Supplemental Digital Content is available in the text.
Keywords: adrenergic beta-antagonists, coronary artery bypass, coronary artery disease, myocardial infarction
Abstract
Background—
Conflicting results from recent observational studies have raised questions concerning the benefit of β-blockers for patients undergoing coronary artery bypass grafting (CABG). Furthermore, the efficacy of long-term β-blocker therapy in CABG patients after hospital discharge is uncertain.
Methods and Results—
The study included 5926 consecutive patients who underwent CABG and were discharged alive. The prevalence and consistency of β-blocker use were determined in patients with and without a history of myocardial infarction (MI). β-Blockers were always used in 1280 patients (50.9%) with and 1642 patients (48.1%) without previous MI after CABG. Compared with always users (n=2922, 49.3%), the risk of all-cause death was significantly higher among inconsistent β-blocker users (hazard ratio [HR], 1.96; 95% confidence interval [CI], 1.50–2.57), and never using β-blockers was associated with increased risk of both all-cause death (HR, 1.42; 95% CI, 1.01–2.00) and the composite of adverse cardiovascular events (HR, 1.29; 95% CI, 1.10–1.50). In the cohort without MI, the HR for all-cause death was 1.70 (95% CI, 1.17–2.48) in inconsistent users and 1.23 (95% CI, 0.76–1.99) in never users. In the MI cohort, mortality was higher for inconsistent users (HR, 2.14; 95% CI, 1.43–3.20) and for never users (HR, 1.59; 95% CI, 1.07–2.63). Consistent results were obtained in equivalent sensitivity analyses.
Conclusions—
In patients with or without previous MI undergoing CABG, the consistent use of β-blockers was associated with a lower risk of long-term mortality and adverse cardiovascular events. Strategies should be developed to understand and improve discharge prescription of β-blockers and long-term patient adherence.
Cumulative data from observational and clinical trials demonstrate that β-blockers reduce overall mortality and decrease subsequent cardiovascular events in patients after acute myocardial infarction (MI).1,2 Clinical guidelines recommend the use of β-blockers in the perioperative period to reduce the risk of atrial fibrillation and have identified preoperative β-blocker use as a quality performance measure for patients undergoing coronary artery bypass grafting (CABG).3–5
Clinical Perspective on p 2201
Conflicting results from recent large observational studies among patients with stable coronary artery disease have raised questions concerning the benefit of β-blockers for patients with coronary artery disease and patients undergoing CABG.6–8 Recent analyses of the Society of Thoracic Surgeons National Adult Cardiac database demonstrated no perioperative mortality advantage in patients receiving β-blockers before CABG, which challenged this traditional practice and its use as a quality metric.9–11 To date, however, very limited evidence has been available on whether long-term therapy with β-blockers is beneficial in CABG patients after hospital discharge.3,12,13 Furthermore, much less attention has been paid to understanding the combined influence of use at discharge and long-term adherence to β-blockers as a secondary preventive medication.
We sought to explore the association between long-term β-blocker use patterns and all-cause mortality, together with adverse cardiovascular outcomes after the isolated CABG.
Methods
Study Population
All consecutive patients treated with isolated CABG between January 2004 and December 2008 at Fuwai Hospital (Beijing, China) were included (Figure I in the online-only Data Supplement). Patients were excluded if they were <18 years of age, had physician-documented contraindications to β-blocker therapy,9 or underwent CABG combined with valvular or other cardiac surgery. Considering the potential influence of MI history before CABG, this study assessed the use of β-blocker and clinical outcomes in 2 separate cohorts: patients without previous MI and patients with known previous MI. A history of MI was defined as last documented previous MI at any time (within or beyond 21 days) before this CABG surgery.9
All procedures were performed with standard bypass techniques (Methods in the online-only Data Supplement).14 Whenever possible, the internal thoracic artery was preferentially used for revascularization of the left anterior descending artery. Complete revascularization was performed when possible with arterial conduits or saphenous vein grafts.
Data Source and Medication Use
The institutional review board at Fuwai Hospital approved the use of clinical data for this study. The CABG registry database and the efforts made to ensure the accuracy and completeness of these data have been described previously.15,16 All data relating to hospital admissions, procedures, and outcomes were collected according to definitions of the Society of Thoracic Surgeons National Adult Cardiac Database (http://www.sts.org/).
Data on discharge medication were collected by review of the hospital discharge summaries and were considered to be the first expected record of β-blocker administration. We determined adherence for a uniform period of 1 year after CABG surgery for all patients to ensure equal and accurate long-term adherence behavior profiles. Patients were encouraged to return for a routine outpatient visit at 3 months, 6 months, and 1 year after hospital discharge. At each visit, patients were asked to collect and read all of their current medications to the interviewer, including drug name, dose, and schedule.17 We categorized each cohort (patients with and without previous MI) into 3 comparison groups based on the patients’ patterns of β-blocker use at hospital discharge and during the first year after CABG: (1) always users, patients discharged with β-blockers and reporting use at each interval; (2) never users, patients discharged without β-blockers and never reporting use during the interval; and (3) inconsistent users, patients who met criteria for neither of the previous 2 patterns. For purposes of defining use patterns, patients had to have information on discharge medication and at least 2 consecutive records during the first year of the study period.
Follow-Up Process and Outcomes
The clinical outcomes were ascertained after the 1-year observational interval that was used for determining β-blocker adherence. As part of the standard institutional procedures, research nurses followed up patients who did not have a visit by telephone or mail contacts. Coronary angiography was recommended only if ischemic symptoms or signs were present during follow-up. In the event that patients reported any adverse events after hospital discharge, their medical records from outpatient clinics were reviewed for further confirmation. All adverse events of interest were carefully verified and adjudicated by independent clinicians.
The primary outcome was all-cause death. The secondary outcome was a composite of major adverse cardiac and cerebrovascular events (MACCEs), including all-cause death, nonfatal MI, nonfatal stroke, or repeat revascularization. Definitions of all outcome components are given in the Methods section in the online-only Data Supplement.18
Statistical Analysis
Continuous and categorical variables were reported as mean±SD and percentages, respectively. We used the Kaplan–Meier method to create survival curves and the log-rank test to examine differences in survival. Differences in risk-adjusted, long-term rates of study outcomes among patients with different patterns of β-blockers use were assessed by use of multivariable Cox proportional hazards regression with adjustment for all patient-level variables in Table I in the online-only Data Supplement. We tested for differences in the association of β-blockers with outcomes in patients with and without previous MI, and we calculated the hazard ratios (HRs) associated with pattern of β-blocker use.
