Abstract
England’s King Richard III, whose skeleton was recently discovered lying ignobly beneath a parking lot, suffered from a lateral curvature of his spinal column called scoliosis. We now know that his scoliosis was not caused by “imbalanced bodily humors”, rather vertebral defects arise from defects in embryonic elongation and segmentation. This review highlights recent advances in our understanding of post-gastrulation biomechanics of the posteriorly advancing tailbud and somite morphogenesis. These processes are beginning to be deciphered from the level of gene networks to a cross-scale physical model incorporating cellular mechanics, the extracellular matrix, and tissue fluidity.
Introduction
The posterior leading edge of the growing vertebrate embryo, named the tailbud, consists of motile progenitors of the axial skeleton, musculature, blood, vasculature and spinal cord, as well as bipotential neural/mesodermal stem cells [1–3]. Musculoskeletal progenitors enter the paraxial mesoderm, which consists of two columns of cells that flank the notochord. Concomitant with axis elongation the paraxial mesoderm stiffens and is segmented into somites whose metameric organization patterns the vertebral column. The somites also give rise to the skeletal muscle of the trunk, tail and limbs, as well as tendons and the dermis. Body elongation and somite morphogenesis are powerful cross-scale models for studying how cellular processes including cell proliferation, cell migration, and cell adhesion organize the biomechanical landscape of a complex tissue.
Cell Proliferation
In zebrafish, the progenitors of the paraxial mesoderm undergo two rounds of cell division during gastrulation. After gastrulation and during body elongation the bipotential stem cells located in the dorsal posterior tailbud do not proliferate due to absence of expression of the cell cycle regulator cdc25a [4••]. Upon migration into the ventral posterior tailbud, the cells begin to express both cdc25a and mesoderm specific transcription factors such as spadetail/tbx16, tbx6l and mesogenin [4••, 5••, 6••, 7•]. These mesodermal progenitor cells undergo one round of cell division before both daughter cells differentiate [4••]. This modest level of proliferation in the tailbud is consistent with studies in zebrafish and chick that found that trunk and tail elongation is driven more by cell migration than cell proliferation [8–12].
Cell migration
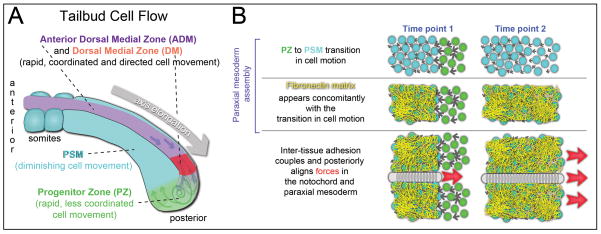
Cell migration in the tailbud has been best described in zebrafish and chick with initial studies finding that cell motion is more disordered among the mesodermal progenitors in the posterior tailbud than in the presomitic mesoderm (PSM) [13,14]. More recent systematic studies have elaborated on these findings [12,15••,16••]. In both organisms, the instantaneous cell velocities are higher in the posterior tailbud, and there is extensive cell mixing. The PSM grows posteriorly as motile posterior progenitors decrease their cell motion and assimilate into the tissue. Thus, elongation of the paraxial mesoderm is not due to directed migration within the PSM. During trunk elongation in the chick, new cells are added to the posterior mesodermal progenitors from a more posterior pool of cells [12]. By contrast, during zebrafish trunk elongation, new cells enter the dorsal tailbud as a coherent posterior flow of cells dorsal to the notochord. As cells move from the dorsal to ventral posterior tailbud, this flow loses coherence resulting in an increase in cell mixing (Figure 1A) [15••,16••]. Computer simulations suggest that this cell mixing may help synchronize the segmentation clock that generates the segmental prepattern in the PSM [17•]. As mesodermal progenitors enter the posterior PSM, cell motion declines concomitantly with the assembly of an extracellular matrix (ECM) composed of Fibronectin and Laminin [15••,18]. While cell-Fibronectin interactions are not required for this transition in cell migration, Cadherin 2 dependent cell-cell adhesion promotes coherent cell motion throughout the zebrafish tailbud [15••,16••].
Figure 1. Tissue mechanics during zebrafish trunk elongation.
(A) Cell motion in the tailbud can be quantified using metrics from fluid mechanics and thus described as cell flow. The first major transition in cell flow occurs as mesodermal progenitors migrate from the Dorsal Medial Zone into the Progenitor Zone (PZ) where they begin to express mesoderm specific transcription factors such as tbx16, tbx6 (in the mouse and chick) /tbx6l (in zebrafish) and mesogenin. The second transition in tissue fluidity occurs as Progenitor Zone cells assimilate into the Presomitic Mesoderm (PSM). (B) During assembly of the PSM, rapidly moving PZ cells (green; time point 1), reduce their instantaneous velocities, (time point 2). This transition coincides with the assembly of a Fibronectin matrix on the surface of the paraxial mesoderm, but the Fibronectin matrix is not necessary for the transition in cell motion. The Fibronectin matrix is required for normal elongation of the bilateral columns of paraxial mesoderm. In addition, the Fibronectin matrix mechanically couples the paraxial mesoderm and elongating notochord.
