Abstract
One distinctive feature of the Trypanosoma brucei life cycle is the presence of two discrete populations that are based on differential expression of variant surface glycoproteins (VSGs). Both are adapted to the environmental pressures they face and more importantly, both contribute directly to transmission. Metacyclics in the tsetse fly enable transmission to a new mammalian host, whereas bloodstream trypanosomes must avoid immune destruction to the extent that sufficient numbers are available for transmission, when the insect vector takes a blood meal. At present, there are few investigations on the molecular aspects of parasite biology in the tsetse vector and specifically about the activation of metacyclic VSG gene expression. Here we used an established in vitro differentiation system based on the overexpression of the RNA-binding protein 6 (RBP6), to monitor two metacyclic VSGs (VSG 397 and VSG 653) during development from procyclics to infectious metacyclic forms. We observed that activation of these two mVSGs was simultaneous both at the transcript and protein level, and manifested by the appearance of only one of the mVSGs in individual cells.
Keywords: Trypanosoma brucei, VSG, metacyclics, surface coat, gene expression, gene activation
Graphical Abstract
Metacyclic VSG activation in T. brucei during development results in a single mVSG being expressed in individual metacyclic cells.
The protozoan parasite Trypanosoma brucei undergoes a complex life cycle between the mammalian host and the blood-feeding tsetse fly vector (Glossina spp.), which involves changes in cell morphology, surface coat composition, metabolism, signaling pathways and gene expression. Within the bloodstream of the mammal, the parasites exist as proliferative slender forms, which establish and maintain parasitaemia and the variant surface glycoprotein (VSG) coat is a well-studied example of antigenic variation [1]. Although there are hundreds of VSG genes in the genome, bloodstream-form VSG expression is restricted to 1 of about 15 specialized telomeric bloodstream expression sites (BES), which is transcribed polycistronically by RNA polymerase I (Pol I). Although the mechanistic details limiting VSG expression to a single BES remain to be worked out, transcription attenuation and epigenetic silencing play important roles and recent evidence suggests that there is regulation at the level of transcription initiation, involving class I transcription factor A (CITFA), the multi-subunit essential Pol I initiation factor [2]. Finally, the active BES is recruited to and transcribed in an extranucleolar expression site body (ESB), thus providing a potential solution for singular expression [3].
The VSG coat is lost when T. brucei cells are ingested by tsetse flies [4] and it reappears only after a complex developmental program that culminates in the tsetse salivary glands with the generation of metacyclic trypanosomes [5]. The non-dividing metacyclic forms (MFs) in the fly saliva have acquired a mVSG coat de novo, as a critical step towards becoming infectious to mammals. The mVSGs expressed in MF T. brucei have been studied primarily using immunochemistry [6,7] and electron microscopy (EM) [5], as well as molecular analyses of MF which were amplified for several days in laboratory animals or fortuitously cloned and cultured in vitro as bloodstream form (BF) trypanosomes that express VSG from a metacyclic expression site (MES) [8-11]. Similar to BES, MESs are genome units positioned adjacent to chromosome telomeres [10,11] and are transcribed by Pol I [10-12]. The repertoire of mVSGs expressed in metacyclics [7] was estimated in the past to be 27 or fewer genes [13] and the prevailing view is that only one gene from this catalogue is randomly activated in any single cell [5,8]. However, a recent survey of the VSG catalogue in the Lister 427 strain of T. brucei revealed a much more limited number of MESs with only six genomic loci containing a VSG gene downstream of a typical metacyclic promoter [14,15], although the possibility that the MES repertoire in this T. brucei strain has been eroded cannot be excluded. One of these MES appears to be the result of a recombination event and was classified as atypical [14].
