Abstract
Recently, low-level laser (light) therapy (LLLT) has been used to improve muscle performance. This study aimed to evaluate the effectiveness of near-infrared light-emitting diode therapy (LEDT) and its mechanisms of action to improve muscle performance in an elite athlete. The kinetics of oxygen uptake (VO2), blood and urine markers of muscle damage (creatine kinase – CK and alanine) and fatigue (lactate) were analyzed. Additionally, some metabolic parameters were assessed in urine using proton nuclear magnetic resonance spectroscopy (1H NMR). A LED cluster with 50 LEDs (λ = 850 nm; 50mW 15 s; 37.5 J) was applied on legs, arms and trunk muscles of a single runner athlete 5 min before a high-intense constant workload running exercise on treadmill. The athlete received either Placebo-1-LEDT; Placebo-2-LEDT; or Effective-LEDT in a randomized double-blind placebo-controlled trial with washout period of 7 d between each test. LEDT improved the speed of the muscular VO2 adaptation (~−9 s), decreased O2 deficit (~−10 L), increased the VO2 from the slow component phase (~+348 ml min−1) and increased the time limit of exercise (~+589 s). LEDT decreased blood and urine markers of muscle damage and fatigue (CK, alanine and lactate levels). The results suggest that a muscular pre-conditioning regimen using LEDT before intense exercises could modulate metabolic and renal function to achieve better performance.
Keywords: Fatigue, LEDT, LLLT, muscle damage, NMR, oxygen uptake, photobiomodulation
Introduction
Low-level laser (light) therapy (LLLT), also known as photobiomodulation, has been known since 1967 and uses visible or near-infrared light to promote tissue healing, and reduce pain and inflammation (Chow, Johnson, Lopes-Martin, and Bjordal, 2009; Enwemeka et al, 2004; Huang, Sharma, Carroll, and Hamblin, 2011). LLLT can be delivered by different light sources such as laser diodes and LEDs (light-emitting diodes; Huang, Sharma, Carroll, and Hamblin, 2011). These light sources are different in monochromaticity and coherence since diode lasers are more coherent, have a tiny spectral bandwidth and promote less divergence of the light beams compared to the light emitted by LEDs (Huang, Chen, Carroll, and Hamblin, 2009). However, lasers and LEDs are considered to produce equivalent effects on the tissue if the dose of light delivered/applied is in accordance with the possible biphasic dose–response, previously reported (Enwemeka, 2005; Huang, Chen, Carroll, and Hamblin, 2009; Huang, Sharma, Carroll, and Hamblin, 2011); and light–tissue interaction depends on light absorption by specific structures in the cells that are known as chromophores (Karu, 2010; Karu and Kolyakov, 2005; Karu, Pyatibrat, and Afanasyeva, 2004; Karu, Pyatibrat, Kolyakov, and Afanasyeva, 2008).
Recently, LLLT and LED therapy (LEDT) have been used to promote better muscle performance when applied to muscles immediately before or after intense exercise (Borsa, Larkin, and True, 2013; Ferraresi, Hamblin, and Parizotto, 2012; Leal-Junior et al, 2013). LLLT and LEDT can stimulate mitochondrial metabolism promoting a higher energy supply in the cells, and especially in skeletal muscles cells (Ferraresi, Hamblin, and Parizotto, 2012; Ferraresi and Parizotto, 2013; Hayworth et al, 2010; Karu, 1999; Silveira et al, 2009). Because mitochondrial metabolism is affected by light therapy, it is expected that oxygen consumption (VO2) kinetics would also be affected since the mitochondria use oxygen as a final electron receptor in the electron transport chain (ETC).
Currently, only two studies (Da Silva Alves et al, 2014; De Marchi et al, 2012) have investigated the acute effects of light therapy on VO2. However, these studies were limited to irradiation of only a few areas of the muscles involved in the exercise. In addition, these studies did not evaluate the kinetics of VO2, a better way to assess the oxidative adaptation caused by exercise stimulus (Barstow, 1994). Thus, the analysis of VO2 kinetics may show how efficient the process of oxygen delivery is (by the cardiorespiratory system) and oxygen utilization (by mitochondria). Among the current methods of analysis, exponential VO2 data modeling seem to provide the best information about the VO2 response at the onset of exercise (i.e. speed – time constant “τ” and magnitude of the steady state – amplitude “a”; Barstow, Casaburi, and Wasserman, 1993; Rossiter et al, 1999).
Several studies have also reported the effects of light therapy on muscle performance during intense exercise (Borsa, Larkin, and True, 2013; Ferraresi, Hamblin, and Parizotto, 2012; Leal-Junior et al, 2013) but almost all of them have been limited to mainly analyzing markers of muscle fatigue (lactate) and muscle damage (creatine kinase – CK) only in blood (Leal-Junior et al, 2013). However, metabolic analysis based on proton nuclear magnetic resonance spectroscopy (1H NMR) in urine can be more specific and can provide detailed information about energy metabolism (Enea et al, 2010; Pechlivanis et al, 2010). Furthermore, if combined, both analyses (urine and blood) could offer a “balanced” analysis of the production and removal processes of lactate and other metabolites from protein degradation that could show the degree of muscle damage.
Moreover, the majority of these previous studies involving light therapy and muscle performance only applied a limited number of irradiation points on muscles (Borsa, Larkin, and True, 2013; Leal-Junior et al, 2013). However, if the idea is to improve the performance of the entire muscle group, the number of irradiation points should cover all the muscle area involved in that specific exercise (Ferraresi et al, 2011; Ferraresi, Hamblin, and Parizotto, 2012; Ferraresi and Parizotto, 2013).
The present study investigated the acute effects of LEDT on muscular mitochondrial oxidation (through VO2 kinetics analysis), blood markers of fatigue and damage associated with 1H NMR metabolic analysis in urine. Muscle performance was assessed on a single elite runner who received a muscular pre-conditioning regimen using LEDT before high-intensity running exercises performed on a treadmill. As in previous studies, the muscular pre-conditioning was immediately applied before the exercises (Da Silva Alves et al, 2014; De Marchi et al, 2012), but for the current study the irradiation covered the entire muscle groups activated during the exercise (Ferraresi et al, 2011; Ferraresi, Hamblin, and Parizotto, 2012; Ferraresi and Parizotto, 2013).