Fgf and Wnt regulate transitions in tissue fluidity
Fgf and Wnt are expressed in gradients from the tip of the tailbud. Fgf signaling promotes the rapid movement and mixing of cells in the posterior chick tailbud and misregulation of Fgf slows body elongation [12]. Similarly, temporally controlled inhibition of Fgf signaling in zebrafish leads to a shorter body axis. These phenotypes contrast with inhibition of Wnt signaling which caused non-linear body elongation. Quantification of cell motion indicates that reduction of Wnt signaling leads to a premature loss of coherence in cell motion in the dorsal tailbud while Fgf inhibition causes both a loss of coherence and a reduction in flux of cells into the posterior tailbud. Computer modeling of cell motion suggests that loss of coherence combined with high flux, as after Wnt inhibition, leads to jamming within the flow of cells. When the jam resolves, bilaterally symmetric flow can be disrupted leading to a bend in the body axis. In contrast, the loss of flux following Fgf inhibition compensates for the loss of coherence to prevent jamming, and the trunk elongates linearly albeit more slowly than wild type [16••].
The notochord
The notochord, which is located between the two columns of the paraxial mesoderm, also contributes to body elongation. It is assembled from axial mesoderm precursors during trunk elongation as cells intercalate along the dorsal midline [19]. This elongation persists in the absence of normal paraxial mesoderm growth but causes the notochord to buckle as it presses against the posterior tailbud [15••]. As tail formation begins at the 16 somite stage, notochord vacuoles begin to enlarge via endosomal trafficking [20]. An ECM consisting of collagen, elastin and laminin forms around the notochord and prevents radial expansion [19]. Thus, vacuole maturation causes elongation of the notochord along the anterior-posterior axis in a process called directed dilation [19]. Failure of vacuole maturation or of peri-notochord ECM integrity can lead to a shorter body axis, scoliosis and fusion of vertebrae [20,21].
ECM and the Mechanics of trunk elongation
Fibronectin is a prominent ECM protein in early vertebrate embryos, and cell-Fibronectin interactions are required for embryonic axis elongation and segmentation [15••,18,22–33]. Live imaging of both the Fibronectin matrix and cell migration during avian embryogenesis found that the ECM undergoes complex movements that closely mirror the pattern of cell migration [12,34–36]. These latter studies suggest that rather than acting primarily as a substrate for cell migration, the ECM may have a greater structural/mechanical function in force transmission or force generation during morphogenesis [36]. Indeed, cell-Fibronectin interactions in the paraxial mesoderm are required for trunk elongation but not cell migration. The Fibronectin matrix coats the zebrafish paraxial mesoderm and mechanically couples the bilateral halves of the paraxial mesoderm to the notochord and periderm [15••]. In Xenopus, the boundary between the paraxial mesoderm and notochord is induced by Eph/Ephrin signaling which increases cytoskeletal contractility and prevents Cadherin clustering along the boundary between the two tissues [37]. Eph/Ephrin also induces membrane blebbing along the tissue boundary, and in zebrafish, reduction of Fibronectin matrix increases cellular blebbing along the tissue boundary of the paraxial mesoderm [15••,37]. Cellular blebbing is driven by intracellular hydrostatic pressure [38]. Thus the appearance of blebbing behavior on the surface of the paraxial mesoderm suggests linkages between Eph/Ephrin signaling and cell-Fibronectin interactions in generating local cellular mechanics along the surface of the tissue.
Fibronectin forms a dense matrix on the surface of the paraxial mesoderm and the ability to assemble this ECM is an intrinsic property of both the zebrafish and chick paraxial mesoderm [39,40]. In zebrafish, removal of the Fibronectin receptors Integrin α5 and αV strongly reduces the amount of Fibronectin matrix, and the fibers that do form exhibit an abnormal medial-lateral anisotropy [15••]. Fibronectin fibrillogenesis is driven by cytoskeletal contractile forces transmitted to Fibronectin via linkage to the cytoplasmic domain of Integrins [41]. In 2D cell culture, mechanical stress is applied to the ECM in the direction of cell motion prior to migration [42], and ECM fibers often align along the direction of cell motion in both cell culture and the Xenopus gastrula [43,44]. The anisotropy of Fibronectin fibers in embryos lacking Integrin α5 and αV suggests a medial-lateral alignment of stresses on the surface of the paraxial mesoderm. This phenotype implies that a countervailing anterior-posterior stress produces a Fibronectin matrix with no bias in fiber alignment in wild-type embryos [15••].
