Abstract
Background
Serotonin (5-HT3) receptor antagonists are commonly used to decrease nausea and vomiting for surgery patients. We conducted a systematic review on the comparative efficacy of 5-HT3 receptor antagonists.
Methods
Searches were done in MEDLINE, Embase, and the Cochrane Central Register of Controlled Trials to identify studies comparing 5-HT3 receptor antagonists with each other, placebo, and/or combined with other antiemetic agents for patients undergoing surgical procedures. Screening search results, data abstraction, and risk of bias assessment were conducted by two reviewers independently. Random-effects pairwise meta-analysis and network meta-analysis (NMA) were conducted. PROSPERO registry number: CRD42013003564.
Results
Overall, 450 studies and 80,410 patients were included after the screening of 7,608 citations and 1,014 full-text articles. Significantly fewer patients experienced nausea with any drug relative to placebo, except for ondansetron plus metoclopramide in a NMA including 195 RCTs and 24,230 patients. Significantly fewer patients experienced vomiting with any drug relative to placebo except for palonosetron plus dexamethasone in NMA including 238 RCTs and 12,781 patients. All agents resulted in significantly fewer patients with postoperative nausea and vomiting versus placebo in a NMA including 125 RCTs and 16,667 patients.
Conclusions
Granisetron plus dexamethasone was often the most effective antiemetic, with the number needed to treat ranging from two to nine.
Electronic supplementary material
The online version of this article (doi:10.1186/s12916-015-0371-y) contains supplementary material, which is available to authorized users.
Keywords: Network meta-analysis, Postoperative nausea, Postoperative vomiting, Serotonin receptor antagonists, Systematic review
Background
Postoperative nausea and/or vomiting can be defined as nausea and/or vomiting within 24 h of surgery [1, 2]. Between 20 % and 65 % of patients undergoing surgery experience postoperative nausea and/or vomiting [2, 3] and the anesthetic agents administered during the procedure have been identified as a contributing factor. Nausea and vomiting are associated with decreased quality of life and patient satisfaction [4, 5]. Vomiting can also cause complications such as aspiration pneumonia [6] and a longer hospital stay [7].
Serotonin (5-HT3) receptor antagonists reduce nausea and vomiting by inhibiting vagal nerves in the central nervous system and intestinal mucosa [8]. These agents are recommended by clinical practice guidelines for patients undergoing surgery and at risk for nausea and/or vomiting [9, 10].
We were commissioned by Health Canada to conduct a systematic review and network meta-analysis to assess the comparative efficacy of 5-HT3 receptor antagonists.
Methods
Protocol
A protocol based on the Preferred Reporting Items for Systematic reviews and Meta-Analysis for Protocols (PRISMA-P) guidelines was developed [11]. We revised our protocol using feedback from the research team and the research users, including Health Canada, a department of the federal government, who posed the original query. The final protocol was registered with PROSPERO (CRD42013003564) and published in an open-access journal [12]. As described in our protocol [12], our initial objective was to include data for patients undergoing surgery and chemotherapy in the overall analysis for both safety and efficacy outcomes. However, due to the extensive number of studies that met the inclusion criteria, we subdivided the analysis and presentation of results in separate papers for chemotherapy and surgery, as well efficacy and safety outcomes [13]. This paper focuses on the efficacy of 5-HT3 receptor antagonist for patients undergoing surgery. Our methods are described briefly below.
Eligibility criteria
We included studies involving patients of any age undergoing any type of surgery and who were given a 5-HT3 receptor antagonist for nausea and/or vomiting (Additional file 1: Appendix 1). Randomized controlled trials (RCTs), quasi-RCTs, non-RCTs, interrupted time series, controlled before–after studies, and observational (cohort) studies were eligible for inclusion. We limited our systematic review to trials published in English due to resource constraints, and excluded studies that were identified as fraudulent or were retracted [14]. The primary outcome was the number of patients who vomited, and secondary outcomes were the number of patients with nausea and the number of patients with both postoperative nausea and vomiting (PONV).
Information sources
Information sources included electronic databases (MEDLINE, EMBASE, and the Cochrane Central Register of Controlled Trials from inception until January 11, 2013), trial protocol registries, and conference proceedings.
Study selection and data collection
Two reviewers screened the literature search results and potentially relevant full-text articles, independently. The same process was followed for data abstraction and methodological quality/risk of bias appraisal. We contacted authors as necessary; for example, to obtain additional information.
Appraisal of methodological quality and risk of bias
We used the Cochrane Effective Practice and Organization of Care (EPOC) risk-of-bias tool to assess risk of bias for experimental and quasi-experimental studies [15], and the Newcastle–Ottawa Scale (NOS) [16] for cohort studies.
Synthesis of included studies
A random-effects pairwise meta-analysis on the odds ratio (OR) scale was performed to combine studies addressing the same clinical outcome and treatment comparison. We decided to apply a random-effects model, as we expected methodological and clinical heterogeneity across the included studies that compared the same pairs of interventions. For studies with dichotomous outcomes where zero events were reported in one treatment arm, we added 0.5 to all cells. Between-study heterogeneity (τ2) was examined using the restricted maximum likelihood (REML) [17] method, and quantified using the I2 statistic [18]. The R 3.1.2 [19] and metafor package [20] were employed to conduct all pairwise meta-analyses.
For a connected network diagram, we conducted a random effects network meta-analysis to make inferences on the comparative efficacy of the 5-HT3 receptor antagonists [21]. Treatment nodes were selected by the clinicians and statisticians on the research team. If a study compared different doses of a particular intervention, we included only the recommended dose in the analysis [9, 10, 22–30].
Prior to conducting a network meta-analysis, we evaluated the transitivity assumption by examining the comparability of the distributions of potential treatment-effect modifiers across comparisons [31]. These included age (children versus adults), timing of administration (all time points versus during surgery), and risk of bias (all versus removing high risk of bias for randomization, allocation concealment, and blinding of outcome assessor). We evaluated transitivity in each network, by visually comparing the mode of the categorical potential effect modifiers across treatment comparisons [32]. We also assessed statistical inconsistency between different sources of evidence in the network using a global χ2 test derived from the design-by-treatment interaction model [21]. In the presence of statistically significant inconsistency, we applied the loop-specific approach [33, 34] to locally assess the network and identify the treatment comparisons responsible for inconsistency. In the network meta-analysis and design-by-treatment interaction models, we assumed common within-network heterogeneity, whereas in loop-specific method we assumed common within-loop heterogeneity. We assumed common heterogeneity across treatment comparisons since the included treatments are of the same nature and it was clinically reasonable to share a common heterogeneity parameter. In all approaches, we estimated the magnitude of between-study heterogeneity using the REML method [17]. Important heterogeneity and/or inconsistency would have been explored using network meta-regression analyses adjusting for potential effect modifiers. For each outcome, we carried out subgroup analyses using time of administration of antiemetics (all time periods versus during surgery) and age (all ages versus children), and sensitivity analyses excluding studies with high risk of incomplete outcome data bias. Although our primary analyses were restricted to RCTs only, as a secondary analysis, we included quasi-RCTs and non-RCTs to examine the robustness of the network meta-analysis results.
We present the network meta-analysis summary of treatment effects along with their 95 % confidence interval (CI) and 95 % predictive interval (PrI). The PrI captures both the uncertainty around the summary treatment effect and between-study variance, and shows the interval within which the treatment effect is expected to lie when a future study is conducted [35, 36]. To visually assess the presence of reporting bias (including publication bias and small-study effects), we used the comparison-adjusted funnel plot [32]. We also ranked the effectiveness of the 5-HT3 agonist receptors using the surface under the cumulative ranking (SUCRA) curve [37]. Network meta-analyses were conducted in Stata 13.0 [38] using the mvmeta command [39].
Results
Literature search
The literature search yielded 7,608 citations in total that met the search criteria, of which 450 full text articles met eligibility criteria for inclusion (444 primary publications and six companion reports reporting on nine studies, Fig. 1; Additional file 1: Appendix 2). Five unpublished conference abstracts were included in the review [40–44]. We excluded 77 studies because we suspected or confirmed that their results were fraudulent [14] and 535 studies from previous reviews that did not fulfill our eligibility criteria (reasons for exclusion presented in Additional file 1: Appendix 3).
Fig. 1.
