Abstract
HIV-associated mortality has been significantly reduced with antiretroviral therapy (ART), and HIV infection has become a chronic disease that frequently coexists with many disorders, including substance abuse (Azar et al. 2010; Phillips et al. 2001). Alcohol and drugs of abuse may modify host-pathogen interactions at various levels including behavioral, metabolic, and immune consequences of HIV infection, as well as the ability of the virus to integrate into the genome and replicate in host cells. Identifying mechanisms responsible for these interactions is complicated by many factors, such as the tissue specific responses to viral infection, multiple cellular mechanisms involved in inflammatory responses, neuroendocrine and localized responses to infection, and kinetics of viral replication. An integrated physiological analysis of the biomedical consequences of chronic alcohol and drug use or abuse on disease progression is possible using rhesus macaques infected with simian immunodeficiency virus (SIV), a relevant model of HIV infection. This review will provide an overview of the data gathered using this model to show that chronic administration of two of the most commonly abused substances, alcohol and cannabinoids (Δ9-Tetrahydrocannabinol; THC), affect host-pathogen interactions.
Keywords: HIV, SIV, Alcohol, Cannabinoids, Immune System, Metabolism
Introduction
Alcohol use disorders (AUDs) are the most common and costly form of substance abuse, and cannabis is the most commonly used illicit drug in the United States (The NSDUH Report: Substance Use and Mental Health Estimates from the 2013 National Survey on Drug Use and Health: Overview of Findings 2014). It is estimated that more than one million people are currently living with HIV in the U.S. (Centers for Disease Control and Prevention 2014). The rates of chronic alcohol consumption and abuse are higher in persons living with HIV/AIDS (PLWHA) than the general public, and rates of heavy alcohol use are reported by 40 to 50% of patients surveyed (Braitstein et al. 2001; Chitsaz et al. 2013; Furler et al. 2004). Similarly, PLWHA have a higher prevalence of cannabis use than that of the general population (Braitstein et al. 2001; Chitsaz et al. 2013; Furler et al. 2004; Harris et al. 2014). Alcohol and illicit drug use are significant predictors of non-adherence to ART (Bell et al. 1998; Bing et al. 2001; Khalsa and Royal 2004; Lee et al. 2001; Nair et al. 2004). PLWHA with alcohol and drug use disorders are consistently found to be non-adherent to ART and have a higher likelihood of virologic non-suppression and adverse disease outcomes (Ghosn et al. 2014; Kino and Chrousos 2003; Slawson et al. 2014). While cannabis use has not been linked to decreased ART adherence (Slawson et al. 2014), both alcohol and cannabis use in PLWHA are associated with high-risk sexual behaviors (Hendershot et al. 2007; Raj et al. 2009; Shuper et al. 2009), increased vaginal (Theall et al. 2008) and seminal viral shedding (Ghosn et al. 2014), and heightened risk of HIV transmission.
By impairing cognition and executive function, alcohol and cannabis use and abuse can lead to an increase in risky behavior and increased transmission of HIV. Because HIV infection has become a more chronic disease, comorbid conditions can alter the disease course by affecting metabolic (Kino and Chrousos 2003), immune, and neurobehavioral function (Lee et al. 2001) function. The use and abuse of illicit drugs is strongly discouraged in this patient population. However, the greater likelihood of alcohol and cannabis use, which is frequent in the general population and perceived by the lay public as having low health risks, has the potential to significantly impact the incidence of comorbid conditions and altering disease progression (Bell et al. 1998; Bing et al. 2001; Galvan et al. 2002; Khalsa and Royal 2004; Nair et al. 2004). Preclinical and clinical studies have provided evidence that alcohol and cannabinoids including Δ9-THC produce multisystemic biological effects that can modulate HIV disease progression. Considerable knowledge regarding these interactions has been derived from studies using non-human primates (NHP) infected with simian immunodeficiency virus (SIV).
Much debate exists regarding the interaction of HIV disease progression and the two most frequently used and abused drugs (alcohol and cannabis), as many of the interactive effects that have been identified range from detrimental to beneficial. While the chronic use of alcohol has been consistently shown to accelerate disease progression in both animal and clinical studies, chronic use and administration of cannabinoids has not been shown to adversely affect disease progression. Systematic analysis of the organ systems impacted by alcohol and cannabinoids has been mostly done using the SIV-infected NHP model. In this review, we compare and contrast the detrimental, neutral, and beneficial effects of chronic alcohol and cannabinoid administration on SIV disease progression. The majority of investigations examining the impact of cannabinoids on disease progression have been limited to Δ9- tetrahydrocannabinol (THC), the most psychoactive component in marijuana and the main component of Marinol®, a drug approved by the Food and Drug Administration for treatment of HIV-associated anorexia. Throughout the review we identify critical domains of drug-disease interaction worthy of further study.
The SIV model for the controlled study of the impact of chronic alcohol and cannabinoids on disease progression
The study of how drugs and alcohol impact disease progression in the clinical setting is obscured by multiple factors, including the difficulty in selecting a homogenous patient population, the ethical limitations in administering psychoactive drugs to an already fragile patient population, and the confounding effect of prescription, experimental, and illicit drugs frequently used by these individuals. Thus, much of our knowledge has been derived from integration of cellular and preclinical studies, and interpreted in light of what we know of human disease progression (Fig 1). The SIV-infected NHP model circumvents many of these limitations. The SIV-infected NHP is well recognized as the best animal model for studying the pathogenesis of HIV-like infection (Gardner and Luciw 1988; Lackner and Veazey 2007; Letvin 1990). Based on the genetic, antigenic, and biologic properties, SIVs are the closest known relatives of human HIVs. Experimental infection of NHP with SIV produces a disease that is remarkably similar to human AIDS (Arthur et al. 1986). Intravenous inoculation with SIV results in peak viral load between days 14 and 21, with viral antigenemia being well established after ~30 days. This initial increase in viremia is followed by a SIV-infected, yet asymptomatic period ultimately culminating in a clinical AIDS stage, which is characterized by diarrhea, weight loss, lymphopenia, thrombocytopenia, and lymphadenopathy/lymphoid hyperplasia progressing to immunosuppression (Baskin et al. 1988; Letvin 1990; McClure et al. 1990). Immunologically, like HIV, SIV infection is characterized by a marked reduction in CD4+ cells and in the CD4+/CD8+ cell ratio, as well as a substantial increase in the rates of lymphocyte turnover (Mohri et al. 1998). The cause of death in the majority of untreated SIV-infected animals is the result of enteric disease, diarrhea and weight loss. Additional causes of death include lymphoma, opportunistic infections, and generalized wasting without diarrhea. The SIV-infected NHP allows for an integrated biological approach for comparing and contrasting the impact of chronic alcohol and Δ9-THC administration on SIV disease progression with particular focus on host defense and immune function, neurobehavioral function, and metabolism.
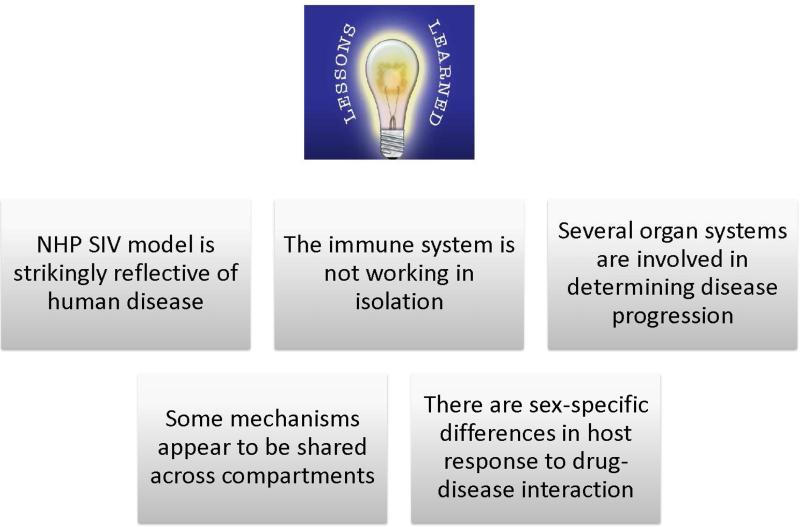
Figure 1.
Salient lessons learned using an integrated biological systems approach to examine the impact of alcohol and cannabinoids on SIV disease progression. The non-human primate model helped identify some of the underlying mechanisms that determine disease progression and how these are affected by alcohol and drugs of abuse under controlled conditions. This model has also allowed us to contrast the different phenotypes of disease progression produced by alcohol and the cannabinoids.
Salient Biomedical and Behavioral Consequences of Alcohol and Cannabinoid Use and their Interaction with HIV/SIV Disease Progression
Alcohol & disease progression
Clinical and preclinical studies have identified significant comorbidity for chronic alcohol use and HIV infection. Alcohol-induced multi-systemic pathophysiological alterations in nutritional, metabolic, oxidative, and neuroendocrine pathways have all been identified as modulators of disease progression (Grant et al. 1995). Studies from our group in which SIV-infected NHP received intragastric chronic binge alcohol (CBA) demonstrated that alcohol elevates the most reliable predictor of disease progression, viral set point (Bagby et al. 2006); accelerates disease progression to end-stage disease; increases and sustains elevations of viral load in the lung during bacterial infection (Nelson and Bagby 2011); induces changes in the ratio of intestinal CD4+ and CD8+ T lymphocytes in a direction that could increase disease transmission (Poonia et al. 2006); increases infectivity (Amedee et al. 2014b); disrupts nitrogen balance (Molina et al. 2006), decreases skeletal muscle (SKM) functional capacity (LeCapitaine et al. 2011); accentuates wasting at end-stage disease (Molina et al. 2008), and unmasks neurobehavioral deficits (Winsauer et al. 2002) (Table 1). Similar detrimental effects of alcohol self-administration in SIV-infected macaques have been reported in other studies. Specifically, higher viral loads in cerebral spinal fluid (Kumar et al. 2005) and increased levels of HIV target cells in various tissues (Marcondes et al. 2008) have been reported in alcohol-exposed animals relative to controls. Although few clinical studies have examined the role of hazardous alcohol consumption on disease progression, studies have reported a dose-dependent decrease in overall survival in PLWHA (Deveaux et al. 2007), as well as an increased likelihood of greater CD4+ cell count declines than those observed in non-drinkers (Baum et al. 2010; Miguez et al. 2003).
Table 1.
Impact of CBA on SIV Disease Pathogenesis
| ↑ Blood T cell proliferation (% of KI67+CD3+ cells) during primary stage of SIV infection |
| ↑ Plasma viral load at set point |
| ↑ Disease progression to end-stage SAIDS |
| ↔ Blood CD4+ lymphocyte count and CD4+/CD8+ ratio decline |
| ↓ % Gut CD3+CD8+ lymphocytes |
| ↑ % Gut CD3+CD4+ lymphocytes |
| ↑ % Gut memory CD4+ lymphocytes (CD95+CD28+) |
| ↓ % Effector memory CD8+ lymphocytes (CD95+CD28-) & activated Ki67+ CD8+ cells |
| ↑ Levels of SIV replication (viral RNA) |
| ↑ Plasma viral load not due to A cell- or humoral-mediated adaptive immune responses |
| ↓ Body weight below predicted body weight at end-stage more often |
| ↑ Nutritional and metabolic dysfunction during SIV infection and promotes SKM inflammation |
| ↓ BMI & exacerbates SKM wasting at SAIDS |
| ↓ Costimulatory molecule CD83 expression of bone marrow & circulating pools of myeloid dendritic cells |
| ↓ Dendritic cell initiation of T-cell expansion in response to infection |
| ↑ Skeletal muscle pro-oxidative milieu & ubiquitin proteasome activity, and ↓ insulin signaling |
| ↑ Local lung SIV replication in response to pneumococcal pneumonia |
| Δ A Myogenic gene expression & ↓ myogenic differentiation potential of primary SKM satellite cells |
| ↔ Effectiveness of NRTI ART suppression of viral load |
| ↑ Expression of fibrotic & inflammatory genes in SKM of SIV-infected macaques |
| ↑ SIV infectivity following rectal inoculation |
| ↑ Immune activation (BrdU+CD4+ & CD8+ cells) leading to CD8+ T cell immunosenescence |
Cannabinoids & disease progression
Marijuana is the most prevalent illicit drug of abuse and contains over 60 different cannabinoids. Δ9-THC is the most psychoactive component of marijuana (Dewey 1986; Hollister 1986). An endogenous cannabinoid system consisting of anandamide and 2-arachidonylglycerol, derivatives of arachidonic acid, has also been described (Felder et al. 1996). These two endogenous cannabinoid ligands have short half-lives, function as neuromodulators at or near their site of synthesis, and share behavioral and physiological effects with Δ9-THC (Pertwee et al. 1993). Cannabinoids bind to two major receptor subtypes (CB1 and CB2) with distinct localization (CB1 mainly in the central nervous system and CB2 mainly on B lymphocytes and natural killer cells), while exerting neurobehavioral, immunomodulatory, and metabolic effects. Marijuana use is frequent in PLWHA, both recreationally and therapeutically (SAMHSA 2004) in its synthetic form (Δ9-THC; MARINOL® (dronabinol) (ElSohly et al. 2001). In contrast to the consistent reports of non-adherence to ART by alcohol-using PLWHA, high-frequency cannabis use has not been reported to interfere with adherence to ART (Slawson et al. 2014). This is an important contrast in behavioral consequences of the use of these two most frequently abused substances that is likely to have an impact on the overall biomedical consequences and comorbidities in PLWHA.