Mechanical stiffening of the axis
Tissue stiffening within the Xenopus gastrula and neurula has been quantified using explant culture. During this developmental time period, the tissues stiffen by more than four fold with the paraxial mesoderm being twice as stiff as the notochord or neural plate and an order of magnitude stiffer than the endoderm [45,46]. Partial knockdown of Fibronectin does not affect the stiffening, but manipulation of the actin cytoskeleton does [46,47]. The morphology of the Xenopus laevis paraxial mesoderm is distinguished by medial-laterally elongated cells in the PSM whereas the other model systems have mesenchymal PSM [48]. Mechanical stiffening of the paraxial mesoderm has not been directly measured in other model species. Thus, it is unclear whether the apparent discrepancy between the role of Fibronectin in paraxial mesoderm mechanics in Xenopus and zebrafish is real and perhaps due to differences in tissue morphology.
The cells and ECM appear to exist as an integrated mechanical unit whose fluidity is modulated throughout tissue morphogenesis. Tissues and cell aggregates can be modeled as viscoelastic materials which have both flow and elastic characteristics. The response of viscoelastic materials to stress (force per unit area) changes with time. Under stress, viscoelastic fluids will eventually deform (flow) until stress equals zero while viscoelastic solids deform over time but retain some elastic form and ability to bear stress. Prior in vitro analyses of cell aggregates found that modulating levels of Fibronectin and Itgα5β1 leads to tissue phase transitions between a viscoelastic-fluid and a viscoelastic-solid. The reduction in matrix fibers may reduce apparent tissue viscosity by diminishing global interconnectivity within the cell aggregates [49]. The assembly of the paraxial mesoderm during trunk elongation can be thought of as a transition from a viscoelastic fluid to a viscoelastic solid (Figure 1B).
Somite morphogenesis
The segmentation clock creates metameric stripes of gene expression that initiate somite morphogenesis (Figure 2). In mouse, chick and zebrafish, somite morphogenesis entails a mesenchymal to epithelial transition, giving rise to an epithelial somite with a core of mesenchyme. The internal mesenchyme is not necessary for morphological segmentation as somites containing only anterior and posterior border cells can form in the absence of convergence-extension [50]. In Xenopus laevis and some other amphibians, somite morphogenesis involves a 90° rotation of elongated cells to give rise to muscle cells that span the anterior-posterior length of the somite [48]. There is a 10 fold variation in the number of cells per somite with zebrafish somites containing 100–150 cells while chick somites comprise ~1000 cells. The larger number of cells would take longer to sort during somite morphogenesis. in silico studies have modeled the mechanics of segmentation at the tissue, cellular and molecular scales [51•,52]. One of the studies suggests that the temporal delay after segmental patterning and before somite morphogenesis is functional. The delay facilitates error correction as cells sort along the segment boundary according to changes in cell adhesion [51].
