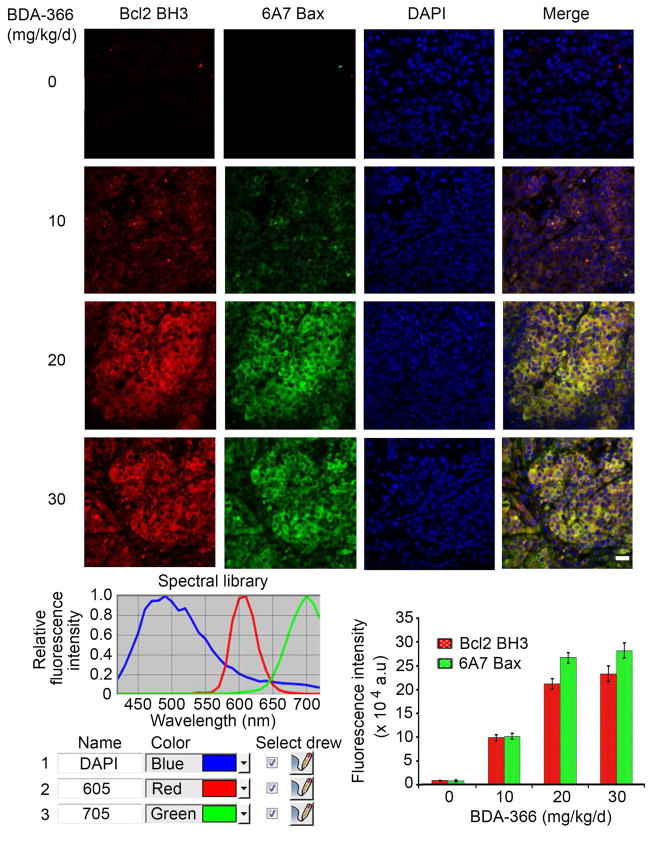
BDA-366 induces Bcl2 conformational change in tumor tissues
Quantum dot-based immunohistofluorescence (QD-IHF) technology has recently been developed as a valuable tool for simultaneous and concurrent immunostaining of multiple biomarkers in formalin-fixed paraffin-embedded (FFPE) tissues, thereby allowing for quantification of several biomarkers simultaneously on the same tissue slide (Li et al., 2013). To determine whether BDA-366 induces Bcl2 conformation change by exposure of its BH3 domain in tumor tissues and whether the BH3 domain-exposed Bcl2 activates Bax, conformational changes in both Bcl2 and Bax were simultaneously analyzed by QD-IHF on the same tissue slide. The antibody 6A7 can selectively recognize Bax after the conformational change associated with membrane insertion that occurs in apoptotic cells (Hsu et al., 1997; Hsu and Youle, 1998). QD images showed that treatment of H460 xenograft mice with BDA-366 for 14 days resulted in a dose-dependent exposure of the Bcl2 BH3 domain in tumor tissues (Figure 6). Intriguingly, BDA-366-induced Bcl2 conformational change was associated with an increased level of 6A7 binding to Bax (i.e. an increase in the level of the active form of Bax; Figure 6), suggesting that the BH3-exposed Bcl2 may activate Bax leading to apoptosis in tumor tissues (Figure 4C). There were no significant changes in total Bcl2 and Bax levels in tumor tissues after BDA-366 treatment (Figure S5).
Figure 6. BDA-366 induces BH3 domain exposure in Bcl2 in association with Bax activation in tumor tissues.
H460 lung cancer xenografts were treated with increasing doses of BDA-366 for 14 days (n = 6 mice per group, 3 sections per animal). Bcl2 and Bax conformation changes were analyzed in tumor tissues at the end of experiments by QD-IHF using anti-Bcl2/BH3 domain antibody or anti-6A7 Bax antibody, respectively, and quantified as described in “Supplemental Experimental Procedures”. Scale bar represents 10 um. Error bars represent ± S.D. See also Figure S5.
