Abstract
The initial events of visual transduction occur on disc membranes which are sequestered within the photoreceptor outer segment. In rod cells, the discs are stacked in the outer segment. Discs are formed at the base of the rod outer segment (ROS) from evaginations of the plasma membrane. As new discs form, older discs move toward the apical tip of the rod, from which they are eventually shed and subsequently phagocytosed by the adjacent pigment epithelium. Thus, disc membranes within a given rod cell are not of uniform age. We have recently shown that disc membranes are not homogeneous with respect to cholesterol content (Boesze-Battaglia, K., Hennessey, T., and Albert, A. D. (1989) J. Biol. Chem. 264, 8151–8155). In the present study, freshly isolated bovine retinas were incubated with [3H]leucine for 4 h in order to allow sufficient time for the radiolabeled proteins to become incorporated into the basal-most (newest) discs. Osmotically intact discs were then isolated. After the addition of digitonin, the discs were fractionated based on cholesterol content, and radioactivity (indicative of newly synthesized protein) was measured. Discs which exhibited high cholesterol content also exhibited high radioactivity. These results demonstrate that the cholesterol heterogeneity of ROS disc membranes is related to the age, and thus the position, of the discs in the ROS.
The retinal rod photoreceptor is a highly polarized, postmitotic cell type which has proven particularly useful in studying various aspects of membrane assembly and signal transduction. The portion of the cell designated the rod outer segment (ROS)1 consists of an orderly stack of flattened membrane saccules (discs) enveloped by the plasma membrane of the cell. The ROS membranes primarily consist of lipids and proteins, in approximately equal amounts by weight (1, 2). Phospholipids represent almost 90 mol % of the total ROS lipids, while cholesterol accounts for less than 10 mol % (2, 3). In striking contrast to the complexity of the ROS lipid molecular species composition, rhodopsin (the visual pigment) accounts for about 95% of the total ROS membrane protein (4, 5).
The ROS undergoes dynamic renewal: newly formed discs are added at the base of the ROS, with compensatory shedding of packets of older discs at the apical tip, thereby maintaining the ROS at a relatively constant length (6, 7). This renewal process takes about 10 days in mammals. Rhodopsin, once assembled into a disc, remains associated with that disc throughout its lifetime in the ROS. Thus, the turnover of rhodopsin parallels the basal-to-apical transit time of the discs in the ROS, and the relative location of a disc along the length of the ROS reflects the age of its protein constituents (i.e. the basal-most discs contain the most recently synthesized proteins) (8, 9). In contrast, glycerolipid constituents are not constrained to remain with the discs into which they were initially assembled; rather, individual lipid classes have distinct turnover rates which are considerably more rapid than those of the membrane proteins. Therefore, lipid turnover does not parallel the rate of bulk membrane turnover in the ROS (10–17).
There is little information regarding the dynamics of ROS membrane cholesterol incorporation and turnover. Freeze-fracture electron microscopy studies have indicated the presence of cholesterol-enriched domains within the ROS plasma membrane and (in some species) basal discs (18–20). This finding suggests a heterogeneity in the cholesterol content of newly assembled versus older discs. Recently, we have demonstrated the heterogeneity of ROS membrane cholesterol content by biochemical means, based on the ability of digitonin to differentially perturb the buoyant density of subpopulations of discs isolated from bovine ROS on sucrose gradients (21). Discs found at higher densities had cholesterol/phospholipid ratios approaching 0.30, while those discs found at lower densities had cholesterol/phospholipid ratios as low as 0.05. In the present study, we demonstrate the coincidence of newly synthesized ROS proteins with relatively cholesterol-enriched discs, thus biochemically demonstrating a relationship between cholesterol content and the age and location of discs in the ROS.