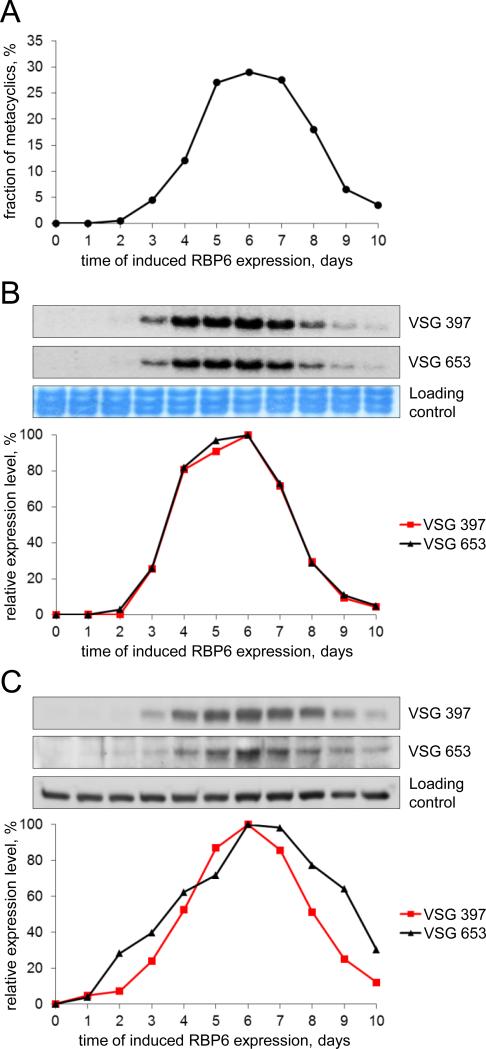
The onset of mVSG expression has not been studied previously at the molecular level, although it was shown that mVSG genes are activated in situ without detectable gene rearrangements and that the control of their expression appears to be at the level of transcription [9,10]. To begin to address the activation of mVSG gene expression, we took advantage of a recently developed in vitro system based on the inducible expression of the RNA-binding protein 6 [15]. This system recapitulates many of the aspects of trypanosome development in the fly, including the generation of metacyclics with a dense coat of VSG on their surface. The metacyclics generated in this in vitro system express five main VSG transcripts, coding for VSG 397, 653, 1954, 531 and 639 [15], which are the VGSs present in the five canonical MES identified in the Lister 427 strain of T. brucei [14]. For the studies reported here, we slightly modified the conditions for induced RBP6 expression compared to the original description [15] by increasing the concentration of glucose in the culture medium to 4.5 g/L, which is similar to what is used in the media for BF trypanosomes. This lead to a slight increase in the number of metacyclics in the culture (not shown) but, more importantly, resulted in a narrower time frame of appearance and disappearance of metacyclics in the culture (Fig. 1A). Purified MF cells die within 2-3 days when cultured in either conditioned or fresh medium, providing an explanation for the decrease in numbers of these non-dividing cells at later time points during induction. We chose to monitor the expression of two dominant mVSGs, namely, Tb427VSG-397 and Tb427VSG-653 [14,15]. The VSG 397 gene has consistently been the most highly expressed VSG in our T. brucei Lister 427 MF cells and VSG 653 has always been a much smaller fraction of the pool of detected VSG mRNA [15]. The abundance of the mRNAs for VSG 397 and VSG 653 during a time course of induced RBP6 expression was determined by Northern blotting with gene-specific probes (Fig. 1B). The relative steady-state levels for both mVSG mRNAs had almost identical profiles and their peak (days 4-7) shortly preceded the peak of percentage of MFs in the culture (days 5-8). To determine the abundance of the two mVSG proteins during the 10-day induction experiment, we generated anti-peptide antibodies against VSG 397 in rabbits and VSG 653 in rats and used them for Western blotting of cell lysates (Fig. 1C). The relative protein abundance profiles of VSG 397 and VSG 653 closely mirrored the fraction of MFs in the culture (Fig. 1A) and shortly followed mVSG mRNA abundance (Fig. 1B) on different days of RBP6 induction. Very similar results were obtained in experiments without using extra glucose in the culture medium (data not shown). These data showed for the first time that mVSGs expressed from different MESs appear at the same time in the trypanosome population and strongly support the idea for simultaneous activation of MESs. Additionally, we did not observe a significant lag between mVSG mRNA and mVSG protein increase in abundance, in accord with the model for control of mVSG expression primarily at the level of transcription [9,10]. To monitor the onset of mVSG expression at the single-cell level, we performed indirect immunofluorescence (IF) assays with the VSG 397 and VSG 653 antisera on paraformaldehyde-fixed cells from mixed cell type cultures on different days of RBP6 induction (Fig. S1). We observed fluorescence only from a small fraction of the cells for either VSG 397 (ranging from 1% to 2.5%) or VSG 653 (ranging from 0.5% to 1.5%), suggesting that mVSG activation appears to be a sudden-onset process in individual cells. We found no evidence for a gradual activation of mVSG expression in all cells competent for development to metacyclics, which would have manifested itself as a weak fluorescence signal in a larger percentage of cells early during the induction of RBP6 expression and the signal becoming stronger in a similar fraction of the cells at a later time points in the experiment.