Figure 2. Somite morphogenesis.
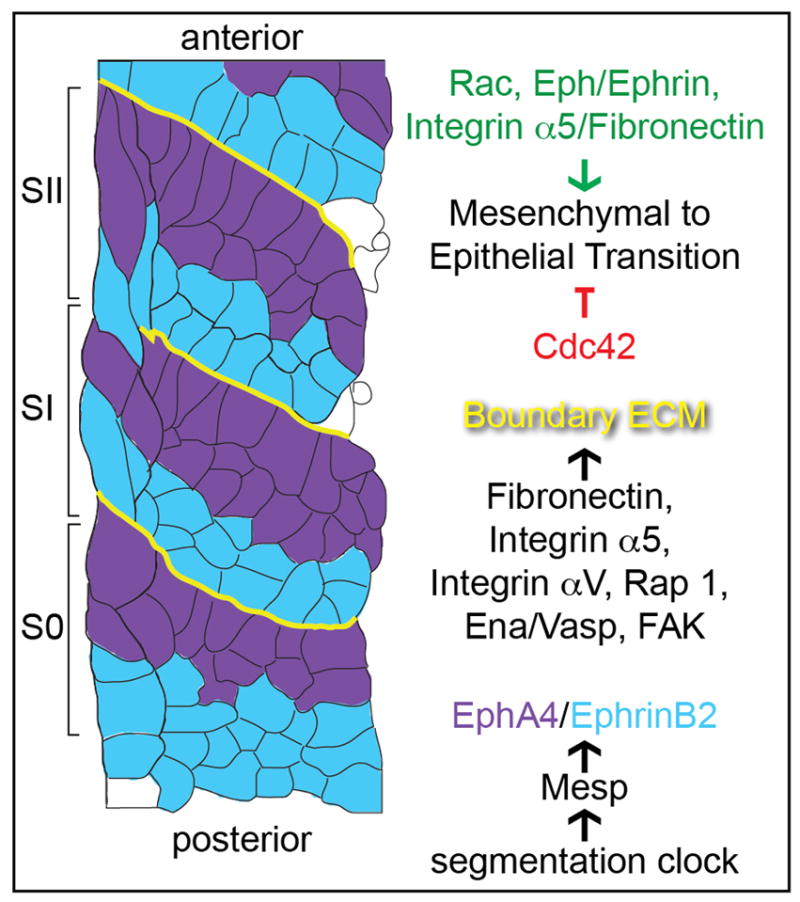
The S0 is the region in the anterior presomitic mesoderm that will form the next somites. The SI is the most recently formed somite and the SII is the preceding somite. In the presomitic mesoderm, the segmentation clock creates a segmental prepattern and stripes of expression of the transcription factor Mesp, which in turn sets up stripes of EphA4 and EphrinB2 expression flanking the somite boundary. Eph/Ephrin signaling activates Integrin α5 which then initiates assembly of Fibronectin matrix along the somite boundary. Integrin αV, Rap1, Ena/Vasp and FAK also promote Fibronectin matrix assembly. Upon initiation of boundary formation, the somite boundary cells undergo a mesenchymal to epithelial transition. This transition is inhibited by Cdc42 in the presomitic mesoderm and promoted by Rac1 in the forming somite. Eph/Ephrin signaling and Integrin α5/Fibronectin also promote the mesenchymal to epithelial transition.
In response to patterning by the segmentation clock, the receptor tyrosine kinase ephA4 is segmentally expressed along the posterior of the nascent somite border while its membrane bound ligand ephrinB2 is transcribed along the anterior of the border in mice, chick and zebrafish [53–57]. This juxtaposed expression of the receptor and ligand activates EphA4 along nascent somite borders [39]. In turn, Eph/Ephrin signaling can induce the mesenchymal to epithelial transition, inside-out Itgα5 activation and FN matrix assembly [39,53,54,57,58]. Formation of the Fibronectin matrix is necessary for the completion of somite epithelialization and cell sorting [28,29,39,59].
A number of other proteins have been implicated in somite morphogenesis, though an integrated understanding of the system at the molecular level remains elusive. rap1b is a GTPase known to regulate Integrin activation and epithelial cell morphology, and it functions with itgα5 to promote Fibronectin assembly at the zebrafish somite border [60]. In Xenopus, the cytoskeletal regulator Ena/Vasp is necessary for Fibronectin matrix assembly and cell rearrangement. In chick, the small GTPase Cdc42 restricts the mesenchymal to epithelial transition, while proper Rac1 levels are necessary for epithelialization [61]. Focal Adhesion Kinase (FAK), which mediates Integrin signaling is also required for boundary matrix formation [62].
Conclusion
The field of developmental biomechanics and biophysics has been reinvigorated by recent advances in instrumentation and conceptually by interdisciplinary research combining biology, physics and engineering. In fact, in September of 2014 the Lorentz Center at Leiden University in the Netherlands hosted a weeklong interdisciplinary workshop focusing specifically on the mechanobiology of somitogenesis. More broadly, integrating gene network function and biomechanics across multiple spatial and temporal scales promises to be a fertile field of inquiry that will contribute to our fundamental understanding of organogenesis, homeostasis and tissue engineering.
