Abstract
The tumor suppressor p53 plays a central role in anti-tumorigenesis and cancer therapy. It has been described as “the guardian of the genome”, because it is essential for conserving genomic stability by preventing mutation, and its mutation and inactivation are highly related to all human cancers. Two important p53 regulators, MDM2 and MDMX, inactivate p53 by directly inhibiting its transcriptional activity and mediating its ubiquitination in a feedback fashion, as their genes are also the transcriptional targets of p53. On account of the importance of the p53-MDM2- MDMX loop in the initiation and development of wild type p53-containing tumors, intensive studies over the past decade have been aiming to identify small molecules or peptides that could specifically target individual protein molecules of this pathway for developing better anti-cancer therapeutics. In this chapter, we review the approaches for screening and discovering efficient and selective MDM2 inhibitors with emphasis on the most advanced synthetic small molecules that interfere with the p53-MDM2 interaction and are currently on Phase I clinical trials. Other therapeutically useful strategies targeting this loop, which potentially improve the prospects of cancer therapy and prevention, will also be discussed briefly.
Keywords: p53, MDM2, MDMX, Drug discovery, Drug design, Drug development, Cancer therapy
Introduction
The p53-MDM2-MDMX-Loop
The tumor suppressor p53 is inarguably the most recognized and studied protein involving human cancers. Its vital importance in preventing human cancer development and progression is simply reflected by the fact that mutations of its gene TP53 are detected in approximately 50 % of all types of human cancers, and the functions and stability of the p53 protein are often abrogated via posttranslational mechanisms in the rest of human cancers that harbor wild type TP53 [1–3]. Cancers often deactivate p53, because it can trigger cell growth arrest, apoptosis, autophagy, and/or senescence, which are detrimental to cancer cells [4, 5], and impede cell migration, metabolism, and/or angiogenesis, which are favorable to cancer cell progression and metastasis [5]. These physiological functions of p53 are executed primarily through its transcription-dependent and independent activities [5]. However, because these functions are also deleterious to normally growing stem cells and developing tissues [6], p53 is tightly monitored by two closely related proteins called MDM2 (sometime called HDM2 for its human analog) [7–9] and MDMX (also known as MDM4) [10] in higher eukaryotes [11].
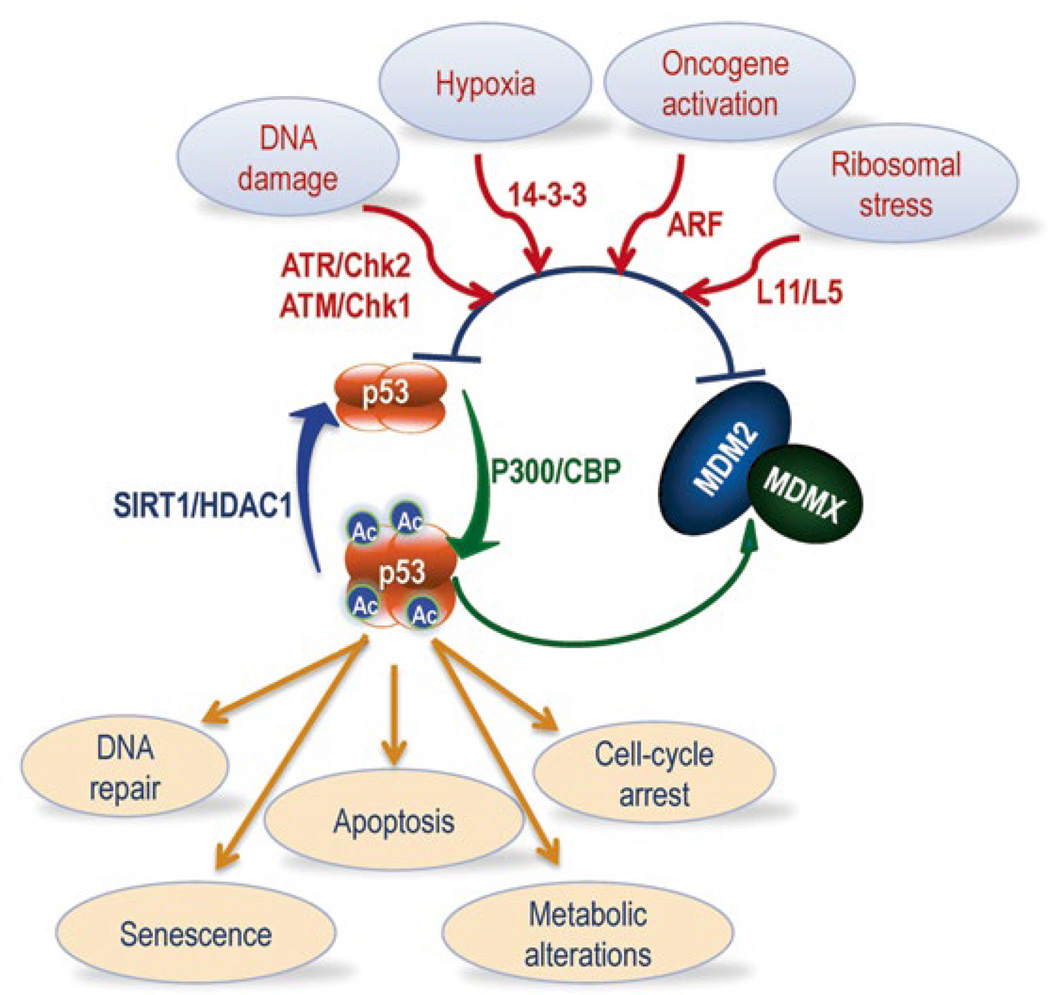
MDM2 and MDMX execute their oncogenic activity mainly by negatively regulating the stability and activity of the p53 protein in a feedback fashion (Fig. 16.1). They work together to block the transcriptional activity of p53 [5, 8, 9, 12] and to mediate p53 rapid degradation via ubiquitin-dependent proteolysis [13, 14], as MDM2 possesses an E3 ubiquitin ligase activity [15], and p53 stimulates MDM2 and MDMX mRNA expression [7, 9, 16–18]. This dual action of MDM2 and MDMX on p53 leads to the barely detectable level and activity of p53 in most normal mammalian cells or tissues. MDM2 and MDMX can also inhibit p53 independently of each other. Often, MDMX negates p53 transcriptional activity, while MDM2 can inhibit both of the p53 protein stability and activity [19]. Hence, in order to activate p53, eukaryotic cells have developed mechanisms to block this negative feedback regulation in response to a variety of cellular, genotoxic, or non-genotoxic stresses [20–22]. These mechanisms include posttranslational modifications of either p53 or MDM2/MDMX, such as acetylation [23], phosphorylation [24–27], and protein-protein interactions, such as ribosomal proteins-MDM2 interaction, or Arf-MDM2 interaction [20, 28], ultimately leading to p53 activation that prevents cells from undergoing transformation and neoplasia. Interestingly, two different modifications, acetylation and ubiquitylation, often occur at a similar set of lysine residues within p53, and thus are mutually exclusive. For example, acetylation of p53 by p300/CBP prevents its degradation by MDM2 and activates its activity whereas MDM2 inhibits p53 acetylation by p300/CBP [29–31]. Conversely, deacetylation of p53 by an NAD-dependent deacetylase, SIRT1 [32– 34], or a class I histone deacetylase, HDAC1 [35], favors MDM2-mediated p53 degradation, leading to p53 inactivation. Remarkably, cancers often take advantages of this feedback loop to promote their own growth, as human breast cancers, osteosarcomas, lymphomas, leukemia or melanoma express high levels of MDM2 or MDMX through distinct mechanisms without p53 mutation [17, 36]. Also, the high level of deacetylases is often detected in cancers [37–40]. Therefore, it is likely that deacetylases might play a role in maintaining p53 in a deacetylated status in cancer cells, consequently facilitating MDM2/MDMX-mediated degradation.
Fig. 16.1. The p53-MDM2-MDMX feedback loop.
Two p53 suppressors, MDM2 and MDMX, which are highly expressed in tumors, often work together as one complex to inactivate p53 by mediating its ubiquitination and degradation as well as to directly inhibit p53 transcriptional activity in a feedback fashion. This feedback regulation is however untied through various mechanisms in response to a variety of stress signals, including DNA damage, hypoxia, oncogene activation and ribosomal stress, leading to p53 activation and consequently cellular responses, such as DNA repair, cell-cycle arrest, senescence, apoptosis and metabolic alterations. On mechanism is through p53 acetylation by p300/CBP and deacetylation by SIRT1 or HDAC1. Deacetylation of p53 by SIRT1 facilitates MDM2/MDMX-mediated p53 degradation, while p53 acetylation by p300/CBP prevents p53 ubiquitination by MDM2/MDMX, thus stabilizing p53 in response to these stress signals
The physiological relevance of the MDM2/MDMX-p53 regulation has been also demonstrated in mouse genetic studies [22, 41, 42]. For example, embryonic lethality of either MDM2 or MDMX-null mice can be completely rescued by the simultaneous deletion of the TP53 gene. In addition, compared to wild-type adult mice, genetically engineered mice expressing reduced levels of MDM2 and MDMX are small in size, have reduced organ weight, and are radiosensitive [43]. The p53 dependence of these phenotypes has been shown by reversing the phenotypes when crossed with p53-null mice. Tissue-specific deletion of either the MDM2 gene or the MDMX gene showed differences between cell types for their dependency on MDM2 or MDMX to keep p53 in check. Together, these genetic studies demonstrate that MDM2 and MDMX are critical for the regulation of p53 functions during embryonic development as well as in adult mice, and the changes of MDM2 and MDMX levels can dictate abnormality and tumorigenesis.
Targeting the p53-MDM2-MDMX Loop for Cancer Therapy
The highly cancer-pertinent and well-defined p53-MDM2-MDMX pathway offers multiple molecular targets for screening small molecules as potential therapies for wild type p53-harboring cancers. Over the past decade, a number of new small molecules in addition to the previously reported Nutlin-3 [44] have been identified to target either MDM2 or MDMX or both in this pathway, some of which as listed in Table 16.1 have been further advanced to the stage of clinical trials.
Table 16.1.
Inhibitors targeting MDM2-MDMX loop discovered by different strategies
| Small molecule | Status | ID | Scaffold | Discover methods |
Ki or IC50 | In vivo test | Reference |
|---|---|---|---|---|---|---|---|
| M2-p53 interactiona |
Preclinical | Nutlin-3c | Imidazolines | HTS, SPR assay |
~0.1 µM | Retinoblastoma | [44] |
| Phase I | RG7112 (RO5045337) |
18 nM | Advanced malignancies, except leukemia |
[100] | |||
| M2-p53 interaction |
Preclinical | MI63, MI-219 | Spirooxindoles | Structure-based design |
~1–50 nM | SJSA-1 xenograft | [94] |
| Phase I | MI-773 (SAR405838) |
Advanced cancer | [63] | ||||
| M2-p53 interaction |
NSC333003c | Benzothiazoles | Virtual screening |
~20 µM | [150] | ||
| M2-p53 interaction |
Preclinical | TPD222669 | Benzodiazepines | HTS ThermoFlor |
0.08 µM | A3 7 5 xenograft | [151] |
| M2-p53 interaction |
Preclinical | YH265 | Pyrazoles | HCS, Biosensor | 1.8 µM | NCI60 cancer cells | [83, 84] WO2011106650 |
| M2-p53 interaction |
Phase I | CGM097 (Novartis) |
<50 nM | Selected advanced and refractory solid tumors |
[63] | ||
| M2-p53 interaction |
Phase I | MK-8242 (Merck) |
Piperidines | [63] | |||
| M2-p53 interaction |
Preclinical | PXN727 (Priaxon) |
Isoquinoline-1-ones, pyrrolinones |
Virtual screening |
8 nM-1 µM | LNCaP xenograft | http://www.priaxon.com/content/_docs/docl264519272209.pdf |
| M2-p53 interaction |
Tetra-substituted heteroaryls |
Structure-based design |
1 nM-50 µM | [63] | |||
| M2-p53 interaction |
c | Substituted pyrazoles |
Structure-based design |
M2: ~0.02 µM; MX: ~10 µM |
|||
| M2-p53 interaction |
c | Indole-2-carboxylic acids |
Structure-based design |
130–400 nM | |||
| M2-p53 interaction |
Indolinones | Structure-based design |
1.2nM-3.7nM | ||||
| M2-p53 interaction |
Piperidines | Structure-based design |
0.02–0.27 µM | ||||
| M2-p53 interaction |
Preclinical | AM-8553 | Piperidinones | Structure-based design |
150 nM | SJSA xenograft | [96] |
| M2-p53 interaction |
Preclinical | Pyrrolidone | Virtual screening, FP assay |
0.26 µM | A549 xenograft | [152] | |
| MX-p53 interactionb |
Preclinical |
SJ-172550c SJ000558295 |
Imidazolines | HTS,FP assay HTS.FP |
~1 µM M2:9.1 µM; MX: ~9.0 µM |
Retinoblastoma cells |
[59] [63] |
| Preclinical | SJ000558304 | HTS.FP | M2:>150nM; MX: ~2 µM |
||||
| MX-p53 interaction |
XI-006 | Benzofuroxans | HCS, luciferase reporter assay |
MCF-7 cells | [60] | ||
| Dual inhibitor | Preclinical | RO-2443, RO-5963c |
Indolyl Hydantoins | HTS,TR-FRET Assay |
M2 and MX: <50 nM |
MCF7, HCT, RKO cells |
[61] |
| Dual inhibitor? | Phase I | RO5503781 | Indolyl Hydantoins? | Soft tissue sarcoma; leukemia |
[103] NCT01462175 |
||
| Dual inhibitor | Novartis | Isoquinolinones | TR-FRET | M2: 0.8 nM; MX: 1.36 µM |
[63] | ||
| Dual inhibitor | Imidazoles, imidazolines |
Virtual screening, MCR |
M2: 15 nM; MX: 1 µM |
HCT116 cells | [65] | ||
| M E3 ligase inhibitor |
Preclinical | MEL23, MEL24 | Cell-based assay |
7.5 µM, 9.2 µM | U20S, HCT116 et al. |
[49] | |
| M2 E3 ligase inhibitor |
Preclinical | MPD | Cell-based assay |
1–50 µM | [48] | ||
| M2 E3 ligase inhibitor |
Preclinical | HLI98 | Cell-based assay |
−50 µM | [47] | ||
| M2-proteasome interaction |
Phase I | JNJ-26854165 | Cyclic alkyl amines | Cell-based assay |
>0.5 µM | Advanced stage solid tumors |
[45] WO2008132175 |
| p53 N terminal binder? |
Preclinical | RITA | Cell-based assay |
[153, 154] | |||
| Peptidic compound | |||||||
| Dual inhibitor | Preclinical | SAH-p53-8 | Phage library | M2: 55 nM; MX: 2.3 nM |
Cutaneous melanoma |
[87,88, 114] | |
| Peptidic compound | |||||||
| Dual inhibitor | β53–16 | Phage library | M2: ~50 nM; MX: >100 nM |
[155] | |||
| Peptide | |||||||
| Dual inhibitor | (D)PMI-gammac | DWWPLAFEALLR | Mirror image phage display |
M2 and MX: <50 nM |
U87 xenograft | [79] | |
| Peptide | |||||||
| Dual inhibitor | PDIc | LTFRYWC1RQLS | Phage library | 220 pM | [80] | ||
M2 indicates MDM2
MX indicates MDMX
3D co-crystal structures are available via PDB protein data bank
For example, a small molecule named JNJ-26854165 was shown to bind the RING domain of MDM2 and prevent the interaction of the MDM2-p53 complex with the proteasome and has been put on phase I clinical trial for advanced or refractory tumor [45, 46]. Also, the other three small compounds, called HLI98, MPD and MEL, respectively, were discovered to inhibit the E3 ubiquitin ligase activity of HDM2, preventing p53 degradation [47–49]. Furthermore, NSC279287, NSC66811 and terphenyl compounds are small molecules that can also disrupt the MDM2-p53 interaction [50–52]. Additional small molecule inhibitors of the MDM2-p53 interaction have been revealed later on, such as TDP521252, TDP665759, PXN727, PXN822 and isoindolinones, which are currently under pre-clinical development [53–57]. By contrast, the two molecules SJ172550 and XI-006 were recently identified to restore WT p53 activity by targeting MDMX. SJ172550 interferes with the MDMX-p53 interaction [58, 59], while XI-006 inhibits MDMX transcription [60]. Interestingly, the indolyl hydantoin class of compounds, RO-2443 and RO-5963, demonstrated as dual inhibitors of the MDM2/MDMX-p53 binding display the potential for further chemical optimization to achieve more desirable pharmacological characteristics with improved potency [61]. More interestingly, naturally-derived molecules have been found to inhibit the p53-MDM2 interaction. Some of them have been shown to decrease MDM2 expression and activity in vitro and in vivo. These newly identified natural MDM2 inhibitors include a plethora of diverse chemical frameworks, ranging from flavonoids, steroids, and sesquiterpenes to alkaloids. Their complex and unique molecular architectures could stimulate further development of synthetic analogs in the near future (see review for more information in [62]). Remarkably, although several advanced synthetic small molecule inhibitors, such as RG7112, MI-773, CGM097, and MK-8242, which interfere with p53-MDM2 interactions based on imidazoline Nutlin, spirooxindole MI-219 and undisclosed scaffolds, have entered Phase I clinical trials, the game of drug screening for small molecules that target this pathway will not be ending, as more patents and patent applications related to MDM2 and/or MDMX inhibitors and more deals among biotech/ pharmco companies/universities and institutions have been either submitted or undergoing over the past several years (see review articles in [63–65] for more information). In addition to the aforementioned direct inhibitors, indirectly interrupting the MDM2-p53 negative feedback loop by enhancing p53 acetylation, which prevents MDM2-mediated degradation of p53 [66, 67], has also been explored for the development of anti-cancer molecule-target therapy. For instance, several studies demonstrated that attaining p53 acetylation through inhibition of its deacetylase SIRT1 [68–70] or activation of the acetyltransferase p300 [71] by small molecules leads to the inhibition of tumor growth.