In contrast to the detrimental impact of chronic alcohol administration to SIV-infected macaques, chronic administration of Δ9-THC to male rhesus macaques results in decreased early mortality from SIV infection, attenuation of plasma and CSF viral load, and modest retention of body mass (Molina et al. 2011b). However, a similar protective effect was not observed in female macaques (Amedee et al. 2014a), suggesting a possible drug-sex steroid interaction and highlighting the need for further studies focusing on sex-specific cannabinoid effects on SIV disease. Among the possible mechanisms responsible for modulation of disease progression in male macaques are attenuation of localized tissue inflammatory responses and viral replication (Molina et al. 2011a) as discussed below and summarized in Table 2.
Table 2.
Impact of Cannabinoids on SIV Disease Pathogenesis
| ↓ Early mortality from SIV infection |
| ↓ Plasma & CSF viral load |
| Retention of body mass |
| ↓ SIV (10 TCID (50)) viral replication in MT4-R5 cells |
| ↓ CB-1 and CB-2 receptor levels in the hippocampus |
| ↓ Brain MCP-1 expression |
| ↑ CD4+ and CD8+ T lymphocyte CXCR4 expression |
| ↔ Blood CD4+ lymphocyte count and CD4+/CD8+ ratio decline |
| ↓ Gut viral load |
| ↑ Duodenal integrin beta 7 (+) (B7) CD4 (+) and CD8(+) central memory T cells |
| ↑ Gut Th2 cytokine expression |
| Δ Gut expression of genes involved in apoptosis, cell survival, proliferation, morphogenesis, and energy and substrate metabolic processes |
| ↓ Gut epithelial crypt cell apoptosis |
| ↑ Total CXCR4 expression in peripheral and duodenal CD4+ and CD8+ T lymphocytes |
| ↑ Gut miR-10a, miR-99b, miR-145, miR-149 and miR-187 (target proinflammatory molecules) expression |
| ↓ NOX4+ crypt epithelial cells |
Principal Domains of Alcohol and Cannabinoid Interaction with HIV/SIV Disease
Because alcohol and marijuana are the most frequently used and abused substances by the general population and PLWHA, understanding how they impact disease progression is of great relevance. While both substances are associated with increased risky behavior, our studies, along with those from other investigators, have identified contrasting effects on disease progression in three major domains; immune function, neurobehavioral impairments, and metabolic activity (Fig. 2 & 3). Here, we discuss some of the most relevant findings that support the sensitivity of these domains to modulation by alcohol and cannabinoids.
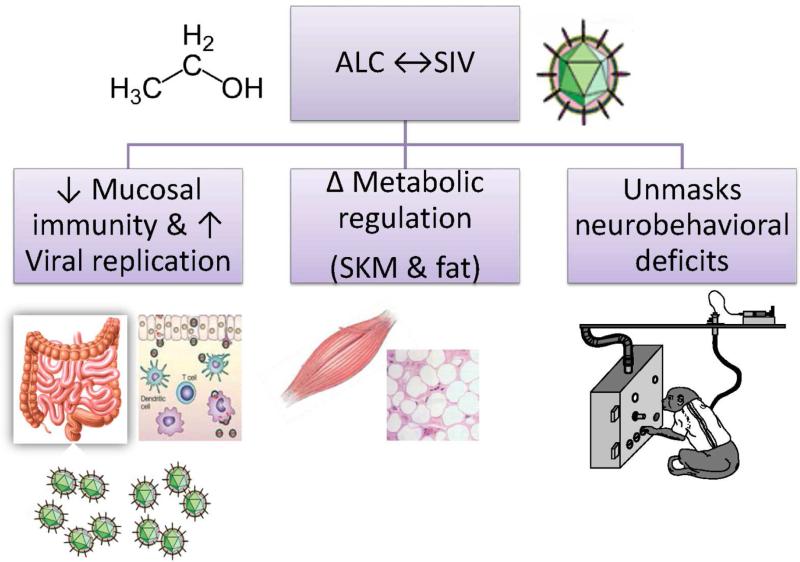
Figure 2.
The most relevant effects identified during the investigation of the interaction of chronic binge alcohol administration with SIV disease progression were decreased mucosal immunity and increased viral replication, changes in metabolic regulation, and increased disruption of neurobehavioral functions.
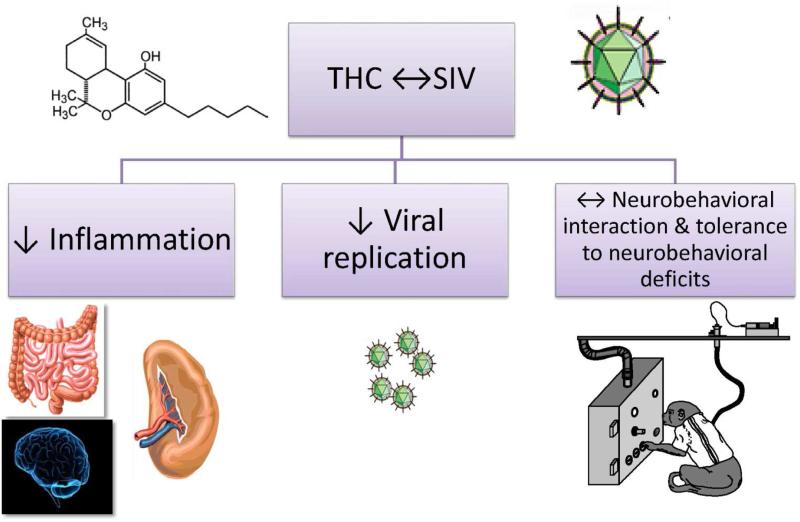
Figure 3.
The most relevant effects identified during the investigation of the interaction of chronic Δ9-THC administration with SIV disease progression were suppressed inflammation and decreased viral replication.
Alcohol Immunomodulation & SIV/HIV disease
Chronic alcohol consumption and abuse, particularly in the form of binge drinking, has been shown to result in significant alterations in immune function, thereby increasing host susceptibility to bacterial and viral infections, including HIV. In vitro studies have reported that peripheral blood mononuclear cells isolated from individuals after consuming alcohol support higher levels of HIV replication as compared to replication levels in cells collected prior to alcohol ingestion (Bagasra et al. 1996). Similarly, a tenfold increase in HIV levels was observed in cultured lymphocytes that were pre-treated with alcohol in vitro, prior to HIV infection (Liu et al. 2003). More recently, cord blood monocyte-derived macrophages (CBMDM) exposed to alcohol in vitro were shown to support higher levels of HIV when compared to controls, and a concomitant suppression of several innate HIV restriction factors (Mastrogiannis et al. 2014). Treatment of CBMDM with 20 – 40 mM alcohol significantly decreased the levels of several miRNAs known to restrict HIV replication and dose-dependently suppressed mRNA levels for the anti-viral factors APOBE3G, APOBEC3H, INF-alpha, and IFN-ß. These innate immune factors play important roles in the initial host responses to infection (Ivashkiv and Donlin 2014). In particular, the balance of type-1 interferon responses is critical to the control of viral dynamics in tissue reservoirs, where the absence of type-1 interferons during acute infection has been shown to accelerate disease progression (Sandler et al. 2014). Recent studies from our group support the hypothesis that CBA disrupts the innate mucosal immune responses to increase acquisition of SIV. Following a single intra-rectal inoculation with SIVmac251, we observed an increased susceptibility to SIV infection in animals exposed to CBA as compared to controls, with 100% of CBA animals infected vs. 67% of the controls (p=0.004) (Amedee et al. 2014b). How CBA affects specific innate responses following HIV/SIV exposure and their contribution to viral acquisition and early replication remains to be examined.
Our studies have also demonstrated that CBA increases viral load at set point and decreases time to end-stage disease in SIV-infected (SIVmac251 and SIVB670) rhesus macaques (Bagby et al. 2006). The higher viral load and accelerated disease progression occured despite CBA-administered animals having greater virus-specific cellular immune responses and similar humoral responses compared to a sucrose-treated group (Pahar et al. 2013). A possible mechanism for the enhanced viral replication may be alcohol-mediated alterations in the gut mucosal immune system, as there were higher percentages of central memory and naïve CD4+ T cells in duodenal tissues of CBA macaques prior to SIV inoculation (Poonia et al. 2006a). After infection, CBA animals had higher levels of SIV in the gut mucosa sampled at viral set point (approximately 10 weeks post-inoculation) (Poonia et al. 2006b). The CBA-induced immunological changes in this mucosal tissue provide an environment conducive to early and rapid virus replication. The increase in viral replication following CBA could lead to a decreased capacity to control viral reactivation during opportunistic infections. In support of this hypothesis, results from our studies show that S. pneumoniae inoculation results in prolonged viral replication in the infected lungs of CBA-administered SIV-infected macaques, as compared to that of SIV-infected controls (Nelson et al. 2013). Increased localized HIV replication, particularly in alveolar macrophages and CD4+ cells, has been reported to increase viral mutations and promote viral escape from latent reservoirs (Nelson and Bagby 2011). In more recent studies, we have also observed compartmentalized changes in the female genital tract of CBA macaques, where we observed decreased levels of lactobacillus morphotypes, increased levels of gram-positive cocci, and an increased number of inflammatory cells in vaginal fluids before and after SIV infection. These changes were associated with a higher incidence of cell-associated SIV shedding in vaginal secretions (Loganantharaj et al. 2014), and suggest that CBA may increase the risk of genital virus shedding and sexual transmission. This is supported by reports that moderate to heavy alcohol consumption by HIV+ women on ART increased the incidence of vaginal HIV-1 shedding compared to women that did not report alcohol consumption (Theall et al. 2008). Studies from other laboratories have also reported alcohol-induced alterations in SIV pathophysiology that could increase vulnerability to disease progression. Alcohol consumption has been reported to reduce circulating memory CD4+ T cells and increase levels of monocytes expressing the viral co-receptor CCR5 during the acute stages of infection (Marcondes et al. 2008). Moreover, others have reported higher plasma viral load, continued CNS viral replication, and greater magnitude of CD4+ cell loss in alcohol-consuming NHP when compared to non-consuming SIV-infected macaques (Kumar et al. 2005).
Alcohol-mediated alterations in immune function may have additional detrimental effects on vulnerable organ compartments. For example, alcohol administration has been shown to affect immune cell populations in gut, liver, and brain during the acute phase of infection (Marcondes et al. 2008). The resulting tissue inflammation has been proposed to favor a milieu with increased susceptibility to infection, but may also enhance or promote tissue injury. We propose that initial immune activation and increased viral replication seen in the CBA-administered SIV-infected macaques is conducive to immunosenescence over time. Recent studies from our group have identified an increase in the percentage of blood and lymph node CD8+ T immunosenescent cells isolated from non-ART treated CBA administered SIV-infected macaques at 12-14 months after SIV infection that is not seen at this time in sucrose controls (unpublished studies). Moreover, ongoing studies indicate that circulating PBMCs isolated from PLWHA with AUDIT scores greater than 8 have higher percentages of activated immunosenescent cells than those with AUDIT scores less than 8. These findings are important because they reflect the clinical relevance of the findings from the NHP model and highlight immunosenescence as a potential factor contributing to HIV disease progression.
Cannabinoids, Immunomodulation & SIV/HIV
Cannabinoids have been shown to alter several aspects of immune function (Friedman et al. 1995; Klein et al. 2003) including cytokine production and lymphocyte phenotype, function, and survival (Friedman et al. 1995; Klein et al. 2003; Zhu et al. 1998). Clinical studies have shown that chronic use of cannabis is associated with increased incidence of infection (Morahan et al. 1979; Shay et al. 2003; Specter et al. 1991; Tindall et al. 1988). Similarly, in the NHP, chronic administration is associated with suppression of lymphocyte (Daul and Heath 1975) and alveolar macrophage (Cabral et al. 1991) function. THC has been reported to decrease cell-mediated immunity by suppressing the production of IFN- and IL-12, and increasing T helper 2 (Th2) activity mediated by enhanced IL-4 expression (Newton et al. 1994). In addition, THC has been shown to suppress T-cell proliferation and shift the balance of Th1/Th2 cytokines (Pross et al. 1990). The cannabinoid-dependent modulation of the activation and balance of human Th1/Th2 cells has been proposed to occur through a CB2 receptor-dependent pathway (Yuan et al. 2002). The possibility that this altered balance affects the course and progression of HIV infection has not been examined and warrants closer attention. To date, no clinical studies have rigorously addressed the impact of chronic THC use on the course of HIV infection. However, reports of studies from the Sydney AIDS project indicated that less frequent oral sexual activity and more frequent use of marijuana in the three months prior to enrollment were the only lifestyle differences between seropositive subjects who progressed to AIDS and those that did not progress (Tindall et al. 1988). However, more recent reports on data collected from two linked longitudinal observational studies of injection drug users suggest that at least daily cannabis use was associated with significantly lower viral load than in non-users during the early period following seroconversion (Milloy et al. 2014). Therefore, much remains to be understood about the interaction of cannabinoids with disease progression, particularly as it is a drug that increasingly has gained acceptance in society with an associated perception of safety or low risk. These factors are likely to further increase use and abuse of cannabis by PLWHA. A better understanding of the potential benefits, detriments, or limitations of cannabis-mediated modulation of disease warrants further research in this area. Specifically, whether chronic cannabis consumption interacts with ART metabolism and/or effectiveness remains to be determined.