Methods
One elite runner athlete, 28 years old, 1.80m height, 63.6 kg weight and 19.6 kg m−2 body mass index with 4 years of experience in high-level running competitions was enrolled in this single-subject randomized double-blind placebo-controlled trial.
The athlete was declared to be healthy without any type of skeletal muscle disorder, or neurological, metabolic, respiratory or cardiovascular disease. After explanation of the study purposes and procedures, he signed a consent form and was submitted to a protocol of exercise to measure his aerobic capacity, kinetics of VO2, muscle performance and metabolic analysis of blood and urine. This study was conducted in compliance with the Declaration of Helsinki, approved by the Human Ethics Committee of the Federal University São Carlos (217/2012; Brazil) and registered at Clinical Trials.gov (NCT01770977).
Cardiopulmonary exercise test [CPX]: identifying the load target for the exercise
The CPX was performed on a treadmill (Master ATL, Inbramed, Porto Alegre, Brazil) using a ramping protocol consisting of 5 min of incremental increase in speed from 0.8 km h−1 to 18 km h−1, followed by an incremental grade increase (0.5% each 30 s). This test was concluded when the athlete presented signs and/or symptoms of maximal exertion fatigue. The gas analyzer system was calibrated before the test following standard procedures (Balady et al, 2010). Ventilation and metabolic parameters were monitored and registered breath-by-breath (CPX-D/BreezeSuite 6.4.1, Medical Graphics, St Paul, MN). Electrocardiogram was continuously monitored (Active, Ecafix, Sao Paulo, Brazil) and heart rate (HR) was recorded by a digital telemetry system (Polar® S810i; Polar Electro Oy, Kempele, Finland). Blood pressure was assessed every 2 min. Using the ventilatory method, three independent evaluators determined the gas exchange threshold (GET) and the respiratory compensation point (RCP). The highest averaged VO2 value observed at the last 30 s of exercise was considered the VO2 peak. CPX was performed in a room with humidity between 35 and 40% and temperature between 24 and 25 °C (Table 1).
Table 1.
Results of functional parameters at gas exchange threshold (GET) and peak of cardiopulmonary exercise test (CPX) performed on treadmill ramp protocol to set the workload for all constant workload exercise tests (CWETs).
| CPX | GET | Peak |
|---|---|---|
| Time (min) | 9 | 17.00 |
| VO2 (mL kg−1.min−1) | 48.6 | 67.60 |
| VO2 (L min−1) | 2.96 | 4.40 |
| VCO2 (L min−1) | 2.54 | 4.48 |
| HR (bpm) | 154 | 182 |
HR, heart rate; VO2, oxygen uptake; VCO2, carbon dioxide output.
Constant workload exercise test [CWET]: running until voluntarily exhaustion
The CWET workload (speed and slope) was based on values of VO2 acquired in the CPX test (previously described). A high workload corresponding to 95% of the VO2 at RCP was employed (Whipp, 1994; Whipp and Casaburi, 1982). After 2 min of standing rest on the treadmill, the CWET was started with a single incremental adjustment to target workload (18.0 km h−1, 4%) until the athlete presented signs and/or symptoms of exercise fatigue and decided to stop the exercise voluntarily. The monitoring and acquisition procedures of heart rate, ventilatory and metabolic parameters were similar to the CPX test.
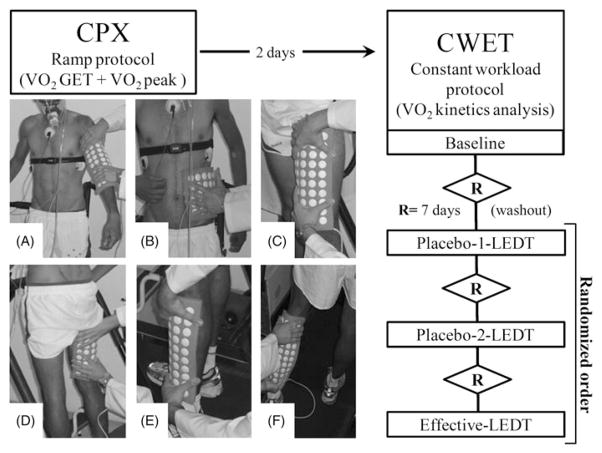
CWET was performed four times in a room with humidity temperature controlled the same as the CPX test. The first CWET was carried out 2 d after the CPX test just to achieve familiarization with all procedures and methods and no data were acquired/analyzed since this was a simulated test. After 7 d, three further CWETs were performed: (1) placebo-1-LEDT; (2) placebo-2-LEDT; and (3) effective-LEDT. Among each CWET, an additional period of 7 d was employed as washout period (Figure 1). CPX test and all CWETs were carried out always at the same period of the day (afternoon). Placebo or effective-LEDT was applied on body muscles in accordance with a randomized procedure.
Figure 1.
Workflow of the study procedures and muscle groups irradiated by light-emitting diode therapy (LEDT): (A) biceps brachii and triceps brachii; (B) external oblique and latissimus dorsi; (C) femoral quadriceps; (D) hamstrings; (E) tibialis anterior and peroneus longus; (F) gastrocnemius and soleus. The order of each therapy (Placebo-1-LEDT, Placebo-2-LEDT and effective-LEDT) was randomized. The sequence of LEDT application was not randomized and had the following sequence: F, E, D, C, B, A.
LED therapy (LEDT): randomization, blind procedures, placebo and therapy
Near-infrared LEDT was applied with an array of multi-diode containing 50 LEDs (850±20 nm) specially built for research by the Federal University of São Carlos and University of São Paulo (Figure 1). The features of each LED and parameters of LEDT are presented in Table 2. All parameters of this device for LEDT were calibrated using Thorlabs® (Dachau, Germany) optical meter model PM100D and photodiode power sensor model S130C. All parameters of LEDT used were based on literature reports (Borsa, Larkin, and True, 2013; Ferraresi, Hamblin, and Parizotto, 2012; Leal-Junior et al, 2013).
Table 2.