Acknowledgments
We thank Abeer Obaid for comments on the manuscript. SAH is supported by grants from the Eunice Kennedy Shriver National Institute of Child Health (1R21HD076173-01A1) and Human Development and the National Institute of General Medical Sciences (1R01GM107385-01A1 and 1R33GM114257-01).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References Cited
- 1.Martin BL, Kimelman D. Canonical Wnt signaling dynamically controls multiple stem cell fate decisions during vertebrate body formation. Dev Cell. 2012;22 :223–232. doi: 10.1016/j.devcel.2011.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Takemoto T, Uchikawa M, Yoshida M, Bell DM, Lovell-Badge R, Papaioannou VE, Kondoh H. Tbx6-dependent Sox2 regulation determines neural or mesodermal fate in axial stem cells. Nature. 2011;470:394–398. doi: 10.1038/nature09729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tzouanacou E, Wegener A, Wymeersch FJ, Wilson V, Nicolas JF. Redefining the progression of lineage segregations during mammalian embryogenesis by clonal analysis. Dev Cell. 2009;17:365–376. doi: 10.1016/j.devcel.2009.08.002. [DOI] [PubMed] [Google Scholar]
- ••4.Bouldin CM, Snelson CD, Farr GH, 3rd, Kimelman D. Restricted expression of cdc25a in the tailbud is essential for formation of the zebrafish posterior body. Genes Dev. 2014;28:384–395. doi: 10.1101/gad.233577.113. This study characterizes the proliferation of paraxial mesoderm progenitors during gastrulation, and proliferation of stem cells in the posterior tail bud. These latter cells are quiescent while in the stem cell niche. When a cell exits the niche, it divides once and both daughter cells differentiate as mesodermal cells. Thus, the stem cells do not self-replenish and natural depletion of the stem cells should limit axis elongation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ••5.Fior R, Maxwell AA, Ma TP, Vezzaro A, Moens CB, Amacher SL, Lewis J, Saude L. The differentiation and movement of presomitic mesoderm progenitor cells are controlled by Mesogenin 1. Development. 2012;139:4656–4665. doi: 10.1242/dev.078923. This thorough paper elucidates the role of the transcription factor mesogenin 1 in regulating differentiation of zebrafish paraxial mesoderm cells as well as the characteristic cell motion in the posterior tailbud. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ••6.Chalamalasetty RB, Garriock RJ, Dunty WC, Jr, Kennedy MW, Jailwala P, Si H, Yamaguchi TP. Mesogenin 1 is a master regulator of paraxial presomitic mesoderm differentiation. Development. 2014;141:4285–4297. doi: 10.1242/dev.110908. This manuscript is an extensive examination of Mesogenin 1 function in mouse paraxial mesoderm development. Microarrays and CHIP-seq were used to define Mesogenin 1 regulated genes with tbx6 and snail1 shown to be direct targets. Expression of mesogenin 1 in bipotential neural/mesodermal stem cells is sufficient to specify the cells as paraxial mesoderm. [DOI] [PMC free article] [PubMed] [Google Scholar]
- •7.Yabe T, Takada S. Mesogenin causes embryonic mesoderm progenitors to differentiate during development of zebrafish tail somites. Dev Biol. 2012;370:213–222. doi: 10.1016/j.ydbio.2012.07.029. Like the paper by Fior et al. but using a complementary experimental design, this manuscript shows that mesogenin 1 functions in conjunction with spadetail/tbx16 to regulate paraxial mesoderm differentiation. [DOI] [PubMed] [Google Scholar]
- 8.Kanki JP, Ho RK. The development of the posterior body in zebrafish. Development. 1997;124:881–893. doi: 10.1242/dev.124.4.881. [DOI] [PubMed] [Google Scholar]
- 9.Harrington MJ, Chalasani K, Brewster R. Cellular mechanisms of posterior neural tube morphogenesis in the zebrafish. Dev Dyn. 2010;239:747–762. doi: 10.1002/dvdy.22184. [DOI] [PubMed] [Google Scholar]
- 10.Quesada-Hernandez E, Caneparo L, Schneider S, Winkler S, Liebling M, Fraser SE, Heisenberg CP. Stereotypical cell division orientation controls neural rod midline formation in zebrafish. Curr Biol. 2010;20:1966–1972. doi: 10.1016/j.cub.2010.10.009. [DOI] [PubMed] [Google Scholar]
- 11.Zhang L, Kendrick C, Jülich D, Holley SA. Cell cycle progression is required for zebrafish somite morphogenesis but not segmentation clock function. Development. 2008;135:2065–2070. doi: 10.1242/dev.022673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Benazeraf B, Francois P, Baker RE, Denans N, Little CD, Pourquie O. A random cell motility gradient downstream of FGF controls elongation of an amniote embryo. Nature. 2010;466:248–252. doi: 10.1038/nature09151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Delfini MC, Dubrulle J, Malapert P, Chal J, Pourquie O. Control of the segmentation process by graded MAPK/ERK activation in the chick embryo. Proc Natl Acad Sci U S A. 2005;102:11343–11348. doi: 10.1073/pnas.0502933102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mara A, Schroeder J, Chalouni C, Holley SA. Priming, Initiation and Synchronization of the Segmentation Clock by deltaD and deltaC. Nat Cell Biol. 2007;9:523–530. doi: 10.1038/ncb1578. [DOI] [PubMed] [Google Scholar]