Most of the immunomodulatory effects of cannabinoids have been shown to be mediated by CB2 receptors (Rom and Persidsky 2013). Cannabinoid receptor agonists have been shown to inhibit viral replication in macrophages (Ramirez et al. 2013), reduce CXCR4-tropic virus infection in primary CD4+ T cells, and decrease CXCR4-mediated activation of G-protein activity and MAPK phosphorylation (Costantino et al. 2012). In addition to the attenuation of inflammation in the spleen and brain, our recent studies have identified the gastrointestinal tract as the site of cannabinoid disease modulation. Those studies showed that chronic Δ9-THC administration modulated duodenal T-cell populations, favored a pro-Th2 cytokine balance, and decreased intestinal apoptosis (Molina et al. 2014). More recently, we have also identified the contribution of epigenetic mechanisms to cannabinoid-mediated disease modulation. Our most recent findings show selective upregulation of miR-10a, miR-24, miR-99b, miR-145, miR-149 and miR-187 expression in the duodenum of THC-treated SIV-infected macaques. These miRs, particularly miR-99b, have been shown to target proinflammatory molecules including NOX4, which may serve to protect the intestinal epithelium from oxidative stress-induced damage (Chandra et al. 2014). Others have shown a role for CB2-mediated attenuation of pro-inflammatory cytokine-mediated mucosal damage without direct effects on human colonic mucosal epithelial barrier function (Harvey et al. 2013). Further examination of cannabinoid-mediated epigenetic modulation of gut barrier function may lead to specific miRs that could be targeted to diminish gastrointestinal inflammation and viral replication.
Neurobehavioral Interactions between Alcohol and SIV/HIV disease
The central nervous system (CNS) is an early target in HIV pathology with increasingly severe neurological deficits developing during the course of the disease (Snider et al. 1983). Early epidemiological data indicated that 50-75% of HIV-infected adults were diagnosed with neurological problems and 20% developed AIDS dementia (Grant et al. 1995). Viral replication in the CNS is thought to establish a state of chronic immune activation that can lead to several forms of cognitive impairment. According to the Cipher Protocol (Rodger et al. 2011), this cognitive impairment typically leads to mental slowing, memory loss, and difficulties in complex tasks requiring executive function. In 2007, the American Academy of Neurology partitioned HIV-associated neurocognitive disorders (HAND) into 3 categories: Asymptomatic Neurocognitive Impairment (ANI, mild to moderate neuropsychological [NP] impairment), Mild Neurocognitive Impairment (MNI, documented mild to moderate NP impairment in at least two cognitive domains with mild everyday difficulties), and HIV Associated Dementia (HAD, moderate to severe NP impairment in two cognitive domains with moderate to severe everyday life difficulties). Furthermore, HAD, a subcortical dementia, is characterized by progressive motor abnormalities (tremor, gait ataxia, and loss of fine motor movements), cognitive impairments (mental slowing, forgetfulness, and poor concentration), and behavioral disorders (including mania at the outset, apathy, and emotional ability). Although severe HAD is rarely seen in patients receiving effective combination antiretroviral therapy (cART), varying degrees of NP impairment are increasingly recognized in HIV patients both on and off cART, even in the asymptomatic period of HIV infection associated with higher CD4 counts (Cysique and Brew 2009). These clinical observations underscore the need for continued research on the neurobiology of HIV.
One of the most important attributes of the SIV-infected NHP model is that some of the same behavioral and neuropathological sequelae that are observed in the human neuroAIDS syndrome have also been shown to occur in SIV-infected monkeys (da Cunha et al. 1994; Heyes et al. 1992; Murray et al. 1992; Prospero-Garcia et al. 1996). One common finding between humans and monkeys has also been that motor skill deficits occur earlier and more frequently than cognitive impairments (Gold et al. 1998; Rausch et al. 1994; Raymond et al. 1999). Although all of the findings from HIV-infected humans and SIV-infected monkeys have not been this congruent and straightforward, the vast majority of findings in monkeys have strong parallels with the findings in humans (Cheney et al. 2008). Moreover, the NHP model of SIV has become an important tool for directly investigating cognitive and motor deficits produced by infection and have allowed investigators to eliminate many of the variables that confound human studies, such as the effects of cART, education level, age, socioeconomic status, and polydrug abuse.
We used the SIV-infected NHP model and a complex learning task to determine the effects of CBA administration on SIV disease progression. Chronic alcohol consumption, like HIV and SIV, is known to produce NP changes in animals and man, and in all likelihood could compromise functioning of some of the same structures in the brain that are affected by immunodeficiency viruses. In animal studies, for example, chronic ethanol administration leads to learning deficits, loss of dendritic spines, reduction in dendritic branching, and cell death in areas of the brain such as the hippocampus and thalamus (Grant 1987; Grant et al. 1995). In our task, which assessed executive function and the capacity for new learning, CBA clearly produced greater disruptions of the task in SIV-infected monkeys than in monkeys who received either CBA or SIV alone. However, the greater NP impairments in the SIV-infected group were only evident during alcohol intoxication. This suggests that CBA unmasks CNS pathology that is otherwise not apparent in these subjects. Interestingly, these data fit well with data from 46% of the cross-sectional studies and 66.6% of the longitudinal studies indicating that asymptomatic individuals with HIV infection do not appear to have clinically relevant cognitive impairments (Fein et al. 1995; Newman et al. 1995). Our findings also fit well with the notion that alcohol can serve as a cofactor that may unmask otherwise subclinical deficits, as chronic alcohol has since been shown to enhance deficits in motor and visuomotor speed and coordination (Fama et al. 2007; Meyerhoff 2001), attention and learning, and memory in HIV patients (Rothlind et al. 2005). Chronic alcohol and HIV infection have also been shown to have a synergistic detrimental impact on brain volume in areas such as the central white matter, subcortical brain structures, and the frontal cortex (Sassoon et al. 2007). These morphological changes, combined with reported alterations in brain inflammatory responses and oxidative stress in HIV subjects, correlates well with the occurrence of impaired cognitive function. Whether these findings are a consequence of enhanced neuroinflammation or greater viral load remains to be determined and is the focus on ongoing investigations.
Neurobehavioral Interactions between Cannabinoid and SIV/HIV disease
Prior to conducting any research with the SIV-infected NHP model, there was good reason to suspect that the disruptive effects of chronic THC on neurobehavioral processes would be greater in the presence of an immunodeficiency viral infection, just as the disruptive effects of alcohol on neurobehavioral processes were greater in the presence of HIV in humans (Green et al. 2004; Rothlind et al. 2005) and of SIV in NHP (Winsauer et al. 2002). For instance, THC is known to affect reaction time (Hunault et al. 2009; Wilson et al. 1994) and working memory (Hampson and Deadwyler 1999; Hampson and Deadwyler 2000; Lichtman et al. 1995), which are thought to involve subcortical structures and the hippocampus, respectively. These areas are also known to express CB-1 receptors, which are known to mediate the NP impairments produced by the cannabinoids (Rodriguez de Fonseca et al. 1994; Romero et al. 1998; Villares 2007; Winsauer et al. 2011b) Thus, even though chronic THC alone had not been shown to produce marked neuropathological alterations that lead to permanent NP impairments, and the long-term consequences of use or abuse on CNS function had not been as well studied in animals or man (Carlini 2004; Landfield et al. 1988) there was little reason to expect anything other than additive NHP impairments for the comorbidity of chronic cannabinoids and HIV or SIV.
To examine this interaction specifically, we used a NHP SIV model similar to that employed in the study of the interaction of CBA and SIV infection (Winsauer et al. 2002). The only exceptions were that we administered THC daily for approximately one month prior to SIV inoculation instead of three months as we had done with CBA. Of note, and in contrast to our results with CBA, chronic THC did not produce additive NP impairments with SIV infection. In fact, NHP that received chronic THC (as opposed to vehicle) developed tolerance to its behaviorally disruptive effects and this tolerance was maintained during the initial 7-12 months of the study irrespective of SIV infection. These findings were associated with postmortem histopathology data indicating that chronic THC may have reduced the frequency of CNS pathology as well as opportunistic infections. Finally, while chronic THC did decrease CB-1 and CB-2 levels in the hippocampus, it did not adversely impact viral load in plasma, CSF, or the brain during the early stages of infection, or increase the levels of inflammatory cytokines. Though this study was limited by small numbers, the notable difference in neuropathology and in opportunistic infection rates between vehicle- and THC-treated SIV-infected subjects strongly suggests a protective effect of cannabinoids on disease progression
Another important consideration when studying the neurobehavioral interaction of cannabinoids and HIV is the sex of the individual. Cannabinoids have clearly been shown to exert both sex-dependent biological and behavioral effects. In humans, marijuana abuse is more prevalent, and tends to persist longer, in males than females, which suggests potential sex differences in both the reinforcing and subjective effects of Δ9-THC (ADAMP 2003; SAMHSA 2009; SAMSHA 2005). There are also behavioral and pharmacodynamic data from studies with animals to support a direct interaction of the cannabinoids with the gonadal hormones (Fattore and Fratta 2010). In a rodent study, for example, a within-subject design was used to show that estradiol administration can attenuate the disruptive effects of Δ9-THC in ovariectomized (OVX) female rats responding in a complex learning task (Daniel et al. 2002). Along with these purely behavioral data, there are biochemical data indicating that ovarian hormones can either inhibit or antagonize cannabinoid signaling in areas of the brain important for cognitive functioning, such as the hippocampus and striatum (Winsauer et al. 2011a). Moreover, estrogens are capable of producing the attenuation of cannabinoid signaling in multiple ways, including reducing guanosine-5'-O-(3-thio)triphosphate (GTPγS) binding or coupling of CB1 receptors to signaling pathways, modification of CB1 receptor mRNA and protein expression, altering the relative affinity of agonists for CB1 receptors, and affecting co-chaperones such as the activator of heat-shock 90kDa protein ATPase homolog 1 (AHA-1) that move CB1 receptors to the cell surface from subcellular locations (Filipeanu et al. 2011; Gonzalez et al. 2000; Mize and Alper 2000; Riebe et al. 2010; Rodriguez de Fonseca et al. 1994). These data, which support an antagonistic interaction between estrogens and cannabinoids, are in agreement with results obtained from our most recent study using the SIV-infected NHP model. In this study, female NHP became tolerant to the neurobehavioral impairments produced by chronically administered THC (as opposed to vehicle), but were not protected from early mortality or some of the other metabolic and immune effects of SIV infection (Amedee et al. 2014a). Further studies are needed to explore the sex-dependent effects of the cannabinoids on HIV disease progression, as well as the implications on viral transmission. Clearly, the SIV-infected NHP model provides a unique opportunity to evaluate sex differences in the protective and detrimental effects of cannabinoids on disease course and progression.
Alcohol Metabolic Modulation & SIV/HIV disease
The increased survival of PLWHA on ART is complicated by severe metabolic side effects (Carr and Cooper 2000; Carr et al. 1998). Metabolic alterations, particularly defects related to lipid metabolism, with or without an associated fat “redistribution” or lipodystrophy, have been shown to lead to a higher risk for insulin resistance. Such resistance is often manifested as an increased frequency of glucose intolerance or frank diabetes mellitus in PLWHA (Barbaro 2006; Hadigan et al. 2000; Jones et al. 2005). The mechanisms underlying dysglycemia in PLWHA remain poorly understood (Gutierrez and Balasubramanyam 2012). However, chronic subclinical inflammation is thought to contribute to adipose tissue dysregulation and metabolic derangements seen in ART-treated PLWHA (Kamin and Grinspoon 2005; Kolter 2003).
Alcohol-using PLWHA have higher odds of presenting with lipodystrophy (Cheng et al. 2009) and altered adipokine profiles, which have been linked to alterations in metabolism in obese (Rasouli and Kern 2008) and ART-treated PLWHA (Sevastianova et al. 2008). Reports on the effects of chronic alcohol use and abuse on fat mass and metabolism have been equivocal. Some have reported decreased fat mass (Addolorato et al. 2000; Leggio et al. 2009), while others have demonstrated a high incidence of dyslipidemia and increased fat mass in alcoholics with more than 20% of patients meeting criteria for metabolic syndrome (Jarvis et al. 2007). The concept that alcohol induces insulin resistance by promoting adipose tissue dysregulation is gaining momentum. Recent publications suggest that chronic alcohol abuse may not only alter fat mass, but may also disrupt adipokine profiles including that of leptin (Nicolas et al. 2001) and adiponectin (Chen et al. 2007; Rogers et al. 2008; You et al. 2005; You and Rogers 2009). Results from our ongoing studies in male and female CBA-administered SIV-infected macaques treated with ART have identified decreased circulating levels of high molecular weight (HMW) adiponectin (unpublished observations), which has been shown to precede development of insulin resistance (Kadowaki et al. 2006). Whether CBA and SIV lead to enhanced adipose inflammation has not been determined. However, a compromised gut barrier, which has been reported in alcoholics and in SIV-infected macaques could lead to increased translocation of gut bacterial toxins and promote subclinical systemic inflammation and dyslipidemia as recently reported in PLWHA (Brenchley 2006).
A U-shaped relationship was described for levels of alcohol consumed and incidence of Type-II diabetes mellitus (T2DM) (Conigrave and Rimm 2003; Howard et al. 2004; Klatsky 2007). In a large meta-analysis, protective effects of alcohol consumption on the incidence of T2DM in both men and women were greatest with the consumption of about two drinks per day. Higher levels of alcohol consumption (above 50 g/day for women and 60 g/day for men) were associated with an increased risk for T2DM (Baliunas et al. 2009). However, in overweight women and men, a positive relationship between alcohol consumption and T2DM was observed irrespective of the amount of alcohol consumed (Beulens et al. 2012). Preclinical studies have also demonstrated that the administration of moderate amounts of alcohol to rodents with metabolic disorders increase the risk of metabolic complications and diabetes (Adaramoye and Oloyede 2012). Acute alcohol administration, on the other hand, impairs insulin sensitivity in short-term experiments in rodent models (Lindtner et al. 2013) and humans (Boden et al. 1993; Yki-Jarvinen and Nikkila 1985).
The deleterious effects of alcohol on metabolic regulation may result from impaired secretion of insulin, reduced insulin sensitivity or increased resistance alone or some combination of these effects (Bau et al. 2007; de la Monte et al. 2012; Eisner et al. 2014; Gao et al. 2010; Lang et al. 2005; Lang et al. 2001; Lieber 2004; Nguyen et al. 2012; Purohit et al. 2009; Ronis et al. 2007). Together with the direct effects of alcohol on metabolic pathways, malnutrition also contributes to metabolic dysregulation among alcoholics (Bunout 1999).