Fig. 1.
Timeline of mVSG mRNA and protein expression and metacyclic cell proportion in cultures of T. brucei Lister 427 (29-13) induced to express RBP6. Cells were grown and induced with doxycycline as described previously [15] in the presence of 4.5 g/L glucose. (A) The percentage of metacyclic cells on each day of induced RBP6 expression was obtained as in [15] and cell samples were collected and processed for isolating total RNA or lysed with SDS-containing buffer. (B) Northern blots with equal amount of total RNA per sample (top) and probes specific for VSG 397 and VSG 653 were quantified with ImageQuantTL software (GE) (bottom). Large rRNAs stained with methylene blue are the loading controls. Day 0 of induction is set as 0% mVSG mRNA expression and the time point with highest value is set as 100% mVSG mRNA expression. (C) Western blots with equal cell number equivalents (top) processed with rabbit serum against the VSG 397 peptide CSDDAATYTSGSIAGTHALG (dilution 1:2,000) and rat serum against the VSG 653 peptide CRLHSGADNEGVVQNAGADN (dilution 1:1,000) were quantified with GeneTools software (Syngene) (bottom). A cross-reacting band served as a loading control. Day 0 of induction is set as 0% mVSG expression and the time point with highest value is set as 100% mVSG expression.
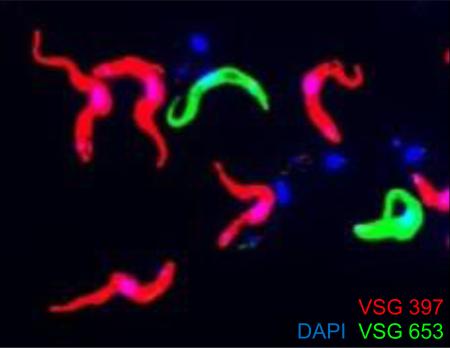
It was demonstrated in the past that monospecific antisera and monoclonal antibodies against mVSGs react only with a fraction of the metacyclic population [5-7,13]. Although this showed that metacyclics are heterogeneous with respect to their surface coat and not all MF cells express the same mVSG, it does not prove that similarly to BF, MF also express only a single mVSG on their surface. To our knowledge, immunofluorescence or immunogold-EM assays, where more than one mVSG is detected in the same cell sample at the same time, have not been previously reported. To demonstrate that individual metacyclics express only one mVSG, we performed IF to simultaneously detect VSG 397 and VSG 653 in a sample of purified MF cells produced in vitro. The purification step allows us to study mature MF with fully-formed mVSG coat, a pre-requisite for successful column purification. Our experiment clearly showed that the vast majority of fluorescent cells were labeled only by one of the two antisera used in the assay (Fig. 2A). Out of 400 cells analyzed, 55% were VSG 397 positive and 8% were VSG 653 positive. A significant proportion of the MF were not labeled with either mVSG antisera, because they must be expressing mVSG coats (to be present in the purified cell sample) from different VSG genes, and not VSG 397 or VSG 653 (Fig. 2A). We observed a single cell (out of 400) which exhibited double fluorescence as a result of having both VSG 397 and VSG 653 on its surface (Fig. S2). At present, it is very difficult to interpret this result. We learned from experiments in BF that cells expressing VSGs from more than one strong promoter have reduced growth [16,17] and that selection for double VSG expressors from two BESs produce T. brucei cell lines that rapidly switch between the two expression sites [16]. In addition, T. brucei cells stably expressing two VSGs at comparable levels can only be generated by placing the second VSG gene in the active expression site in tandem with the expressed VSG gene, leading to the production of both VSG mRNAs at half the normal VSG mRNA levels [18]. Thus, further studies will be required to explain the appearance of two different mVSGs on the surface of a single cell.