Parameters of light-emitting diode (LED) therapy (LEDT) and regions of LEDT irradiation on body before constant workload exercise tests (CWETs).
| Number of LEDs: 50 |
| Wavelength: 850±20 nm (infrared) |
| Frequency: continuous output |
| Optical output: 50mW |
| LED spot size: 0.2 cm2 |
| Power density: 250mW cm−2 |
| Treatment time over each muscle group: 15 s |
| Energy per diode at 15 s: 0.75 J |
| Energy density per diode at 15 s: 3.75 J cm−2 |
| Number of irradiation points per muscle group: 50 |
| Total energy delivered per muscle group: 37.5 J |
| Muscle groups irradiated before CWETs: (A) biceps brachii and triceps brachii; (B) external oblique and latissimus dorsi; (C) femoral quadriceps; (D) hamstrings; (E) tibialis anterior and peroneus longus; (F) gastrocnemius and soleus |
| Total energy delivered on body: 450 J |
| Total power output: 2500mW |
| Application mode: device held coupled in skin contact |
LEDT (placebo or effective) was applied on six main muscle groups (A, B, C, D, E and F) used during a run, as described in Table 2 and illustrated in Figure 1. All muscle groups received LEDT (placebo or effective) 5 min before each constant workload exercise test (CWET) in accordance with randomization procedures (Borsa, Larkin, and True, 2013; Ferraresi, Hamblin, and Parizotto, 2012; Leal-Junior et al, 2009; Leal-Junior et al, 2013). The randomization scheme was generated at Randomization.com (http://www.randomization.com) using balanced permutations in one block with three different therapies: (1) Placebo-1-LEDT; (2) Placebo-2-LEDT; and (3) Effective-LEDT. Thus, this study had the following order of treatments: 1) Placebo-1-LEDT; (2) Placebo-2-LEDT; and (3) Effective-LEDT (Figure 1). Neither subject nor evaluators knew if LEDT was effective or placebo during data collection and analysis. A hidden button for placebo or effective LEDT in the LED device was employed to ensure the double-blind procedures. This button was switched previously without the knowledge of either evaluator or the subject. In addition, this button was switched to “on” (effective LEDT) or “off” (Placebo) by the only researcher who just participated in the randomization procedure and LEDT application, having no access to data collection and analysis. As the light therapy used was infrared, nobody could identify if the LEDT was effective or placebo while the time display was on.
Analysis of VO2 kinetics: data modeling
As the initial VO2 phase is mainly attributed to the increase in cardiac output and pulmonary blood flow (Arena, Tevald, Peberdy, and Turner, 2004), the first 20 s were excluded from the VO2 data (Whipp et al, 1982). Next, a bi-exponential model was employed to fit the VO2 data for all CWETs where:
“t” is time; “a0” is the mean VO2 value during warm-up; “a1” is muscle VO2 amplitude; “a2” is the “extra” VO2 amplitude from the slow component phase (VO2 amplitude increases in this phase mainly from the recruitment of type II muscle fibers with lower phosphorylation efficiency (Borrani et al, 2001)); “τ1” is the speed of muscular VO2 adjustment; “TD1” is the time delay to start the muscular phase (Rossiter et al, 1999); and “TD2” is time delay to start the slow component phase, which arises after some minutes of constant workload exercise above that of the volunteer’s GET (Barstow, 1994; Gaesser and Poole, 1996; Whipp, 1994). Additionally, the total muscular oxygen deficit was calculated (O2def=(τ+TD1) a1; Whipp and Casaburi, 1982).
Blood lactate and creatine kinase analysis
Blood samples for lactate measurement were collected by puncturing the athlete’s ear lobe using sterile lancets before and 5 min after each CWET test. The puncture site was cleaned with alcohol, dried and the first drop of blood was discarded. The blood collected (25 μL) was quickly transferred to Eppendorf tubes containing 50 μL of 1% sodium fluoride (NaF) and analyzed by electroenzymatic lactimeter (YSI 1500 – Yellow Springs, Ohio, USA; Simoes et al, 2013). Lactate was used to infer muscle fatigue.
CK was analyzed from 4.5mL of blood collected from the antecubital vein, before and 5 min after each CWET test. This blood was collected in heparinized tubes, centrifuged at 3000 × g for 10 min at 4 °C and the heparinized plasma was immediately pipetted into Eppendorf tubes and stored at −80 °C until analysis on the Reflotron Plus® biochemical analyzer (Roche, Mannheim, Germany; Hornery, Farrow, Mujika, and Young, 2007), using 30 μL of heparinized plasma in accordance with the manufacturer’s guidelines.
Metabolic analysis based on proton nuclear magnetic resonance spectroscopy (1H NMR) in urine
Urinary samples were collected before and 30±5 min after each placebo or effective intervention (Enea et al, 2010; Pechlivanis et al, 2010). The runner was instructed to discard the first urinary flow and to urinate about 10mL into a Falcon tube. Immediately this tube was frozen in liquid nitrogen and stored at −80 °C until analysis. In addition, during each intervention day, the athlete received general instructions to ingest the same amounts of food and water (Enea et al, 2010; Pechlivanis et al, 2010).
For 1H NMR analysis, urine samples were thawed, and 1.0mL of urine was centrifuged (10 min at 10 000 × g) to eliminate cellular fragments and sediments. Afterwards 0.9mL of urine was added to 0.1mL of phosphate buffer solution in D2O (1.5 M, pH = 7.00) containing 2mM of NaN3 (sodium azide) and 0.1% of TSP-d4 (sodium 3-trimethylsilyl [2,2,3,3-2D4] propionate), and pH was adjusted to 7.00±0.01 after a 20-minute delay with HCl or NaOH. At last, 0.6mL of urine was transferred to a 5-mm NMR tube (Enea et al, 2010).
1H NMR analysis of urine samples were recorded on a Bruker AVANCE III spectrometer at a proton frequency of 600 MHz equipped with a 5mm TCI CryoProbe (z-gradient). The one-dimensional (1D) NOESY pulse sequence with water pre-saturation was used throughout. Spectra of CK and alanine (for muscle damage), creatinine (for energy metabolism) and glycine, dimethylamine (DMA) and trimethylamine N-oxide (TMAO; for renal function) were qualitatively and quantitatively assigned using Chenomx NMR suite 7.5 software, evaluation edition (Chenomx Inc., Edmonton, Canada), with reference to the literature (Wishart et al, 2009).
Statistical analysis
All results were presented in descriptive form (i.e. in absolute, percentage values and normalized per time of exercise performed (Tlim)). Tables and graphs show all differences between each test. There is no statistical test performed since this study enrolled a single runner athlete.