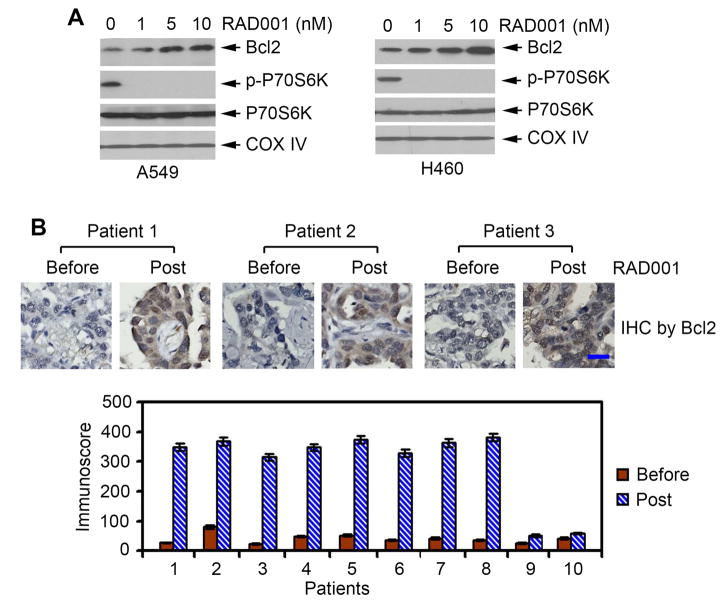
mTOR inhibition up-regulates Bcl2 in lung cancer cell lines and in tumor tissues from patients with NSCLC
Rapamycin and its derivative RAD001 (i.e. everolimus) are potent allosteric inhibitors of mTOR (Legrier et al., 2007). RAD001 is well tolerated but shows limited antitumor activity in patients with lung cancer (Besse et al., 2014; Ramalingam et al., 2013; Tarhini et al., 2010). Previous reports indicate that expression of Bcl2 is associated with resistance of cancer cells to mTOR inhibitors (Aguirre et al., 2004; Majumder et al., 2004). To further test whether mTOR inhibition regulates Bcl2 expression, A549 and H460 cells were treated with increasing concentrations of RAD001 for 24 hr. Inhibition of mTOR by RAD001 resulted in up-regulation of Bcl2 in a dose-dependent manner in both A549 and H460 lung cancer cells (Figure 7A). To test whether a similar effect of RAD001 on Bcl2 expression occurs in patients, we analyzed Bcl2 expression by IHC in baseline and post-treatment tissue samples obtained from 10 NSCLC patients treated with RAD001 (5 or 10mg/day) for 28 days as part of a neoadjuvant clinical study of everolimus in patients with resectable NSCLC (Owonikoko et al., 2015). There was increased expression of Bcl2 in post-treatment tumor tissues compared to baseline samples (Figure 7B). These findings suggest that up-regulation of Bcl2 by mTOR inhibition may negatively affect the sensitivity of lung cancer to mTOR inhibitor. Thus, inhibition of Bcl2 may enhance the potency of mTOR inhibitor against lung cancer.
Figure 7. Treatment of lung cancer cells or patients with RAD001 up-regulates Bcl2.
(A) A549 or H460 cells were treated with increasing concentrations of RAD001 for 24h. Bcl2 and p-p70S6K were analyzed by Western blot. (B) 10 patients with NSCLC were treated with RAD001 (5mg or 10mg/day) for 28 days. Bcl2 in tumor tissues was analyzed by IHC using anti-Bcl2 antibody and quantified by analyzing immunoscore. Scale bar represents 10 um. Error bars represent ± S.D.
BDA-366 synergizes with mTOR inhibitor in suppression of lung cancer in vitro and in vivo
To test whether combined Bcl2 and mTOR inhibition shows the predicted synergistic activity against lung cancer cells, H460 cells were treated with BDA-366 (100nM), RAD001 (1nM) or the combination. SRB assay showed that either RAD001 or BDA-366 alone partially inhibited lung cancer cell growth. Intriguingly, the combination of RAD001 and BDA-366 resulted in significantly more growth inhibition (Figure S6A), indicating that combined Bcl2 and mTOR inhibition has greater activity than either single agent alone in inhibiting lung cancer cell growth. To more accurately analyze the degree of synergy between RAD001 and BDA-366, a combination index (CI) value was calculated as described in “Supplemental Experimental Procedures”. The CI value was less than 0.3 (i.e. 0.278), indicating that RAD001 and BDA-366 exhibit strong synergistic growth inhibition of lung cancer cells.
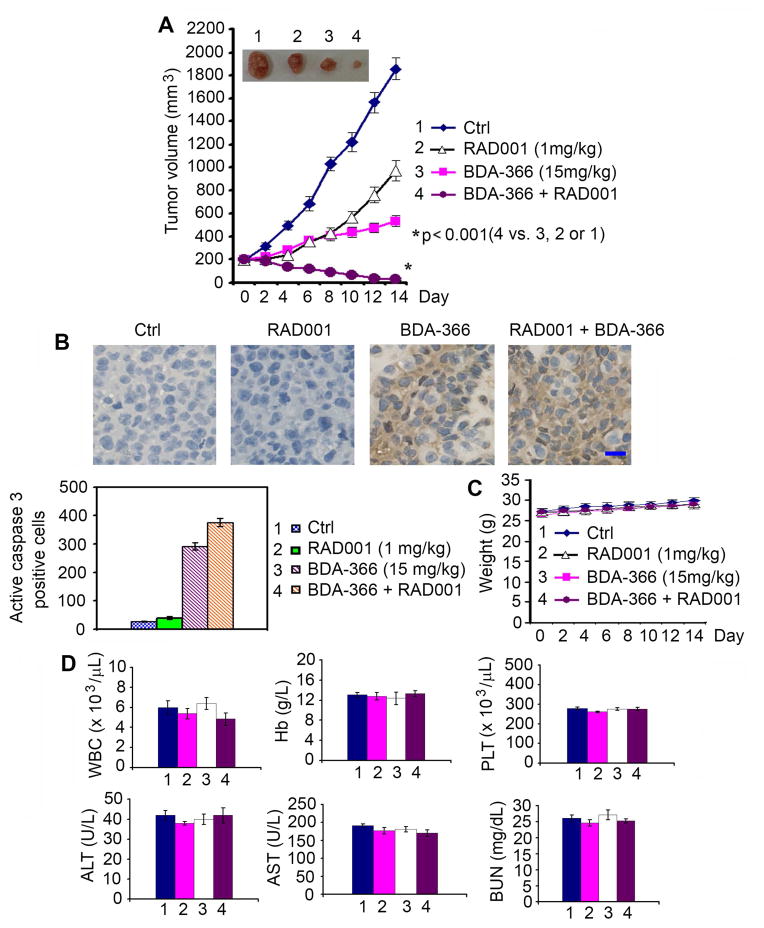
To test whether co-targeting Bcl2 and mTOR also synergistically represses lung cancer in vivo, mice with NSCLC (i.e. H460) xenografts were treated with RAD001 (1mg/kg/d), BDA-366 (15mg/kg/d) or the combination for 14 days. Intriguingly, the combination of BDA-366 and RAD001 exhibited a significantly greater efficacy than BDA-366 or RAD001 alone in suppressing lung tumor growth in vivo (Figure 8A), leading to sustained tumor repression. One of the 6 tumor-bearing mice treated with combined BDA-366 and RAD001 achieved complete tumor regression, which is still persisting as of the time of euthanization (4 months from the end of treatment on day 14). Importantly, RAD001 alone did not cause significant apoptosis in tumor tissues but significantly enhanced the apoptosis induced by BDA-366 in tumor tissues (Figure 8B). No weight loss or organ toxicities were observed in mice with these treatments, including combined treatment (Figures 8C, D and S6B).
