Abstract
Objective
Recent studies have demonstrated that earlier menarche is associated with increased risks of prediabetes and diabetes in white women; however, the associations have not been fully explored in Asian populations. We investigated the associations between age at menarche and prediabetes and/or diabetes in Korean middle-aged women.
Methods
This was a cross-sectional study of 2,039 premenopausal and postmenopausal women aged 44 to 56 years who visited the health promotion center for medical checkups. Participants were divided into three groups based on age at menarche: early (<13 y), average (13-16 y), and late (>16 y).
Results
The mean (SD) age at menarche was 14.6 (1.6) years. Of 2,039 women, 820 and 85 women had prediabetes (impaired fasting glucose and/or 5.7%-6.4% glycated hemoglobin) and diabetes, respectively. On logistic regression analysis, earlier menarche was significantly associated with prediabetes (odds ratio [OR], 1.80; 95% CI, 1.24-2.61; P = 0.002), diabetes (OR, 2.43; 95% CI, 1.04-5.69; P = 0.04), and dysglycemia (OR, 1.85; 95% CI, 1.28-2.66; P = 0.001), after adjusting for a number of confounding factors, compared with average age at menarche. On linear regression analysis, earlier age at menarche was significantly associated with increased fasting insulin, homeostatic model assessment for insulin resistance, homeostatic model assessment for β-cell function, body mass index, and waist circumference.
Conclusions
Age at menarche is inversely associated with various forms of dysglycemia. A history of earlier menarche may be helpful in predicting prediabetes and subsequent diabetes in Korean women.
Key Words: Diabetes, Prediabetes, Dysglycemia, Glycated hemoglobin, Menarche
Diabetes is an increasing global health and economic problem.1 Upon diagnosis of diabetes (even prediabetes), some individuals already experience various complications including microvascular and macrovascular complications.2-4 Thus, it has become more important to identify individuals at risk for diabetes earlier in life so that they may benefit from early intervention.
Age at menarche has been suggested to be associated with future risk of adverse health consequences, including higher body mass index (BMI),5 metabolic syndrome,6 cardiovascular disease,7 and mortality.7,8 A series of studies have shown an inverse correlation between age at menarche and risk of diabetes.9-13 However, relatively few inconsistent results on the association between age at menarche and prediabetes have been reported.11,14 In addition, most studies reporting an association on these issues have been conducted in western countries, whereas the association remains unclear in nonwhite populations, including Asian populations. The mechanisms that underlie these connections have yet to be fully identified. Studies have demonstrated associations between earlier age at menarche and elevated fasting glucose, fasting insulin, and insulin resistance5,8; however, the results have been inconclusive. Furthermore, some studies have suggested that the association between age at menarche and diabetes is completely mediated by obesity,9,10 whereas other studies have found no such relationship.11,12 Therefore, these issues must be revisited.
In this study, we determined whether there was an independent association between age at menarche and prediabetes or diabetes, and we clarified if these associations were mediated by adiposity in Korean women.
METHODS
Participants
This was a cross-sectional study of middle-aged women who visited the health promotion center at Kangbuk Samsung Hospital, Sungkyunkwan University (Seoul, Korea), for a routine medical checkup between November 2012 and March 2013. Inclusion criteria included age from 44 to 56 years, no serious illness (malignancy), and ability to understand a questionnaire. A total of 2,204 women were enrolled, and the participation rate was 71% (2,204 of 3,123). A questionnaire survey and blood analysis (as part of routine checkup) were completed by all participants. After the exclusion of individuals with no information or incomplete information on age at menarche (n = 140) and with any missing blood analysis data (n = 25), 2,039 women were available for analysis. This study was approved by the Internal Review Board of Kangbuk Samsung Hospital, and all participants provided a written informed consent form.
Data collection
At the time of the health checkup, a standard questionnaire was used to obtain information on age, educational level (high school or less, more than high school), household income (<4,000,000 KRW/mo, ≥4,000,000 KRW/mo), family status (married, living with a partner, living alone), current history of diabetes (yes, no), current use of insulin or hypoglycemic agents (yes, no), menopause status (premenopausal, postmenopausal), parity (number of pregnancies), smoking status (never, past or current), and alcohol intake (never, ever). Physical activity was assessed using the short “last-7-days” self-administered format of the International Physical Activity Questionnaire (http://www.ipaq.ki.se) and categorized into three levels: low, moderate, and high physical activity.
