Abstract
Introduction
Multiple techniques are used for femoral tunnel drilling in ACL reconstruction, including the Mini-Two Incision method (MT) and Anteromedial Portal technique (AM). Both techniques allow for independent placement of the femoral tunnel, though there are no reports comparing kinematics and cartilage health after these reconstructions. We hypothesized that both techniques would result in the restoration of normal knee kinematics and show no evidence of early cartilage degeneration.
Methods
A total of 20 patients were evaluated one year after ACL reconstruction, including 10 patients after MT and 10 patients after AM. MR-imaging was acquired bilaterally with the knee loaded in extension and flexion to evaluate kinematics of the reconstructed knee compared to the normal knee. Quantitative cartilage imaging was obtained and compared to 10 matched control subjects. The Marx Activity Rating Scale and KOOS survey were administered.
Results
The tibia was positioned significantly more anteriorly in extension and flexion relative to the contralateral knee for the MT group. The tibial position in the AM group was not significantly different from the patient’s contralateral knee. T1ρ values in the central-medial tibia were significantly elevated in the MT group compared to the Control group. KOOS Symptom scores were significantly better for the MT group compared to the AM group.
Conclusions
We have observed in vivo differences in knee kinematics and early cartilage degeneration between patients following MT and AM ACL reconstruction. Both techniques allow for anatomic ACL reconstruction, though the MT group shows significant early differences compared to the patient’s normal knee.
Keywords: ACL reconstruction, femoral tunnel drilling, kinematics, cartilage
1. Introduction
Anterior cruciate ligament (ACL) tears are one of the most common knee ligament injuries that require surgical reconstruction [1]. The development of post-traumatic medial compartment arthritis is well-documented regardless of treatment, with several studies reporting increased rates of degenerative changes after ACL reconstruction [2–6]. There has been an increased emphasis on utilizing new methods to position the femoral tunnel in order to improve the current trend of achieving less than satisfactory mid-term and long-term results after ACL reconstruction.
The Anteromedial Portal Technique (AM) has gained popularity as it allows for all-inside placement of the femoral tunnel, independent of tibial tunnel placement. Potential pitfalls still remain with this method [7]. The tunnel length and orientation are dependent on the flexion angle of the knee at the time of drilling, and the knee must be hyperflexed past 100 degrees of flexion to achieve an acceptable tunnel position and length, which frequently limits optimal visualization of the femoral ACL footprint while drilling [8]. There is also a risk of lateral cortex disruption while placing the femoral tunnel, since the endpoint of reaming is not well-defined [7]. A short femoral tunnel and damage to the articular cartilage are other potential downsides [7,9].
A Mini-Two Incision Technique (MT) has been developed to allow for more reliable placement of the femoral tunnel [7]. The MT utilizes a 1–2 centimeter incision over the lateral femur to limit trauma to the soft tissue and iliotibial band [7]. The femoral tunnel is then drilled from the inside-out with a retrograde drill under arthroscopic visualization, which can lead to a reliable and anatomic position of the femoral tunnel [7]. Ultimate tunnel length is calculated prior to drilling, and a cadaveric study has shown that the tunnel created with this method is on average significantly longer than the tunnel drilled through the anteromedial portal [9]. This procedure is performed with the knee at 90 degrees of flexion, which allows for improved identification of anatomic landmarks.
Advanced imaging methodologies can provide an early assessment of knee function through information on progressive cartilage degradation and abnormal kinematics. In particular, the T1ρ relaxation time, which describes spin-lattice relaxation in a rotating spatial frame, can provide detailed information on the extracellular matrix of cartilage [10]. This parameter reflects the proteoglycan content of cartilage and can detect early changes in the cartilage matrix prior to the development of radiographic abnormalities [11] Kinematic MR imaging reconstructs static images in different positions to understand the complex rotational and translational movements of the knee [12,13]. Accelerated medial compartment degenerative changes are commonly encountered after ACL injury, and quantitative MR imaging provides a focused assessment of abnormalities.
The purpose of this study is to evaluate MR-based in vivo kinematics, early cartilage degeneration, as measured by T1ρ values, and patient reported outcomes between the MT reconstruction and the AM reconstruction. We hypothesized that both the MT and AM reconstruction would restore normal knee kinematics and produce similar patient reported outcomes. The primary outcome was dynamic anterior tibial translation. Additionally, we hypothesized that both surgical techniques would lead to similar cartilage health as compared to healthy control patients.
2. Methods
2.1 Subjects
A total of twenty patients were recruited for participation, with ten patients undergoing ACL reconstruction with the MT method and ten patients with the AM technique, with data collected 12 months after ACL reconstruction. Quantitative cartilage imaging was obtained of both knees for a matched group of ten control subjects who had no history of previous knee injury. Patient demographics for each of the groups are displayed in Table 1. Inclusion criteria were an acute, isolated ACL injury requiring ACL reconstruction, and patient age of 18–50 years. Patients were excluded if there was an associated ligamentous injury requiring surgical treatment, an inability to obtain a follow-up MRI study, meniscal repair, meniscal debridement constituting greater than 20% of the meniscus, a history of inflammatory arthropathy, a history of prior surgery to either knee, or a history of previous ligamentous injury to the contralateral knee. The Committee on Human Research at our institution approved all procedures, and documented informed consent was obtained prior to enrollment.
Table 1.