Outcomes
VO2 kinetics and performance
The parameters measured during CWET were: “a0”; “a1”; “a2”; “τ1”; “TD1”; “TD2”; time limit of exercise (Tlim); and deficit of oxygen (O2def).
Placebo-1-LEDT and Placebo-2-LEDT had very similar results as shown in Figure 2, suggesting a very similar response to the same exercise and in accordance with the literature (Faisal, Beavers, and Hughson, 2010). Comparing both placebo-LEDT, Placebo-1-LEDT had approximately an additional of 200 ml min−1 of “extra” VO2 amplitude in slow component (“a2”; Figure 2) and Placebo-2-LEDT had higher “Tlim” of exercise (+144 s or +17%), lower “O2def” (−4L or −2.8%) and “τ1” (i.e. the speed of muscular VO2 adjustment; −2 s or 5.9% faster).
Figure 2.
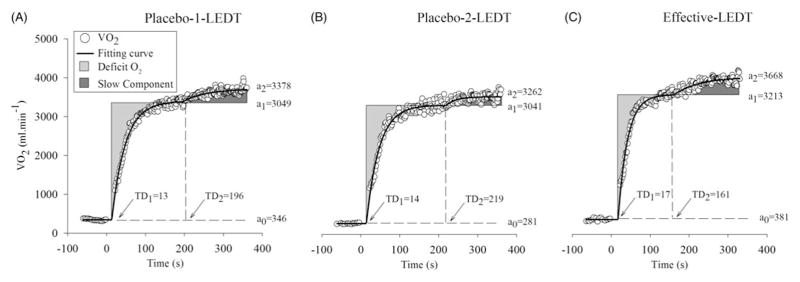
Values of VO2; a0, a1 and a2; TD1 and TD2 during constant workload exercise tests (CWETs): Placebo-1-LEDT, Placebo-2-LEDT and Effective-LEDT. Abbreviations: VO2 (oxygen uptake); a0 (mean VO2 value during warm-up), a1 (muscle VO2 amplitude in muscular phase); a2 (“extra” VO2 amplitude in slow component phase); TD1 (time delay to start muscular phase); TD2 (time delay to start the slow component phase); LEDT (light-emitting diode therapy).
On the other hand, effective-LEDT compared to Placebo-1-LEDT increased “Tlim” (+589 s or +70%), decreased “O2def” (−12 L or −8.4%) and “τ1” (−10 s or 29.4% more fast). Compared to Placebo-2-LEDT, effective-LEDT presented higher “Tlim” of exercise (+445 s or +45%), lower “O2def” (−8L or −5.8%) and “τ1” (−8 s or 25% more fast). In addition, effective-LEDT presented an earlier appearance of the slow component phase (lower “TD2”), faster muscular oxidative adaptation (lower “τ” value), higher muscle VO2 amplitude (“a1”) and the highest “extra” VO2 amplitude in the slow component phase (“a2”; Figure 2).
Placebo-1-LEDT, placebo-2-LEDT and effective LEDT presented 839, 983 and 1428 s for “Tlim”; 143, 139 and 131 L for “O2def”; and 34, 32 and 24 s for “τ1”, respectively (Figure 3A, B and C, respectively).
Figure 3.
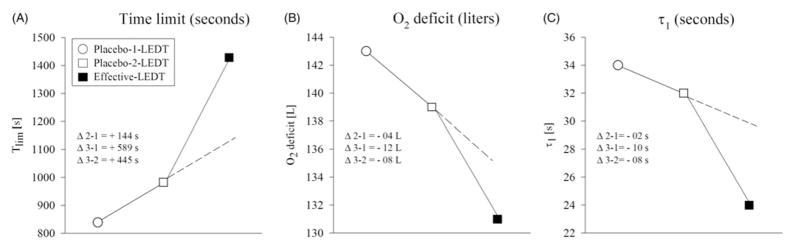
Comparisons between constant workload exercise tests (CWET): Placebo-1-LEDT, Placebo-2-LEDT and effective-LEDT. Abbreviations: Tlim (time limit of exercise); “τ1” (speed of muscular VO2 adjustment); L (liters); LEDT (light-emitting diode therapy). Note in this figure that there are dotted lines plotted to demonstrate a tendency or linearity for the expected oscillation or small adaptation after multiple constant workload exercise tests. However, after effective-LEDT, there was a clearly break in this tendency or linearity, suggesting beneficial effects of LEDT on VO2 kinetics.
Blood and urine analysis
Concentrations of each metabolite (in blood and/or urine through 1H NMR analysis) are expressed in absolute rate, percentage of change and normalized per time of exercise (Tlim) of each CWET (Table 3).
Table 3.
Results of blood creatine kinase (U L−1) and blood lactate (mmol L−1), and metabolic analyses based on 1H NMR (mM) in urine.
| Concentration | Placebo1-LEDT
|
Placebo2-LEDT
|
Effective-LEDT
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Before | After | Δ% | Δ%/Tlim (839 s) × 10−2 | Before | After | Δ% | Δ%/Tlim (983 s) × 10−2 | Before | After | Δ% | Δ%/Tlim (1428 s) × 10−2 | |
| CK (blood) | 105 | 156 | 48.57 | 5.78 | 113.00 | 169.00 | 49.56 | 5.04 | 110.00 | 149.00 | 35.45 | 2.48 |
| Lactate (blood) | 1.50 | 10.20 | 580 | 69.12 | 0.84 | 9.69 | 1053 | 107.12 | 1.74 | 9.60 | 451.72 | 31.63 |
| Lactate (urine) | 0.32 | 39.70 | 12 306 | 1466.74 | 1.20 | 52.20 | 4250 | 432.34 | 0.63 | 41.10 | 6423 | 449.84 |
| Creatinine (urine) | 10.80 | 11.80 | 9.26 | 1.10 | 61.50 | 70.00 | 13.82 | 1.40 | 20.90 | 19.70 | −5.74 | −0.40 |
| Alanine (urine) | 0.31 | 0.54 | 74.19 | 8.84 | 1.59 | 2.37 | 49.06 | 4.98 | 0.711 | 1.14 | 60.34 | 4.22 |
| Glycine (urine) | 0.84 | 0.81 | −3.57 | −0.42 | 3.69 | 3.22 | −12.74 | −1.29 | 1.11 | 1.37 | 23.42 | 1.64 |
| DMA (urine) | 0.33 | 0.39 | 18.18 | 2.16 | 2.05 | 2.38 | 16.10 | 1.63 | 0.80 | 0.73 | −8.75 | −0.61 |
| TMAO (urine) | 0.19 | 0.13 | −31.58 | −3.76 | 5.43 | 4.77 | −12.15 | −1.23 | 0.35 | 0.27 | −22.86 | −1.60 |
Data were expressed as absolute concentration, percentage changes and normalized per time of exercise performed (Tlim) value and percentage. LEDT, light-emitting diode therapy; CK, creatine kinase; 1H NMR, proton nuclear magnetic resonance; DMA, dimethylamine; TMAO, trimethylamine N-oxide; mM, milimolar; Δ%, delta in percentage, Tlim, time limit of exercise.