Figure 8. Combination of BDA-366 and RAD001 synergistically represses lung cancer in vivo.
Nu/Nu mice with H460 lung cancer xenografts were treated with BDA-366, RAD001 or their combination by i.p. for 14 days (n = 6 mice per group). Tumor volume (A), active caspase 3 in tumor tissues (B), body weight (C) and blood (D) were analyzed. Scale bar represents 10 um. Error bars represent ± S.D. See also Figure S6.
DISCUSSION
Bcl2 is a major antiapoptotic Bcl2 family member that is directly involved in the suppression of apoptosis at the decision stage (Deng et al., 2006). Mimicking the BH3 domain to induce apoptosis has recently been used as a strategy for the development of Bcl2 inhibitors as anticancer drugs (Chonghaile and Letai, 2008; Kang and Reynolds, 2009; Oltersdorf et al., 2005). The BH3 mimetic agents function as competitive inhibitors by binding to the hydrophobic cleft of Bcl2/Bcl-XL (Chonghaile and Letai, 2008; Oltersdorf et al., 2005). Four Bcl2 inhibitors, including oblimersen sodium (G3139), gossypol (AT-101), obatoclax (GX15-070) and ABT-263, have already been tested in human clinical trials but showed limited clinical efficacy (Chonghaile and Letai, 2008; Kang and Reynolds, 2009).
The BH4 domain is required for the survival activity of Bcl2, and removal of this domain can convert Bcl2 from a survival to a killer molecule (Cheng et al., 1997), suggesting that the BH4 domain constitutes a promising structure-based target for the disruption of Bcl2’s survival function or conversion of Bcl2 into a death molecule. In this study, we have identified a class of Bcl2 antagonist (i.e. BDA-366) that targets the BH4 domain and is distinct from previous BH3 mimetics. BDA-366 directly binds to purified Bcl2 protein selectively at the BH4 domain with high affinity, with an inhibitory constant (Ki) value at the nanomolar level. BDA-366 failed to bind other Bcl2 family members (i.e. Bcl-XL, Mcl-1 and Bfl-1/A1), demonstrating the specificity of Bcl2/BDA-366 binding. The binding of BDA-366 with the BH4 domain resulted in Bcl2 conformational change and exposure of the BH3 domain in vitro and in vivo. This conformational change could activate Bax in a cell-free system and also in tumor tissues, suggesting that it is likely responsible for the death-inducing effect of BDA-336. These findings suggest a mechanistic model of using a small molecule inhibitor to convert an antiapoptotic Bcl2 family member(s) into a death molecule.
The multi-domain proapoptotic Bax provides the required gateway to apoptotic cell death (Wei et al., 2001). Since BDA-366 is dependent on Bax for apoptosis induction, this suggests its mechanism of action may be predominantly through the Bcl2 family. However, direct activation of Bax appears unlikely because BDA-366 failed to induce Bax conformational change in vitro.
BDA-366 demonstrated potent antitumor activity in lung cancer xenografts derived from either a lung cancer cell line or a patient-derived SCLC tumor. We determined the MTD of BDA-366 with a 12-day treatment to be between 30 and 40 mg/kg/d. Dose-response experiments revealed that doses of BDA-366 between 10 and 30mg/kg/d potently suppress lung cancer growth in vivo without platelet reduction or other significant normal tissue toxicity, indicating that this dose range should be effective and safe in murine lung cancer models. Since BDA-366 effectively suppressed the growth of PDX raised from a patient with refractory SCLC, there is a good possibility that BDA-366 may provide clinical utility in patients in the future.