Our studies have provided evidence that CBA accentuates metabolic derangements in SIV-infected macaques (Molina et al. 2008; Molina et al. 2006). CBA-administered SIV-infected macaques not treated with ART present with decreased total caloric intake, altered nutrient selection, and decreased nitrogen intake and balance, as disease progresses. Moreover, they show a marked decrease in skeletal muscle mass (SAIDS wasting) and dysfunctional skeletal muscle phenotype, which we found were associated with accelerated time to end-stage disease, relative to isocaloric sucrose SIV-infected animals (Molina et al. 2006). Our findings indicate that the mechanisms underlying (and preceding) accentuated SAIDS wasting include the amplification of localized skeletal muscle inflammation, profound depletion of skeletal muscle anti-oxidant capacity (oxidative stress), increased proteasomal activity, and decreased myoblast differentiation potential (LeCapitaine et al. 2011; Simon et al. 2014).
A mechanism that is likely an underlying factor for alcohol-induced disruption of metabolic regulation and muscle wasting is impaired anabolic cell signaling (Ronis et al. 2007). Specifically, studies have shown that chronic alcohol consumption markedly impairs insulin and insulin-like growth factor (IGF) cell signaling, and this has been considered an important mechanism contributing to alcoholic myopathy (Lang et al. 2005; Nguyen et al. 2012). The results from our studies and those of others collectively indicate that enhanced skeletal muscle inflammation promotes degradation and impairs accretion or repair of skeletal muscle, which we predict translates into decreased strength in PLWHA. Reduction and cessation of alcohol consumption result in improved muscle strength (Fernandez-Sola et al. 2000; Martin et al. 1985).
However, whether targeted nutritional or physical (exercise) interventions are effective in improving metabolic regulation, restoring muscle mass, and improving strength in alcohol-using PLWHA remains to be determined.
Cannabinoid metabolic modulation & SIV/HIV
Reports on the effects of marijuana on body mass index (BMI) are inconsistent. Two representative national surveys reported lower BMI and lower prevalence of obesity among marijuana users than that of non-users (Penner et al. 2013). In the National Epidemiological Survey on Alcohol and Related Conditions (NESARC) and the National Comorbidity Survey-Replication (NCS-R) studies, the adjusted prevalence of obesity was 22.0% and 25.3% in participants reporting no use of cannabis in the past 12 months, compared to 14.3% and 17.2% in participants reporting the use of cannabis 3 days a week or more, respectively (Le Foll et al. 2013). Similarly, in a 21-year follow-up study from the Mater-University Study of Pregnancy and its Outcomes (MUSP), the prevalence of obesity was lower among recent cannabis users (Hayatbakhsh et al. 2010). These findings would appear incongruent with the fact that cannabis and its derivatives have been approved by the Food and Drug Administration (FDA) for treatment of anorexia (Merroun et al. 2009), nausea, anxiety, constipation, tiredness and depression in PLWHA and in cancer patients (Cinti 2009; Woolridge et al. 2005). Few clinical studies have provided insight into the impact of cannabinoids on metabolic comorbidities in PLWHA. Short-term use of cannabis, either oral or smoked, does not substantially elevate viral load in HIV-infected individuals receiving stable ART containing nelfinavir or indinavir (Abrams et al. 2003). Among human clinical studies that have looked at the effects of marijuana or dronabinol in HIV patients, the body weights of participants on the treatment significantly increased in comparison with those on lower doses or those not taking cannabinoids (Abrams et al. 2003; Haney et al. 2007; Struwe et al. 1993). However, dual-energy x-ray absorptiometry (DEXA) demonstrated that most of the weight gained was due to increased adipose tissue accumulation (Abrams et al. 2003), supporting the findings from others showing that BMI was slightly lower (Smit and Crespo 2001) even though marijuana users had higher intakes of energy and nutrients than non-users. This inability of cannabinoids to augment lean body mass and to increase fat mass deposition should be critically examined, and is particularly important in light of the well-described lipodystrophy seen in AIDS patients on ART (Carr and Cooper 2000; Carr et al. 1998).
In our studies using SIV-infected male rhesus macaques, chronic daily administration of Δ9-THC (0.32 mg/kg twice a day) produced a greater retention of body mass than chronic daily administration of vehicle (Molina et al. 2011a; Molina et al. 2011b). Conversely, Δ9-THC-treated female macaques failed to gain body weight during the course of the study (Amedee et al. 2014a). Changes in body composition due to HIV wasting have been shown to be sex-specific and women lose more body fat while men lose more lean body mass (Grinspoon et al. 1997; Kotler et al. 1985). This could be partially explained by the increased sensitivity of males to cannabinoid receptor 1 (CB1R) agonist/antagonist effects on food intake and body weight (Diaz et al. 2009) together with the effects of estrogen on food consumption (Czaja 1975).
Limited preclinical and clinical studies have examined the effects of cannabinoids on the incidence and progression of diabetes and insulin resistance in HIV patients. Marijuana use has been associated with lower prevalence of obesity (Le Strat and Le Foll 2011) and diabetes mellitus (Penner et al. 2013; Rajavashisth et al. 2012). In an experimental model of autoimmune diabetes, Δ9-THC reduced the severity of autoimmune responses, preserved pancreatic insulin content, and led to lower blood glucose levels in the diabetic group of animals (Li et al. 2001). The serum cholesterol, HDL and LDL levels were lower in the Δ9-THC treated diabetic rats compared to untreated rats. This could partly be explained by the anti-inflammatory effects rendered by Δ9-THC (Ehrhart et al. 2005; Molina et al. 2011a). Cannabidiol, a non-psychotropic component of marijuana, has also been shown to significantly reduce the incidence of diabetes in non-obese diabetic mice (Weiss et al. 2006). Furthermore, current marijuana users had lower fasting insulin levels and insulin resistance, and higher high density lipoprotein-C (HDL-C) levels than non-users in a study conducted by National Health and Nutrition Examination Survey (NHANES). The same effects were not observed in past users, however, suggesting a temporal pattern of cannabinoid-mediated modulation of insulin sensitivity (Penner et al. 2013). In another large population of subjects recruited by the National Institute on Drug Abuse, chronic cannabis smokers had more visceral adiposity and adipose tissue insulin resistance compared to controls, but there were no differences in hepatic steatosis, glucose insulin insensitivity, pancreatic ß-cell function, or dyslipidemia (Muniyappa et al. 2013). Thus, the potential that chronic cannabinoid use interacts with metabolic processes that can impact on the incidence of insulin resistance or metabolic syndrome cannot be ignored (Coskun and Bolkent 2014; Horvath et al. 2012).
The molecular mechanisms by which cannabinoids, especially Δ9-THC, might modulate insulin sensitivity are still unknown. Human and animal studies have shown that endogenous cannabinoid receptors play a critical role in energy expenditure and metabolism. Activation of the peripheral endogenous cannabinoid system has been associated with human obesity (Engeli et al. 2005) and with activation of both peripheral and central CB1R (Di Marzo 2008). Δ9-THC, a partial CB-1 receptor agonist (Pertwee 2008) and its acute administration stimulates appetite. However, reports in the literature indicate that chronic cannabis consumption is associated with lower incidence of obesity (Le Foll et al. 2013) and lower fasting insulin levels and index of insulin resistance (Penner et al. 2013). This may be the result of down-regulation and desensitization of the CB1R (Sim-Selley 2003; Winsauer et al. 2011b), activation of peroxisome proliferator-activated receptor- γ (PPAR-γ) (O'Sullivan 2007), or alternatively, modulation of adipocyte adiponectin transcription (Teixeira et al. 2010). However, understanding the molecular mechanisms through which cannabinoids, both endogenous and exogenous, may modulate metabolic comorbidities in PLWHA is only in its early stages.
Perspectives and emerging areas in need of further research
With the use of effective ART, morbidity and mortality have been substantially reduced in HIV-infected patients. HIV infection has become a chronic disease during which comorbid conditions such as drug abuse have greater potential of altering the course of disease progression by affecting metabolic, immune and neurobehavioral function. The greater prevalence of alcohol and marijuana use in this patient population and the known biomedical consequences of their use and abuse, urgently call for continued research to understand the detrimental, neutral, and/or potentially beneficial effects (in the case of cannabinoids) of these drugs on disease progression. This review highlights salient metabolic, immune, and neurobehavioral processes that are susceptible to alcohol and cannabis modulation (Fig. 4). Overall, studies from our group as well as many others in the field have provided evidence that both alcohol and the cannabinoids may impact disease progression directly through pro-inflammatory and anti-inflammatory mechanisms, respectively. However, epigenetic mechanisms of alcohol and cannabinoid action, the role of stem cells in tissue repair and regeneration from combined injury (alcohol and HIV-associated inflammation), compartment-specific alterations in host defense that impact on infectivity, antiretroviral metabolism, distribution, and effectiveness, as well as the possible impact of these drugs on viral reservoir reactivation also need investigation. We believe the current advancement in experimental approaches, analytical techniques, and animal models such as the SIV-infected NHP, facilitate translation of science-derived knowledge to improve patient care and provide a unique opportunity for discoveries that will inform future treatment and prevention strategies.
Figure 4.
Summary of the principal mechanisms of alcohol- and cannabinoid-induced modulation of SIV disease progression in male rhesus macaques.
Acknowledgements
The authors are grateful for the outstanding veterinarian and technical support provided by Jason Dufour, DVM, Leslie Birke, DVM, Jean Carnal, Connie Porretta, Curtis Vande Stouwe, Rhonda Martinez, Amy Weinberg, Peter Lewis, and Jamie Hubbell. Analytical support for the studies was provided by the LSUHSC Comprehensive Alcohol Research Center Analytical Core Laboratory, and particularly Dr. Robert Siggins.
Funding
The authors have received support from: NIH/NIAAA P60 AA09803, NIH/NIDA 5 R01 DA030053.
Footnotes
Conflicts of Interest
The authors declare no conflicts of interest.