Treatment-related differences in long-term outcomes among β-blocker users were also analyzed in high-risk clinical subsets (patients >65 years of age and those with congestive heart failure, left ventricular ejection fraction <50%, chronic obstructive pulmonary disease, and unstable angina). The differential association of β-blocker use across all subgroups was tested by use of a test for interaction.
To further assess the robustness of our findings, additional sensitivity analyses were conducted. First, to reduce the impact of treatment selection bias and potential confounding in an observational study, we also performed rigorous adjustment for baseline differences by use of propensity score matching.19 To estimate the propensity score, a logistic regression model predicting the use of β-blockers was developed with the use of the same covariates listed above for the regression-based analyses. For each comparison (inconsistent users versus always users, never users versus always users), a separate propensity score for β-blocker use was derived. Model discrimination was assessed with C statistics, and model calibration was assessed with Hosmer-Lemeshow statistics. In the propensity score–matched cohort, the risks of each outcome were compared by use of Cox regression models. Second, to ensure that our findings were applicable to longer-term adherence (beyond 1 year), we changed the observation interval from hospital discharge to the end of follow-up with β-blocker use as a time-dependent covariate that incorporates changes in β-blocker use over time.6 Third, to help further disentangle biological drug effects from contraindication effects, we evaluated the impact of β-blocker use in selected patients without any potential contraindications to β-blocker therapy (Methods in the online-only Data Supplement).2,20–22
All reported P values are 2 sided, and values of P<0.05 were considered to indicate statistical significance. All statistical analyses were performed with SAS software version 9.2 (SAS Institute).
Results
Patient Characteristics
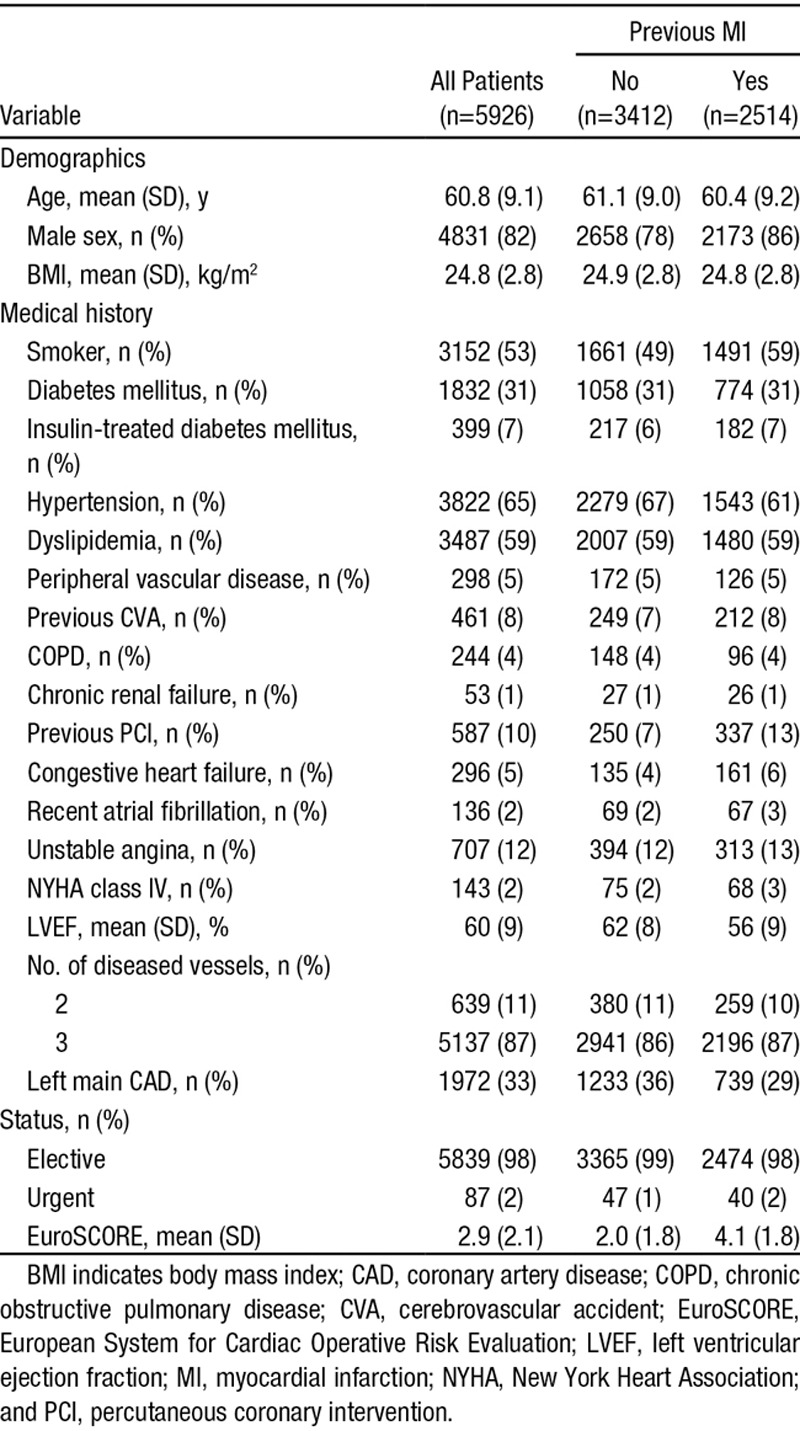
Between January 2004 and December 2008, a total of 7390 patients were hospitalized for undergoing CABG during the study period, whereas 1309 met the criteria for exclusion and 155 died during the interval or were lost to follow-up (Figure I in the online-only Data Supplement). Of this total, 5926 patients were included in the present analyses, and 2514 (42.4%) had a documented history of MI before surgery. Patients with previous MI had a higher-risk clinical profile than those without previous MI (Table 1).
Table 1.
Baseline Demographic and Clinical Characteristics in the Overall Cohort
The baseline and procedural characteristics of study patients according to pattern of β-blockers use are illustrated in Table II in the online-only Data Supplement. Consistent β-blocker use after hospital discharge was noted in 2922 patients (49.3%), whereas 1323 patients (22.3%) never used β-blockers. Compared with always users, patients who never used β-blockers were less likely to demonstrate preoperative comorbidities, including diabetes mellitus (29% versus 33%; P=0.01), dyslipidemia (42% versus 66%; P<0.001), and previous percutaneous coronary intervention (8% versus 11%; P=0.01), congestive heart failure (2% versus 6%; P<0.001), and unstable angina (8% versus 15%; P<0.001). Trends in the use of specific β-blockers in different patient groups and over time are shown in Figures II and III in the online-only Data Supplement.