Cancer cells overexpressing Bcl2 are resistant to mTOR inhibitor and down-regulation of Bcl2 restores sensitivity to mTOR inhibition (Aguirre et al., 2004; Majumder et al., 2004). Here, we discovered that inhibition of mTOR by RAD001 resulted in Bcl2 up-regulation in lung cancer cell lines and in tumor tissues from NSCLC patients treated with RAD001. It is possible that Bcl2 expression induced by mTOR inhibitor therapy may negatively affect the efficacy of mTOR inhibitor in lung therapy. This may help explain why RAD001 only had limited efficacy in lung cancer patients (Ramalingam et al., 2013; Tarhini et al., 2010). Our findings provide a strong rationale to propose that combined Bcl2 and mTOR inhibition should have superior therapeutic benefits over each agent alone. As expected, BDA-366 in combination with RAD001 exhibited strong synergistic activity against lung cancer in vitro and in vivo without significant normal tissue toxicity. Based on our data, we propose that co-targeting Bcl2 and mTOR offers a more effective strategy for lung cancer treatment.
In summary, we have identified a class of Bcl2 antagonist (BDA-366) that selectively targets the BH4 domain of Bcl2. The binding of BDA-366 with the BH4 domain results in conversion of Bcl2 from an antiapoptotic molecule into a death protein through a conformational change that exposes its BH3 death domain. The BH4 antagonist BDA-366 exhibits potent efficacy against human lung cancer in vivo without platelet reduction. Development of the BH4 antagonist as a class of anti-cancer agent offers additional strategy for lung cancer therapy.
EXPERIMENTAL PROCEDURES
Detailed experimental information and reagents used in this study is provided in the Supplementary Experimental Procedures.
Cell lines and cell culture
Normal small airway epithelial cells (SAEC) and lung cancer cell lines were purchased from the American Type Culture Collection (ATCC, Manassas, VA). SAEC cells were cultured in small airway epithelial growth media (SAGM™ BulletKit®, ATCC). NSCLC cell lines (i.e. H157, H358, H460, HCC827, H292, H1299, H1975 and Calu-1) were maintained in RPMI 1640 with 5% fetal bovine serum and 5% bovine serum. A549 cells were cultured in F-12K medium with 10% fetal bovine serum. WT and Bcl2 BH deletion mutants (i.e. ΔBH1, ΔBH2, ΔBH3 and ΔBH4) were created and stably expressed in H1299 cells as we previously described (Hou et al., 2007; Wang et al., 2008). SCLC cell lines DMS53, DMS114, DMS153, H128, H146 and H69 were cultured in Weymouth’s medium supplemented with 5% FBS and 5% bovine serum (BS) as described (Park et al., 2013). Wild type and Bax knockout (Bax−/−) MEF cells were obtained from Dr. Douglas R. Green (St Jude Children’s Research Hospital, Memphis, TN) and maintained in DMEM medium with 10% fetal bovine serum.
Human patient samples from clinical trial and establishment of patient-derived xenografts
Informed consent was obtained from all human subjects, and use of human samples for immunohistochemistry and patient-derived xenograft was approved by the institutional review board (IRB) of Emory University. Paired samples of NSCLC tissues were collected as part of a completed phase IB clinical trial evaluating the pharmacodynamic effects of RAD001 in adult patients with resectable NSCLC. Enrolled eligible patients received RAD001 (5 or10mg) orally once daily for 28 days (Owonikoko et al., 2015). Pretreatment biopsy samples and post treatment surgical resection specimens were analyzed for Bcl2 expression. Patient-derived xenograft was obtained as part of an IRB-approved phase II co-clinical trial of arsenic trioxide in patients with relapsed SCLC who have failed standard platinum-based chemotherapy. Tumor samples were obtained by image guided biopsy and were directly implanted into Nu/Nu nude mice without intervening propagation in plastic culture plates. Direct serial propagation of growing tumor occurred for up to 5 generations. The animal propagation protocol was approved by the Emory IACUC and the Emory Animal Ethics Committee.
Treatment of lung cancer xenografts
Lung cancer xenografts were generated as described as previously (Park et al., 2013). 6-week-old male Nu/Nu nude mice were purchased from Harlan and housed under pathogen-free conditions in microisolator cages. All animal treatments were undertaken in accordance with protocols approved by the Institutional Animal Care and Use Committee (IACUC) at Emory University. 3×106 lung cancer H460 cells were injected into subcutaneous tissue in the flank region of nude mice. The tumors were allowed to grow to an average volume of ~250 mm3 prior to initiation of therapy as described (Oltersdorf et al., 2005). Mice with lung cancer xenografts were treated with BDA-366, RAD001 or the combination intraperitoneally (i.p.) at the indicated dose. During treatment, tumor volume (V) was measured by caliper measurements once every 2 days and calculated with the formula: V=(LxW2)/2 (L: length; W: width) as described (Park et al., 2013). At the end of experiments, mice were euthanized by CO2 inhalation. Harvested tumor tissues were used for further analysis.
Statistical analysis
The statistical significance of differences between groups was analyzed with two-sided unpaired student’s t-test or Fisher’s exact test. Results were considered statistically significant at p<0.05. The IC50 values were calculated using SPSS Statistics software 18 (IBM). All data are presented as mean ± standard deviation (S.D.).
Supplementary Material
SIGNIFICANCE.