These exciting studies not only consolidate the p53-MDM2-MDMX pathway as a valid target, but also provide multiple candidates for their future development into clinically applicable anti-cancer drugs. Here, we review the strategies that have been employed to identify and discover MDM2/MDMX-targeted inhibitors, including high-throughput screening (HTS) using biochemical, physicochemical and/or cell-based assays, combinatorial chemistry, and computational aided drug design. We will also provide the status of small molecules that are currently in clinical trials. Finally, we will discuss other potential and therapeutically useful approaches targeting the p53-MDM2-MDMX loop.
Discovery of Inhibitors Targeting the p53-MDM2-MDMX Loop
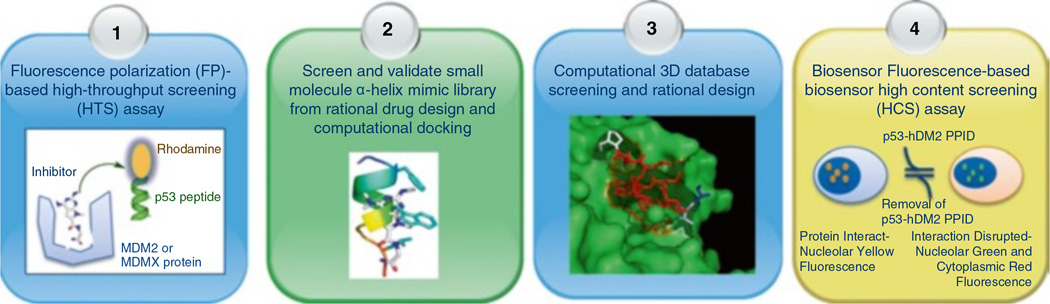
As briefly described above, numerous small molecules, such as synthetic small-molecules, small peptides, peptidomimetics, or natural products, have been revealed to target the p53-MDM2-MDMX pathway. These molecules are identified via a variety of drug discovery strategies that have been undertaken to block p53-MDM2/MDMX interactions, or modulate MDM2’s E3 ubiquitin ligase activity, and/or inhibit MDM2-mediated ubiquitination of p53 (Table 16.1). However, the main strategies are high-throughput screening (HTS) using biochemical, physicochemical and/or cell-based assays, combinatorial chemistry and computational aided drug design including structure-based rational drug design and virtual screening. Because of the complexity of the p53 pathway as a drug target, combining different drug screening or design approaches, such as crystal structure-guided molecular design, biochemical assays and cell-based phenotypic approaches, would be more successful in developing more effective and selective drugs targeting this pathway. In this section, we provide a detailed description of several frequently used strategies as follows (Fig. 16.2).
Fig. 16.2. Drug discovery strategies for identification of antagonists of MDM2 and MDMX.
Some representative strategies for Drug discovery by searching MDM2 and/or MDMX antagonists are shown here. For details, see the description in sections “High-throughput Screening”, “α-Helix Mimetic Based Chemistry or Combinational Chemistry”, and “Computational Aided Drug Design”
High-Throughput Screening
A number of screening strategies are based on the available 3D structures of either the MDM2-p53 or MDMX-p53 complex, as the X-ray crystal structure of human MDM2 (aa, 17–125) bound by a 15-residue p53 peptide (aa, 15–29) (PDB: 1YCR) and the structure of human MDMX (aa, 1–140) with p53 peptide (PDB: 2Z5X, 2Z5T) revealed crucial binding information in these complexes [72]. Although designing non-peptidic small-molecule inhibitors disrupting the binding of p53-MDM2/MDMX has been challenging, the structures provide a good starting point for developing affinity-based assays for high throughput screenings in order to identify lead compounds. Such screening approaches include surface plasmon resonance, fluorescence polarization, and fluorescence resonance energy transfer as well as fluorescence-correlation spectroscopy experiments. A secondary NMR-based and isothermal calorimetric approach is often used to further confirm the identified molecules and to determine the (apparent) dissociation constant (KD) between p53 and MDM2 or MDMX in the presence of the molecules or the affinity between the proteins and the molecules. Following these steps, identified compounds are routinely tested for their ability to inhibit proliferation of wild type p53-, but not mutant p53- or null, containing cells in order to determine if their cellular effects are due to their specific inhibition of MDM2 or MDMX. Now let us walk you through each of these comprehensive approaches below.
Surface Plasmon Resonance
The first potent small molecule inhibitor of MDM2 capable of activating p53 in cells and in vivo was cis-imidazoline or Nutlin as reported by the Hoffman Roche team led by Vassilev, L. T. in 2004, 8 years after the first published p53-MDM2 crystal structure [44, 72]. It was discovered by screening a diverse library of synthetic compounds using surface plasmon resonance (SPR). The SPR assays were performed on a Biacore Series S Sensor chip CM5 derived from immobilization of a Penta-His antibody for capture of the His-tagged p53 protein (aa, 1–312). The assays were conducted with MDM2 fragments (aa, 25–108). Nutlins were one class of lead structures identified and optimized for potency and selectivity. These compounds displaced the recombinant p53 protein from its complex with MDM2 at inhibitory concentration (IC50) values in the 100–300 nM range. Nutlin-1 and Nutlin-2 are racemic mixtures, and Nutlin-3a is an active enantiomer isolated from racemic Nutlin-3. The IC50 of Nutlin-3a is 90 nM, which is 150 times more potent than the enantiomer-b of Nutlin-3. X-ray crystal structure of the MDM2-Nutlin-2 complex showed that Nutlin binds to the p53 binding site in MDM2 [PDB: 1RV1]. Nutlin-2 mimics the interaction between the p53 peptide and MDM2 with a bromophenyl moiety sitting deeply in the Trp23 pocket and the other bromophenyl group occupying the Leu26 pocket. The ethyl ether side chain is directed towards the Phe19 pocket. Computational modeling of Nutlin-3 with MDMX suggested that Nutlin-3 might also block the MDMX-p53 interaction. However, Nutlin does not bind to MDMX as efficiently as to MDM2 due to structural differences in the p53-binding pockets of the MDM2 and MDMX proteins [73]. Consistent with these observations, Nutlin-3a binds MDMX with at least a 40-fold weaker equilibrium binding constant (Kd) than that for MDM2 [61]. Recent model simulations also indicate that the inherent plasticity of MDM2 is higher than that of MDMX, enabling it to bind both p53 and Nutlin. The less flexible MDMX interacts with the more mobile p53, because the peptide can adapt different conformations to dock to MDMX, albeit with a reduced affinity. Nutlin, however, is rigid and hence can only interact with MDMX with low affinity [74]. This series of studies sets up the first successful example of targeting the MDM2-p53 interaction for anti-cancer drug discovery.
Fluorescence Polarization
However, MDMX inhibitors were not reported until more than half a decade later when a high throughput screen campaign of a library consisting of around 300,000 chemicals resulted in the identification of SJ-172550 by using a fluorescence polarization (FP) assay [59]. The FP assay was carried out using FITC labeled p53 peptide (a.a.15–29) and MDM2-GST (a.a. 1–188) or MDMX-GST (a.a. 1–185). The specificity of this FP assay was confirmed by the competitive displacement of unlabeled p53 peptides and Nutlin. Over 1,150 active compounds from the FP assay were further subjected to a cytotoxicity cell-based assay using retinoblastoma cells with MDMX amplification. Further studies of SJ-172550 using p53-deficient (SJmRbl-8) cells and the BJ cells, a human foreskin fibroblast cell line, as an additional control to estimate general cytotoxicity of the compounds, indicated its p53-dependent cytotoxicity [59]. It was through this series of screening that this compound was finally selected as the most potent compound. Interestingly, it contains a substituted imidazoline group similar to that in Nutlins. Although this compound could effectively kill retinoblastoma cells, a series of biochemical and structural modeling studies suggested that SJ-172550 alkylated Cys76 of MDMX protein and could lock MDMX into a conformation to block p53 binding. Disappointingly, this covalent binding hinders the further development of SJ-172550 [58]. Regardless of this obstacle, the same research group reported two novel small molecules, SJ000558295 (IC50: MDM2 = 9.1 µM; MDMX = 9.0 µM) and SJ000558304 (IC50: MDM2 = 14.4 µM; MDMX = 9.0 µM), as MDM2 and MDMX inhibitors, as shown in a recent international PCT application (WO2012045018). Among the two, SJ000558304 was with a reportedly improved binding affinity for MDMX over MDM2 in contrast to Nutlin-3. The affinity of these compounds to MDMX was further confirmed by nuclear magnetic resonance (NMR), monitoring the compound-induced NMR chemical shift perturbations. These compounds displayed desired pharmacokinetic properties and may be eventually developed into a therapy for pediatric cancers and various adult tumors that overexpress MDMX or have similar genetic lesions, though more studies are necessary to achieve this clinical goal.
Time Resolved-Fluorescence Resonance Energy Transfer
Because MDM2 inhibitors do not inhibit the p53-MDMX interaction, and their effectiveness can be compromised in tumors overexpressing MDMX, developing dual MDM2/MDMX antagonists is necessary to restore p53 apoptotic activity in the presence of high levels of MDMX and may offer a more effective therapeutic modality for MDMX-overexpressing cancers. To reach this goal, Hoffmann-La Roche identified indolyl hydantoins as dual MDM2/MDMX antagonists from a diverse small-molecule library using a Time Resolved-Fluorescence Resonance Energy Transfer assay (TR-FRET) [61]. The TR-FRET assay was performed using GST-tagged MDM2 or GST-MDMX and biotinylated p53 peptide, europium (Eu)-conjugated streptavidin and allophycocyanin (APC)-conjugated anti-GST antibody. Binding signals were monitored by reading excitation at 340 nm and emission fluorescence at 615 nm and 665 nm. A series of indolyl hydantoin compounds emerged as potent, dual MDM2/MDMX antagonists. The binding potency and binding mode were further measured and verified by Trp fluorescence quenching, ITC, NMR and X-ray crystallography (PDB: 3U15, 3VBG). For example, RO-2443 showed a remarkably similar inhibitory activity against both MDM2 (IC50 = 33 nM) and MDMX (IC50 = 41 nM) binding to p53. For its size, RO-2443 appeared highly potent and likely to fit into, at most, two of the three sub-pockets on the surface of MDMX or MDM2. These pockets were defined by the original structure of a p53 peptide bound to MDM2, which showed three key residues of the p53 peptide, Phe19, Trp23, and Leu26, important for MDM2 binding. Crystal structures of MDMX bound to RO-2443 revealed that the inhibitor blocks the interaction of p53 by inducing the formation of dimeric complexes of MDMX. Further modifications of RO-2443 in part to improve its physicochemical properties resulted in RO-5963. As expected, RO-5963 restored p53 transcriptional activity and overcame the apoptotic resistance of MDMX-overexpressing cell line, SJSA-X, to Nutlin-3. Therefore, this type of MDM2/MDMX dual inhibitors could be potentially developed into more potent anti-cancer drugs, though more studies are apparently necessary to translate them into clinical therapy.
Fluorescence Correlation Spectroscopy-Based Assay
The researchers from the University of Tokyo identified new MDMX inhibitors after screening almost 40,000 commercially available compounds by employing a fluorescence correlation spectroscopy (FCS)-based high-throughput screening (HTS) assay [75]. In this assay, an MDMX fragment (aa 26–106) was fused to a green fluorescent protein (GFP), and a p53 peptide was labeled with a fluorescence quencher. The MDMX-p53 interaction was evaluated by detecting the quenching of the fluorescence of GFP; the inhibition of the interaction was detected by the recovery of GFP fluorescence. Two hundred and fifty five compounds were selected after the first screening, and six of these compounds were further confirmed by SPR binding assay to have IC50 values less than 5 µM. The hit compound 1 increased p53 and p21 levels and triggered apoptosis in wild type p53-containing MV4-11 leukemia cells and more selectively killed MV4-11 compared to H1299 p53-null and WI38 normal cells. This study presents another new compound that can inhibit MDMX-p53 binding and activate p53 as a promising anti-cancer drug candidate.
ThermoFluor Microcalorimetry
3DP (3-Dimensional Pharmaceuticals) (Yardley, PA, USA), which was later merged with Johnson & Johnson, reported a MDM2 complex containing a benzodiazepinedione (BDP) based inhibitor (PDB: 1T4E) [76, 77]. The scaffold was found by HTS with the temperature-dependent protein-unfolding assay using the ThermoFluor microcalorimetry technology. In this technology, fluorescent dyes were used to monitor protein unfolding as a function of temperature for the identification of compounds that bind to MDM2. Detection of compound binding to MDM2 was measured by the resultant increase in thermal stability. Thermal stability was quantified as a change in midpoint transition temperature in the presence of the compound at a single concentration. The sensitivity of this assay was verified by the shift in Tm on addition of peptides known to bind to MDM2, with higher-affinity peptides generating larger shifts. From the initial screening, 1,216 compounds out of 338,000 compounds from combinatorial libraries were selected, which included 116 compounds belonging to a benzodiazepinedione library. The affinity of the selected compounds was determined using an FP-based assay to monitor the binding of p53 peptide to MDM2. TDP222669 with a benzodiazepinedione scaffold and a carboxylic acid group was proved to be the most potent inhibitor with a binding affinity of 80 nM. The crystal structure of its complex with an MDM2 fragment demonstrated that it occupies the same pocket as the peptide side chains Phe19, Trp23, and Leu26 of p53 in the MDM2 binding cleft. The MDM2 interactions with the inhibitor are largely nonspecific van der Waals contacts and, similar to the p53 peptide; the bound conformation of the inhibitor is amphipathic. Unfortunately, despite the initial tremendous effort, this series of compounds series seems to have been abandoned, most likely because of their insufficient drug-like properties.