Demographic Information for MT and AM groups
| Mini-Two Incision |
Anteromedial Portal |
Control Group |
p value | |
|---|---|---|---|---|
| Number of patients | 10 | 10 | 10 | -- |
| Age at Time of Study (years)a | 30.5 ± 9.5 | 33.9 ± 5.1 | 31.2 ± 5.0 | 0.51b |
| Gender | 6 Female | 6 Female | 6 Female | -- |
| Height (m)a | 1.70 ± 0.09 | 1.74 ± 0.09 | 1.66 ± 0.1 | 0.16b |
| Mass (kg)a | 76.8 ± 26.5 | 71.1 ± 7.60 | 67.4 ± 11.7 | 0.49b |
| BMI (kg/m2)a | 26.2 ± 7.4 | 23.6 ± 3.3 | 24.3 ± 2.6 | 0.47b |
| Follow-up to Study (months)a | 14.4 ± 2.0 | 12.6 ± 0.6 | -- | 0.01c |
| Graft choice | 7 Autograft 3 Allograft |
6 Autograft 4 Allograft |
-- | 1.0d |
| Meniscal debridements | 2 (lateral) | 3 (lateral) | -- | 1.0d |
Listed as mean value ± standard deviation
p-value from Analysis of Variance
p-value from two-tailed, unpaired Student’s t-test
p-value from Fisher’s exact test
2.2 Treatment Determination
The reconstruction method and graft choice were determined by a pre-operative conversation between the patient and the operating surgeon. Patients were not randomized to the two treatment groups. The patients were assigned to each treatment group by the surgeon they chose to perform the operation. This study design allows the surgeons to perform their most common reconstructive technique, and while not randomized, achieves the majority of the advantages of expertise-based assignment as described by Devereaux et al [14]. All patients were treated with a soft tissue graft with the same suspensory fixation on the femoral side and interference screw and sheath fixation on the tibial side. For those patients receiving hamstring autograft reconstruction, the graft was harvested first through a standard anteromedial tibial incision. For allograft reconstruction, all patients received a soft tissue allograft, such as a two-strand hamstring or posterior tibialis tendon graft. All patients had the same intra-operative ACL reconstruction except for the femoral tunnel which was drilled with one of the procedures as described below. The tibial tunnel was placed at the center of the tibial ACL footprint. Through the anteromedial tibial incision, an ACL tibial guide was used to drill an appropriately sized tunnel based on graft diameter. All grafts were secured using a variable length suspensory button on the femoral side (Tightrope, Arthrex Inc., Naples, FL, USA) with a non-metallic interference screw/sheath fixation on the tibial side (Intrafix, DePuy Mitek, IN, USA).
2.3 RECONSTRUCTION METHODS
2.3.1 Anteromedial Portal Reconstruction
With the knee positioned in 90 degrees of knee flexion, the femoral footprint was chosen as the border between the anteromedial and posterolateral bundles of the ACL. The knee was then hyperflexed 130–140 degrees, and a guidewire was advanced through the desired location. First, a drill was used with the appropriate graft size reamer to create a footprint. After confirming 1 mm or less back wall, a 4.5mm drill bit was used to drill through the lateral femoral cortex. The tunnel length was measured, with a minimum tunnel length 30 mm. If the tunnel was too short, the trajectory was altered to ensure a minimal femoral tunnel length of 30mm. The remainder of the tunnel was drilled over a guidewire, leaving a 6 mm osseous bridge at the lateral cortex.
2.3.2 Mini-Two Incision Reconstruction
The femoral footprint was visualized arthroscopically while viewing from the anteromedial portal with the knee flexed to 90 degrees. The desired femoral tunnel position was defined as a point midway between the insertion of the anteromedial and posterolateral bundles of the ACL. A femoral aiming device (Arthrex, Naples, FL, USA) was positioned intra-articularly at the desired center of the femoral tunnel with bullet of the guide positioned on the skin overlying the lateral cortex of the femur. A 1–2 cm incision was made over the distal lateral thigh, and dissection was carried down through the ilitotibial band to allow the bullet of the guide to be seated on the lateral femoral cortex. A retrograde drill guide pin (FlipCutter; Arthrex, Naples, FL, USA) was inserted from outside-in. Once the guide pin entry position was verified to be correct, the bullet of the guide was impacted 7 mm into the lateral femoral cortex to ensure a 7 mm cortical bridge. The guide pin was converted to drilling capability, and the femoral tunnel was created in retrograde fashion, leaving a 7 mm osseous bridge at the lateral femoral cortex.
2.4 Rehabilitation
All patients participated in a standard post-operative ACL rehabilitation program. Immediate post-operative recovery emphasized control of pain and swelling, and regaining motor control. The operative knee was kept in a hinged knee brace at all times, which was locked in extension while walking until quadriceps control and normal gait were achieved. The primary focus for the first six weeks was on return of normal range of motion and quadriceps control. Return to running was allowed at approximately 4 months, when core stability was appropriately achieved, and return to sport at 6–8 months, as long as the patient had achieved appropriate functional milestones.
2.5 Patient-Reported Outcome Measures
Two surveys, the Knee Injury and Osteoarthritis Outcome Score (KOOS) and Marx Activity Scale, were administered to patients to evaluate patient-centric outcomes. The Marx Activity Scale was obtained for the Control group to help match activity level. These outcome measures have been validated in the setting of ACL reconstruction and in general following sports-related injuries [15,16].
2.6 Quantitative Cartilage Imaging
Magnetic resonance imaging of the bilateral knees was obtained with a 3T GE Signa MR Scanner (General Electric, Milwaukee, WI, USA) and an eight-channel phased array knee coil (Invivo, Orlando, FL, USA). Prior to starting a scan, patients were instructed to sit for at least 30 minutes to limit variability introduced from pre-scan physical activity. Cartilage imaging of the reconstructed knee and control group was acquired through a sagittal, 3D fat-saturated CUBE sequence (TR=1500 ms; TE=23 ms; FOV=16 cm; slice/gap=0.5/0 mm; matrix=512×512) and sagittal 3D MAPSS sequence (TR=7.2 ms; TE=2.1 ms; FOV=16 cm; slice/gap=3/0 mm; matrix=256×256; time of spin-lock=0/10/40/80 ms; frequency of spin-lock=500 Hz) [10]. Cartilage imaging was not obtained for one patient in the MT group. We have previously shown good reproducibility of T1ρ quantification with global CV of 1.6% and regional CV of 1.7–8.7% [10].