Discussion
This preliminary study investigated the effects of muscular pre-conditioning using LEDT on the VO2 kinetics and metabolic changes in blood and urine during high-intensity running exercise performed by an elite runner athlete. Several studies have used near-infrared LLLT or LEDT to improve muscle performance (Borsa, Larkin, and True, 2013; Ferraresi, Hamblin, and Parizotto, 2012; Leal-Junior et al, 2013). The main effects reported by these studies are: decreased muscle fatigue; lower blood lactate levels and less muscle damage (blood CK levels); increased torque; workload; aerobic capacity; oxidative stress defense; and micro-circulation when applied to muscles (Borsa, Larkin, and True, 2013; Da Silva Alves et al, 2014; De Marchi et al, 2012; Ferraresi et al, 2011; Ferraresi, Hamblin, and Parizotto, 2012; Vieira et al, 2012).
However, there is still no consensus about how all the parameters of near-infrared radiation such as wavelength, optical output, treatment time, energy, energy density, power, power density and number of radiation points should be optimized to increase muscle performance in exercise. Therefore, based on possible biphasic dose–response relationships previously reported (Huang, Chen, Carroll, and Hamblin, 2009; Huang, Sharma, Carroll, and Hamblin, 2011), the current study applied a similar total energy per muscle group (37.5 J) as has been previously reported. We used 50 radiation points to deliver the light to each muscle group in order to cover the entire muscular area employed during running exercise, and the optical output (50mW; Ferraresi, Hamblin, and Parizotto, 2012; Ferraresi et al, 2011; Vieira et al, 2012), treatment time (15 s; De Marchi et al, 2012) and the wavelength (Leal-Junior et al, 2009) were also described in the literature.
VO2 kinetics and performance
The dotted lines plotted in Figure 3 demonstrated the expected oscillation or a progressive adaptation between each constant workload exercise test (even if this is not described in the literature; Faisal, Beavers, and Hughson, 2010). Note in each of these lines there is an abrupt break in linearity (or a tendency for a break) after effective-LEDT (Figure 3). These preliminary results may suggest an accelerated (here assessed by “τ1”), and more efficient adenosine tri-phosphate (ATP) turnover from aerobic (by oxidative phosphorylation) and anaerobic alactic metabolism (Cr-P system) mainly in slow-twitch muscle fibers before the appearance of the slow component phase (Hepple, 2002). Furthermore, our results suggest a higher influence of aerobic ATP turnover in fast-twitch muscle fibers (glycolytic and oxidative) during the slow component phase (Jones et al, 2011) which probably produced an extended “Tlim” of exercise compared to Placebo-LEDT.
During the slow component phase there is a progressive increase in blood lactate levels due to progressive recruitment of fast-twitch fibers (Borrani et al, 2001) that produces metabolic acidosis (Barstow, 1994; Gaesser and Poole, 1996; Whipp, 1994). In this context, it is known that near-infrared LEDT can improve mitochondrial function, with possible mechanisms consisting of a lactate shuttle through mitochondrial monocarboxylate transporters and lactate oxidation to ATP synthesis during exercise (Ferraresi et al, 2011; Ferraresi and Parizotto, 2013; Vieira et al, 2012).
It has been reported that light therapy can promote an improvement of complex IV activity (cytochrome c oxidase; Karu, 2010) as well as the other complexes of the mitochondrial electric transport chain (ETC; Silveira et al, 2009) in non-injured skeletal muscles (Hayworth et al, 2010). Furthermore, some studies have reported better vasodilatation, microcirculation and tissue perfusion after effective-LEDT applied to the human body (Mak and Cheing, 2012). Thereby improved blood transport and oxygen delivery to muscle cells, together with increased mitochondrial metabolism, could modulate the VO2 response during exercise, explaining the results of the VO2 kinetics.
Blood lactate, blood creatine kinase and metabolic analysis based on 1H NMR in urine
Lactate levels in blood normalized for time of exercise performed (Tlim) were much lower after effective-LEDT, suggesting a better clearance (lactate removal; De Marchi et al, 2012), or a smaller lactate production possibly by inhibition of lactate dehydrogenase (LDH) enzyme activity or increased lactate oxidation (consumption) in mitochondria (Ferraresi et al, 2011; Ferraresi, Hamblin, and Parizotto, 2012; Ferraresi and Parizotto, 2013). However, if NIR can improve vasodilatation and microcirculation (Mak and Cheing, 2012) and consequently provide better lactate clearance or removal, it is important to measure lactate concentration in urine (Enea et al, 2010; Pechlivanis et al, 2010) and blood analysis in order to show the real lactate balance during exercise.
We found effective-LEDT had the lowest levels of lactate in blood and urine normalized for time of exercise performed. These results may suggest that effects of light therapy could be better related to the decrease in lactate production (and/or increase in its consumption by mitochondria; Ferraresi et al, 2011; Ferraresi, Hamblin and Parizotto, 2012; Ferraresi and Parizotto, 2013) than to lactate removal or clearance as reported previously (De Marchi et al, 2012; Leal-Junior et al, 2013).