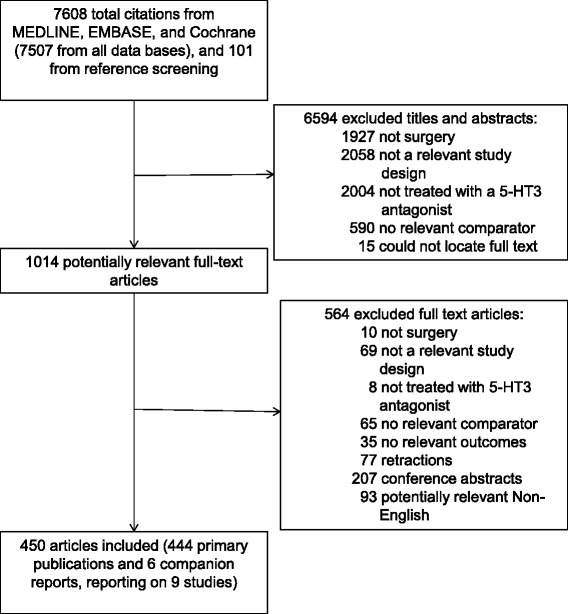
Study flow. Details the flow of information through the different phases of the review, mapping out the number of records identified, included and excluded, and the reasons for their exclusion
Study and patient characteristics
The majority of the included studies had an RCT design (97 %) with a short duration of follow-up of 12 to 24 h (72 %). Most studies were published between 1995 and 2013 (94 %), and were mainly conducted in Asia (39 %), North America (27 %), or Europe (24 %) (Table 1, Additional file 1: Appendix 4).
Table 1.
Study characteristics
| Characteristic | No. of studies * (n = 444) | Percentage of studies |
|---|---|---|
| Year of publication | ||
| 1990–1994 | 25 | 5.63 |
| 1995–1999 | 141 | 31.76 |
| 2000–2004 | 110 | 24.77 |
| 2005–2009 | 107 | 24.10 |
| 2010–2013 | 61 | 13.74 |
| Geographic region | ||
| Asia | 171 | 38.51 |
| North America | 118 | 26.58 |
| Europe | 108 | 24.32 |
| Australasia | 13 | 2.93 |
| Multi-continent | 12 | 2.70 |
| Africa | 11 | 2.48 |
| South America | 9 | 2.03 |
| Not reported | 2 | 0.45 |
| Study design | ||
| Randomized clinical trial | 429 | 96.62 |
| Cohort study | 9 | 2.03 |
| Non-randomized clinical trial | 5 | 1.13 |
| Controlled before–after study | 1 | 0.23 |
| Study conduct period | ||
| 1990–1999 | 11 | 2.48 |
| 2000–2009 | 45 | 10.14 |
| 2010–2013 | 8 | 1.80 |
| Not reported | 380 | 85.59 |
| Duration of follow-up ** | ||
| 0 to ≤6 | 13 | 2.93 |
| >6 to ≤12 | 9 | 2.03 |
| >12 to ≤24 | 319 | 71.85 |
| >24 to ≤48 | 52 | 11.71 |
| >48 to ≤72 | 14 | 3.15 |
| >72 to ≤1 week | 12 | 2.70 |
| >1 week | 3 | 0.68 |
| Not reported | 22 | 4.95 |
| Interventions examined: frequency *** | ||
| Serotonin antagonists Reported as administered alone (administered with dexamethasone) | ||
| Ondansetron | 336 (46) | 75.68 (10.36) |
| Granisetron | 57 (15) | 12.84 (3.38) |
| Tropisetron | 35 (2) | 7.88 (0.45) |
| Dolasetron | 33 (3) | 7.43 (0.68) |
| Palonosetron | 14 (3) | 3.15 (0.68) |
| Ramosetron | 10 (1) | 2.25 (0.23) |
| Comparator antiemetics | ||
| Butyrophenone | 72 | 16.22 |
| Benzamide | 72 | 16.22 |
| Dexamethasone | 40 | 9.01 |
| Phenothiazine | 13 | 2.93 |
| Antihistamine | 11 | 2.48 |
| NK-1 | 5 | 1.13 |
| Anticholinergic | 2 | 0.45 |
| Serotonin antagonists given with other antiemetic | ||
| Serotonin antagonist + dexamethasone | 70 | 15.77 |
| Serotonin antagonist + butyrophenone | 15 | 3.38 |
| Serotonin antagonist + benzamide | 5 | 1.13 |
| Serotonin antagonist + antihistamine | 3 | 0.68 |
| Serotonin antagonist + NK-1 | 2 | 0.45 |
| Serotonin antagonist + phenothiazine | 2 | 0.45 |
| Placebo or no treatment | ||
| 293 | 65.99 | |
| Outcomes examined: frequency **** | ||
| Vomiting | 347 | 78.15 |
| Nausea | 308 | 69.40 |
| PONV | 268 | 60.36 |
| Setting | ||
| Not reported | 270 | 60.81 |
| Hospital | 113 | 25.45 |
| Multi-center | 31 | 6.98 |
| Medical center | 30 | 6.76 |
* Includes unpublished data; ** Duration is in hours unless otherwise noted; *** Multiple interventions and comparators examined across the studies; **** Multiple interventions and outcomes reported per study
NK-1 neurokinin 1 receptor antagonist, PONV postoperative nausea and vomiting
The 5-HT3 receptor antagonists we examined were ondansetron (0.1 − 48 mg/day; 76 %), granisetron (0.1 − 3 mg/day; 13 %), tropisetron (0.1 − 5 mg/day; 8 %), dolasetron (12.5 − 200 mg/day; 7 %), palonosetron (0.025 − 0.25 mg/day; 3 %), and ramosetron (0.1 − 0.6 mg/day; 2 %) (Table 1, Additional file 1: Appendix 5). We also included studies comparing combinations of 5-HT3 drugs administered concomitantly with other antiemetics, e.g., dexamethasone (2–20 mg/day; 16 %), butyrophenone (3 %), and benzamide (1 %).
Overall, 286 studies with dichotomous outcome data were included in our analyses. Studies with continuous outcome data and studies investigating the same 5-HT3 treatment in different doses were not included in the analysis.
The median study size was 118 (interquartile range, 75–180) patients, whereas most patients were women (72 %), and adults (59 %), with an American Society of Anesthesiologists (ASA) physical status [45] of I or II (60 %) undergoing obstetrical and gynecological (30 %) surgery (Table 2, Additional file 1: Appendix 6). The included studies often did not report patients’ history of PONV (56 %). Similarly, a history of motion sickness was reported in only 33 % of the studies; comorbidities were rarely reported (5 %).
Table 2.
Patient characteristics
| Total no. of patients | 80,410 | |
| Mean, Median sample size | 181, 118 | |
| Mean % female | 72 | |
| No. of studies (n = 444) * | Percentage of studies | |
| Age category | ||
| Children only (aged <18 yr) | 75 | 16.89 |
| Adults only (aged ≥18 yr to ≤65 yr) | 262 | 59.01 |
| Children and adults (aged ≤65 yr) | 17 | 3.83 |
| Adults and elderly (aged ≥18 yr) | 76 | 17.12 |
| All ages | 9 | 2.03 |
| Not reported | 5 | 1.13 |
| American Society of Anesthesiologists (ASA) physical status | ||
| I | 15 | 3.38 |
| I or II | 266 | 59.91 |
| I or II or III | 87 | 19.59 |
| II or III | 4 | 0.90 |
| Not reported | 72 | 16.22 |
| Surgery type | ||
| Obstetric and gynecological | 134 | 30.18 |
| Gastrointestinal | 51 | 11.49 |
| Eye | 35 | 7.88 |
| General dentistry, oral and maxillofacial surgery, and orthodontics | 35 | 7.88 |
| Otolaryngological | 20 | 4.50 |
| Breast | 18 | 4.05 |
| Orthopedic | 16 | 3.60 |
| Neurological | 15 | 3.38 |
| Endocrine | 9 | 2.03 |
| Cardiovascular | 3 | 0.68 |
| Urological | 1 | 0.23 |
| Miscellaneous (includes multiple surgery types, abdominal surgery, and plastic surgery unspecified) | 103 | 23.20 |
| Not reported | 4 | 0.90 |
| History of motion sickness | ||
| Yes | 147 | 33.11 |
| No or not reported | 297 | 66.89 |
| History of postoperative nausea and vomiting | ||
| Yes | 197 | 44.37 |
| No or not reported | 247 | 55.63 |
| Comorbidities ** | ||
| Not reported | 415 | 93.47 |
| Diabetes mellitus | 9 | 2.03 |
| Cardiovascular | 8 | 1.80 |
| Obesity | 5 | 1.13 |
| Cancer | 4 | 0.90 |
| Migraines | 3 | 0.68 |
| Gallbladder | 2 | 0.45 |
| Liver disease | 2 | 0.45 |
| Asthma | 1 | 0.23 |
| Disorder of the ear | 1 | 0.23 |
| Mental health | 1 | 0.23 |
| Osteoarthritis | 1 | 0.23 |
| Urological | 1 | 0.23 |
* Includes unpublished data; ** Some studies considered more than one comorbidity
Methodological quality and risk of bias
Most of the included RCTs and quasi-RCTs had an unclear or high risk of bias on the following items: allocation concealment (59 %), baseline outcome characteristics (89 %), incomplete outcome data (60 %), and selective outcome reporting bias (97 %). When assessing potential for funding bias, we considered a study at a high or unclear risk of bias when it was funded by a private industry or when an author on the publication was employed by the company sponsoring the study, which occurred in 92 % of the RCTs and quasi-RCTs (Additional file 1: Appendices 7 and 8). Of the nine observational studies included in the analysis, eight used a somewhat representative sample, two did not describe ascertainment of exposure, all failed to demonstrate that the outcome was not present at the start of the study, six did not control for confounders, three did not describe the assessment of outcome, and all neglected to report follow-up (Additional file 1: Appendix 9). The visual inspection of the comparison adjusted funnel plots showed that there is no evidence for small-study effects and publication bias (Additional file 1: Appendix 10).