- ••15.Dray N, Lawton AK, Nandi A, Jülich D, Emonet T, Holley SA. Cell-Fibronectin interactions propel vertebrate trunk elongation via tissue mechanics. Curr Biol. 2013;23:1335–1341. doi: 10.1016/j.cub.2013.05.052. This study finds that the body elongation defect in zebrafish embryos lacking the primary Fibronectin receptors, integrin α5 and integrin αV, is not due to abnormal cell migration. Rather, loss of paraxial mesoderm assembly and inter-tissue adhesion between the paraxial mesoderm and notochord causes this elongation phenotype. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ••16.Lawton AK, Nandi A, Stulberg MJ, Dray N, Sneddon MW, Pontius W, Emonet T, Holley SA. Regulated tissue fluidity steers zebrafish body elongation. Development. 2013;140:573–582. doi: 10.1242/dev.090381. This study builds on the pioneering work of Kanki and Ho (ref 8) by imaging and tracking all cells in the zebrafish tailbud over a 2–3 hours period. By utilizing metrics from fluid mechanics to analyze the motion transitions in tissue fluidity are identified. Both experiments and computer simulations suggest that regulation of the coherence of cell motion is necessary for linear body elongation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- •17.Uriu K, Morelli LG. Collective cell movement promotes synchronization of coupled genetic oscillators. Biophys J. 2014;107:514–526. doi: 10.1016/j.bpj.2014.06.011. This in silico study examines the effect of cell mixing in the posterior tailbud on synchronization of the segmentation clocks in neighboring cells. The authors find that cell movement with a correlation length of 2–3 cell diameters, as observed by Lawton et al., actually enhances synchronization. Thus, the cell mixing promotes information flow by dispersing anomalous patterns of oscillations. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Latimer A, Jessen JR. Extracellular matrix assembly and organization during zebrafish gastrulation. Matrix Biol. 2010;29:89–96. doi: 10.1016/j.matbio.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 19.Stemple DL. Structure and function of the notochord: an essential organ for chordate development. Development. 2005;132:2503–2512. doi: 10.1242/dev.01812. [DOI] [PubMed] [Google Scholar]
- 20.Ellis K, Bagwell J, Bagnat M. Notochord vacuoles are lysosome-related organelles that function in axis and spine morphogenesis. J Cell Biol. 2013;200:667–679. doi: 10.1083/jcb.201212095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gray RS, Wilm TP, Smith J, Bagnat M, Dale RM, Topczewski J, Johnson SL, Solnica-Krezel L. Loss of col8a1a function during zebrafish embryogenesis results in congenital vertebral malformations. Dev Biol. 2014;386:72–85. doi: 10.1016/j.ydbio.2013.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.George EL, Georges-Labouesse EN, Patel-King RS, Rayburn H, Hynes RO. Defects in mesoderm, neural tube and vascular development in mouse embryos lacking fibronectin. Development. 1993;119:1079–1091. doi: 10.1242/dev.119.4.1079. [DOI] [PubMed] [Google Scholar]
- 23.Georges-Labouesse EN, George EL, Rayburn H, Hynes RO. Mesodermal development in mouse embryos mutant for fibronectin. Dev Dyn. 1996;207:145–156. doi: 10.1002/(SICI)1097-0177(199610)207:2<145::AID-AJA3>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 24.Goh KL, Yang JT, Hynes RO. Mesodermal defects and cranial neural crest apoptosis in alpha5 integrin-null embryos. Development. 1997;124:4309–4319. doi: 10.1242/dev.124.21.4309. [DOI] [PubMed] [Google Scholar]
- 25.Takahashi S, Leiss M, Moser M, Ohashi T, Kitao T, Heckmann D, Pfeifer A, Kessler H, Takagi J, Erickson HP, et al. The RGD motif in fibronectin is essential for development but dispensable for fibril assembly. J Cell Biol. 2007;178:167–178. doi: 10.1083/jcb.200703021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang JT, Rayburn H, Hynes RO. Embryonic mesodermal defects in alpha 5 integrin-deficient mice. Development. 1993;119:1093–1105. doi: 10.1242/dev.119.4.1093. [DOI] [PubMed] [Google Scholar]
