Summary
Interleukin-33 (IL-33) is a nuclear-associated cytokine of the IL-1 family originally described as a potent inducer of allergic type 2 immunity. IL-33 signals via the receptor ST2, which is highly expressed on group 2 innate lymphoid cells (ILC2s) and T helper 2 (Th2) cells, thus underpinning its association with helminth infection and allergic pathology. Recent studies have revealed ST2 expression on subsets of regulatory T cells, and for a role for IL-33 in tissue homeostasis and repair that suggests previously unrecognized interactions within these cellular networks. IL-33 can participate in pathologic fibrotic reactions, or, in the setting of microbial invasion, can cooperate with inflammatory cytokines to promote responses by cytotoxic NK cells, Th1 cells and CD8+ T cells. Here, we highlight the regulation and function of IL-33 and ST2, and review their roles in homeostasis, damage and inflammation, suggesting a conceptual framework for future studies.
Introduction
In 1989, a primary response gene was identified in serum-stimulated fibroblasts that resembled the extracellular portion of the interleukin-1 (IL-1) receptor. This protein, named T1 (Werenskiold et al., 1989), ST2 (Tominaga, 1989), Der4 (Lanahan et al., 1992), or Fit1 (Bergers et al., 1994), was the soluble form of the IL-33 receptor. Now designated IL-1-receptor-like 1 (IL1RL1), we will here use the common designation ST2. The ligand for ST2 was identified in 2005 during a search for unknown IL-1 family members (Schmitz et al., 2005), which revealed a sequence originally identified as a canine gene induced in endothelial cells in response to subarachnoid hemorrhage (Onda et al., 1999). T helper 2 (Th2) cells and mast cells expressed ST2, and IL-33 promoted Th2-associated allergic immunity when administered to mice(Coyle et al., 1999; Löhning et al., 1998; Schmitz et al., 2005; Townsend et al., 2000; Xu et al., 1998; Yanagisawa et al., 1997). Although IL-33 is most frequently characterized as an epithelial cytokine that promotes type 2 immune responses, recent studies have extended its biology to include roles in basal tissue regulation, organ-specific injury and repair (which promote fibrosis when dysregulated), and immunity to viruses, microbes and neoplasms. Despite this pleiotropic spectrum, surprising gaps remain in our knowledge regarding the molecular control and diverse functionalities of this cytokine. Here, we review the literature and propose a progressive model for conceptualizing the role of IL-33 in homeostasis and inflammation. In the homeostatic stage, constitutive pools of IL-33 become active locally to sustain basal physiology by activating ST2+ resident cells in a cell- and tissue-specific manner. In the amplification stage, tissue-specific pools of IL-33 increase and ST2+ cells increase in numbers in attempting to maintain homeostasis, usually in response to perturbations like parasites or allergens and resulting in overt manifestations of type 2 immunity. When continued over long periods, pathologic fibrosis can occur. In the conversion stage, inflammation from damaged tissues leads to the acquisition of IL-33 responsiveness through induced expression of ST2 on inflammatory cells, including NK cells, Th1 cells and CD8+ T cells, and resulting in overt manifestations of type 1 immunity. This facilitates clearance of pathogens but often at the cost of tissue damage. Consideration of this model may have implications in considering therapeutic strategies for altering IL-33 activities in vivo.
Molecular characterization of IL-33
IL-33 is a member of the IL-1 family, which includes IL-1α, IL-1β, IL-18, IL-36α, IL-36β, IL-36γ and IL-37, and the receptor antagonists IL-1Ra, IL-36Ra, and possibly IL-38 (Garlanda et al., 2013a). IL-33 is localized in the cell nucleus but also functions as a cell-free cytokine (Carriere et al., 2007), and in this way is similar to IL-1α and HMGB1 (Garlanda et al., 2013a; Moussion et al., 2008). Human and mouse IL-33 consist of seven coding exons, which produce a ~31 kDa protein of 270 and 266 amino acids, respectively. Exons 1–3 encode the N-terminal domains required for IL-33 nuclear localization, whereas exons 4–7 encode the C-terminal IL-1-like cytokine domain (Carriere et al., 2007). The nuclear localization domain (amino acids 1–65 in human) includes a chromatin-binding motif (amino acids 40–58) (Roussel et al., 2008), which mediates interaction of IL-33 with histone dimers and promotes chromatin compaction; mutation at R48 results in diffuse cytoplasmic localization of IL-33 and abolishes IL-33-mediated transcriptional repression in Gal4 reporter assays (Roussel et al., 2008). IL-33 has also been shown to bind the p65 subunit of NF-κB via the RelA interaction domain (amino acids 66–111) and inhibit NF-κB transcriptional activity in HEK293RI cells (Ali et al., 2011). Despite these data, evidence that nuclear IL-33 regulates normal transcriptional activity as part of its functionality requires further study.
The remainder of the IL-33 protein (amino acids 109–266 in mouse) encodes the IL-1-like cytokine domain, a 12-stranded β-trefoil fold similar to IL-1α, IL-1β, IL-1Rα and IL-18 (Lingel et al., 2009). This domain binds to the IL-33 receptor ST2, thus facilitating recruitment of IL-1RAcP to form the heterotrimeric signaling complex. Unlike IL-1β and IL-18, the N-terminal portion of IL-33 does not require cleavage by caspase 1 for release from the cell or to initiate signaling via ST2 (Cayrol and Girard, 2009; Luthi et al., 2009; Talabot-Ayer et al., 2009). Apoptosis-associated caspase-3 and caspase-7 cleave and inactivate IL-33 at a conserved residue, Asp178 (Asp175 in mouse), within the IL-1-like domain(Cayrol and Girard, 2009; Luthi et al., 2009). N-terminal processing of full-length IL-33 can also occur via a short stretch of amino acids between the nuclear-binding domain and the IL-1-like cytokine domain. These residues are targeted by extracellular proteases common at inflammatory sites, including neutrophil cathepsin G, neutrophil elastase and mast cell serine proteases, resulting in IL-3395–270, IL-3399–270, and IL-33109–270. The resulting 19 kDa cytokine forms of IL-33 exhibit 10- to 30-fold higher bioactivity, and are functionally similar to the 18 kDa recombinant IL-33112–270 available commercially(Lefrancais and Cayrol, 2012; Lefrancais et al., 2014; 2012). The relative contributions of full-length and cleaved IL-33 in vivo are incompletely resolved.
Whether or not it is necessary for underlying function, the nuclear localization of full-length IL-33 is highly regulated, since mislocalization has drastic consequences. Gene-targeted knock-in mice in which the IL-33 nuclear domain was replaced by DsRed reporter sequence express a fusion protein consisting of the cytokine domain but lacking nuclear localization. Heterozygous mice are born normally but develop high serum IL-33 and lethal inflammation characterized by splenomegaly with multi-organ infiltration by myeloid cells, particularly eosinophils, neutrophils and monocytes/macrophages (Bessa et al., 2014). These pathologic changes resemble those induced by constitutive over-expression of full-length IL-33 (Talabot-Ayer et al., 2015).
Regulation of IL-33 mRNA is also not well understood. In the mouse, IL-33 transcription initiates from one of two non-coding exons which are associated with constitutive or induced production of IL-33 (Polumuri et al., 2012; Talabot-Ayer et al., 2012). There are also a number of potential high-quality miRNA binding sites within the long 3′ UTR of IL-33 (www.microrna.org; A. Savage, unpublished observations). Among these, miR-9, miR-145, miR-214 and miR-499-5p have been linked to vascular and coronary function (Gidlöf et al., 2013; Hu et al., 2014; Jin et al., 2015; Shimizu et al., 2013; Turczy ska et al., 2012; Wang et al., 2010), and could be of interest because IL-33 from cardiac fibroblasts can promote cardioprotection (Sanada et al., 2007).
IL-33 release and/or secretion
Because IL-33 lacks a traditional signal sequence (Garlanda et al., 2013a) or a non-canonical processing and export pathway, cell death by necrosis and/or active necroptosis may be dominant mechanisms by which the cytokine reaches the extracellular milieu (Cayrol and Girard, 2014; Kaczmarek et al., 2013), leading to its designation as an ‘alarmin’. Indeed, receptor-interacting protein kinase 1 (Ripk1) knockout mice succumb to perinatal necroptosis-driven inflammation and exhibit marked increases in extracellular IL-33 (Rickard et al., 2014). However, it is unclear if cell death can account entirely for the biologic effects of IL-33. IL-33 release from living cells was reported in human bronchial epithelium exposed to Alternaria(Kouzaki et al., 2011) and from mechanically-stressed fibroblasts in vitro and in vivo (Kakkar et al., 2012). Several studies suggest extracellular ATP can promote IL-33 production or secretion in models of allergic inflammation(Byers et al., 2013; Hudson et al., 2008; Kouzaki et al., 2011). Human IL-33 transcripts lacking exon 3, 4 and/or 5 have been recovered from cell lines and primary cells (Hong et al., 2011; Tsuda et al., 2012). However, these splice forms appear to be minor components in primary cells and further work is required to determine their physiologic relevance. Other nuclear alarmins such as HMGB1 and IL-1α may be useful in understanding IL-33 regulation and activity. HMGB1 is ubiquitously expressed and, while it can be liberated from the cell by necrosis, active release from inflammatory macrophages may constitute an additional release mechanism (Harris et al., 2012). In contrast, IL-1α appears to require necrosis to access the extracellular space (Rider et al., 2013). Further study is necessary to elucidate these various pathways and how they are controlled.
The IL-33 receptor subunit ST2
The receptor complex for IL-33 consists of the specific subunit ST2 and the shared signaling chain, IL-1RAcP (Figure 1). ST2 is in a conserved locus on human chromosome 2 and mouse chromosome 1 with other IL-1 receptors, including those for IL-1 (IL1R1, decoy receptor IL1R2), IL-18 (IL18R1, IL18RAP), and IL-36 (IL1RL2) (Garlanda et al., 2013a). IL-1 family receptors are present throughout vertebrates together with their cytokine ligands, suggesting their co-evolutionary development. Although ST2 exhibits a similar distribution, its ligand IL-33 apparently arose later in evolution, as it is present in mammals but absent in non-mammalian vertebrates (Sattler et al., 2013). Despite this disconnect, there is no evidence for unidentified ST2 ligands in humans or mice. Further, ST2 is the only well-documented receptor for IL-33. In support of this, mice with loss of IL-33 nuclear localization signals or constitutive over-expression of IL-33 develop systemic inflammation that is abrogated by crossing onto the ST2-deficient background(Bessa et al., 2014; Talabot-Ayer et al., 2015).
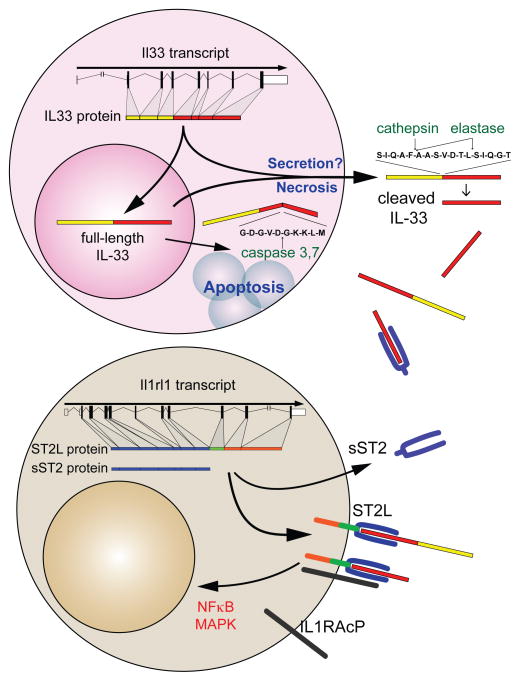
Figure 1. IL-33-ST2 molecular characteristics.
IL-33 is transcribed from seven coding exons and transported to the nucleus. During lytic cell death associated with necrosis or necroptosis, or possibly via direct secretion from intact cells, full-length IL-33 is released from the nucleus into the extracellular environment. Activation of apoptotic pathways leads to inactivation of IL-33 via caspase 3- or 7-mediated cleavage. Once released, full-length IL-33 can be further processed by serine proteases, such as cathepsin-G and elastase, into forms with increased activity. The IL-33 receptor ST2 is produced in two forms, a short soluble (sST2) or longer membrane-bound form (ST2L). sST2 is constitutively expressed, but can be induced during tissue damage, and binds IL-33 and restricts its availability. In contrast, both full-length and actively processed IL-33 bind to ST2L in combination with IL-1RAcP on target cells and induces canonical NFκB and MAPK signaling pathways leading to cellular activation and proliferation.
Soluble ST2
An additional layer of complexity in the biology of IL-33 is the occurrence of two ST2 isoforms, ST2L and sST2 (Yanagisawa et al., 1993). ST2L is the full-length protein and includes the extracellular Ig-like domains, a short extracellular spacer, the transmembrane domain and an intracellular TIR domain(Liu et al., 2013). sST2 is a short form which lacks the final three exons, resulting in a soluble secreted protein consisting of the extracellular cytokine-binding domains. sST2 is present constitutively in human serum (Kuroiwa et al., 2000; Oshikawa et al., 2001), where it acts as a decoy receptor by binding free IL-33. sST2 is increased by diverse inflammatory stimuli and in cardiovascular, rheumatologic and allergic diseases, potentially restricting the deleterious effects of elevated systemic IL-33 (Hayakawa et al., 2007; Kumar et al., 1997; Oshikawa et al., 2002; Weinberg et al., 2002; Yanagisawa et al., 1993). In humans, genome wide association studies (GWAS) have identified ST2 variants associated with altered levels of serum sST2(Ho et al., 2013), which could influence susceptibility to IL-33-mediated responses.
ST2L signaling
The crystal structure of the ectodomain of ST2 complexed with IL-33 has been solved (Liu et al., 2013). The ectodomain of ST2 consists of 3 Ig-like domains and resembles IL-1R1. The two distal domains (D1D2) pack together and connect via a flexible linker to the membrane-proximal third (D3) domain; IL-33 binds between the D1D2 and D3 domains (Liu et al., 2013). The binary IL-33-ST2 complex recruits IL-1RAcP, a shared signaling component of receptors for IL-1α and β and IL-36α, β and γ, to initiate signaling. IL-1RAcP signaling induces recruitment to the receptor complex of MyD88, IRAK, IRAK4 and TRAF6; activation of MAP kinases Erk1/2, p38 and JNK; activation of AP-1 transcription factors; and degradation of IκBα leading to translocation of NFκB to the nucleus (Andrade et al., 2011; Schmitz et al., 2005). IL-33 signaling may also require JAK2 activation (Funakoshi-Tago et al., 2011). As these signaling pathways are largely conserved with TLR, IL-1 and IL-18 signaling, the unique biologic effects of IL-33 are likely mediated by ST2L expression. ST2L signaling pathways can be inhibited by the IL-1 receptor-like molecule SIGIRR (TIR8), such that in Th2 cells, SIGIRR negatively regulates ST2 signaling and inhibits type 2 inflammatory processes (Bulek et al., 2009). IL-23 signaling impairs IL-33-mediated signaling in intestinal Tregs by restricting phosphorylation and activation of the Th2-associated transcription factor GATA3 (Schiering et al., 2014). IFN-γ potently inhibits IL-33 activation of ILC2 in vitro and in vivo and may be critical in suppressing this pathway during infections by bacteria and viruses (see below; Molofsky et al., 2015). Whether other described inhibitors of MyD88-dependent pathways, such as A20 (TNFAIP3), IRAK-M, or SOCS family members restrict IL-33/ST2 signaling is unknown (Garlanda et al., 2013b; Tamiya et al., 2011; Turer et al., 2008). The regulation, interaction, and signaling of IL-33 and ST2 are summarized in Figure 1.