Height, body weight, and waist circumference (WC) were measured by trained healthcare providers, with the participant barefoot and wearing a light hospital gown. BMI was calculated as the ratio of weight in kilograms to height in meters squared. Blood samples were drawn from an antecubital vein after an overnight fast to determine glucose, insulin, and glycated hemoglobin (HbA1c) levels. The hexokinase method was used to measure fasting glucose levels (Hitachi Modular D2400; Roche, Tokyo, Japan). Fasting insulin levels were determined by electrochemiluminescence immunoassay (Hitachi Modular E170; Roche). HbA1c (reference range, 4.4%-6.4% [25-46 mmol/mol]) was measured by immunoturbidimetric assay using a Cobra Integra 800 automatic analyzer (Roche Diagnostics, Basel, Switzerland). Homeostatic model assessment for insulin resistance (HOMA-IR) and homeostatic model assessment for β-cell function (HOMA-β) were calculated as markers of insulin resistance and secretion15: HOMA-IR = (fasting insulin [μIU/mL] × fasting glucose [mmol/L]) / 22.5; HOMA-β = (20 × fasting insulin [μIU/mL] / fasting glucose [mmol/L] − 3.5).
Age at menarche
Age at menarche was defined as the first menstrual period. Participants were asked, “At what age did your first menstrual period begin?” Participants were divided into three categories based on age at menarche: early (<13 y), average (13-16 y), and late (>16 y) age at menarche.
Diabetes, prediabetes, and dysglycemia
Diagnoses of diabetes and prediabetes were made in accordance with the 2010 guidelines of the American Diabetes Association, in which HbA1c was newly adopted as one of the criteria.16 Diabetes was defined as self-reported history of physician-diagnosed diabetes, fasting plasma glucose (FPG) level of 126 mg/dL (7.0 mmol/L) or higher, HbA1c level of 6.5% (48 mmol/mol) or higher, or self-reported use of insulin or hypoglycemic medication. Prediabetes was defined as IFG with FPG levels of 100 to 125 mg/dL (5.6-6.9 mmol/L) or HbA1c levels of 5.7% to 6.4% (39-46 mmol/mol). Dysglycemia was indicated by the presence of either diabetes or prediabetes (IFG or elevated HbA1c). Oral glucose tolerance testing was not performed.
Statistical analysis
Basic characteristics of the study population were described and stratified by age at menarche (<13, 13-16, and >16 y). Continuous variables are expressed as means (SDs), whereas categorical variables are expressed as numbers (percentages). One-way analysis of variance was used to compare the means of continuous variables, and χ2 test was used to compare the proportions of categorical variables. Three outcome variables, such as prediabetes, diabetes, and dysglycemia (combined prediabetes and diabetes), were fitted for analysis. Logistic regression analyses were performed to assess the association between categorized age at menarche and prediabetes, diabetes, or dysglycemia, after adjusting for potential confounders. Model 1 adjusted for age. Model 2 additionally adjusted for education, income, spouse, menopause status, parity, smoking, alcohol intake, and physical activity. Model 3 additionally adjusted for BMI. Model 4 additionally adjusted for WC. We excluded individuals with diabetes from the model for prediabetes and individuals with prediabetes from the model for diabetes. Linear regression analyses were conducted to evaluate independent associations between age at menarche and diabetes risk markers such as FPG, fasting plasma insulin, HbA1c, HOMA-IR, HOMA-β, BMI, and WC. Natural logarithmic transformations were applied for all non–normally distributed variables. Confounders were adjusted as described for logistic regression models 1 and 2. All statistical tests were two-tailed, and P < 0.05 was considered to indicate significance. Statistical analyses were performed using SPSS version 21.0 (SPSS Inc, Chicago, IL).
RESULTS
Among the 2,039 women (mean [SD] age, 48.9 [3.5] y; range, 44-56 y), 55.6% had normal plasma glucose levels, 40.2% had prediabetes, and 4.2% had diabetes. Figure shows that the prevalences of diabetes, prediabetes, and dysglycemia were higher in women with earlier age at menarche. In addition, 1,443 (70.8%) women had been premenopausal at enrollment, and 596 (29.2%) had been postmenopausal at enrollment. The mean (SD) [range] age at menarche was normally distributed at 14.6 (1.6) [15] years; the mean (SD) [median] age at menopause was 47.4 (6.2) [49] years.
FIG. 1.