- 27.Giros A, Grgur K, Gossler A, Costell M. alpha5beta1 Integrin-Mediated Adhesion to Fibronectin Is Required for Axis Elongation and Somitogenesis in Mice. PLoS One. 2011;6:e22002. doi: 10.1371/journal.pone.0022002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jülich D, Geisler R, Holley SA Consortium TS. Integrina5 and Delta/Notch Signalling have Complementary Spatiotemporal Requirements during Zebrafish Somitogenesis. Dev Cell. 2005:575–586. doi: 10.1016/j.devcel.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 29.Koshida S, Kishimoto Y, Ustumi H, Shimizu T, Furutani-Seiki M, Kondoh H, Takada S. Integrinalpha5-Dependent Fibronectin Accumulation for Maintenance of Somite Boundaries in Zebrafish Embryos. Dev Cell. 2005;8:587–598. doi: 10.1016/j.devcel.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 30.Trinh LA, Stainier DY. Fibronectin regulates epithelial organization during myocardial migration in zebrafish. Dev Cell. 2004;6:371–382. doi: 10.1016/s1534-5807(04)00063-2. [DOI] [PubMed] [Google Scholar]
- 31.Yang JT, Bader BL, Kreidberg JA, Ullman-Cullere M, Trevithick JE, Hynes RO. Overlapping and independent functions of fibronectin receptor integrins in early mesodermal development. Dev Biol. 1999;215:264–277. doi: 10.1006/dbio.1999.9451. [DOI] [PubMed] [Google Scholar]
- 32.Kragtorp KA, Miller JR. Integrin alpha5 is required for somite rotation and boundary formation in Xenopus. Dev Dyn. 2007;236:2713–2720. doi: 10.1002/dvdy.21280. [DOI] [PubMed] [Google Scholar]
- 33.Rifes P, Carvalho L, Lopes C, Andrade RP, Rodrigues G, Palmeirim I, Thorsteinsdottir S. Redefining the role of ectoderm in somitogenesis: a player in the formation of the fibronectin matrix of presomitic mesoderm. Development. 2007;134:3155–3165. doi: 10.1242/dev.003665. [DOI] [PubMed] [Google Scholar]
- 34.Czirok A, Rongish BJ, Little CD. Extracellular matrix dynamics during vertebrate axis formation. Dev Biol. 2004;268:111–122. doi: 10.1016/j.ydbio.2003.09.040. [DOI] [PubMed] [Google Scholar]
- 35.Zamir EA, Czirok A, Cui C, Little CD, Rongish BJ. Mesodermal cell displacements during avian gastrulation are due to both individual cell-autonomous and convective tissue movements. Proc Natl Acad Sci U S A. 2006;103:19806–19811. doi: 10.1073/pnas.0606100103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zamir EA, Rongish BJ, Little CD. The ECM moves during primitive streak formation--computation of ECM versus cellular motion. PLoS Biol. 2008;6:e247. doi: 10.1371/journal.pbio.0060247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fagotto F, Rohani N, Touret AS, Li R. A molecular base for cell sorting at embryonic boundaries: contact inhibition of cadherin adhesion by ephrin/ Eph-dependent contractility. Dev Cell. 2013;27:72–87. doi: 10.1016/j.devcel.2013.09.004. [DOI] [PubMed] [Google Scholar]
- 38.Charras G, Paluch E. Blebs lead the way: how to migrate without lamellipodia. Nat Rev Mol Cell Biol. 2008;9:730–736. doi: 10.1038/nrm2453. [DOI] [PubMed] [Google Scholar]
- 39.Jülich D, Mould AP, Koper E, Holley SA. Control of extracellular matrix assembly along tissue boundaries via Integrin and Eph/Ephrin signaling. Development. 2009;136:2913–2921. doi: 10.1242/dev.038935. [DOI] [PubMed] [Google Scholar]
- 40.Dias AS, de Almeida I, Belmonte JM, Glazier JA, Stern CD. Somites without a clock. Science. 2014;343:791–795. doi: 10.1126/science.1247575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schwarzbauer JE, DeSimone DW. Fibronectins, their fibrillogenesis, and in vivo functions. Cold Spring Harb Perspect Biol. 2011:3. doi: 10.1101/cshperspect.a005041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Trepat X, Wasserman MR, Angelini TE, Millet E, Weitz DA, Butler JP, Fredberg JJ. Physical forces during collective cell migration. Nat Phys. 2009;5:426–430. [Google Scholar]
- 43.Czirok A, Zach J, Kozel BA, Mecham RP, Davis EC, Rongish BJ. Elastic fiber macro-assembly is a hierarchical, cell motion-mediated process. J Cell Physiol. 2006;207:97–106. doi: 10.1002/jcp.20573. [DOI] [PubMed] [Google Scholar]
- 44.Goto T, Davidson L, Asashima M, Keller R. Planar cell polarity genes regulate polarized extracellular matrix deposition during frog gastrulation. Curr Biol. 2005;15:787–793. doi: 10.1016/j.cub.2005.03.040. [DOI] [PubMed] [Google Scholar]
- 45.Moore SW, Keller RE, Koehl MA. The dorsal involuting marginal zone stiffens anisotropically during its convergent extension in the gastrula of Xenopus laevis. Development. 1995;121:3131–3140. doi: 10.1242/dev.121.10.3131. [DOI] [PubMed] [Google Scholar]