Follow-Up and Outcomes
The median follow-up was 3.0 years (interquartile range, 1.6–5.5 years) with a completion rate of 98.2% in the overall cohort. Concomitant medication use for secondary prevention at hospital discharge and at 1 year after CABG is listed in Table III in the online-only Data Supplement. During overall follow-up, 315 patients (5.3%) died, of whom 170 (54.0%) died of a cardiac cause. The rates of all-cause death and MACCEs were not significantly different between the non-MI cohort and the MI cohort (for all-cause death: 5.6% versus 6.7%; HR, 0.89; 95% confidence interval [CI], 0.65–1.21; P=0.45; and for MACCEs: 19.7% versus 20.5%; HR, 0.94; 95% CI, 0.80–1.12; P=0.49; Table IV in the online-only Data Supplement).
The observed long-term rates of death and the composite of MACCE were significantly higher in the inconsistent users and never users than in the always users. (Figure 1 and Table V in the online-only Data Supplement). Compared with always users, the observed frequencies of MI and stroke were similar for both the inconsistent users and never users (log-rank P=0.54 and 0.77 for MI; log-rank P=0.21 and 0.08 for stroke; Figure IV in the online-only Data Supplement). After adjustment for baseline differences with multivariable regression analysis, the risks of all-cause death and cardiac death remained consistently higher in the inconsistent users (Table 2). Never using β-blockers was associated with a higher risk of death and MACCEs, with HRs of 1.42 (95% CI, 1.01–2.00) and 1.29 (95% CI, 1.10–1.50), respectively.
Figure 1.
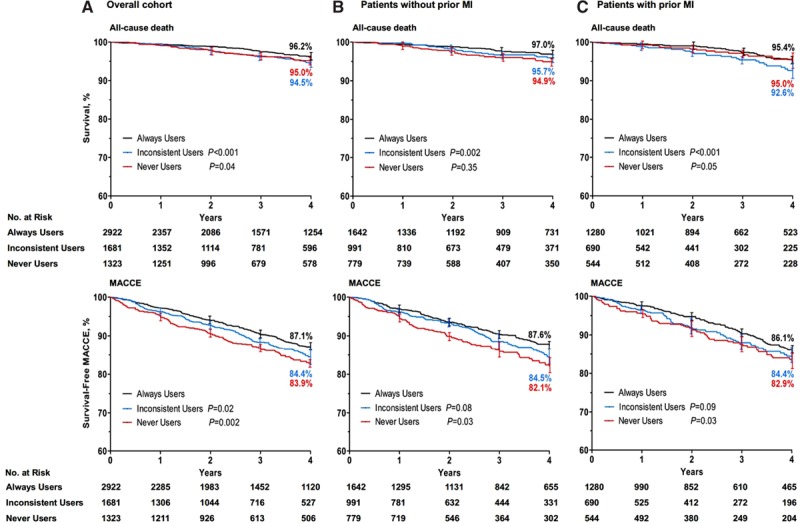
Kaplan–Meier curves of outcomes associated with β-blocker use after coronary artery bypass graft surgery. Shown are rates of all-cause death and major adverse cardiac and cerebrovascular events (MACCEs) in the overall population (A), patients without pervious myocardial infarction (MI; B), and patients with pervious MI (C). The P values were calculated with the log-rank test on the basis of all available follow-up data with always users as reference.
Table 2.
Long-Term Outcomes According to Use Pattern Classification in the Overall Population and Patients With and Without Previous MI
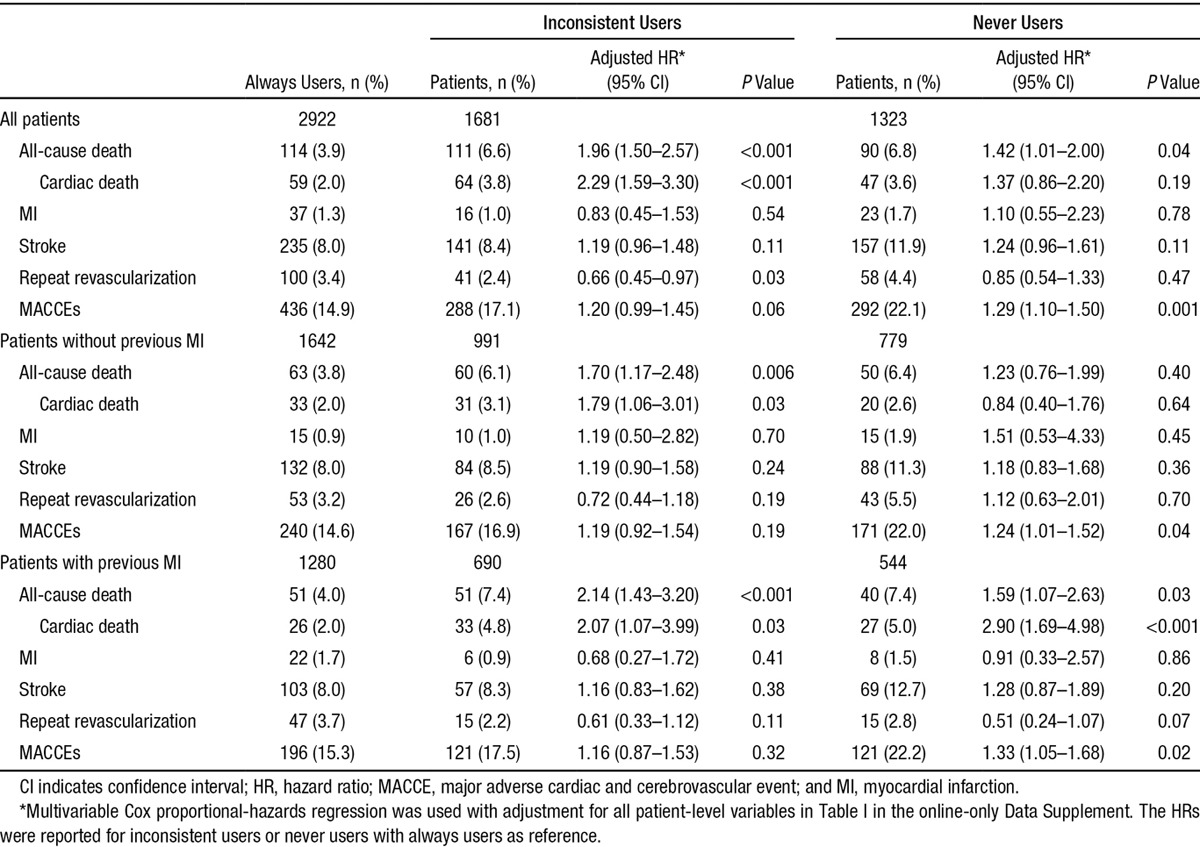
In the cohort without previous MI, the adjusted HRs for death (1.70; 95% CI, 1.17–2.48) and cardiac death (1.79; 95% CI, 1.06–3.01) were significantly higher among inconsistent users. Patients with previous MI had higher HRs for all-cause mortality associated with inconsistent use of β-blockers (2.14; 95% CI, 1.43–3.20) but not for MACCEs (1.16; 95% CI, 0.87–1.53). In the previous MI cohort, compared with always using β-blockers, never using β-blockers was associated with a higher risk of death (HR, 1.59; 95% CI, 1.07–2.63) and MACCEs (HR, 1.33; 95% CI, 1.05–1.68). The HR associated with inconsistent use of β-blockers for all-cause mortality was higher for patients with MI (2.14; 95% CI, 1.43–3.20) than for patients without MI (1.70; 1.17–2.48; P=0.005 for interaction). Although the association of never using β-blockers with all-cause mortality was similar for patients with or without previous MI (P=0.95 for interaction), a greater association with respect to cardiac death was observed (P=0.001 for interaction).