CK has been used as a “gold standard” to measure muscle damage after intense exercise and frequently is related to higher oxidative stress (De Marchi et al, 2012). Near-infrared radiation seems to modulate oxidative stress and prevent muscle damage if applied before intense exercises (De Marchi et al, 2012; Leal- Junior et al, 2009). In accordance with these previous studies, the CK level in blood normalized for time of exercise had much lower levels in effective-LEDT CWET, than in Placebo. Alanine was quantified in urine to show the renal clearance function of amino acids released from damaged muscle. CK levels in blood are associated with muscle damage, and aminoacids from protein breakdown are released into the bloodstream and excreted in urine (Enea et al, 2010). The increased amount of alanine in the urine after high-intensity exercise suggests that muscle damage occurred (Enea et al, 2010). In effective-LEDT, the amounts of alanine in urine normalized for time of exercise was lower than in Placebo-2-LEDT, which had the second higher “Tlim” of exercise, suggesting a protective effect of effective-LEDT against muscle damage as reported previously (Borsa, Larkin, and True, 2013; Ferraresi, Hamblin, and Parizotto, 2012; Leal-Junior et al, 2009, 2013).
The other important metabolite identified in the urine was creatinine. This metabolite is frequently associated with renal dysfunction in seriously ill patients suffering increased protein breakdown (catabolism process; Bairaktari et al, 2002). However, the creatinine in the urine of healthy people subjected to intense exercise suggests that phosphocreatine is being biochemically broken down (Pechlivanis et al, 2010). Phosphocreatine (associated with higher cellular energy states) is produced by the re-phosphorylation of creatine by ATP; therefore, if more mitochondrial ATP is available (LEDT effect; Ferraresi et al, 2011; Pechlivanis et al, 2010) a higher energy state in muscles will be expected, which in turn could explain the apparently faster ATP turnover rate during the beginning of exercise. Thus, creatinine production and its excretion were decreased only in effective-LEDT, suggesting better alactic energy metabolism.
Other important metabolites identified in urine were glycine, DMA and TMAO, all of which are related to renal function (Bairaktari et al, 2002). High levels of lactic acidosis may cause acute renal dysfunction through tubular acidosis (Bairaktari et al, 2002). In agreement with the literature, the concentration of glycine normalized for “Tlim” of exercise in Placebo-1-LEDT and Placebo-2-LEDT were sharply decreased, suggesting reversible renal dysfunction (Bairaktari et al, 2002). Additionally, glycine was increased in effective-LEDT suggesting a minor renal dysfunction. On the other hand, increased excretion of DMA and TMAO suggested renal dysfunction (Bairaktari et al, 2002; Enea et al, 2010). DMA was excreted in both placebo interventions, and TMAO was more excreted in Placebo-2-LEDT, suggesting worse renal function.
Our study design aimed to have 2 d of placebo therapy in order to demonstrate the expected small oscillation between repeated exercise tests, unlike other studies that performed just one test during cross-over procedures (Da Silva Alves et al, 2014). This strategy, for our understanding, should be understood as a valuable caution to warrant that all changes in performance and VO2 kinetics possibly is attributable to the effective LEDT and should be greater than the normal oscillations between repeated exercise tests, as demonstrated in both days of placebo therapy.
However, the main possible concern about our results is the order of the therapies, once that effective LEDT was randomized to the last day of exercise test. At this point, it is very important to remark that due to our strategy of performing 2 d of exercise testing using placebo, the expected changes in VO2 response for a next placebo visit was plotted (Figure 3 – dotted lines), although being hard to measure the behavior of this oscillation (but for sure not higher than ~−9 s in Δτ – Figure 3(C); Faisal, Beavers, and Hughson, 2010).
Regarding this same figure, it is essential to note an abrupt inflection of the response when effective LEDT was applied before the exercise test (even considering this normal oscillation or the already discussed “learning” influences), showing superior responses. In other words, we were extremely careful to warrant that the LEDT effects should be greater than the small oscillation between tests, that are normal physiological responses and does not mean better or worse performances. In addition, the elite runner enrolled in this study had 4 years of running experience. Exercises like those performed in this study are part of the participant’s daily training program. Therefore, the “learning” effect to running is unlikely, although this is not conclusive from this pilot study.
As an elite athlete the VO2 adjustment is optimized close to its maximum and the small changes observed (Δτ = −2 s – Figure 3C) between the placebo visits is due to small oscillations expected despite the error inherent to the modeling process (from intra-breath noise); and that there is insignificant influences of the circadian rhythm on VO2 kinetics (Faisal, Beavers, and Hughson, 2010).
The present study has shown potential effects of LEDT on performance and VO2 kinetics during intense and constant workload exercises (that simulate endurance training protocols) associated with metabolic analysis in blood and urine using 1H NMR analysis. However, generalization of our findings should be avoided because this pilot study enrolled just one single elite runner participant. We encourage more researchers to reproduce this study with more athletes to corroborate our findings, and to investigate other muscle adaptations such as gene expression and muscular VO2.
Conclusion
Light-emitting diode therapy (LEDT) using a multi-diode array of LEDs with near-infrared wavelength and the dose applied in this study possibly can improve VO2 kinetics; increase time of exercise; decrease muscle damage, fatigue and renal dysfunction during intense running exercise performed on a treadmill.
Acknowledgments
The authors would like to thank the runner athlete who participated of this initial research and all universities and departments involved.
Footnotes
Declaration of interest
The authors declare have no conflict of interest. Cleber Ferraresi would like to thank FAPESP for his PhD’s degree scholarship (number 2010/07194-7). M. R. Hamblin was supported by US NIH grant R01AI050875.