Sources and production of IL-33
IL-33 protein is constitutively present in healthy mice and humans, primarily in nuclei of non-hematopoietic cells (Schmitz et al., 2005), with particular abundance in specialized populations of epithelial and endothelial cells(Moussion et al., 2008; Pichery et al., 2012). IL-33 was first identified in human lymph node high endothelial venules (HEV; originally named NF-HEV or DVS-27) and its expression was induced in canine cerebral vasculature after subarachnoid hemorrhage(Baekkevold et al., 2003; Onda et al., 1999). IL-33 is highly expressed in lymph node and spleen fibroblastic reticular cells (FRC) in mice and humans, although not in mouse HEV (Moussion et al., 2008; Pichery et al., 2012). In contrast to humans, mouse endothelial expression of IL-33 appears restricted to adipose tissue, liver and female reproductive tract (Carlock et al., 2014; Marvie et al., 2010; Pichery et al., 2012). IL-33 expression in endothelial cells in vitro has been linked to cellular quiescence and confluence, and may require Notch signals (Küchler et al., 2008; Sundlisaeter et al., 2012). Human and mouse share constitutive IL-33 expression at epithelial surfaces, including in skin, stomach, intestine, salivary gland, vagina and lung, where expression is particularly high in alveolar type 2 cells (Mohapatra et al., 2015; Moussion et al., 2008; Pastorelli et al., 2010; Schmitz et al., 2005); species-specific differences may exist (Sundnes et al., 2015). This epithelial expression pattern partially overlaps with other cytokines that target ILC2s, including TSLP and IL-25, suggesting potentially shared functions (Bulek et al., 2010; Mohapatra et al., 2015). During inflammation additional populations express IL-33, such as epithelial progenitor cells in models of COPD (Byers et al., 2013). In myeloid cells, IL-33 transcription can be induced by allergic challenge (Hardman et al., 2013) and TLR signaling. Mouse peritoneal macrophages express IL-33 mRNA in response to TLR activation by a process dependent on TBK1, RIG-I and IRF3 (Polumuri et al., 2012). TLR2-dependent induction of IL-33 mRNA in human monocytes and mouse macrophages was dependent on TRAF6 and IRF7 (Sun et al., 2014). Experimental demonstration of pathways that induce IL-33 protein in hematopoietic cells in vivo, as well as the physiologic relevance of hematopoietic sources of IL-33, require further study.
IL-33 in tissue homeostasis
IL-33 expression is regulated by diverse stimuli and in a cell- and tissue-specific manner, reflecting interactions in tissue between constitutive and induced components. Here, it may be useful to consider cell types that constitutively express high amounts of ST2, thus revealing cells and tissues that likely orchestrate initial responses to IL-33. Non-hematopoietic cells, including endothelial cells, epithelial cells and fibroblasts, are reported to express ST2L and respond to IL-33, although the in vivo consequences of signaling in these populations are not well described. In hematopoietic cells, IL-33 acts primarily on immune cells associated with type 2 and regulatory immune responses, including ILC2s, Th2 cells, eosinophils, mast cells, and basophils, as well as subsets of dendritic cells, myeloid-derived suppressor cells and Tregs (Cayrol and Girard, 2014). ILC2s, some Tregs, and mast cells are the primary tissue-resident cells that constitutively express high levels of ST2, positioning these cells as initial targets of IL-33. Mast cells are present in most tissues, can be activated by IL-33 to release mediators (Lunderius-Andersson et al., 2012), and may interact with ILC2s to maintain epithelial integrity(Roediger et al., 2013). ILC2s are systemically distributed in tissues, including skin, lung, gastrointestinal tract, uterus and adipose tissue. When activated they comprise the major innate source of type 2 cytokines such as IL-5, IL-13, IL-9 and GM-CSF, in addition to epithelial growth factors such as amphiregulin (Cheng and Locksley, 2015; von Moltke and Locksley, 2014; Moro et al., 2010; Neill et al., 2010; Price et al., 2010). Studies comparing responses to IL-33 in ILC2-replete Rag-knockout and ILC2-deficient Rag x γc – knockout mice suggest that ILC2s comprise the major innate target of exogenous IL-33 (Brestoff et al., 2014; Mchedlidze et al., 2013; Moro et al., 2010). IL-33-mediated ILC2 activation typically leads to tissue accumulation of eosinophils and alternatively activated macrophages (AAM), and is associated with elevated numbers of tissue Tregs.
Certain subsets of Tregs, including those in adipose tissue, express high amounts of the Th2-associated transcription factor GATA3 as well as ST2, and require IL-33 for their maintenance and function (Molofsky et al., 2015; Schiering et al., 2014; Vasanthakumar et al., 2015)). These tissue Tregs are highly suppressive and express IL-10 together with other markers associated with activation, including ICOS, KLRG1 and GITR. In vitro, IL-33 promotes proliferation of ST2+ Tregs and further enhances GATA3 expression, which stabilizes FoxP3 expression while increasing ST2 expression in a feed-forward reinforcing manner (Schiering et al., 2014; Vasanthakumar et al., 2015). Although IL-33 has direct effects on Treg stabilization and function, IL-33 also promotes ILC2 and/or dendritic cell subsets that enhance Treg numbers and function by indirect mechanisms (Besnard et al., 2015; Duan et al., 2012; Matta et al., 2014; Molofsky et al., 2015). Tregs generated during the perinatal period express ST2 and are highly suppressive and proliferative (Yang et al., 2015). These perinatal Tregs restrict the tissue autoimmune manifestations of Aire deficiency, a model of the human autoimmune disorder, APECED (autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy), hinting at a possible role for IL-33 in supporting perinatal tissue tolerance.
Although further work is needed, these data suggest a model whereby ILC2s, a subset of Tregs and possibly mast cells are positioned in tissues through a developmentally regulated process, where they can respond to local fluctuations in extracellular IL-33. In certain tissues, additional resident hematopoietic or tissue cells may also respond to IL-33, although this remains poorly explored. Serum sST2 serves to localize endogenous IL-33, where it might mediate processes involved in normal growth and development while sustaining tolerance by the developing adaptive immune system (Figure 2A).
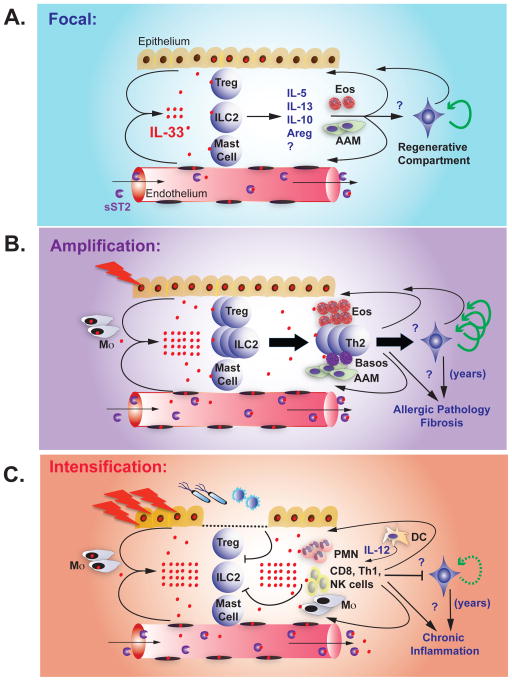
Figure 2. Progressive stages of IL-33-ST2.
A. Homeostasis. IL-33 is present in the nuclei of a subset of epithelial and endothelial cells at rest. Poorly understood signals promote IL-33 release or focal cellular necrosis. IL-33 acts on tissue-resident ST2-expressing ILC2, Treg, and possibly mast cells, inducing the production of IL-5, IL-13, IL-10, amphiregulin (Areg), and other signals that promote eosinophils (Eos) and alternatively activated macrophages (AAM). These cells and signals feed back on the tissue and may regulate remodeling and limit inflammation, in part by activating tissue progenitor cells. sST2 in the blood prevents systemic IL-33 effects.
B. Amplification. During tissue allergic insults and injury, epithelial and endothelial cells release IL-33, likely via necrosis, and IL-33 expression is further induced. Increased extracellular IL-33 activates and expands tissue ILC2 and Treg, and promotes recruitment and survival of additional immune cells (eosinophils, AAM, Th2 cells, basophils). These cells and signals feed back on the tissue to promote remodeling and limit inflammation, in part by activating tissue progenitor cells. In acute injury (infarction, tissue damage, helminth infection) these pathways help resolve injury and limit helminth infection. Chronic stimuli, such as allergens and repetitive tissue damage, lead to multiple cycles of IL-33 release that promote chronic allergic pathology, tissue fibrosis, increased Th2 cells, loss of Treg and other regulatory components, and increased tissue stores of IL-33. sST2 production is increased, but IL-33 concentrations may exceed blood buffering capacity in chronic damage and lead to systemic effects.
C. Conversion. Infectious or inflammatory triggers elicit tissue damage and epithelial breaches in the context of pathogen-associated molecular patterns. Pre-formed tissue IL-33 stores are released, likely via necrosis, and IL-33 is further induced in tissue cells. Inflammation and foreign antigen induces dendritic cell activation and IL-12 production, trafficking of inflammatory leukocytes from blood, and increased responsiveness to IL-33 through upregulation of ST2 on additional cell types. Activated inflammatory cells and cytokines, including IFN-γ, repress the Treg and type 2 immune response and facilitate killing of microbial and viral pathogens. In chronic inflammatory states, such as COPD and possibly autoimmune disease, IL-33 pools are increased and promotes repetitive cycles of IL-33 release, increasing tissue damage. sST2 production is increased, but IL-33 levels may exceed blood buffering capacity in chronic damage and lead to systemic effects.
Adipose tissue
The best-studied model of IL-33 function in tissue homeostasis is in white adipose tissue (Figure 2). White adipose tissue (WAT) is the major storage site for high-energy triglycerides, which are released as free fatty acids by lipolysis during periods of energy need. WAT of lean mice is populated with ST2+ ILC2s and Tregs that promote WAT eosinophils and AAM (Brestoff and Artis, 2015; Hams et al., 2013; Mathis, 2013; Molofsky et al., 2013b; Moro et al., 2010). IL-33 is abundant in adipose tissue, where expression has been confirmed in endothelial cells and fibroblast-like reticular cells, although further study is needed (Kolodin et al., 2015; Molofsky et al., 2015; Pichery et al., 2012; Wood et al., 2009; Zeyda et al., 2013). In mice, loss of IL-33 or ST2 leads to worsening obesity and insulin resistance, a hallmark of type 2 diabetes (Brestoff et al., 2014; Lee et al., 2015; Miller et al., 2010; Vasanthakumar et al., 2015). These changes are associated with a shift in the function and population of normal WAT immune cells, characterized by diminished ILC2 activation and decreased numbers of eosinophils, AAM and Tregs (Kolodin et al., 2015; Molofsky et al., 2015; Vasanthakumar et al., 2015)). Each of these cell types has been shown to protect in mouse models of obesity-induced insulin resistance (Brestoff and Artis, 2015; Mathis, 2013; Wu et al., 2011), supporting the concept whereby IL-33 sustains the architecture and function of ST2+ resident cells that limit infiltration of adipose tissue by inflammatory lymphocytes and myeloid cells that lead to insulin resistance and metabolic syndrome.
Although WAT is responsible for lipid storage, brown adipose tissue (BAT) expresses high amounts of uncoupling protein 1 (UCP1) and converts energy into heat, an important component of cold adaptation. BAT is abundant in newborns and decreases in adults (Frontini and Cinti, 2010). Recent studies have shown that white adipose tissue can convert to a brown-like depot, termed ‘beige’ or ‘brite’ adipose (Bartelt and Heeren, 2014; Harms and Seale, 2013). This occurs predominantly in subcutaneous WAT depots and may facilitate BAT-mediated adaptive thermogenesis (Bartelt and Heeren, 2014; Harms and Seale, 2013). Both BAT and beige adipose dissipate energy and influence glucose and VLDL-triglyceride metabolism (Bartelt et al., 2011; Chondronikola et al., 2014), potentially protecting against metabolic disorders such as type 2 diabetes. Type 2 immune cells have been implicated in promoting cold-induced adipose tissue beiging (Brestoff and Artis, 2015; Qiu et al., 2014; Rao et al., 2014), and IL-33 can act on ILC2s to promote beiging even at room temperature (Brestoff et al., 2014; Lee et al., 2015). In one study, ILC2-derived IL-13 was shown to act on adipose precursors to promote a beige fate (Lee et al., 2015), whereas a second study demonstrated ILC2s induce beiging by secreting the endogenous opioid methionine-enkephalin (Brestoff et al., 2014). Fundamental questions remain, including the relevant source(s) and direct target(s) of adipose tissue IL-33 and the signals and mechanisms that promote adipose tissue IL-33 production and release.
Female reproductive tissues
IL-33 and ST2 are expressed in uterine endometrial cells and increase with decidualization before fetal implantation (Salker et al., 2012). IL-33 was reported in placental macrophages and shown to promote the growth of trophoblasts (Fock et al., 2013). Ovarian IL-33 and ST2 increase with ovulation (Carlock et al., 2014), where IL-33 is expressed by endothelial cells surrounding developing follicles (Wu et al., 2015). IL-33 is required for recruitment of macrophages to mediate ovarian atresia post-ovulation, and IL-33-deficient mice show a 33% reduction in reproductive lifespan (Wu et al., 2015). These findings suggest IL-33 may promote the physiologic clearance of atretic ovarian follicles and possibly promote uterine and placental remodeling in the course of the estrous cycle and pregnancy. ILC2s are present in the uterus (Nussbaum et al., 2013), where they may coordinate aspects of the IL-33 response.