2.7 MR-Kinematic Imaging
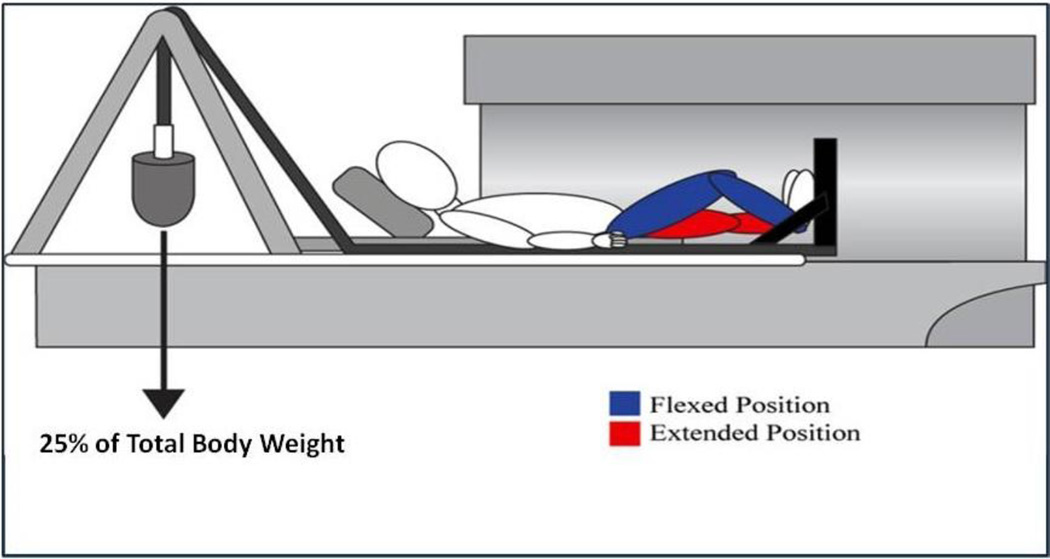
A previously developed and validated protocol was used for MR kinematic measurements [12,13]. A load equal to 25% of total body weight was applied to the patient’s foot through a low-friction pulley system (Figure 1). The knee was imaged first in full extension and then in approximately 30 degrees of flexion. For each position, sagittal T1-weighted 2D fat-suppressed fast spin echo (FSE) images (TR=5000 ms; TE=40 ms; FOV=16 cm; slice/gap=3.5/0 mm; matrix=512×512). Previous studies established an inter-observer and intra-observer reliability of less than 0.6 to 0.9 mm for tibial translation and 1.5 degrees for rotational measurements [17].
Fig. 1.
Schematic of positioning for kinematic MR imaging
2.8 Image Segmentation
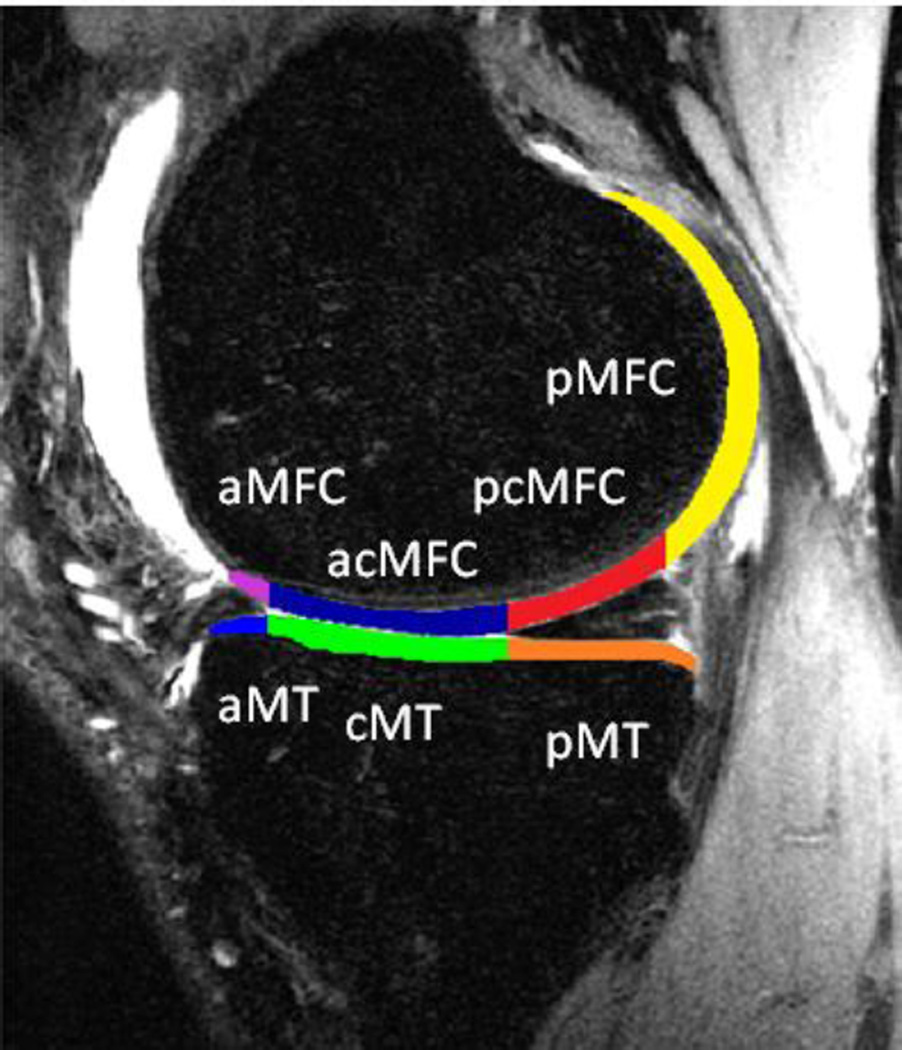
The femoral and tibial cartilage regions of interest were defined on sagittal 3D high-resolution CUBE images using an in-house software program based on a spline-based semi-automated (automated edge detection with manual correction) segmentation algorithm in MATLAB (MathWorks, Natick, MA, USA) [18]. The tibial and femoral cartilage was divided into sub-compartments to evaluate for regional variability in cartilage changes (Figure 2). The sub-compartments were defined according to the boundaries of the meniscus with three compartments in the medial and lateral tibial cartilage (anterior, central and posterior) and four compartments in the medial and lateral femoral cartilage (anterior, anterior-central, posterior-central, and posterior). The CUBE images were registered and re-sampled to align with the 3D T1ρ maps. The segmentation was transferred to the T1ρ map, and mean values were calculated for each global region and each sub-compartment.
Fig. 2.
Cartilage regions of interest, relative to the meniscus with sub-compartments defined as Anterior Medial Tibia (aMT), Central Medial Tibia (cMT), Posterior Medial Tibia (pMT), Anterior Medial Femoral Condyle (aMFC), Anterior/Central Medial Femoral Condyle (acMFC), Posterior/Central Medial Femoral Condyle (pcMFC), and Posterior Medial Femoral Condyle (pMFC)
Kinematic images were segmented according to previously reported methods [12]. The posterior aspect of the femoral condyles and the tibia were defined as Bezier splines for the extended and flexed states. The inter-meniscal contact area was defined as regions of cartilage in contact between the anterior and posterior horns of the medial and lateral menisci. These regions were defined with Bezier splines.
The femoral condyles were fit as spheres using a least-squares algorithm. The center of each sphere was connected with a line, which was used as the medial-lateral axis with the origin as the central point. The axis of the femoral shaft was defined on the mid-sagittal slice, and the anterior-posterior axis for the femoral coordinate system was set as the cross-product of these two axes.