Screening of Phage Display Library
Although most of the screening against the MDM2/MDMX-p53 interaction have been based on biochemical and biophysical assays as discussed above, a high-affinity peptide inhibitor of p53 interaction with MDM2 and MDMX was also identified by screening a duodecimal peptide library displayed on M13 phage using site-specific biotinylated p53-binding domains of human MDM2 and MDMX [78]. The peptide inhibitor (TSFAEYWNLLSP), termed PMI, bound to MDM2 and MDMX with low nanomolar affinities-approximately 2 orders of magnitude stronger than the wild-type p53 peptide of the same length (Kd: 3.6 nM for MDM2; 4.15 nM for MDMX). The crystal structure of MDM2 or MDMX in complex with PMI was solved at 1.6 Å resolution (PDB: 3EQS, 3EQY). An extensive, tightened intramolecular H-bonding network in the PMI-bound complex was identified by comparative structural analysis. The H-bonding network contributed to the conformational stability of this complex, thus enhancing binding to MDM2 and MDMX proteins. Importantly, the C-terminal residue Pro of PMI induced the formation of a hydrophobic cleft in MDMX previously unseen in the structures of p53-bound MDM2 or MDMX [78].
Small peptides are often capable of efficiently blocking protein interactions with high affinity and supreme specificity. However, their poor in vivo stability and membrane permeability impede the thriving of peptide-based therapeutics. To overcome their instability in vivo, aided by native chemical ligation and mirror image phage display, several proteolysis-resistant D-peptide inhibitors termed D-PMI-α, β, γ were identified to bind MDM2 with Kd values of 50–200 nM [79]. Mirror image phage display is a straightforward technique to identify proteolysis-resistant D-peptide ligands of a native protein through phage library screening against the D enantiomer of the L target. The screening of the duodecimal peptide phage library was carried out against N79K-biotin-d-25–109MDM2 immobilized on streptavidin- agarose resin. Bound phage particles were competitively eluted with 1 mM D-15–29p53, and subsequently amplified in host strain Escherichia coli ER2738. The D-peptides from the screening was confirmed by SPR and FP assays, and structural studies coupled with mutational analysis also verified the mode of action of the D-peptide as an MDM2-dependent p53 activator (PDB: 3IWY). Despite being resistant to proteolysis, both (D) PMI-alpha and (D) PMI-gamma failed to actively traverse the cell membrane and, when conjugated to a cationic cell-penetrating peptide, were indiscriminately cytotoxic independently of p53 status. This issue was solved when encapsulated in liposomes decorated with an integrin-targeting cyclic-RGD peptide. By using this approach, (D) PMI-alpha was found to exert potent p53-dependent growth inhibitory activity against human glioblastoma in cell cultures and nude mouse xenograft models. The findings validate D-peptide antagonists of MDM2 as a class of p53 activators for targeted molecular therapy of malignant neoplasms harboring WT p53 and elevated levels of MDM2. Remarkably, this group led by Lu, W has continued to modify this type of D-peptides and recently reported a superactive D-peptide antagonist of MDM2, termed D-PMI-δ (Kd = 220 pM) [80]. Their X-ray crystallographic studies (PDB: 3EQS, 3LNJ) validated D-PMI-δ as an exceedingly potent inhibitor of the p53-MDM2 interaction, which could be a highly attractive anti-cancer drug candidate. The field will be excited to see its future success as the first peptide drug for cancer therapy.
In Vitro Autoubiquitination and MDM2-Catalyzed p53 Ubiquitination Assay
Beside the aforementioned N-terminal p53-binding pocket of MDM2 or MDMX, the C-terminal RING finger domain with an intrinsic E3 ubiquitin ligase activity of the MDM2 protein has also been utilized as a target for anti-cancer drug screening. There have been at least two types of high throughput assays that are aimed at the discovery of inhibitors that might modulate ligase activity of MDM2. One of them measures the ubiquitination of p53 by MDM2, and the other measures the self- ubiquitination of MDM2. The Vousden team first identified MDM2 ubiquitin ligase inhibitors, 7-nitro-5-deazaflavin compounds (HLI98s), through an HTS of small- molecule libraries of 10,000 compounds using an in vitro MDM2 autoubiquitination assay [47]. HLI98 compounds specifically inhibited the RING finger domain of MDM2, and not the regions that interact with p53. When primary human fibroblasts were treated with HLI98 compounds, both p53 and MDM2 levels increased. Ubiquitylated p53 was not detected, which is consistent with the ability of the compounds to inhibit ubiquitylation instead of proteasome function. HLI98 compounds showed some selectivity for MDM2 compared with other RING finger E3s in cell lines. The compounds did not stabilize p53 in the absence of MDM2 in mouse embryonic fbroblasts (MEFs), indicating that they do not inhibit other reported E3s that target p53 for degradation, such as PIRH2 and COP1. Although the compound stabilized p53 and MDM2, and activated p53-dependent transcription and apoptosis, it also had p53-independent cytotoxicity. Furthermore, as expected, the compound worked much better in cancer cells containing wild-type p53 than in those containing mutant p53, because targeting MDM2 should, in theory, have little or no effect on human cancers with mutant p53. However, in vivo antitumor activity of HLI98, using human xenograft models has not been reported. Therefore, the anti- cancer potential of this compound remains to be further investigated.
A novel electrochemiluminescent assay system was developed to monitor the inhibition of MDM2’s E3 ubiquitin ligase activity for a high-throughput screening of natural product extracts [81]. This assay was used to screen more than 144,000 natural product extracts. Interestingly, sempervirine was identified to inhibit MDM2 auto-ubiquitination and MDM2-mediated p53 degradation, and to induce p53 level in cancer cells. Consequently, this natural product preferentially induced apoptosis in transformed cells expressing wild-type p53, suggesting that it could be a potential lead natural product for anticancer therapeutics. In addition to this, three new alkaloids, isolissoclinotoxin B, diplamine B, and lissoclinidine B, have also been identified from Lissoclinum cf. badium. Lissoclinidine B was found to inhibit ubiquitylation and degradation of p53 and selectively kill transformed cells harboring wild-type p53, suggesting this compound could also be used for the development of new anti-cancer treatments.
Additionally, Murray MF et al. developed a TR-FRET assay for measuring the ubiquitination of p53 by MDM2 and executed an HTS campaign with >600,000 compounds [82]. An assay for MDM2 autoubiquitination was also developed using the same TR-FRET format to find compounds selective for p53 ubiquitination versus MDM2 autoubiquitination. The most selective compound identified displayed an IC50 of 8 µM in the MDM2/p53 assay and no discernible inhibition up to 100 µM in the autoubiquitination assay. These studies aimed at targeting the RING finger domain of MDM2 could be promising, though much more remain to be done before an ideal candidate with a more specific inhibitory activity toward MDM2 could be eventually identified.
Cell-Based Auto-ubiquitination Assay
It has been believed that MDM2 can regulate itself in cells through a so-called auto- ubiquitination and auto-degradation mechanism. Based on this notion, Herman, A. G. et al. developed a high-throughput cellular MDM2 auto-ubiquitination assay to discover a class of small-molecule MDM2-MDMX ligase inhibitors [49]. In this assay, the compounds were screened using wild-type MDM2 or mutant MDM2 (C464A)-luciferase cell lines. The C464A mutation disrupts a metal-binding site in the RING domain, thereby eliminating the MDM2 E3 ligase activity. Compounds that specifically increase the luminescence of MDM2 (wt)-luciferase fusion protein rather than MDM2 (C464A) would likely inhibit MDM2 E3 ligase activity or proteasomal degradation of MDM2. Two of such compounds, MEL23 and MEL24, were identified. They were found effectively to inhibit the E3 ligase activity of the MDM2-MDMX hetero-complex, thereby inhibiting MDM2 and p53 ubiquitination in cells, reducing viability of cells with wild-type p53, and synergizing with DNA- damaging agents to cause cell death. Also, the activity of MEL compounds was specific to MDM2, and independent of p53 transcription. As MDM2-MDMX ligase inhibitors, MEL compounds may be used as molecular tools to validate novel targets of MDM2-MDMX and to inhibit MDM2’s E3 ligases, which could be beneficial in diverse applications, as it remains to be determined if they could be druggable candidates or not.
Fluorescence-Based Biosensor High Content Screening Assay
Fluorescence-based biosensor high content screening (HCS) is a cell- based assay that was developed recently by a research team from University of Pittsburgh. Compared to biochemical assay-based HTS screening, an HTS application in living cells by a biosensor assay can be used to only identify the “drug like” compounds that are able to block the p53-MDM2 interaction in the nucleus of cancer cells [83]. In this novel imaging-based biosensor HCS assay, the recombinant adenovirus biosensors used to express the N-terminal domains of p53 and MDM2 were fused to green or red fluorescent proteins (GRP or RFP) and co-infected in U2OS cells. By monitoring the p53-MDM2 interactions through changes in the subcellular localization of the MDM2 component of the biosensor and analyzing the multiparameter data from images of the 3 fluorescent channels, this team was able to identify and eliminate compounds that were cytotoxic or fluorescent artifacts [83]. They identified compounds with methylbenzo-naphthyridin-5-amine (MBNA) after screening a collection of over 220,000 compounds using this novel biosensor HCS assay. The MBNAs were proved to induce p53 protein levels, increase the expression of p53 target genes, cause G1 arrest during the cell cycle, induce apoptosis, and inhibit cell proliferation with an IC50 ~ 4µM in p53-WT HCT116 cells. However, unlike Nutlin-3, MBNAs also increased the percentage of apoptosis in p53 null cells and exhibited similar potencies for growth inhibition in isogenic cell lines null of p53 or p21. Therefore, these compounds might target other cellular proteins and have p53-independent cytotoxicity.
Reported by the same group, compounds containing the substituted pyrazole scaffold were also identified by employing this biosensor HCS assay [84]. The potency of the best compound YH265 in the biosensor assay was determined at 1.8 µM (WO2011106650). The affinity (Ki) of the selected compounds toward MDM2 and MDMX was further demonstrated in vitro FP assays. The affinity toward MDM2 and MDMX ranged from 0.02 to 10 µM. The ability of these compounds to dissociate MDM2-p53 or MDMX-p53 complexes was confirmed based on fluorescent polarization analysis, AIDA NMR and HSQC NMR analysis. Selected compounds were also tested on the NCI60 cell panel, and the results showed that the compounds could arrest the growth of various cancer cells in culture. The crystal structure of a compound analogous to YH265, but based on an imidazole rather than pyrazole scaffold, bound to MDMX has been solved (PDB: MDMX: 3LBJ, MDM2: 3LBK) and represents the only MDMX small molecule co-crystal structure so far [see a recent review of available structural information on MDM2/X-inhibitor interaction for more details [77, 85, 86]]. The YH265 analogs could be hot candidates for further development, although this set of cell-based biosensor screening might yield more candidate compounds that target the MDM2-MDMX-p53 loop.
α-Helix Mimetic Based Chemistry or Combinational Chemistry
HTS is not the only strategy that has been employed to identify small molecules or peptides that might target the MDM2-MDMX-p53 loop. Other approaches, such as rational design to imitate the structure of the N-terminal MDM2/MDMX-binding domain of p53, have also been explored. For instance, using a chemical approach termed “hydrocarbon stapling”, Federico Bernal et al. designed Stabilized Alpha Helix of p53 (SAH-p53) [17, 87, 88] peptides based on the peptide sequence of the p53 transactivation domain α-helix [87, 88]. They replaced natural amino acids at positions S20 and P27 with synthetic olefinic residues and generated the structurally reinforcing hydrocarbon staple by olefin metathesis. Other residues within the MDM2/MDMX-binding domain of p53 not required for MDM2/MDMX interaction were also modified to improve peptide solubility and uptake. The newly designed SAH- p53 peptide was found to preferably bind to MDMX compared to MDM2 (Kd for MDMX: 2.3 ± 0.2 nM; Kd for MDM2: 55 ± 11 nM) and subsequently reactivate the p53 pathway and suppress tumor growth by targeting MDMX. Also this novel MDMX-binding peptide restored the sensitivity of Nutlin in cancer cells with high levels of MDMX expression and in JEG-3 xenograft mice with little toxicity.
Similarly, a peptidomimetic strategy was also employed to synthesize small molecules with a peptidomimetic 1,4-thienodiazepine-2, 5-dione scaffold as an α-helix mimetic of the MDM2-binding peptide of p53 to disturb the p53-MDM2 interaction [89]. This strategy takes advantage of an Ugi-deprotection-cyclization reaction that has been exploited in combinatorial chemistry because it combines four separate components to make one scaffold, providing an easy access to create diversity around a single scaffold. A small library of 18 diverse thienodiazepine-2, 5-diones selected from a large virtual library was prepared in one pot by solution phase synthesis via an Ugi-deprotection-cyclization reaction. The compounds were found to antagonize the p53-MDM2 interaction in an FP assay, exhibiting a dose dependent effect to compete with a p53-like peptide. Also, in an NMR competition assay, two compounds were found to dissociate the MDM2-p53 complex with Kd values of 30 ± 20 µM and 10 ± 6 µM, respectively [89]. Further studies are needed to show its clinical application for cancer therapy.
Recently, a library of 900 compounds based on a pyrrolopyrimidine scaffold as an α-helix mimetic, was prepared by using solid phase parallel synthesis in hope to discover small molecules able to disrupt the interaction between p53 and MDMX/ MDM2 [90]. The structural basis for their pyrrolopyrimidine-based molecules is Hamilton’s terphenyls, which are among the most prominent and most-imitated scaffolds in the field. However, this work is much more than a simple variation of the terphenyl scaffold. It rises above many other imitators through a clever set of features, including the use of a scaffold known by medicinal chemists to have favorable aqueous solubility and cell permeability, a unique and simple synthesis route that is immediately amenable to library generation, an FP screening approach for inhibitor discovery rather than reliance on rational design. Two compounds from the library were identified as sub-micromolar p53-MDMX and p53-MDM2 inhibitors in the FP binding assay, a relatively novel profile, especially for a small molecule. Their activity was also shown in cells for dose-dependent increase of p53 and triggering of apoptosis. Although the properties of these pyrrolopyrimidine derivatives established them as promising lead compounds for further structural elaboration toward p53-MDM2/MDMX inhibitors with improved drug-like attributes, much more need to be done to verify the pharmacological activity of these small molecules and to further develop them into a possible anti-cancer therapy. This approach might also be useful for targeting other protein-protein interactions.
Finally, a fragment-based strategy, involving “multicomponent reaction chemistry” (MCR), identified imidazolines as dual MDM2/MDMX inhibitors [91]. The crystal structures of p53-MDM2 (PDB: 1YCR) and imidazole antagonist PB12 bound to MDM2 (PDB: 3LBK) were used as templates to identify a fragmentation/anchor. The anchor was imported into a database containing MCR-accessible scaffolds to generate a virtual library of compounds, which subsequently were docked into the binding pocket of the target protein. Results from docking then were used to select compounds for synthesis and complementary screening by an NMR- based binding assay. Building upon the success of the imidazole and using multicomponent reactions, compounds with imidazoline scaffolds were identified with low micromolar activity in HCT116 cancer cells, claiming dual inhibition of the p53-MDM2 as well as p53-MDMX protein interactions with a Kd < 1 µM (US20080280769) [63]. With alternative structures to Nutlins, which require a rather lengthy synthetic route with more than eight individual steps, imidazolines are accessible via a straightforward multicomponent reaction in just one or two steps.