Here we report a small molecule (BDA-366) that selectively targets the BH4 domain of Bcl2 and converts Bcl2 from a survival molecule to a cell death inducer through a conformational change. Our results provide mechanistic insights into Bcl2 conversion by BDA-366 and illuminate a direction for the development of BH4-based anticancer agents. The inhibition of mTOR by RAD001 up-regulates Bcl2 in vitro and in vivo. Accordingly, a synergistic anti-tumor effect through combined treatment with BDA-366 and RAD001 was observed in vitro and in vivo. This study has significant translational value and may foster more effective strategy against lung cancer.
Highlights.
Small molecule BDA-366 specifically targets the BH4 domain of Bcl2
BDA-366 induces a conformational change of Bcl2 via exposure of its BH3 domain
BDA-366 converts Bcl2 from antiapoptotic molecule into a death protein
BDA-366 exhibits potent efficacy against human lung cancer in vitro and in vivo
Acknowledgments
We thank Anthea Hammond for editing of the manuscript. This work was supported by NCI, National Institutes of Health grant R01CA136534.
Footnotes
Disclosure of Potential Conflicts of Interest
The authors disclose no potential conflicts of interest
AUTHOR CONTRIBUTIONS
The project was conceived by X.D.. Experiments were performed by B.H., D.P., R.L., M.X.. PDX model was provided by T.K.O. and G.Z.. Histopathological study was performed by G.L.S. Chemical analysis was performed by C.D. and J.Z.. A.T.M. performed screening of small molecules. Z.G.C. and D.M.S. provided QD-IHF technology. T.K.O., S.S.R., F.R.K. and W.J.C. provided patient samples. The manuscript was written by X.D. and edited by T.K.O., S.S.R., B.H. and A.H.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aguirre D, Boya P, Bellet D, Faivre S, Troalen F, Benard J, Saulnier P, Hopkins-Donaldson S, Zangemeister-Wittke U, Kroemer G, Raymond E. Bcl-2 and CCND1/CDK4 expression levels predict the cellular effects of mTOR inhibitors in human ovarian carcinoma. Apoptosis. 2004;9:797–805. doi: 10.1023/B:APPT.0000045781.46314.e2. [DOI] [PubMed] [Google Scholar]
- Bartel C, Bytzek AK, Scaffidi-Domianello YY, Grabmann G, Jakupec MA, Hartinger CG, Galanski M, Keppler BK. Cellular accumulation and DNA interaction studies of cytotoxic trans-platinum anticancer compounds. J Biol Inorg Chem. 2012;17:465–474. doi: 10.1007/s00775-011-0869-5. [DOI] [PubMed] [Google Scholar]
- Besse B, Leighl N, Bennouna J, Papadimitrakopoulou VA, Blais N, Traynor AM, Soria JC, Gogov S, Miller N, Jehl V, Johnson BE. Phase II study of everolimus-erlotinib in previously treated patients with advanced non-small-cell lung cancer. Ann Oncol. 2014;25:409–415. doi: 10.1093/annonc/mdt536. [DOI] [PubMed] [Google Scholar]
- Bruncko M, Oost TK, Belli BA, Ding H, Joseph MK, Kunzer A, Martineau D, McClellan WJ, Mitten M, Ng SC, et al. Studies leading to potent, dual inhibitors of Bcl-2 and Bcl-xL. J Med Chem. 2007;50:641–662. doi: 10.1021/jm061152t. [DOI] [PubMed] [Google Scholar]
- Castelli M, Reiners JJ, Kessel D. A mechanism for the proapoptotic activity of ursodeoxycholic acid: effects on Bcl-2 conformation. Cell Death Differ. 2004;11:906–914. doi: 10.1038/sj.cdd.4401433. [DOI] [PubMed] [Google Scholar]
- Cheng EH, Kirsch DG, Clem RJ, Ravi R, Kastan MB, Bedi A, Ueno K, Hardwick JM. Conversion of Bcl-2 to a Bax-like death effector by caspases. Science. 1997;278:1966–1968. doi: 10.1126/science.278.5345.1966. [DOI] [PubMed] [Google Scholar]
- Chonghaile TN, Letai A. Mimicking the BH3 domain to kill cancer cells. Oncogene. 2008;27(Suppl 1):S149–157. doi: 10.1038/onc.2009.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Moissac D, Zheng H, Kirshenbaum LA. Linkage of the BH4 domain of Bcl-2 and the nuclear factor kappaB signaling pathway for suppression of apoptosis. J Biol Chem. 1999;274:29505–29509. doi: 10.1074/jbc.274.41.29505. [DOI] [PubMed] [Google Scholar]
- Deng X, Gao F, Flagg T, Anderson J, May WS. Bcl2’s flexible loop domain regulates p53 binding and survival. Mol Cell Biol. 2006;26:4421–4434. doi: 10.1128/MCB.01647-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng X, Gao F, May WS. Protein phosphatase 2A inactivates Bcl2’s antiapoptotic function by dephosphorylation and up-regulation of Bcl2-p53 binding. Blood. 2009;113:422–428. doi: 10.1182/blood-2008-06-165134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng X, Xiao L, Lang W, Gao F, Ruvolo P, May WS., Jr Novel role for JNK as a stress-activated Bcl2 kinase. J Biol Chem. 2001;276:23681–23688. doi: 10.1074/jbc.M100279200. [DOI] [PubMed] [Google Scholar]