The tibiae were registered in flexion and extension using an iterative closest point algorithm. The tibial axis was defined as the midpoint of an axis running between the posterior aspect of the medial and lateral tibial plateau. The inferior-superior axis of the tibia was defined from the mid-sagittal slice, and the anterior-posterior axis is the cross product of the two calculated axes. The femoral coordinate system was registered to the tibia, and the position of the tibia relative to the femoral coordinate system was calculated. For kinematic changes, the change in tibial position and rotational alignment from the extended to flexed position was calculated.
The points defined for the contact area were connected with triangles, and the area of these triangles was summed to calculate the contact area for the medial and lateral tibiofemoral compartments. The contact centroid was defined as the centroid of these triangles. From the tibial coordinate system, translation of the contact centroid was calculated for the flexed and extended states.
2.9 Statistical Analyses
Statistical analyses were performed in Excel (Microsoft, Redmond, WA, USA) and Stata version 13 (StataCorp, College Station, TX, USA). For demographic variables, an analysis of variance (ANOVA) was used to compare the two surgical groups and the control group. A two-tailed unpaired t-test was used for continuous variables between the surgical groups, and a Fisher’s exact test was utilized for categorical variables. A paired t-test was used to compare kinematic measurements between the patient’s reconstructed and contralateral knees, including the tibial translation, tibial rotation, absolute position of the tibia in flexion and extension, and location of the cartilage contact centroids. An ANOVA with a Bonferroni correction was used to compare the T1ρ values for the MT, AM and Control groups. Statistical significance was set at an alpha of 0.05.
3. Results
Demographic results for the MT, AM, and Control groups are shown in Table 1. There was no significant difference between the groups with respect to patient age, height, weight, or body mass index. All patients underwent ACL reconstruction with a soft-tissue graft, with a similar number of autografts and allografts. The time of image acquisition was significantly later for the MT group, though this difference is likely not clinically significant.
The dynamic tibiofemoral kinematics showed no side-to-side difference between the reconstructed and normal knee for either surgical technique with regards to the amount of tibial translation in moving from knee extension to flexion. Similarly, there were no significant differences in the change in tibial rotation when moving from extension to flexion.
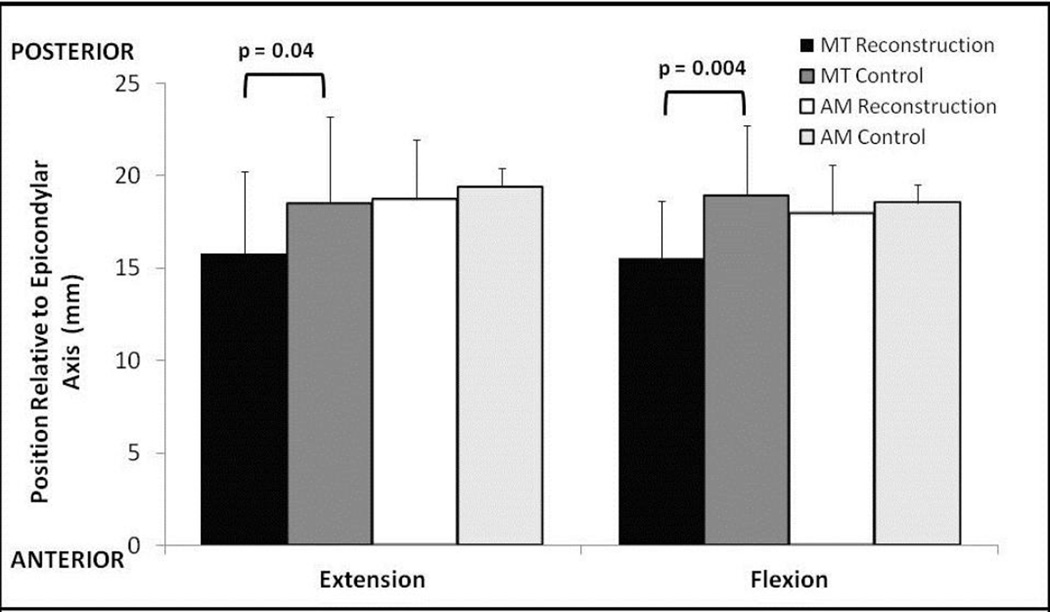
When comparing the absolute position of the knee, the tibiae in the MT group were significantly more anterior in both the extended and flexed positions as compared to the patient’s contralateral knee (Figure 3). In the extended position, the tibia for the MT reconstruction knee was significantly anterior by 2.73 mm when compared with the uninjured side. The difference was increased in the flexed position, with the tibia positioned significantly anterior by 3.39 mm when compared with the contralateral side. No significant difference was observed with regards to tibial position in the AM group. A similar finding was present with regards to the rotational alignment of the tibia. The MT group was significantly more externally rotated relative to the control knee by 3.19° in extension (p=0.046) and 4.39° in flexion (p=0.017). The AM group showed no significant difference between the side-to-side difference in external rotation of the reconstructed and normal tibiae, which was 1.45° in extension (p=0.22) and 1.20° in flexion (p=0.34).
Fig. 3.