Reference List
- Abrams DI, et al. Short-term effects of cannabinoids in patients with HIV-1 infection: a randomized, placebo- controlled clinical trial. Annals of internal medicine. 2003;139:258–266. doi: 10.7326/0003-4819-139-4-200308190-00008. [DOI] [PubMed] [Google Scholar]
- ADAMP NIoJ . Among Arrestees. Washington DC: 2003. Drug and Alcohol Use & Related Matters. [Google Scholar]
- Adaramoye OA, Oloyede GK. Effect of moderate ethanol administration on biochemical indices in streptozotocin- diabetic Wistar rats. The West Indian medical journal. 2012;61:3–9. doi: 10.7727/wimj.2011.111. [DOI] [PubMed] [Google Scholar]
- Addolorato G, et al. Body composition changes induced by chronic ethanol abuse: evaluation by dual energy X-ray absorptiometry. The American journal of gastroenterology. 2000;95:2323–2327. doi: 10.1111/j.1572-0241.2000.02320.x. doi:10.1111/j.1572-0241.2000.02320.x. [DOI] [PubMed] [Google Scholar]
- Amedee AM, et al. Chronic Delta-Tetrahydrocannabinol Administration May Not Attenuate Simian Immunodeficiency Virus Disease Progression in Female Rhesus Macaques. AIDS research and human retroviruses. 2014a doi: 10.1089/aid.2014.0108. doi:10.1089/AID.2014.0108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amedee AM, Veazey R, Molina P, Nelson S, Bagby GJ. Chronic binge alcohol increases susceptibility to rectal simian immunodeficiency virus infection in macaques. AIDS. 2014b;28:2485–2487. doi: 10.1097/QAD.0000000000000413. doi:10.1097/qad.0000000000000413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arthur LO, Gilden RV, Marx PA, Gardner MB. Simian acquired immunodeficiency syndrome. Progress in allergy. 1986;37:332–352. doi: 10.1159/000318452. [DOI] [PubMed] [Google Scholar]
- Azar MM, Springer SA, Meyer JP, Altice FL. A systematic review of the impact of alcohol use disorders on HIV treatment outcomes, adherence to antiretroviral therapy and health care utilization. Drug Alcohol Depend. 2010;112:178–193. doi: 10.1016/j.drugalcdep.2010.06.014. doi:10.1016/j.drugalcdep.2010.06.014. PMCID: PMC2997193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagasra O, et al. Increased human immunodeficiency virus type 1 replication in human peripheral blood mononuclear cells induced by ethanol: potential immunopathogenic mechanisms. The Journal of infectious diseases. 1996;173:550–558. doi: 10.1093/infdis/173.3.550. [DOI] [PubMed] [Google Scholar]
- Bagby GJ, Zhang P, Purcell JE, Didier PJ, Nelson S. Chronic binge ethanol consumption accelerates progression of simian immunodeficiency virus disease. Alcohol Clin Exp Res. 2006;30:1781–1790. doi: 10.1111/j.1530-0277.2006.00211.x. [DOI] [PubMed] [Google Scholar]
- Baliunas DO, Taylor BJ, Irving H, Roerecke M, Patra J, Mohapatra S, Rehm J. Alcohol as a risk factor for type 2 diabetes: A systematic review and meta-analysis. Diabetes care. 2009;32:2123–2132. doi: 10.2337/dc09-0227. doi:10.2337/dc09-0227. PMCID: PMC2768203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbaro G. Highly active antiretroviral therapy-associated metabolic syndrome: pathogenesis and cardiovascular risk. American journal of therapeutics. 2006;13:248–260. doi: 10.1097/01.mjt.0000162013.66614.16. doi:10.1097/01.mjt.0000162013.66614.16. [DOI] [PubMed] [Google Scholar]
- Baskin GB, Murphey-Corb M, Watson EA, Martin LN. Necropsy findings in rhesus monkeys experimentally infected with cultured simian immunodeficiency virus. (SIV)/delta Veterinary pathology. 1988;25:456–467. doi: 10.1177/030098588802500609. [DOI] [PubMed] [Google Scholar]
- Bau PF, Bau CH, Rosito GA, Manfroi WC, Fuchs FD. Alcohol consumption, cardiovascular health, and endothelial function markers. Alcohol. 2007;41:479–488. doi: 10.1016/j.alcohol.2007.08.004. doi:10.1016/j.alcohol.2007.08.004. [DOI] [PubMed] [Google Scholar]
- Baum MK, Rafie C, Lai S, Sales S, Page JB, Campa A. Alcohol use accelerates HIV disease progression. AIDS research and human retroviruses. 2010;26:511–518. doi: 10.1089/aid.2009.0211. doi:10.1089/aid.2009.0211. PMCID: PMC2875959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell JE, Brettle RP, Chiswick A, Simmonds P. HIV encephalitis, proviral load and dementia in drug users and homosexuals with AIDS. Effect of neocortical involvement Brain : a journal of neurology. 1998;121(Pt 11):2043–2052. doi: 10.1093/brain/121.11.2043. [DOI] [PubMed] [Google Scholar]
- Beulens JW, et al. Alcohol consumption and risk of type 2 diabetes in European men and women: influence of beverage type and body size The EPIC-InterAct study. Journal of internal medicine. 2012;272:358–370. doi: 10.1111/j.1365-2796.2012.02532.x. doi:10.1111/j.1365-2796.2012.02532.x. [DOI] [PubMed] [Google Scholar]
- Bing EG, et al. Psychiatric disorders and drug use among human immunodeficiency virus-infected adults in the United States. Archives of general psychiatry. 2001;58:721–728. doi: 10.1001/archpsyc.58.8.721. [DOI] [PubMed] [Google Scholar]
- Boden G, Chen X, DeSantis RA, Kendrick Z. Ethanol inhibits insulin action on lipolysis and on insulin release in elderly men. The American journal of physiology. 1993;265:E197–202. doi: 10.1152/ajpendo.1993.265.2.E197. [DOI] [PubMed] [Google Scholar]
- Braitstein P, Kendall T, Chan K, Wood E, Montaner JS, O'Shaughnessy MV, Hogg RS. Mary-Jane and her patients: sociodemographic and clinical characteristics of HIV-positive individuals using medical marijuana and antiretroviral agents. Aids. 2001;15:532–533. doi: 10.1097/00002030-200103090-00016. [DOI] [PubMed] [Google Scholar]
- Brenchley JM, et al. Microbial translocation is a cause of systemic immune activation in chronic. HIV infection Nature medicine. 2006;12:1365–1371. doi: 10.1038/nm1511. doi:10.1038/nm1511. [DOI] [PubMed] [Google Scholar]
- Bunout D. Nutritional and metabolic effects of alcoholism: their relationship with alcoholic liver disease. Nutrition. 1999;15:583–589. doi: 10.1016/s0899-9007(99)00090-8. [DOI] [PubMed] [Google Scholar]
- Cabral GA, Stinnett AL, Bailey J, Ali SF, Paule MG, Scallet AC, Slikker W., Jr. Chronic marijuana smoke alters alveolar macrophage morphology and protein expression. Pharmacology, biochemistry, and behavior. 1991;40:643–649. doi: 10.1016/0091-3057(91)90376-d. [DOI] [PubMed] [Google Scholar]
- Carlini EA. The good and the bad effects of (-) trans-delta-9-tetrahydrocannabinol (Delta 9-THC) on humans Toxicon. official journal of the International Society on Toxinology. 2004;44:461–467. doi: 10.1016/j.toxicon.2004.05.009. doi:10.1016/j.toxicon.2004.05.009. [DOI] [PubMed] [Google Scholar]
- Carr A, Cooper DA. Adverse effects of antiretroviral therapy. Lancet. 2000;356:1423–1430. doi: 10.1016/S0140-6736(00)02854-3. doi:10.1016/S0140- 6736(00)02854-3. [DOI] [PubMed] [Google Scholar]
- Carr A, Samaras K, Burton S, Law M, Freund J, Chisholm DJ, Cooper DA. A syndrome of peripheral lipodystrophy, hyperlipidaemia and insulin resistance in patients receiving HIV protease inhibitors. AIDS. 1998;12:F51–58. doi: 10.1097/00002030-199807000-00003. [DOI] [PubMed] [Google Scholar]
- Chandra LC, et al. Journal of Virology. 2014. Chronic administration of Δ9-Tetrahydrocannabinol induces intestinal anti-inflammatory microRNA expression during acute SIV infection of rhesus macaques. PMCID: PMCJournal - In Process. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Sebastian BM, Nagy LE. Chronic ethanol feeding to rats decreases adiponectin secretion by subcutaneous adipocytes. American journal of physiology Endocrinology and metabolism. 2007;292:E621–628. doi: 10.1152/ajpendo.00387.2006. doi:10.1152/ajpendo.00387.2006. PMCID: PMC1794258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheney PD, Riazi M, Marcario JM. Behavioral and neurophysiological hallmarks of simian immunodeficiency virus infection in macaque monkeys. Journal of neurovirology. 2008;14:301–308. doi: 10.1080/13550280802116322. doi:10.1080/13550280802116322. [DOI] [PubMed] [Google Scholar]
- Cheng DM, Libman H, Bridden C, Saitz R, Samet JH. Alcohol consumption and lipodystrophy in HIV-infected adults with alcohol problems. Alcohol. 2009;43:65–71. doi: 10.1016/j.alcohol.2008.09.004. doi:10.1016/j.alcohol.2008.09.004. PMCID: PMC2635495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chitsaz E, et al. Contribution of substance use disorders on HIV treatment outcomes and antiretroviral medication adherence among HIV-infected persons entering jail. AIDS Behav. 2013;17(Suppl 2):S118–127. doi: 10.1007/s10461-013-0506-0. doi:10.1007/s10461-013-0506-0. PMCID: PMC3818019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cinti S. Medical marijuana in HIV-positive patients: what do we know? J Int Assoc Physicians AIDS Care (Chic) 2009;8:342–346. doi: 10.1177/1545109709351167. doi:10.1177/1545109709351167. [DOI] [PubMed] [Google Scholar]
- Conigrave KM, Rimm EB. Alcohol for the prevention of type 2 diabetes mellitus? Treatments in endocrinology. 2003;2:145–152. doi: 10.2165/00024677-200302030-00001. [DOI] [PubMed] [Google Scholar]
- Coskun ZM, Bolkent S. Oxidative stress and cannabinoid receptor expression in type-2 diabetic rat pancreas following treatment with Delta(9) THC Cell biochemistry and function. 2014;32:612–619. doi: 10.1002/cbf.3058. doi:10.1002/cbf.3058. [DOI] [PubMed] [Google Scholar]
- Costantino CM, Gupta A, Yewdall AW, Dale BM, Devi LA, Chen BK. Cannabinoid receptor 2-mediated attenuation of CXCR4-tropic HIV infection in primary CD4+ T cells. PloS one. 2012;7:e33961. doi: 10.1371/journal.pone.0033961. doi:10.1371/journal.pone.0033961. PMCID: PMC3309010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cysique LA, Brew BJ. Neuropsychological functioning and antiretroviral treatment in HIV/AIDS: a review. Neuropsychology review. 2009;19:169–185. doi: 10.1007/s11065-009-9092-3. doi:10.1007/s11065-009-9092-3. [DOI] [PubMed] [Google Scholar]
- Czaja JA. Food rejection by female rhesus monkeys during the menstrual cycle and early pregnancy. Physiology & behavior. 1975;14:579–587. doi: 10.1016/0031-9384(75)90185-7. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention Monitoring selected national HIV prevention and care objectives by using HIV surveillance data—United States and 6 dependent areas—2012. HIV Surveillance Supplemental Report. 2014;19(No.3) [Google Scholar]
- da Cunha A, Eiden LE, Rausch DM. Neuronal substrates for SIV encephalopathy. Advances in neuroimmunology. 1994;4:265–271. doi: 10.1016/s0960-5428(06)80266-4. [DOI] [PubMed] [Google Scholar]
- Daniel JM, Winsauer PJ, Brauner IN, Moerschbaecher JM. Estrogen improves response accuracy and attenuates the disruptive effects of delta9-THC in ovariectomized rats responding under a multiple schedule of repeated acquisition and performance. Behavioral neuroscience. 2002;116:989–998. [PubMed] [Google Scholar]
- Daul CB, Heath RG. The effect of chronic marihuana usage on the immunological status of rheusus monkeys. Life sciences. 1975;17:875–881. doi: 10.1016/0024-3205(75)90438-5. [DOI] [PubMed] [Google Scholar]
- de la Monte S, Derdak Z, Wands JR. Alcohol, insulin resistance and the liver-brain axis. Journal of gastroenterology and hepatology. 2012;27(Suppl 2):33–41. doi: 10.1111/j.1440-1746.2011.07023.x. doi:10.1111/j.1440-1746.2011.07023.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deveaux L, et al. Reduction in human immunodeficiency virus risk among youth in developing countries. Archives of pediatrics & adolescent medicine. 2007;161:1130–1139. doi: 10.1001/archpedi.161.12.1130. doi:10.1001/archpedi.161.12.1130. [DOI] [PubMed] [Google Scholar]
- Dewey WL. Cannabinoid pharmacology. Pharmacological reviews. 1986;38:151–178. [PubMed] [Google Scholar]
- Di Marzo V. The endocannabinoid system in obesity and type 2 diabetes. Diabetologia. 2008;51:1356–1367. doi: 10.1007/s00125-008-1048-2. doi:10.1007/s00125-008-1048-2. [DOI] [PubMed] [Google Scholar]
- Diaz S, Farhang B, Hoien J, Stahlman M, Adatia N, Cox JM, Wagner EJ. Sex differences in the cannabinoid modulation of appetite, body temperature and neurotransmission at POMC synapses. Neuroendocrinology. 2009;89:424–440. doi: 10.1159/000191646. doi:10.1159/000191646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrhart J, et al. Stimulation of cannabinoid receptor 2 (CB2) suppresses microglial activation. Journal of neuroinflammation. 2005;2:29. doi: 10.1186/1742-2094-2-29. doi:10.1186/1742-2094-2-29. PMCID: PMC1352348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisner V, Lenaers G, Hajnoczky G. Mitochondrial fusion is frequent in skeletal muscle and supports excitation- contraction coupling. The Journal of cell biology. 2014;205:179–195. doi: 10.1083/jcb.201312066. doi:10.1083/jcb.201312066. PMCID: PMC4003250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ElSohly MA, deWit H, Wachtel SR, Feng S, Murphy TP. Delta9-tetrahydrocannabivarin as a marker for the ingestion of marijuana versus Marinol: results of a clinical study. Journal of analytical toxicology. 2001;25:565–571. doi: 10.1093/jat/25.7.565. [DOI] [PubMed] [Google Scholar]