Vomiting
The network meta-analysis for vomiting included 238 RCTs with a total of 12,781 patients. The network geometry and included drugs can be found in Fig. 2a, whereas the statistically significant results are available in Table 3 and the overall results in Additional file 1: Appendix 11. The following treatment comparisons were statistically significant using both the CIs and PrIs: all agents (except for palonosetron plus dexamethasone and granisetron plus droperidol intravenous (IV)) versus placebo, ondansetron plus droperidol IV versus ondansetron, granisetron plus dexamethasone versus ondansetron, ondansetron plus dexamethasone versus dolasetron, ondansetron plus droperidol IV versus dolasetron, granisetron plus dexamethasone versus dolasetron, palonosetron plus dexamethasone versus ondansetron plus dexamethasone, palonosetron plus dexamethasone versus ondansetron plus droperidol IV, and palonosetron plus dexamethasone versus granisetron plus dexamethasone (Fig. 3). According to the SUCRA (Additional file 1: Appendix 12), the most effective agents for vomiting were ondansetron plus droperidol IV (85 % probability) and granisetron plus dexamethasone (84 % probability). The within-network heterogeneity in the network meta-analysis model was estimated at 0.15, and the evaluation of the network inconsistency using the design-by-treatment interaction model suggested that there was no evidence of statistical inconsistency (χ2 = 49.27, degrees of freedom = 44, P = 0.271, heterogeneity variance = 0.15).
Fig. 2.
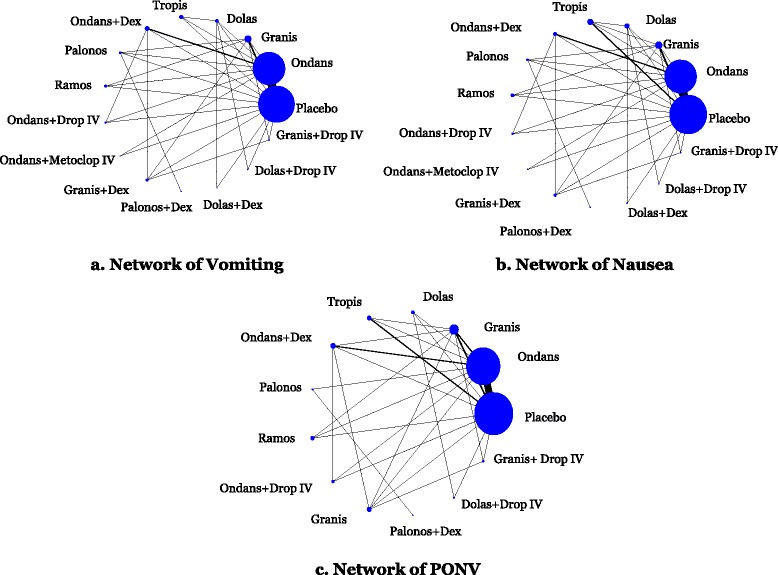
Network geometry. Network meta-analysis diagrams for vomiting, nausea, and PONV. Nodes are weighted according to the number of patients included in the corresponding treatments, and edges are weighted according to the number of studies included in the respective comparisons
Table 3.
Statistically significant results of network meta-analysis for all time periods of drug administration
| All ages | Children only | |||||
|---|---|---|---|---|---|---|
| Treatment comparison | No. of studies | MA estimate: OR (95 % CI) * | NMA estimate: OR (95 % CI) | No. of studies | MA estimate: OR (95 % CI) * | NMA estimate: OR (95 % CI) |
| Vomiting | 238 RCTs and 12,781 patients | 46 RCTs and 1,830 patients | ||||
| Ondansetron vs. placebo | 146 | 0.35 (0.32–0.39) | 0.36 (0.33–0.40) | 34 | 0.30 (0.24–0.38) | 0.30 (0.24–0.38) |
| Granisetron vs. placebo | 27 | 0.24 (0.16–0.34) | 0.26 (0.21–0.34) | 4 | 0.21 (0.08–0.56) | 0.23 (0.12–0.48) |
| Dolasetron vs. placebo | 7 | 0.42 (0.21–0.83) | 0.44 (0.30–0.63) | 3 | 0.41 (0.23–0.75) | 0.39 (0.19–0.78) |
| Tropisetron vs. placebo | 15 | 0.32 (0.22–0.48) | 0.32 (0.23–0.43) | 3 | 0.18 (0.09–0.36) | 0.18 (0.08–0.41) |
| Ondansetron + DEX vs. placebo | 12 | 0.16 (0.09–0.27) | 0.16 (0.12–0.23) | 5 | 0.06 (0.03–0.17) | 0.07 (0.03–0.15) |
| Palonosetron vs. placebo | 4 | 0.53 (0.38–0.73) | 0.38 (0.24–0.60) | NA | NA | NA |
| Ramosetron vs. placebo | 5 | 0.42 (0.26–0.68) | 0.28 (0.18–0.43) | NA | NA | NA |
| Ondansetron + DROP vs. placebo | 2 | 0.15 (0.07–0.31) | 0.14 (0.08–0.26) | 1 | 0.13 (0.05–0.33) | 0.11 (0.04–0.33) |
| Ondansetron + METO vs. placebo | 2 | 0.16 (0.06–0.43) | 0.15 (0.06–0.42) | 2 | 0.16 (0.06–0.43) | 0.18 (0.06–0.53) |
| Granisetron + DEX vs. placebo | 5 | 0.16 (0.08–0.31) | 0.15 (0.09–0.24) | 2 | 0.08 (0.03–0.27) | 0.09 (0.02–0.31) |
| Dolasetron + DEX vs. placebo | 1 | 0.06 (0.01–0.30) | 0.18 (0.06–0.49) | NA | NA | NA |
| Dolasetron + DROP vs. placebo | 1 | 0.16 (0.07–0.35) | 0.19 (0.07–0.52) | NA | NA | NA |
| Granisetron + DROP vs. placebo | 2 | 0.30 (0.05–1.66) | 0.31 (0.11–0.82) | NA | NA | NA |
| Granisetron vs. ondansetron | 12 | 0.52 (0.34–0.81) | 0.73 (0.56–0.94) | NA | NA | 0.78 (0.37–1.63) |
| Ondansetron + DEX vs. ondansetron | 15 | 0.50 (0.33–0.75) | 0.46 (0.33–0.63) | 3 | 0.24 (0.12–0.47) | 0.23 (0.11–0.49) |
| Ondansetron + DROP vs. ondansetron | 5 | 0.43 (0.24–0.78) | 0.39 (0.21–0.71) | 1 | 0.31 (0.12–0.77) | 0.37 (0.13–1.09) |
| Granisetron + DEX vs. ondansetron | NA | NA | 0.41 (0.25–0.67) | NA | NA | 0.28 (0.08–1.04) |
| Dolasetron vs. granisetron | NA | NA | 1.66 (1.07–2.57) | NA | NA | 1.65 (0.61–4.47) |
| Ondansetron + DEX vs. granisetron | NA | NA | 0.63 (0.42–0.94) | NA | NA | 0.30 (0.11–0.83) |
| Granisetron + DEX vs. granisetron | 7 | 0.39 (0.20–0.77) | 0.57 (0.35–0.92) | 1 | 0.14 (0.02–1.23) | 0.36 (0.09–1.50) |
| Ondansetron + DEX vs. dolasetron | NA | NA | 0.38 (0.23–0.62) | NA | NA | 0.18 (0.07–0.50) |
| Ondansetron + DROP vs. dolasetron | NA | NA | 0.32 (0.16–0.65) | NA | NA | 0.29 (0.08–1.04) |
| Granisetron + DEX vs. dolasetron | NA | NA | 0.34 (0.19–0.63) | NA | NA | 0.22 (0.05–0.95) |
| Ondansetron + DEX vs. tropisetron | NA | NA | 0.52 (0.33–0.82) | NA | NA | 0.40 (0.13–1.22) |
| Ondansetron + DROP vs. tropisetron | NA | NA | 0.45 (0.23–0.88) | NA | NA | 0.64 (0.16–2.48) |
| Granisetron + DEX vs. tropisetron | NA | NA | 0.47 (0.26–0.84) | NA | NA | 0.48 (0.10–2.25) |
| Palonosetron vs. ondansetron + DEX | NA | NA | 2.32 (1.33–4.07) | NA | NA | NA |
| Ramosetron vs. ondansetron + DEX | NA | NA | 1.71 (1.01–2.90) | NA | NA | NA |
| Palonosetron + DEX vs. ondansetron + DEX | NA | NA | 8.68 (1.19–63.20) | NA | NA | NA |