Absolute position of the tibial in flexion and extension for the reconstructed and normal knees for each surgical technique. In the MT reconstructed group, the tibia is positioned significantly more anterior in both extension and flexion
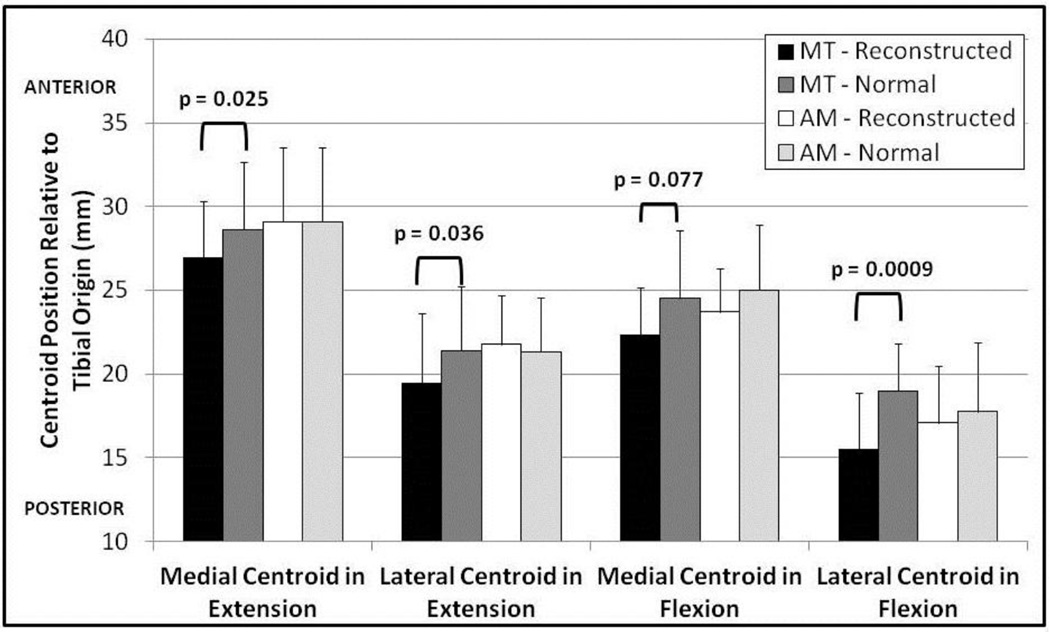
The contact centroid in both the medial and lateral compartments was shifted significantly in the MT group (Figure 4). The medial and lateral contact centroids were significantly posterior in extension (medial by 1.67 mm; lateral by 1.91 mm), relative to the normal knee. The lateral centroid was located significantly more posteriorly in flexion (4.19 mm). Contact centroid position showed no significant difference in the anteroposterior direction between the reconstructed and normal knees in the AM group in either the medial or lateral compartment for the flexed or extended states.
Fig. 4.
Position of the cartilage contact centroid relative to the posterior aspect of the tibia for the reconstructed and normal knees for both surgical techniques. The contact centroid is significantly more posterior for the MT group in the medial and lateral compartments in extension and the lateral compartment in flexion
Cartilage contact area was significantly decreased in the MT group in the medial compartment in extension (Table 2). Contact area in the lateral compartment showed no difference between the reconstructed and control groups for the MT patients. The AM group had significantly higher contact area in the lateral compartment in extension. The remainder of the contact areas for the AM group showed no difference between the reconstructed and normal knees.
Table 2.
Cartilage contact areas of reconstructed knees relative to the patient’s normal knee
| Mini-Two Incisiona |
p valueb | Anteromedial Portala |
p valueb | |
|---|---|---|---|---|
| EXTENSION | ||||
| Medial Compartment | 93.0% ± 6.7 | 0.048 | 104.9% ± 22.7 | 0.67 |
| Lateral Compartment | 104.9% ± 13.0 | 0.70 | 121.8% ± 25.4 | 0.011 |
| FLEXION | ||||
| Medial Compartment | 83.0% ± 8.0 | 0.052 | 120.9% ± 33.6 | 0.18 |
| Lateral Compartment | 98.5% ± 13.3 | 0.74 | 121.1% ± 27.4 | 0.12 |
Contact area expressed as means of each reconstructed knee divided by the corresponding normal knee ± standard deviations
p value from a two-tailed paired t-test between reconstructed and normal knees
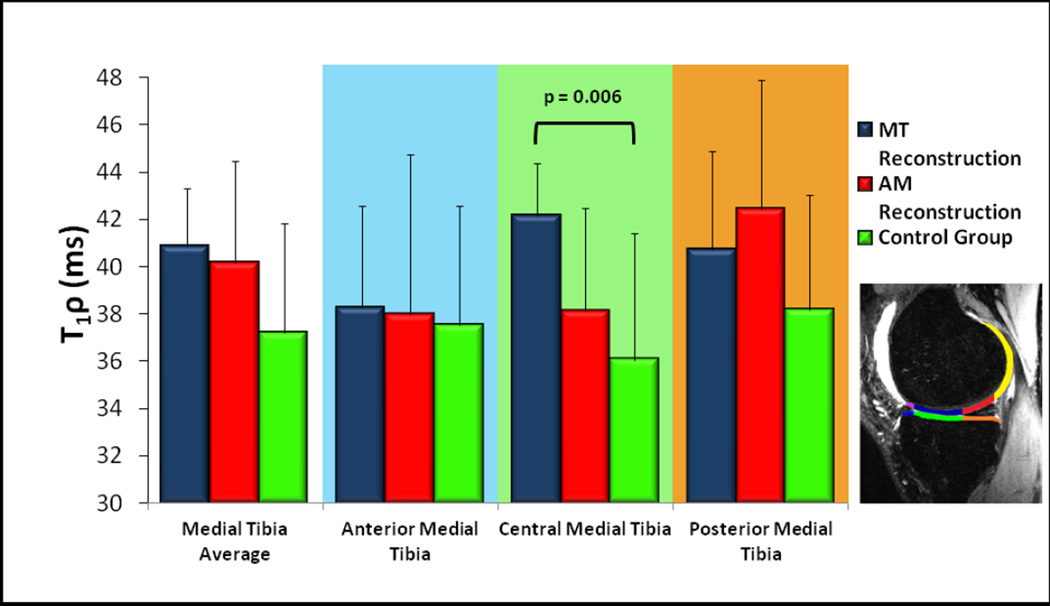
For the MT group, early cartilage changes were present in the central medial tibia sub-compartment with elevation of T1ρ values. The T1ρ values in this region were significantly higher as compared to control subjects, and increased, though not significantly, relative to AM subjects (Figure 5). The mean T1ρ value in the Control group was 36.1 ± 5.3ms, while the mean value was 42.1 ± 2.3ms for the MT group and 38.1 ± 4.4ms for the AM group. The remainder of the sub-compartments in the medial and lateral tibial plateaus and femoral condyles did not show a significant difference in T1ρ values.
Fig. 5.
Early cartilage degeneration as measured by T1ρ for the medial tibial plateau with subcompartments according to the inlay diagram. The central-medial tibia values are significantly higher for the MT reconstruction group relative to the control group
Patient reported outcome measures are listed in Table 3. The results from the Marx survey showed no significant differences between the two surgical groups. Additionally, the Marx activity ratings for the Control group (Running = 2.5 ± 1.4, Cutting = 1 ± 1.2, Decelerating = 1.1 ± 1.0, Pivoting = 0.8 ± 1.3) were not significantly different when compared to the two surgical groups with Analysis of Variance. The Symptoms sub-score of the KOOS survey was significantly higher in the MT group, indicating that these patients endorsed fewer symptoms. The remainder of the KOOS scores showed no significant difference between the two surgical groups.