Prespecified Subgroup Analysis
We also assessed the relative treatment effects in subsets of patients with major high-risk clinical factors. The greater benefit of always using β-blockers was consistent across all prespecified subgroups (Figure 2 and Table VI in the online-only Data Supplement). A significantly different effect of inconsistent use of β-blockers for mortality was found between patients with and those without abnormal left ventricular ejection fraction (<50%), with HRs of 1.95 (95% CI, 0.96–3.97) and 2.06 (95% CI, 1.53–2.78), respectively (P<0.001 for interaction). The association of never use of β-blockers and increased risk of MACCEs was greater among patients ≥65 years of age (HR, 1.58; 95% CI, 1.25–2.00) than among patients <65 years of age (HR, 1.15; 95% CI, 0.88–1.51; P<0.001 for interaction).
Figure 2.
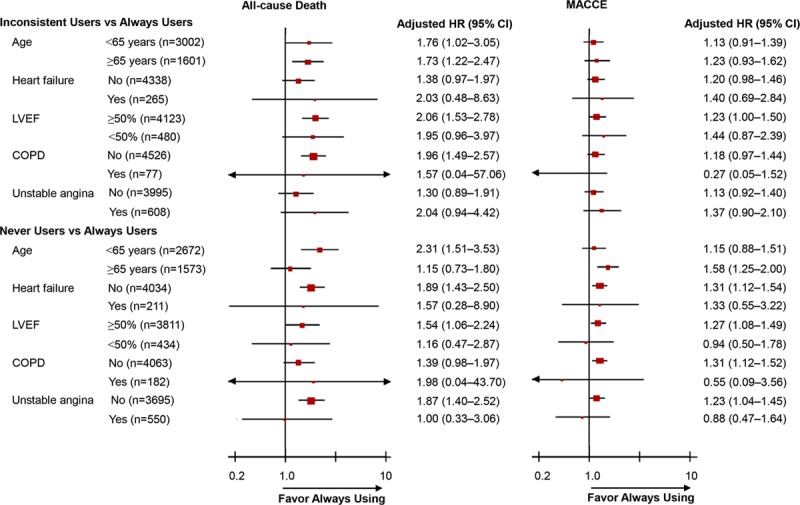
Hazard ratios (HRs) associated with β-blockers in prespecified subgroups of patients. Subgroup analyses were performed with the use of Cox proportional hazards regression with the always user group as reference and with adjustment for all patient-level variables in Table I in the online-only Data Supplement. The HRs were reported for inconsistent users or never users with always users as reference. CI indicates confidence interval; COPD, chronic obstructive pulmonary disease; LVEF, left ventricular ejection fraction; and MACCE, major adverse cardiac and cerebrovascular events.
Sensitivity Analyses
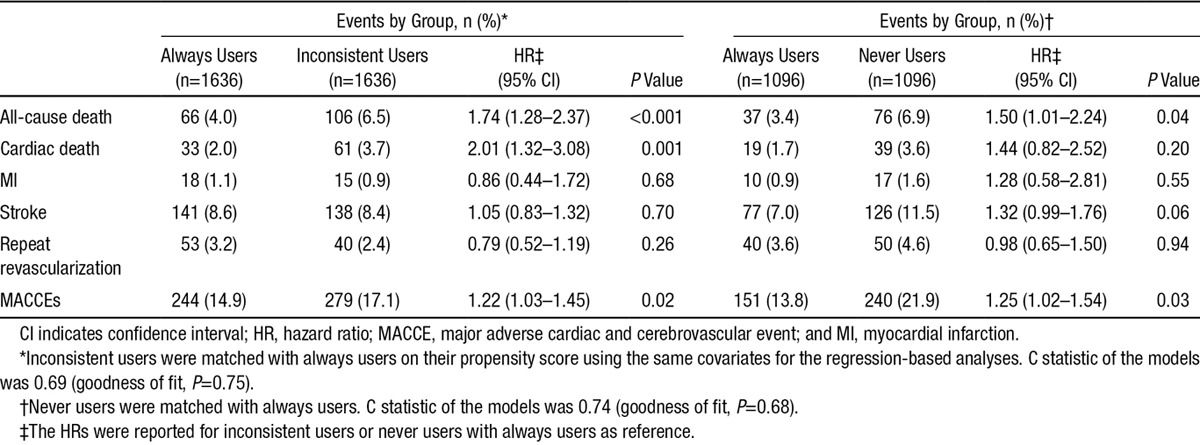
There was no significant difference among propensity-matched groups with regard to baseline characteristics (Table VII in the online-only Data Supplement). Clinical outcomes for the matched cohorts (1636 matched pairs of inconsistent users and always user control subjects, and 1096 matched pairs of never users and always user control subjects) are summarized in Table 3. All-cause death and MACCEs were significantly more common in the inconsistent users (6.5% for death and 17.1% for MACCEs) compared with the always users (4.0% for death and 14.9% for MACCEs; Figure V in the online-only Data Supplement). Moreover, patients never using β-blockers had a higher rate of all-cause death compared with always users (6.9% versus 3.4%), with an HR of 1.50 (95% CI, 1.01–2.24). Trends in other outcomes similar to the main analyses were observed, with a tendency for increased risks associated with never use of β-blockers. Similar results were seen for the primary and the secondary outcomes with β-blocker use as a time-dependent covariate for multivariable regression analysis (Table VIII in the online-only Data Supplement). The sensitivity analysis that was performed in the cohort of patients without contraindications to β-blocker therapy (n=5638), excluding patients with left ventricular ejection fraction <35%, hypotension or cardiogenic shock, second- and third-degree heart block, and chronic obstructive pulmonary disease, yielded largely similar results (Table IX in the online-only Data Supplement).
Table 3.