MATERIALS AND METHODS
Retinas utilized for in vitro incubations were obtained from freshly enucleated bovine eyes (Tarpoff Packing Co., Granite City, IL) maintained on ice in darkness prior to [3H]leucine labeling (within 1 h postmortem). Fresh bovine retinas (2 per 4 ml of medium, in 25-ml Erlenmeyer flasks) were incubated essentially as previously described by Fliesler and Schroepfer (22), using a modified Ringer’s bicarbonate-glucose medium supplemented with 3% (w/v) sucrose and 50% (v/v) RPMI 1640 culture medium (GIBCO/Bethesda Research Laboratories) and containing 0.20 mCi of [4,5-3H]leucine (ICN Biologicals; 128 Ci/mmol). Incubations were performed in a Dubnoff metabolic incubator under dim red light for 4 h at 37 °C under a humidified 95% O2, 5% CO2 atmosphere, with continuous gentle reciprocal agitation. Following incubation, retinas were rinsed gently by serial transfer (3 × 20 ml each) through chilled Dulbecco’s phosphate-buffered saline (Gibco/Bethesda Research Laboratories) to remove unincorporated radiolabel and then frozen at −80 °C in 10 ml of 45% (w/w) sucrose prior to utilization for ROS disc membrane preparation.
Frozen bovine retinas (J. Lawson Inc., Lincoln, NE) were used for preparation of ROS disc membranes. Typically, 4 radiolabeled retinas were mixed with 50 unlabeled retinas immediately prior to preparation of ROS disc membranes. Procedures for preparation and digitonin treatment of ROS discs, isolation of disc subpopulations on sucrose density gradients, quantitative analyses of lipids, and protein determination were as previously described in detail (see Ref. 21 and references therein). For radioactivity determination, 1–2 ml of the fractions were washed in 0.01 M NaCl to remove sucrose. Assay of radioactivity in disc membrane subpopulations was performed by dissolving aliquots of membrane fractions in 0.03 ml of Solusol (National Diagnostics, Manville, NJ) and 2 ml of Liquiscint and measuring the radioactivity with a Beckman model LS-230 scintillation spectrometer (Beckman Instruments, Inc., Palo Alto, CA); tritium counting efficiency was 38%.
A sham incubation was performed to show that discs from the labeled retinas behaved as did discs from the unincubated retinas. Freshly isolated retinas were incubated as described above, but with unlabeled leucine. Discs isolated from these retinas were treated with digitonin and separated into subpopulations by sucrose density gradient centrifugation as described above. These discs were then assayed for phospholipid and cholesterol content.
In a separate experiment, bovine retinas were incubated in the presence of [3H]leucine as described above; following serial rinsing, the retinas were vortexed in TM buffer (10 mM Tris acetate, pH 7.4, containing 5 mM MgCl2; final volume, 40 ml) and centrifuged (1 h at 15,000 rpm, Sorvall SS-34 rotor, 4 °C). The supernatant (containing soluble components and residual unincorporated isotopic substrate) was discarded, the pellet was homogenized in 50% (w/v) sucrose in TM buffer, and ROS membranes were prepared by discontinuous sucrose gradient centrifugation as previously described (23). ROS membranes were harvested from the sucrose gradient (1.11/1.13 g/cm3 interface), washed twice by centrifugation in TM buffer as described above, and resuspended by probe sonication in 1.0 ml of ice-cold distilled water. Aliquots were taken in duplicate for assay of total radioactivity, Cl3CCOOH precipitation and specific activity determination, and extraction of total lipids, as previously described (24). Lipid extracts were evaporated to dryness under argon and redissolved in 1.0 ml of CHCl3, and aliquots were taken for assay of total lipid-soluble radioactivity.
RESULTS AND DISCUSSION
The lipid composition of ROS disc membranes has been established through numerous studies (for reviews, see Refs. 1 and 2). These studies, however, provide a compositional average of all ROS discs. The method used here can now be employed to investigate changes in the lipid composition as the disc membranes move from their site of assembly to their site of disposal at the apical tip of the ROS.
In the present study, bovine retinas were incubated with [3H]leucine under conditions which have been shown previously to result in incorporation of newly synthesized radiolabeled proteins (including opsin) into nascent ROS discs (25). These retinas were then mixed with unlabeled retinas so as to introduce a radiolabeled marker for basal (i.e. new) discs into the total disc population, and ROS discs were prepared. The discs were then fractionated into subpopulations on the basis of their cholesterol content, employing digitonin-induced perturbation of buoyant density in conjunction with sucrose density gradient centrifugation (21). Sucrose density gradients from digitonin-treated and control (i.e. untreated) ROS discs were then analyzed for cholesterol, phospholipid, total protein, and radiolabeled protein content. As was previously found (21), greater than 90% of the cholesterol, phospholipid, and protein was recovered from the gradients. Also consistent with this earlier study, the phospholipid/protein molar ratio remained constant across the gradient (21).