References
- Arena R, Tevald M, Peberdy MA, Turner T. Prognostic value of phase 1 of oxygen uptake on-kinetics response in the heart failure population: A pilot study. Journal of Cardiopulmonary Rehabilitation. 2004;24:401–404. doi: 10.1097/00008483-200411000-00007. [DOI] [PubMed] [Google Scholar]
- Bairaktari E, Seferiadis K, Liamis G, Psihogios N, Tsolas O, Elisaf M. Rhabdomyolysis-related renal tubular damage studied by proton nuclear magnetic resonance spectroscopy of urine. Clinical Chemistry. 2002;48:1106–1109. [PubMed] [Google Scholar]
- Balady GJ, Arena R, Sietsema K, Myers J, Coke L, Fletcher GF, Forman D, Franklin B, Guazzi M, Gulati M, Keteyian SJ, Lavie CJ, Macko R, Mancini D, Milani RV American Heart Association Exercise, Cardiac Rehabilitation, Prevention Committee of the Council on Clinical Cardiology; Council on Epidemiology and Prevention; Council on Peripheral Vascular Disease; Interdisciplinary Council on Quality of Care and Outcomes Research. Clinician’s Guide to cardiopulmonary exercise testing in adults: A scientific statement from the American Heart Association. Circulation. 2010;122:191–225. doi: 10.1161/CIR.0b013e3181e52e69. [DOI] [PubMed] [Google Scholar]
- Barstow TJ. Characterization of VO2 kinetics during heavy exercise. Medicine and Science in Sports and Exercise. 1994;26:1327–1334. [PubMed] [Google Scholar]
- Barstow TJ, Casaburi R, Wasserman K. O2 uptake kinetics and the O2 deficit as related to exercise intensity and blood lactate. Journal of Applied Physiology. 1993;75:755–762. doi: 10.1152/jappl.1993.75.2.755. [DOI] [PubMed] [Google Scholar]
- Borrani F, Candau R, Millet GY, Perrey S, Fuchslocher J, Rouillon JD. Is the VO2 slow component dependent on progressive recruitment of fast-twitch fibers in trained runners? Journal of Applied Physiology. 2001;90:2212–2220. doi: 10.1152/jappl.2001.90.6.2212. [DOI] [PubMed] [Google Scholar]
- Borsa PA, Larkin KA, True JM. Does phototherapy enhance skeletal muscle contractile function and postexercise recovery? A systematic review. Journal of Athletic Training. 2013;48:57–67. doi: 10.4085/1062-6050-48.1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow RT, Johnson MI, Lopes-Martins RA, Bjordal JM. Efficacy of low-level laser therapy in the management of neck pain: A systematic review and meta-analysis of randomised placebo or active-treatment controlled trials. Lancet. 2009;374:1897–1908. doi: 10.1016/S0140-6736(09)61522-1. [DOI] [PubMed] [Google Scholar]
- Da Silva Alves MA, Pinfildi CE, Neto LN, Lourenco RP, De Azevedo PH, Dourado VZ. Acute effects of low-level laser therapy on physiologic and electromyographic responses to the cardiopulmonary exercise testing in healthy untrained adults. Lasers in Medical Science. 2014;29:1945–1951. doi: 10.1007/s10103-014-1595-3. [DOI] [PubMed] [Google Scholar]
- De Marchi T, Leal-Junior EC, Bortoli C, Tomazoni SS, Lopes-Martins RA, Salvador M. Low-level laser therapy (LLLT) in human progressive-intensity running: Effects on exercise performance, skeletal muscle status, and oxidative stress. Lasers in Medical Science. 2012;27:231–236. doi: 10.1007/s10103-011-0955-5. [DOI] [PubMed] [Google Scholar]
- Enea C, Seguin F, Petitpas-Mulliez J, Boildieu N, Boisseau N, Delpech N, Diaz V, Eugene M, Dugue B. (1)H NMR-based metabolomics approach for exploring urinary metabolome modifications after acute and chronic physical exercise. Analytical and Bioanalytical Chemistry. 2010;396:1167–1176. doi: 10.1007/s00216-009-3289-4. [DOI] [PubMed] [Google Scholar]
- Enwemeka CS. Light is light. Photomedicine and Laser Surgery. 2005;23:159–160. doi: 10.1089/pho.2005.23.159. [DOI] [PubMed] [Google Scholar]
- Enwemeka CS, Parker JC, Dowdy DS, Harkness EE, Sanford LE, Woodruff LD. The efficacy of low-power lasers in tissue repair and pain control: A meta-analysis study. Photomedicine and Laser Surgery. 2004;22:323–329. doi: 10.1089/pho.2004.22.323. [DOI] [PubMed] [Google Scholar]
- Faisal A, Beavers KR, Hughson RL. O2 uptake and blood pressure regulation at the onset of exercise: interaction of circadian rhythm and priming exercise. American Journal of Physiology - Heart and Circulatory Physiology. 2010;299:H1832–42. doi: 10.1152/ajpheart.00762.2010. [DOI] [PubMed] [Google Scholar]
- Ferraresi C, De Brito Oliveira T, De Oliveira Zafalon L, De Menezes Reiff RB, Baldissera V, De Andrade Perez SE, Matheucci E, Junior, Parizotto NA. Effects of low level laser therapy (808 nm) on physical strength training in humans. Lasers in Medical Science. 2011;26:349–358. doi: 10.1007/s10103-010-0855-0. [DOI] [PubMed] [Google Scholar]
- Ferraresi C, Hamblin MR, Parizotto NA. Low-level laser (light) therapy (LLLT) on muscle tissue: performance, fatigue and repair benefited by the power of light. Photonics & Lasers in Medicine. 2012;1:267–286. doi: 10.1515/plm-2012-0032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferraresi C, Parizotto NA. Low-level laser therapy and light-emitting diode therapy on muscle tissue. In: Hamblin R, Huang YY, editors. Handbook of photomedicine. Boca Raton, Florida, USA: Taylor & Francis; 2013. pp. 611–630. [Google Scholar]
- Gaesser GA, Poole DC. The slow component of oxygen uptake kinetics in humans. Exercise and Sport Sciences Reviews. 1996;24:35–71. [PubMed] [Google Scholar]
- Hayworth CR, Rojas JC, Padilla E, Holmes GM, Sheridan EC, Gonzalez-Lima F. In vivo low-level light therapy increases cytochrome oxidase in skeletal muscle. Photochemistry and Photobiology. 2010;86:673–680. doi: 10.1111/j.1751-1097.2010.00732.x. [DOI] [PubMed] [Google Scholar]
- Hepple RT. The role of O2 supply in muscle fatigue. Canadian Journal of Appied Physiology. 2002;27:56–69. doi: 10.1139/h02-004. [DOI] [PubMed] [Google Scholar]