| Ondansetron + DROP vs. palonosetron | NA | NA | 0.37 (0.17–0.78) | NA | NA | NA |
| Granisetron + DEX vs. palonosetron | NA | NA | 0.39 (0.20–0.75) | NA | NA | NA |
| Palonosetron + DEX vs. ondansetron + DROP | NA | NA | 10.13 (1.31–78.58) | NA | NA | NA |
| Palonosetron + DEX vs. ondansetron + METO | NA | NA | 9.38 (1.03–85.06) | NA | NA | NA |
| Palonosetron + DEX vs. granisetron + DEX | NA | NA | 9.60 (1.28–72.03) | NA | NA | NA |
| Nausea | 195 RCTs and 24,230 patients | 11 RCTs and 1,326 patients | ||||
| Ondansetron vs. placebo | 121 | 0.46 (0.40–0.52) | 0.46 (0.41–0.52) | 10 | 0.44 (0.29–0.68) | 0.45 (0.30–0.66) |
| Granisetron vs. placebo | 21 | 0.35 (0.23–0.52) | 0.35 (0.26–0.47) | NA | NA | NA |
| Dolasetron vs. placebo | 9 | 0.59 (0.48–0.73) | 0.60 (0.43–0.86) | 1 | 0.29 (0.11–0.73) | 0.26 (0.09–0.74) |
| Tropisetron vs. placebo | 15 | 0.51 (0.40–0.66) | 0.48 (0.35–0.65) | NA | NA | NA |
| Ondansetron + DEX vs. placebo | 8 | 0.21 (0.14–0.34) | 0.28 (0.19–0.41) | 2 | 0.21 (0.08–0.57) | 0.22 (0.08–0.61) |
| Palonosetron vs. placebo | 3 | 0.48 (0.33–0.68) | 0.30 (0.17–0.53) | NA | NA | NA |
| Ramosetron vs. placebo | 5 | 0.35 (0.18–0.68) | 0.32 (0.22–0.47) | NA | NA | NA |
| Ondansetron + DROP vs. placebo | 2 | 0.31 (0.06–1.55) | 0.26 (0.14–0.49) | 1 | 0.07 (0.00–1.26) | 0.07 (0.00–1.38) |
| Granisetron + DEX vs. placebo | 4 | 0.21 (0.11–0.39) | 0.20 (0.12–0.34) | 1 | 0.09 (0.02–0.49) | 0.10 (0.02–0.53) |
| Dolasetron + DEX vs. placebo | 1 | 0.28 (0.05–1.53) | 0.21 (0.07–0.61) | NA | NA | NA |
| Dolasetron + DROP vs. placebo | 1 | 0.17 (0.08–0.36) | 0.19 (0.07–0.54) | NA | NA | NA |
| Granisetron + DROP vs. placebo | 2 | 0.22 (0.08–0.61) | 0.21 (0.07–0.62) | NA | NA | NA |
| Ondansetron + DEX vs. ondansetron | 14 | 0.68 (0.48–0.96) | 0.61 (0.42–0.89) | 1 | 0.53 (0.11–2.60) | 0.48 (0.16–1.43) |
| Granisetron + DEX vs. ondansetron | NA | NA | 0.43 (0.25–0.73) | NA | NA | 0.21 (0.04–1.23) |
| Dolasetron vs. granisetron | NA | NA | 1.73 (1.10–2.72) | NA | NA | NA |
| Granisetron + DEX vs. granisetron | 6 | 0.59 (0.39–0.88) | 0.56 (0.33–0.95) | NA | NA | NA |
| Ondansetron + DEX vs. dolasetron | NA | NA | 0.47 (0.28–0.78) | NA | NA | 0.81 (0.19–3.50) |
| Palonosetron vs. dolasetron | NA | NA | 0.50 (0.26–0.97) | NA | NA | NA |
| Ramosetron vs. dolasetron | NA | NA | 0.53 (0.31–0.88) | NA | NA | NA |
| Ondansetron + DROP vs. dolasetron | NA | NA | 0.44 (0.21–0.89) | NA | NA | 0.28 (0.01–6.17) |
| Granisetron + DEX vs. dolasetron | NA | NA | 0.33 (0.17–0.62) | NA | NA | 0.36 (0.05–2.66) |
| Dolasetron + DEX vs. dolasetron | 2 | 0.33 (0.15–0.72) | 0.35 (0.13–0.97) | NA | NA | NA |
| Dolasetron + DROP vs. dolasetron | 1 | 0.35 (0.17–0.73) | 0.32 (0.11–0.89) | NA | NA | NA |
| Ondansetron + DEX vs. tropisetron | NA | NA | 0.59 (0.36–0.95) | NA | NA | NA |
| Granisetron + DEX vs. tropisetron | NA | NA | 0.41 (0.22–0.76) | NA | NA | NA |
| Postoperative nausea and vomiting | 125 RCTs and 16,667 patients | 14 RCTs and 2,394 patients | ||||
| Ondansetron vs. placebo | 89 | 0.30 (0.26–0.35) | 0.31 (0.27–0.36) | 11 | 0.33 (0.18–0.60) | 0.34 (0.19–0.62) |
| Granisetron vs. placebo | 16 | 0.23 (0.14–0.37) | 0.23 (0.16–0.32) | 2 | 0.54 (0.26–1.12) | 0.32 (0.08–1.23) |
| Dolasetron vs. placebo | 5 | 0.27 (0.14–0.52) | 0.25 (0.14–0.43) | NA | NA | NA |
| Tropisetron vs. placebo | 8 | 0.39 (0.28–0.53) | 0.36 (0.24–0.54) | NA | NA | NA |
| Ondansetron + DEX vs. placebo | 8 | 0.12 (0.07–0.20) | 0.15 (0.10–0.22) | 1 | 0.20 (0.06–0.66) | 0.27 (0.04–1.61) |
| Palonosetron vs. placebo | NA | NA | 0.11 (0.03–0.40) | NA | NA | NA |
| Ramosetron vs. placebo | 4 | 0.30 (0.15–0.59) | 0.26 (0.16–0.41) | NA | NA | NA |
| Ondansetron + DROP vs. placebo | 2 | 0.13 (0.05–0.34) | 0.11 (0.05–0.24) | 1 | 0.12 (0.04–0.34) | 0.12 (0.02–0.71) |
| Granisetron + DEX vs. placebo | 4 | 0.06 (0.02–0.17) | 0.09 (0.05–0.16) | NA | NA | NA |
| Palonosetron + DEX vs. placebo | NA | NA | 0.12 (0.02–0.64) | NA | NA | NA |
| Dolasetron + DROP vs. placebo | 1 | 0.17 (0.08–0.36) | 0.12 (0.04–0.35) | NA | NA | NA |
| Granisetron + DROP vs. placebo | 2 | 0.17 (0.07–0.38) | 0.16 (0.06–0.40) | NA | NA | NA |
| Ondansetron + DEX vs. ondansetron | 11 | 0.48 (0.33–0.72) | 0.46 (0.31–0.69) | 1 | 1.00 (0.36–2.75) | 0.78 (0.13–4.65) |
| Ondansetron + DROP vs. ondansetron | 3 | 0.39 (0.20–0.75) | 0.35 (0.16–0.77) | NA | NA | NA |
| Granisetron + DEX vs. ondansetron | 1 | 0.22 (0.04–1.21) | 0.30 (0.17–0.53) | NA | NA | NA |
| Granisetron + DEX vs. granisetron | 8 | 0.48 (0.29–0.77) | 0.41 (0.24–0.69) | NA | NA | NA |
| Granisetron + DEX vs. dolasetron | NA | NA | 0.37 (0.17–0.82) | NA | NA | NA |
| Ondansetron + DEX vs. tropisetron | NA | NA | 0.41 (0.23–0.73) | NA | NA | NA |
| Ondansetron + DROP vs. tropisetron | NA | NA | 0.31 (0.13–0.75) | NA | NA | NA |
| Granisetron + DEX vs. tropisetron | NA | NA | 0.26 (0.13–0.52) | NA | NA | NA |
| Granisetron + DEX vs. ramosetron | NA | NA | 0.36 (0.18–0.75) | NA | NA | NA |
* Meta-analysis was not conducted for treatment comparisons where only 1 trial was included. In that situation, the direct estimate was obtained from the single trial
CI confidence interval, DEX dexamethasone, DROP droperidol (intravenous), MA meta-analysis, METO metoclopramide (intravenous), NA not applicable, NMA network meta-analysis, OR odds ratio
Fig. 3.