- Enyedy IJ, Ling Y, Nacro K, Tomita Y, Wu X, Cao Y, Guo R, Li B, Zhu X, Huang Y, et al. Discovery of small-molecule inhibitors of Bcl-2 through structure-based computer screening. J Med Chem. 2001;44:4313–4324. doi: 10.1021/jm010016f. [DOI] [PubMed] [Google Scholar]
- Gavathiotis E, Reyna DE, Bellairs JA, Leshchiner ES, Walensky LD. Direct and selective small-molecule activation of proapoptotic BAX. Nature chemical biology. 2012;8:639–645. doi: 10.1038/nchembio.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamblett KJ, Senter PD, Chace DF, Sun MM, Lenox J, Cerveny CG, Kissler KM, Bernhardt SX, Kopcha AK, Zabinski RF, et al. Effects of drug loading on the antitumor activity of a monoclonal antibody drug conjugate. Clin Cancer Res. 2004;10:7063–7070. doi: 10.1158/1078-0432.CCR-04-0789. [DOI] [PubMed] [Google Scholar]
- Hao J, Madigan MC, Khatri A, Power CA, Hung TT, Beretov J, Chang L, Xiao W, Cozzi PJ, Graham PH, et al. In vitro and in vivo prostate cancer metastasis and chemoresistance can be modulated by expression of either CD44 or CD147. PLoS One. 2012;7:e40716. doi: 10.1371/journal.pone.0040716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirotani M, Zhang Y, Fujita N, Naito M, Tsuruo T. NH2-terminal BH4 domain of Bcl-2 is functional for heterodimerization with Bax and inhibition of apoptosis. J Biol Chem. 1999;274:20415–20420. doi: 10.1074/jbc.274.29.20415. [DOI] [PubMed] [Google Scholar]
- Hou Y, Gao F, Wang Q, Zhao J, Flagg T, Zhang Y, Deng X. Bcl2 impedes DNA mismatch repair by directly regulating the hMSH2-hMSH6 heterodimeric complex. J Biol Chem. 2007;282:9279–9287. doi: 10.1074/jbc.M608523200. [DOI] [PubMed] [Google Scholar]
- Hsu YT, Wolter KG, Youle RJ. Cytosol-to-membrane redistribution of Bax and Bcl-X(L) during apoptosis. Proc Natl Acad Sci U S A. 1997;94:3668–3672. doi: 10.1073/pnas.94.8.3668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu YT, Youle RJ. Bax in murine thymus is a soluble monomeric protein that displays differential detergent-induced conformations. J Biol Chem. 1998;273:10777–10783. doi: 10.1074/jbc.273.17.10777. [DOI] [PubMed] [Google Scholar]
- Hunter JJ, Bond BL, Parslow TG. Functional dissection of the human Bc12 protein: sequence requirements for inhibition of apoptosis. Mol Cell Biol. 1996;16:877–883. doi: 10.1128/mcb.16.3.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. CA Cancer J Clin. 2007;57:43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- Kang MH, Reynolds CP. Bcl-2 inhibitors: targeting mitochondrial apoptotic pathways in cancer therapy. Clin Cancer Res. 2009;15:1126–1132. doi: 10.1158/1078-0432.CCR-08-0144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelekar A, Thompson CB. Bcl-2-family proteins: the role of the BH3 domain in apoptosis. Trends Cell Biol. 1998;8:324–330. doi: 10.1016/s0962-8924(98)01321-x. [DOI] [PubMed] [Google Scholar]
- Konopleva M, Contractor R, Tsao T, Samudio I, Ruvolo PP, Kitada S, Deng X, Zhai D, Shi YX, Sneed T, et al. Mechanisms of apoptosis sensitivity and resistance to the BH3 mimetic ABT-737 in acute myeloid leukemia. Cancer Cell. 2006;10:375–388. doi: 10.1016/j.ccr.2006.10.006. [DOI] [PubMed] [Google Scholar]
- Lassus P, Rodriguez J, Lazebnik Y. Confirming specificity of RNAi in mammalian cells. Sci STKE. 2002;2002:pl13. doi: 10.1126/stke.2002.147.pl13. [DOI] [PubMed] [Google Scholar]
- Legrier ME, Yang CP, Yan HG, Lopez-Barcons L, Keller SM, Perez-Soler R, Horwitz SB, McDaid HM. Targeting protein translation in human non small cell lung cancer via combined MEK and mammalian target of rapamycin suppression. Cancer Res. 2007;67:11300–11308. doi: 10.1158/0008-5472.CAN-07-0702. [DOI] [PubMed] [Google Scholar]
- Li R, Hu Z, Sun SY, Chen ZG, Owonikoko TK, Sica GL, Ramalingam SS, Curran WJ, Khuri FR, Deng X. Niclosamide overcomes acquired resistance to erlotinib through suppression of STAT3 in non-small cell lung cancer. Molecular cancer therapeutics. 2013;12:2200–2212. doi: 10.1158/1535-7163.MCT-13-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang C, Feng P, Ku B, Dotan I, Canaani D, Oh BH, Jung JU. Autophagic and tumour suppressor activity of a novel Beclin1-binding protein UVRAG. Nat Cell Biol. 2006;8:688–699. doi: 10.1038/ncb1426. [DOI] [PubMed] [Google Scholar]