In summary, using the p53 peptide imitating strategy, several promising mimetic peptides or small molecules that could inhibit the interaction of MDM2 and/or MDMX with p53 have been discovered. Although none of them has yet been developed to the stage of preclinical studies, as more studies are necessary to demonstrate their druggable potential, this approach provides solid evidence for the proof of principle and would eventually offer some promising candidate molecules for anti-cancer drug development.
Computational Aided Drug Design
Medicinal chemistry is not merely limited to the mimetic-based design of inhibitors, but it also exploits advances in bioinformatics. Two major approaches in medicinal chemistry developed by taking advantage of information technology (IT)-based computational power are the straightforward in silico compound- selection (virtual screening) and structure-based de novo design. These tools have also been applied to the design of optimal MDM2/MDMX inhibitors. The compounds were designed to generally mimic the interactions provided by the important amino acid side chains, such as Phe19, Trp23 and Leu26, within the p53 peptide, to disrupt the p53-MDM2 and/or p53-MDMX bindings. In addition, it is believed that Leu22 is important for the p53-MDM2/X complex, and it could assist in the development of molecules with higher binding affinity than the p53 TAD peptide [92]. Based on structural analysis, the p53 residues Phe19 and Trp23 interact in a similar way to MDM2 and MDMX, but not Leu26. The interaction contributions by the MDM2 residues Leu54, His96 and Ile99 are different from the equivalent MDMX residues Met53, Pro95 and Leu98, which could account for the differential binding of the p53 peptide to MDM2/X.
Structure-Based de novo Design
Using a structure-based de novo design strategy, the Wang laboratory at the University of Michigan discovered the spiro-oxindoles MI-63 and MI-219 that are more potent and selective than Nutlin. In a similar fashion to the Nutlins, spiro-oxindoles bind MDM2 by mimicking the interactions of crucial hydrophobic residues (Phe19, Trp23 and Leu26) in the p53 peptide [93, 94]. The design started from a search for chemical moieties that can mimic the interaction of Trp23, the most critical for binding to MDM2. The results showed that oxindole can nicely mimic the side chain of Trp23 for interaction with MDM2. Further substructure search identified the natural alkaloids such as spirotryprostatin A and alstonisine, which both contain a spirooxindole substructure. Computational modeling studies predicted the oxindole can closely mimic the Trp23 side chain in p53 in both hydrogen-bonding formation and hydrophobic interactions with MDM2, and the spiropyrrolidine ring provides a rigid scaffold from which two hydrophobic groups can be projected to mimic the side chain of Phe19 and Leu26. Initial compounds were designed with different hydrophobic groups and different stereochemistry, and they were docked into the MDM2 binding cleft using the GOLD program. X-ray structure of the MDM2-p53 complex, mutagenesis and alanine scanning of p53 peptides suggested that a fourth residue, Leu22, also appears to play an important role in the overall interaction between p53 and MDM2. Structure-based optimization to capture the additional interaction between Leu22 in p53 and MDM2 yielded MI-63, and improvements to ensure sufficient water solubility ultimately yielded MI-219 with desirable pharmacological properties, such as 55 % oral bioavailability in mice. MI-219 was greater than 10,000-fold more selective for MDM2 over its closely related homolog MDMX (MDM2: Ki: 5 nM). Consistent with the high binding affinity for MDM2 and disruption of the MDM2-p53 complex, spiro-oxindoles induced accumulation of p53 in p53 wild-type cancer cells, and it showed anti-tumor activity in human tumour xenograft models. Extensive modifications have been made on spirooxindoles and MI-147 is one of the most potent of this class of compounds, with a Ki of 0.6 nM in an FP-based competitive binding assay [95]. Additional modifications yielded new compounds with high affinities to MDM2 and improved pharmacokinetic properties. One such compound in this class, MI-773, has completed Investigational New Drug (IND) -enabling studies and currently Sanofi-Aventis is recruiting participants for Phase I clinical trials with MI-773 [63] (reference spirooxindole SAR405838). This series of studies sets a perfect example for de novo drug design if the 3D structure of a protein or protein-protein complex is available.
In another study reported by Rew, Y. et al. from Amgen, a new class of potent MDM2-p53 inhibitors bearing a piperidinone scaffold has been successfully designed and optimized using the structure-based de novo design strategy [96, 97]. On the basis of the analysis of known MDM2 inhibitors, assisted by computer modeling, SAR studies and crystal structure (PDB: 4ERE), the affinity of these compounds for MDM2 was improved through conformational control of both the piperidinone ring and the appended N-alkyl substituent. Optimization afforded AM-8553, with a KD of 0.4 nM, especially with excellent pharmacokinetic properties. AM-8553 has an oral bioavailability of 100 % in rats and 12 % in mice. On the basis of its low human hepatocyte intrinsic clearance, AM-8553 was projected to have a long human half-life (>12 h). It is effective in inhibition of tumor growth in a dose-dependent manner and is capable of achieving partial tumor regression (R = 27 %) against SJSA-1 xenograft tumors in mice at 200 mg/kg once daily dosing [96].
Virtual Screening
Sulphonamide I (NSC279287) is the first example that was identified by screening the NCBI database using a computational pharmacophore model of MDM2 binding [50]. The HINT molecular modeling program was used to generate predictive QSAR regression equations for p53-MDM2 inhibition based on the template of the interaction between p53-based peptide inhibitors and MDM2, which led to the development of the pharmacophore model to mimic the portions of p53 necessary to bind to MDM2. In an ELISA-based p53-MDM2 binding assay, sulphonamide I showed an IC50 of approximately 32 µM compared to a value of 13 µM for the p53 peptide (16QETFSDLWKLLP27). The efficacy of NSC279287 to induce a p53 response was verified in a p53 reporter gene assay using MDM2-over-expressing osteosarcoma cells.
Another approach combined pharmacophore and structure-based screening that used computational database screening of a subset of the NCI database of 150,000 compounds, identifying 354 potential MDM2 inhibitors. A 3D-pharmacophore model was derived from the X-ray crystal structure of the p53 peptide associated with MDM2, with several known small-molecule inhibitors. The pharmacophore model consisted of three elements that mimic the three key hydrophobic binding residues in p53 (Phe19, Trp23, Leu26), together with three associated distance constraints. Computational docking was performed using the GOLD program to dock each hit to the p53-binding site in MDM2. Their binding affinities were ranked using Chemscore and X-score. In a fluorescence-based binding assay, the quinolinole NSC66811 was identified to inhibit MDM2 with a significantly lower Ki (120 nM) than the natural p53 peptide [51].
Molecular dynamics accounting for protein flexibility was applied to a pharmacophore model derived from the MDM2 structure. Out of 35,000 small-molecule compounds, computational analysis identified five non-peptidic MDM2 inhibitors with novel scaffolds. Based on fluorescence polarization binding assays, the most potent of them displayed a Ki of 110 nM [98]. However, besides conveying results on biochemical experiments, there were no reports on their effect at the cellular level.
A strategy to find compounds that interact with new targets based on existing knowledge of ligand-target interactions has been employed based on chemogenomics knowledge-based methodologies, which relies on homology and conserved residues that are involved in molecular recognition. Based on MDM2-p53 interactions as template, this approach was applied to the MDM4-p53 complex, where combination of virtual screening strategies resulted in unique compounds with varying degrees of selectivity for both MDM2-p53 and MDM4-p53 systems [99].
Development of Inhibitors Targeting the p53-MDM2-MDMX Loop
Until now several of the aforementioned MDM-p53 targeted inhibitors that directly activate wild-type p53 in cancer cells have been successfully developed for the clinical trials (Table 16.1), but additional clinical data is necessary to verify if these non-genotoxic inhibitors can effectively activate p53 and improve the clinical efficacy in patients carrying wild-type p53. Other strategies that target the p53-MDM2- MDMX loop other than disrupting the MDM2-p53 interactions have also been considered, but encounter major challenges.
Clinical Candidates of MDM2-p53 Inhibitors
RG7112 and RO5503781 (Hoffmann-La Roche)
Driven by the success of Nutlin (see “Surface Plasmon Resonance”), Hoffmann-La Roche has undertaken an extensive optimization of lead compounds that culminated in the selection of RG7112 (RO5045337), a Nutlin-3 derivative, and RO5503781, whose structure has not been disclosed, for clinical application. RG7112 is the first MDM2 inhibitor that advances to clinical trials. It is a more potent binder of MDM2 compared to Nutlin (Kd = 10.7 vs 90 nM), but similarly to Nutlin, it is inactive against MDMX [100]. The crystal structure of the RG7112-MDM2 complex revealed that RG7112 binds in the same fashion as Nutlin to the p53 pocket of MDM2 by mimicking three critical p53 amino acid residues Trp23, Leu26 and Phe19 [PDB: 4IPF]. The compound differs from Nutlin by the introduction of 4, 5-dimethyl substitution on the imidazoline ring. The substitutions possibly add greater structural rigidity to the imidazoline scaffold, which might block metabolic conversion to the inactive imidazole form. In December of 2007, Hoffmann-La Roche initiated Phase I studies of RG7112, and until now, five clinical studies in patients with liposarcomas (before debulking surgery), solid tumors, and soft tissue sarcomas, advanced solid tumors (http://www.clinicaltrials.gov; NCT01677780, NCT01143740, NCT01164033, NCT00559533, NCT00623870) have been completed. The clinical biomarker studies in 20 patients with liposarcomas (a frequently HDM2-amplified tumor) confirmed the ability of RG7112 to activate p53 and its major functions, cell cycle arrest and apoptosis, in human tumors [101]. The clinical results provided not only proof of mechanism, but also evidence for the clinical activity of the single agent RG7112. In phase I leukemia trial of RG7112, among 116 patients treated with RG7112 for 10 days followed by 18 days of rest, 6 AML patients with complete remission (CR), 5 of 31 (16 %) at the MTD (the maximum tolerated dose), and additional patients showed significant decreases in blasts [102]. Notably, RG7112 caused >10 % adverse events related to hematological toxicities. Currently, Hoffmann-La Roche is recruiting patients participating in previous cancer studies for extension studies of RG7112 (NCT01677780). Clinical studies of RG7112 in combination with doxorubicin in patients with soft tissue sarcoma, or in combination with cytarabine in patients with acute myelogenous leukemia (NCT01605526 and NCT01635296) are currently underway. Another compound RO5503781 is also entering phase I studies for advanced malignancies, except leukemia (NCT01462175), and in combination with cytarabine for patients with acute myelogenous leukemia (AML) in 2013 (NCT01773408). Although it has been almost a decade since Nutlin was discovered to take these Nutlin-derived inhibitors of MDM2-p53 binding to their phase I or II clinical trials, these progresses are still quite impressive, offering a big promise for targeting the MDM2-p53 loop as a way to develop effective and non-genotoxic anti-cancer therapy.
SAR405838 (Sanofi-Aventis)
Another anti-MDM2-p53-binding small molecule that has also been developed into phase I clinical trial is a derivative of Spirooxindoles, which represent one of the most promising chemical scaffolds for MDM inhibitors (see “Structure-Based de novo Design”) and are actively being developed by several companies, including Hoffmann-La Roche, Sanofi-Aventis and Daiichi Sankyo. The first candidate SAR405838 is an analog of MI-773 and currently entering the phase I trial for patients with advanced cancer prescreening with wild-type p53 (NCT01636479) [63, 103]. Spirooxindoles MI-series were initially designed by the Wang group at the University of Michigan, further developed by Ascenta Therapeutics and licensed by Sanofi-Aventis. As discussed above, initially, MI-219 was identified in 2008 as a potent, specific, cell-permeable, and orally active MDM2 inhibitor through structure based de novo design. However, due to its high dose required, short half-life and quick metabolism, MI-219 is not an ideal candidate for the clinical development. Further optimization of MI-219 led to a more potent analogue MI-319 [104]. This compound exhibited potent activity against follicular lymphoma that retains wild-type p53 both in vitro and in vivo. In addition, MI-319 in combination with cisplatin induced cell growth inhibition and apoptosis in pancreatic cancer cells irrespective of their p53 mutational status. An even more potent analogue of MI-319, MI-773, was later claimed in 2012. This series of Spirooxindole compounds has a more complicated stero-chemistry compared to Nutlin. Thus, the newly developed optimal enantiomer, MI-77301, displayed 10 times higher binding affinity against MDM2 than did MI-773 (Kd = 62 vs 8.2 nM). The antitumor activity of MI-77301 was more pronounced in a set of wild type p53 xenograft models than MI-773, including SJSA-1 osteosarcoma, human prostate, melanoma, colorectal tumor, LNCAP human prostate tumor and human acute lymphoblastic leukemia (WO2012065022), although there is a less pronounced difference between the two compounds in vitro cell-based cytotoxicity assays. Apparently, this group of anti-MDM2- p53-binding small molecules is also appealing in terms of their clinical development into anti-cancer therapy.
CGM097 (Novartis)
Novartis, another pharmaceutical giant, has also joined the race to develop anti-cancer drugs that can specifically target the MDM2/MDMX-p53 loop. Very recently, this company sponsored a Phase I trial of a small molecule called CGM097 in patients with advanced solid tumor with wild type p53 (http://clinicaltrials.gov/ show/NCT01760525). Ninety-two individuals will be orally dosed with CGM097. Trial dates were designed from 1st March 2013 to 1st June 2016. Although the structure of CGM097 is not disclosed, this compound appears to have a dual inhibitory activity toward both MDM2 and MDMX to different extents, as Novartis has recently showed several small molecular scaffolds as inhibitors of MDM2 and/or MDMX in patents, such as 3-imidazolylindoles, substituted dihydroimidazole derivatives, tetra-substituted heteroaryl compounds and substituted isoquinolinones and quinazolinones [63]. The most potent compound among the 3- imidazolylindoles, prepared by a van Leusen multicomponent reaction, has an IC50 of 15 nM for MDM2 in an FP binding assay and an IC50 of 1.32 µM for MDMX in a TR-FRET binding assay. Compounds derived from dihydroimidazole demonstrated IC50 values from 70 to 2 µM. This scaffold closely resembles Roche’s Nutlins. The potency (IC50) of disclosed tetra-substituted heteroaryl and isoquinolinones and quinazolinones as obtained from TR-FRET based assay ranging in the low nanomolar for MDM2, but in the low micromolar for MDMX. The cancer drug discovery field will keep its eyes wide open for the birth of this MDM2/MDMX dual inhibitor as an anti-cancer therapy.
MK-8242 (Merck)
The competition became more intensive when Merck recently claimed the US patent 7884107 on February 2011, based on a provisional application from 2006. This patent describes a substituted piperidine as a specific MDM2 inhibitor with an IC50 value of 0.02 µM as determined by an FP assay. In the same year, Merck launched two Phase I trials (NCT01451437 and NCT01463696) with MK-8242, an MDM2 inhibitor of undisclosed structure in advanced solid tumor, alone and in combination with cytarabine in participants with acute myelogenous leukemia. Hopefully, these clinical trials together with those trials as described above will eventually lead to some effective therapies for human cancers that harbor wild type p53 by targeting the p53-MDM2-MDMX loop.