Table 3.
Patient Reported Outcome Measures
| Mini-Two Incisiona | Anteromedial Portala | p valueb | |
|---|---|---|---|
| Marx Activity Scale | |||
| Running | 1.5 ± 1.4 | 2.4 ± 1.3 | 0.14 |
| Cutting | 1.3 ± 1.3 | 1.9 ± 1.4 | 0.33 |
| Decelerating | 1.5 ± 1.6 | 1.8 ± 1.4 | 0.66 |
| Pivoting | 1.4 ± 1.8 | 1.9 ± 1.4 | 0.50 |
| Knee Injury and Osteoarthritis Outcome Score (KOOS) | |||
| Pain | 87.8 ± 12.0 | 80.9 ± 10.5 | 0.19 |
| Symptoms | 88.7 ± 9.4 | 73.9 ± 16.3 | 0.023 |
| Activities of Daily Living | 95.9 ± 5.4 | 91.5 ± 6.2 | 0.11 |
| Sports and Recreation | 84.0 ± 13.7 | 70.5 ± 18.2 | 0.077 |
| Quality of Life | 64.4 ± 21.1 | 49.4 ± 14.3 | 0.079 |
Expressed as means ± standard deviations.
p value from a two-tailed unpaired t-test.
4. Discussion
The AM and MT methods for ACL reconstruction have both been proposed in order to allow for a more anatomic ACL reconstruction. The results of this study suggest possible clinical differences between the two cohorts using the different techniques. We originally hypothesized that both techniques would lead to the restoration of kinematic parameters that are similar to the patient’s contralateral knee and there would be no evidence of early cartilage degeneration.
The MT group showed significant differences in tibial position as compared to the patient’s contralateral tibia. Non-anatomic positioning of the tibia following ACL reconstruction has been hypothesized to contribute to persistently abnormal knee function and possibly to the development of degenerative changes [19]. Multiple studies have demonstrated even with the restoration of normal anteroposterior translation, the tibia may be more anterior than in the normal knee after ACL reconstruction [19–23]. The anterior position of the tibia in the MT group in the present study (2.73 mm in extension; 3.79 mm in flexion) compares to previous reports of abnormal tibial position after ACL reconstruction, with average side-to-side differences of 1.8–4.4 mm [19,20,23]. Tashman et al reported the rotational alignment of the tibia after ACL reconstruction during running with the use of dynamic fluoroscopy [24]. The tibiae in reconstructed knees were significantly more externally rotated, by 3.8 degrees, similar to the amount of external rotation seen in the MT group (3.19° in extension; 4.39° in flexion). The AM group in the present study shows a restoration of stability in both translation and rotation, as well as the return of the tibiae to absolute positions that are similar to the contralateral knee. Both surgical techniques restore dynamic translation and rotational function, though only the AM group showed a similar tibia position compared to the patient’s normal knee.
The cartilage contact centroids were shifted relative to the normal knee in the MT group, and this difference was not present in the AM group. The anterior position of the tibia resulted in a corresponding posterior shift of both the medial and lateral contact centroids. Additionally, the area of cartilage contact was significantly decreased in the MT group in the medial compartment in extension. The contact area of the lateral compartment in the AM group was significantly increased, which we have reported previously [13]. Li et al determined the cartilage contact points in patients with an ACL deficient knee with MRI and dual fluoroscopy [25]. The cartilage contact point in the medial compartment was shifted posteriorly by 4.2 mm in the ACL-deficient knee. Andriacchi proposed a framework for the pathophysiology of the knee after an ACL injury and the development of osteoarthritis [26]. Within this framework, an abnormal change in the mechanics of the knee, the Initiation Phase, shifts the load bearing area to an unconditioned region of cartilage. This shift may damage the articular surface and the collagen network. After the initiation of these degenerative changes, progressive damage ensues from shear forces. The change in the tibial position, contact centroid location, and contact area could be an in vivo demonstration of this Initiation Phase.
Elevated T1ρ values were present in the central sub-compartment of the medial tibia in the MT group even as early as one year following reconstruction, and there were no observed elevations of T1ρ in the AM group. These early cartilage degenerative changes in the MT group correspond with the observed change in tibial position, centroid location, and decreased contact area. Post-traumatic medial compartment arthritis is a well-known consequence of ACL injury, often occurring 10–15 years after the initial trauma [27,28]. The advantage of quantitative imaging studies is that cartilage changes can be determined much earlier than radiographic changes. Previous studies have demonstrated elevated T1ρ values in the medial compartment at one and two years after surgical reconstruction [29,30]. Li et al. reported T1ρ relaxation times approximately 6.5 ms longer in patients with osteoarthritis relative to patients without knee pathology [31]. Finite element modeling has also demonstrated an increased rate of cartilage thinning following ACL injury, especially in the medial aspect of the joint [32]. The cartilage changes in the present study correspond with side-to-side differences in tibial position and cartilage contact position for the MT group. In the AM group, which had a tibial position and cartilage contact centroid that were not significantly different from the contralateral knee, the medial compartment T1ρ values were not significantly different from the healthy control group.
The analysis of the Marx Activity and KOOS scores show limited differences between the two surgical groups, with the only statistically significant difference seen as a better score in the KOOS-Symptoms category for the MT group. Patient reported outcome measures have been previously reported to be improved with an anatomic ACL reconstruction [33]. The lack of a difference in this study, despite kinematic differences and evidence of early cartilage degeneration, could be due to an inability of these subjective outcome measures to reflect early differences. Alternatively, the differences between these groups may reflect observations that do not influence patient-centered outcomes.
While both surgical techniques allow for anatomic positioning of the femoral tunnel, there are specific differences in these approaches that could explain the findings observed in these two groups. A previous study demonstrated differences in femoral tunnel geometry between the AM and MT techniques in patients with double-bundle ACL reconstruction [34]. This difference, combined with suspensory fixation on the femoral side, may result in altered kinematics. Additionally, though an anatomic reconstruction was the goal for both groups in this study, variable placement in the femoral and tibial tunnel may lead to alterations in knee kinematics. Future studies will attempt to clarify specific surgical factors that may be responsible for the observed differences in these groups.