- Lim LY, Vidnovic N, Ellisen LW, Leong CO. Mutant p53 mediates survival of breast cancer cells. Br J Cancer. 2009;101:1606–1612. doi: 10.1038/sj.bjc.6605335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin B, Kolluri SK, Lin F, Liu W, Han YH, Cao X, Dawson MI, Reed JC, Zhang XK. Conversion of Bcl-2 from protector to killer by interaction with nuclear orphan receptor Nur77/TR3. Cell. 2004;116:527–540. doi: 10.1016/s0092-8674(04)00162-x. [DOI] [PubMed] [Google Scholar]
- Liu Y, Sun SY, Owonikoko TK, Sica GL, Curran WJ, Khuri FR, Deng X. Rapamycin induces Bad phosphorylation in association with its resistance to human lung cancer cells. Mol Cancer Ther. 2012;11:45–56. doi: 10.1158/1535-7163.MCT-11-0578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiuri MC, Le Toumelin G, Criollo A, Rain JC, Gautier F, Juin P, Tasdemir E, Pierron G, Troulinaki K, Tavernarakis N, et al. Functional and physical interaction between Bcl-X(L) and a BH3-like domain in Beclin-1. EMBO J. 2007;26:2527–2539. doi: 10.1038/sj.emboj.7601689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majumder PK, Febbo PG, Bikoff R, Berger R, Xue Q, McMahon LM, Manola J, Brugarolas J, McDonnell TJ, Golub TR, et al. mTOR inhibition reverses Akt-dependent prostate intraepithelial neoplasia through regulation of apoptotic and HIF-1-dependent pathways. Nat Med. 2004;10:594–601. doi: 10.1038/nm1052. [DOI] [PubMed] [Google Scholar]
- Monaco G, Decrock E, Akl H, Ponsaerts R, Vervliet T, Luyten T, De Maeyer M, Missiaen L, Distelhorst CW, De Smedt H, et al. Selective regulation of IP3-receptor-mediated Ca2+ signaling and apoptosis by the BH4 domain of Bcl-2 versus Bcl-Xl. Cell Death Differ. 2012;19:295–309. doi: 10.1038/cdd.2011.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oltersdorf T, Elmore SW, Shoemaker AR, Armstrong RC, Augeri DJ, Belli BA, Bruncko M, Deckwerth TL, Dinges J, Hajduk PJ, et al. An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Nature. 2005;435:677–681. doi: 10.1038/nature03579. [DOI] [PubMed] [Google Scholar]
- Owonikoko TK, Ramalingam SS, Miller DL, Force SD, Sica GL, Mendel J, Chen Z, Rogatko A, Tighiouart M, Harvey RD, et al. A translational, pharmacodynamic and pharmacokinetic phase IB clinical study of everolimus in resectable non-small cell lung cancer. Clin Cancer Res. 2015 doi: 10.1158/1078-0432.CCR-14-1998. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park D, Magis AT, Li R, Owonikoko TK, Sica GL, Sun SY, Ramalingam SS, Khuri FR, Curran WJ, Deng X. Novel small-molecule inhibitors of Bcl-XL to treat lung cancer. Cancer Res. 2013;73:5485–5496. doi: 10.1158/0008-5472.CAN-12-2272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattingre S, Tassa A, Qu X, Garuti R, Liang XH, Mizushima N, Packer M, Schneider MD, Levine B. Bcl-2 antiapoptotic proteins inhibit Beclin 1-dependent autophagy. Cell. 2005;122:927–939. doi: 10.1016/j.cell.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Ramalingam SS, Owonikoko TK, Behera M, Subramanian J, Saba NF, Kono SA, Gal AA, Sica G, Harvey RD, Chen Z, et al. Phase II study of docetaxel in combination with everolimus for second- or third-line therapy of advanced non-small-cell lung cancer. J Thorac Oncol. 2013;8:369–372. doi: 10.1097/JTO.0b013e318282709c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed JC, Zha H, Aime-Sempe C, Takayama S, Wang HG. Structure-function analysis of Bcl-2 family proteins. Regulators of programmed cell death. Adv Exp Med Biol. 1996;406:99–112. [PubMed] [Google Scholar]
- Rong YP, Aromolaran AS, Bultynck G, Zhong F, Li X, McColl K, Matsuyama S, Herlitze S, Roderick HL, Bootman MD, et al. Targeting Bcl-2-IP3 receptor interaction to reverse Bcl-2’s inhibition of apoptotic calcium signals. Mol Cell. 2008;31:255–265. doi: 10.1016/j.molcel.2008.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rong YP, Bultynck G, Aromolaran AS, Zhong F, Parys JB, De Smedt H, Mignery GA, Roderick HL, Bootman MD, Distelhorst CW. The BH4 domain of Bcl-2 inhibits ER calcium release and apoptosis by binding the regulatory and coupling domain of the IP3 receptor. Proc Natl Acad Sci U S A. 2009;106:14397–14402. doi: 10.1073/pnas.0907555106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sartorius UA, Krammer PH. Upregulation of Bcl-2 is involved in the mediation of chemotherapy resistance in human small cell lung cancer cell lines. Int J Cancer. 2002;97:584–592. doi: 10.1002/ijc.10096. [DOI] [PubMed] [Google Scholar]