In the mammary gland, cells and cytokines associated with type 2 immunity mediate ductal branching and outgrowth during pubescent morphogenesis (Gjorevski and Nelson, 2011; Khaled et al., 2007; Sternlicht et al., 2006), a process that occurs within an adipose tissue matrix (Gjorevski and Nelson, 2011). The cytokines IL-4, IL-5 and IL-13, the epithelial growth factor amphiregulin, and eosinophil and AAM infiltration are all necessary for normal ductal outgrowth (Aupperlee et al., 2014; Colbert et al., 2005; Khaled et al., 2007); all of these findings are consistent with a role for ILC2s, which promote adipose tissue eosinophils and AAM (Molofsky et al., 2013b), produce a majority of tissue IL-5 and IL-13 (Nussbaum et al., 2013), and are a source of amphiregulin (Monticelli et al., 2011). Definitive studies, including the potential role of mammary gland IL-33, ILC2s, and Tregs, are needed.
Central nervous system
Prenatally, IL-33 is expressed in discrete regions of the central nervous system (CNS), including eye and spinal cord (Molofsky et al., 2013a; Pichery et al., 2012) and postnatally in the thalamus, cerebellum, spinal cord, and optic nerve (Hudson et al., 2008; Pichery et al., 2012; Wicher et al., 2013; Yasuoka et al., 2011). IL-33 is abundant in macroglia, including grey-matter astrocytes and oligodendrocytes (Hudson et al., 2008; Yasuoka et al., 2011). Microglia, the primary immune cells of the CNS, can express ST2 and are positioned to respond to glial signals (Yasuoka et al., 2011). In models of CNS damage, IL-33 promotes microglial and macrophage alternative activation and limits glial scarring (Luo et al., 2015; Pomeshchik et al., 2015). Astrocytes and microglia also participate in neural circuit formation during development, and immune molecules can fundamentally shape and alter these circuits(Clarke and Barres, 2013). A role for glial-derived IL-33 in shaping CNS neural circuits remains unknown.
Despite the widespread constitutive pool of nuclear IL-33 and strategically positioned ST2-expressing cells, both IL-33-deficient and ST2-deficient mice are grossly normal and suffer no overt developmental abnormalities. As previously noted, IL-33 is also expressed at barrier surfaces that contact the environment and commensals, including the skin, lung and gastrointestinal tract (Pichery et al., 2012). The potential homeostatic functions of IL-33 at these sites and others during normal growth and development have not been systematically explored.
IL-33 in type 2 immune responses
At the time of its discovery as a cytokine binding to the ST2 receptor, IL-33 was administered to mice exogenously by a variety of routes and methods (Humphreys et al., 2008; Kondo et al., 2008; Miller et al., 2008; Schmitz et al., 2005). In each case, massive infiltrations of tissues by eosinophils, epithelial goblet cell hyperplasia and elevations of typical type 2 cytokines were noted. These responses also occurred in Rag-deficient mice, indicating that innate cells were the primary target of IL-33 (Kondo et al., 2008). Importantly, these observations formed the cornerstone of experiments that later contributed to the discovery and characterization of ILC2s (Moro et al., 2010; Neill et al., 2010; Price et al., 2010). As such, these first experiments revealed that ILC2s are a dominant ST2+ cell responsive to IL-33 in the intact animal, and have prompted further investigations into the role of IL-33 in type 2 immune responses that accompany helminth infections, allergy and asthma.
IL-33 and helminth infection
In the mouse, infection with the helminth Nippostrongylus brasiliensis causes IL-33 release (Moro et al., 2010) and activation of ILC2s (Moro et al., 2010; Neill et al., 2010; Price et al., 2010) which cooperate with Th2 cells to mediate intestinal worm expulsion (Neill et al., 2010; Oliphant et al., 2014; Price et al., 2010). IL-33 signaling is not absolutely required for the generation of CD4+ Th2 cells (Hoshino et al., 1999; Townsend et al., 2000), N. brasiliensis intestinal clearance (Neill et al., 2010; Senn et al., 2000), or IgE responses (Hung et al., 2013; Townsend et al., 2000), although it expedites these processes in part via efficient ILC2 activation (Hung et al., 2013; Neill et al., 2010). During infection with Schistosoma mansoni, IL-33 signaling was required for optimal granuloma formation, lung eosinophilia and Th2 cytokine production (Townsend et al., 2000). IL-33 promotes Th2 cells necessary to clear Trichuris muris (Humphreys et al., 2008), and IL-33 signaling limited Trichinella spiralis encystation in muscle (Scalfone et al., 2013). IL-33 can cooperate with other epithelial cytokines, including IL-25 (Neill et al., 2010), to mediate helminth clearance. In vitro, ILC2s are synergistically activated by IL-33 when combined with other cytokines such as IL-2, IL-7, IL-9 and TSLP (Martinez-Gonzalez et al., 2015; Oliphant et al., 2014; Turner et al., 2013; Wilhelm et al., 2011), suggesting environmental perturbations are integrated to mediate these responses. Thus, IL-33 is a partially redundant but important component of type 2 immune responses in the context of helminth infection.
Th1 cell-associated inflammatory responses can counter-regulate IL-33-associated Th2 cell immunity. Thus, IL-1β can impair intestinal IL-33 upregulation during helminth infection and limit the ability to clear chronic gastrointestinal infection (Zaiss et al., 2013). Similarly, mice lacking the IL-1β receptor, IL-1R, or the adaptor MyD88, had more robust intestinal granuloma formation when infected with Heligmosomoides polygyrus (Reynolds et al., 2014). In models of cerebral malaria, IL-33 administration protected against Th1 cell-associated lethality by inducing ILC2s and Tregs (Besnard et al., 2015). IFN-γ potently inhibits IL-33-mediated ILC2 activation in vitro and in vivo following Listeria infection (Molofsky et al., 2015). IL-33 has been implicated in protection or pathology in a variety of additional bacterial and parasitic infections, as recently reviewed, (Rostan et al., 2015), although the mechanisms of action in these models are not well defined.
IL-33 in allergic pathology
Large-scale GWAS implicate both IL-33 and ST2 in susceptibility to asthma; other loci associated with ILC2 and Th2 cell function, including IL-13, TSLP and RORα were also noted (Gudbjartsson et al., 2009; Moffatt et al., 2010; Torgerson et al., 2011). IL-33 was required for ovalbumin- and papain-induced type 2 airway inflammation (Oboki et al., 2010). Additional allergic challenges, including Alternaria (Bartemes et al., 2012; Kouzaki et al., 2011; Snelgrove et al., 2014), house dust mite extract (Willart et al., 2012), and chitin polymers (Van Dyken et al., 2014) were found to induce lung IL-33 that promoted Th2 immune responses and airway hyperreactivity. IL-33 induction occurred in both hematopoietic and non-hematopoietic cells, particularly in alveolar type 2 cells (Kouzaki et al., 2011; McSorley et al., 2014; Mohapatra et al., 2015). Activation of lung ILC2s (Bartemes et al., 2012; Beamer et al., 2013; Doherty et al., 2012; Halim et al., 2012; Van Dyken et al., 2014), Th2 cells (Endo et al., 2015; Kurowska-Stolarska et al., 2008), or both (Iijima et al., 2014), were required to mediate the allergic phenotype, depending on the model system. ILC2s may indirectly support the generation of Th2 cells via IL-13 induction of dendritic cell migration or through direct ILC2/Th2 interactions (Halim et al., 2014; Martinez-Gonzalez et al., 2015; Oliphant et al., 2014). Additionally, Th2 and Th9 cells produce γc receptor-binding cytokines such as IL-2, IL-4 and IL-9, which can potentiate ILC2 activation (Mirchandani et al., 2014; Wilhelm et al., 2011). IL-33 can also act directly on ST2+ Th2 cells in vitro (Guo et al., 2009; Schmitz et al., 2005) and in vivo (Endo et al., 2015; Kurowska-Stolarska et al., 2008) to promote cytokine production.
IL-33 plays a role in other models of allergic disease, including allergic rhinitis and chronic rhinosinusitus through activation of ILC2s, basophils and mast cells (Haenuki et al., 2012; Kato, 2015; Mjösberg et al., 2011; Nakanishi et al., 2013). In humans, chronic eosinophilic rhinosinusitis with nasal polyps is associated with increased epithelial IL-33 and IL-13+ ILC2s (Shaw et al., 2013). IL-33 is expressed in skin keratinocytes and is elevated in humans and mouse models of atopic dermatitis (Kim and Artis, 2015). In mice, overexpression of IL-33 in the skin can cause atopic dermatitis-like immune pathology (Imai et al., 2013). The role of endogenous IL-33 in murine atopic dermatitis models has been inconsistent (Kim et al., 2013; Salimi et al., 2013), suggesting IL-33 may be one of several signals that together promote disease (Kim and Artis, 2015). IL-33 may contribute to gastrointestinal food allergy (Chu et al., 2013; Muto et al., 2014) and other eosinophilic gastrointestinal diseases, although further study is required to delineate these roles. Together, these findings indicate that IL-33 can promote pathologic type 2 immune responses, particularly at barrier surfaces such as the lung and skin. Similar to helminth infections, IL-33 frequently acts in combination with additional signals such as IL-2, IL-9, TSLP, IL-25, leukotrienes, prostaglandins, or TL1A, to promote ILC2 and/or Th2 functions (von Moltke and Locksley, 2014). Inhibition of the IL-33 pathway may be a promising therapeutic approach for limiting these pathologic responses (Fahy, 2015).
IL-33 promotes both protective and pathologic type 2 immune responses in the setting of tissue injury (Figure 2b), which may represent extensions of the role of IL-33 during tissue homeostasis, here termed amplification. Indeed, type 2 immunity plays active roles in both wound healing and helminth immunity (Gause et al., 2013). Helminths induce adaptive Th2 cells and low-affinity IgE antibody production, but these responses typically cause little acute tissue pathology, likely due to the concomitant activation of regulatory cells, such as Treg (Finlay et al., 2014; McSorley and Maizels, 2012). Although helminths actively elicit regulatory responses in part through secretion of immune-modulatory products (Finlay et al., 2014; McSorley and Maizels, 2012), IL-33 itself has direct and indirect effects in stimulating these regulatory pathways (Molofsky et al., 2015). In contrast, allergic pathologic states are characterized by chronic elevations in tissue IL-33 and activated ILC2s with Th2 cells, and are associated with tissue pathology and a failure to activate or maintain regulatory responses, including Treg. In these chronic settings, Th2 cells likely become activated during repetitive rounds of antigenic stimulation, further driving maladaptive cycles of immune dysfunction.
IL-33 in tissue damage, repair and fibrosis
Work in models of cardiovascular disease demonstrated IL-33 induction following vascular and cardiac stress that was correlated with improved outcomes (Miller et al., 2008; Onda et al., 1999; Sanada et al., 2007; Sánchez-Más et al., 2014). IL-33 reduced atherosclerosis (Miller et al., 2008), limited pressure overload-induced cardiac hypertrophy (Sanada et al., 2007), and improved cardiac function and cardiomyocyte survival after myocardial infarction (Seki et al., 2009). IL-33 administration expands ILC2s and Tregs and protects in models of allograft rejection, tissue injury and pathology driven by excess type 1 immune responses (Brunner et al., 2011; Duan et al., 2012; Liang et al., 2013; Schiering et al., 2014; Turnquist et al., 2011; Yin et al., 2013). ST2+ Tregs are also increased during helminth infection (Layland et al., 2010; Molofsky et al., 2015) and in models of muscle damage (Burzyn et al., 2013), suggesting damage-induced IL-33 may participate in these responses. However, elevated serum IL-33 can also be associated with autoimmune disease and chronic pathology (Palmer and Gabay, 2011). Human patients with systemic sclerosis have elevated IL-33 levels that correlated with the extent of skin and lung fibrosis (Yanaba et al., 2011). In mouse models of liver damage, IL-33 activates ILC2s to expand, produce IL-13 and promote hepatic stellate cell activation and liver fibrosis (Mchedlidze et al., 2013). IL-33 can also cause skin fibrosis in an ILC2- and eosinophil-dependent manner (Rankin et al., 2010). These findings suggest IL-33 promotes tissue repair, likely coordinated by ILC2s and Tregs, but that these pathways can become maladaptive when chronic or excessive, resulting in allergic pathology and fibrosis. These changes may reflect the outgrowth of pathologic Th2 effector cells, loss of regulatory cells, and/or damage or depletion of tissue niches for critical precursor cells required to sustain tissue homeostasis.
IL-33-ST2 in infection and non-allergic inflammation
During inflammatory, infectious and neoplastic insults, other white blood cells, including neutrophils, macrophages, NK cells, NKT cells, Th1 cells and CD8+ T cells, are recruited to damaged tissues and gain responsiveness to IL-33, a process we term conversion (Figure 2C). These cells express little ST2 at rest, but transient expression, particularly in inflammatory lymphocytes, can be induced in response to signals such as IL-12 (Bourgeois et al., 2009; Smithgall et al., 2008; Sun et al., 2009; Yang et al., 2011). Th1 cells required STAT4 signaling and the Th1-associated transcription factor Tbet to induce transient up-regulation of ST2 (Baumann et al., 2015). In these inflammatory settings, IL-33 drives cell expansion and IFN-γ production, and is required for optimal protection against viral infection (Bonilla et al., 2012; Nabekura et al., 2015; Villarreal and Weiner, 2014). After immune stimulation, dendritic cells (DC) which reside near high endothelial venules (HEV) in lymphoid organs are major sources of IL-12 (Reinhardt et al., 2006). Inflammatory DCs also interact with fibroblastic reticular cells (FRC), which mediate lymph node remodeling and enlargement during infection (Acton et al., 2014). HEV and FRC are primary sources of lymph node IL-33 in mice and humans (Moussion et al., 2008; Pichery et al., 2012), and release of IL-33 in these cells might reflect mechanical forces inherent in the rapid inflammation-induced changes in lymph node size, akin to findings in fibroblasts (Kakkar et al., 2012). In contrast, ILC2s, mast cells and ST2+ Tregs are rare in lymphoid tissues (Molofsky et al., 2015; Nussbaum et al., 2013), potentially limiting competition for IL-33 and allowing IL-12 to drive low amounts of ST2 expression on inflammatory lymphocytes that enables competence to detect IL-33. As such, IL-33 may have a role as a vaccine adjuvant, as revealed in studies in which IL-33 promoted protective CD4+ and CD8+ T cells against human papilloma virus(Villarreal et al., 2014). The success of this approach may depend on the route of the vaccine and its ability to promote a Th1 cell-prone environment, including IL-12 production (Villarreal and Weiner, 2015).
In mice exposed chronically to tobacco smoke, the IL-33 tissue pool in lung is massively increased and ST2 expression becomes up-regulated on inflammatory leukocytes; in this setting, IL-33 enhances inflammation and worsens disease(Kearley et al., 2015). In mouse models of virally driven COPD, lung precursors increased expression of IL-33; similar findings were noted in humans with COPD (Byers et al., 2013). These studies suggest states of chronic inflammation, such as COPD, can increase tissue IL-33 pools and IL-33 may cooperate with persistent inflammatory signals to further promote inflammation and tissue damage. There are likely additional signals that directly repress tissue ILC2s and Tregs during chronic inflammation (Kearley et al., 2015).