The limitations of our study must be considered while interpreting the findings. First, data was acquired for the MT group at a significantly later time point than the AM group. This difference is approximately two months, which should not influence the kinematic measurements, though this could contribute to the elevation of T1ρ values in the central-medial tibia of the MT group. Additionally, cartilage imaging was not available for one patient in the MT group. A sensitivity analysis showed that if the missing value was one standard deviation below the mean value for the control group (30.72 ms), the significant difference between these two groups remains (40.99 ms vs 36.06 ms; p=0.038). The quantitative MR cartilage comparisons are limited with reference to a control group due to initial injuries or subject variability. We are currently conducting a prospective study with the acquisition of pre-operative and post-reconstruction data of bilateral knees. We will also continue to follow this cohort to detect mid-term changes in kinematics and cartilage health following ACL reconstructions.
This study is notable for the matched demographics of the two surgical groups, as well as a control group matched to age, gender, BMI, and activity level. The surgical treatment was not randomized, but each patient underwent reconstruction with a high-volume senior surgeon with extensive experience with the respective technique. This study combines validated kinematic measurements with quantitative cartilage imaging to provide an in vivo evaluation of early status after ACL reconstruction.
5. Conclusion
In conclusion, this study reports the one year results of two different surgical reconstruction techniques following ACL injury, with an observed difference in kinematic outcomes and early cartilage matrix alteration following the mini-two incision ACL reconstruction. Further investigations are needed to evaluate the effects of femoral tunnel placement and orientation on the tibial position, as well as on early cartilage changes and long-term functional outcomes.
Highlights.
Imaging-based evaluation of two methods of anatomic ACL reconstruction
Retrospective study of two age and gender-matched groups
A side-to-side difference in tibial position was observed in the MT group
Medial tibial cartilage degenerative changes were observed in the MT group
Acknowledgments
Funding for this project was provided by grants from the Orthopaedic Research and Education Foundation (OREF #12-034) and the National Institute of Health/National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIH/NIAMS P50 AR060752).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Beynnon BD, Johnson RJ, Abate JA, Felming BC, Nichols CE. Treatment of anterior cruciate ligament injuries, Part 1. Am Jour Sports Med. 2005;33:1579–1602. doi: 10.1177/0363546505279913. [DOI] [PubMed] [Google Scholar]
- 2.Johnson RJ, Kettelkamp DB, Clark W, Leaverton P. Factors affecting late results after meniscectomy. J Bone Joint Surg Am. 1974;56:719–729. [PubMed] [Google Scholar]
- 3.Kannus P, Jarvinen M. Conservatively treated tears of the anterior cruciate ligament: long-term results. J Bone Joint Surg Am. 1987;69:1007–1012. [PubMed] [Google Scholar]
- 4.Kessler MA, Behrend H, Henz S, Stutz G, Rukavina A, Kuster MS. Function, osteoarthritis and activty after ACL-rupture, 11 years follow-up results of conservative versus reconstructive treatment. Knee Surg Sports Traumatol Arthrosc. 2008;16:442–448. doi: 10.1007/s00167-008-0498-x. [DOI] [PubMed] [Google Scholar]
- 5.Fink C, Hoser C, Hackl W, Navarro RA, Benedetto KP. Long-term outcome of operative or nonoperative treatment of anterior cruciate ligament rupture - Is sports activity a determining variable? Int J Soprts Med. 2001;22:304–309. doi: 10.1055/s-2001-13823. [DOI] [PubMed] [Google Scholar]
- 6.Fithian DC, Paxton EW, Stone ML, Luetzow WF, Csintalan RP, Phelan D, et al. Prospective trial of a treatment algorithm for the management of the anterior cruciate ligament-injured knee. Am Jour Sports Med. 2005;33:335–346. doi: 10.1177/0363546504269590. [DOI] [PubMed] [Google Scholar]
- 7.Lubowitz JH, Ahmad CH, Anderson K. All-inside anterior cruciate ligament graft-link technique: second-generation no-incision anterior cruciate ligament reconstruction. Arthroscopy. 2011;27:717–727. doi: 10.1016/j.arthro.2011.02.008. [DOI] [PubMed] [Google Scholar]
- 8.Basdekis G, Abisafi C, Christel P. Influence of knee flexion angle on femoral tunnel characteristics when drilled through the anteromedial portal during anterior cruciate ligament reconstruction. Arthroscopy. 2008;24:459–464. doi: 10.1016/j.arthro.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 9.Lubowitz JH, Konicek J. Anterior cruciate ligament femoral tunnel length: cadaveric analysis comparing anteromedial portal versus outside-in technique. Arthroscopy. 2010;26:1357–1362. doi: 10.1016/j.arthro.2010.02.014. [DOI] [PubMed] [Google Scholar]
- 10.Li X, Han ET, Busse RF, Majumdar S. In Vivo T1ρ mapping in cartilage using 3D magnetization-prepared angle-modulated partitioned k-space spoiled gradient echo snapshots (3D MAPSS) Magn Reson Med. 2008;59:298–307. doi: 10.1002/mrm.21414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lozano J, Li X, Link TM, Safran M, Majumdar S, Ma CB. Detection of posttraumatic cartilage injury using quantitative T1rho magnetic resonance imaging. Jour Bone Joint Surg Am. 2006;88-A:1349–1352. doi: 10.2106/JBJS.E.01051. [DOI] [PubMed] [Google Scholar]
- 12.Haughom B, Schairer W, Souza RB, Carpenter D, Ma CB, Li X. Abnormal tibiofemoral kinematics following ACL reconstruction are associated with early cartilage matrix degeneration measured by MRI T1rho. The Knee. 2012;19:482–487. doi: 10.1016/j.knee.2011.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schairer WW, Haughom BD, Morse LJ, Li X, Ma CB. Magnetic resonance imaging evaluation of knee kinematics after anterior cruciate ligament reconstruction with anteromedial and transtibial femoral drilling techniques. Arthroscopy. 2011;27:1663–1670. doi: 10.1016/j.arthro.2011.06.032. [DOI] [PubMed] [Google Scholar]