Fig. 2.
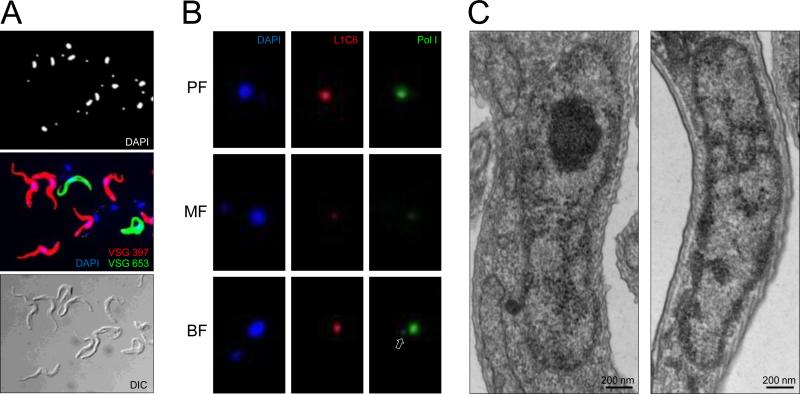
Coat formation by a single mVSG in metacyclics is followed by dispersal of the nucleolus. (A) Metacyclic cells were purified from culture also containing procyclics and epimastigotes on 0.1 mm diameter zirconia/silica beads column prepared in a Pasteur pipette in BBSG buffer (50 mM bicine, 50 mM NaCl, 5 mM KCl, 70 mM glucose). IF assay was performed with paraformaldehyde-fixed cells with the anti-VSG 397 rabbit serum (diluted 1:5,000) and anti-VSG 653 rat serum (diluted 1:500) and Alexa Fluor 488-conjugated goat anti-rabbit and Alexa Fluor 594-conjugated goat anti-rat secondary antibodies (Invitrogen), diluted 1:1,000. Note that some metacyclics are not recognized by either anti-VSG antibodies. (B) IF assay with affinity-purified anti-Pol I antibodies and L1C6 mouse monoclonal antibody was performed on PF, purified MF, and T.brucei Lister 427 single marker BF cells as described previously [3]. Arrow points to the ESB in BF. (C) Transmission EM of nucleus of a cell without a VSG coat (left) and a VSG-coated cell (right). The nucleolus is easily identifiable in the nonmetacyclic nucleus as an electron-dense sphere and it is not discernible in the metacyclic nucleus.
Similarly to the polycistronic BESs, the monocistronic MESs are transcribed by Pol I [12]. BF VSG transcription by Pol I takes place in a specialized nuclear compartment, called the expression site body (ESB), clearly distinguishable from the nucleolus [3]. To investigate whether the ESB is also a feature of MES transcription, we performed IF assay with antibodies against the largest subunit of Pol I. We detected Pol I concentrated in the nucleolus of PF and BF, and a clearly visible ESB in 46% of BF cells (out of 200 analyzed), but detected very little signal above background in MF cells that still localizes to the nucleolus (Fig. 2B). Proteomics data for MF suggested that the abundance of Pol I subunits in these cells is approximately half the abundance of these proteins in PF (Christiano et al., in preparation). Lower Pol I amounts in MF, combined with more diffuse localization throughout the nucleus, is the likely explanation for this observation. We also failed to detect an ESB in Pol I IF experiments performed not only in purified metacyclics, but also with samples from cultures containing cells at different stages of development from procyclics to metacyclics (data not shown). At present, we cannot exclude the possibility that ESB is temporarily formed during metacyclic VSG coat synthesis, yet not clearly detected in our assays. In addition to lower Pol I amounts and limited to none Pol I enrichment in the nucleolus in MF, we observed that the nucleolus itself had undergone a major transformation in MF. Transmission EM did not reveal the nucleolus as an electron dense compartment of the nucleus in MF (Fig. 2C), in contrast to cells without a VSG coat in the cultures. Remnants of the nucleolus were detected with the nucleolar-specific L1C6 monoclonal antibody, usually as foci that are much smaller than the nucleolus (Fig. 2B). Our results are very similar to data obtained for changing nucleolar morphology during development of T. cruzi cells to MF [19,20]. T. cruzi metacyclic trypomastigotes similarly undergo nucleolar dispersal [20], combined with general decrease in transcriptional activity in this non-dividing life-cycle stage [19]. A similar drop in transcription has been observed for T. brucei during the transition from proliferating slender to non-dividing stumpy BF [21].