IL-33 has diverse effects in cancer models. Direct overexpression of IL-33 in neoplasms promoted NK and CD8+ T cell infiltration and restriction of cancer growth and metastasis (Gao et al., 2015). However, IL-33 can signal on cancer cells that themselves express ST2L, thereby promoting survival and metastasis (Kim et al., 2014; Levescot et al., 2014; Yu et al., 2015). In a model of breast cancer, exogenous IL-33 drove a mixed regulatory/type 2 immune infiltrate that promoted tumor growth and metastasis (Jovanovic et al., 2013). Elevated levels of blood ILC2s were identified in patients with gastric cancer and were associated with a suppressive immune state (Bie et al., 2014). Together, the effects of IL-33 on cancer appear context-specific, and may depend on the types of immune cells in the tumor environment, the amount of available IL-33, the amount of sST2 and the tumor cell-intrinsic expression of ST2L.
Integrating the spectrum of IL-33 activities
Understanding of IL-33 biology has evolved and expanded from its predominant role in allergic pathology to unexpected participation in basic physiologic processes connected with development and metabolism, to tissue repair and fibrosis, and to roles in classic inflammation. A framework for conceptualizing the spectrum of IL-33 biology hypothesizes a stepwise process by which the size of the tissue IL-33 nuclear pool, the presence of supporting cytokines, and the types of responding cells create a dynamic element embedded within the functionality of tissues (Figure 3).
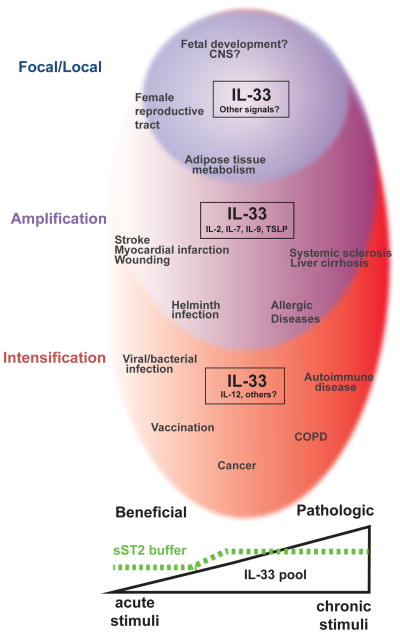
Figure 3. Spectrum of IL-33 biology.
A model of the spectrum of IL-33 effects on tissue during beneficial and pathologic immune responses, with IL-33 cellular pools increasing from left to right. (Homeostasis, Blue) IL-33 is present in a restricted subset of cells and cooperates with unknown signals to maintain tissue integrity, limit excess inflammation and promote tissue adaptation to remodeling and other physiologic stressors. (Amplification, Purple) During acute tissue injury and damage, IL-33 synergizes with other epithelial cytokines and lymphokines to promote tissue homeostasis and repair (stroke, myocardial infarction, wounding). Helminths elicit similar responses. With repetitive tissue damage, IL-33 pools increase, regulatory mechanisms are suppressed, inflammation is amplified and fibrosis ensues (sclerosis, cirrhosis, allergic disease). (Conversion, Red) Generation of IL-12 and other inflammatory signals promotes IL-33 signaling on inflammatory cells that are normally unresponsive, while repressing type 2-associated responses. Activation of this pathway promotes beneficial responses to infection and possibly vaccination, and may support anti-cancer immune responses in certain settings. With chronic unresolved inflammation, however, tissue IL-33 increases and ultimately contributes to tissue damage (COPD, autoimmune diseases).
Stage 1: Homeostasis
The role of IL-33 during tissue homeostasis is most informed from studies in adipose tissue, but similar mechanisms may occur in female reproductive organs and elsewhere (Figure 2A). At rest, IL-33 is maintained as a reservoir in the nuclei of certain endothelial and epithelial cells, and perhaps certain fibroblast reticular stromal cells. In response to poorly defined cues, which may be mechanical, hormonal or metabolic, active IL-33 is translocated to the extracellular space, perhaps through regulated secretion or cellular turnover associated with focal areas of cell death. The primary targets of constitutive IL-33 production are likely ILC2s and subsets of Tregs, which are positioned during development and whose activation leads to accumulation of AAMs and eosinophils to create a tissue environment that suppresses inflammation and promotes a reparative state characterized by tolerance. At these homeostatic concentrations, effects of IL-33 are restricted to local tissue by the constitutive presence of the decoy receptor, sST2, in serum. Similar pathways may be elicited during localized tissue injury (Brunner et al., 2011; Duan et al., 2012; Liang et al., 2013; Schiering et al., 2014; Turnquist et al., 2011; Yin et al., 2013), where the target of these and related activities may include resident precursor-like stromal cells (Burzyn et al., 2013; Goh et al., 2013; Heredia et al., 2013; Lee et al., 2015). While speculative at this time, the ability of IL-33, ILC2 and Tregs to regulate the tissue regenerative compartment remains an important area for further study.
Stage 2: Amplification
Migratory helminths, which are highly adapted to their hosts, elicit the second stage of IL-33 biology characterized by increased numbers and amounts of IL-33 in involved tissues (Figure 2B). This is accompanied by expansion of ST2+ immune cells, particularly ILC2s and Th2 cells which help to restrict the helminth, but also Tregs, which repress the pro-inflammatory effects of chronic infestation and together promote the healing of involved tissue. sST2 levels may increase to contain effects of the expanded IL-33 pool, which can be buffered over many years before pathologic effects of fibrosis and other tissue injuries become apparent. Over time, niches for key precursor cells may become disturbed, such that tissue architecture can no longer be sustained. Tissue tolerance and metabolic alterations necessary to sustain the massive egg-laying capacity of long-lived helminths may represent adaptations that have co-evolved with these parasites, which are widespread among vertebrates (Finlay et al., 2014; Maizels et al., 2012; McSorley and Maizels, 2012). During allergic pathology, such as asthma and atopic dermatitis, expansion of ILC2s becomes dissociated from Treg stimulation, enabling the activation of adaptive immunity and associated accumulation of Th2 cells and the development of high-affinity IgE capable of activating mast cells and basophils. Why Tregs are poorly induced in allergy to environmental allergens remains perplexing, but may reflect developmental anomalies related to changes in the early postnatal environment in westernized countries, the so-called ‘hygiene’ hypothesis. As such, further research investigating the early postnatal development, tissue positioning and differentiation of ILC2s and Tregs might be particularly informative. Indeed, perinatal Tregs may have specialized properties that endow them with functionalities related to tissue-specific tolerance (Yang et al., 2015).
Stage 3: Conversion
Inflammatory and infectious conditions associated with loss of epithelial integrity, microbial invasion at damaged barriers, and damage to precursor populations define the third stage of IL-33 (Figure 2C). Inflammatory cells such as NK cells, Th1 CD4+ T cells, and CD8+ T cells acquire ST2 and thus IL-33 sensitivity in response to IL-12 and other inflammatory mediators, such that classical immune inflammatory pathways become engaged, a process we term conversion (Villarreal and Weiner, 2014). Regulatory and type 2 responses are also actively repressed by inflammatory signals such as IFN-γ (Molofsky et al., 2015). In certain tissues (lymph nodes, spleen), a relative lack of otherwise constitutively ST2+ immune cells may establish permissive conditions for acquiring responsiveness to IL-33, which is greatly expanded during the amplification of the fibroblastic reticular cell network and (in human) HEV in the enlarging lymph node. Here, IL-33 becomes critical in mediating host defense against damaged barriers in the setting of bacterial or viral infection. In the chronic phases of inflammation, nuclear pools of IL-33 become greatly expanded in tissue, such that periodic challenges lead to massive release of active IL-33 that overwhelms local sST2 levels and systemic effects of IL-33 begin to dominate. Although such a dramatic shift from type 2-associated to type 1-associated inflammation seems unusual, IL-18, another member of the IL-1 family, can mediate similarly disparate responses (Fabbi et al., 2015; Smith, 2011; Voehringer, 2012). Expression of novel IL-1 family receptors by cells constitutes an economical mechanism to rapidly re-direct conserved signaling networks to alternative effector programs in response to life-threatening challenges.
While much of this model remains conjectural, it provides a framework for organizing the complexities of IL-33 biology while exposing areas in need of further investigation. Details of IL-33 production and access to the extracellular environment, of sST2 production and regulation, and of factors determining the expression of ST2 on distinct populations of ILC2s and Tregs remain unclear. The precise role of ST2 on myeloid cells is largely undefined, and the mechanisms by which IL-12 and potentially other signals enable the expansion of ST2 expression and IL-33 responsiveness onto inflammatory cytotoxic cells remains understudied. The next few years will be marked by further understanding of the remarkable spectrum of biologic processes affected by IL-33 and its role in homeostasis, repair, host defense and immunopathology.
Acknowledgments
We thank laboratory members for critical review of the manuscript. This work was supported by AI026918, AI030663, HL107202, and K08DK101604 (ABM) from the NIH, the UCSF Diabetes Family Fund (ABM), the Sandler Asthma Basic Research Center at UCSF and the Howard Hughes Medical Institute.
Footnotes
The authors have no conflicting financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Acton SE, Farrugia AJ, Astarita JL, Mourão-Sá D, Jenkins RP, Nye E, Hooper S, van Blijswijk J, Rogers NC, Snelgrove KJ, et al. Dendritic cells control fibroblastic reticular network tension and lymph node expansion. Nature. 2014;514:498–502. doi: 10.1038/nature13814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali S, Mohs A, Thomas M, Klare J, Ross R, Schmitz ML, Martin MU. The dual function cytokine IL-33 interacts with the transcription factor NF-κB to dampen NF-κB-stimulated gene transcription. J Immunol. 2011;187:1609–1616. doi: 10.4049/jimmunol.1003080. [DOI] [PubMed] [Google Scholar]
- Andrade MV, Iwaki S, Ropert C, Gazzinelli RT, Cunha-Melo JR, Beaven MA. Amplification of cytokine production through synergistic activation of NFAT and AP-1 following stimulation of mast cells with antigen and IL-33. Eur J Immunol. 2011;41:760–772. doi: 10.1002/eji.201040718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aupperlee MD, Zhao Y, Tan YS, Leipprandt JR, Bennett J, Haslam SZ, Schwartz RC. Epidermal growth factor receptor (EGFR) signaling is a key mediator of hormone-induced leukocyte infiltration in the pubertal female mammary gland. Endocrinology. 2014;155:2301–2313. doi: 10.1210/en.2013-1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baekkevold ES, Roussigné M, Yamanaka T, Johansen FE, Jahnsen FL, Amalric F, Brandtzaeg P, Erard M, Haraldsen G, Girard JP. Molecular characterization of NF-HEV, a nuclear factor preferentially expressed in human high endothelial venules. Am J Pathol. 2003;163:69–79. doi: 10.1016/S0002-9440(10)63631-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartelt A, Heeren J. Adipose tissue browning and metabolic health. Nat Rev Endocrinol. 2014;10:24–36. doi: 10.1038/nrendo.2013.204. [DOI] [PubMed] [Google Scholar]
- Bartelt A, Bruns OT, Reimer R, Hohenberg H, Ittrich H, Peldschus K, Kaul MG, Tromsdorf UI, Weller H, Waurisch C, et al. Brown adipose tissue activity controls triglyceride clearance. Nat Med. 2011;17:200–205. doi: 10.1038/nm.2297. [DOI] [PubMed] [Google Scholar]
- Bartemes KR, Iijima K, Kobayashi T, Kephart GM, McKenzie AN, Kita H. IL-33-responsive lineage- CD25+ CD44(hi) lymphoid cells mediate innate type 2 immunity and allergic inflammation in the lungs. J Immunol. 2012;188:1503–1513. doi: 10.4049/jimmunol.1102832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann C, Bonilla WV, Fröhlich A, Helmstetter C, Peine M, Hegazy AN, Pinschewer DD, Löhning M. T-bet– and STAT4–dependent IL-33 receptor expression directly promotes antiviral Th1 cell responses. Proc Natl Acad Sci USA. 2015;112:4056–4061. doi: 10.1073/pnas.1418549112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beamer CA, Girtsman TA, Seaver BP, Finsaas KJ, Migliaccio CT, Perry VK, Rottman JB, Smith DE, Holian A. IL-33 mediates multi-walled carbon nanotube (MWCNT)-induced airway hyper-reactivity via the mobilization of innate helper cells in the lung. Nanotoxicology. 2013;7:1070–1081. doi: 10.3109/17435390.2012.702230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergers G, Reikerstorfer A, Braselmann S, Graninger P, Busslinger M. Alternative promoter usage of the Fos-responsive gene Fit-1 generates mRNA isoforms coding for either secreted or membrane-bound proteins related to the IL-1 receptor. The EMBO Journal. 1994;13:1176. doi: 10.1002/j.1460-2075.1994.tb06367.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besnard AG, Guabiraba R, Niedbala W, Palomo J, Reverchon F, Shaw TN, Couper KN, Ryffel B, Liew FY. IL-33-mediated protection against experimental cerebral malaria is linked to induction of type 2 innate lymphoid cells, M2 macrophages and regulatory T cells. PLoS Pathog. 2015;11:e1004607. doi: 10.1371/journal.ppat.1004607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bessa J, Meyer CA, de Vera Mudry MC, Schlicht S, Smith SH, Iglesias A, Cote-Sierra J. Altered subcellular localization of IL-33 leads to non-resolving lethal inflammation. J Autoimmun. 2014:1–9. doi: 10.1016/j.jaut.2014.02.012. [DOI] [PubMed] [Google Scholar]