Association of β-Blocker Use at Discharge and During Follow-Up With Long-Term Outcomes in a Matched Cohort
Discussion
In this long-term observational study of 5926 consecutive patients undergoing isolated CABG, consistent use of β-blockers as a long-term therapy after hospital discharge was associated with a lower risk of all-cause death and composite cardiovascular events among patients with or without previous MI. The positive relationship between adherence to β-blockers and survival after CABG was influenced by the compliance at hospital discharge and long-term patient adherence. The present study supports the National Quality Forum standards of β-blocker use at hospital discharge as a quality indicator.
Preoperative and continued postoperative administration of β-blockers is recommended for all patients without contraindications because β-blockers have been shown to reduce the incidence of postoperative atrial fibrillation and to provide a survival benefit in CABG patients who receive them preoperatively.3,13,23,24 However, results from recent observational studies in ischemic heart disease have aroused widespread controversy about the efficacy of β-blocker use for decreasing mortality and preventing major cardiac complications.6–11 Furthermore, the evidence supporting the use of β-blockers in CABG patients after hospital discharge is less clear.3,12,13
Early randomized trials evaluating treatment with metoprolol for 2 years after CABG did not find significant changes in mortality or exercise capacity.25,26 In the Project of Ex-vivo Vein Graft Engineering via Transfection (PREVENT) IV trial, greater use of indicated secondary preventive medications after CABG was associated with a lower 2-year rate of death or MI, whereas individual β-blocker use was not associated with clinical outcomes.22 However, a beneficial effect of β-blockers has been reported in high-risk subgroups. In a group of elderly patients undergoing coronary revascularization, β-blocker therapy at hospital discharge was effective in reducing 1-year mortality.2 In patients with chronic obstructive pulmonary disease undergoing CABG, β-blocker administration significantly improved survival at the midterm follow-up.27
In the present study, we evaluated the effect of the pattern of β-blocker use in patients treated with CABG. Our analysis showed that either inconsistent use or never use of β-blockers was associated with increased incidence of all-cause death and subsequent cardiovascular events. In a previous study assessing long-term adherence to evidence-based secondary prevention therapies in coronary artery disease,28 consistent use of β-blockers was associated with improved clinical outcomes, as further evidenced by our observation. Furthermore, the relationship between adherence to evidence-based pharmacotherapy and long-term mortality was reported by Rasmussen and colleagues,29 who found that the survival advantages associated with adherence to β-blockers after acute MI were class specific, which might be mediated by drug effects.
The use of β-blockers after MI in the United States increased from 41.8% to 71.6% between 1995 and 2004, in association with a 3% reduction in mortality each year.30 Whereas the effect of β-blockers for reducing mortality among patients with heart failure or a recent MI is well established, this effect in patients with stable ischemic heart disease has been questioned lately.6–8 In the Reduction of Atherothrombosis for Continued Health (REACH) registry, the effect of β-blockers was not associated with history of MI or the presence of coronary artery disease.6 On the basis of these findings, β-blockers are no longer recommended in stable patients after MI or acute coronary syndrome as long-term medical therapy by the latest guidelines from the European Society of Cardiology.31 However, there was a differential association of β-blockers with previous MI for all-cause death or cardiac death in our analysis. Similarly, the association of β-blocker use with cardiovascular events was modified significantly by a recent MI among patients with ischemic heart disease undergoing noncardiac surgery and patients with new-onset coronary heart disease.7,8 Another recently published analysis of the Society of Thoracic Surgeons National Adult Cardiac database assessing the impact of preoperative β-blockers on outcomes in patients undergoing CABG excluded patients with previous MI within 21 days with a consideration that the use of β-blockers is well established after acute MI.9 Besides, our findings showed that patients with a history of MI shared similar risk of cardiac death (HR, 0.88; 95% CI, 0.59–1.31) and new-onset MI (HR, 1.41; 95% CI, 0.91–2.18) after CABG compared with those without previous MI, indicating the protective impact associated with CABG as a highly sophisticated method of restoring blood flow to the myocardium.
The effect of β-blockers on outcomes has been reported to be influenced by both the initial prescription at the time of hospital discharge, known as physician performance,2,32,33 and long-term patient adherence after the surgery.17,29,34 However, little evidence evaluating the combined effect of these 2 individual components is available. In our present study, inconsistent users and never users accounted for 28.4% and 22.3% of the overall patients, respectively. A total of 68.8% of patients received a β-blocker prescription at discharge, which is lower than the proportion (90.8%) from the Get With The Guidelines database,35 supporting the need to improve discharge processes to increase prescription rates of β-blockers after CABG in the real-world setting. We observed that a further 28.6% of patients who were discharged with a β-blocker discontinued taking it. Another study reported a β-blocker discontinuation rate of 19.6% at 1 year after MI,17 whereas 30.4% of patients with coronary artery disease never received β-blockers during a 7-year follow-up, as reported by Newby and colleagues,28 which was associated with increased mortality risk. Future research should examine the factors that predict poor adherence to β-blockers and should develop interventions to improve both the discharge prescription and long-term adherence to proven secondary preventive medications.
Limitations
Our study was based on a single-center experience and therefore may not be representative of the entire Chinese population. However, with systematic and standardized clinical training for the cardiologists and surgeons at Fuwai Hospital, satisfactory in-hospital outcomes could be achieved, which are consistent with those of most outstanding cardiac centers worldwide. Second, the observational nature of our study and the presence of unmeasured confounders that may have contributed to the strength of the association requiring 2 records to determine the use pattern of β-blockers must be considered. Therefore, it is possible that the observed association between β-blocker use and improved clinical outcomes may rather reflect better concomitant medical care or higher socioeconomic or educational status in always users. Third, medication use was collected by patient self-report, whereas self-report measures with confirmed congruence have been used commonly in previous studies.17,22,28 Fourth, some prespecified subgroup comparisons such as patients with chronic obstructive pulmonary disease were based on rather small numbers of individuals; therefore, a type II error cannot be excluded. Finally, the physiological response and reasons for refusal of β-blockers were not available in our present analysis.
Conclusions
In patients undergoing CABG, the consistent use of β-blockers was associated with a lower risk of long-term mortality and composite cardiac and cerebrovascular events. The survival advantages associated with adherence to β-blockers after CABG were class specific but were not significantly affected by the presence of a history of MI. Considerable attention must also be focused on understanding and improving β-blocker use at the time of hospital discharge and long-term patient adherence.
Acknowledgments
We thank follow-up staff, including Yuyan Zhou, Lingyi Xu, and Ying Zhang. We are indebted to Ning Su, MD, at Tianjin Medical University; Guang Hao, PhD, at State Key Laboratory of Cardiovascular Disease; and Jing Li, PhD, at National Clinical Research Center of Cardiovascular Diseases for assistance in the preparation and revision of the manuscript.