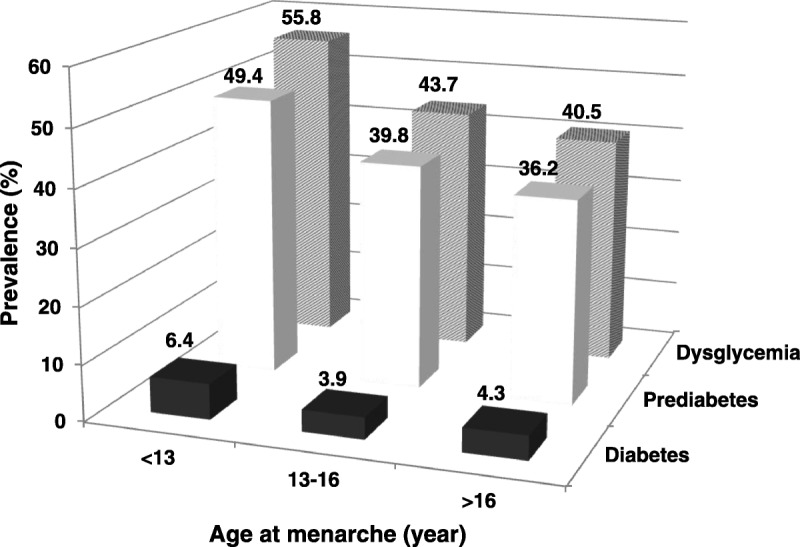
Prevalence of diabetes, prediabetes, and dysglycemia, by age at menarche.
The characteristics of the population stratified by age at menarche are shown in Table 1. In summary, women with earlier age at menarche were associated with higher education level (P < 0.001), marriage (P < 0.001), higher household income (P < 0.001), lower parity (P = 0.005), and premenopause (P < 0.001). Women with later age at menarche were more likely to consume alcohol (P = 0.009), had lower BMI (P = 0.001), and had lower WC (P = 0.03) than those with earlier age at menarche. In addition, women with earlier age at menarche more frequently had prediabetes and had higher fasting plasma insulin (P = 0.008), HOMA-IR (P = 0.01), HOMA-β (P = 0.03), and HbA1c (P = 0.02).
TABLE 1.
Baseline characteristics of the study population, by age at menarche
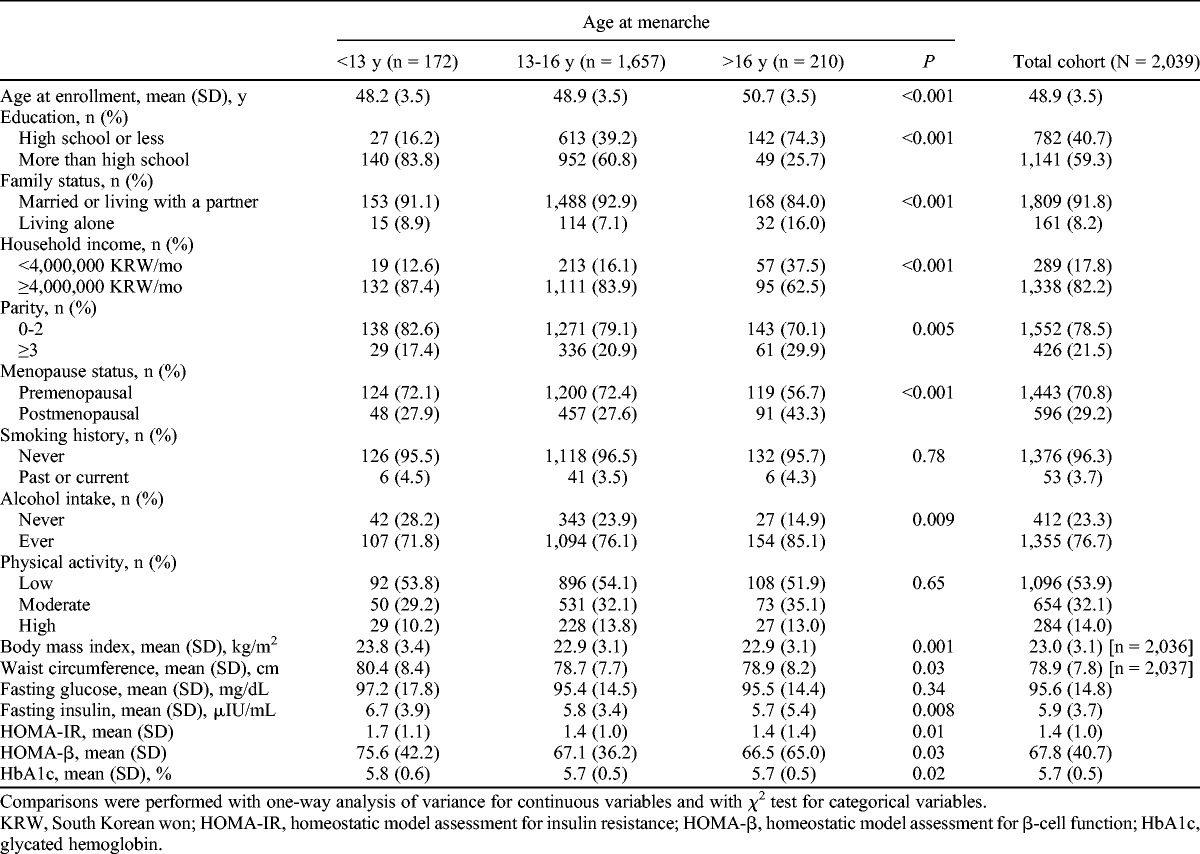
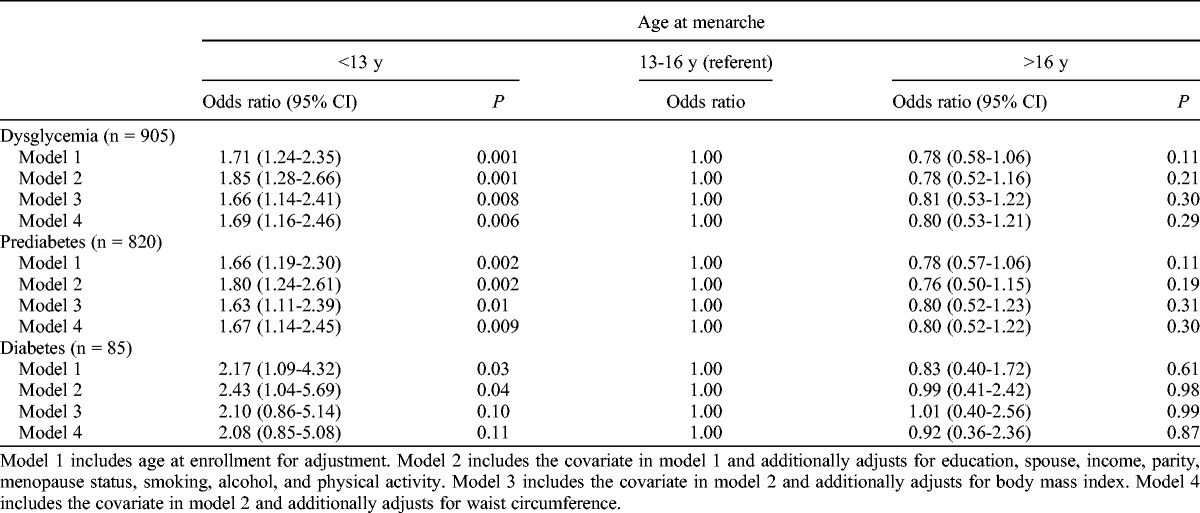
Table 2 presents the odds ratios (ORs) for diabetes, prediabetes, and dysglycemia (combined diabetes and prediabetes) by age at menarche. After adjustment for multivariate confounders, including age, education, spouse, income, parity, menopause status, smoking, alcohol, and physical activity, earlier age at menarche (<13 y) was associated with increased odds for diabetes (model 2: OR, 2.43; 95% CI, 1.04-5.69) compared with average age at menarche (13-16 y). The association was no longer significant after additional adjustment for current BMI (model 3: OR, 2.10; 95% CI, 0.86-5.14) or current WC (model 4: OR, 2.08; 95% CI, 0.85-5.08) as the CIs crossed unity. Earlier age at menarche was also associated with 80% and 85% increased risks of prediabetes and dysglycemia, respectively, after adjusting for multiple confounders (model 2: OR, 1.80; 95% CI, 1.24-2.61 for prediabetes; OR, 1.85; 95% CI, 1.28-2.66 for dysglycemia). These results were slightly attenuated but remained significant after additional adjustment for current BMI (model 3: OR, 1.63; 95% CI, 1.11-2.39 for prediabetes; OR, 1.66; 95% CI, 1.14-2.41 for dysglycemia) or WC (model 4: OR, 1.67; 95% CI, 1.14-2.45 for prediabetes; OR, 1.69; 95% CI, 1.16-2.46 for dysglycemia).
TABLE 2.
Odds ratios for dysglycemia, prediabetes, and diabetes, by age at menarche
Linear regression analysis (Table 3) showed that women with earlier age at menarche had significantly greater mean values for fasting insulin, HOMA-IR, and HOMA-β compared with those with average age at menarche, after adjusting for multiple covariates (model 2). The corresponding regression coefficients (β) were 0.13 (P = 0.01), 0.14 (P = 0.02), and 0.12 (P = 0.02), respectively. Those with later age at menarche did not have significant findings for fasting insulin, HOMA-IR, or HOMA-β values. Age at menarche was inversely associated with HbA1c in the age-adjusted model (model 1: β = 0.02, P = 0.007), but the significance was attenuated in a multivariate-adjusted model (model 2: β = 0.01, P = 0.11). No significant associations between age at menarche and fasting glucose were found in either unadjusted or multivariate-adjusted model.