- Bie Q, Zhang P, Su Z, Zheng D, Ying X, Wu Y, Yang H, Chen D, Wang S, Xu H. Polarization of ILC2s in peripheral blood might contribute to immunosuppressive microenvironment in patients with gastric cancer. J Immunol Res. 2014;2014:923135. doi: 10.1155/2014/923135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonilla WV, Fröhlich A, Senn K, Kallert S, Fernandez M, Johnson S, Kreutzfeldt M, Hegazy AN, Schrick C, Fallon PG, et al. The alarmin interleukin-33 drives protective antiviral CD8+ T cell responses. Science. 2012;335:984–989. doi: 10.1126/science.1215418. [DOI] [PubMed] [Google Scholar]
- Bourgeois E, Van LP, Samson M, Diem S, Barra A, Roga S, Gombert JM, Schneider E, Dy M, Gourdy P, et al. The pro-Th2 cytokine IL-33 directly interacts with invariant NKT and NK cells to induce IFN-gamma production. Eur J Immunol. 2009;39:1046–1055. doi: 10.1002/eji.200838575. [DOI] [PubMed] [Google Scholar]
- Brestoff JR, Artis D. Immune regulation of metabolic homeostasis in health and disease. Cell. 2015;161:146–160. doi: 10.1016/j.cell.2015.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brestoff JR, Kim BS, Saenz SA, Stine RR, Monticelli LA, Sonnenberg GF, Thome JJ, Farber DL, Lutfy K, Seale P, et al. Group 2 innate lymphoid cells promote beiging of white adipose tissue and limit obesity. Nature. 2014:1–17. doi: 10.1038/nature14115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunner SM, Schiechl G, Falk W, Schlitt HJ, Geissler EK, Fichtner-Feigl S. Interleukin-33 prolongs allograft survival during chronic cardiac rejection. Transpl Int. 2011;24:1027–1039. doi: 10.1111/j.1432-2277.2011.01306.x. [DOI] [PubMed] [Google Scholar]
- Bulek K, Swaidani S, Aronica M, Li X. Epithelium: the interplay between innate and Th2 immunity. Immunol Cell Biol. 2010;88:257–268. doi: 10.1038/icb.2009.113. [DOI] [PubMed] [Google Scholar]
- Bulek K, Swaidani S, Qin J, Lu Y, Gulen MF, Herjan T, Min B, Kastelein RA, Aronica M, Kosz-Vnenchak M, et al. The essential role of single Ig IL-1 receptor-related molecule/Toll IL-1R8 in regulation of Th2 immune response. J Immunol. 2009;182:2601–2609. doi: 10.4049/jimmunol.0802729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burzyn D, Kuswanto W, Kolodin D, Shadrach JL, Cerletti M, Jang Y, Sefik E, Tan TG, Wagers AJ, Benoist C, et al. A special population of regulatory T cells potentiates muscle repair. Cell. 2013;155:1282–1295. doi: 10.1016/j.cell.2013.10.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byers DE, Alexander-Brett J, Patel AC, Agapov E, Dang-Vu G, Jin X, Wu K, You Y, Alevy Y, Girard JP, et al. Long-term IL-33-producing epithelial progenitor cells in chronic obstructive lung disease. J Clin Invest. 2013;123:3967–3982. doi: 10.1172/JCI65570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlock CI, Wu J, Zhou C, Tatum K, Adams HP, Tan F, Lou Y. Unique temporal and spatial expression patterns of IL-33 in ovaries during ovulation and estrous cycle are associated with ovarian tissue homeostasis. J Immunol. 2014;193:161–169. doi: 10.4049/jimmunol.1400381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carriere V, Roussel L, Ortega N, Lacorre DA, Americh L, Aguilar L, Bouche G, Girard JP. IL-33, the IL-1-like cytokine ligand for ST2 receptor, is a chromatin-associated nuclear factor in vivo. Proc Natl Acad Sci USA. 2007;104:282–287. doi: 10.1073/pnas.0606854104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cayrol C, Girard JP. The IL-1-like cytokine IL-33 is inactivated after maturation by caspase-1. Proc Natl Acad Sci USA. 2009;106:9021–9026. doi: 10.1073/pnas.0812690106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cayrol C, Girard JP. IL-33: an alarmin cytokine with crucial roles in innate immunity, inflammation and allergy. Curr Opin Immunol. 2014;31:31–37. doi: 10.1016/j.coi.2014.09.004. [DOI] [PubMed] [Google Scholar]
- Cheng LE, Locksley RM. Allergic inflammation--innately homeostatic. Cold Spring Harb Perspect Biol. 2015;7:1–13. doi: 10.1101/cshperspect.a016352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chondronikola M, Volpi E, Børsheim E, Porter C, Annamalai P, Enerbäck S, Lidell ME, Saraf MK, Labbe SM, Hurren NM, et al. Brown adipose tissue improves whole-body glucose homeostasis and insulin sensitivity in humans. Diabetes. 2014;63:4089–4099. doi: 10.2337/db14-0746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu DK, Llop-Guevara A, Walker TD, Flader K, Goncharova S, Boudreau JE, Moore CL, Seunghyun In T, Waserman S, Coyle AJ, et al. IL-33, but not thymic stromal lymphopoietin or IL-25, is central to mite and peanut allergic sensitization. J Allergy Clin Immunol. 2013;131:187–200. e1–8. doi: 10.1016/j.jaci.2012.08.002. [DOI] [PubMed] [Google Scholar]
- Clarke LE, Barres BA. Emerging roles of astrocytes in neural circuit development. Nat Rev Neurosci. 2013;14:311–321. doi: 10.1038/nrn3484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colbert DC, McGarry MP, O’neill K, Lee NA, Lee JJ. Decreased size and survival of weanling mice in litters of IL-5−/− mice are a consequence of the IL-5 deficiency in nursing dams. Contemp Top Lab Anim Sci. 2005;44:53–55. [PubMed] [Google Scholar]
- Coyle AJ, Lloyd C, Tian J, Nguyen T, Erikkson C, Wang L, Ottoson P, Persson P, Delaney T, Lehar S, et al. Crucial role of the interleukin 1 receptor family member T1/ST2 in T helper cell type 2-mediated lung mucosal immune responses. J Exp Med. 1999;190:895–902. doi: 10.1084/jem.190.7.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty TA, Khorram N, Chang JE, Kim HK, Rosenthal P, Croft M, Broide DH. STAT6 regulates natural helper cell proliferation during lung inflammation initiated by Alternaria. Am J Physiol Lung Cell Mol Physiol. 2012;303:L577–L588. doi: 10.1152/ajplung.00174.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan L, Chen J, Zhang H, Yang H, Zhu P, Xiong A, Xia Q, Zheng F, Tan Z, Gong F, et al. Interleukin-33 ameliorates experimental colitis through promoting Th2/Foxp3+ regulatory T-cell responses in mice. Mol Med. 2012;18:753–761. doi: 10.2119/molmed.2011.00428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo Y, Hirahara K, Iinuma T, Shinoda K, Tumes DJ, Asou HK, Matsugae N, Obata-Ninomiya K, Yamamoto H, Motohashi S, et al. The interleukin-33-p38 kinase axis confers memory T helper 2 cell pathogenicity in the airway. Immunity. 2015;42:294–308. doi: 10.1016/j.immuni.2015.01.016. [DOI] [PubMed] [Google Scholar]
- Fabbi M, Carbotti G, Ferrini S. Context-dependent role of IL-18 in cancer biology and counter-regulation by IL-18BP. J Leukoc Biol. 2015;97:665–675. doi: 10.1189/jlb.5RU0714-360RR. [DOI] [PubMed] [Google Scholar]
- Fahy JV. Type 2 inflammation in asthma — present in most, absent in many. Nat Rev Immunol. 2015;15:57–65. doi: 10.1038/nri3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finlay CM, Walsh KP, Mills KHG. Induction of regulatory cells by helminth parasites: exploitation for the treatment of inflammatory diseases. Immunol Rev. 2014;259:206–230. doi: 10.1111/imr.12164. [DOI] [PubMed] [Google Scholar]
- Fock V, Mairhofer M, Otti GR, Hiden U, Spittler A, Zeisler H, Fiala C, Knöfler M, Pollheimer J. Macrophage-derived IL-33 is a critical factor for placental growth. J Immunol. 2013;191:3734–3743. doi: 10.4049/jimmunol.1300490. [DOI] [PubMed] [Google Scholar]
- Frontini A, Cinti S. Distribution and development of brown adipocytes in the murine and human adipose organ. Cell Metab. 2010;11:253–256. doi: 10.1016/j.cmet.2010.03.004. [DOI] [PubMed] [Google Scholar]
- Funakoshi-Tago M, Tago K, Sato Y, Tominaga SI, Kasahara T. JAK2 is an important signal transducer in IL-33-induced NF-κB activation. Cellular Signalling. 2011;23:363–370. doi: 10.1016/j.cellsig.2010.10.006. [DOI] [PubMed] [Google Scholar]
- Gao X, Wang X, Yang Q, Zhao X, Wen W, Li G, Lu J, Qin W, Qi Y, Xie F, et al. Tumoral expression of IL-33 inhibits tumor growth and modifies the tumor microenvironment through CD8+ T and NK cells. J Immunol. 2015;194:438–445. doi: 10.4049/jimmunol.1401344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garlanda C, Dinarello CA, Mantovani A. The interleukin-1 family: back to the future. Immunity. 2013a;39:1003–1018. doi: 10.1016/j.immuni.2013.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garlanda C, Riva F, Bonavita E, Mantovani A. Negative regulatory receptors of the IL-1 family. Semin Immunol. 2013b;25:408–415. doi: 10.1016/j.smim.2013.10.019. [DOI] [PubMed] [Google Scholar]
- Gause WC, Wynn TA, Allen JE. Type 2 immunity and wound healing: evolutionary refinement of adaptive immunity by helminths. Nat Rev Immunol. 2013;13:607–614. doi: 10.1038/nri3476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gidlöf O, Smith JG, Miyazu K, Gilje P, Spencer A, Blomquist S, Erlinge D. Circulating cardio-enriched microRNAs are associated with long-term prognosis following myocardial infarction. BMC Cardiovasc Disord. 2013;13:12. doi: 10.1186/1471-2261-13-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gjorevski N, Nelson CM. Integrated morphodynamic signalling of the mammary gland. Nature Reviews Molecular Cell Biology. 2011;12:581–593. doi: 10.1038/nrm3168. [DOI] [PubMed] [Google Scholar]
- Goh YPS, Henderson NC, Heredia JE, Red Eagle A, Odegaard JI, Lehwald N, Nguyen KD, Sheppard D, Mukundan L, Locksley RM, et al. Eosinophils secrete IL-4 to facilitate liver regeneration. Proc Natl Acad Sci USA. 2013;110:9914–9919. doi: 10.1073/pnas.1304046110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gudbjartsson DF, Bjornsdottir US, Halapi E, Helgadottir A, Sulem P, Jonsdottir GM, Thorleifsson G, Helgadottir H, Steinthorsdottir V, Stefansson H, et al. Sequence variants affecting eosinophil numbers associate with asthma and myocardial infarction. Nat Genet. 2009;41:342–347. doi: 10.1038/ng.323. [DOI] [PubMed] [Google Scholar]
- Guo L, Wei G, Zhu J, Liao W, Leonard WJ, Zhao K, Paul W. IL-1 family members and STAT activators induce cytokine production by Th2, Th17, and Th1 cells. Proc Natl Acad Sci USA. 2009;106:13463–13468. doi: 10.1073/pnas.0906988106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haenuki Y, Matsushita K, Futatsugi-Yumikura S, Ishii KJ, Kawagoe T, Imoto Y, Fujieda S, Yasuda M, Hisa Y, Akira S, et al. A critical role of IL-33 in experimental allergic rhinitis. J Allergy Clin Immunol. 2012;130:184–94. e11. doi: 10.1016/j.jaci.2012.02.013. [DOI] [PubMed] [Google Scholar]
- Halim TYF, Krauß RH, Sun AC, Takei F. Lung natural helper cells are a critical source of th2 cell-type cytokines in protease allergen-induced airway inflammation. Immunity. 2012;36:451–463. doi: 10.1016/j.immuni.2011.12.020. [DOI] [PubMed] [Google Scholar]
- Halim TYF, Steer CA, Mathä L, Gold MJ, Martinez-Gonzalez I, McNagny KM, McKenzie ANJ, Takei F. Group 2 innate lymphoid cells are critical for the initiation of adaptive T helper 2 cell-mediated allergic lung inflammation. Immunity. 2014;40:425–435. doi: 10.1016/j.immuni.2014.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hams E, Locksley RM, McKenzie ANJ, Fallon PG. Cutting edge: IL-25 elicits innate lymphoid type 2 and type II NKT cells that regulate obesity in mice. J Immunol. 2013;191:5349–5353. doi: 10.4049/jimmunol.1301176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardman CS, Panova V, McKenzie ANJ. IL-33 citrine reporter mice reveal the temporal and spatial expression of IL-33 during allergic lung inflammation. Eur J Immunol. 2013;43:488–498. doi: 10.1002/eji.201242863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harms M, Seale P. Brown and beige fat: development, function and therapeutic potential. Nat Med. 2013;19:1252–1263. doi: 10.1038/nm.3361. [DOI] [PubMed] [Google Scholar]
- Harris HE, Andersson U, Pisetsky DS. HMGB1: a multifunctional alarmin driving autoimmune and inflammatory disease. Nat Rev Rheumatol. 2012;8:195–202. doi: 10.1038/nrrheum.2011.222. [DOI] [PubMed] [Google Scholar]
- Hayakawa H, Hayakawa M, Kume A, Tominaga SI. Soluble ST2 blocks interleukin-33 signaling in allergic airway inflammation. J Biol Chem. 2007;282:26369–26380. doi: 10.1074/jbc.M704916200. [DOI] [PubMed] [Google Scholar]
- Heredia JE, Mukundan L, Chen FM, Mueller AA, Deo RC, Locksley RM, Rando TA, Chawla A. Type 2 innate signals stimulate fibro/adipogenic progenitors to facilitate muscle regeneration. Cell. 2013;153:376–388. doi: 10.1016/j.cell.2013.02.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho JE, Chen WY, Chen MH, Larson MG, McCabe EL, Cheng S, Ghorbani A, Coglianese E, Emilsson V, Johnson AD, et al. Common genetic variation at the IL1RL1 locus regulates IL-33/ST2 signaling. J Clin Invest. 2013;123:4208–4218. doi: 10.1172/JCI67119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong J, Bae S, Jhun H, Lee S, Choi J, Kang T, Kwak A, Hong K, Kim E, Jo S, et al. Identification of constitutively active interleukin 33 (IL-33) splice variant. J Biol Chem. 2011;286:20078–20086. doi: 10.1074/jbc.M111.219089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshino K, Kashiwamura S, Kuribayashi K, Kodama T, Tsujimura T, Nakanishi K, Matsuyama T, Takeda K, Akira S. The absence of interleukin 1 receptor-related T1/ST2 does not affect T helper cell type 2 development and its effector function. J Exp Med. 1999;190:1541–1548. doi: 10.1084/jem.190.10.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu B, Song JT, Qu HY, Bi CL, Huang XZ, Liu XX, Zhang M. Mechanical stretch suppresses microRNA-145 expression by activating extracellular signal-regulated kinase 1/2 and upregulating angiotensin-converting enzyme to alter vascular smooth muscle cell phenotype. PLoS ONE. 2014;9:e96338. doi: 10.1371/journal.pone.0096338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson CA, Christophi GP, Gruber RC, Wilmore JR, Lawrence DA, Massa PT. Induction of IL-33 expression and activity in central nervous system glia. Journal of Leukocyte Biology. 2008;84:631–643. doi: 10.1189/jlb.1207830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphreys NE, Xu D, Hepworth MR, Liew FY, Grencis RK. IL-33, a potent inducer of adaptive immunity to intestinal nematodes. J Immunol. 2008;180:2443–2449. doi: 10.4049/jimmunol.180.4.2443. [DOI] [PubMed] [Google Scholar]