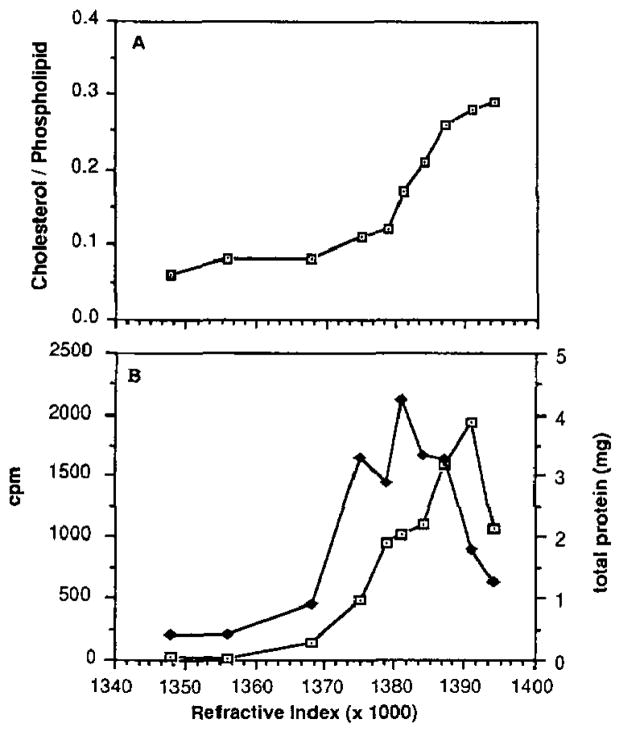
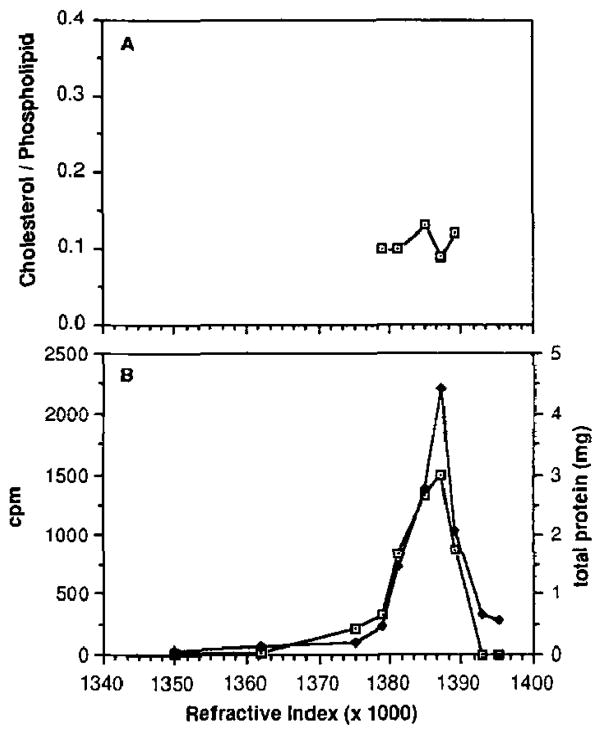
The data for digitonin-treated and control discs are shown in Figs. 1 and 2, respectively. Both digitonin-treated and control discs were derived from the same disc preparation. The experiment shown is representative of three independent experiments. The values for the cholesterol/phospholipid ratio of the digitonin-treated discs (Fig. 1A) ranged from 0.05 to 0.30, while those for control discs (Fig. 2A) showed virtually no variation from a ratio of about 0.12. These values are in good agreement with previously published results (2, 21). The data indicate that discs which are relatively enriched in cholesterol correspond to higher density (shown as higher refractive index values).