TABLE 3.
Associations of age at menarche with metabolic risk markers of diabetes, by linear regression analyses
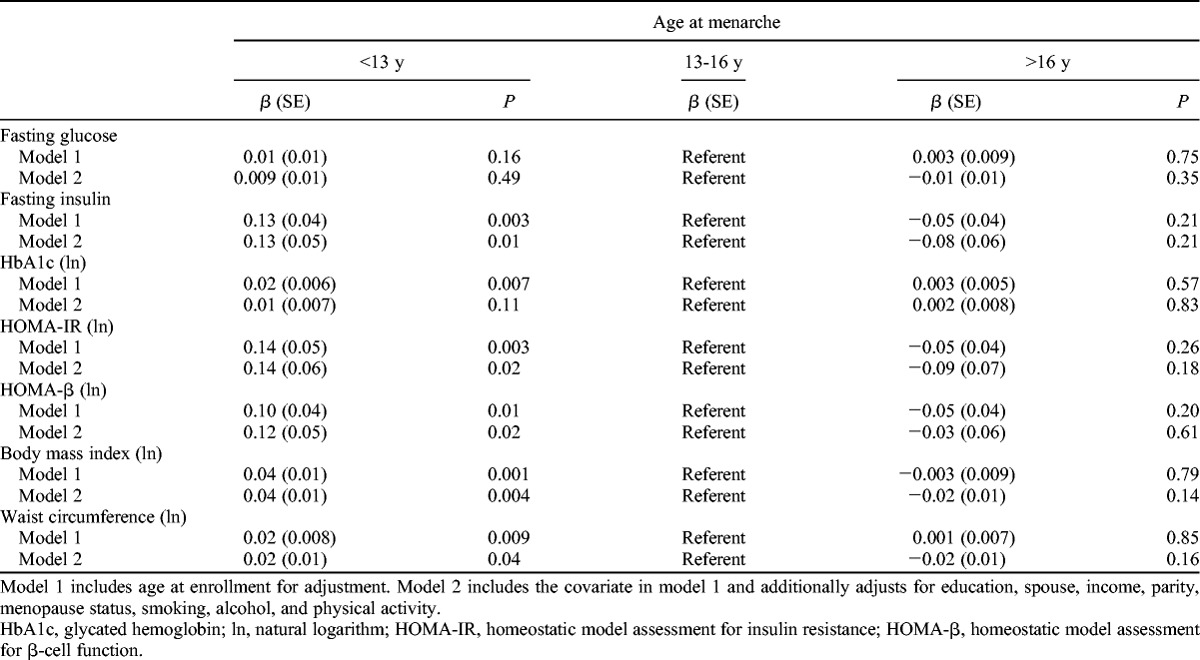
Age at menarche was also inversely associated with measures of body adiposity in linear regression models. Compared with women with average age at menarche, women with earlier age at menarche had higher BMI (β = 0.04, P = 0.004) and WC (β = 0.02, P = 0.04) even after adjusting for multiple covariates (model 2).
DISCUSSION
We found that earlier age at menarche was significantly associated with various forms of dysglycemia, even after adjusting for potential confounders. This is the first study to demonstrate a significant association between earlier age at menarche and increased risk of dysglycemia even at the prediabetes level in an Asian population. These associations were slightly attenuated after additional adjustment for adult adiposity, including BMI and WC. The significance remained in the prediabetes and dysglycemia models, whereas the association was no longer significant in the diabetes model. We also confirmed that earlier age at menarche was associated with elevated fasting insulin, HOMA-IR, HOMA-β, and adult adiposity (ie, BMI and WC). We did not find a significant association between age at menarche and levels of fasting glucose or HbA1c.