In conclusion, our data support a model for mVSG activation in cells developing into MFs as a sudden-onset event during T. brucei development. Individual mVSGs are expressed synchronously in developing MFs, and only a single mVSG is expressed in a single cell for the vast majority of cells. Our results also suggest that the rapid formation of the mVSG coat is followed by disassembly of the nucleolus, suggestive of overall decrease in transcriptional activity by Pol I in the non-dividing metacyclic T. brucei.
Supplementary Material
Highlights.
A single mVSG is expressed in individual metacyclic cells.
Activation of the expression of mVSGs is synchronous.
Formation of the mVSG coat is accompanied by disassembly of the nucleolus.
Acknowledgments
We thank Miguel Navarro for the anti-RNA Pol I and protocol for immunofluorescence and Keith Gull for the anti-nucleolar L1C6 and anti-RNA Pol I antibodies. Research reported in this publication was supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under Award Numbers AI028798 to E.U., and AI043594 and AI110325 to C.T. K.R.-B. was supported by a training grant from the National Institute of Allergy and Infectious Diseases of the National Institutes of Health (T32 AI007404 to C.T.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Abbreviations
- BES
bloodstream expression site
- BF
bloodstream form
- ESB
expression site body
- MES
metacyclic expression site
- MF
metacyclic form
- mVSG
metacyclic VSG
- PF
procyclic form
- Pol I
RNA polymerase I
- VSG
variant surface glycoprotein
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
We declare that we have no conflict of interest.
References
- 1.Horn D. Antigenic variation in African trypanosomes. Mol Biochem Parasitol. 2014;195:123–9. doi: 10.1016/j.molbiopara.2014.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nguyen TN, Müller LS, Park SH, Siegel TN, Günzl A. Promoter occupancy of the basal class I transcription factor A differs strongly between active and silent VSG expression sites in Trypanosoma brucei. Nucleic Acids Res. 2014;42:3164–76. doi: 10.1093/nar/gkt1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Navarro M, Gull K. A pol I transcriptional body associated with VSG mono-allelic expression in Trypanosoma brucei. Nature. 2001;414:759–63. doi: 10.1038/414759a. [DOI] [PubMed] [Google Scholar]
- 4.Turner CM, Barry JD, Vickerman K. Loss of variable antigen during transformation of Trypanosoma brucei rhodesiense from bloodstream to procyclic forms in the tsetse fly. Parasitol Res. 1988;74:507–11. doi: 10.1007/BF00531626. [DOI] [PubMed] [Google Scholar]
- 5.Tetley L, Turner CM, Barry JD, Crowe JS, Vickerman K. Onset of expression of the variant surface glycoproteins of Trypanosoma brucei in the tsetse fly studied using immunoelectron microscopy. J Cell Sci. 1987;87:363–72. doi: 10.1242/jcs.87.2.363. [DOI] [PubMed] [Google Scholar]
- 6.Le Ray D, Barry JD, Vickerman K. Antigenic heterogeneity of metacyclic forms of Trypanosoma brucei. Nature. 1978;273:300–2. doi: 10.1038/273300a0. [DOI] [PubMed] [Google Scholar]
- 7.Barry JD, Crowe JS, Vickerman K. Instability of the Trypanosoma brucei rhodesiense metacyclic variable antigen repertoire. Nature. 1983;306:699–701. doi: 10.1038/306699a0. [DOI] [PubMed] [Google Scholar]
- 8.Barry JD, Graham SV, Fotheringham M, Graham VS, Kobryn K, Wymer B. VSG gene control and infectivity strategy of metacyclic stage Trypanosoma brucei. Mol Biochem Parasitol. 1998;91:93–105. doi: 10.1016/s0166-6851(97)00193-x. [DOI] [PubMed] [Google Scholar]