Source of Funding
This study was funded by the Key Project in the National Science and Technology Pillar Program during the Twelfth 5-Year Plan Period of China (2011BAI11B02, 2011BAI11B21).
Disclosures
None.
Supplementary Material
Footnotes
The online-only Data Supplement is available with this article at http://circ.ahajournals.org/lookup/suppl/doi:10.1161/CIRCULATIONAHA.114.014209/-/DC1.
CLINICAL PERSPECTIVE
This large, population-based cohort study assessed the effect of long-term β-blocker therapy for secondary prevention of long-term outcomes after isolated coronary artery bypass grafting (CABG). The prevalence and consistency of β-blocker use were determined in patients with and without a history of myocardial infarction. Each cohort (patients with and without previous myocardial infarction) was categorized into 3 comparison groups based on patients’ patterns of β-blocker use at hospital discharge and during the first year after CABG: always users, inconsistent users, and never users. We found that 68.8% of patients received a β-blocker prescription at discharge, and a further 28.6% of patients who were discharged with a β-blocker discontinued taking it. During the first year after CABG, consistent β-blocker use was noted in 2922 patients (49.3%), whereas 1323 patients (22.3%) never used β-blockers. At a median follow-up of 3.0 years, the consistent use of β-blockers was associated with a lower risk of all-cause mortality and adverse cardiovascular events in the overall population and in patients with and without previous myocardial infarction. Moreover, the results demonstrated that the positive relationship between adherence to β-blockers and survival after CABG was influenced by compliance at hospital discharge and long-term patient adherence. Recognizing the beneficial effect associated with β-blocker use after CABG provides support for existing guidelines and the National Quality Forum standards of β-blocker use at hospital discharge as a performance measure and a quality indicator. Furthermore, strategies should be developed to understand and improve discharge prescription of β-blockers and long-term patient adherence after CABG.
References
- 1.CAPRICORN Investigators. Effect of carvedilol on outcome after myocardial infarction in patients with left-ventricular dysfunction: the CAPRICORN randomised trial. Lancet. 2001;357:1385–1390. doi: 10.1016/s0140-6736(00)04560-8. [DOI] [PubMed] [Google Scholar]
- 2.Chen J, Radford MJ, Wang Y, Marciniak TA, Krumholz HM. Are beta-blockers effective in elderly patients who undergo coronary revascularization after acute myocardial infarction? Arch Intern Med. 2000;160:947–952. doi: 10.1001/archinte.160.7.947. [DOI] [PubMed] [Google Scholar]
- 3.Hillis LD, Smith PK, Anderson JL, Bittl JA, Bridges CR, Byrne JG, Cigarroa JE, Disesa VJ, Hiratzka LF, Hutter AM, Jr, Jessen ME, Keeley EC, Lahey SJ, Lange RA, London MJ, Mack MJ, Patel MR, Puskas JD, Sabik JF, Selnes O, Shahian DM, Trost JC, Winniford MD American College of Cardiology Foundation; American Heart Association Task Force on Practice Guidelines; American Association for Thoracic Surgery; Society of Cardiovascular Anesthesiologists; Society of Thoracic Surgeons. 2011 ACCF/AHA guideline for coronary artery bypass graft surgery: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines: developed in collaboration with the American Association for Thoracic Surgery, Society of Cardiovascular Anesthesiologists, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2011;58:e123–e210. doi: 10.1016/j.jacc.2011.08.009. doi: 10.1016/j.jacc.2011.08.009. [DOI] [PubMed] [Google Scholar]
- 4.Guru V, Anderson GM, Fremes SE, O’Connor GT, Grover FL, Tu JV Canadian CABG Surgery Quality Indicator Consensus Panel. The identification and development of Canadian coronary artery bypass graft surgery quality indicators. J Thorac Cardiovasc Surg. 2005;130:1257–1264. doi: 10.1016/j.jtcvs.2005.07.041. [DOI] [PubMed] [Google Scholar]
- 5.Shahian DM, Edwards FH, Ferraris VA, Haan CK, Rich JB, Normand SL, DeLong ER, O’Brien SM, Shewan CM, Dokholyan RS, Peterson ED Society of Thoracic Surgeons Quality Measurement Task Force. Quality measurement in adult cardiac surgery, part 1: conceptual framework and measure selection. Ann Thorac Surg. 2007;83(suppl):S3–S12. doi: 10.1016/j.athoracsur.2007.01.053. doi: 10.1016/j.athoracsur.2007.01.053. [DOI] [PubMed] [Google Scholar]
- 6.Bangalore S, Steg G, Deedwania P, Crowley K, Eagle KA, Goto S, Ohman EM, Cannon CP, Smith SC, Zeymer U, Hoffman EB, Messerli FH, Bhatt DL REACH Registry Investigators. β-Blocker use and clinical outcomes in stable outpatients with and without coronary artery disease. JAMA. 2012;308:1340–1349. doi: 10.1001/jama.2012.12559. doi: 10.1001/jama.2012.12559. [DOI] [PubMed] [Google Scholar]
- 7.Andersson C, Mérie C, Jørgensen M, Gislason GH, Torp-Pedersen C, Overgaard C, Køber L, Jensen PF, Hlatky MA. Association of β-blocker therapy with risks of adverse cardiovascular events and deaths in patients with ischemic heart disease undergoing noncardiac surgery: a Danish nationwide cohort study. JAMA Intern Med. 2014;174:336–344. doi: 10.1001/jamainternmed.2013.11349. doi: 10.1001/jamainternmed.2013.11349. [DOI] [PubMed] [Google Scholar]
- 8.Andersson C, Shilane D, Go AS, Chang TI, Kazi D, Solomon MD, Boothroyd DB, Hlatky MA. β-blocker therapy and cardiac events among patients with newly diagnosed coronary heart disease. J Am Coll Cardiol. 2014;64:247–252. doi: 10.1016/j.jacc.2014.04.042. doi: 10.1016/j.jacc.2014.04.042. [DOI] [PubMed] [Google Scholar]