Fig. 1. Sucrose density gradient profiles of digitonin-treated ROS disc membranes from bovine retinas.
Data (presented as a function of refractive index) are from a single experiment, representative of three independent experiments. A, cholesterol/phospholipid molar ratios of individual fractions. B, [3H]leucine incorporation (⊡) versus total protein content (◆) in each fraction. The radioactivity is expressed as counts/min and is the total radioactivity derived from 2-ml aliquots of each fraction.
Fig. 2. Sucrose density gradient profiles of control (untreated) ROS disc membranes from bovine retinas.
Data (presented as a function of refractive index) are from a single experiment, representative of three independent experiments. A, cholesterol/phospholipid molar ratios of individual fractions. The cholesterol/phospholipid ratio was not plotted for the five fractions in which the sterol was undetectable. B, [3H]leucine incorporation (⊡) versus total protein content (◆) in each fraction. The radioactivity is expressed as counts/min and is the total radioactivity derived from 2-ml aliquots of each fraction.
Fig. 2B shows the distribution profile of total protein and radiolabel (corresponding to newly synthesized protein) among the control disc subpopulations, as a function of density. The profiles are coincident across the density gradient. In contrast, the profiles for digitonin-treated discs (Fig. 1B) are not coincident: the radiolabeled material was recovered at a higher density than the total protein. Almost 50% of the total radioactivity is coincident with only about 20% of the total membrane protein in the digitonin-treated discs. The peak in the radiolabel corresponds to a cholesterol/phospholipid ratio of 0.28, characteristic of the most cholesterol-enriched fraction on the gradient, while the total protein maximum corresponds to a cholesterol/phospholipid ratio of 0.17. Although there is typically some broadening of the total protein mass profile of digitonin-treated discs to lower density, in control samples the new and total protein are always recovered in superimposable peaks, while in digitonin-treated discs the new protein is always recovered at a higher density than is the total protein. Since radiolabeled (i.e. newly synthesized) protein is indicative of newly assembled discs, these results demonstrate that the cholesterol-enriched disc population corresponds to the newest (and therefore basal-most) discs in the ROS.
Biochemical analysis of 3H-labeled ROS membrane preparations (data not shown) revealed that only 2–3% of the radiolabel derived from [3H]leucine was incorporated into lipid-soluble components. In reasonable agreement with this result, approximately 93% of the label was recovered in acid-precipitable material (not corrected for losses during sample workup). Taken together, these data indicate that most of the tritium in the ROS preparations represents newly synthesized protein rather than lipid.
To determine if the different histories of the radiolabeled (incubated) and the “carrier” (unlabeled) discs caused them to behave differently on the sucrose density gradients, a large-scale incubation was performed (see “Materials and Methods”) and discs were then isolated from these retinas. After digitonin treatment and sucrose density gradient centrifugation, the disc subpopulations exhibited cholesterol/phospholipid molar ratios varying from 0.08 to 0.29. These values are in excellent agreement with those given in Fig. 1A and with our previously published data (21). Also, as reported earlier (21), the protein/phospholipid ratio remained essentially constant. These data indicate that labeling incubation procedure, under the given conditions, does not affect the density gradient behavior of digitonin-treated or control disc membranes with respect to cholesterol, phospholipid, or protein when compared to the sucrose density gradient profiles of unincubated discs. We therefore believe it reasonable to assume that the labeled discs are accurately reflecting the behavior of the total disc preparation.
The relationship of age and spatial distribution to disc function has been difficult to investigate using biochemical techniques because it has not been possible to isolate sufficient quantities of discs from a specific spatial location along the ROS. However, a limited number of studies have been carried out on single rod cells. These studies have assessed changes in ROS function at different positions along the length of the outer segment. Electrophysiological measurements have indicated a decrease in the amplitude of the single photon response from the base to the apical tip of the ROS (26). Microspectrophotometric measurements also have indicated differences in rhodopsin regenerability as a function of spatial location within the ROS (27). These results were species-dependent, since rats and toads exhibited opposite regeneration patterns.