- Engeli S, et al. Activation of the peripheral endocannabinoid system in human obesity. Diabetes. 2005;54:2838–2843. doi: 10.2337/diabetes.54.10.2838. PMCID: PMC2228268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fama R, et al. Upper and lower limb motor impairments in alcoholism, HIV infection, and their comorbidity. Alcohol Clin Exp Res. 2007;31:1038–1044. doi: 10.1111/j.1530-0277.2007.00385.x. doi:10.1111/j.1530-0277.2007.00385.x. [DOI] [PubMed] [Google Scholar]
- Fattore L, Fratta W. How important are sex differences in cannabinoid action? British journal of pharmacology. 2010;160:544–548. doi: 10.1111/j.1476-5381.2010.00776.x. doi:10.1111/j.1476-5381.2010.00776.x. PMCID: PMC2931555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fein G, Biggins CA, MacKay S. Alcohol abuse and HIV infection have additive effects on frontal cortex function as measured by auditory evoked potential P3A latency. Biological psychiatry. 1995;37:183–195. doi: 10.1016/0006-3223(94)00119-N. doi:10.1016/0006- 3223(94)00119-n. [DOI] [PubMed] [Google Scholar]
- Felder CC, et al. Isolation and measurement of the endogenous cannabinoid receptor agonist, anandamide, in brain and peripheral tissues of human and rat. FEBS letters. 1996;393:231–235. doi: 10.1016/0014-5793(96)00891-5. [DOI] [PubMed] [Google Scholar]
- Fernandez-Sola J, Nicolas JM, Sacanella E, Robert J, Cofan M, Estruch R, Urbano-Marquez A. Low-dose ethanol consumption allows strength recovery in chronic alcoholic myopathy. QJM : monthly journal of the Association of Physicians. 2000;93:35–40. doi: 10.1093/qjmed/93.1.35. [DOI] [PubMed] [Google Scholar]
- Filipeanu CM, Guidry JJ, Leonard ST, Winsauer PJ. Delta9-THC increases endogenous AHA1 expression in rat cerebellum and may modulate CB1 receptor function during chronic use. Journal of neurochemistry. 2011;118:1101–1112. doi: 10.1111/j.1471-4159.2011.07391.x. doi:10.1111/j.1471-4159.2011.07391.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman H, Klein TW, Newton C, Daaka Y. Marijuana, receptors and immunomodulation. Advances in experimental medicine and biology. 1995;373:103–113. doi: 10.1007/978-1-4615-1951-5_15. [DOI] [PubMed] [Google Scholar]
- Furler MD, Einarson TR, Millson M, Walmsley S, Bendayan R. Medicinal and recreational marijuana use by patients infected with HIV AIDS Patient Care. STDS. 2004;18:215–228. doi: 10.1089/108729104323038892. doi:10.1089/108729104323038892. [DOI] [PubMed] [Google Scholar]
- Galvan FH, et al. The prevalence of alcohol consumption and heavy drinking among people with HIV in the United States: results from the HIV Cost and Services Utilization Study. J Stud Alcohol. 2002;63:179–186. doi: 10.15288/jsa.2002.63.179. [DOI] [PubMed] [Google Scholar]
- Gao L, et al. Chronic ethanol consumption up-regulates protein-tyrosine phosphatase-1B (PTP1B) expression in rat skeletal muscle Acta pharmacologica. Sinica. 2010;31:1576–1582. doi: 10.1038/aps.2010.161. doi:10.1038/aps.2010.161. PMCID: PMC4002945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner MB, Luciw PA. Simian immunodeficiency viruses and their relationship to the human immunodeficiency viruses. Aids. 1988;2(Suppl 1):S3–10. doi: 10.1097/00002030-198800001-00002. [DOI] [PubMed] [Google Scholar]
- Ghosn J, et al. HIV-1 DNA levels in peripheral blood mononuclear cells and cannabis use are associated with intermittent HIV shedding in semen of men who have sex with men on successful antiretroviral regimens. Clin Infect Dis. 2014;58:1763–1770. doi: 10.1093/cid/ciu187. doi:10.1093/cid/ciu187. [DOI] [PubMed] [Google Scholar]
- Gold LH, et al. Longitudinal analysis of behavioral, neurophysiological, viral and immunological effects of SIV infection in rhesus monkeys. J Med Primatol. 1998;27:104–112. doi: 10.1111/j.1600-0684.1998.tb00234.x. [DOI] [PubMed] [Google Scholar]
- Gonzalez S, et al. Sex steroid influence on cannabinoid CB(1) receptor mRNA and endocannabinoid levels in the anterior pituitary gland. Biochemical and biophysical research communications. 2000;270:260–266. doi: 10.1006/bbrc.2000.2406. doi:10.1006/bbrc.2000.2406. [DOI] [PubMed] [Google Scholar]
- Grant I. Alcohol and the brain: neuropsychological correlates. Journal of consulting and clinical psychology. 1987;55:310–324. doi: 10.1037//0022-006x.55.3.310. [DOI] [PubMed] [Google Scholar]
- Grant I, Heaton RK, Atkinson JH. Neurocognitive disorders in HIV-1 infection. HNRC Group. HIV Neurobehavioral Research Center Current topics in microbiology and immunology. 1995;202:11–32. [PubMed] [Google Scholar]
- Green JE, Saveanu RV, Bornstein RA. The effect of previous alcohol abuse on cognitive function in HIV infection. The American journal of psychiatry. 2004;161:249–254. doi: 10.1176/appi.ajp.161.2.249. [DOI] [PubMed] [Google Scholar]
- Grinspoon S, et al. Body composition and endocrine function in women with acquired immunodeficiency syndrome wasting. The Journal of clinical endocrinology and metabolism. 1997;82:1332–1337. doi: 10.1210/jcem.82.5.3907. doi:10.1210/jcem.82.5.3907. [DOI] [PubMed] [Google Scholar]
- Gutierrez AD, Balasubramanyam A. Dysregulation of glucose metabolism in HIV patients: epidemiology, mechanisms, and management. Endocrine. 2012;41:1–10. doi: 10.1007/s12020-011-9565-z. doi:10.1007/s12020-011-9565-z. PMCID: PMC3417129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadigan C, Corcoran C, Stanley T, Piecuch S, Klibanski A, Grinspoon S. Fasting hyperinsulinemia in human immunodeficiency virus-infected men: relationship to body composition, gonadal function, and protease inhibitor use. The Journal of clinical endocrinology and metabolism. 2000;85:35–41. doi: 10.1210/jcem.85.1.6264. doi:10.1210/jcem.85.1.6264. [DOI] [PubMed] [Google Scholar]
- Hampson RE, Deadwyler SA. Cannabinoids, hippocampal function and memory. Life sciences. 1999;65:715–723. doi: 10.1016/s0024-3205(99)00294-5. [DOI] [PubMed] [Google Scholar]
- Hampson RE, Deadwyler SA. Cannabinoids reveal the necessity of hippocampal neural encoding for short-term memory in rats. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2000;20:8932–8942. doi: 10.1523/JNEUROSCI.20-23-08932.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haney M, Gunderson EW, Rabkin J, Hart CL, Vosburg SK, Comer SD, Foltin RW. Dronabinol and marijuana in HIV-positive marijuana smokers. Caloric intake, mood, and sleep. J Acquir Immune Defic Syndr. 2007;45:545–554. doi: 10.1097/QAI.0b013e31811ed205. doi:10.1097/QAI.0b013e31811ed205. [DOI] [PubMed] [Google Scholar]
- Harris GE, et al. Patterns and correlates of cannabis use among individuals with HIV/AIDS in Maritime Canada. The Canadian journal of infectious diseases & medical microbiology = Journal canadien des maladies infectieuses et de la microbiologie medicale / AMMI Canada. 2014;25:e1–7. doi: 10.1155/2014/301713. PMCID: PMC3950986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey BS, Nicotra LL, Vu M, Smid SD. Cannabinoid CB2 receptor activation attenuates cytokine-evoked mucosal damage in a human colonic explant model without changing epithelial permeability. Cytokine. 2013;63:209–217. doi: 10.1016/j.cyto.2013.04.032. doi:10.1016/j.cyto.2013.04.032. [DOI] [PubMed] [Google Scholar]
- Hayatbakhsh MR, O'Callaghan MJ, Mamun AA, Williams GM, Clavarino A, Najman JM. Cannabis use and obesity and young adults. The American journal of drug and alcohol abuse. 2010;36:350–356. doi: 10.3109/00952990.2010.500438. doi:10.3109/00952990.2010.500438. [DOI] [PubMed] [Google Scholar]
- Hendershot CS, Stoner SA, George WH, Norris J. Alcohol use, expectancies, and sexual sensation seeking as correlates of HIV risk behavior in heterosexual young adults. Psychol Addict Behav. 2007;21:365–372. doi: 10.1037/0893-164X.21.3.365. doi:10.1037/0893-164X.21.3.365. PMCID: PMC2749924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyes MP, et al. Relationship of neurologic status in macaques infected with the simian immunodeficiency virus to cerebrospinal fluid quinolinic acid and kynurenic acid. Brain research. 1992;570:237–250. doi: 10.1016/0006-8993(92)90587-y. [DOI] [PubMed] [Google Scholar]
- Hollister LE. Health aspects of cannabis. Pharmacological reviews. 1986;38:1–20. [PubMed] [Google Scholar]
- Horvath B, Mukhopadhyay P, Hasko G, Pacher P. The endocannabinoid system and plant-derived cannabinoids in diabetes and diabetic complications. The American journal of pathology. 2012;180:432–442. doi: 10.1016/j.ajpath.2011.11.003. doi:10.1016/j.ajpath.2011.11.003. PMCID: PMC3349875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard AA, Arnsten JH, Gourevitch MN. Effect of alcohol consumption on diabetes mellitus: a systematic review. Annals of internal medicine. 2004;140:211–219. doi: 10.7326/0003-4819-140-6-200403160-00011. [DOI] [PubMed] [Google Scholar]
- Hunault CC, et al. Cognitive and psychomotor effects in males after smoking a combination of tobacco and cannabis containing up to 69 mg delta-9-tetrahydrocannabinol (THC) Psychopharmacology. 2009;204:85–94. doi: 10.1007/s00213-008-1440-0. doi:10.1007/s00213-008-1440-0. [DOI] [PubMed] [Google Scholar]
- SAMHSA SAaMHSA . Treatment Episode Data Set (TEDS) Series vol. U.S. Department of Health and Human Services; Ann Arbor, MI.: 2009. [Google Scholar]
- Ivashkiv LB, Donlin LT. Regulation of type I interferon responses Nature reviews. Immunology. 2014;14:36–49. doi: 10.1038/nri3581. doi:10.1038/nri3581. PMCID: PMC4084561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis CM, Hayman LL, Braun LT, Schwertz DW, Ferrans CE, Piano MR. Cardiovascular risk factors and metabolic syndrome in alcohol-and nicotine-dependent men and women. The Journal of cardiovascular nursing. 2007;22:429–435. doi: 10.1097/01.JCN.0000297387.21626.88. doi:10.1097/01.JCN.0000297387.21626.88. [DOI] [PubMed] [Google Scholar]
- Jones CY, et al. Insulin resistance in HIV-infected men and women in the nutrition for healthy living cohort. J Acquir Immune Defic Syndr. 2005;40:202–211. doi: 10.1097/01.qai.0000165910.89462.2f. [DOI] [PubMed] [Google Scholar]
- Kadowaki T, Yamauchi T, Kubota N, Hara K, Ueki K, Tobe K. Adiponectin and adiponectin receptors in insulin resistance, diabetes, and the metabolic syndrome. The Journal of clinical investigation. 2006;116:1784–1792. doi: 10.1172/JCI29126. doi:10.1172/jci29126. PMCID: PMCPmc1483172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamin DS, Grinspoon SK. Cardiovascular disease in HIV-positive patients. AIDS. 2005;19:641–652. doi: 10.1097/01.aids.0000166087.08822.bc. [DOI] [PubMed] [Google Scholar]
- Khalsa JH, Royal W. Do drugs of abuse impact on HIV disease? Journal of neuroimmunology. 2004;147:6–8. doi: 10.1016/j.jneuroim.2003.10.007. [DOI] [PubMed] [Google Scholar]
- Kino T, Chrousos GP. AIDS-related insulin resistance and lipodystrophy syndrome Current drug targets. Immune, endocrine and metabolic disorders. 2003;3:111–117. doi: 10.2174/1568008033340289. [DOI] [PubMed] [Google Scholar]
- Klatsky AL. Alcohol, cardiovascular diseases and diabetes mellitus. Pharmacological research : the official journal of the Italian Pharmacological Society. 2007;55:237–247. doi: 10.1016/j.phrs.2007.01.011. doi:10.1016/j.phrs.2007.01.011. [DOI] [PubMed] [Google Scholar]
- Klein TW, Newton C, Larsen K, Lu L, Perkins I, Nong L, Friedman H. The cannabinoid system and immune modulation. Journal of leukocyte biology. 2003;74:486–496. doi: 10.1189/jlb.0303101. doi:10.1189/jlb.0303101. [DOI] [PubMed] [Google Scholar]
- Kolter DP. Current concepts of metabolic abnormalities in HIV patients: focus on lipodystrophy. The AIDS reader. 2003;13:S5–13. [PubMed] [Google Scholar]
- Kotler DP, Wang J, Pierson RN. Body composition studies in patients with the acquired immunodeficiency syndrome. The American journal of clinical nutrition. 1985;42:1255–1265. doi: 10.1093/ajcn/42.6.1255. [DOI] [PubMed] [Google Scholar]
- Kumar R, et al. Increased viral replication in simian immunodeficiency virus/simian-HIV-infected macaques with self-administering model of chronic alcohol consumption. J Acquir Immune Defic Syndr. 2005;39:386–390. doi: 10.1097/01.qai.0000164517.01293.84. [DOI] [PubMed] [Google Scholar]
- Landfield PW, Cadwallader LB, Vinsant S. Quantitative changes in hippocampal structure following long-term exposure to delta 9-tetrahydrocannabinol: possible mediation by glucocorticoid systems. Brain research. 1988;443:47–62. doi: 10.1016/0006-8993(88)91597-1. [DOI] [PubMed] [Google Scholar]
- Lackner AA, Veazey RS. Current concepts in AIDS pathogenesis: insights from the SIV/macaque model. Annual review of medicine. 2007;58:461–476. doi: 10.1146/annurev.med.58.082405.094316. doi:10.1146/annurev.med.58.082405.094316. [DOI] [PubMed] [Google Scholar]
- Lang CH, Frost RA, Summer AD, Vary TC. Molecular mechanisms responsible for alcohol-induced myopathy in skeletal muscle and heart. The international journal of biochemistry & cell biology. 2005;37:2180–2195. doi: 10.1016/j.biocel.2005.04.013. doi:10.1016/j.biocel.2005.04.013. [DOI] [PubMed] [Google Scholar]