- Hung LY, Lewkowich IP, Dawson LA, Downey J, Yang Y, Smith DE, Herbert DR. IL-33 drives biphasic IL-13 production for noncanonical Type 2 immunity against hookworms. Proc Natl Acad Sci USA. 2013;110:282–287. doi: 10.1073/pnas.1206587110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iijima K, Kobayashi T, Hara K, Kephart GM, Ziegler SF, McKenzie AN, Kita H. IL-33 and thymic stromal lymphopoietin mediate immune pathology in response to chronic airborne allergen exposure. J Immunol. 2014;193:1549–1559. doi: 10.4049/jimmunol.1302984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai Y, Yasuda K, Sakaguchi Y, Haneda T, Mizutani H, Yoshimoto T, Nakanishi K, Yamanishi K. Skin-specific expression of IL-33 activates group 2 innate lymphoid cells and elicits atopic dermatitis-like inflammation in mice. Proc Natl Acad Sci USA. 2013;110:13921–13926. doi: 10.1073/pnas.1307321110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Y, Yang CJ, Xu X, Cao JN, Feng QT, Yang J. MiR-214 regulates the pathogenesis of patients with coronary artery disease by targeting VEGF. Mol Cell Biochem. 2015;402:111–122. doi: 10.1007/s11010-014-2319-5. [DOI] [PubMed] [Google Scholar]
- Jovanovic IP, Pejnovic NN, Radosavljevic GD, Pantic JM, Milovanovic MZ, Arsenijevic NN, Lukic ML. Interleukin-33/ST2 axis promotes breast cancer growth and metastases by facilitating intratumoral accumulation of immunosuppressive and innate lymphoid cells. Int J Cancer. 2013;134:1669–1682. doi: 10.1002/ijc.28481. [DOI] [PubMed] [Google Scholar]
- Kaczmarek A, Vandenabeele P, Krysko DV. Necroptosis: the release of damage-associated molecular patterns and its physiological relevance. Immunity. 2013;38:209–223. doi: 10.1016/j.immuni.2013.02.003. [DOI] [PubMed] [Google Scholar]
- Kakkar R, Hei H, Dobner S, Lee RT. Interleukin 33 as a mechanically responsive cytokine secreted by living cells. J Biol Chem. 2012;287:6941–6948. doi: 10.1074/jbc.M111.298703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato A. Immunopathology of chronic rhinosinusitis. Allergol Int. 2015;64:121–130. doi: 10.1016/j.alit.2014.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kearley J, Silver JS, Sanden C, Liu Z, Berlin AA, White N, Mori M, Pham TH, Ward CK, Criner GJ, et al. Cigarette smoke silences innate lymphoid cell function and facilitates an exacerbated type I interleukin-33-dependent response to infection. Immunity. 2015;42:566–579. doi: 10.1016/j.immuni.2015.02.011. [DOI] [PubMed] [Google Scholar]
- Khaled WT, Read EKC, Nicholson SE, Baxter FO, Brennan AJ, Came PJ, Sprigg N, McKenzie ANJ, Watson CJ. The IL-4/IL-13/Stat6 signalling pathway promotes luminal mammary epithelial cell development. Development. 2007;134:2739–2750. doi: 10.1242/dev.003194. [DOI] [PubMed] [Google Scholar]
- Kim BS, Artis D. Group 2 innate lymphoid cells in health and disease. Cold Spring Harb Perspect Biol. 2015;7:a016337. doi: 10.1101/cshperspect.a016337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim BS, Siracusa MC, Saenz SA, Noti M, Monticelli LA, Sonnenberg GF, Hepworth MR, Van Voorhees AS, Comeau MR, Artis D. TSLP elicits IL-33-independent innate lymphoid cell responses to promote skin inflammation. Science Transl Med. 2013;5:170ra16–170ra16. doi: 10.1126/scitranslmed.3005374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JY, Lim S-C, Kim G, Yun HJ, Ahn S-G, Choi HS. Interleukin-33/ST2 axis promotes epithelial cell transformation and breast tumorigenesis via upregulation of COT activity. Oncogene. 2014:1–11. doi: 10.1038/onc.2014.418. [DOI] [PubMed] [Google Scholar]
- Kolodin D, van Panhuys N, Li C, Magnuson AM, Cipolletta D, Miller CM, Wagers A, Germain RN, Benoist C, Mathis D. Antigen- and cytokine-driven accumulation of regulatory T cells in visceral adipose tissue of lean mice. Cell Metab. 2015;21:543–557. doi: 10.1016/j.cmet.2015.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo Y, Yoshimoto T, Yasuda K, Futatsugi-Yumikura S, Morimoto M, Hayashi N, Hoshino T, Fujimoto J, Nakanishi K. Administration of IL-33 induces airway hyperresponsiveness and goblet cell hyperplasia in the lungs in the absence of adaptive immune system. Int Immunol. 2008;20:791–800. doi: 10.1093/intimm/dxn037. [DOI] [PubMed] [Google Scholar]
- Kouzaki H, Iijima K, Kobayashi T, O’Grady SM, Kita H. The danger signal, extracellular ATP, is a sensor for an airborne allergen and triggers IL-33 release and innate Th2-type responses. J Immunol. 2011;186:4375–4387. doi: 10.4049/jimmunol.1003020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Tzimas MN, Griswold DE, Young PR. Expression of ST2, an interleukin-1 receptor homologue, is induced by proinflammatory stimuli. Biochem Biophys Res Commun. 1997;235:474–478. doi: 10.1006/bbrc.1997.6810. [DOI] [PubMed] [Google Scholar]
- Kuroiwa K, Li H, Tago K, Iwahana H, Yanagisawa K, Komatsu N, Oshikawa K, Sugiyama Y, Arai T, Tominaga SI. Construction of ELISA system to quantify human ST2 protein in sera of patients. Hybridoma. 2000;19:151–159. doi: 10.1089/02724570050031194. [DOI] [PubMed] [Google Scholar]
- Kurowska-Stolarska M, Kewin P, Murphy G, Russo RC, Stolarski B, Garcia CC, Komai-Koma M, Pitman N, Li Y, Niedbala W, et al. IL-33 induces antigen-specific IL-5+ T cells and promotes allergic-induced airway inflammation independent of IL-4. J Immunol. 2008;181:4780–4790. doi: 10.4049/jimmunol.181.7.4780. [DOI] [PubMed] [Google Scholar]
- Küchler AM, Pollheimer J, Balogh J, Sponheim J, Manley L, Sorensen DR, De Angelis PM, Scott H, Haraldsen G. Nuclear interleukin-33 is generally expressed in resting endothelium but rapidly lost upon angiogenic or proinflammatory activation. Am J Pathol. 2008;173:1229–1242. doi: 10.2353/ajpath.2008.080014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanahan A, Williams JB, Sanders LK, Nathans D. Growth factor-induced delayed early response genes. Mol Cell Biol. 1992;12:3919–3929. doi: 10.1128/mcb.12.9.3919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Layland LE, Mages J, Loddenkemper C, Hoerauf A, Wagner H, Lang R, da Costa CUP. Pronounced phenotype in activated regulatory T cells during a chronic helminth infection. J Immunol. 2010;184:713–724. doi: 10.4049/jimmunol.0901435. [DOI] [PubMed] [Google Scholar]
- Lee MW, Odegaard JI, Mukundan L, Qiu Y, Molofsky AB, Nussbaum JC, Yun K, Locksley RM, Chawla A. Activated type 2 innate lymphoid cells regulate beige fat biogenesis. Cell. 2015;160:74–87. doi: 10.1016/j.cell.2014.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefrancais E, Cayrol C. Mechanisms of IL-33 processing and secretion: differences and similarities between IL-1 family members. Eur Cytokine Netw. 2012;23:120–127. doi: 10.1684/ecn.2012.0320. [DOI] [PubMed] [Google Scholar]
- Lefrancais E, Duval A, Mirey E, Roga S, Espinosa E, Cayrol C, Girard JP. Central domain of IL-33 is cleaved by mast cell proteases for potent activation of group-2 innate lymphoid cells. Proc Natl Acad Sci USA. 2014;111:15502–15507. doi: 10.1073/pnas.1410700111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefrancais E, Roga S, Gautier V, Gonzalez-de-Peredo A, Monsarrat B, Girard JP, Cayrol C. IL-33 is processed into mature bioactive forms by neutrophil elastase and cathepsin G. Proc Natl Acad Sci USA. 2012;109:1673–1678. doi: 10.1073/pnas.1115884109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levescot A, Flamant S, Basbous S, Jacomet F, Féraud O, Anne Bourgeois E, Bonnet ML, Giraud C, Roy L, Barra A, et al. BCR-ABL-induced deregulation of the IL-33/ST2 pathway in CD34+ progenitors from chronic myeloid leukemia patients. Cancer Research. 2014;74:2669–2676. doi: 10.1158/0008-5472.CAN-13-2797. [DOI] [PubMed] [Google Scholar]
- Liang Y, Jie Z, Hou L, Aguilar-Valenzuela R, Vu D, Soong L, Sun J. IL-33 induces nuocytes and modulates liver injury in viral hepatitis. J Immunol. 2013;190:5666–5675. doi: 10.4049/jimmunol.1300117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lingel A, Weiss TM, Niebuhr M, Pan B, Appleton BA, Wiesmann C, Bazan JF, Fairbrother WJ. Structure of IL-33 and its interaction with the ST2 and IL-1RAcP receptors--insight into heterotrimeric IL-1 signaling complexes. Structure. 2009;17:1398–1410. doi: 10.1016/j.str.2009.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Hammel M, He Y, Tainer JA, Jeng US, Zhang L, Wang S, Wang X. Structural insights into the interaction of IL-33 with its receptors. Proc Natl Acad Sci USA. 2013;110:14918–14923. doi: 10.1073/pnas.1308651110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Löhning M, Stroehmann A, Coyle AJ, Grogan JL, Lin S, Gutierrez-Ramos JC, Levinson D, Radbruch A, Kamradt T. T1/ST2 is preferentially expressed on murine Th2 cells, independent of interleukin 4, interleukin 5, and interleukin 10, and important for Th2 effector function. Proc Natl Acad Sci USA. 1998;95:6930–6935. doi: 10.1073/pnas.95.12.6930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunderius-Andersson C, Enoksson M, Nilsson G. Mast Cells respond to cell injury through the recognition of IL-33. Front Immunol. 2012;3:82. doi: 10.3389/fimmu.2012.00082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Y, Zhou Y, Xiao W, Liang Z, Dai J, Weng X, Wu X. Interleukin-33 ameliorates ischemic brain injury in experimental stroke through promoting Th2 response and suppressing Th17 response. Brain Res. 2015;1597:86–94. doi: 10.1016/j.brainres.2014.12.005. [DOI] [PubMed] [Google Scholar]
- Luthi AU, Cullen SP, McNeela EA, Duriez PJ, Afonina IS, Sheridan C, Brumatti G, Taylor RC, Kersse K, Vandenabeele P, et al. Suppression of interleukin-33 bioactivity through proteolysis by apoptotic caspases. Immunity. 2009;31:84–98. doi: 10.1016/j.immuni.2009.05.007. [DOI] [PubMed] [Google Scholar]
- Maizels RM, Hewitson JP, Smith KA. Susceptibility and immunity to helminth parasites. Curr Opin Immunol. 2012;24:459–466. doi: 10.1016/j.coi.2012.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Gonzalez I, Steer CA, Takei F. Lung ILC2s link innate and adaptive responses in allergic inflammation. Trends Immunol. 2015;36:189–195. doi: 10.1016/j.it.2015.01.005. [DOI] [PubMed] [Google Scholar]
- Marvie P, Lisbonne M, L’helgoualc’h A, Rauch M, Turlin B, Preisser L, Bourd-Boittin K, Théret N, Gascan H, Piquet-Pellorce C, et al. Interleukin-33 overexpression is associated with liver fibrosis in mice and humans. J Cell Mol Med. 2010;14:1726–1739. doi: 10.1111/j.1582-4934.2009.00801.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathis D. Immunological goings-on in visceral adipose tissue. Cell Metab. 2013;17:851–859. doi: 10.1016/j.cmet.2013.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matta BM, Lott JM, Mathews LR, Liu Q, Rosborough BR, Blazar BR, Turnquist HR. IL-33 is an unconventional alarmin that stimulates IL-2 secretion by dendritic cells to selectively expand IL-33R/ST2+ regulatory T cells. J Immunol. 2014;193:4010–4020. doi: 10.4049/jimmunol.1400481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mchedlidze T, Waldner M, Zopf S, Walker J, Rankin AL, Schuchmann M, Voehringer D, McKenzie ANJ, Neurath MF, Pflanz S, et al. Interleukin-33-dependent innate lymphoid cells mediate hepatic fibrosis. Immunity. 2013;39:357–371. doi: 10.1016/j.immuni.2013.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McSorley HJ, Blair NF, Smith KA, McKenzie ANJ, Maizels RM. Blockade of IL-33 release and suppression of type 2 innate lymphoid cell responses by helminth secreted products in airway allergy. Mucosal Immunol. 2014;7:1068–1078. doi: 10.1038/mi.2013.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McSorley HJ, Maizels RM. Helminth infections and host immune regulation. Clin Microbiol Rev. 2012;25:585–608. doi: 10.1128/CMR.05040-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AM, Asquith DL, Hueber AJ, Anderson LA, Holmes WM, McKenzie AN, Xu D, Sattar N, McInnes IB, Liew FY. Interleukin-33 induces protective effects in adipose tissue inflammation during obesity in mice. Circ Res. 2010;107:650–658. doi: 10.1161/CIRCRESAHA.110.218867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AM, Xu D, Asquith DL, Denby L, Li Y, Sattar N, Baker AH, McInnes IB, Liew FY. IL-33 reduces the development of atherosclerosis. J Exp Med. 2008;205:339–346. doi: 10.1084/jem.20071868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirchandani AS, Besnard AG, Yip E, Scott C, Bain CC, Cerovic V, Salmond RJ, Liew FY. Type 2 innate lymphoid cells drive CD4+ Th2 cell responses. J Immunol. 2014;192:2442–2448. doi: 10.4049/jimmunol.1300974. [DOI] [PubMed] [Google Scholar]