The ability to investigate disc properties as a function of their spatial distribution has several important consequences. Cellular events such as fusion (28, 29) and enzyme activity (30) have been shown to be influenced by the properties of the surrounding lipid bilayer. The formation of the various photolytic intermediates of rhodopsin are dependent on the composition of the surrounding bilayer (31–34). In a filipin-binding study comparing normal and dystrophic RCS rats, it was observed that the normal rats exhibited a difference in filipin binding between base and tip while the dystrophic rats showed no variation in filipin binding. This suggested cholesterol heterogeneity in normal, but not dystrophic, RCS rats (20). A characteristic of dystrophism in these animals is the formation of unusually long ROS due to the inability of the discs to be shed and phagocytosed normally.
It has recently been shown that the composition of the ROS plasma membrane is distinct from the average composition of the disc membrane with respect to both protein (35–37) and lipid composition (38). The ability to isolate newly synthesized discs now allows the comparison of the composition of these discs with that of the ROS plasma membrane. The disc membrane is formed from the plasma membrane. Therefore, it is anticipated that this study will provide insight with respect to the process of lipid and protein sorting during disc biogenesis. For example the cholesterol/phospholipid ratio in the ROS plasma membrane is 0.4 (38). This is similar to the cholesterol/phospholipid ratio of 0.3 found in the newly synthesized discs (21). Studies are presently underway to examine in greater detail the protein and lipid compositions of the plasma membrane and newly synthesized discs.
Footnotes
This work was supported in part by Public Health Service Grants EY06045 (to S. J. F.) and EY03328 (to A. D. A.), an unrestricted departmental grant from Research to Prevent Blindness, Inc. (to S. J. F.), and Grants-in-Aid Award PN79010 from Sigma Xi (to K. B. B.).
The abbreviation used is: ROS, retinal outer segment.
References
- 1.Daemen FJM. Biochim Biophys Acta. 1973;300:255–288. doi: 10.1016/0304-4157(73)90006-3. [DOI] [PubMed] [Google Scholar]
- 2.Fliesler S, Anderson RE. Prog Lipid Res. 1983;22:79–131. doi: 10.1016/0163-7827(83)90004-8. [DOI] [PubMed] [Google Scholar]
- 3.Fliesler SJ, Schroepfer GJ., Jr Biochim Biophys Acta. 1982;711:138–142. doi: 10.1016/0005-2760(82)90020-0. [DOI] [PubMed] [Google Scholar]
- 4.Papermaster D, Dreyer W. Biochemistry. 1974;13:2438–2444. doi: 10.1021/bi00708a031. [DOI] [PubMed] [Google Scholar]
- 5.Krebs W, Kuhn H. Exp Eye Res. 1977;25:511–526. doi: 10.1016/0014-4835(77)90180-4. [DOI] [PubMed] [Google Scholar]
- 6.Young RW. Invest Ophthalmol. 1976;15:700–725. [PubMed] [Google Scholar]
- 7.Bok D. Invest Ophthalmol & Visual Sci. 1986;26:1659–1694. [PubMed] [Google Scholar]
- 8.Young RW, Droz B. J Cell Biol. 1968;39:169–184. doi: 10.1083/jcb.39.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hall MO, Bok D, Bacharach ADE. J Mol Biol. 1969;45:397–406. doi: 10.1016/0022-2836(69)90114-4. [DOI] [PubMed] [Google Scholar]
- 10.Anderson RE, Maude M. Arch Biochem Biophys. 1972;151:270–276. doi: 10.1016/0003-9861(72)90497-3. [DOI] [PubMed] [Google Scholar]