Network meta-analysis results for vomiting. All treatments are compared to placebo. The black horizontal lines represent the 95 % confidence intervals (CI) of the summary treatment effects and red horizontal lines the 95 % predictive intervals (PrI). Results are presented on the odds ratio scale
In order to account for the treatment effect modifier ‘age’ (Additional file 1: Appendix 13), a subgroup analysis was conducted for 46 RCTs involving a total of 1,830 children (Table 3, Additional file 1: Appendix 11). The following treatment comparisons were statistically significant for vomiting: ondansetron versus placebo, granisetron versus placebo, dolasetron versus placebo, tropisetron versus placebo, ondansetron plus dexamethasone versus placebo, ondansetron plus droperidol lV versus placebo, ondansetron plus metoclopramide IV versus placebo, granisetron plus dexamethasone versus placebo, ondansetron plus dexamethasone versus ondansetron, ondansetron plus dexamethasone versus dolasetron, ondansetron plus dexamethasone versus granisetron, and granisetron plus dexamethasone versus dolasetron (Additional file 1: Appendix 11). According to the SUCRA, the most effective agents for vomiting in children were ondansetron plus dexamethasone (83 % probability) and granisetron plus dexamethasone (82 % probability).
In order to account for the treatment effect modifier ‘timing of administration’ (Additional file 1: Appendix 14), a subgroup analysis was conducted for 220 RCTs involving 10,811 patients when the agents were administered during surgery (Additional file 1: Appendix 15). The results were the same as for the primary analysis, except that ondansetron plus droperidol IV was statistically superior to granisetron, and ondansetron plus droperidol IV and granisetron plus dexamethasone were superior to ramosetron. According to the SUCRA for this subgroup analysis, the most effective agents for vomiting were ondansetron plus droperidol IV (88 % probability) and granisetron plus dexamethasone (84 % probability).
In order to account for the treatment effect modifier ‘risk of bias’, a sensitivity analysis was conducted in which 11 RCTs were removed because of high risk of incomplete outcome data bias (Additional file 1: Appendix 15); the same results were observed, except granisetron plus dexamethasone was associated with significantly less vomiting compared with ramosetron (OR, 0.52;, 95 % CI, 0.27–0.99). In another sensitivity analysis, in which four cohort studies [46–49], two non-randomized controlled trials [50, 51], and one controlled before–after study [52] were added to the included studies, all of the results were the same, except that the differences between ondansetron plus dexamethasone and granisetron or ramosetron and between ondansetron plus metoclopramide IV and palonosetron plus dexamethasone were no longer statistically significant (Additional file 1: Appendix 15).
Nausea
The network meta-analysis for nausea included 195 RCTs with a total of 24,230 patients. The network geometry and included drugs can be found in Fig. 2b. We present the statistically significant treatment effect estimates derived through the network meta-analysis model in Table 3 and overall results in Additional file 1: Appendix 11. Using both the CIs and PrIs, the only treatment comparisons that were statistically significant for nausea were granisetron versus placebo (OR, 0.35; 95 % PrI, 0.13–0.91), ondansetron plus dexamethasone versus placebo (OR, 0.28; 95 % PrI, 0.10–0.76), palonosetron versus placebo (OR, 0.30; 95 % PrI, 0.10–0.89), ramosetron versus placebo (OR, 0.32; 95 % PrI, 0.12–0.86), ondansetron plus droperidol IV versus placebo (OR, 0.26; 95 % PrI, 0.09–0.80), granisetron plus dexamethasone versus placebo (OR, 0.20; 95 % PrI, 0.07–0.57), dolasetron plus droperidol IV versus placebo (OR, 0.19; 95 % PrI, 0.05–0.77), and granisetron plus droperidol IV versus placebo (OR, 0.21; 95 % PrI, 0.05–0.87) (Additional file 1: Appendices 11 and 16). According to the SUCRA (Additional file 1: Appendix 17), the most effective agents for nausea were granisetron plus dexamethasone (82 % probability) and dolasetron plus droperidol IV (78 % probability). The within-network heterogeneity in the network meta-analysis model was estimated at 0.24, and the design-by-treatment interaction model suggested that there was no statistically significant inconsistency (χ2 = 26.65, degrees of freedom = 41, P = 0.959, heterogeneity variance = 0.27).
In order to account for the treatment effect modifier ‘age’ (Additional file 1: Appendix 13), a subgroup analysis was conducted for 11 RCTs involving 1,326 children (Table 3, Additional file 1: Appendix 11). The following treatment comparisons were statistically significant for nausea: ondansetron versus placebo, dolasetron versus placebo, ondansetron plus dexamethasone versus placebo, and granisetron plus dexamethasone versus placebo (Additional file 1: Appendix 11). According to the SUCRA, the most effective agents for nausea in children were granisetron plus dexamethasone (84 % probability) and ondansetron plus droperidol IV (81 % probability).
In order to account for the treatment effect modifier ‘timing of administration’ (Additional file 1: Appendix 14), a subgroup analysis was conducted for 175 RCTs involving 21,844 patients when the agents were administered during surgery (Additional file 1: Appendix 18). All of the results were the same as for the primary analysis, except that the difference between tropisetron and ondansetron plus dexamethasone was no longer statistically significant. According to the SUCRA for this subgroup analysis, the most effective agents for nausea were granisetron plus dexamethasone (82 % probability) and dolasetron plus droperidol IV (77 % probability).
In order to account for the treatment effect modifier ‘risk of bias’, a sensitivity analysis was conducted in which 10 RCTs were removed because of high risk of incomplete outcome data bias [53–62], and the results were unchanged from the primary analysis (Additional file 1: Appendix 18). In another sensitivity analysis, in which two cohort studies [46, 49] and two non-randomized controlled trials [50, 51] were added to the included studies, all of the results were the same, except that the differences between palonosetron and dolasetron and between dolasetron plus dexamethasone and dolasetron were no longer statistically significant (Additional file 1: Appendix 18).
Postoperative nausea and vomiting
The network meta-analysis for PONV included 125 RCTs with 16,667 patients. The network geometry and included drugs can be found in Fig. 2c, statistically significant results are presented in Table 3, and the overall results in Additional file 1: Appendix 11. According to both the CIs and PrIs, the following treatment comparisons were statistically significant for PONV: all agents versus placebo except for tropisetron, granisetron plus dexamethasone versus ondansetron, and granisetron plus dexamethasone versus tropisetron (Additional file 1: Appendix 19). According to the SUCRA, the most effective agents for PONV were granisetron plus dexamethasone (86 % probability) and ondansetron plus droperidol IV (78 % probability; Additional file 1: Appendices 11 and 20). The within-network heterogeneity in the network meta-analysis model was estimated at 0.25, and the design-by-treatment interaction model suggested that there was no statistically significant inconsistency (χ2 = 26.58, degrees of freedom = 32, P = 0.737, heterogeneity variance = 0.26).
In order to account for the treatment effect modifier ‘age’ (Additional file 1: Appendix 13), a subgroup analysis was conducted for 14 RCTs involving a total of 2,394 children (Table 3, Additional file 1: Appendix 11). The following treatment comparisons were statistically significant: ondansetron versus placebo and ondansetron plus droperidol IV versus placebo. According to the SUCRA, the most effective agents for PONV in children were ondansetron plus droperidol IV (85 % probability) and ondansetron plus dexamethasone (59 % probability).
In order to account for the treatment effect modifier ‘timing of administration’ (Additional file 1: Appendix 14), a subgroup analysis was conducted for 116 RCTs involving 12,415 patients in which the agents were administered during surgery (Additional file 1: Appendix 21). All of the results were the same as for the primary analysis, except that ondansetron plus dexamethasone was statistically superior to ramosetron. According to the SUCRA for this subgroup analysis, the most effective agents for PONV were granisetron plus dexamethasone (84 % probability) and ondansetron plus droperidol IV (79 % probability).
In order to account for the treatment effect modifier ‘risk of bias’, a sensitivity analysis was conducted in which 10 RCTs were removed because of high risk of incomplete outcome data bias (Additional file 1: Appendix 21) [53–55, 58, 59, 61–65]; the results were unchanged from the primary analysis, except that the risk of PONV was significantly higher with tropisetron than with granisetron. In another sensitivity analysis, two cohort studies [46, 49] and three non-randomized controlled trials [50, 51, 66] were added to the included studies, and the same results were observed (Additional file 1: Appendix 11).
Discussion
Administration of most 5-HT3 antagonists led to significantly fewer patients experiencing nausea, vomiting, and PONV relative to placebo. However, some of the corresponding PrIs were not statistically significant, suggesting that the statistically significant treatment effects might change should a new study become available. For all age groups and across all outcomes, the most effective agents were granisetron plus dexamethasone. For adults, the most effective agents were ondansetron plus droperidol IV; and for children, the most effective agents were ondansetron plus dexamethasone.