Other Therapeutically Useful Approaches Targeting the p53-MDM2- MDMX Loop
Modulation of MDM2 and MDMX Expression
In addition to disrupting the interaction between MDM2/MDMX and p53, other strategies have also been explored for the possibility of developing anti-cancer therapy by negatively affecting the p53-MDM2-MDMX loop. Because MDM2 and MDMX are p53 target genes, disruption of MDM2/MDMX-p53 interaction would lead to elevated MDM2 or MDMX levels and thus might be less effective in cancer cells that express high levels of MDM2 and/or MDMX. Therefore, down-regulation of MDM2 or MDMX protein in cancer cells is a straightforward approach and has been proved to activate the p53 pathway and inhibit tumor growth using small interfering RNA (siRNA), short hairpin RNA (shRNA) or miRNA in tumor xenografts in nude mice [16, 105, 106]. Unfortunately, siRNA therapy is hampered by the issues of delivery and cellular uptake, and thus it is less feasible. However, interestingly, a benzofuroxan derivative (NSC207895, XI006) that selectively inhibits MDMX expression has been identified through a reporter-based screening [60]. This small molecule caused p53-dependent trans-activation of pro-apoptotic genes in MCF-7 cells. NSC207895 was shown to exhibit the additive effect with Nutlin-3a in activating p53, inducing apoptosis and decreasing the viability of MCF-7 cells. NSC207895 seemed to repress the MDMX promoter activity and decrease MDMX transcription, although the underlying molecular mechanism of this promoter- specific targeting has not yet been deciphered. In addition, NSC207895 was highly correlated to known DNA-damaging agents, such as methyl methanesulphonate (MMS) and camptothecin, in a cross-species chemogenomic profiling screen. As DNA-damaging agents also induce MDMX degradation, it is possible that the effects of NSC207895 on MDMX protein levels and p53 activation might involve more than the repression of MDMX transcription. Besides this small molecule, several naturally derived compounds have also been shown to exert their anticancer activities by inhibiting MDM2 expression, independent of p53, such as Genistein, gambogic acid, curcumin, and berberine (disruption of MDM2-DAXX-HAUSP complex). Since this natural product field has been expanding dramatically, we refer readers to a comprehensive review on natural products that block MDM2 expression in a p53-dependent or -independent manner [62].
Interfering with MDM2 Interactions with Other Proteins
MDM2 regulates the stability of p53 protein by not only directly interacting with it and mediating its ubiquitylation, but also associating with different subunits of the 26S proteasome, such as S2, S4, S5a, S6a, S6b, to facilitate p53 proteasomal turnover. Thus targeting the interaction between MDM2 and the 26S proteasomal subunits is also an attractive approach. Indeed, a novel tryptamine derivative, orally bioavailable small molecule JNJ-26854165, was invented by Forschungszentrum Karlsruhe and Janssen Pharmaceutical using a high-throughput screening approach based on the characterization of MDM2-proteasome interaction (WO2008132175) [45, 46, 107]. JNJ-26854165 specifically bound to the ring domain of MDM2 and inhibited the binding of the MDM2-p53 complex to the proteasome, consequently blocking the degradation of p53. However, in addition to p53, degradation of other human MDM2 client proteins may be inhibited through disturbance of the ubiquitin-proteasome proteolysis (UPS)-pathway. Preclinical studies of this small molecule have revealed induction of apoptosis and anti-proliferation in p53 wild-type and p53-mutant cancer cells and a general genotoxic effect in various tumor models including NCI-H1373 non-small cell lung cancer xenografts, PC-3M orthotopic prostate and p53 mutant HT-29 colon xenografts, and U87 glioblastoma xenografts, indicating that JNJ-26854165 is not p53-specific. To date, a phase I clinical study of JNJ-26854165 (NCT00676910) to determine safety and dosing in patients with advanced stage or refractory solid tumors has been completed. Although JNJ-26854165 was initially well tolerated at clinically effective doses in patients, its cardiotoxic effects and MDM2-independent mechanism were subsequently observed. Hence this compound is no longer in competition.
It has been shown that Arf, a tumor suppressor protein, can bind to MDM2 and enhance the degradation of MDM2 and MDMX, consequently activating p53 [108–110]. This Arf activity may represent an efficient strategy for therapeutics. Indeed, a group at the St. Jude Children’s Research Hospital characterized the interaction domains of Arf and MDM2 [111–114]. They further exploited the possibility of identifying and/or designing compounds that mimic, inhibit and/or enhance the effect of Arf on MDM2. Although this research is still at its embryonic stage, it might produce surprising and promising results useful for anti-cancer drug discovery.
Recently, several ribosomal proteins (RP), such as RPL11, RPL5, RPL23, RPS7, RPS14, RPL26, RPS27, RPS3, and other nucleolar proteins have been identified as native MDM2 and/or MDMX inhibitors in response to ribosomal stress. This type of stress often occurs upon perturbation of ribosomal biogenesis caused by chemicals, nutrient deprivation, DNA damaging agents, or genetic alterations [115]. Interestingly, these ribosomal proteins upon this stress can uncouple p53 from its key negative regulator MDM2, consequently leading to p53 activation and protecting cells from tumor formation. Biochemical characterization by several groups including our lab [116, 117] and particularly genetic studies by the Zhang group at University of North Carolina [117–119] have demonstrated the specific interaction of RPL11 with the Zinc domain of MDM2, which is particularly important for p53 activation in response to ribosomal stress and whose defect would lead to cancer development. Specifically, cancer-related zinc finger cysteine mutations of MDM2 can disrupt the binding of MDM2 with RPL11 and RPL5. Furthermore, our biochemical studies uncovered several non-cysteine amino acids within the Zinc finger of MDM2 and basic amino acids in the MDM2 binding domain of RPL11, which are important for their specific interactions [116]. These findings identify the Zinc finger domain of MDM2 as a new and potential target site for future anticancer drug discovery.
SIRT1 Inhibitors
SIRT1, a NAD-dependent deacetylase, has attracted much attention due to its relation to cellular longevity [120, 121] and its role in negating p53 activity and stability [33], but it is also highly expressed in cancers [122]. It would readily maintain the tumor suppressor p53 in a deacetylated and inactive status in cancer cells, consequently favoring its MDM2/MDMX-mediated degradation [123]. The role of the HIC-1-SIRT1-p53 loop in lung cancer development has been confirmed in animal models [124]. These studies offer an opportunity for developing SIRT1 inhibitors as a potential anti-cancer therapy because they would not affect p53 function in normal cells and thus be less toxic to these cells. Indeed, there are several sirtuin inhibitors with proven activity in preclinical cancer models. The current state of the art in research on SIRT1 inhibitors has been extensively reviewed by other scientists [125–127], and thus we will not further replicate the review here. Among these SIRT1 inhibitors, Inauhzin (INZ), as a newly discovered SIRT1-specific inhibitor in our lab, can re-activate p53 in several wild type p53-containing cancer cells without genotoxic, and suppresses tumor growth by targeting SIRT1 and activating p53 [69]. Moreover, INZ is more potent, but much less toxic to normal cells or tissues than Tenovin [70] and other SIRT1 inhibitors [69]. Amazingly, INZ can sensitize the anti-cancer effect of chemotherapy and Nutlin-3 as tested in colon and lung cancer models [128, 129]. Thus, this small molecule presents as a promising contender for a molecule-targeted anti-cancer therapy that indirectly targets the MDM2-p53 pathway. We are currently undertaking systematic optimization [130], new target identification [131], and intensive preclinical studies of this compound, in hoping to eventually develop it into clinical trials in the near future.
Combination Strategies
Drug resistance and toxicity have been the two major obstacles for effective chemotherapy against cancer. To overcome and minimize the emergence of resistance caused by single-agent cancer therapy and to achieve maximal therapeutic response as well as to reduce the toxicity of genotoxic chemotherapy, combinations of different types of chemotherapeutic and molecule-targeted agents have been a common practice in clinic. This strategy has also been applied to the clinical trials of MDM2 or MDMX inhibitors, as combination of non-genotoxic p53 activators or MDM2 antagonists with clinically used chemotherapeutic agents [132], such as cisplatin, doxorubicin or cytarabine, has been shown to augment efficacy in the preclinical studies [133–135] and are currently being tested in several Phase I clinical trials (ClinicalTrials.gov: NCT01773408, NCT01605526 and NCT01605526). However, the radiosensitivity of the hematopoietic system of mice to p53 activation suggests that such an approach would potentially exacerbate the toxicity that is associated with MDM2 antagonists. Thus, combining non-genotoxic specific molecule-targeted therapeutic agents with p53 agonists could be a safer alternative. Moreover, this approach targeting more than one highly cancer-related molecule of one or multiple cellular signaling pathway(s) in cancers would be more important and effective for cancer treatment. A number of laboratories have been working on combinatorial therapy with the MDM2 antagonists, as shown in Table 16.2. Combinatorial therapy in a systematic manner seems more effective than empirically determined combination regimens, as p53 mutations confer absolute resistance or de novo p53- mutated multi-drug resistance emerges to MDM2 antagonists [135, 136], and more than 50 % of human cancers carry p53 mutants [137]. Conversely, the association of mutated Flt3 (Flt-ITD) with heightened sensitivity to MDM2 inhibitors was found in AML patients; BRAF mutation was associated with MDM2 inhibitor Nutlin 3a sensitivity [138], suggesting that therapeutic blockage of Flt3 using Flt3 inhibitor or targeting BRAF as well as the p53 pathway could generate additive or synergistic effects. Indeed, co-treatment of melanomas with the p53-activating stapled peptide SAH-p53-8 and BRAF inhibitor PLX4032 significantly enhanced cytotoxicity when compared with single-agent treatment [17]. Apparently, the development of a combinatorial anti-cancer therapy with anti-MDM2 or anti-MDMX inhibitors holds an encouraging future in this field.
Table 16.2.
Combination of non-genotoxic p53 activators or MDM2 antagonists with targeted therapeutic agents
| Drug 1 | Target 1 | Drug 2 | Target 2 | In vitro evidence | In vivo evidence | Status | Reference |
|---|---|---|---|---|---|---|---|
| Nutlin | M2-p53 interaction |
Inauhzin | SIRT1 inhibitor | H460 and HCT116 cells | HCT116 xenografts |
Preclinical | [128] |
| Nutlin-3 | M2-p53 interaction |
Vorinostat, NaB, MS-275, Apicidin |
HDAC1 inhibitor | A549 and A2780 | Preclinical | [156] | |
| Nutlin-3 | M2-p53 interaction |
Sorafenib | Multi-kinase inhibitor | RCC, acute myeloid leukemic |
[157–159] | ||
| Nutlin | M2-p53 interaction |
17AAG | Hsp90 inhibitor | RKO, AGS, HCT116 MCF7, U20S | Xenografts? | [160] | |
| Nutlin-3 | M2-p53 interaction |
Dasatinib | Multi-kinase inhibitor | Primary B-CLL cell & cell line |
[161] | ||
| Nutlin-3 | M2-p53 interaction |
Imatinib | BCR/ABL tyrosine kinase inhibitor |
B210, IL-3 | [162] | ||
| Nutlin-3 | M2-p53 interaction |
VX-680 | Aurora kinase inhibitors |
A549, HCT116 | [163] | ||
| MI-219 | M2-p53 interaction |
ZnCl2, TPEN | Zinc chelator | HCT116, MCF-7 | [164] | ||
| Nutlin-3 | M2-p53 interaction |
1396-11, XIAP antisense oligonucleotide (ASO) |
XIAP inhibitor | OCI-AML3,MOLM-13, HL-60 |
Phase I clinical trial |
[165] | |
| Nutlin-3 | M2-p53 interaction |
Bortezomib | Proteasome inhibitor | MCL, HCT116, U20S | [166–170] | ||
| Nutlin-3 | M2-p53 interaction |
Velcade | Proteasome inhibitor | Myeloma (MM) | [171] | ||
| Nutlin-3 | M2-p53 interaction |
Valproic acid (VPA) |
HDAC1 inhibitors | AML cell lines | MOLM-13 orthotopic and xenograft |
[172] | |
| Nutlin-3a | M2-p53 interaction |
ABT-737 | Bcl-2 protein inhibitor | OCI-AML-3 and MOLM-13 |
[173] | ||
| Nutlin-3 | M2-p53 interaction |
MK-0457 | Aurora kinase inhibitors |
OCI-AML-3 and MOLM-13 | [174] | ||
| Nutlin | M2-p53 interaction |
PI-103 | PI3K/mTOR inhibitor | AML cells | Preclinical | [175] | |
| Nutlin-3 | M2-p53 interaction |
Mithramycin | Spl DNA binding inhibitor |
Gynecologic cancer cells | Xenografts | Preclinical | [176] |
| Nutlin-3 | M2-p53 interaction |
5-FU cisplatin | Suicide inhibitor, crosslinking of DNA |
Gastric cancer cell, OVCa cells |
Xenografts | [177, 178] | |
| Paclitaxel | AKT-M2-p53 | Beta-lapachone (LPC) |
(Topo I) | Retinoblastoma (Y79 cells) | [179] | ||
| (PTX) Nutlin-3a | M2-p53 interaction |
Roscovitine and DRB |
CDK inhibitors | ARN8 cells | [180] | ||
| Nutlin-3 | M2-p53 interaction |
SiMDMX | MDMX | A549, U20S, SJSA MCF-7, JEG3 |
[181] | ||
| Nutlin-3 | M2-p53 interaction |
Doxil | Intercalating DNA | RKO, HT-29 | [182] | ||
| MI-63 | M2-p53 interaction |
Gemcitabine | RNR inhibitor | MCL cell | Xenografts | Preclinical | [183] |
Summary and Outlook
There is a great impetus in finding new types of chemotherapeutics to combat the drastically growing cancers with ever increasing number of cases of resistance to existing anti-cancer drugs. Remarkable progress in the past decade has been made in the discovery of novel chemotherapeutic drugs that selectively activate p53 to trigger rapid elimination of tumors by targeting the p53-MDM2-MDMX loop. As discussed above, a number of small-molecule drugs that inhibit the interaction of MDM2-p53 and block p53 degradation have been successfully developed through comprehensive medicinal chemistry, and some of them are currently in clinical trials. Experimental therapeutics and preclinical studies have demonstrated that MDM2 antagonists can be used alone or in combination regimens with cytotoxic drugs or other molecule-targeted drugs. The clinical results of MDM2 antagonists, such as RG7112, analogs of Nutlins, provide not only the proof of the concept, but also the usefulness of this therapeutic strategy for the treatment of human cancers in the near future. Combined screening of biomarkers, such as p53 status, MDM2 or MDMX gene amplification or other additional markers, would effectively select patients for clinical trials of MDM2-and MDMX-targeted single or combination therapies, ultimately improving the prospects for cancer therapy and prevention.
Compared to the progress in the discovery of MDM2 antagonists, the development of selective MDMX inhibitors seems slower, more complicated and challenging. Although the first crystal structure of MDMX-small molecule inhibitor complex was disclosed recently [86], a clinically useful MDMX inhibitor has not yet been reported. However, it is predicated that selective MDMX antagonists or dual inhibitors of MDM2 and MDMX will be of high interest and represent as a promising new approach to fight certain types of cancers that highly express MDMX, such as retinoblastoma [139] or melanoma [17]. Additionally, it has been indicated that systemic inhibition of MDMX is not only feasible as a therapeutic strategy for restoring p53 function in tumors that retain wild-type p53, but also significantly safer than inhibiting MDM2 [18, 22]. Hence, we will certainly not wait for too long to see a promising anti-MDMX small molecule in clinical trials.
A study using an MDM2 RING finger (C462A) mutant knockin mouse model by the Zhang group has shown that the stability of MDM2 is in fact not regulated by autoubiquitination in vivo [140], nor it is capable of blocking p53 activity by binding alone. This finding suggests that the MDM2 RING finger E3 ubiquitin ligase function plays an important role in suppressing p53, although this mutant is also defective in binding to MDMX [141, 142]. It also implies that it is possible to achieve the goal of reactivating p53 in cancers retaining wild type p53 by inhibiting MDM2 E3 ubiquitin ligase activity. However, the development of MDM2 E3 ubiquitin ligase inhibitors has been hampered by the biological complexity of the ubiquitination process. Unlike kinases, which can be inhibited via their ATP binding site, it is not known exactly how RING E3 ubiquitin ligases function, and hence it is unclear how to target their activity. Structural information is urgently needed for better understanding at the atomic and molecular levels how exactly MDM2 functions as an E3 ligase. This information would facilitate structure-based drug design to target MDM2 E3 ubiquitin ligase activity in the future [143].