There are only a few studies on the association between age at menarche and risk of dysglycemia (including prediabetes), and they have shown inconsistent results.11,14,17 The German Cooperative Health Research in the Region of Augsburg F4 Study (1,503 women aged 32-81 y)11 found an inverse association between age at menarche and prediabetes and diabetes, even after adjusting for potential confounders and current BMI. A smaller study of 121 women with polycystic ovary syndrome17 reported that earlier age at menarche was associated with glucose intolerance (impaired glucose tolerance or type 2 diabetes). Our results are in agreement with these previous reports suggesting that earlier age at menarche is associated with prediabetes and diabetes. In contrast, the Rancho Bernardo Study (997 women aged 50-92 y) reported no association between age at menarche and abnormal glucose tolerance (IFG or impaired glucose tolerance) and type 2 diabetes, although glucose level was associated with early age at menarche.14
A series of studies demonstrating an association between age at menarche and risk of diabetes9-13 showed that the association was attenuated after adjusting for adult adiposity to varying degrees. The European Prospective Investigation into Cancer and Nutrition Norfolk cohort study (13,308 women aged 40-75 y) reported that earlier menarche was inversely associated with diabetes, but this effect seemed to be completely mediated by adult BMI or WC.9 The Nurses’ Health Study (101,415 women aged 34-59 y) showed increased risk of diabetes in the early menarche group; this association was completely attenuated by adulthood BMI. However, among younger women in the Nurses’ Health Study II (100,547 women aged 26-46 y), those with earlier age at menarche had increased risk of diabetes before and after adjusting for adiposity.10 The Atherosclerosis Risk in Communities study (8,491 women aged 45 to 65 y)12 and the European Prospective Investigation into Cancer and Nutrition InterAct study13 commonly showed that age at menarche was inversely associated with diabetes after adjusting for potential confounders; these associations were partially attenuated by adult adiposity but remained significant.
It is remarkable that the significant relationship between age at menarche and diabetes has been derived mainly from white populations. Three studies of Asian cohorts of women have also investigated the impact of age at menarche on diabetes risk.18-20 The Shanghai Women’s Health Study (69,385 Chinese women aged 40-70 y) showed that later age at menarche was related to a reduced risk of diabetes after adjusting for birth cohort, education, and household income, but the significance disappeared after further adjustment for baseline BMI.18 The Singapore Chinese Health Study (34,022 Chinese women aged 45-74 y) also showed that later age at menarche was significantly associated with a lower prevalence of diabetes in a graded manner even after adjusting for multiple confounders and baseline BMI.19 In another Chinese study among 3,304 postmenopausal women aged 21 to 92 years, age at menarche was not associated with risk of diabetes.20 Our results correspond with those of two former Chinese studies reporting that age at menarche is inversely associated with risk of diabetes; one remarkable difference was that later age at menarche in our study was not significantly associated with dysglycemia compared with the average menarche group. That the associations observed between earlier menarche and dysglycemia persisted in our study after adjusting for adulthood adiposity suggest that early age at menarche mediates this risk independently. Although the diabetes-only model did not reach statistical significance after additional adjustment for adiposity, it exhibited a similar trend toward an inverse association between age at menarche and diabetes. The disappearance of significance may be attributed to the small number of individuals with diabetes in our study.
Our findings on the association between early age at menarche and elevated fasting insulin, insulin resistance (HOMA-IR), and β-cell function (HOMA-β), compared with average age at menarche, are in agreement with previous studies.5,21 These findings provide further evidence that early age at menarche is associated with a risk for diabetes; however, the mechanisms underlying such an association are unclear.
Age at menarche is a key indicator of female maturation and the beginning of reproductive life. There has been a downward secular trend in age at menarche in the United States and Europe during the last century22,23 and in China during the past few decades.24 In South Korea, there has also been a decreasing secular trend in age at menarche, indicating a value of 0.68 years per decade (95% CI, 0.64-0.71) between 1920 and 1985 in a study using the Korean National Health and Nutrition Examination Survey III data,25 whereas diabetes prevalence increased during the same period,26 suggesting that earlier age at menarche may be associated with a risk of diabetes. The mechanism underlying this association is unclear. Several studies have reported that women who experience relatively early menarche tend to be more obese in later life5,27,28 and that obesity is associated with increased risk of insulin resistance and type 2 diabetes.29 Estrogen is responsible for the growth spurt and the onset of menstruation during puberty. Estrogen modulates growth hormone secretory activity in a biphasic manner at relatively low levels and stimulates insulin-like growth factor-1 (IGF-1) production through enhanced growth hormone secretion, whereas it inhibits IGF-1 production at high levels accompanying menarche.30,31 In addition, low IGF-1 levels are associated with increased risk of diabetes.32 Therefore, these findings lead to the conclusion that early exposure to estrogens probably influences the future development of diabetes.
The strengths of our study were the large sample size (comprising women of a nonwhite population) and direct measures of anthropometric indices. Moreover, women with prediabetes and diabetes were included in our study, allowing us to extend recent observations by others. In addition, our study population was a relatively homogeneous cohort of women; thus, the findings are less likely to be confounded by factors associated with socioeconomic status.