- Hornery DJ, Farrow D, Mujika I, Young W. An integrated physiological and performance profile of professional tennis. British Journal of Sports Medicine. 2007;41:531–336. doi: 10.1136/bjsm.2006.031351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YY, Chen AC, Carroll JD, Hamblin MR. Biphasic dose response in low level light therapy. Dose Response. 2009;7:358–383. doi: 10.2203/dose-response.09-027.Hamblin. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YY, Sharma SK, Carroll J, Hamblin MR. Biphasic dose response in low level light therapy – An update. Dose Response. 2011;9:602–618. doi: 10.2203/dose-response.11-009.Hamblin. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones AM, Grassi B, Christensen PM, Krustrup P, Bangsbo J, Poole DC. Slow component of V.O kinetics: Mechanistic bases and practical applications. Medicine and Science in Sports and Exercise. 2011;43:2046–2062. doi: 10.1249/MSS.0b013e31821fcfc1. [DOI] [PubMed] [Google Scholar]
- Karu T. Primary and secondary mechanisms of action of visible to near-IR radiation on cells. Journal of Photochemistry and Photobiology B. 1999;49:1–17. doi: 10.1016/S1011-1344(98)00219-X. [DOI] [PubMed] [Google Scholar]
- Karu TI. Multiple roles of cytochrome c oxidase in mammalian cells under action of red and IR-A radiation. IUBMB Life. 2010;62:607–610. doi: 10.1002/iub.359. [DOI] [PubMed] [Google Scholar]
- Karu TI, Kolyakov SF. Exact action spectra for cellular responses relevant to phototherapy. Photomedicine and Laser Surgery. 2005;23:355–361. doi: 10.1089/pho.2005.23.355. [DOI] [PubMed] [Google Scholar]
- Karu TI, Pyatibrat LV, Afanasyeva NI. A novel mitochondrial signaling pathway activated by visible-to-near infrared radiation. Photochemistry and Photobiology. 2004;80:366–372. doi: 10.1562/2004-03-25-RA-123. [DOI] [PubMed] [Google Scholar]
- Karu TI, Pyatibrat LV, Kolyakov SF, Afanasyeva NI. Absorption measurements of cell monolayers relevant to mechanisms of laser phototherapy: Reduction or oxidation of cytochrome c oxidase under laser radiation at 632.8 nm. Photomedicine and Laser Surgery. 2008;26:593–599. doi: 10.1089/pho.2008.2246. [DOI] [PubMed] [Google Scholar]
- Leal-Junior EC, Lopes-Martins RA, Rossi RP, De Marchi T, Baroni BM, De Godoi V, Marcos RL, Ramos L, Bjordal JM. Effect of cluster multi-diode light emitting diode therapy (LEDT) on exercise-induced skeletal muscle fatigue and skeletal muscle recovery in humans. Lasers in Medical Science. 2009;41:572–577. doi: 10.1002/lsm.20810. [DOI] [PubMed] [Google Scholar]
- Leal-Junior EC, Vanin AA, Miranda EF, De Carvalho PD, Dal Corso S, Bjordal JM. Effect of phototherapy (low-level laser therapy and light-emitting diode therapy) on exercise performance and markers of exercise recovery: A systematic review with meta-analysis. Lasers in Medical Science. 2013 doi: 10.1007/s10103-013-1465-4. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Mak MC, Cheing GL. Immediate effects of monochromatic infrared energy on microcirculation in healthy subjects. Photomedicine and Laser Surgery. 2012;30:193–199. doi: 10.1089/pho.2011.3012. [DOI] [PubMed] [Google Scholar]
- Pechlivanis A, Kostidis S, Saraslanidis P, Petridou A, Tsalis G, Mougios V, Gika HG, Mikros E, Theodoridis GA. (1)H NMR-based metabonomic investigation of the effect of two different exercise sessions on the metabolic fingerprint of human urine. Journal of Proteome Research. 2010;9:6405–6416. doi: 10.1021/pr100684t. [DOI] [PubMed] [Google Scholar]
- Rossiter HB, Ward SA, Doyle VL, Howe FA, Griffiths JR, Whipp BJ. Inferences from pulmonary O2 uptake with respect to intramuscular [phosphocreatine] kinetics during moderate exercise in humans. Journal of Physiology. 1999;518:921–932. doi: 10.1111/j.1469-7793.1999.0921p.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silveira PC, Silva LA, Fraga DB, Freitas TP, Streck EL, Pinho R. Evaluation of mitochondrial respiratory chain activity in muscle healing by low-level laser therapy. Journal of Photochemistry and Photobiology B. 2009;95:89–92. doi: 10.1016/j.jphotobiol.2009.01.004. [DOI] [PubMed] [Google Scholar]
- Simoes RP, Castello-Simoes V, Mendes RG, Archiza B, Santos DA, Machado HG, Bonjorno JC, Jr, Oliveira CR, Reis MS, Catai AM, Arena R, Borghi-Silva A. Lactate and heart rate variability threshold during resistance exercise in the young and elderly. International Journal of Sports Medicine. 2013;34:991–996. doi: 10.1055/s-0033-1337946. [DOI] [PubMed] [Google Scholar]
- Vieira WH, Ferraresi C, Perez SE, Baldissera V, Parizotto NA. Effects of low-level laser therapy (808 nm) on isokinetic muscle performance of young women submitted to endurance training: A randomized controlled clinical trial. Lasers in Medical Science. 2012;27:497–504. doi: 10.1007/s10103-011-0984-0. [DOI] [PubMed] [Google Scholar]
- Whipp BJ. The slow component of O2 uptake kinetics during heavy exercise. Medicine and Science in Sports and Exercise. 1994;26:1319–1326. [PubMed] [Google Scholar]
- Whipp BJ, Casaburi R. Characterizing O2 uptake response kinetics during exercise. International Journal of Sports Medicine. 1982;3:97–99. doi: 10.1055/s-2008-1026070. [DOI] [PubMed] [Google Scholar]
- Whipp BJ, Ward SA, Lamarra N, Davis JA, Wasserman K. Parameters of ventilatory and gas exchange dynamics during exercise. Journal of Applied Physiology. 1982;52:1506–1513. doi: 10.1152/jappl.1982.52.6.1506. [DOI] [PubMed] [Google Scholar]
- Wishart DS, Knox C, Guo AC, Eisner R, Young N, Gautam B, Hau DD, Psychogios N, Dong E, Bouatra S, Mandal R, Sinelnikov I, Xia J, Jia L, Cruz JA, Lim E, Sobsey CA, Shrivastava S, Huang P, Liu P, Fang L, Peng J, Fradette R, Cheng D, Tzur D, Clements M, Lewis A, De Souza A, Zuniga A, Dawe M, Xiong Y, Clive D, Greiner R, Nazyrova A, Shaykhutdinov R, Li L, Vogel HJ, Forsythe I. HMDB: A knowledgebase for the human metabolome. Nucleic Acids Research. 2009;37:D603–D610. doi: 10.1093/nar/gkn810. [DOI] [PMC free article] [PubMed] [Google Scholar]