We also conducted a systematic review and network meta-analysis on the safety of these medications [13]. Our network meta-analysis results suggested that granisetron plus dexamethasone increases the risk of arrhythmia. However, a statistically significant increase in the risk of delirium was not observed in another network meta-analysis. In a meta-analysis including three studies for ondansetron versus placebo, no statistically significant results were observed for mortality. Only two studies reported prolongation of the QT interval; meta-analysis was not feasible because the studies compared different interventions.
Our network meta-analysis results for vomiting and PONV are similar to those of a previous network meta-analysis that examined only these two outcomes [67]. The only difference was that, unlike the current study, the earlier analysis showed that granisetron was significantly better than ondansetron and dolasetron for PONV. However, we included 378 studies involving a total of 68,167 patients that were not included in the earlier review. Although we are aware of other systematic reviews and meta-analyses of 5-HT3 receptor antagonists [14, 68], the previous researchers did not conduct a network meta-analysis, and therefore the results cannot be compared. Notably, because of our comprehensive literature search and broad eligibility criteria, we included 205 studies involving a total of 43,075 patients that were not included in those previous reviews (Additional file 1: Appendix 22).
The included studies were limited by having an unclear or high risk of bias on important components, including allocation concealment, selective outcome reporting bias, and potential for funding bias. Further, this systematic review process had some inherent limitations. Slight changes to the original protocol [12] were necessary, such as preparing a separate paper for patients undergoing chemotherapy, as well as one focused on the safety of these agents for patients with surgery [13]. Furthermore, it was assumed that the effects of the different doses and durations were identical across the treatments, and that they defined the same node they belong to. We are currently exploring these assumptions in another paper [69]. Although study designs above and beyond RCTs were included, the network meta-analysis was limited to the RCTs in order to increase the confidence of the results. We also were unable to present the results from the hundreds of meta-analyses conducted, as well as the raw data; these are available from the corresponding author upon request. Although the analyses were adjusted to account for the treatment effect modifiers (age, timing of administration, and risk of bias), the results might be influenced by effect modifiers that we were unaware of. However, the statistical evaluation of the transitivity assumption using the design-by-treatment interaction model suggested there was no evidence of inconsistency. Finally, 77 studies were excluded because they contained data known to be fraudulent or were retracted [14].
Conclusions
In conclusion, granisetron plus dexamethasone was often the most effective antiemetic across the effectiveness outcomes considered here, with the number needed to treat ranging from two to nine. A study that examines the administration of these agents at different dosages would provide further clarity to this important issue and our team is currently working on such an initiative [70].
Acknowledgements
This systematic review was funded by the Canadian Institutes for Health Research/Drug Safety and Effectiveness Network (CIHR/DSEN). We thank Laure Perrier for conducting the literature searches, Becky Skidmore for peer-reviewing the literature search, and Drs. Maggie Chen and Joseph Beyene for providing feedback on conceptualization of the review. We also thank Vladi Struchkov, Vera Nincic, Roberta Cardoso, Christy Johnson, and Derek Roberts for screening some of the citations, and/or abstracting some of the data, and/or appraising quality for a few of the included studies. Finally, we thank Wing Hui for abstracting some of the data, Ana Guzman for formatting the manuscript, and Peggy Robinson for copyediting the manuscript.
Abbreviations
- 5-HT3
Serotonin
- CI
Confidence interval
- IV
Intravenous.
- OR
Odds ratio
- PONV
Postoperative nausea and vomiting
- PrI
Predictive interval
- RCTs
Randomized control trials
- REML
Restricted maximum likelihood
- SUCRA
Surface under the cumulative ranking
Additional file
Appendices 1–22.
Footnotes
Competing interests
This systematic review was funded by the Canadian Institutes of Health Research/Drug Safety and Effectiveness Network (CIHR/DSEN). ACT and BH are funded by a CIHR/DSEN New Investigator Award in Knowledge Synthesis. BRH receives funding from the Alberta Heritage Foundation for Medical Research. AAV is funded by the Banting Postdoctoral Fellowship Program from the CIHR. DM is funded by a University of Ottawa Research Chair. SES is funded by a Tier 1 Canada Research Chair in Knowledge Translation. RR owns stock in a company (GlaxoSmithKline Inc) that manufactures one of the interventions included in our study. All of the other authors declare no conflicts of interest.
Authors’ contributions
ACT conceived, designed, and helped obtain funding for the study, guided the analysis, interpreted the results, and drafted the manuscript. CS coordinated the review, screened citations and full-text articles, abstracted data, appraised quality, cleaned the data, and edited the manuscript. EB, LS, and HA screened citations and full-text articles, abstracted data, appraised quality, cleaned the data, and edited the manuscript. AAV analyzed the data, interpreted the data, wrote the synthesis section, and edited the manuscript. PAK, AV, JI, HM, ER, RR, JH, CN, and KM screened citations and full-text articles, abstracted data, appraised quality, and edited the manuscript. JA provided methodological support and screened citations and full-text articles. BH, BRH, and DM helped conceive the study and edited the manuscript. SES conceived and designed the study, obtained the funding, interpreted the results, and edited the manuscript. All authors read and approved the final paper and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Contributor Information
Andrea C. Tricco, Email: triccoa@smh.ca
Charlene Soobiah, Email: soobiahc@smh.ca.
Erik Blondal, Email: blondale@smh.ca.
Areti A. Veroniki, Email: veronikia@smh.ca
Paul A. Khan, Email: pakahn@hotmail.com
Afshin Vafaei, Email: vafaeia@smh.ca.
John Ivory, Email: john.d.ivory@gmail.com.
Lisa Strifler, Email: striflerl@smh.ca.
Huda Ashoor, Email: ashoorh@smh.ca.
Heather MacDonald, Email: hrmacdonald@gmail.com.
Emily Reynen, Email: ereynen@gmail.com.
Reid Robson, Email: reidcrobson@gmail.com.
Joanne Ho, Email: jmho2001@gmail.com.
Carmen Ng, Email: carmen_hm_ng@yahoo.ca.
Jesmin Antony, Email: antonyj@smh.ca.
Kelly Mrklas, Email: kmrklas@gmail.com.
Brian Hutton, Email: bhutton@ohri.ca.
Brenda R. Hemmelgarn, Email: brenda.hemmelgarn@albertahealthservices.ca
David Moher, Email: dmoher@ohri.ca.
Sharon E. Straus, Email: sharon.straus@utoronto.ca
References
- 1.Habib AS, Chen YT, Taguchi A, Hu XH, Gan TJ. Postoperative nausea and vomiting following inpatient surgeries in a teaching hospital: a retrospective database analysis. Curr Med Res Opin. 2006;22:1093–9. doi: 10.1185/030079906X104830. [DOI] [PubMed] [Google Scholar]
- 2.Sarin P, Urman RD, Ohno-Machado L. An improved model for predicting postoperative nausea and vomiting in ambulatory surgery patients using physician-modifiable risk factors. J Am Med Inform Assoc. 2012;19:995–1002. doi: 10.1136/amiajnl-2012-000872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Apfel CC, Laara E, Koivuranta M, Greim CA, Roewer N. A simplified risk score for predicting postoperative nausea and vomiting: conclusions from cross-validations between two centers. Anesthesiology. 1999;91:693–700. doi: 10.1097/00000542-199909000-00022. [DOI] [PubMed] [Google Scholar]
- 4.Ballatori E, Roila F, Ruggeri B, Betti M, Sarti S, Soru G, et al. The impact of chemotherapy-induced nausea and vomiting on health-related quality of life. Support Care Cancer. 2007;15:179–85. doi: 10.1007/s00520-006-0109-7. [DOI] [PubMed] [Google Scholar]
- 5.Osoba D, Zee B, Warr D, Latreille J, Kaizer L, Pater J. Effect of postchemotherapy nausea and vomiting on health-related quality of life. The Quality of Life and Symptom Control Committees of the National Cancer Institute of Canada Clinical Trials Group. Support Care Cancer. 1997;5:307–13. doi: 10.1007/s005200050078. [DOI] [PubMed] [Google Scholar]
- 6.Apfel CC, Korttila K, Abdalla M, Kerger H, Turan A, Vedder I, et al. A factorial trial of six interventions for the prevention of postoperative nausea and vomiting. N Engl J Med. 2004;350:2441–51. doi: 10.1056/NEJMoa032196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chung F, Mezei G. Factors contributing to a prolonged stay after ambulatory surgery. Anesth Analg. 1999;89:1352–9. doi: 10.1097/00000539-199912000-00004. [DOI] [PubMed] [Google Scholar]
- 8.Constenla M. 5-HT3 receptor antagonists for prevention of late acute-onset emesis. Ann Pharmacother. 2004;38:1683–91. doi: 10.1345/aph.1D191. [DOI] [PubMed] [Google Scholar]
- 9.Gan TJ, Meyer T, Apfel CC, Chung F, Davis PJ, Eubanks S, et al. Consensus guidelines for managing postoperative nausea and vomiting. Anesth Analg. 2003;97:62–71. doi: 10.1213/01.ANE.0000068580.00245.95. [DOI] [PubMed] [Google Scholar]
- 10.McCracken G, Houston P, Lefebvre G. Guideline for the management of postoperative nausea and vomiting. J Obstet Gynaecol Can. 2008;30:600–7. doi: 10.1016/s1701-2163(16)32895-x. [DOI] [PubMed] [Google Scholar]
- 11.Shamseer L, Moher D, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ. 2015;349:g7647. doi: 10.1136/bmj.g7647. [DOI] [PubMed] [Google Scholar]
- 12.Tricco AC, Soobiah C, Antony J, Hemmelgarn B, Moher D, Hutton B, et al. Safety of serotonin (5-HT3) receptor antagonists in patients undergoing surgery and chemotherapy: protocol for a systematic review and network meta-analysis. Syst Rev. 2013;2:46. doi: 10.1186/2046-4053-2-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tricco AC, Soobiah C, Blondal E, Veroniki AA, Khan A, Vafaei A, et al. Comparative safety of serotonin (5-HT3) receptor antagonists in patients undergoing surgery: a systematic review and network meta-analysis. BMC Med. 2015; ahead of print. [DOI] [PMC free article] [PubMed]
- 14.Carlisle JB. The analysis of 168 randomised controlled trials to test data integrity. Anaesthesia. 2012;67:521–37. doi: 10.1111/j.1365-2044.2012.07128.x. [DOI] [PubMed] [Google Scholar]
- 15.Effective Practice and Organization of Care (EPOC). Suggested risk of bias criteria for EPOC reviews. EPOC Resources for review authors. Oslo: Norwegian Knowledge Centre for the Health Services; 2014. http://epoc.cochrane.org/sites/epoc.cochrane.org/files/uploads/14%20Suggested%20risk%20of%20bias%20criteria%20for%20EPOC%20reviews%202013%2008%2012_0.pdf. Accessed: Jan 2015.