- Schoenwaelder SM, Jarman KE, Gardiner EE, Hua M, Qiao J, White MJ, Josefsson EC, Alwis I, Ono A, Willcox A, et al. Bcl-xL-inhibitory BH3 mimetics can induce a transient thrombocytopathy that undermines the hemostatic function of platelets. Blood. 2011;118:1663–1674. doi: 10.1182/blood-2011-04-347849. [DOI] [PubMed] [Google Scholar]
- Song L, Coppola D, Livingston S, Cress D, Haura EB. Mcl-1 regulates survival and sensitivity to diverse apoptotic stimuli in human non-small cell lung cancer cells. Cancer Biol Ther. 2005;4:267–276. doi: 10.4161/cbt.4.3.1496. [DOI] [PubMed] [Google Scholar]
- Souers AJ, Leverson JD, Boghaert ER, Ackler SL, Catron ND, Chen J, Dayton BD, Ding H, Enschede SH, Fairbrother WJ, et al. ABT-199, a potent and selective BCL-2 inhibitor, achieves antitumor activity while sparing platelets. Nat Med. 2013;19:202–208. doi: 10.1038/nm.3048. [DOI] [PubMed] [Google Scholar]
- Tarhini A, Kotsakis A, Gooding W, Shuai Y, Petro D, Friedland D, Belani CP, Dacic S, Argiris A. Phase II study of everolimus (RAD001) in previously treated small cell lung cancer. Clin Cancer Res. 2010;16:5900–5907. doi: 10.1158/1078-0432.CCR-10-0802. [DOI] [PubMed] [Google Scholar]
- Trisciuoglio D, De Luca T, Desideri M, Passeri D, Gabellini C, Scarpino S, Liang C, Orlandi A, Del Bufalo D. Removal of the BH4 domain from Bcl-2 protein triggers an autophagic process that impairs tumor growth. Neoplasia. 2013;15:315–327. doi: 10.1593/neo.121392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tse C, Shoemaker AR, Adickes J, Anderson MG, Chen J, Jin S, Johnson EF, Marsh KC, Mitten MJ, Nimmer P, et al. ABT-263: a potent and orally bioavailable Bcl-2 family inhibitor. Cancer Res. 2008;68:3421–3428. doi: 10.1158/0008-5472.CAN-07-5836. [DOI] [PubMed] [Google Scholar]
- Vichai V, Kirtikara K. Sulforhodamine B colorimetric assay for cytotoxicity screening. Nat Protoc. 2006;1:1112–1116. doi: 10.1038/nprot.2006.179. [DOI] [PubMed] [Google Scholar]
- Wang JL, Liu D, Zhang ZJ, Shan S, Han X, Srinivasula SM, Croce CM, Alnemri ES, Huang Z. Structure-based discovery of an organic compound that binds Bcl-2 protein and induces apoptosis of tumor cells. Proc Natl Acad Sci U S A. 2000;97:7124–7129. doi: 10.1073/pnas.97.13.7124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Gao F, May WS, Zhang Y, Flagg T, Deng X. Bcl2 negatively regulates DNA double-strand-break repair through a nonhomologous end-joining pathway. Mol Cell. 2008;29:488–498. doi: 10.1016/j.molcel.2007.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei MC, Zong WX, Cheng EH, Lindsten T, Panoutsakopoulou V, Ross AJ, Roth KA, MacGregor GR, Thompson CB, Korsmeyer SJ. Proapoptotic BAX and BAK: a requisite gateway to mitochondrial dysfunction and death. Science. 2001;292:727–730. doi: 10.1126/science.1059108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie M, Yen Y, Owonikoko TK, Ramalingam SS, Khuri FR, Curran WJ, Doetsch PW, Deng X. Bcl2 induces DNA replication stress by inhibiting ribonucleotide reductase. Cancer research. 2014;74:212–223. doi: 10.1158/0008-5472.CAN-13-1536-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin XM, Oltvai ZN, Korsmeyer SJ. BH1 and BH2 domains of Bcl-2 are required for inhibition of apoptosis and heterodimerization with Bax. Nature. 1994;369:321–323. doi: 10.1038/369321a0. [DOI] [PubMed] [Google Scholar]
- Zhang H, Nimmer P, Rosenberg SH, Ng SC, Joseph M. Development of a high-throughput fluorescence polarization assay for Bcl-x(L) Anal Biochem. 2002;307:70–75. doi: 10.1016/s0003-2697(02)00028-3. [DOI] [PubMed] [Google Scholar]
- Zhong F, Harr MW, Bultynck G, Monaco G, Parys JB, De Smedt H, Rong YP, Molitoris JK, Lam M, Ryder C, et al. Induction of Ca(2)+-driven apoptosis in chronic lymphocytic leukemia cells by peptide-mediated disruption of Bcl-2-IP3 receptor interaction. Blood. 2011;117:2924–2934. doi: 10.1182/blood-2010-09-307405. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.