- 9.Brinkman W, Herbert MA, O’Brien S, Filardo G, Prince S, Dewey T, Magee M, Ryan W, Mack M. Preoperative β-blocker use in coronary artery bypass grafting surgery: national database analysis. JAMA Intern Med. 2014;174:1320–1327. doi: 10.1001/jamainternmed.2014.2356. doi: 10.1001/jamainternmed.2014.2356. [DOI] [PubMed] [Google Scholar]
- 10.Brinkman WT, Herbert MA, Prince SL, Magee MJ, Dewey TM, Smith RL, Edgerton JR, Head SJ, Ryan WH, Mack MJ. Preoperative beta-blocker usage: is it really worthy of being a quality indicator? Ann Thorac Surg. 2011;92:788–795. doi: 10.1016/j.athoracsur.2011.03.088. doi: 10.1016/j.athoracsur.2011.03.088. [DOI] [PubMed] [Google Scholar]
- 11.LaPar DJ, Crosby IK, Kron IL, Kern JA, Fonner E, Jr, Rich JB, Speir AM, Ailawadi G. Preoperative beta-blocker use should not be a quality metric for coronary artery bypass grafting. Ann Thorac Surg. 2013;96:1539–1544. doi: 10.1016/j.athoracsur.2013.05.059. [DOI] [PubMed] [Google Scholar]
- 12.Smith SC, Jr, Benjamin EJ, Bonow RO, Braun LT, Creager MA, Franklin BA, Gibbons RJ, Grundy SM, Hiratzka LF, Jones DW, Lloyd-Jones DM, Minissian M, Mosca L, Peterson ED, Sacco RL, Spertus J, Stein JH, Taubert KA. AHA/ACCF secondary prevention and risk reduction therapy for patients with coronary and other atherosclerotic vascular disease: 2011 update: a guideline from the American Heart Association and American College of Cardiology Foundation. J Am Coll Cardiol. 2011;58:2432–2446. doi: 10.1016/j.jacc.2011.10.824. [DOI] [PubMed] [Google Scholar]
- 13.Kulik A, Ruel M, Jneid H, Ferguson TB, Hiratzka LF, Ikonomidis JS, Lopez-Jimenez F, McNallan SM, Patel M, Roger VL, Sellke FW, Sica DA, Zimmerman L American Heart Association Council on Cardiovascular Surgery and Anesthesia. Secondary prevention after coronary artery bypass graft surgery: a scientific statement from the American Heart Association. Circulation. 2015;131:927–964. doi: 10.1161/CIR.0000000000000182. doi: 10.1161/CIR.0000000000000182. [DOI] [PubMed] [Google Scholar]
- 14.Eagle KA, Guyton RA, Davidoff R, Edwards FH, Ewy GA, Gardner TJ, Hart JC, Herrmann HC, Hillis LD, Hutter AM, Jr, Lytle BW, Marlow RA, Nugent WC, Orszulak TA, Antman EM, Smith SC, Jr, Alpert JS, Anderson JL, Faxon DP, Fuster V, Gibbons RJ, Gregoratos G, Halperin JL, Hiratzka LF, Hunt SA, Jacobs AK, Ornato JP American College of Cardiology/American Heart Association Task Force on Practice Guidelines Committee to Update the 1999 Guidelines for Coronary Artery Bypass Graft Surgery; American Society for Thoracic Surgery; Society of Thoracic Surgeons. ACC/AHA 2004 guideline update for coronary artery bypass graft surgery: summary article: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Update the 1999 Guidelines for Coronary Artery Bypass Graft Surgery). J Am Coll Cardiol. 2004;44:e213–e310. doi: 10.1016/j.jacc.2004.07.021. doi: 10.1016/j.jacc.2004.07.021. [DOI] [PubMed] [Google Scholar]
- 15.Li Y, Zheng Z, Hu S Chinese Coronary Artery Bypass Grafting Registry Study. The Chinese Coronary Artery Bypass Grafting Registry Study: analysis of the national multicentre database of 9248 patients. Heart. 2009;95:1140–1144. doi: 10.1136/hrt.2008.146563. doi: 10.1136/hrt.2008.146563. [DOI] [PubMed] [Google Scholar]
- 16.Zhang H, Yuan X, Osnabrugge RL, Meng D, Gao H, Zhang S, Rao C, Hu S, Zheng Z. Influence of diabetes mellitus on long-term clinical and economic outcomes after coronary artery bypass grafting. Ann Thorac Surg. 2014;97:2073–2079. doi: 10.1016/j.athoracsur.2014.02.047. doi: 10.1016/j.athoracsur.2014.02.047. [DOI] [PubMed] [Google Scholar]
- 17.Ho PM, Spertus JA, Masoudi FA, Reid KJ, Peterson ED, Magid DJ, Krumholz HM, Rumsfeld JS. Impact of medication therapy discontinuation on mortality after myocardial infarction. Arch Intern Med. 2006;166:1842–1847. doi: 10.1001/archinte.166.17.1842. doi: 10.1001/archinte.166.17.1842. [DOI] [PubMed] [Google Scholar]
- 18.Cannon CP, Brindis RG, Chaitman BR, Cohen DJ, Cross JT, Jr, Drozda JP, Jr, Fesmire FM, Fintel DJ, Fonarow GC, Fox KA, Gray DT, Harrington RA, Hicks KA, Hollander JE, Krumholz H, Labarthe DR, Long JB, Mascette AM, Meyer C, Peterson ED, Radford MJ, Roe MT, Richmann JB, Selker HP, Shahian DM, Shaw RE, Sprenger S, Swor R, Underberg JA, Van de Werf F, Weiner BH, Weintraub WS American College of Cardiology Foundation/American Heart Association Task Force on Clinical Data Standards; American College of Emergency Physicians; Emergency Nurses Association; National Association of Emergency Medical Technicians; National Association of EMS Physicians; Preventive Cardiovascular Nurses Association; Society for Cardiovascular Angiography and Interventions; Society of Cardiovascular Patient Care; Society of Thoracic Surgeons. 2013 ACCF/AHA key data elements and definitions for measuring the clinical management and outcomes of patients with acute coronary syndromes and coronary artery disease: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Clinical Data Standards (Writing Committee to Develop Acute Coronary Syndromes and Coronary Artery Disease Clinical Data Standards). Circulation. 2013;127:1052–1089. doi: 10.1161/CIR.0b013e3182831a11. doi: 10.1161/CIR.0b013e3182831a11. [DOI] [PubMed] [Google Scholar]
- 19.Li F, Zaslavsky AM, Landrum MB. Propensity score weighting with multilevel data. Stat Med. 2013;32:3373–3387. doi: 10.1002/sim.5786. doi: 10.1002/sim.5786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ferguson TB, Jr, Coombs LP, Peterson ED Society of Thoracic Surgeons National Adult Cardiac Surgery Database. Preoperative beta-blocker use and mortality and morbidity following CABG surgery in North America. JAMA. 2002;287:2221–2227. doi: 10.1001/jama.287.17.2221. [DOI] [PubMed] [Google Scholar]