- 16.Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm. Accessed: Jan 2015
- 17.Raudenbush SW. Analyzing effect sizes: random-effects models. In: Cooper H, Hedges LV, Valentine JC, editors. The Handbook of Research Synthesis and Meta-analysis. 2. New York: The Russell Sage Foundation; 2009. pp. 295–316. [Google Scholar]
- 18.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–58. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 19.R Core Team. R: A language and environment for statistical computing. http://www.R-project.org. Accessed Jan 2015.
- 20.Viechtbauer W. Conducting meta-analyses in R with the metafor package. J Stat Softw. 2010;36:1–48. [Google Scholar]
- 21.White IR, Barrett JK, Jackson D, Higgins J. Consistency and inconsistency in network meta‐analysis: model estimation using multivariate meta‐regression. Res Synth Methods. 2012;3:111–25. doi: 10.1002/jrsm.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen C-C, Lin C-S, Ko Y-P, Hung Y-C, Lao H-C, Hsu Y-W. Premedication with mirtazapine reduces preoperative anxiety and postoperative nausea and vomiting. Anesth Analg. 2008;106:109–13. doi: 10.1213/01.ane.0000289636.09841.bc. [DOI] [PubMed] [Google Scholar]
- 23.Omran HASA, Nasr DAM. Effect of premedication with mirtazapine versus ondansetron on postoperative nausea and vomiting in breast surgery. Egyp J Anaesth. 2011;27:135–9. doi: 10.1016/j.egja.2011.06.003. [DOI] [Google Scholar]
- 24.Homesley HD, Hahne WF, McLees B, Heck K, Barrett RJ, Lentz SS, et al. Randomized comparison of the antiemetic efficacy of a serotonin type 3 receptor antagonist (MDL 72,222) with a high-dose metoclopramide regimen. Am J Clin Oncol. 1993;16:175–9. doi: 10.1097/00000421-199304000-00020. [DOI] [PubMed] [Google Scholar]
- 25.Gan TJ, Meyer TA, Apfel CC, Chung F, Davis PJ, Habib AS, et al. Society for Ambulatory Anesthesia guidelines for the management of postoperative nausea and vomiting. Anesth Analg. 2007;105:1615–28. doi: 10.1213/01.ane.0000295230.55439.f4. [DOI] [PubMed] [Google Scholar]
- 26.Golembiewski J, Chernin E, Chopra T. Prevention and treatment of postoperative nausea and vomiting. Am J Health Syst Pharm. 2005;62:1247–60. doi: 10.2146/ajhp050374. [DOI] [PubMed] [Google Scholar]
- 27.Kovac AL. Prevention and treatment of postoperative nausea and vomiting. Drugs. 2000;59:213–43. doi: 10.2165/00003495-200059020-00005. [DOI] [PubMed] [Google Scholar]
- 28.Yun MJ, Kim YH, Kim AR. Comparison of azasetron and ondansetron for preventing postoperative nausea and vomiting in patients undergoing gynecological laparoscopic surgery. Yonsei Med J. 2010;51:88–92. doi: 10.3349/ymj.2010.51.1.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.MIMS. Ramosetron. http://www.mims.com/Thailand/drug/search/Ramosetron. Accessed: Jan 2015.
- 30.MIMS. Azasetron. http://www.mims.com/Thailand/drug/search/Azasetron?AllFlag=ALL. Accessed: Jan 2015.
- 31.Salanti G. Indirect and mixed-treatment comparison, network, or multiple-treatments meta-analysis: many names, many benefits, many concerns for the next generation evidence synthesis tool. Res Synth Methods. 2012;3:80–97. doi: 10.1002/jrsm.1037. [DOI] [PubMed] [Google Scholar]
- 32.Chaimani A, Higgins JP, Mavridis D, Spyridonos P, Salanti G. Graphical tools for network meta-analysis in STATA. PLoS One. 2013;8:e76654. doi: 10.1371/journal.pone.0076654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Song F, Altman DG, Glenny A-M, Deeks JJ. Validity of indirect comparison for estimating efficacy of competing interventions: empirical evidence from published meta-analyses. BMJ. 2003;326:472. doi: 10.1136/bmj.326.7387.472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Veroniki AA, Vasiliadis HS, Higgins JP, Salanti G. Evaluation of inconsistency in networks of interventions. Int J Epidemiol. 2013;42:332–45. doi: 10.1093/ije/dys222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Riley RD, Higgins JPT, Deeks JJ. Interpretation of random effects meta-analyses. BMJ. 2011;342:d549. doi: 10.1136/bmj.d549. [DOI] [PubMed] [Google Scholar]
- 36.Higgins JP, Thompson SG, Spiegelhalter DJ. A re-evaluation of random-effects meta-analysis. J R Stat Soc Ser A Stat Soc. 2009;172:137–59. doi: 10.1111/j.1467-985X.2008.00552.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Salanti G, Ades AE, Ioannidis JPA. Graphical methods and numerical summaries for presenting results from multiple-treatment meta-analysis: an overview and tutorial. J Clin Epidemiol. 2011;64:163–71. doi: 10.1016/j.jclinepi.2010.03.016. [DOI] [PubMed] [Google Scholar]
- 38.StataCorp . Stata Statistical Software: Release 13. College Station, TX: StataCorp LP; 2013. [Google Scholar]
- 39.White IR. Multivariate random-effects meta-regression: updates to mvmeta. Stata J. 2011;11:255–70. [Google Scholar]
- 40.Apfel CC, Candiotti K, Gan TJ, Ahmed R, Cox D. Palonosetron or ondansetron for rescue treatment of PONV after failed prophylaxis with ondansetron. Anesth Analg. 2011;112:S16. [Google Scholar]
- 41.Gaynes B, Fiscella R, Larson B, Kiesling D. Intravenous ondansetron versus placebo for post-operative nausea and vomiting following cataract surgery. In: American Academy of Optometry. 1996: 170p.
- 42.Gurha P, Kaur RD, Sanjay RR. Prophylactic intravenous palonosetron, granisetron and ondansetron in the prevention of post operative nausea and vomiting in laparoscopic surgeries. Br J Anaesth. vol. 108: Oxford Univ Press; 2012: 376–376.
- 43.Samra SK, Williams B, Ciarralo C, Turner C, Smythe P. Lack of effect of droperidol, ondansetron, and dolasetron administration on PONV in a placebo controlled trial. Anesthesiology. 2003;99:A305. [Google Scholar]
- 44.White PF, Scuderi PE. Prevention of postoperative nausea and vomiting (PONV): A dose-ranging study involving palonosetron, a potent 5-HT3 receptor antagonist. Anesthesiology. 2005;103:A703. [Google Scholar]
- 45.American Society of Anesthesiologists. ASA Physical Status Classification System. http://www.asahq.org/resources/clinical-information/asa-physical-status-classification-system. Accessed: Jan 2015.