- Mjösberg JM, Trifari S, Crellin NK, Peters CP, van Drunen CM, Piet B, Fokkens WJ, Cupedo T, Spits H. Human IL-25- and IL-33-responsive type 2 innate lymphoid cells are defined by expression of CRTH2 and CD161. Nat Immunol. 2011;12:1055–1062. doi: 10.1038/ni.2104. [DOI] [PubMed] [Google Scholar]
- Moffatt MF, Gut IG, Demenais F, Strachan DP, Bouzigon E, Heath S, von Mutius E, Farrall M, Lathrop M, Cookson WOCM, et al. A large-scale, consortium-based genomewide association study of asthma. N Engl J Med. 2010;363:1211–1221. doi: 10.1056/NEJMoa0906312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohapatra A, Van Dyken SJ, Schneider C, Nussbaum JC, Liang H-E, Locksley RM. Group 2 innate lymphoid cells utilize the IRF4-IL-9 module to coordinate epithelial cell maintenance of lung homeostasis. Mucosal Immunol. 2015 doi: 10.1038/mi.2015.59. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molofsky AV, Glasgow SM, Chaboub LS, Tsai HH, Murnen AT, Kelley KW, Fancy SPJ, Yuen TJ, Madireddy L, Baranzini S, et al. Expression profiling of Aldh1l1- precursors in the developing spinal cord reveals glial lineage- specific genes and direct Sox9- Nfe2l1 interactions. Glia. 2013a;61:1518–1532. doi: 10.1002/glia.22538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molofsky AB, Nussbaum JC, Liang HE, Van Dyken SJ, Cheng LE, Mohapatra A, Chawla A, Locksley RM. Innate lymphoid type 2 cells sustain visceral adipose tissue eosinophils and alternatively activated macrophages. J Exp Med. 2013b;210:535–549. doi: 10.1084/jem.20121964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molofsky AB, Van Gool F, Liang H-E, Van Dyken SJ, Nussbaum JC, Lee J, Bluestone JA, Locksley RM. Interleukin-33 and IFN-γ counter-regulate Group 2 innate lymphoid cell activation during immune perturbation. Immunity. 2015 doi: 10.1016/j.immuni.2015.05.019. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monticelli LA, Sonnenberg GF, Abt MC, Alenghat T, Ziegler CGK, Doering TA, Angelosanto JM, Laidlaw BJ, Yang CY, Sathaliyawala T, et al. Innate lymphoid cells promote lung-tissue homeostasis after infection with influenza virus. Nat Immunol. 2011;12:1045–1054. doi: 10.1031/ni.2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moro K, Yamada T, Tanabe M, Takeuchi T, Ikawa T, Kawamoto H, Furusawa JI, Ohtani M, Fujii H, Koyasu S. Innate production of T(H)2 cytokines by adipose tissue-associated c-Kit(+)Sca-1(+) lymphoid cells. Nature. 2010;463:540–544. doi: 10.1038/nature08636. [DOI] [PubMed] [Google Scholar]
- Moussion C, Ortega N, Girard JP. The IL-1-like cytokine IL-33 is constitutively expressed in the nucleus of endothelial cells and epithelial cells in vivo: a novel ‘alarmin’? PLoS ONE. 2008;3:e3331. doi: 10.1371/journal.pone.0003331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muto T, Fukuoka A, Kabashima K, Ziegler SF, Nakanishi K, Matsushita K, Yoshimoto T. The role of basophils and proallergic cytokines, TSLP and IL-33, in cutaneously sensitized food allergy. Int Immunol. 2014;26:539–549. doi: 10.1093/intimm/dxu058. [DOI] [PubMed] [Google Scholar]
- Nabekura T, Girard J-P, Lanier LL. IL-33 Receptor ST2 Amplifies the Expansion of NK Cells and Enhances Host Defense during Mouse Cytomegalovirus Infection. J Immunol. 2015 doi: 10.4049/jimmunol.1500424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakanishi W, Yamaguchi S, Matsuda A, Suzukawa M, Shibui A, Nambu A, Kondo K, Suto H, Saito H, Matsumoto K, et al. IL-33, but not IL-25, is crucial for the development of house dust mite antigen-induced allergic rhinitis. PLoS ONE. 2013;8:e78099. doi: 10.1371/journal.pone.0078099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neill DR, Wong SH, Bellosi A, Flynn RJ, Daly M, Langford TKA, Bucks C, Kane CM, Fallon PG, Pannell R, et al. Nuocytes represent a new innate effector leukocyte that mediates type-2 immunity. Nature. 2010;464:1367–1370. doi: 10.1038/nature08900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nussbaum JC, Van Dyken SJ, von Moltke J, Cheng LE, Mohapatra A, Molofsky AB, Thornton EE, Krummel MF, Chawla A, Liang HE, et al. Type 2 innate lymphoid cells control eosinophil homeostasis. Nature. 2013;502:245–248. doi: 10.1038/nature12526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oboki K, Ohno T, Kajiwara N, Arae K, Morita H, Ishii A, Nambu A, Abe T, Kiyonari H, Matsumoto K, et al. IL-33 is a crucial amplifier of innate rather than acquired immunity. Proc Natl Acad Sci USA. 2010;107:18581–18586. doi: 10.1073/pnas.1003059107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliphant CJ, Hwang YY, Walker JA, Salimi M, Wong SH, Brewer JM, Englezakis A, Barlow JL, Hams E, Scanlon ST, et al. MHCII-mediated dialog between group 2 innate lymphoid cells and CD4(+) T cells potentiates type 2 immunity and promotes parasitic helminth expulsion. Immunity. 2014;41:283–295. doi: 10.1016/j.immuni.2014.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onda H, Kasuya H, Takakura K, Hori T, Imaizumi T, Takeuchi T, Inoue I, Takeda J. Identification of genes differentially expressed in canine vasospastic cerebral arteries after subarachnoid hemorrhage. J Cereb Blood Flow Metab. 1999;19:1279–1288. doi: 10.1097/00004647-199911000-00013. [DOI] [PubMed] [Google Scholar]
- Oshikawa K, Kuroiwa K, Tago K, Iwahana H, Yanagisawa K, Ohno S, Tominaga SI, Sugiyama Y. Elevated soluble ST2 protein levels in sera of patients with asthma with an acute exacerbation. Am J Respir Crit Care Med. 2001;164:277–281. doi: 10.1164/ajrccm.164.2.2008120. [DOI] [PubMed] [Google Scholar]
- Oshikawa K, Yanagisawa K, Tominaga S, Sugiyama Y. Expression and function of the ST2 gene in a murine model of allergic airway inflammation. Clin Exp Allergy. 2002;32:1520–1526. doi: 10.1046/j.1365-2745.2002.01494.x. [DOI] [PubMed] [Google Scholar]
- Palmer G, Gabay C. Interleukin-33 biology with potential insights into human diseases. Nat Rev Rheumatol. 2011;7:321–329. doi: 10.1038/nrrheum.2011.53. [DOI] [PubMed] [Google Scholar]
- Pastorelli L, Garg RR, Hoang SB, Spina L, Mattioli B, Scarpa M, Fiocchi C, Vecchi M, Pizarro TT. Epithelial-derived IL-33 and its receptor ST2 are dysregulated in ulcerative colitis and in experimental Th1/Th2 driven enteritis. Proc Natl Acad Sci USA. 2010;107:8017–8022. doi: 10.1073/pnas.0912678107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pichery M, Mirey E, Mercier P, Lefrancais E, Dujardin A, Ortega N, Girard JP. Endogenous IL-33 is highly expressed in mouse epithelial barrier tissues, lymphoid organs, brain, embryos, and inflamed tissues: in situ analysis using a novel Il-33-LacZ gene trap reporter strain. J Immunol. 2012;188:3488–3495. doi: 10.4049/jimmunol.1101977. [DOI] [PubMed] [Google Scholar]
- Polumuri SK, Jayakar GG, Shirey KA, Roberts ZJ, Perkins DJ, Pitha PM, Vogel SN. Transcriptional regulation of murine IL-33 by TLR and non-TLR agonists. J Immunol. 2012;189:50–60. doi: 10.4049/jimmunol.1003554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomeshchik Y, Kidin I, Korhonen P, Savchenko E, Jaronen M, Lehtonen S, Wojciechowski S, Kanninen K, Koistinaho J, Malm T. Interleukin-33 treatment reduces secondary injury and improves functional recovery after contusion spinal cord injury. Brain Behav Immun. 2015;44:68–81. doi: 10.1016/j.bbi.2014.08.002. [DOI] [PubMed] [Google Scholar]
- Price AE, Liang HE, Sullivan BM, Reinhardt RL, Eisley CJ, Erle DJ, Locksley RM. Systemically dispersed innate IL-13-expressing cells in type 2 immunity. Proc Natl Acad Sci USA. 2010;107:11489–11494. doi: 10.1073/pnas.1003988107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu Y, Nguyen KD, Odegaard JI, Cui X, Tian X, Locksley RM, Palmiter RD, Chawla A. Eosinophils and type 2 cytokine signaling in macrophages orchestrate development of functional beige fat. Cell. 2014;157:1292–1308. doi: 10.1016/j.cell.2014.03.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rankin AL, Mumm JB, Murphy E, Turner S, Yu N, McClanahan TK, Bourne PA, Pierce RH, Kastelein R, Pflanz S. IL-33 induces IL-13-dependent cutaneous fibrosis. J Immunol. 2010;184:1526–1535. doi: 10.4049/jimmunol.0903306. [DOI] [PubMed] [Google Scholar]
- Rao RR, Long JZ, White JP, Svensson KJ, Lou J, Lokurkar I, Jedrychowski MP, Ruas JL, Wrann CD, Lo JC, et al. Meteorin-like is a hormone that regulates immune-adipose interactions to increase beige fat thermogenesis. Cell. 2014;157:1279–1291. doi: 10.1016/j.cell.2014.03.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhardt RL, Hong S, Kang SJ, Wang ZE, Locksley RM. Visualization of IL-12/23p40 in vivo reveals immunostimulatory dendritic cell migrants that promote Th1 differentiation. J Immunol. 2006;177:1618–1627. doi: 10.4049/jimmunol.177.3.1618. [DOI] [PubMed] [Google Scholar]
- Reynolds LA, Harcus Y, Smith KA, Webb LM, Hewitson JP, Ross EA, Brown S, Uematsu S, Akira S, Gray D, et al. MyD88 signaling inhibits protective immunity to the gastrointestinal helminth parasite Heligmosomoides polygyrus. J Immunol. 2014;193:2984–2993. doi: 10.4049/jimmunol.1401056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rickard JA, O’Donnell JA, Evans JM, Lalaoui N, Poh AR, Rogers T, Vince JE, Lawlor KE, Ninnis RL, Anderton H, et al. RIPK1 regulates RIPK3-MLKL-driven systemic inflammation and emergency hematopoiesis. Cell. 2014;157:1175–1188. doi: 10.1016/j.cell.2014.04.019. [DOI] [PubMed] [Google Scholar]
- Rider P, Carmi Y, Voronov E, Apte RN. Interleukin-1α. Semin Immunol. 2013;25:430–438. doi: 10.1016/j.smim.2013.10.005. [DOI] [PubMed] [Google Scholar]
- Roediger B, Kyle R, Yip KH, Sumaria N, Guy TV, Kim BS, Mitchell AJ, Tay SS, Jain R, Forbes-Blom E, et al. Cutaneous immunosurveillance and regulation of inflammation by group 2 innate lymphoid cells. Nat Immunol. 2013;14:564–573. doi: 10.1038/ni.2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rostan O, Arshad MI, Piquet-Pellorce C, Robert-Gangneux F, Gangneux JP, Samson M. Crucial and diverse role of the Interleukin-33/ST2 axis in infectious diseases. Infect Immun. 2015;83:1738–1748. doi: 10.1128/IAI.02908-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roussel L, Erard M, Cayrol C, Girard JP. Molecular mimicry between IL- 33 and KSHV for attachment to chromatin through the H2A–H2B acidic pocket. EMBO Reports. 2008;9:1006–1012. doi: 10.1038/embor.2008.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salimi M, Barlow JL, Saunders SP, Xue L, Gutowska-Owsiak D, Wang X, Huang LC, Johnson D, Scanlon ST, McKenzie ANJ, et al. A role for IL-25 and IL-33-driven type-2 innate lymphoid cells in atopic dermatitis. J Exp Med. 2013;210:2939–2950. doi: 10.1084/jem.20130351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salker MS, Nautiyal J, Steel JH, Webster Z, Šućurović S, Nicou M, Singh Y, Lucas ES, Murakami K, Chan YW, et al. Disordered IL-33/ST2 activation in decidualizing stromal cells prolongs uterine receptivity in women with recurrent pregnancy loss. PLoS ONE. 2012;7:e52252. doi: 10.1371/journal.pone.0052252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanada S, Hakuno D, Higgins LJ, Schreiter ER, McKenzie ANJ, Lee RT. IL-33 and ST2 comprise a critical biomechanically induced and cardioprotective signaling system. J Clin Invest. 2007;117:1538–1549. doi: 10.1172/JCI30634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sattler S, Smits HH, Xu D, Huang FP. The evolutionary role of the IL-33/ST2 system in host immune defence. Arch Immunol Ther Exp (Warsz) 2013;61:107–117. doi: 10.1007/s00005-012-0208-8. [DOI] [PubMed] [Google Scholar]
- Sánchez-Más J, Lax A, Asensio-López MDC, Fernandez-Del Palacio MJ, Caballero L, Santarelli G, Januzzi JL, Pascual-Figal DA. Modulation of IL-33/ST2 system in postinfarction heart failure: correlation with cardiac remodelling markers. Eur J Clin Invest. 2014;44:643–651. doi: 10.1111/eci.12282. [DOI] [PubMed] [Google Scholar]
- Scalfone LK, Nel HJ, Gagliardo LF, Cameron JL, Al-Shokri S, Leifer CA, Fallon PG, Appleton JA. Participation of MyD88 and interleukin-33 as innate drivers of Th2 immunity to Trichinella spiralis. Infect Immun. 2013;81:1354–1363. doi: 10.1128/IAI.01307-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiering C, Krausgruber T, Chomka A, Fröhlich A, Adelmann K, Wohlfert EA, Pott J, Griseri T, Bollrath J, Hegazy AN, et al. The alarmin IL-33 promotes regulatory T-cell function in the intestine. Nature. 2014;513:564–568. doi: 10.1038/nature13577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz J, Owyang A, Oldham E, Song Y, Murphy E, McClanahan TK, Zurawski G, Moshrefi M, Qin J, Li X, et al. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity. 2005;23:479–490. doi: 10.1016/j.immuni.2005.09.015. [DOI] [PubMed] [Google Scholar]
- Seki K, Sanada S, Kudinova AY, Steinhauser ML, Handa V, Gannon J, Lee RT. Interleukin-33 prevents apoptosis and improves survival after experimental myocardial infarction through ST2 signaling. Circ Heart Fail. 2009;2:684–691. doi: 10.1161/CIRCHEARTFAILURE.109.873240. [DOI] [PubMed] [Google Scholar]