- 11.Bibb C, Young RW. J Cell Biol. 1974;61:327–343. doi: 10.1083/jcb.61.2.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bibb C, Young RW. J Cell Biol. 1974;62:378–389. doi: 10.1083/jcb.62.2.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Masland RH, Mills JW. J Cell Biol. 1979;83:159–178. doi: 10.1083/jcb.83.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Anderson RE, Kelleher PA, Maude MB, Maida TM. Neurochem Int. 1980;1:29–42. doi: 10.1016/0197-0186(80)90048-0. [DOI] [PubMed] [Google Scholar]
- 15.Anderson RE, Kelleher PA, Maude MB. Biochim Biophys Acta. 1980;620:227–235. doi: 10.1016/0005-2760(80)90204-0. [DOI] [PubMed] [Google Scholar]
- 16.Anderson RE, Maude MB, Kelleher PA. Biochim Biophys Acta. 1980;620:236–246. doi: 10.1016/0005-2760(80)90205-2. [DOI] [PubMed] [Google Scholar]
- 17.Mercurio AM, Holtzman E. J Neurocytol. 1982;11:263–293. doi: 10.1007/BF01258247. [DOI] [PubMed] [Google Scholar]
- 18.Andrews LD, Cohen AI. J Cell Biol. 1979;81:215–228. doi: 10.1083/jcb.81.1.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Andrews LD, Cohen AI. J Cell Biol. 1981;97:749–755. doi: 10.1083/jcb.97.3.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Caldwell R, McLaughlin B. J Comp Neurol. 1985;236:523–537. doi: 10.1002/cne.902360408. [DOI] [PubMed] [Google Scholar]
- 21.Boesze-Battaglia K, Hennessey T, Albert AD. J Biol Chem. 1989;264:8151–8155. [PMC free article] [PubMed] [Google Scholar]
- 22.Fliesler SJ, Schroepfer GJ., Jr J Neurochem. 1986;46:448–460. doi: 10.1111/j.1471-4159.1986.tb12989.x. [DOI] [PubMed] [Google Scholar]
- 23.Fliesler SJ, Basinger SF. Proc Natl Acad Sci U S A. 1985;82:1116–1120. doi: 10.1073/pnas.82.4.1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fliesler SJ, Basinger SF. J Biol Chem. 1987;262:17516–17523. [PubMed] [Google Scholar]
- 25.O’Brien PJ, Muellenberg CG, Bungenberg de Jong JJ. Biochemistry. 1972;11:64–70. doi: 10.1021/bi00751a012. [DOI] [PubMed] [Google Scholar]
- 26.Schnapf JL. J Physiol. 1983;343:147–159. doi: 10.1113/jphysiol.1983.sp014886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Williams TP. The Visual System. Alan R. Liss; New York: 1983. pp. 61–71. [Google Scholar]
- 28.Asano K, Asano A. Biochemistry. 1988;27:1321–1329. doi: 10.1021/bi00404a035. [DOI] [PubMed] [Google Scholar]
- 29.Bentz J, Ellens H. Colloids Surf. 1974;30:65–112. [Google Scholar]
- 30.Yeagle PL, Young J, Rice D. Biochemistry. 1988;27:6449–6452. doi: 10.1021/bi00417a037. [DOI] [PubMed] [Google Scholar]
- 31.Baldwin PA, Hubbell WL. Biochemistry. 1985;24:2633–2639. doi: 10.1021/bi00332a007. [DOI] [PubMed] [Google Scholar]
- 32.Straume M, Litman BJ. Biochemistry. 1987;26:5121–5126. doi: 10.1021/bi00390a034. [DOI] [PubMed] [Google Scholar]
- 33.Straume M, Litman BJ. Biochemistry. 1987;26:5113–5120. doi: 10.1021/bi00390a033. [DOI] [PubMed] [Google Scholar]
- 34.Straume M, Litman BJ. Biochemistry. 1988;27:7733–7740. doi: 10.1021/bi00420a022. [DOI] [PubMed] [Google Scholar]
- 35.Molday L, Molday R. Biochim Biophys Acta. 1987;897:335–340. doi: 10.1016/0005-2736(87)90430-5. [DOI] [PubMed] [Google Scholar]
- 36.Molday RS, Molday LL. J Cell Biol. 1987;105:2589–2601. doi: 10.1083/jcb.105.6.2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cook NJ, Molday LL, Reid D, Kaupp VB, Molday RS. J Biol Chem. 1989;264:6996–6999. [PubMed] [Google Scholar]
- 38.Boesze-Battaglia K, Albert AD. Exp Eye Res. 1989;49:598–600. doi: 10.1016/s0014-4835(89)80064-8. [DOI] [PubMed] [Google Scholar]