- 21.Foody JM, Ferdinand FD, Galusha D, Rathore SS, Masoudi FA, Havranek EP, Nilasena D, Radford MJ, Krumholz HM. Patterns of secondary prevention in older patients undergoing coronary artery bypass grafting during hospitalization for acute myocardial infarction. Circulation. 2003;108(suppl 1):II24–II28. doi: 10.1161/01.cir.0000087654.26917.00. doi: 10.1161/01.cir.0000087654.26917.00. [DOI] [PubMed] [Google Scholar]
- 22.Goyal A, Alexander JH, Hafley GE, Graham SH, Mehta RH, Mack MJ, Wolf RK, Cohn LH, Kouchoukos NT, Harrington RA, Gennevois D, Gibson CM, Califf RM, Ferguson TB, Jr, Peterson ED PREVENT IV Investigators. Outcomes associated with the use of secondary prevention medications after coronary artery bypass graft surgery. Ann Thorac Surg. 2007;83:993–1001. doi: 10.1016/j.athoracsur.2006.10.046. doi: 10.1016/j.athoracsur.2006.10.046. [DOI] [PubMed] [Google Scholar]
- 23.Connolly SJ, Cybulsky I, Lamy A, Roberts RS, O’brien B, Carroll S, Crystal E, Thorpe KE, Gent M Beta-Blocker Length Of Stay (BLOS) Study. Double-blind, placebo-controlled, randomized trial of prophylactic metoprolol for reduction of hospital length of stay after heart surgery: the Beta-Blocker Length Of Stay (BLOS) study. Am Heart J. 2003;145:226–232. doi: 10.1067/mhj.2003.147. doi: 10.1067/mhj.2003.147. [DOI] [PubMed] [Google Scholar]
- 24.Halonen J, Hakala T, Auvinen T, Karjalainen J, Turpeinen A, Uusaro A, Halonen P, Hartikainen J, Hippeläinen M. Intravenous administration of metoprolol is more effective than oral administration in the prevention of atrial fibrillation after cardiac surgery. Circulation. 2006;114(suppl):I1–I4. doi: 10.1161/CIRCULATIONAHA.105.000851. doi: 10.1161/CIRCULATIONAHA.105.000851. [DOI] [PubMed] [Google Scholar]
- 25.MACB Study Group. Effect of metoprolol on death and cardiac events during a 2-year period after coronary artery bypass grafting. Eur Heart J. 1995;16:1825–1832. [PubMed] [Google Scholar]
- 26.Sjöland H, Caidahl K, Lurje L, Hjalmarson A, Herlitz J. Metoprolol treatment for two years after coronary bypass grafting: effects on exercise capacity and signs of myocardial ischaemia. Br Heart J. 1995;74:235–241. doi: 10.1136/hrt.74.3.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Angeloni E, Melina G, Roscitano A, Refice S, Capuano F, Lechiancole A, Comito C, Benedetto U, Sinatra R. β-Blockers improve survival of patients with chronic obstructive pulmonary disease after coronary artery bypass grafting. Ann Thorac Surg. 2013;95:525–531. doi: 10.1016/j.athoracsur.2012.07.080. doi: 10.1016/j.athoracsur.2012.07.080. [DOI] [PubMed] [Google Scholar]
- 28.Newby LK, LaPointe NM, Chen AY, Kramer JM, Hammill BG, DeLong ER, Muhlbaier LH, Califf RM. Long-term adherence to evidence-based secondary prevention therapies in coronary artery disease. Circulation. 2006;113:203–212. doi: 10.1161/CIRCULATIONAHA.105.505636. doi: 10.1161/CIRCULATIONAHA.105.505636. [DOI] [PubMed] [Google Scholar]
- 29.Rasmussen JN, Chong A, Alter DA. Relationship between adherence to evidence-based pharmacotherapy and long-term mortality after acute myocardial infarction. JAMA. 2007;297:177–186. doi: 10.1001/jama.297.2.177. doi: 10.1001/jama.297.2.177. [DOI] [PubMed] [Google Scholar]
- 30.Setoguchi S, Glynn RJ, Avorn J, Mittleman MA, Levin R, Winkelmayer WC. Improvements in long-term mortality after myocardial infarction and increased use of cardiovascular drugs after discharge: a 10-year trend analysis. J Am Coll Cardiol. 2008;51:1247–1254. doi: 10.1016/j.jacc.2007.10.063. doi: 10.1016/j.jacc.2007.10.063. [DOI] [PubMed] [Google Scholar]
- 31.Windecker S, Kolh P, Alfonso F, Collet JP, Cremer J, Falk V, Filippatos G, Hamm C, Head SJ, Juni P, Kappetein AP, Kastrati A, Knuuti J, Landmesser U, Laufer G, Neumann FJ, Richter DJ, Schauerte P, Sousa Uva M, Stefanini GG, Taggart DP, Torracca L, Valgimigli M, Wijns W, Witkowski A. 2014 ESC/EACTS guidelines on myocardial revascularization: the Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J. 2014. 2014;35:2541–2619. doi: 10.1093/eurheartj/ehu278. [DOI] [PubMed] [Google Scholar]
- 32.Chan AY, McAlister FA, Norris CM, Johnstone D, Bakal JA, Ross DB Alberta Provincial Program for Outcome Assessment in Coronary Heart Disease (APPROACH) Investigators. Effect of beta-blocker use on outcomes after discharge in patients who underwent cardiac surgery. J Thorac Cardiovasc Surg. 2010;140:182–187. doi: 10.1016/j.jtcvs.2010.03.015. [DOI] [PubMed] [Google Scholar]
- 33.Hlatky MA, Solomon MD, Shilane D, Leong TK, Brindis R, Go AS. Use of medications for secondary prevention after coronary bypass surgery compared with percutaneous coronary intervention. J Am Coll Cardiol. 2013;61:295–301. doi: 10.1016/j.jacc.2012.10.018. doi: 10.1016/j.jacc.2012.10.018. [DOI] [PubMed] [Google Scholar]
- 34.Jackevicius CA, Li P, Tu JV. Prevalence, predictors, and outcomes of primary nonadherence after acute myocardial infarction. Circulation. 2008;117:1028–1036. doi: 10.1161/CIRCULATIONAHA.107.706820. doi: 10.1161/CIRCULATIONAHA.107.706820. [DOI] [PubMed] [Google Scholar]
- 35.Hiratzka LF, Eagle KA, Liang L, Fonarow GC, LaBresh KA, Peterson ED Get With the Guidelines Steering Committee. Atherosclerosis secondary prevention performance measures after coronary bypass graft surgery compared with percutaneous catheter intervention and nonintervention patients in the Get With the Guidelines database. Circulation. 2007;116(suppl):I207–I212. doi: 10.1161/CIRCULATIONAHA.106.681247. doi: 10.1161/CIRCULATIONAHA.106.681247. [DOI] [PubMed] [Google Scholar]