Nevertheless, several limitations also need to be acknowledged. First, we were unable to obtain information on childhood adiposity, which could influence both age at menarche and diabetes. Second, because age at menarche was reported by recall, misclassification may have occurred. However, previous studies have shown that recalled age at menarche in middle-aged women is highly correlated (r = 0.67-0.79) with original childhood information.33,34 Third, this study was restricted to mainly middle-class to upper-middle-class women in a metropolitan area. Because socioeconomic status is an important determinant of diabetes, the prevalence of women with diabetes may be low in this relatively healthy population. Thus, our results may not be generalizable to other socioeconomic groups. Fourth, we did not clinically distinguish between type 1 and type 2 diabetes. In contrast to type 2 diabetes, type 1 diabetes is associated with delayed menarche35; thus, it is possible that cases of type 1 diabetes in our study could have masked the subtle influence of early menarche on diabetes risk. Finally, cause-effect relationships could not be evaluated because of the cross-sectional design of this study.
CONCLUSIONS
Our study provides convincing evidence that earlier age at menarche is associated with increased risk of dysglycemia, independently of adulthood BMI, among Korean middle-aged women. Therefore, ascertaining a history of earlier menarche may help to identify women with future risk of prediabetes and subsequent diabetes.
Footnotes
Funding/support: This study was supported by funding (grant 2012-NG63001-00) from the Korea National Institute of Health.
Financial disclosure/conflicts of interest: None reported.
REFERENCES
- 1. Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care 2004; 27: 1047- 1053. [DOI] [PubMed] [Google Scholar]
- 2. Spijkerman AM, Dekker JM, Nijpels G, et al. Microvascular complications at time of diagnosis of type 2 diabetes are similar among diabetic patients detected by targeted screening and patients newly diagnosed in general practice: the Hoorn Screening Study. Diabetes Care 2003; 26: 2604- 2608. [DOI] [PubMed] [Google Scholar]
- 3. Spijkerman AM, Henry RM, Dekker JM, et al. Prevalence of macrovascular disease amongst type 2 diabetic patients detected by targeted screening and patients newly diagnosed in general practice: the Hoorn Screening Study. J Intern Med 2004; 256: 429- 436. [DOI] [PubMed] [Google Scholar]
- 4. Nichols GA, Arondekar B, Herman WH. Complications of dysglycemia and medical costs associated with nondiabetic hyperglycemia. Am J Manag Care 2008; 14: 791- 798. [PubMed] [Google Scholar]
- 5. Chen L, Zhang C, Yeung E, et al. Age at menarche and metabolic markers for type 2 diabetes in premenopausal women: the BioCycle Study. J Clin Endocrinol Metab 2011; 96: E1007- E1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Stöckl D, Meisinger C, Peters A, et al. Age at menarche and its association with the metabolic syndrome and its components: results from the KORA F4 Study. PLoS One 2011; 6: e26076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lakshman R, Forouhi NG, Sharp SJ, et al. Early age at menarche associated with cardiovascular disease and mortality. J Clin Endocrinol Metab 2009; 94: 4953- 4960. [DOI] [PubMed] [Google Scholar]
- 8. Jacobsen BK, Heuch I, Kvåle G. Association of low age at menarche with increased all-cause mortality: a 37-year follow-up of 61,319 Norwegian women. Am J Epidemiol 2007; 166: 1431- 1437. [DOI] [PubMed] [Google Scholar]
- 9. Lakshman R, Forouhi N, Luben R, et al. Association between age at menarche and risk of diabetes in adults: results from the EPIC-Norfolk cohort study. Diabetologia 2008; 51: 781- 786. [DOI] [PubMed] [Google Scholar]
- 10. He C, Zhang C, Hunter DJ, et al. Age at menarche and risk of type 2 diabetes: results from 2 large prospective cohort studies. Am J Epidemiol 2010; 171: 334- 344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Stöckl D, Döring A, Peters A, et al. Age at menarche is associated with prediabetes and diabetes in women (aged 32-81 years) from the general population: the KORA F4 Study. Diabetologia 2012; 55: 681- 688. [DOI] [PubMed] [Google Scholar]
- 12. Dreyfus JG, Lutsey PL, Huxley R, et al. Age at menarche and risk of type 2 diabetes among African-American and white women in the Atherosclerosis Risk in Communities (ARIC) study. Diabetologia 2012; 55: 2371- 2380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Elks CE, Ong K, Scott RA, et al. Age at menarche and type 2 diabetes risk: the EPIC-InterAct study. Diabetes Care 2013; 36: 3526- 3534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Saquib N, Kritz Silverstein D, Barrett Connor E. Age at menarche, abnormal glucose tolerance and type 2 diabetes mellitus: the Rancho Bernardo Study. Climacteric 2005; 8: 76- 82. [DOI] [PubMed] [Google Scholar]