- Lang CH, Kimball SR, Frost RA, Vary TC. Alcohol myopathy: impairment of protein synthesis and translation initiation. The international journal of biochemistry & cell biology. 2001;33:457–473. doi: 10.1016/s1357-2725(00)00081-9. [DOI] [PubMed] [Google Scholar]
- Le Foll B, Trigo JM, Sharkey KA, Le Strat Y. Cannabis and Delta9-tetrahydrocannabinol (THC) for weight loss? Medical hypotheses. 2013;80:564–567. doi: 10.1016/j.mehy.2013.01.019. doi:10.1016/j.mehy.2013.01.019. [DOI] [PubMed] [Google Scholar]
- Le Strat Y, Le Foll B. Obesity and cannabis use: results from 2 representative national surveys. American journal of epidemiology. 2011;174:929–933. doi: 10.1093/aje/kwr200. doi:10.1093/aje/kwr200. [DOI] [PubMed] [Google Scholar]
- LeCapitaine NJ, et al. Disrupted anabolic and catabolic processes may contribute to alcohol-accentuated SAIDS-associated wasting. The Journal of infectious diseases. 2011;204:1246–1255. doi: 10.1093/infdis/jir508. doi:10.1093/infdis/jir508. PMCID: PMC3173505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee LM, Karon JM, Selik R, Neal JJ, Fleming PL. Survival after AIDS diagnosis in adolescents and adults during the treatment era, United States, 1984-1997. Jama. 2001;285:1308–1315. doi: 10.1001/jama.285.10.1308. [DOI] [PubMed] [Google Scholar]
- Leggio L, et al. Is cortisol involved in the alcohol-related fat mass impairment? A longitudinal clinical study. Alcohol Alcohol. 2009;44:211–215. doi: 10.1093/alcalc/agn116. doi:10.1093/alcalc/agn116. [DOI] [PubMed] [Google Scholar]
- Letvin NL. Animal models for AIDS. Immunology today. 1990;11:322–326. doi: 10.1016/0167-5699(90)90127-u. [DOI] [PubMed] [Google Scholar]
- Li X, Kaminski NE, Fischer LJ. Examination of the immunosuppressive effect of delta9-tetrahydrocannabinol in streptozotocin-induced autoimmune diabetes. International immunopharmacology. 2001;1:699–712. doi: 10.1016/s1567-5769(01)00003-0. [DOI] [PubMed] [Google Scholar]
- Lichtman AH, Dimen KR, Martin BR. Systemic or intrahippocampal cannabinoid administration impairs spatial memory in rats. Psychopharmacology. 1995;119:282–290. doi: 10.1007/BF02246292. [DOI] [PubMed] [Google Scholar]
- Lieber CS. The discovery of the microsomal ethanol oxidizing system and its physiologic and pathologic role. Drug metabolism reviews. 2004;36:511–529. doi: 10.1081/dmr-200033441. doi:10.1081/DMR-200033441. [DOI] [PubMed] [Google Scholar]
- Lindtner C, et al. Binge drinking induces whole-body insulin resistance by impairing hypothalamic insulin action. Science translational medicine. 2013;5:170ra114. doi: 10.1126/scitranslmed.3005123. doi:10.1126/scitranslmed.3005123. PMCID: PMC3740748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Zha J, Nishitani J, Chen H, Zack JA. HIV-1 infection in peripheral blood lymphocytes (PBLs) exposed to alcohol. Virology. 2003;307:37–44. doi: 10.1016/s0042-6822(02)00031-4. doi:10.1089/aid.2014.0065. PMCID: PMC4118703. [DOI] [PubMed] [Google Scholar]
- Loganantharaj N, et al. The effects of chronic binge alcohol on the genital microenvironment of simian immunodeficiency virus-infected female rhesus macaques. AIDS research and human retroviruses. 2014;30:783–791. doi: 10.1089/aid.2014.0065. doi:10.1089/aid.2014.0065. PMCID: PMC4118703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcondes MC, Watry D, Zandonatti M, Flynn C, Taffe MA, Fox H. Chronic alcohol consumption generates a vulnerable immune environment during early SIV infection in rhesus macaques. Alcohol Clin Exp Res. 2008;32:1583–1592. doi: 10.1111/j.1530-0277.2008.00730.x. doi:10.1111/j.1530-0277.2008.00730.x. PMCID: PMC2579901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin F, Ward K, Slavin G, Levi J, Peters TJ. Alcoholic skeletal myopathy, a clinical and pathological study. The Quarterly journal of medicine. 1985;55:233–251. [PubMed] [Google Scholar]
- Mastrogiannis DS, et al. Alcohol enhances HIV infection of cord blood monocyte-derived macrophages. Current HIV research. 2014;12:301–308. doi: 10.2174/1570162x12666140721124923. PMCID: PMC4153785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure HM, Anderson DC, Ansari AA, Fultz PN, Klumpp SA, Schinazi RF. Nonhuman primate models for evaluation of AIDS therapy. Annals of the New York Academy of Sciences. 1990;616:287–298. doi: 10.1111/j.1749-6632.1990.tb17849.x. [DOI] [PubMed] [Google Scholar]
- Merroun I, et al. Influence of intracerebroventricular or intraperitoneal administration of cannabinoid receptor agonist (WIN 55,212-2) and inverse agonist (AM 251) on the regulation of food intake and hypothalamic serotonin levels. The British journal of nutrition. 2009;101:1569–1578. doi: 10.1017/S0007114508083530. doi:10.1017/S0007114508083530. [DOI] [PubMed] [Google Scholar]
- Meyerhoff DJ. Effects of alcohol and HIV infection on the central nervous system. Alcohol research & health : the journal of the National Institute on Alcohol Abuse and Alcoholism. 2001;25:288–298. [PMC free article] [PubMed] [Google Scholar]
- Miguez MJ, Shor-Posner G, Morales G, Rodriguez A, Burbano X. HIV treatment in drug abusers: impact of alcohol use. Addiction biology. 2003;8:33–37. doi: 10.1080/1355621031000069855. doi:10.1080/1355621031000069855. [DOI] [PubMed] [Google Scholar]
- Milloy MJ, et al. High-intensity cannabis use associated with lower plasma human immunodeficiency virus-1 RNA viral load among recently infected people who use injection drugs. Drug and alcohol review. 2014 doi: 10.1111/dar.12223. doi:10.1111/dar.12223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mize AL, Alper RH. Acute and long-term effects of 17beta-estradiol on G(i/o) coupled neurotransmitter receptor function in the female rat brain as assessed by agonist-stimulated [35S]GTPgammaS binding. Brain research. 2000;859:326–333. doi: 10.1016/s0006-8993(00)01998-3. [DOI] [PubMed] [Google Scholar]
- Mohri H, Bonhoeffer S, Monard S, Perelson AS, Ho DD. Rapid turnover of T lymphocytes in SIV-infected rhesus macaques. Science (New York, NY) 1998;279:1223–1227. doi: 10.1126/science.279.5354.1223. [DOI] [PubMed] [Google Scholar]
- Molina PE, Amedee A, LeCapitaine NJ, Zabaleta J, Mohan M, Winsauer P, Vande Stouwe C. Cannabinoid neuroimmune modulation of SIV disease. Journal of neuroimmune pharmacology : the official journal of the Society on NeuroImmune Pharmacology. 2011a;6:516–527. doi: 10.1007/s11481-011-9301-8. doi:10.1007/s11481-011-9301-8. PMCID: PMC3208744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina PE, et al. Modulation of gut-specific mechanisms by chronic delta(9)-tetrahydrocannabinol administration in male rhesus macaques infected with simian immunodeficiency virus: a systems biology analysis. AIDS research and human retroviruses. 2014;30:567–578. doi: 10.1089/aid.2013.0182. doi:10.1089/aid.2013.0182. PMCID: PMC4046212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina PE, Lang CH, McNurlan M, Bagby GJ, Nelson S. Chronic alcohol accentuates simian acquired immunodeficiency syndrom-associated wasting Alcohol. Clin Exp Res. 2008;32:138–147. doi: 10.1111/j.1530-0277.2007.00549.x. PMCID: PMC3178106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina PE, et al. Chronic alcohol accentuates nutritional, metabolic, and immune alterations during asymptomatic simian immunodeficiency virus infection Alcohol. Clin Exp Res. 2006;30:2065–2078. doi: 10.1111/j.1530-0277.2006.00252.x. doi:10.1111/j.1530-0277.2006.00252.x. [DOI] [PubMed] [Google Scholar]
- Molina PE, et al. Cannabinoid administration attenuates the progression of simian immunodeficiency virus. AIDS research and human retroviruses. 2011b;27:585–592. doi: 10.1089/aid.2010.0218. doi:10.1089/AID.2010.0218. PMCID: PMC3131805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morahan PS, Klykken PC, Smith SH, Harris LS, Munson AE. Effects of cannabinoids on host resistance to Listeria monocytogenes and herpes simplex virus. Infection and immunity. 1979;23:670–674. doi: 10.1128/iai.23.3.670-674.1979. PMCID: PMC414217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muniyappa R, et al. Metabolic effects of chronic cannabis smoking. Diabetes care. 2013;36:2415–2422. doi: 10.2337/dc12-2303. doi:10.2337/dc12-2303. PMCID: PMC3714514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray EA, Rausch DM, Lendvay J, Sharer LR, Eiden LE. Cognitive and motor impairments associated with SIV infection in rhesus monkeys. Science (New York, NY) 1992;255:1246–1249. doi: 10.1126/science.1546323. [DOI] [PubMed] [Google Scholar]
- Nair MP, Mahajan S, Hewitt R, Whitney ZR, Schwartz SA. Association of drug abuse with inhibition of HIV-1 immune responses: studies with long-term of HIV-1 non-progressors. Journal of neuroimmunology. 2004;147:21–25. doi: 10.1016/j.jneuroim.2003.10.038. [DOI] [PubMed] [Google Scholar]
- Nelson S, Bagby GJ. Alcohol and HIV Infection. Transactions of the American Clinical and Climatological Association. 2011;122:244–253. PMCID: PMC3116369. [PMC free article] [PubMed] [Google Scholar]
- Newman SP, Lunn S, Harrison MJ. Do asymptomatic HIV-seropositive individuals show cognitive deficit? Aids. 1995;9:1211–1220. doi: 10.1097/00002030-199511000-00001. [DOI] [PubMed] [Google Scholar]
- SAMSHA SAaMHSA . Results from the 2004 National Survey on Druge Use and Health: National Findings. Rockville, MD: 2005. vol NSDUH Series H-36. [Google Scholar]
- Nelson S, Happel KI, Zhang P, Myers L, Dufour JP, Bagby GJ. Effect of bacterial pneumonia on lung simian immunodeficiency virus (SIV) replication in alcohol consuming SIV-infected rhesus macaques. Alcohol Clin Exp Res. 2013;37:969–977. doi: 10.1111/acer.12070. doi:10.1111/acer.12070. PMCID: PMC3661707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton CA, Klein TW, Friedman H. Secondary immunity to Legionella pneumophila and Th1 activity are suppressed by delta-9-tetrahydrocannabinol injection. Infection and immunity. 1994;62:4015–4020. doi: 10.1128/iai.62.9.4015-4020.1994. PMCID: PMC303061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen VA, Le T, Tong M, Silbermann E, Gundogan F, de la Monte SM. Impaired insulin/IGF signaling in experimental alcohol-related myopathy. Nutrients. 2012;4:1058–1075. doi: 10.3390/nu4081058. doi:10.3390/nu4081058. PMCID: PMC3448087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolas JM, et al. Increased circulating leptin levels in chronic alcoholism. Alcohol Clin Exp Res. 2001;25:83–88. [PubMed] [Google Scholar]
- The NSDUH Report: Substance Use and Mental Health Estimates from the 2013 National Survey on Drug Use and Health: Overview of Findings. Rockville, MD.: 2014. [PubMed] [Google Scholar]
- O'Sullivan SE. Cannabinoids go nuclear: evidence for activation of peroxisome proliferator-activated receptors. British journal of pharmacology. 2007;152:576–582. doi: 10.1038/sj.bjp.0707423. doi:10.1038/sj.bjp.0707423. PMCID: PMC2190029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pahar B, et al. Effects of alcohol consumption on antigen-specific cellular and humoral immune responses to SIV in rhesus macaques. J Acquir Immune Defic Syndr. 2013;64:332–341. doi: 10.1097/QAI.0b013e31829f6dca. doi:10.1097/QAI.0b013e31829f6dca. PMCID: PMC3812314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penner EA, Buettner H, Mittleman MA. The impact of marijuana use on glucose, insulin, and insulin resistance among US adults. The American journal of medicine. 2013;126:583–589. doi: 10.1016/j.amjmed.2013.03.002. doi:10.1016/j.amjmed.2013.03.002. [DOI] [PubMed] [Google Scholar]
- Pertwee RG. The diverse CB1 and CB2 receptor pharmacology of three plant cannabinoids: delta9-tetrahydrocannabinol, cannabidiol and delta9-tetrahydrocannabivarin. British journal of pharmacology. 2008;153:199–215. doi: 10.1038/sj.bjp.0707442. doi:10.1038/sj.bjp.0707442. PMCID: PMC2219532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertwee RG, Stevenson LA, Griffin G. Cross-tolerance between delta-9-tetrahydrocannabinol and the cannabimimetic agents, CP 55,940, WIN 55,212-2 and anandamide. British journal of pharmacology. 1993;110:1483–1490. doi: 10.1111/j.1476-5381.1993.tb13989.x. PMCID: PMC2175863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips SJ, Freedberg KA, Traphagen ET, Horton NJ, Samet JH. Screening for alcohol problems in HIV-infected primary care patients. J Gen Int Med. 2001;16:165. [Google Scholar]
- Poonia B, Nelson S, Bagby GJ, Veazey RS. Intestinal lymphocyte subsets and turnover are affected by chronic alcohol consumption: implications for SIV/HIV infection. J Acquir Immune Defic Syndr. 2006;41:537–547. doi: 10.1097/01.qai.0000209907.43244.ee. doi:10.1097/01.qai.0000209907.43244.ee. [DOI] [PubMed] [Google Scholar]
- Poonia B, Nelson S, Bagby GJ, Zhang P, Quniton L, Veazey RS. Chronic alcohol consumption results in higher simian immunodeficiency virus replication in mucosally inoculated rhesus macaques. AIDS research and human retroviruses. 2006;22:589–594. doi: 10.1089/aid.2006.22.589. doi:10.1089/aid.2006.22.589. [DOI] [PubMed] [Google Scholar]