Another challenge is that although MDM inhibitors mainly target the p53 pathway and kill wild-type p53-containing tumors by inducing p53-dependent apoptosis of cancer cells, more than half of human cancers lack of functional p53 largely because they harbor mutated forms of p53, which either lose wild type function or gain new oncogenic functions. Several approaches for drug discovery have been explored to identify small molecules that target mutant p53, restoring wild type p53 function and/or inhibiting the negative p53 regulators, such as rational design and high throughput screening of chemical libraries [144]. One example is the compound PRIMA-1 that restores wild-type conformation by binding to the core of mutant p53 and induces massive apoptosis in human tumor cells [145]. The PRIMA-1 analog APR-246 is currently in a clinical trial [146–148]. Very recently, another small molecule rescuer of mutant p53 was reported [149]. Interestingly, this small molecule called NSC319726 can convert R175H or R175W mutant p53 into a wild type form by chelating the Zinc ion and altering the redox environment for the p53 protein. Amazingly this compound can inhibit xenograft tumor growth by inducing apoptosis in an R175 mutant-dependent manner. Although much more needs to be investigated, this serves as a lead compound for further development of another mutant p53-targeted cancer therapy. Therefore, systematic and successful development of mutant p53-reactivating anticancer drugs will have a strong impact on the treatment of cancers, particularly those late stage and malignant ones that harbor mutant p53.
As a Chinese saying indicates, “dripping water wears through rock”, we strongly believe that the persistent, systematic, intensive and joint efforts from troops of cancer researchers on drug discovery by searching small molecules or agents targeting the p53-MDM2-MDMX pathway will ultimately be translated into a clinically useful anti-cancer therapy or therapies.
Acknowledgements
This work was supported in part by NIH-NCI grants CA095441, CA 079721, CA129828, and CA172468, as well as the LLL fund to H.L.
References
- 1.Brown CJ, et al. Awakening guardian angels: drugging the p53 pathway. Nat Rev Cancer. 2009;9(12):862–873. doi: 10.1038/nrc2763. [DOI] [PubMed] [Google Scholar]
- 2.Kruse JP, Gu W. Modes of p53 regulation. Cell. 2009;137(4):609–622. doi: 10.1016/j.cell.2009.04.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hollstein M, et al. p53 mutations in human cancers. Science. 1991;253(5015):49–53. doi: 10.1126/science.1905840. [DOI] [PubMed] [Google Scholar]
- 4.Vogelstein B, Lane D, Levine AJ. Surfing the p53 network. Nature. 2000;408(6810):307–310. doi: 10.1038/35042675. [DOI] [PubMed] [Google Scholar]
- 5.Vousden KH, Prives C. Blinded by the light: the growing complexity of p53. Cell. 2009;137(3):413–431. doi: 10.1016/j.cell.2009.04.037. [DOI] [PubMed] [Google Scholar]
- 6.Hong H, et al. Suppression of induced pluripotent stem cell generation by the p53-p21 pathway. Nature. 2009;460(7259):1132–1135. doi: 10.1038/nature08235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu X, et al. The p53-mdm-2 autoregulatory feedback loop. Genes Dev. 1993;7(7):1126–1132. doi: 10.1101/gad.7.7a.1126. [DOI] [PubMed] [Google Scholar]
- 8.Momand J, et al. The mdm-2 oncogene product forms a complex with the p53 protein and inhibits p53-mediated transactivation. Cell. 1992;69(7):1237–1245. doi: 10.1016/0092-8674(92)90644-r. [DOI] [PubMed] [Google Scholar]
- 9.Barak Y, et al. mdm2 expression is induced by wild type p53 activity. EMBO J. 1993;12(2):461–468. doi: 10.1002/j.1460-2075.1993.tb05678.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shvarts A, et al. MDMX: a novel p53-binding protein with some functional properties of MDM2. EMBO J. 1996;15(19):5349–5357. [PMC free article] [PubMed] [Google Scholar]
- 11.Eischen CM, Lozano G. p53 and MDM2: antagonists or partners in crime? Cancer Cell. 2009;15(3):161–162. doi: 10.1016/j.ccr.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 12.Gu J, et al. Mutual dependence of MDM2 and MDMX in their functional inactivation of p53. J Biol Chem. 2002;277(22):19251–19254. doi: 10.1074/jbc.C200150200. [DOI] [PubMed] [Google Scholar]
- 13.Haupt Y, et al. Mdm2 promotes the rapid degradation of p53. Nature. 1997;387(6630):296–299. doi: 10.1038/387296a0. [DOI] [PubMed] [Google Scholar]
- 14.Kubbutat MH, Jones SN, Vousden KH. Regulation of p53 stability by Mdm2. Nature. 1997;387(6630):299–303. doi: 10.1038/387299a0. [DOI] [PubMed] [Google Scholar]
- 15.Honda R, Tanaka H, Yasuda H. Oncoprotein MDM2 is a ubiquitin ligase E3 for tumor suppressor p53. FEBS Lett. 1997;420(1):25–27. doi: 10.1016/s0014-5793(97)01480-4. [DOI] [PubMed] [Google Scholar]
- 16.Wade M, Li YC, Wahl GM. MDM2, MDMX and p53 in oncogenesis and cancer therapy. Nat Rev Cancer. 2013;13(2):83–96. doi: 10.1038/nrc3430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gembarska A, et al. MDM4 is a key therapeutic target in cutaneous melanoma. Nat Med. 2012;18(8):1239–1247. doi: 10.1038/nm.2863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Biderman L, et al. MdmX is required for p53 interaction with and full induction of the Mdm2 promoter after cellular stress. Mol Cell Biol. 2012;32(7):1214–1225. doi: 10.1128/MCB.06150-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wade M, Wahl GM. Targeting Mdm2 and Mdmx in cancer therapy: better living through medicinal chemistry? Mol Cancer Res. 2009;7(1):1–11. doi: 10.1158/1541-7786.MCR-08-0423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang Y, Lu H. Signaling to p53: ribosomal proteins find their way. Cancer Cell. 2009;16(5):369–377. doi: 10.1016/j.ccr.2009.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wade M, Wang YV, Wahl GM. The p53 orchestra: Mdm2 and Mdmx set the tone. Trends Cell Biol. 2010;20(5):299–309. doi: 10.1016/j.tcb.2010.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Garcia D, et al. Validation of MdmX as a therapeutic target for reactivating p53 in tumors. Genes Dev. 2011;25(16):1746–1757. doi: 10.1101/gad.16722111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tang Y, et al. Acetylation is indispensable for p53 activation. Cell. 2008;133(4):612–626. doi: 10.1016/j.cell.2008.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shieh SY, et al. DNA damage-induced phosphorylation of p53 alleviates inhibition by MDM2. Cell. 1997;91(3):325–334. doi: 10.1016/s0092-8674(00)80416-x. [DOI] [PubMed] [Google Scholar]
- 25.Banin S, et al. Enhanced phosphorylation of p53 by ATM in response to DNA damage. Science. 1998;281(5383):1674–1677. doi: 10.1126/science.281.5383.1674. [DOI] [PubMed] [Google Scholar]
- 26.Maya R, et al. ATM-dependent phosphorylation of Mdm2 on serine 395: role in p53 activation by DNA damage. Genes Dev. 2001;15(9):1067–1077. doi: 10.1101/gad.886901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kapoor M, Lozano G. Functional activation of p53 via phosphorylation following DNA damage by UV but not gamma radiation. Proc Natl Acad Sci U S A. 1998;95(6):2834–2837. doi: 10.1073/pnas.95.6.2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang Y, Xiong Y, Yarbrough WG. ARF promotes MDM2 degradation and stabilizes p53: ARF-INK4a locus deletion impairs both the Rb and p53 tumor suppression pathways. Cell. 1998;92(6):725–734. doi: 10.1016/s0092-8674(00)81401-4. [DOI] [PubMed] [Google Scholar]
- 29.Kobet E, et al. MDM2 inhibits p300-mediated p53 acetylation and activation by forming a ternary complex with the two proteins. Proc Natl Acad Sci U S A. 2000;97(23):12547–12552. doi: 10.1073/pnas.97.23.12547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li M, et al. Acetylation of p53 inhibits its ubiquitination by Mdm2. J Biol Chem. 2002;277(52):50607–50611. doi: 10.1074/jbc.C200578200. [DOI] [PubMed] [Google Scholar]
- 31.Ito A, et al. p300/CBP-mediated p53 acetylation is commonly induced by p53-activating agents and inhibited by MDM2. EMBO J. 2001;20(6):1331–1340. doi: 10.1093/emboj/20.6.1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Luo J, et al. Negative control of p53 by Sir2alpha promotes cell survival under stress. Cell. 2001;107(2):137–148. doi: 10.1016/s0092-8674(01)00524-4. [DOI] [PubMed] [Google Scholar]
- 33.Vaziri H, et al. hSIR2(SIRT1) functions as an NAD-dependent p53 deacetylase. Cell. 2001;107(2):149–159. doi: 10.1016/s0092-8674(01)00527-x. [DOI] [PubMed] [Google Scholar]
- 34.Cheng HL, et al. Developmental defects and p53 hyperacetylation in Sir2 homolog (SIRT1)-deficient mice. Proc Natl Acad Sci U S A. 2003;100(19):10794–10799. doi: 10.1073/pnas.1934713100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Luo J, et al. Deacetylation of p53 modulates its effect on cell growth and apoptosis. Nature. 2000;408(6810):377–381. doi: 10.1038/35042612. [DOI] [PubMed] [Google Scholar]
- 36.Onel K, Cordon-Cardo C. MDM2 and prognosis. Mol Cancer Res. 2004;2(1):1–8. [PubMed] [Google Scholar]
- 37.Nosho K, et al. SIRT1 histone deacetylase expression is associated with microsatellite instability and CpG island methylator phenotype in colorectal cancer. Mod Pathol. 2009;22(7):922–932. doi: 10.1038/modpathol.2009.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jung-Hynes B, et al. Role of sirtuin histone deacetylase SIRT1 in prostate cancer. A target for prostate cancer management via its inhibition? J Biol Chem. 2009;284(6):3823–3832. doi: 10.1074/jbc.M807869200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ozdag H, et al. Differential expression of selected histone modifier genes in human solid cancers. BMC Genomics. 2006;7:90. doi: 10.1186/1471-2164-7-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tseng RC, et al. Distinct HIC1-SIRT1-p53 loop deregulation in lung squamous carcinoma and adenocarcinoma patients. Neoplasia. 2009;11(8):763–770. doi: 10.1593/neo.09470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jones SN, et al. Rescue of embryonic lethality in Mdm2-deficient mice by absence of p53. Nature. 1995;378(6553):206–208. doi: 10.1038/378206a0. [DOI] [PubMed] [Google Scholar]
- 42.Montes de Oca Luna R, Wagner DS, Lozano G. Rescue of early embryonic lethality in mdm2-deficient mice by deletion of p53. Nature. 1995;378(6553):203–206. doi: 10.1038/378203a0. [DOI] [PubMed] [Google Scholar]
- 43.Gannon HS, Jones SN. Using mouse models to explore MDM-p53 signaling in development, cell growth, and tumorigenesis. Genes Cancer. 2012;3(3–4):209–218. doi: 10.1177/1947601912455324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vassilev LT, et al. In vivo activation of the p53 pathway by small-molecule antagonists of MDM2. Science. 2004;303(5659):844–848. doi: 10.1126/science.1092472. [DOI] [PubMed] [Google Scholar]
- 45.Chargari C, et al. Preclinical assessment of JNJ-26854165 (Serdemetan), a novel tryptamine compound with radiosensitizing activity in vitro and in tumor xenografts. Cancer Lett. 2011;312(2):209–218. doi: 10.1016/j.canlet.2011.08.011. [DOI] [PubMed] [Google Scholar]
- 46.Kojima K, et al. The novel tryptamine derivative JNJ-26854165 induces wild-type p53-and E2F1-mediated apoptosis in acute myeloid and lymphoid leukemias. Mol Cancer Ther. 2010;9(9):2545–2557. doi: 10.1158/1535-7163.MCT-10-0337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yang Y, et al. Small molecule inhibitors of HDM2 ubiquitin ligase activity stabilize and activate p53 in cells. Cancer Cell. 2005;7(6):547–559. doi: 10.1016/j.ccr.2005.04.029. [DOI] [PubMed] [Google Scholar]
- 48.Roxburgh P, et al. Small molecules that bind the Mdm2 RING stabilize and activate p53. Carcinogenesis. 2012;33(4):791–798. doi: 10.1093/carcin/bgs092. [DOI] [PubMed] [Google Scholar]
- 49.Herman AG, et al. Discovery of Mdm2-MdmX E3 ligase inhibitors using a cell-based ubiquitination assay. Cancer Discov. 2011;1(4):312–325. doi: 10.1158/2159-8290.CD-11-0104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Galatin PS, Abraham DJ. A nonpeptidic sulfonamide inhibits the p53-mdm2 interaction and activates p53-dependent transcription in mdm2-overexpressing cells. J Med Chem. 2004;47(17):4163–4165. doi: 10.1021/jm034182u. [DOI] [PubMed] [Google Scholar]
- 51.Lu Y, et al. Discovery of a nanomolar inhibitor of the human murine double minute 2 (MDM2)-p53 interaction through an integrated, virtual database screening strategy. J Med Chem. 2006;49(13):3759–3762. doi: 10.1021/jm060023+. [DOI] [PubMed] [Google Scholar]
- 52.Yin H, et al. Terphenyl-based helical mimetics that disrupt the p53/HDM2 interaction. Angew Chem Int Ed Engl. 2005;44(18):2704–2707. doi: 10.1002/anie.200462316. [DOI] [PubMed] [Google Scholar]
- 53.Hardcastle IR, et al. Small-molecule inhibitors of the MDM2-p53 protein-protein interaction based on an isoindolinone scaffold. J Med Chem. 2006;49(21):6209–6221. doi: 10.1021/jm0601194. [DOI] [PubMed] [Google Scholar]
- 54.Hardcastle IR, et al. Isoindolinone inhibitors of the murine double minute 2 (MDM2)-p53 protein-protein interaction: structure-activity studies leading to improved potency. J Med Chem. 2011;54(5):1233–1243. doi: 10.1021/jm1011929. [DOI] [PubMed] [Google Scholar]
- 55.Koblish HK, et al. Benzodiazepinedione inhibitors of the Hdm2:p53 complex suppress human tumor cell proliferation in vitro and sensitize tumors to doxorubicin in vivo. Mol Cancer Ther. 2006;5(1):160–169. doi: 10.1158/1535-7163.MCT-05-0199. [DOI] [PubMed] [Google Scholar]
- 56.Schilling D, et al. Radiosensitization of wildtype p53 cancer cells by the MDM2-inhibitor PXN727 is associated with altered heat shock protein 70 (Hsp70) levels. Cell Stress Chaperones. 2013;18(2):183–191. doi: 10.1007/s12192-012-0369-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cheok CF, et al. Translating p53 into the clinic. Nat Rev Clin Oncol. 2011;8(1):25–37. doi: 10.1038/nrclinonc.2010.174. [DOI] [PubMed] [Google Scholar]