- 15. Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care 2004; 27: 1487- 1495. [DOI] [PubMed] [Google Scholar]
- 16.American Diabetes Association. Standards of medical care in diabetes—2010. Diabetes Care 2010; 33 (suppl 1): S11- S61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gambineri A, Pelusi C, Manicardi E, et al. Glucose intolerance in a large cohort of Mediterranean women with polycystic ovary syndrome: phenotype and associated factors. Diabetes 2004; 53: 2353- 2358. [DOI] [PubMed] [Google Scholar]
- 18. Conway BN, Shu X, Zhang X, et al. Age at menarche, the leg length to sitting height ratio, and risk of diabetes in middle-aged and elderly Chinese men and women. PLoS One 2012; 7: e30625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mueller NT, Odegaard AO, Gross MD, Koh WP, Yuan JM, Pereira MA. Age at menarche and cardiovascular disease mortality in Singaporean Chinese women: the Singapore Chinese Health Study. Ann Epidemiol 2012; 22: 717- 722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Qiu C, Chen H, Wen J, et al. Associations between age at menarche and menopause with cardiovascular disease, diabetes, and osteoporosis in Chinese women. J Clin Endocrinol Metab 2013; 98: 1612- 1621. [DOI] [PubMed] [Google Scholar]
- 21. Feng Y, Hong X, Wilker E, et al. Effects of age at menarche, reproductive years, and menopause on metabolic risk factors for cardiovascular diseases. Atherosclerosis 2008; 196: 590- 597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Anderson SE, Must A. Interpreting the continued decline in the average age at menarche: results from two nationally representative surveys of U.S. girls studied 10 years apart. J Pediatr 2005; 147: 753- 760. [DOI] [PubMed] [Google Scholar]
- 23. Fredriks AM, van Buuren S, Burgmeijer RJ, et al. Continuing positive secular growth change in The Netherlands 1955-1997. Pediatr Res 2000; 47: 316- 323. [DOI] [PubMed] [Google Scholar]
- 24. Graham MJ, Larsen U, Xu X. Secular trend in age at menarche in China: a case study of two rural counties in Anhui Province. J Biosoc Sci 1999; 31: 257- 267. [DOI] [PubMed] [Google Scholar]
- 25. Cho GJ, Park HT, Shin JH, et al. Age at menarche in a Korean population: secular trends and influencing factors. Eur J Pediatr 2010; 169: 89- 94. [DOI] [PubMed] [Google Scholar]
- 26. Cho NH. The epidemiology of diabetes in Korea: from the economics to genetics. Korean Diabetes J 2010; 34: 10- 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. van Lenthe FJ, Kemper CG, van Mechelen W. Rapid maturation in adolescence results in greater obesity in adulthood: the Amsterdam Growth and Health Study. Am J Clin Nutr 1996; 64: 18- 24. [DOI] [PubMed] [Google Scholar]
- 28. Okasha M, McCarron P, McEwen J, Smith GD. Age at menarche: secular trends and association with adult anthropometric measures. Ann Hum Biol 2001; 28: 68- 78. [DOI] [PubMed] [Google Scholar]
- 29. Kahn SE, Hull RL, Utzschneider KM. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature 2006; 444: 840- 846. [DOI] [PubMed] [Google Scholar]
- 30. Ho KY, Evans WS, Blizzard RM, et al. Effects of sex and age on the 24-hour profile of growth hormone secretion in man: importance of endogenous estradiol concentrations. J Clin Endocrinol Metab 1987; 64: 51- 58. [DOI] [PubMed] [Google Scholar]
- 31. Rooman RP, De Beeck LO, Martin M, van Doorn J, Mohan S, Du Caju MV. Ethinylestradiol and testosterone have divergent effects on circulating IGF system components in adolescents with constitutional tall stature. Eur J Endocrinol/Eur Fed Endocr Soc 2005; 152: 597- 604. [DOI] [PubMed] [Google Scholar]
- 32. Teppala S, Shankar A. Association between serum IGF-1 and diabetes among U.S. adults. Diabetes Care 2010; 33: 2257- 2259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Casey VA, Dwyer JT, Coleman KA, Krall EA, Gardner J, Valadian I. Accuracy of recall by middle-aged participants in a longitudinal study of their body size and indices of maturation earlier in life. Ann Hum Biol 1991; 18: 155- 166. [DOI] [PubMed] [Google Scholar]
- 34. Must A, Phillips SM, Naumova EN, et al. Recall of early menstrual history and menarcheal body size: after 30 years, how well do women remember? Am J Epidemiol 2002; 155: 672- 679. [DOI] [PubMed] [Google Scholar]
- 35. Picardi A, Cipponeri E, Bizzarri C, Fallucca S, Guglielmi C, Pozzilli P. Menarche in type 1 diabetes is still delayed despite good metabolic control. Fertil Steril 2008; 90: 1875- 1877. [DOI] [PubMed] [Google Scholar]