- Prospero-Garcia O, Gold LH, Fox HS, Polis I, Koob GF, Bloom FE, Henriksen SJ. Microglia-passaged simian immunodeficiency virus induces neurophysiological abnormalities in monkeys. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:14158–14163. doi: 10.1073/pnas.93.24.14158. PMCID: PMC19510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pross SH, Klein TW, Newton CA, Smith J, Widen R, Friedman H. Differential suppression of T-cell subpopulations by thc (delta-9-tetrahydrocannabinol) International journal of immunopharmacology. 1990;12:539–544. doi: 10.1016/0192-0561(90)90118-7. [DOI] [PubMed] [Google Scholar]
- Purohit V, Gao B, Song BJ. Molecular mechanisms of alcoholic fatty liver. Alcohol Clin Exp Res. 2009;33:191–205. doi: 10.1111/j.1530-0277.2008.00827.x. doi:10.1111/j.1530-0277.2008.00827.x. PMCID: PMC2633431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raj A, et al. The associations of binge alcohol use with HIV/STI risk and diagnosis among heterosexual. African American men Drug Alcohol Depend. 2009;101:101–106. doi: 10.1016/j.drugalcdep.2008.11.008. doi:10.1016/j.drugalcdep.2008.11.008. [DOI] [PubMed] [Google Scholar]
- Rajavashisth TB, Shaheen M, Norris KC, Pan D, Sinha SK, Ortega J, Friedman TC. Decreased prevalence of diabetes in marijuana users: cross-sectional data from the National Health and Nutrition Examination Survey (NHANES) III. BMJ open. 2012;2:e000494. doi: 10.1136/bmjopen-2011-000494. doi:10.1136/bmjopen-2011-000494. PMCID: PMC3289985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez SH, et al. Attenuation of HIV-1 replication in macrophages by cannabinoid receptor 2 agonists. Journal of leukocyte biology. 2013;93:801–810. doi: 10.1189/jlb.1012523. doi:10.1189/jlb.1012523. PMCID: PMC3629438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasouli N, Kern PA. Adipocytokines and the metabolic complications of obesity. The Journal of clinical endocrinology and metabolism. 2008;93:S64–73. doi: 10.1210/jc.2008-1613. doi:10.1210/jc.2008-1613. PMCID: PMC2585759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rausch DM, et al. Cytopathologic and neurochemical correlates of progression to motor/cognitive impairment in SIV-infected rhesus monkeys. Journal of neuropathology and experimental neurology. 1994;53:165–175. doi: 10.1097/00005072-199403000-00008. [DOI] [PubMed] [Google Scholar]
- Raymond LA, et al. Motor evoked potentials in a rhesus macaque model of neuro-AIDS. Journal of neurovirology. 1999;5:217–231. doi: 10.3109/13550289909015808. [DOI] [PubMed] [Google Scholar]
- Riebe CJ, Hill MN, Lee TT, Hillard CJ, Gorzalka BB. Estrogenic regulation of limbic cannabinoid receptor binding. Psychoneuroendocrinology. 2010;35:1265–1269. doi: 10.1016/j.psyneuen.2010.02.008. doi:10.1016/j.psyneuen.2010.02.008. PMCID: PMC2933663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodger DA, et al. A Study in HIV outpatients to Assess Prevalence of Cognitive Disorders, Associated Features and Impact on Risk Behaviours. Cell Stress & Chaperones. 2011;12:e1. doi:doi:10.1379/1466-1268(2007)12[e1:AFTRIC]2.0.CO;2. [Google Scholar]
- Rodriguez de Fonseca F, Cebeira M, Ramos JA, Martin M, Fernandez-Ruiz JJ. Cannabinoid receptors in rat brain areas: sexual differences, fluctuations during estrous cycle and changes after gonadectomy and sex steroid replacement. Life sciences. 1994;54:159–170. doi: 10.1016/0024-3205(94)00585-0. [DOI] [PubMed] [Google Scholar]
- Rogers CQ, Ajmo JM, You M. Adiponectin and alcoholic fatty liver disease. IUBMB life. 2008;60:790–797. doi: 10.1002/iub.124. doi:10.1002/iub.124. [DOI] [PubMed] [Google Scholar]
- Rom S, Persidsky Y. Cannabinoid receptor 2: potential role in immunomodulation and neuroinflammation. Journal of neuroimmune pharmacology : the official journal of the Society on NeuroImmune Pharmacology. 2013;8:608–620. doi: 10.1007/s11481-013-9445-9. doi:10.1007/s11481-013-9445-9. PMCID: PMC3663904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero J, et al. Time-course of the cannabinoid receptor down-regulation in the adult rat brain caused by repeated exposure to delta9-tetrahydrocannabinol. Synapse (New York, NY) 1998;30:298–308. doi: 10.1002/(SICI)1098-2396(199811)30:3<298::AID-SYN7>3.0.CO;2-6. doi:10.1002/(sici)1098-2396(199811)30:3<298::aid-syn7>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Ronis MJ, Wands JR, Badger TM, de la Monte SM, Lang CH, Calissendorff J. Alcohol-induced disruption of endocrine signaling. Alcohol Clin Exp Res. 2007;31:1269–1285. doi: 10.1111/j.1530-0277.2007.00436.x. doi:10.1111/j.1530-0277.2007.00436.x. [DOI] [PubMed] [Google Scholar]
- Rothlind JC, Greenfield TM, Bruce AV, Meyerhoff DJ, Flenniken DL, Lindgren JA, Weiner MW. Heavy alcohol consumption in individuals with HIV infection: effects on neuropsychological performance. Journal of the International Neuropsychological Society : JINS. 2005;11:70–83. doi: 10.1017/S1355617705050095. doi:10.1017/s1355617705050095. PMCID: PMC2376753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAMHSA . Overview of Findings from the 2003 National Survey on Drug Use and Health vol Office of Applied Studies. Rockville, MD: 2004. NSDUH Series H-24, DHHS Publication No. SMA 04-3963. [Google Scholar]
- Sandler NG, et al. Type I interferon responses in rhesus macaques prevent SIV infection and slow disease progression. Nature. 2014;511:601–605. doi: 10.1038/nature13554. doi:10.1038/nature13554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sassoon SA, Fama R, Rosenbloom MJ, O'Reilly A, Pfefferbaum A, Sullivan EV. Component cognitive and motor processes of the digit symbol test: differential deficits in alcoholism, HIV infection, and their comorbidity. Alcohol Clin Exp Res. 2007;31:1315–1324. doi: 10.1111/j.1530-0277.2007.00426.x. doi:10.1111/j.1530-0277.2007.00426.x. [DOI] [PubMed] [Google Scholar]
- Sevastianova K, Sutinen J, Kannisto K, Hamsten A, Ristola M, Yki-Jarvinen H. Adipose tissue inflammation and liver fat in patients with highly active antiretroviral therapy-associated lipodystrophy. American journal of physiology Endocrinology and metabolism. 2008;295:E85–91. doi: 10.1152/ajpendo.90224.2008. doi:10.1152/ajpendo.90224.2008. [DOI] [PubMed] [Google Scholar]
- Shay AH, et al. Impairment of antimicrobial activity and nitric oxide production in alveolar macrophages from smokers of marijuana and cocaine. The Journal of infectious diseases. 2003;187:700–704. doi: 10.1086/368370. doi:10.1086/368370. [DOI] [PubMed] [Google Scholar]
- Shuper PA, Joharchi N, Irving H, Rehm J. Alcohol as a correlate of unprotected sexual behavior among people living with HIV/AIDS: review and meta-analysis. AIDS Behav. 2009;13:1021–1036. doi: 10.1007/s10461-009-9589-z. doi:10.1007/s10461-009-9589-z. [DOI] [PubMed] [Google Scholar]
- Sim-Selley LJ. Regulation of cannabinoid CB1 receptors in the central nervous system by chronic cannabinoids. Critical reviews in neurobiology. 2003;15:91–119. doi: 10.1615/critrevneurobiol.v15.i2.10. [DOI] [PubMed] [Google Scholar]
- Simon L, et al. Chronic binge alcohol consumption alters myogenic gene expression and reduces in vitro myogenic differentiation potential of myoblasts from rhesus macaques. American journal of physiology Regulatory, integrative and comparative physiology. 2014;306:R837–844. doi: 10.1152/ajpregu.00502.2013. doi:10.1152/ajpregu.00502.2013. PMCID: PMC4042206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slawson G, et al. High-Intensity Cannabis Use and Adherence to Antiretroviral Therapy Among People Who Use Illicit Drugs in a Canadian Setting. AIDS Behav. 2014 doi: 10.1007/s10461-014-0847-3. doi:10.1007/s10461-014-0847-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smit E, Crespo CJ. Dietary intake and nutritional status of US adult marijuana users: results from the Third National Health and Nutrition Examination Survey. Public health nutrition. 2001;4:781–786. doi: 10.1079/phn2000114. [DOI] [PubMed] [Google Scholar]
- Snider WD, Simpson DM, Nielsen S, Gold JW, Metroka CE, Posner JB. Neurological complications of acquired immune deficiency syndrome: analysis of 50 patients. Annals of neurology. 1983;14:403–418. doi: 10.1002/ana.410140404. doi:10.1002/ana.410140404. [DOI] [PubMed] [Google Scholar]
- Specter S, Lancz G, Westrich G, Friedman H. Delta-9-tetrahydrocannabinol augments murine retroviral induced immunosuppression and infection. International journal of immunopharmacology. 1991;13:411–417. doi: 10.1016/0192-0561(91)90011-u. [DOI] [PubMed] [Google Scholar]
- Struwe M, et al. Effect of dronabinol on nutritional status in HIV infection. The Annals of pharmacotherapy. 1993;27:827–831. doi: 10.1177/106002809302700701. [DOI] [PubMed] [Google Scholar]
- Teixeira D, Pestana D, Faria A, Calhau C, Azevedo I, Monteiro R. Modulation of adipocyte biology by delta(9)-tetrahydrocannabinol. Obesity (Silver Spring) 2010;18:2077–2085. doi: 10.1038/oby.2010.100. doi:10.1038/oby.2010.100. [DOI] [PubMed] [Google Scholar]
- Theall KP, Amedee A, Clark RA, Dumestre J, Kissinger P. Alcohol consumption and HIV-1 vaginal RNA shedding among women. J Stud Alcohol Drugs. 2008;69:454–458. doi: 10.15288/jsad.2008.69.454. [DOI] [PubMed] [Google Scholar]
- Tindall B, Cooper DA, Donovan B, Barnes T, Philpot CR, Gold J, Penny R. The Sydney AIDS Project: development of acquired immunodeficiency syndrome in a group of HIV seropositive homosexual men. Australian and New Zealand journal of medicine. 1988;18:8–15. doi: 10.1111/j.1445-5994.1988.tb02232.x. [DOI] [PubMed] [Google Scholar]
- Villares J. Chronic use of marijuana decreases cannabinoid receptor binding and mRNA expression in the human brain. Neuroscience. 2007;145:323–334. doi: 10.1016/j.neuroscience.2006.11.012. doi:10.1016/j.neuroscience.2006.11.012. [DOI] [PubMed] [Google Scholar]
- Weiss L, Zeira M, Reich S, Har-Noy M, Mechoulam R, Slavin S, Gallily R. Cannabidiol lowers incidence of diabetes in non-obese diabetic mice. Autoimmunity. 2006;39:143–151. doi: 10.1080/08916930500356674. doi:10.1080/08916930500356674. [DOI] [PubMed] [Google Scholar]
- Wilson WH, Ellinwood EH, Mathew RJ, Johnson K. Effects of marijuana on performance of a computerized cognitive-neuromotor test battery. Psychiatry research. 1994;51:115–125. doi: 10.1016/0165-1781(94)90031-0. [DOI] [PubMed] [Google Scholar]
- Winsauer PJ, et al. Long-term behavioral and pharmacodynamic effects of delta-9-tetrahydrocannabinol in female rats depend on ovarian hormone status. Addiction biology. 2011a;16:64–81. doi: 10.1111/j.1369-1600.2010.00227.x. PMCID: PMC3057667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winsauer PJ, Moerschbaecher JM, Brauner IN, Purcell JE, Lancaster JR, Jr., Bagby GJ, Nelson S. Alcohol unmasks simian immunodeficiency virus-induced cognitive impairments in rhesus monkeys. Alcohol Clin Exp Res. 2002;26:1846–1857. doi: 10.1097/01.ALC.0000042171.80435.F1. doi:10.1097/01.alc.0000042171.80435.f1. [DOI] [PubMed] [Google Scholar]
- Winsauer PJ, et al. Tolerance to chronic delta-9-tetrahydrocannabinol (Delta(9)-THC) in rhesus macaques infected with simian immunodeficiency virus. Experimental and clinical psychopharmacology. 2011b;19:154–172. doi: 10.1037/a0023000. doi:10.1037/a0023000. PMCID: PMC3140653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolridge E, Barton S, Samuel J, Osorio J, Dougherty A, Holdcroft A. Cannabis use in HIV for pain and other medical symptoms. Journal of pain and symptom management. 2005;29:358–367. doi: 10.1016/j.jpainsymman.2004.07.011. doi:10.1016/j.jpainsymman.2004.07.011. [DOI] [PubMed] [Google Scholar]
- Yki-Jarvinen H, Nikkila EA. Ethanol decreases glucose utilization in healthy man. The Journal of clinical endocrinology and metabolism. 1985;61:941–945. doi: 10.1210/jcem-61-5-941. [DOI] [PubMed] [Google Scholar]
- You M, Considine RV, Leone TC, Kelly DP, Crabb DW. Role of adiponectin in the protective action of dietary saturated fat against alcoholic fatty liver in mice. Hepatology. 2005;42:568–577. doi: 10.1002/hep.20821. doi:10.1002/hep.20821. PMCID: PMC1398076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You M, Rogers CQ. Adiponectin: a key adipokine in alcoholic fatty liver. Exp Biol Med (Maywood) 2009;234:850–859. doi: 10.3181/0902-MR-61. doi:10.3181/0902-MR-61. [DOI] [PubMed] [Google Scholar]
- Yuan M, Kiertscher SM, Cheng Q, Zoumalan R, Tashkin DP, Roth MD. Delta 9-Tetrahydrocannabinol regulates Th1/Th2 cytokine balance in activated human T cells. Journal of neuroimmunology. 2002;133:124–131. doi: 10.1016/s0165-5728(02)00370-3. [DOI] [PubMed] [Google Scholar]
- Zhu W, Friedman H, Klein TW. Delta9-tetrahydrocannabinol induces apoptosis in macrophages and lymphocytes: involvement of Bcl-2 and caspase-1. The Journal of pharmacology and experimental therapeutics. 1998;286:1103–1109. [PubMed] [Google Scholar]