- 58.Bista M, et al. On the mechanism of action of SJ-172550 in inhibiting the interaction of MDM4 and p53. PLoS One. 2012;7(6):e37518. doi: 10.1371/journal.pone.0037518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Reed D, et al. Identification and characterization of the first small molecule inhibitor of MDMX. J Biol Chem. 2010;285(14):10786–10796. doi: 10.1074/jbc.M109.056747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang H, et al. A small-molecule inhibitor of MDMX activates p53 and induces apoptosis. Mol Cancer Ther. 2011;10(1):69–79. doi: 10.1158/1535-7163.MCT-10-0581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Graves B, et al. Activation of the p53 pathway by small-molecule-induced MDM2 and MDMX dimerization. Proc Natl Acad Sci U S A. 2012;109(29):11788–11793. doi: 10.1073/pnas.1203789109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Qin JJ, et al. Natural product MDM2 inhibitors: anticancer activity and mechanisms of action. Curr Med Chem. 2012;19(33):5705–5725. doi: 10.2174/092986712803988910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zak K, et al. Mdm2 and MdmX inhibitors for the treatment of cancer: a patent review (2011-present) Expert Opin Ther Pat. 2013;23(4):425–448. doi: 10.1517/13543776.2013.765405. [DOI] [PubMed] [Google Scholar]
- 64.Kamal A, Mohammed AA, Shaik TB. p53-Mdm2 inhibitors: patent review (2009–2010) Expert Opin Ther Pat. 2012;22(2):95–105. doi: 10.1517/13543776.2012.656593. [DOI] [PubMed] [Google Scholar]
- 65.Weber L. Patented inhibitors of p53-Mdm2 interaction (2006–2008) Expert Opin Ther Pat. 2010;20(2):179–191. doi: 10.1517/13543770903514129. [DOI] [PubMed] [Google Scholar]
- 66.Gu W, Roeder RG. Activation of p53 sequence-specific DNA binding by acetylation of the p53 C-terminal domain. Cell. 1997;90(4):595–606. doi: 10.1016/s0092-8674(00)80521-8. [DOI] [PubMed] [Google Scholar]
- 67.Gu W, Shi XL, Roeder RG. Synergistic activation of transcription by CBP and p53. Nature. 1997;387(6635):819–823. doi: 10.1038/42972. [DOI] [PubMed] [Google Scholar]
- 68.Li L, et al. Activation of p53 by SIRT1 inhibition enhances elimination of CML leukemia stem cells in combination with imatinib. Cancer Cell. 2012;21(2):266–281. doi: 10.1016/j.ccr.2011.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhang Q, et al. A small molecule Inauhzin inhibits SIRT1 activity and suppresses tumour growth through activation of p53. EMBO Mol Med. 2012;4(4):298–312. doi: 10.1002/emmm.201100211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lain S, et al. Discovery, in vivo activity, and mechanism of action of a small-molecule p53 activator. Cancer Cell. 2008;13(5):454–463. doi: 10.1016/j.ccr.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kim WJ, et al. The WTX tumor suppressor enhances p53 acetylation by CBP/p300. Mol Cell. 2012;45(5):587–597. doi: 10.1016/j.molcel.2011.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kussie PH, et al. Structure of the MDM2 oncoprotein bound to the p53 tumor suppressor transactivation domain. Science. 1996;274(5289):948–953. doi: 10.1126/science.274.5289.948. [DOI] [PubMed] [Google Scholar]
- 73.Joseph TL, et al. Differential binding of p53 and nutlin to MDM2 and MDMX: computational studies. Cell Cycle. 2010;9(6):1167–1181. doi: 10.4161/cc.9.6.11067. [DOI] [PubMed] [Google Scholar]
- 74.Almerico AM, et al. Molecular dynamics studies on Mdm2 complexes: an analysis of the inhibitor influence. Biochem Biophys Res Commun. 2012;424(2):341–347. doi: 10.1016/j.bbrc.2012.06.138. [DOI] [PubMed] [Google Scholar]
- 75.Tsuganezawa K, et al. A fluorescent-based high-throughput screening assay for small molecules that inhibit the interaction of MdmX with p53. J Biomol Screen. 2013;18(2):191–198. doi: 10.1177/1087057112460729. [DOI] [PubMed] [Google Scholar]
- 76.Grasberger BL, et al. Discovery and cocrystal structure of benzodiazepinedione HDM2 antagonists that activate p53 in cells. J Med Chem. 2005;48(4):909–912. doi: 10.1021/jm049137g. [DOI] [PubMed] [Google Scholar]
- 77.Popowicz GM, Domling A, Holak TA. The structure-based design of Mdm2/ Mdmx-p53 inhibitors gets serious. Angew Chem Int Ed Engl. 2011;50(12):2680–2688. doi: 10.1002/anie.201003863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pazgier M, et al. Structural basis for high-affinity peptide inhibition of p53 interactions with MDM2 and MDMX. Proc Natl Acad Sci U S A. 2009;106(12):4665–4670. doi: 10.1073/pnas.0900947106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Liu M, et al. D-peptide inhibitors of the p53-MDM2 interaction for targeted molecular therapy of malignant neoplasms. Proc Natl Acad Sci U S A. 2010;107(32):14321–14326. doi: 10.1073/pnas.1008930107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhan C, et al. An ultrahigh affinity d-peptide antagonist Of MDM2. J Med Chem. 2012;55(13):6237–6241. doi: 10.1021/jm3005465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sasiela CA, et al. Identification of inhibitors for MDM2 ubiquitin ligase activity from natural product extracts by a novel high-throughput electrochemiluminescent screen. J Biomol Screen. 2008;13(3):229–237. doi: 10.1177/1087057108315038. [DOI] [PubMed] [Google Scholar]
- 82.Murray MF, et al. A high-throughput screen measuring ubiquitination of p53 by human mdm2. J Biomol Screen. 2007;12(8):1050–1058. doi: 10.1177/1087057107308556. [DOI] [PubMed] [Google Scholar]
- 83.Dudgeon DD, et al. Implementation of a 220,000-compound HCS campaign to identify disruptors of the interaction between p53 and hDM2 and characterization of the confirmed hits. J Biomol Screen. 2010;15(7):766–782. doi: 10.1177/1087057110375304. [DOI] [PubMed] [Google Scholar]
- 84.Dudgeon DD, et al. Characterization and optimization of a novel protein-protein interaction biosensor high-content screening assay to identify disruptors of the interactions between p53 and hDM2. Assay Drug Dev Technol. 2010;8(4):437–458. doi: 10.1089/adt.2010.0281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Khoury K, et al. The p53-MDM2/MDMX axis - a chemotype perspective. Med Chem Commun. 2011;2(4):246–260. doi: 10.1039/C0MD00248H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Popowicz GM, et al. Structures of low molecular weight inhibitors bound to MDMX and MDM2 reveal new approaches for p53-MDMX/MDM2 antagonist drug discovery. Cell Cycle. 2010;9(6):1104–1111. doi: 10.4161/cc.9.6.10956. [DOI] [PubMed] [Google Scholar]
- 87.Bernal F, et al. Reactivation of the p53 tumor suppressor pathway by a stapled p53 peptide. J Am Chem Soc. 2007;129(9):2456–2457. doi: 10.1021/ja0693587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bernal F, et al. A stapled p53 helix overcomes HDMX-mediated suppression of p53. Cancer Cell. 2010;18(5):411–422. doi: 10.1016/j.ccr.2010.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Huang Y, et al. 1,4-Thienodiazepine-2,5-diones via MCR (I): synthesis, virtual space and p53-Mdm2 activity. Chem Biol Drug Des. 2010;76(2):116–129. doi: 10.1111/j.1747-0285.2010.00989.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lee JH, et al. Novel pyrrolopyrimidine-based alpha-helix mimetics: cell-permeable inhibitors of protein-protein interactions. J Am Chem Soc. 2011;133(4):676–679. doi: 10.1021/ja108230s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Czarna A, et al. Robust generation of lead compounds for protein-protein interactions by computational and MCR chemistry: p53/Hdm2 antagonists. Angew Chem Int Ed Engl. 2010;49(31):5352–5356. doi: 10.1002/anie.201001343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Fu T, et al. Molecular dynamic simulation insights into the normal state and restoration of p53 function. Int J Mol Sci. 2012;13(8):9709–9740. doi: 10.3390/ijms13089709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ding K, et al. Structure-based design of spiro-oxindoles as potent, specific small- molecule inhibitors of the MDM2-p53 interaction. J Med Chem. 2006;49(12):3432–3435. doi: 10.1021/jm051122a. [DOI] [PubMed] [Google Scholar]
- 94.Shangary S, et al. Temporal activation of p53 by a specific MDM2 inhibitor is selectively toxic to tumors and leads to complete tumor growth inhibition. Proc Natl Acad Sci U S A. 2008;105(10):3933–3938. doi: 10.1073/pnas.0708917105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yu S, et al. Potent and orally active small-molecule inhibitors of the MDM2-p53 interaction. J Med Chem. 2009;52(24):7970–7973. doi: 10.1021/jm901400z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Rew Y, et al. Structure-based design of novel inhibitors of the MDM2-p53 interaction. J Med Chem. 2012;55(11):4936–4954. doi: 10.1021/jm300354j. [DOI] [PubMed] [Google Scholar]
- 97.Lucas BS, et al. An expeditious synthesis of the MDM2-p53 inhibitor AM-8553. J Am Chem Soc. 2012;134(30):12855–12860. doi: 10.1021/ja305123v. [DOI] [PubMed] [Google Scholar]
- 98.Bowman AL, et al. Small molecule inhibitors of the MDM2-p53 interaction discovered by ensemble-based receptor models. J Am Chem Soc. 2007;129(42):12809–12814. doi: 10.1021/ja073687x. [DOI] [PubMed] [Google Scholar]
- 99.Jacoby E, et al. Knowledge-based virtual screening: application to the MDM4/p53 protein-protein interaction. Methods Mol Biol. 2009;575:173–194. doi: 10.1007/978-1-60761-274-2_7. [DOI] [PubMed] [Google Scholar]
- 100.Tovar C, et al. MDM2 small-molecule antagonist RG7112 activates p53 signaling and regresses human tumors in preclinical cancer models. Cancer Res. 2013;73(8):2587–2597. doi: 10.1158/0008-5472.CAN-12-2807. [DOI] [PubMed] [Google Scholar]
- 101.Ray-Coquard I, et al. Effect of the MDM2 antagonist RG7112 on the P53 pathway in patients with MDM2-amplified, well-differentiated or dedifferentiated liposarcoma: an exploratory proof-of-mechanism study. Lancet Oncol. 2012;13(11):1133–1140. doi: 10.1016/S1470-2045(12)70474-6. [DOI] [PubMed] [Google Scholar]
- 102.Andreeff M, et al. Results of the phase 1 trial of RG7112, a small-molecule MDM2 antagonist, in acute leukemia; 54 annual meeting American Society of Hematology; Atlanta. 8–11 Dec 2012; 2012. p. 615. Abstract 675. [Google Scholar]
- 103.Wang S, et al. Targeting the MDM2-p53 protein-protein interaction for new cancer therapeutics. Top Med Chem. 2012;8:57–80. [Google Scholar]
- 104.Mohammad RM, et al. An MDM2 antagonist (MI-319) restores p53 functions and increases the life span of orally treated follicular lymphoma bearing animals. Mol Cancer. 2009;8:115. doi: 10.1186/1476-4598-8-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Menendez S, et al. MDM4 downregulates p53 transcriptional activity and response to stress during differentiation. Cell Cycle. 2011;10(7):1100–1108. doi: 10.4161/cc.10.7.15090. [DOI] [PubMed] [Google Scholar]
- 106.Laurie NA, Schin-Shih C, Dyer MA. Targeting MDM2 and MDMX in retinoblastoma. Curr Cancer Drug Targets. 2007;7(7):689–695. doi: 10.2174/156800907782418266. [DOI] [PubMed] [Google Scholar]
- 107.Smith MA, et al. Initial testing of JNJ-26854165 (Serdemetan) by the pediatric pre-clinical testing program. Pediatr Blood Cancer. 2012;59(2):329–332. doi: 10.1002/pbc.23319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Honda R, Yasuda H. Association of p19(ARF) with Mdm2 inhibits ubiquitin ligase activity of Mdm2 for tumor suppressor p53. EMBO J. 1999;18(1):22–27. doi: 10.1093/emboj/18.1.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Weber JD, et al. Nucleolar Arf sequesters Mdm2 and activates p53. Nat Cell Biol. 1999;1(1):20–26. doi: 10.1038/8991. [DOI] [PubMed] [Google Scholar]
- 110.Kamijo T, et al. Functional and physical interactions of the ARF tumor suppressor with p53 and Mdm2. Proc Natl Acad Sci U S A. 1998;95(14):8292–8297. doi: 10.1073/pnas.95.14.8292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Bothner B, et al. Defining the molecular basis of Arf and Hdm2 interactions. J Mol Biol. 2001;314(2):263–277. doi: 10.1006/jmbi.2001.5110. [DOI] [PubMed] [Google Scholar]
- 112.Sivakolundu SG, et al. Intrinsically unstructured domains of Arf and Hdm2 form bimo-lecular oligomeric structures in vitro and in vivo. J Mol Biol. 2008;384(1):240–254. doi: 10.1016/j.jmb.2008.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.DiGiammarino EL, et al. Solution structure of the p53 regulatory domain of the p19Arf tumor suppressor protein. Biochemistry. 2001;40(8):2379–2386. doi: 10.1021/bi0024005. [DOI] [PubMed] [Google Scholar]
- 114.Weber JD, et al. Cooperative signals governing ARF-mdm2 interaction and nucleolar localization of the complex. Mol Cell Biol. 2000;20(7):2517–2528. doi: 10.1128/mcb.20.7.2517-2528.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zhou X, et al. Scission of the p53-MDM2 loop by ribosomal proteins. Genes Cancer. 2012;3(3–4):298–310. doi: 10.1177/1947601912455200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zhang Q, et al. Hydrophilic residues are crucial for ribosomal protein L11 (RPL11) interaction with zinc finger domain of MDM2 and p53 protein activation. J Biol Chem. 2011;286(44):38264–38274. doi: 10.1074/jbc.M111.277012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Dai MS, et al. Regulation of the MDM2-p53 pathway by ribosomal protein L11 involves a post-ubiquitination mechanism. J Biol Chem. 2006;281(34):24304–24313. doi: 10.1074/jbc.M602596200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Bhat KP, et al. Essential role of ribosomal protein L11 in mediating growth inhibition-induced p53 activation. EMBO J. 2004;23(12):2402–2412. doi: 10.1038/sj.emboj.7600247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Zhang Y, et al. Ribosomal protein L11 negatively regulates oncoprotein MDM2 and mediates a p53-dependent ribosomal-stress checkpoint pathway. Mol Cell Biol. 2003;23(23):8902–8912. doi: 10.1128/MCB.23.23.8902-8912.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Imai S. Is Sirt1 a miracle bullet for longevity? Aging Cell. 2007;6(6):735–737. doi: 10.1111/j.1474-9726.2007.00344.x. [DOI] [PubMed] [Google Scholar]
- 121.Chen D, et al. Increase in activity during calorie restriction requires Sirt1. Science. 2005;310(5754):1641. doi: 10.1126/science.1118357. [DOI] [PubMed] [Google Scholar]
- 122.Stunkel W, et al. Function of the SIRT1 protein deacetylase in cancer. Biotechnol J. 2007;2(11):1360–1368. doi: 10.1002/biot.200700087. [DOI] [PubMed] [Google Scholar]
- 123.Inoue Y, et al. Suppression of p53 activity through the cooperative action of Ski and histone deacetylase SIRT1. J Biol Chem. 2011;286(8):6311–6320. doi: 10.1074/jbc.M110.177683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Chen WY, et al. Tumor suppressor HIC1 directly regulates SIRT1 to modulate p53-dependent DNA-damage responses. Cell. 2005;123(3):437–448. doi: 10.1016/j.cell.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 125.Blum CA, et al. SIRT1 modulation as a novel approach to the treatment of diseases of aging. J Med Chem. 2011;54(2):417–432. doi: 10.1021/jm100861p. [DOI] [PubMed] [Google Scholar]