- 9.Lenardo MJ, Esser KM, Moon AM, Van der Ploeg LH, Donelson JE. Metacyclic variant surface glycoprotein genes of Trypanosoma brucei subsp. rhodesiense are activated in situ, and their expression is transcriptionally regulated. Mol Cell Biol. 1986;6:1991–7. doi: 10.1128/mcb.6.6.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Graham SV, Barry JD. Transcriptional regulation of metacyclic variant surface glycoprotein gene expression during the life cycle of Trypanosoma brucei. Mol Cell Biol. 1995;15:5945–56. doi: 10.1128/mcb.15.11.5945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alarcon CM, Son HJ, Hall T, Donelson JE. A monocistronic transcript for a trypanosome variant surface glycoprotein. Mol Cell Biol. 1994;14:5579–91. doi: 10.1128/mcb.14.8.5579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ginger ML, Blundell PA, Lewis AM, Browitt A, Günzl A, Barry JD. Ex vivo and in vitro identification of a consensus promoter for VSG genes expressed by metacyclic-stage trypanosomes in the tsetse fly. Eukaryot Cell. 2002;1:1000–9. doi: 10.1128/EC.1.6.1000-1009.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Turner CM, Barry JD, Maudlin I, Vickerman K. An estimate of the size of the metacyclic variable antigen repertoire of Trypanosoma brucei rhodesiense. Parasitology. 1988;97:269–76. doi: 10.1017/s0031182000058479. [DOI] [PubMed] [Google Scholar]
- 14.Cross GA, Kim HS, Wickstead B. Capturing the variant surface glycoprotein repertoire (the VSGnome) of Trypanosoma brucei Lister 427. Mol Biochem Parasitol. 2014;195:59–73. doi: 10.1016/j.molbiopara.2014.06.004. [DOI] [PubMed] [Google Scholar]
- 15.Kolev NG, Ramey-Butler K, Cross GA, Ullu E, Tschudi C. Developmental progression to infectivity in Trypanosoma brucei triggered by an RNA-binding protein. Science. 2012;338:1352–3. doi: 10.1126/science.1229641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chaves I, Rudenko G, Dirks-Mulder A, Cross M, Borst P. Control of variant surface glycoprotein gene-expression sites in Trypanosoma brucei. EMBO J. 1999;18:4846–55. doi: 10.1093/emboj/18.17.4846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Batram C, Jones NG, Janzen CJ, Markert SM, Engstler M. Expression site attenuation mechanistically links antigenic variation and development in Trypanosoma brucei. Elife. 2014;3:e02324. doi: 10.7554/eLife.02324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Muñoz-Jordán JL, Davies KP, Cross GA. Stable expression of mosaic coats of variant surface glycoproteins in Trypanosoma brucei. Science. 1996;272:1795–7. doi: 10.1126/science.272.5269.1795. [DOI] [PubMed] [Google Scholar]
- 19.Elias MC, Marques-Porto R, Freymüller E, Schenkman S. Transcription rate modulation through the Trypanosoma cruzi life cycle occurs in parallel with changes in nuclear organisation. Mol Biochem Parasitol. 2001;112:79–90. doi: 10.1016/s0166-6851(00)00349-2. [DOI] [PubMed] [Google Scholar]
- 20.Gluenz E, Taylor MC, Kelly JM. The Trypanosoma cruzi metacyclic-specific protein Met-III associates with the nucleolus and contains independent amino and carboxyl terminal targeting elements. Int J Parasitol. 2007;37:617–25. doi: 10.1016/j.ijpara.2006.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Amiguet-Vercher A, Pérez-Morga D, Pays A, Poelvoorde P, Van Xong H, Tebabi P, et al. Loss of the mono-allelic control of the VSG expression sites during the development of Trypanosoma brucei in the bloodstream. Mol Microbiol. 2004;51:1577–88. doi: 10.1111/j.1365-2958.2003.03937.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.