- 46.Zhou J, Kim HY, Davidson LA. Actomyosin stiffens the vertebrate embryo during crucial stages of elongation and neural tube closure. Development. 2009;136:677–688. doi: 10.1242/dev.026211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhou J, Kim HY, Wang JH, Davidson LA. Macroscopic stiffening of embryonic tissues via microtubules, RhoGEF and the assembly of contractile bundles of actomyosin. Development. 2010;137:2785–2794. doi: 10.1242/dev.045997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Keller R. The origin and morphogenesis of amphibian somites. Curr Top Dev Biol. 2000;47:183–246. doi: 10.1016/s0070-2153(08)60726-7. [DOI] [PubMed] [Google Scholar]
- 49.Caicedo-Carvajal CE, Shinbrot T, Foty RA. Alpha5beta1 integrin-fibronectin interactions specify liquid to solid phase transition of 3D cellular aggregates. PLoS One. 2010;5:e11830. doi: 10.1371/journal.pone.0011830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Henry CA, Hall LA, Hille MB, Solnica-Krezel L, Cooper MS. Somite in zebrafish doubly mutant for knypek and trilobite form without internal mesenchymal cells or compaction. Curr Biol. 2000;10:1063–1066. doi: 10.1016/s0960-9822(00)00677-1. [DOI] [PubMed] [Google Scholar]
- •51.Hester SD, Belmonte JM, Gens JS, Clendenon SG, Glazier JA. A multi-cell, multi-scale model of vertebrate segmentation and somite formation. PLoS computational biology. 2011;7:e1002155. doi: 10.1371/journal.pcbi.1002155. This is an ambitious in silico analysis of somitogenesis that incorporates both the segmentation clock gene network as well as cell adhesion and cell sorting. The study suggests that the experimentally observed temporal delay between segmental determination and morphological boundary formation may serve to allow cell sorting to correct errors prior to somite morphogenesis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Truskinovsky L, Vitale G, Smit TH. A mechanical perspective on vertebral segmentation. International Journal of Engineering Science. 2014;83:124–137. [Google Scholar]
- 53.Barrios A, Poole RJ, Durbin L, Brennan C, Holder N, Wilson SW. Eph/Ephrin signaling regulates the mesenchymal-to-epithelial transition of the paraxial mesoderm during somite morphogenesis. Curr Biol. 2003;13:1571–1582. doi: 10.1016/j.cub.2003.08.030. [DOI] [PubMed] [Google Scholar]
- 54.Durbin L, Brennan C, Shiomi K, Cooke J, Barrios A, Shanmugalingam S, Guthrie B, Lindberg R, Holder N. Eph signaling is required for segmentation and differentiation of the somites. Genes Dev. 1998;12:3096–3109. doi: 10.1101/gad.12.19.3096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nakajima Y, Morimoto M, Takahashi Y, Koseki H, Saga Y. Identification of Epha4 enhancer required for segmental expression and the regulation by Mesp2. Development. 2006;133:2517–2525. doi: 10.1242/dev.02422. [DOI] [PubMed] [Google Scholar]
- 56.Nomura-Kitabayashi A, Takahashi Y, Kitajima S, Inoue T, Takeda H, Saga Y. Hypomorphic Mesp allele distinguishes establishment of rostrocaudal polarity and segment border formation in somitogenesis. Development. 2002:129. doi: 10.1242/dev.129.10.2473. [DOI] [PubMed] [Google Scholar]
- 57.Watanabe T, Sato Y, Saito D, Tadokoro R, Takahashi Y. EphrinB2 coordinates the formation of a morphological boundary and cell epithelialization during somite segmentation. Proc Natl Acad Sci U S A. 2009;106:7467–7472. doi: 10.1073/pnas.0902859106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Durbin L, Sordino P, Barrios A, Gering M, Thisse C, Thisse B, Brennan C, Green A, Wilson S, Holder N. Anteriorposterior patterning is required within segments for somite boundary formation in developing zebrafish. Development. 2000;127:1703–1713. doi: 10.1242/dev.127.8.1703. [DOI] [PubMed] [Google Scholar]
- 59.Martins GG, Rifes P, Amandio R, Rodrigues G, Palmeirim I, Thorsteinsdottir S. Dynamic 3D cell rearrangements guided by a fibronectin matrix underlie somitogenesis. PLoS One. 2009;4:e7429. doi: 10.1371/journal.pone.0007429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lackner S, Schwendinger-Schreck J, Jülich D, Holley SA. Segmental assembly of Fibronectin matrix requires rap1b and integrin α5. Dev Dyn. 2013;242:122–131. doi: 10.1002/dvdy.23909. [DOI] [PubMed] [Google Scholar]
- 61.Nakaya Y, Kuroda S, Katagiri YT, Kaibuchi K, Takahashi Y. Mesenchymal-epithelial transition during somitic segmentation is regulated by differential roles of Cdc42 and Rac1. Dev Cell. 2004;7:425–438. doi: 10.1016/j.devcel.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 62.Kragtorp KA, Miller JR. Regulation of somitogenesis by Ena/VASP proteins and FAK during Xenopus development. Development. 2006;133:685–695. doi: 10.1242/dev.02230. [DOI] [PubMed] [Google Scholar]