- 126.Chakrabarty SP, Balaram H, Chandrasekaran S. Sirtuins: multifaceted drug targets. Curr Mol Med. 2011;11(9):709–718. doi: 10.2174/156652411798062412. [DOI] [PubMed] [Google Scholar]
- 127.Balcerczyk A, Pirola L. Therapeutic potential of activators and inhibitors of sirtuins. Biofactors. 2010;36(5):383–393. doi: 10.1002/biof.112. [DOI] [PubMed] [Google Scholar]
- 128.Zhang Y, et al. Inauhzin and Nutlin3 synergistically activate p53 and suppress tumor growth. Cancer Biol Ther. 2012;13(10):915–924. doi: 10.4161/cbt.20844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Yiwei Z, Zhang Q, Zeng SX, Qian Hao, Hua Lu. Inauhzin sensitizes p53-dependent cytotoxicity and tumor suppression of chemotherapeutic agents. Neoplasia. 2013;15(5):523–534. doi: 10.1593/neo.13142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Zhang Q, et al. Structure and activity analysis of Inauhzin analogs as novel antitumor compounds that induce p53 and inhibit cell growth. PLoS One. 2012;7(10):e46294. doi: 10.1371/journal.pone.0046294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Liao JM, et al. Global effect of inauhzin on human p53-responsive transcriptome. PLoS One. 2012;7(12):e52172. doi: 10.1371/journal.pone.0052172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Azmi AS, et al. Network perspectives on HDM2 inhibitor chemotherapy combinations. Curr Pharm Des. 2011;17(6):640–652. doi: 10.2174/138161211795222612. [DOI] [PubMed] [Google Scholar]
- 133.Azmi AS, et al. MDM2 inhibitor MI-319 in combination with cisplatin is an effective treatment for pancreatic cancer independent of p53 function. Eur J Cancer. 2010;46(6):1122–1131. doi: 10.1016/j.ejca.2010.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Zheng T, et al. Nutlin-3 cooperates with doxorubicin to induce apoptosis of human hepatocellular carcinoma cells through p53 or p73 signaling pathways. J Cancer Res Clin Oncol. 2010;136(10):1597–1604. doi: 10.1007/s00432-010-0817-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Koster R, et al. Disruption of the MDM2-p53 interaction strongly potentiates p53-dependent apoptosis in cisplatin-resistant human testicular carcinoma cells via the Fas/ FasL pathway. Cell Death Dis. 2011;2:e148. doi: 10.1038/cddis.2011.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Michaelis M, et al. Adaptation of cancer cells from different entities to the MDM2 inhibitor nutlin-3 results in the emergence of p53-mutated multi-drug-resistant cancer cells. Cell Death Dis. 2011;2:e243. doi: 10.1038/cddis.2011.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Muller PA, Vousden KH. p53 mutations in cancer. Nat Cell Biol. 2013;15(1):2–8. doi: 10.1038/ncb2641. [DOI] [PubMed] [Google Scholar]
- 138.Long J, et al. Multiple distinct molecular mechanisms influence sensitivity and resistance to MDM2 inhibitors in adult acute myelogenous leukemia. Blood. 2010;116(1):71–80. doi: 10.1182/blood-2010-01-261628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Laurie NA, et al. Inactivation of the p53 pathway in retinoblastoma. Nature. 2006;444(7115):61–66. doi: 10.1038/nature05194. [DOI] [PubMed] [Google Scholar]
- 140.Clegg HV, et al. Mdm2 RING mutation enhances p53 transcriptional activity and p53-p300 interaction. PLoS One. 2012;7(5):e38212. doi: 10.1371/journal.pone.0038212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Huang L, et al. The p53 inhibitors MDM2/MDMX complex is required for control of p53 activity in vivo. Proc Natl Acad Sci U S A. 2011;108(29):12001–12006. doi: 10.1073/pnas.1102309108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Itahana K, et al. Targeted inactivation of Mdm2 RING finger E3 ubiquitin ligase activity in the mouse reveals mechanistic insights into p53 regulation. Cancer Cell. 2007;12(4):355–366. doi: 10.1016/j.ccr.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 143.Di J, Zhang Y, Zheng J. Reactivation of p53 by inhibiting Mdm2 E3 ligase: a novel antitumor approach. Curr Cancer Drug Targets. 2011;11(8):987–994. doi: 10.2174/156800911797264789. [DOI] [PubMed] [Google Scholar]
- 144.Maslon MM, Hupp TR. Drug discovery and mutant p53. Trends Cell Biol. 2010;20(9):542–555. doi: 10.1016/j.tcb.2010.06.005. [DOI] [PubMed] [Google Scholar]
- 145.Bykov VJ, et al. Restoration of the tumor suppressor function to mutant p53 by a low-molecular- weight compound. Nat Med. 2002;8(3):282–288. doi: 10.1038/nm0302-282. [DOI] [PubMed] [Google Scholar]
- 146.Mohell N, et al. Preclinical efficacy and toxicology studies of APR-246, a novel anticancer compound currently in clinical trials for refractory hematological malignancies and prostate cancer. Blood. 2010;116(21) [Google Scholar]
- 147.Lehmann S, et al. Targeting p53 in vivo: a first-in-human study with p53-targeting compound APR-246 in refractory hematologic malignancies and prostate cancer. J Clin Oncol. 2012;30(29):3633–3639. doi: 10.1200/JCO.2011.40.7783. [DOI] [PubMed] [Google Scholar]
- 148.Shalom-Feuerstein R, et al. Impaired epithelial differentiation of induced pluripotent stem cells from ectodermal dysplasia-related patients is rescued by the small compound APR-246/PRIMA-1MET. Proc Natl Acad Sci U S A. 2013;110(6):2152–2156. doi: 10.1073/pnas.1201753109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Yu X, et al. Allele-specific p53 mutant reactivation. Cancer Cell. 2012;21(5):614–625. doi: 10.1016/j.ccr.2012.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Lawrence HR, et al. Identification of a disruptor of the MDM2-p53 protein-protein interaction facilitated by high-throughput in silico docking. Bioorg Med Chem Lett. 2009;19(14):3756–3759. doi: 10.1016/j.bmcl.2009.04.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Parks DJ, et al. 1,4-Benzodiazepine-2,5-diones as small molecule antagonists of the HDM2-p53 interaction: discovery and SAR. Bioorg Med Chem Lett. 2005;15(3):765–770. doi: 10.1016/j.bmcl.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 152.Zhuang C, et al. Discovery, synthesis, and biological evaluation of orally active pyrrolidone derivatives as novel inhibitors of p53-MDM2 protein-protein interaction. J Med Chem. 2012;55(22):9630–9642. doi: 10.1021/jm300969t. [DOI] [PubMed] [Google Scholar]
- 153.Krajewski M, et al. NMR indicates that the small molecule RITA does not block p53-MDM2 binding in vitro. Nat Med. 2005;11(11):1135–1136. doi: 10.1038/nm1105-1135. author reply 1136-7. [DOI] [PubMed] [Google Scholar]
- 154.Issaeva N, et al. Small molecule RITA binds to p53, blocks p53-HDM-2 interaction and activates p53 function in tumors. Nat Med. 2004;10(12):1321–1328. doi: 10.1038/nm1146. [DOI] [PubMed] [Google Scholar]
- 155.Michel J, et al. In Silico Improvement of beta3-peptide inhibitors of p53 x hDM2 and p53 x hDMX. J Am Chem Soc. 2009;131(18):6356–6357. doi: 10.1021/ja901478e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Palani CD, Beck JF, Sonnemann J. Histone deacetylase inhibitors enhance the anticancer activity of nutlin-3 and induce p53 hyperacetylation and downregulation of MDM2 and MDM4 gene expression. Invest New Drugs. 2012;30(1):25–36. doi: 10.1007/s10637-010-9510-7. [DOI] [PubMed] [Google Scholar]
- 157.Zauli G, et al. The sorafenib plus nutlin-3 combination promotes synergistic cytotoxicity in acute myeloid leukemic cells irrespectively of FLT3 and p53 status. Haematologica. 2012;97(11):1722–1730. doi: 10.3324/haematol.2012.062083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Vatsyayan R, et al. Nutlin-3 enhances sorafenib efficacy in renal cell carcinoma. Mol Carcinog. 2013;52(1):39–48. doi: 10.1002/mc.20875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Weisberg E, Sattler M. A novel combination therapy approach for the treatment of acute myeloid leukemia: the multi-kinase inhibitor sorafenib and the HDM2 inhibitor nutlin-3. Haematologica. 2012;97(11):1620–1621. doi: 10.3324/haematol.2012.078451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Vaseva AV, et al. Blockade of Hsp90 by 17AAG antagonizes MDMX and synergizes with Nutlin to induce p53-mediated apoptosis in solid tumors. Cell Death Dis. 2011;2:e156. doi: 10.1038/cddis.2011.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Zauli G, et al. Dasatinib plus Nutlin-3 shows synergistic antileukemic activity in both p53 wild-type and p53 mutated B chronic lymphocytic leukemias by inhibiting the Akt pathway. Clin Cancer Res. 2011;17(4):762–770. doi: 10.1158/1078-0432.CCR-10-2572. [DOI] [PubMed] [Google Scholar]
- 162.Kurosu T, et al. Enhancement of imatinib-induced apoptosis of BCR/ABL-expressing cells by nutlin-3 through synergistic activation of the mitochondrial apoptotic pathway. Apoptosis. 2010;15(5):608–620. doi: 10.1007/s10495-010-0457-0. [DOI] [PubMed] [Google Scholar]
- 163.Cheok CF, et al. Combination of nutlin-3 and VX-680 selectively targets p53 mutant cells with reversible effects on cells expressing wild-type p53. Cell Death Differ. 2010;17(9):1486–1500. doi: 10.1038/cdd.2010.18. [DOI] [PubMed] [Google Scholar]
- 164.Azmi AS, et al. MI-219-zinc combination: a new paradigm in MDM2 inhibitor-based therapy. Oncogene. 2011;30(1):117–126. doi: 10.1038/onc.2010.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Carter BZ, et al. Simultaneous activation of p53 and inhibition of XIAP enhance the activation of apoptosis signaling pathways in AML. Blood. 2010;115(2):306–314. doi: 10.1182/blood-2009-03-212563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Ooi MG, et al. Interactions of the Hdm2/p53 and proteasome pathways may enhance the antitumor activity of bortezomib. Clin Cancer Res. 2009;15(23):7153–7160. doi: 10.1158/1078-0432.CCR-09-1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Tabe Y, et al. MDM2 antagonist nutlin-3 displays antiproliferative and proapoptotic activity in mantle cell lymphoma. Clin Cancer Res. 2009;15(3):933–942. doi: 10.1158/1078-0432.CCR-08-0399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Jin L, et al. MDM2 antagonist Nutlin-3 enhances bortezomib-mediated mitochondrial apoptosis in TP53-mutated mantle cell lymphoma. Cancer Lett. 2010;299(2):161–170. doi: 10.1016/j.canlet.2010.08.015. [DOI] [PubMed] [Google Scholar]
- 169.Cross B, et al. Inhibition of p53 DNA binding function by the MDM2 protein acidic domain. J Biol Chem. 2011;286(18):16018–16029. doi: 10.1074/jbc.M111.228981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Secchiero P, et al. Recent advances in the therapeutic perspectives of Nutlin-3. Curr Pharm Des. 2011;17(6):569–577. doi: 10.2174/138161211795222586. [DOI] [PubMed] [Google Scholar]
- 171.Saha MN, et al. MDM2 antagonist nutlin plus proteasome inhibitor velcade combination displays a synergistic anti-myeloma activity. Cancer Biol Ther. 2010;9(11):936–944. doi: 10.4161/cbt.9.11.11882. [DOI] [PubMed] [Google Scholar]
- 172.McCormack E, et al. Synergistic induction of p53 mediated apoptosis by valproic acid and nutlin-3 in acute myeloid leukemia. Leukemia. 2012;26(5):910–917. doi: 10.1038/leu.2011.315. [DOI] [PubMed] [Google Scholar]
- 173.Kojima K, et al. Concomitant inhibition of MDM2 and Bcl-2 protein function synergistically induce mitochondrial apoptosis in AML. Cell Cycle. 2006;5(23):2778–2786. doi: 10.4161/cc.5.23.3520. [DOI] [PubMed] [Google Scholar]
- 174.Kojima K, et al. Concomitant inhibition of Mdm2-p53 interaction and Aurora kinases activates the p53-dependent postmitotic checkpoints and synergistically induces p53-mediated mitochondrial apoptosis along with reduced endoreduplication in acute myelogenous leukemia. Blood. 2008;112(7):2886–2895. doi: 10.1182/blood-2008-01-128611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Kojima K, et al. The dual PI3 kinase/mTOR inhibitor PI-103 prevents p53 induction by Mdm2 inhibition but enhances p53-mediated mitochondrial apoptosis in p53 wild-type AML. Leukemia. 2008;22(9):1728–1736. doi: 10.1038/leu.2008.158. [DOI] [PubMed] [Google Scholar]
- 176.Ohgami T, et al. Low-dose mithramycin exerts its anticancer effect via the p53 signaling pathway and synergizes with nutlin-3 in gynecologic cancers. Cancer Sci. 2010;101(6):1387–1395. doi: 10.1111/j.1349-7006.2010.01543.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Endo S, et al. Potent in vitro and in vivo antitumor effects of MDM2 inhibitor nutlin-3 in gastric cancer cells. Cancer Sci. 2011;102(3):605–613. doi: 10.1111/j.1349-7006.2010.01821.x. [DOI] [PubMed] [Google Scholar]
- 178.Mir R, et al. Mdm2 antagonists induce apoptosis and synergize with cisplatin overcoming chemoresistance in TP53 wild-type ovarian cancer cells. Int J Cancer. 2013;132(7):1525–1536. doi: 10.1002/ijc.27832. [DOI] [PubMed] [Google Scholar]
- 179.D’Anneo A, et al. Paclitaxel and beta-lapachone synergistically induce apoptosis in human retinoblastoma Y79 cells by downregulating the levels of phospho-Akt. J Cell Physiol. 2010;222(2):433–443. doi: 10.1002/jcp.21983. [DOI] [PubMed] [Google Scholar]
- 180.Cheok CF, Dey A, Lane DP. Cyclin-dependent kinase inhibitors sensitize tumor cells to nutlin-induced apoptosis: a potent drug combination. Mol Cancer Res. 2007;5(11):1133–1145. doi: 10.1158/1541-7786.MCR-07-0161. [DOI] [PubMed] [Google Scholar]
- 181.Hu B, et al. MDMX overexpression prevents p53 activation by the MDM2 inhibitor Nutlin. J Biol Chem. 2006;281(44):33030–33035. doi: 10.1074/jbc.C600147200. [DOI] [PubMed] [Google Scholar]
- 182.Nadler-Milbauer M, et al. Synchronized release of Doxil and Nutlin-3 by remote degradation of polysaccharide matrices and its possible use in the local treatment of colorectal cancer. J Drug Target. 2011;19(10):859–873. doi: 10.3109/1061186X.2011.622401. [DOI] [PubMed] [Google Scholar]
- 183.Jones RJ, et al. HDM-2 inhibition suppresses expression of ribonucleotide reductase subunit M2, and synergistically enhances gemcitabine-induced cytotoxicity in mantle cell lymphoma. Blood. 2011;118(15):4140–4149. doi: 10.1182/blood-2011-03-340323. [DOI] [PMC free article] [PubMed] [Google Scholar]