- 14.Devereaux PJ, Bhandari M, Clarke M, Montori VM, Cook DJ, Yusuf S, et al. Need for expertise based randomised controlled trials. BMJ. 2005;330:88–91. doi: 10.1136/bmj.330.7482.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marx RG, Stump TJ, Jones EC, Wickiewicz TL, Warren RF. Development and evaluation of an activity rating scale for disorders of the knee. Am Jour Sports Med. 2001;29:213–218. doi: 10.1177/03635465010290021601. [DOI] [PubMed] [Google Scholar]
- 16.Roos EM, Roos HP, Lohmander LS, Ekdahl C, Beynnon BD. Knee injury and osteoarthritis outcome score (KOOS) -- development of a self-administered outcome measure. J Orthop Sports Phys Ther. 1998;28:88–96. doi: 10.2519/jospt.1998.28.2.88. [DOI] [PubMed] [Google Scholar]
- 17.Shefelbine SJ, Ma CB, Lee KY, Schrumpf MA, Patel P, Safran MR, et al. MRI analysis of in vivo meniscal and tibiofemoral kinematics in ACL-deficient and normal knees. J Orthop Res. 2006;24:1208–1217. doi: 10.1002/jor.20139. [DOI] [PubMed] [Google Scholar]
- 18.Carballido-Gamio J, Bauer JS, Stahl R, Lee K, Krause S, Link TM, et al. Inter-subject comparison of MRI knee cartilage thickness. Med Image Anal. 2008;12:120–135. doi: 10.1016/j.media.2007.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Almekinders LC, Pandarinath R, Rahusen FT. Knee stability following anterior cruciate ligament rupture and surgery: The contribution of irreducible tibial subluxation. J Bone Joint Surg Am. 2004;86-A:983–987. doi: 10.2106/00004623-200405000-00014. [DOI] [PubMed] [Google Scholar]
- 20.Takeda Y, Sato R, Ogawa T, Fujii J, Naruse A. In vivo magnetic resonance imaging measurement of tibiofemoral relation with different knee flexion angles after single- and double-bundle anterior cruciate ligament reconstructions. Arthroscopy. 2009;25:733–741. doi: 10.1016/j.arthro.2009.01.010. [DOI] [PubMed] [Google Scholar]
- 21.Logan MC, Williams A, Lavelle J, Gedroyc W, Freeman M. Tibiofemoral kinematics following successful anterior cruciate ligament reconstruction using dynamic multiple resonance imaging. Am Jour Sports Med. 2004;32:984–992. doi: 10.1177/0363546503261702. [DOI] [PubMed] [Google Scholar]
- 22.Almekinders LC, de Castro D. Fixed tibial subluxation after successful anterior cruciate ligament reconstruction. Am Jour Sports Med. 2001;29:280–283. doi: 10.1177/03635465010290030301. [DOI] [PubMed] [Google Scholar]
- 23.Papannagari R, Gill TJ, DeFrate LE, Moses JM, Petruska AJ, Li G. In vivo kinematics of the knee after anterior cruciate ligament reconstruction: A clinical and functional evaluation. Am Jour Sports Med. 2006;34:2006–2012. doi: 10.1177/0363546506290403. [DOI] [PubMed] [Google Scholar]
- 24.Tashman S, Collon D, Anderson K, Kolowich P, Anderst W. Abnormal rotational knee motion during running after anterior cruciate ligament reconstruction. Am Jour Sports Med. 2004;32:975–983. doi: 10.1177/0363546503261709. [DOI] [PubMed] [Google Scholar]
- 25.Li G, Moses JM, Papannagari R, Pathare NP, DeFrate LE, Gill TJ. Anterior cruciate ligament deficiency alters the in vivo motion of the tibiofemoral cartilage contact points in both the anteroposterior and mediolateral directions. J Bone Joint Surg Am. 2006;88A:1826–1834. doi: 10.2106/JBJS.E.00539. [DOI] [PubMed] [Google Scholar]
- 26.Andriacchi TP, Mundermann A, Smith RL, Alexander EJ, Dyrby CO, Koo S. A framework for the in vivo pathomechanics of osteoarthritis at the knee. Ann Biomed Eng. 2004;32:447–457. doi: 10.1023/b:abme.0000017541.82498.37. [DOI] [PubMed] [Google Scholar]
- 27.Roos H, Adalberth T, Dahlberg L, Lohmander LS. Osteoarthritis of the knee after injury to the anterior cruciate ligament or meniscus: the influence of time and age. Osteoarth Cartil. 1995;3:261–267. doi: 10.1016/s1063-4584(05)80017-2. [DOI] [PubMed] [Google Scholar]
- 28.Daniel DM, Stone ML, Dobson BE, Fithian DC, Rossman DJ, Kaufman KR. Fate of the ACL-injured patient: A prospective outcome study. Am Jour Sports Med. 1994;22:632–644. doi: 10.1177/036354659402200511. [DOI] [PubMed] [Google Scholar]
- 29.Su F, Hilton JF, Nardo L, Wu S, Liang F, Link TM, et al. Cartilage morphology and T1ρ and T2 quantification in ACL-reconstructed knees: a 2-year follow-up. Osteoarthr and Cartilage. 2013;21:1058–1067. doi: 10.1016/j.joca.2013.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li X, Kuo D, Theologis A, Carballido-Gamio J, Stehling C, Link TM, et al. Cartilage in anterior cruciate ligament-reconstructed knees: MR imaging T1ρ and T2 - Initial experience with 1-year follow-up. Radiology. 2011;258:505–514. doi: 10.1148/radiol.10101006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li X, Ma CB, Link TM, Castillo D-B G, Lozano J, Carballido-Gamio J, et al. In vivo T1p and T2 mapping of articular cartilage in osteoarthritis of the knee using 3T MRI. Osteoarthr and Cartilage. 2007;15:789–797. doi: 10.1016/j.joca.2007.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Andriacchi TP, Briant PL, Bevill SL, Koo S. Rotational changes at the knee after ACL injury cause cartilage thinning. Clin Orthop Relat Res. 2006;442:39–44. doi: 10.1097/01.blo.0000197079.26600.09. [DOI] [PubMed] [Google Scholar]
- 33.Sadoghi P, Kropfl A, Jansson V, Muller PE, Pietschmann MF, Fischmeister MF. Impact of tibial and femoral tunnel position on clinical results after anterior cruciate ligament reconstruction. Arthroscopy. 2011;27:355–364. doi: 10.1016/j.arthro.2010.08.015. [DOI] [PubMed] [Google Scholar]
- 34.Kim JG, Wang JH, Lim HC, Ahn JH. Femoral Graft Bending Angle and Femoral Tunnel Geometry of Transportal and Outside-In Techniques in Anterior Cruciate Ligament Reconstruction: An In Vivo 3-Dimensional Computed Tomography Analysis. Arthroscopy: The Journal of Arthroscopic & Related Surgery. 2012;28:1682–1694. doi: 10.1016/j.arthro.2012.05.884. [DOI] [PubMed] [Google Scholar]