- Senn KA, McCoy KD, Maloy KJ, Stark G, Fröhli E, Rülicke T, Klemenz R. T1-deficient and T1-Fc-transgenic mice develop a normal protective Th2-type immune response following infection with Nippostrongylus brasiliensis. Eur J Immunol. 2000;30:1929–1938. doi: 10.1002/1521-4141(200007)30:7<1929::AID-IMMU1929>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Shaw JL, Fakhri S, Citardi MJ, Porter PC, Corry DB, Kheradmand F, Liu YJ, Luong A. IL-33-responsive innate lymphoid cells are an important source of IL-13 in chronic rhinosinusitis with nasal polyps. Am J Respir Crit Care Med. 2013;188:432–439. doi: 10.1164/rccm.201212-2227OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu C, Kim J, Stepanowsky P, Trinh C, Lau HD, Akers JC, Chen C, Kanegaye JT, Tremoulet A, Ohno-Machado L, et al. Differential expression of miR-145 in children with Kawasaki disease. PLoS ONE. 2013;8:e58159. doi: 10.1371/journal.pone.0058159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DE. The biological paths of IL-1 family members IL-18 and IL-33. J Leukoc Biol. 2011;89:383–392. doi: 10.1189/jlb.0810470. [DOI] [PubMed] [Google Scholar]
- Smithgall MD, Comeau MR, Yoon BRP, Kaufman D, Armitage R, Smith DE. IL-33 amplifies both Th1- and Th2-type responses through its activity on human basophils, allergen-reactive Th2 cells, iNKT and NK Cells. Int Immunol. 2008;20:1019–1030. doi: 10.1093/intimm/dxn060. [DOI] [PubMed] [Google Scholar]
- Snelgrove RJ, Gregory LG, Peiró T, Akthar S, Campbell GA, Walker SA, Lloyd CM. Alternaria-derived serine protease activity drives IL-33-mediated asthma exacerbations. J Allergy Clin Immunol. 2014;134:583–592. e586. doi: 10.1016/j.jaci.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternlicht MD, Kouros Mehr H, Lu P, Werb Z. Hormonal and local control of mammary branching morphogenesis. Differentiation. 2006;74:365–381. doi: 10.1111/j.1432-0436.2006.00105.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun JC, Ma A, Lanier LL. Cutting edge: IL-15-independent NK cell response to mouse cytomegalovirus infection. J Immunol. 2009;183:2911–2914. doi: 10.4049/jimmunol.0901872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun L, Zhu Z, Cheng N, Yan Q, Ye RD. Serum amyloid A induces interleukin-33 expression through an IRF7-dependent pathway. Eur J Immunol. 2014;44:2153–2164. doi: 10.1002/eji.201344310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundlisaeter E, Edelmann RJ, Hol J, Sponheim J, Küchler AM, Weiss M, Udalova IA, Midwood KS, Kasprzycka M, Haraldsen G. The alarmin IL-33 is a notch target in quiescent endothelial cells. Am J Pathol. 2012;181:1099–1111. doi: 10.1016/j.ajpath.2012.06.003. [DOI] [PubMed] [Google Scholar]
- Sundnes O, Pietka W, Loos T, Sponheim J, Rankin AL, Pflanz S, Bertelsen V, Sitek JC, Hol J, Haraldsen G, et al. Epidermal Expression and Regulation of Interleukin-33 during Homeostasis and Inflammation: Strong Species Differences. J Invest Dermatol. 2015 doi: 10.1038/jid.2015.85. in press. [DOI] [PubMed] [Google Scholar]
- Talabot-Ayer D, Calo N, Vigne S, Lamacchia C, Gabay C, Palmer G. The mouse interleukin (Il)33 gene is expressed in a cell type- and stimulus-dependent manner from two alternative promoters. J Leukoc Biol. 2012;91:119–125. doi: 10.1189/jlb.0811425. [DOI] [PubMed] [Google Scholar]
- Talabot-Ayer D, Lamacchia C, Gabay C, Palmer G. Interleukin-33 is biologically active independently of caspase-1 cleavage. J Biol Chem. 2009;284:19420–19426. doi: 10.1074/jbc.M901744200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talabot-Ayer D, Martin P, Vesin C, Seemayer CA, Vigne S, Gabay C, Palmer G. Severe neutrophil-dominated inflammation and enhanced myelopoiesis in IL-33-overexpressing CMV/IL33 mice. J Immunol. 2015;194:750–760. doi: 10.4049/jimmunol.1402057. [DOI] [PubMed] [Google Scholar]
- Tamiya T, Kashiwagi I, Takahashi R, Yasukawa H, Yoshimura A. Suppressors of cytokine signaling (SOCS) proteins and JAK/STAT pathways: regulation of T-cell inflammation by SOCS1 and SOCS3. Arteriosclerosis, Thrombosis, and Vascular Biology. 2011;31:980–985. doi: 10.1161/ATVBAHA.110.207464. [DOI] [PubMed] [Google Scholar]
- Tominaga SI. A putative protein of a growth specific cDNA from BALB/C-3T3 cells is highly similar to the extracellular portion of mouse interleukin 1 receptor. FEBS Letters. 1989;258:301–304. doi: 10.1016/0014-5793(89)81679-5. [DOI] [PubMed] [Google Scholar]
- Torgerson DG, Ampleford EJ, Chiu GY, Gauderman WJ, Gignoux CR, Graves PE, Himes BE, Levin AM, Mathias RA, Hancock DB, et al. Meta-analysis of genome-wide association studies of asthma in ethnically diverse North American populations. Nat Genet. 2011;43:887–892. doi: 10.1038/ng.888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend MJ, Fallon PG, Matthews DJ, Jolin HE, McKenzie AN. T1/ST2-deficient mice demonstrate the importance of T1/ST2 in developing primary T helper cell type 2 responses. J Exp Med. 2000;191:1069–1076. doi: 10.1084/jem.191.6.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuda H, Komine M, Karakawa M, Etoh T, Tominaga SI, Ohtsuki M. Novel splice variants of IL-33: differential expression in normal and transformed cells. J Invest Dermatol. 2012;132:2661–2664. doi: 10.1038/jid.2012.180. [DOI] [PubMed] [Google Scholar]
- Turczyńska KM, Sadegh MK, Hellstrand P, Swärd K, Albinsson S. MicroRNAs are essential for stretch-induced vascular smooth muscle contractile differentiation via microRNA (miR)-145-dependent expression of L-type calcium channels. J Biol Chem. 2012;287:19199–19206. doi: 10.1074/jbc.M112.341073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turer EE, Tavares RM, Mortier E, Hitotsumatsu O, Advincula R, Lee B, Shifrin N, Malynn BA, Ma A. Homeostatic MyD88-dependent signals cause lethal inflammation in the absence of A20. J Exp Med. 2008;205:451–464. doi: 10.1084/jem.20071108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner JE, Morrison PJ, Wilhelm C, Wilson M, Ahlfors H, Renauld JC, Panzer U, Helmby H, Stockinger B. IL-9-mediated survival of type 2 innate lymphoid cells promotes damage control in helminth-induced lung inflammation. J Exp Med. 2013;210:2951–2965. doi: 10.1084/jem.20130071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turnquist HR, Zhao Z, Rosborough BR, Liu Q, Castellaneta A, Isse K, Wang Z, Lang M, Stolz DB, Zheng XX, et al. IL-33 expands suppressive CD11b+ Gr-1(int) and regulatory T cells, including ST2L+ Foxp3+ cells, and mediates regulatory T cell-dependent promotion of cardiac allograft survival. J Immunol. 2011;187:4598–4610. doi: 10.4049/jimmunol.1100519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dyken SJ, Mohapatra A, Nussbaum JC, Molofsky AB, Thornton EE, Ziegler SF, McKenzie ANJ, Krummel MF, Liang HE, Locksley RM. Chitin activates parallel immune modules that direct distinct inflammatory responses via innate lymphoid type 2 and γδ T cells. Immunity. 2014;40:414–424. doi: 10.1016/j.immuni.2014.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasanthakumar A, Moro K, Xin A, Liao Y, Gloury R, Kawamoto S, Fagarasan S, Mielke LA, Afshar-Sterle S, Masters SL, et al. The transcriptional regulators IRF4, BATF and IL-33 orchestrate development and maintenance of adipose tissue-resident regulatory T cells. Nat Immunol. 2015;16:276–285. doi: 10.1038/ni.3085. [DOI] [PubMed] [Google Scholar]
- Villarreal DO, Weiner DB. Interleukin 33: a switch-hitting cytokine. Curr Opin Immunol. 2014;28:102–106. doi: 10.1016/j.coi.2014.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villarreal DO, Weiner DB. IL-33 isoforms: their future as vaccine adjuvants? Expert Rev Vaccines. 2015;14:489–492. doi: 10.1586/14760584.2015.1011135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villarreal DO, Wise MC, Walters JN, Reuschel EL, Choi MJ, Obeng-Adjei N, Yan J, Morrow MP, Weiner DB. Alarmin IL-33 acts as an immunoadjuvant to enhance antigen-specific tumor immunity. Cancer Research. 2014;74:1789–1800. doi: 10.1158/0008-5472.CAN-13-2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voehringer D. Basophil modulation by cytokine instruction. Eur J Immunol. 2012;42:2544–2550. doi: 10.1002/eji.201142318. [DOI] [PubMed] [Google Scholar]
- von Moltke J, Locksley RM. I-L-C-2 it: type 2 immunity and group 2 innate lymphoid cells in homeostasis. Curr Opin Immunol. 2014;31:58–65. doi: 10.1016/j.coi.2014.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K, Long B, Zhou J, Li PF. miR-9 and NFATc3 regulate myocardin in cardiac hypertrophy. J Biol Chem. 2010;285:11903–11912. doi: 10.1074/jbc.M109.098004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg EO, Shimpo M, De Keulenaer GW, MacGillivray C, Tominaga SI, Solomon SD, Rouleau JL, Lee RT. Expression and regulation of ST2, an interleukin-1 receptor family member, in cardiomyocytes and myocardial infarction. Circulation. 2002;106:2961–2966. doi: 10.1161/01.CIR.0000038705.69871.D9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werenskiold AK, Hoffmann S, Klemenz R. Induction of a mitogen-responsive gene after expression of the Ha-ras oncogene in NIH 3T3 fibroblasts. Mol Cell Biol. 1989;9:5207–5214. doi: 10.1128/mcb.9.11.5207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wicher G, Husic E, Nilsson G, Forsberg-Nilsson K. Developmental expression of IL-33 in the mouse brain. Neurosci Lett. 2013;555:171–176. doi: 10.1016/j.neulet.2013.09.046. [DOI] [PubMed] [Google Scholar]
- Wilhelm C, Hirota K, Stieglitz B, Van Snick J, Tolaini M, Lahl K, Sparwasser T, Helmby H, Stockinger B. An IL-9 fate reporter demonstrates the induction of an innate IL-9 response in lung inflammation. Nat Immunol. 2011:1–8. doi: 10.1038/ni.2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willart MAM, Deswarte K, Pouliot P, Braun H, Beyaert R, Lambrecht BN, Hammad H. Interleukin-1α controls allergic sensitization to inhaled house dust mite via the epithelial release of GM-CSF and IL-33. J Exp Med. 2012;209:1505–1517. doi: 10.1084/jem.20112691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood IS, Wang B, Trayhurn P. IL-33, a recently identified interleukin-1 gene family member, is expressed in human adipocytes. Biochem Biophys Res Commun. 2009;384:105–109. doi: 10.1016/j.bbrc.2009.04.081. [DOI] [PubMed] [Google Scholar]
- Wu J, Carlock C, Zhou C, Nakae S, Hicks J, Adams HP, Lou Y. IL-33 is required for disposal of unnecessary cells during ovarian atresia through regulation of autophagy and macrophage migration. J Immunol. 2015;194:2140–2147. doi: 10.4049/jimmunol.1402503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu D, Chan WL, Leung BP, Huang FP, Wheeler R, Piedrafita D, Robinson JH, Liew FY. Selective expression of a stable cell surface molecule on type 2 but not type 1 helper T cells. J Exp Med. 1998;187:787–794. doi: 10.1084/jem.187.5.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanaba K, Yoshizaki A, Asano Y, Kadono T, Sato S. Serum IL-33 levels are raised in patients with systemic sclerosis: association with extent of skin sclerosis and severity of pulmonary fibrosis. Clin Rheumatol. 2011;30:825–830. doi: 10.1007/s10067-011-1686-5. [DOI] [PubMed] [Google Scholar]
- Yanagisawa K, Naito Y, Kuroiwa K, Arai T, Furukawa Y, Tomizuka H, Miura Y, Kasahara T, Tetsuka T, Tominaga S. The expression of ST2 gene in helper T cells and the binding of ST2 protein to myeloma-derived RPMI8226 cells. J Biochem. 1997;121:95–103. doi: 10.1093/oxfordjournals.jbchem.a021577. [DOI] [PubMed] [Google Scholar]
- Yanagisawa K, Takagi T, Tsukamoto T, Tetsuka T, Tominaga S. Presence of a novel primary response gene ST2L, encoding a product highly similar to the interleukin 1 receptor type 1. FEBS Letters. 1993;318:83–87. doi: 10.1016/0014-5793(93)81333-u. [DOI] [PubMed] [Google Scholar]
- Yang Q, Li G, Zhu Y, Liu L, Chen E, Turnquist H, Zhang X, Finn OJ, Chen X, Lu B. IL-33 synergizes with TCR and IL-12 signaling to promote the effector function of CD8+ T cells. Eur J Immunol. 2011;41:3351–3360. doi: 10.1002/eji.201141629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S, Fujikado N, Kolodin D, Benoist C, Mathis D. Immune tolerance. Regulatory T cells generated early in life play a distinct role in maintaining self-tolerance. Science. 2015;348:589–594. doi: 10.1126/science.aaa7017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuoka S, Kawanokuchi J, Parajuli B, Jin S, Doi Y, Noda M, Sonobe Y, Takeuchi H, Mizuno T, Suzumura A. Production and functions of IL-33 in the central nervous system. Brain Res. 2011;1385:8–17. doi: 10.1016/j.brainres.2011.02.045. [DOI] [PubMed] [Google Scholar]
- Yin H, Li X, Hu S, Liu T, Yuan B, Gu H, Ni Q, Zhang X, Zheng F. IL-33 accelerates cutaneous wound healing involved in upregulation of alternatively activated macrophages. Mol Immunol. 2013;56:347–353. doi: 10.1016/j.molimm.2013.05.225. [DOI] [PubMed] [Google Scholar]
- Yu XX, Hu Z, Shen X, Dong LY, Zhou WZ, Hu WH. IL-33 promotes gastric cancer cell invasion and migration via ST2-ERK1/2 pathway. Dig Dis Sci. 2015;60:1265–1272. doi: 10.1007/s10620-014-3463-1. [DOI] [PubMed] [Google Scholar]
- Zaiss MM, Maslowski KM, Mosconi I, Guenat N, Marsland BJ, Harris NL. IL-1β suppresses innate IL-25 and IL-33 production and maintains helminth chronicity. PLoS Pathog. 2013;9:e1003531. doi: 10.1371/journal.ppat.1003531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeyda M, Wernly B, Demyanets S, Kaun C, Hämmerle M, Hantusch B, Schranz M, Neuhofer A, Itariu BK, Keck M, et al. Severe obesity increases adipose tissue expression of interleukin-33 and its receptor ST2, both predominantly detectable in endothelial cells of human adipose tissue. Int J Obes (Lond) 2013;37:658–665. doi: 10.1038/ijo.2012.118. [DOI] [PubMed] [Google Scholar]