- 46.Chen YF, Yeh WL, Lee KH, Li MC, Yang TH, Huang HC, et al. Intravenous ondansetron as antiemetic prophylaxis for postoperative nausea and vomiting after shoulder arthroscopy. Chang Gung Med J. 2011;34:205–12. [PubMed] [Google Scholar]
- 47.Flynn BC, Nemergut EC. Postoperative nausea and vomiting and pain after transsphenoidal surgery: a review of 877 patients. Anesth Analg. 2006;103:162–7. doi: 10.1213/01.ane.0000221185.08155.80. [DOI] [PubMed] [Google Scholar]
- 48.Shakeel M, Trinidade A, Al-Adhami A, Khan I, Supriya M, Kubba H. Retrospective review of three antiemetic regimens versus no therapy in the prevention of postoperative and vomiting following adenotonsillar surgery. J Otolaryngol Head Neck Surg. 2012;41:35–40. [PubMed] [Google Scholar]
- 49.Walker JB. Efficacy of single-dose intravenous dolasetron versus ondansetron in the prevention of postoperative nausea and vomiting. Clin Ther. 2001;23:932–8. doi: 10.1016/S0149-2918(01)80080-1. [DOI] [PubMed] [Google Scholar]
- 50.Biedler A, Wermelt J, Kunitz O, Muller A, Wilhelm W, Dethling J, et al. A risk adapted approach reduces the overall institutional incidence of postoperative nausea and vomiting. Can J Anaesth. 2004;51:13–9. doi: 10.1007/BF03018540. [DOI] [PubMed] [Google Scholar]
- 51.Kim EJ, Ko JS, Kim CS, Lee SM, Choi DH. Combination of antiemetics for the prevention of postoperative nausea and vomiting in high-risk patients. J Korean Med Sci. 2007;22:878–82. doi: 10.3346/jkms.2007.22.5.878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wagner DS, Gauger V, Chiravuri D, Faust K. Ondansetron oral disintegrating tablets for the prevention of postoperative vomiting in children undergoing strabismus surgery. Ther Clin Risk Manag. 2007;3:691–4. [PMC free article] [PubMed] [Google Scholar]
- 53.Alexander R, Fennelly M. Comparison of ondansetron, metoclopramide and placebo as premedicants to reduce nausea and vomiting after major surgery. Anaesthesia. 1997;52:695–8. doi: 10.1111/j.1365-2044.1997.az0130a.x. [DOI] [PubMed] [Google Scholar]
- 54.Aouad MT, Siddik-Sayyid SM, Taha SK, Azar MS, Nasr VG, Hakki MA, et al. Haloperidol vs. ondansetron for the prevention of postoperative nausea and vomiting following gynaecological surgery. Eur J Anaesthesiol. 2007;24:171–8. doi: 10.1017/S0265021506001323. [DOI] [PubMed] [Google Scholar]
- 55.Gan TJ, Gu J, Singla N, Chung F, Pearman MH, Bergese SD, et al. Rolapitant for the prevention of postoperative nausea and vomiting: a prospective, double-blinded, placebo-controlled randomized trial. Anesth Analg. 2011;112:804–12. doi: 10.1213/ANE.0b013e31820886c3. [DOI] [PubMed] [Google Scholar]
- 56.McKenzie R, Sharifi-Azad S, Dershwitz M, Miguel R, Joslyn AF, Tantisira B, et al. A randomized, double-blind pilot study examining the use of intravenous ondansetron in the prevention of postoperative nausea and vomiting in female inpatients. J Clin Anesth. 1993;5:30–6. doi: 10.1016/0952-8180(93)90084-R. [DOI] [PubMed] [Google Scholar]
- 57.Metaxari M, Papaioannou A, Petrou A, Chatzimichali A, Pharmakalidou E, Askitopoulou H. Antiemetic prophylaxis in thyroid surgery: a randomized, double-blind comparison of three 5-HT3 agents. J Anesth. 2011;25:356–62. doi: 10.1007/s00540-011-1119-2. [DOI] [PubMed] [Google Scholar]
- 58.Munro FJ, Fisher S, Dickson U, Morton N. The addition of antiemetics to the morphine solution in patient controlled analgesia syringes used by children after an appendicectomy does not reduce the incidence of postoperative nausea and vomiting. Paediatr Anaesth. 2002;12:600–3. doi: 10.1046/j.1460-9592.2002.00900.x. [DOI] [PubMed] [Google Scholar]
- 59.Sarvela PJ, Halonen PM, Soikkeli AI, Kainu JP, Korttila KT. Ondansetron and tropisetron do not prevent intraspinal morphine- and fentanyl-induced pruritus in elective cesarean delivery. Acta Anaesthesiol Scand. 2006;50:239–44. doi: 10.1111/j.1399-6576.2006.00934.x. [DOI] [PubMed] [Google Scholar]
- 60.Tang J, Watcha MF, White PF. A comparison of costs and efficacy of ondansetron and droperidol as prophylactic antiemetic therapy for elective outpatient gynecologic procedures. Anesth Analg. 1996;83:304–13. doi: 10.1097/00000539-199608000-00018. [DOI] [PubMed] [Google Scholar]
- 61.Jain V, Mitra JK, Rath GP, Prabhakar H, Bithal PK, Dash HH. A randomized, double-blinded comparison of ondansetron, granisetron, and placebo for prevention of postoperative nausea and vomiting after supratentorial craniotomy. J Neurosurg Anesthesiol. 2009;21:226–30. doi: 10.1097/ANA.0b013e3181a7beaa. [DOI] [PubMed] [Google Scholar]
- 62.Jokela R, Koivuranta M, Kangas-Saarela T, Purhonen S, Alahuhta S. Oral ondansetron, tropisetron or metoclopramide to prevent postoperative nausea and vomiting: a comparison in high-risk patients undergoing thyroid or parathyroid surgery. Acta Anaesthesiol Scand. 2002;46:519–24. doi: 10.1034/j.1399-6576.2002.460508.x. [DOI] [PubMed] [Google Scholar]
- 63.Bacic A, Rumboldt Z, Gluncic I, Buklijas J. The impact of the menstrual cycle and ondansetron on postoperative nausea and vomiting. Int J Clin Pharmacol Res. 1998;18:153–8. [PubMed] [Google Scholar]
- 64.Iatrou CA, Dragoumanis CK, Vogiatzaki TD, Vretzakis GI, Simopoulos CE, Dimitriou VK. Prophylactic intravenous ondansetron and dolasetron in intrathecal morphine-induced pruritus: a randomized, double-blinded, placebo-controlled study. Anesth Analg. 2005;101:1516–20. doi: 10.1213/01.ANE.0000181338.35454.6A. [DOI] [PubMed] [Google Scholar]
- 65.Ruiz de Adana J, Tobalina Bonis R, Garcia Galan F, Hernandez Matias A, Fernandez Luengas D, Ortega Deballon P, et al. Antiemetic efficacy of ondansetron in laparoscopic cholecystectomy. A randomized, double-blind, placebo-controlled study. Rev Esp Enferm Dig. 1999;91:639–43. [PubMed]
- 66.Manani G, Cantele P, Dona B, Meroni M, Tiberio I, Ceschin C, et al. Oculo-emetic and oculo-cardiac reflex in the prevention of postoperative nausea and vomitus by diehydrobenzperidol and ondansetron after squint surgery. Acta Anaesthesiol Italica. 1996;47:201–9. [Google Scholar]
- 67.Tang DH, Malone DC. A network meta-analysis on the efficacy of serotonin type 3 receptor antagonists used in adults during the first 24 h for postoperative nausea and vomiting prophylaxis. Clin Ther. 2012;34:282–94. doi: 10.1016/j.clinthera.2012.01.007. [DOI] [PubMed] [Google Scholar]
- 68.Rawlinson A, Kitchingham N, Hart C, McMahon G, Ong SL, Khanna A. Mechanisms of reducing postoperative pain, nausea and vomiting: a systematic review of current techniques. Evid Based Med. 2012;17:75–80. doi: 10.1136/ebmed-2011-100265. [DOI] [PubMed] [Google Scholar]
- 69.Tricco A, Veroniki A, Blondal E, Hamid J, Straus S. Incorporating dosages increases the relevance of network meta-analysis for decision-makers: A case example using data from a systematic review of serotonin (5-HT3) receptor antagonists in patients undergoing surgery. Toronto: Canadian Association of Pharmacy Technicians (CAPT) Conference; 2014. [Google Scholar]
- 70.Veroniki AA, Del Giovane C, Blondal E, Thavorn K, Straus SE, Tricco A. How to model different dose-effects in networks of interventions? 2015 Canadian Agency for Drugs and Technologies in Health (CADTH) Symposium. Saskatoon, Saskatchewan; 2015. http://www.slideshare.net/CADTH_Symposium/cadth-2015-d5-veroniki-modeling-dose-effects-in-nma-cadth-14-apr2015-v3. Accessed: Apr 2015.


