Abstract
Deltorphins are naturally occurring peptides produced by the skin of the giant monkey frog (Phyllomedusa bicolor). They are δ-opioid receptor-selective agonists. Herein, we report the design and synthesis of a peptide, Tyr-D-Ala-(pI)Phe-Glu-Ile-Ile-Gly-NH2 3 (GATE3-8), based on the [D-Ala2]deltorphin II template, which is δ-selective in in vitro radioligand binding assays over the μ- and κ-opioid receptors. It is a full agonist in [35S]GTPγS functional assays and analgesic when administered supraspinally to mice. Analgesia of 3 (GATE3-8) is blocked by the selective δ receptor antagonist naltrindole, indicating that the analgesic action of 3 is mediated by the δ-opioid receptor. We have established a radioligand in which 125I isincorporated into 3 (GATE3-8). The radioligand has a KD of 0.1 nM in Chinese hamster ovary (CHO) cells expressing the δ receptor. Additionally, a series of peptides based on 3 (GATE3-8) was synthesized by incorporating various halogens in the para position on the aromatic ring of Phe3. The peptides were characterized for binding affinity at the μ-, δ-, and κ-opioid receptors, which showed a linear correlation between binding affinity and the size of the halogen substituent. These peptides may be interesting tools for probing δ-opioid receptor pharmacology.
Keywords: Deltorphin, delta opioid receptor, sandmeyer, radioiodination
Opioid analgesics have been used for their pain-relieving properties for centuries. These compounds decrease the sensation of pain by binding opioid receptors found in the central and peripheral nervous system.1 Morphine, the most widely used opioid analgesic, has served as a structural template for the design and synthesis of novel opioids. Unfortunately, morphine and its clinically used analogues have deleterious side-effects, such as respiratory depression and a high potential for addiction. Therefore, it is desirable to design a drug that is able to retain the pain-relieving properties of morphine without causing dangerous side effects.
Three classes of opioid receptors have been cloned: μ (MOR), δ (DOR), and κ (KOR). Subtypes of MOR, DOR, and KOR have also been proposed on the basis of a variety of biochemical and pharmacological approaches.2 Radiolabeled peptides have been useful in the characterization of opioid receptors. Endogenous enkephalin analogue probes with tritium such as [D-Ala2,MePhe4,Gly(ol)5]enkephalin (DAMGO) and [D-Pen2,D-Pen5]enkephalin (DPDPE) are commercially available MOR- and DOR-selective ligands, respectively. However, most tritiated probes have limitations for identifying high-affinity binding sites with low expression levels given the specific activity of 28.8 Ci/mmol of tritium.3 For example, Zhu et al. could not detect binding in brains of DOR knockout mice using [3H]DPDPE and [3H]deltorphin-II, despite findings that both of these compounds retain their analgesic effects when administered supraspinally to this mouse.4
Radioiodinated compounds have numerous other clinically and scientifically relevant uses, which include tissue ablation, tumor imaging, autoradiography, and binding assays.5 High specific activity, such as that of 125I or 131I, has major advantages when visualizing small receptor populations. This is illustrated by our recent studies with [125I]iodobenzoylnaltrexamide ([125I]IBNtxA). [125I]IBNtxA binds with high affinity to the novel opioid target, 6TM/E11.6
Since there are currently no iodinated small molecules or peptides commercially available to target DOR, our primary goal was to synthesize a DOR-selective analgesic opioid peptide bearing an iodine atom. The radioiodinated analogue could be used for radioligand binding assays in rodent brain homogenates, whereas the nonradioactive counterpart could be used as a DOR analgesic model compound in rodents, thus creating a useful probe for DOR pharmacology.
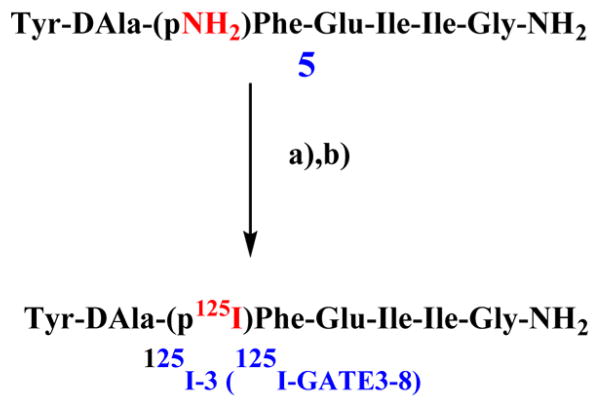
Secretions on the skin of the amphibian Phyllomedusa bicolor contain opioid peptides such as deltorphins, which are highly selective for DOR and analgesic.7 In an attempt to synthesize a selective radioiodinated probe for DOR, we decided to use the [D-ALA2]deltorphin II amino acid sequence as a template. All peptides synthesized were characterized in radioligand binding assays in Chinese hamster ovary (CHO) cell lines stably transfected with opioid receptors. The nonradioactive iodinated deltorphin analogue, Tyr-D-Ala-(p-I)Phe-Glu-Ile-Ile-Gly-NH2 3 (GATE3-8), was further characterized in in vitro [35S]GTPγS functional assays and in vivo tail flick analgesia assays. The radioiodinated analogue, [125I]-3, Tyr-D-Ala-(p-125I)Phe-Glu-Ile-Ile-Gly-NH2 (125I-GATE3-8), was made by the introduction of an amino group at the 4′ position of Phe3 in 5 and was followed by a subsequent Sandmeyer reaction.8 This radio-ligand was further characterized in saturation binding assays and competition assays, confirming its selectivity for DOR.
RESULTS AND DISCUSSION
Five analogues of [D-Ala2]deltorphin II were synthesized using Fmoc-based solid-phase peptide synthesis.9 The Val5–Val6 of [D-Ala2]deltorphin II (Table 1) was replaced with Ile5–Ile6 and pX-Phe3, where X at the 4′ position of Phe was varied (F, Cl, Br, I, and NH2; Table 1 and Figure 2). Valines at positions 5 and 6 were replaced with isoleucine in order to increase hydrophobicity. Isoleucine is more branched than valine, and increasing the hydrophobicity has been shown to increase activity at the δ receptor.10 Additionally the para hydrogen of phenylalanine was substituted with various halogens. The atomic radius of each halogen increases with molecular weight, resulting in an increase in size of the halogen substituent: F < Cl < Br < I (Table 2).
Table 1.
Sequences of Peptides Synthesized
| peptide name | amino acid sequence |
|---|---|
| [D-Ala2]deltorphin II | Tyr-D-Ala-Phe-Glu-Val-Val-Gly-NH2 |
| 1 | Tyr-D-Ala-(pF)Phe-Glu-Ile-Ile-Gly-NH2 |
| 2 | Tyr-D-Ala-(pCl)Phe-Glu-Ile-Ile-Gly-NH2 |
| 3 | Tyr-D-Ala-(pI)Phe-Glu-Ile-Ile-Gly-NH2 |
| 4 | Tyr-D-Ala-(pBr)Phe-Glu-Ile-Ile-Gly-NH2 |
| 5 | Tyr-D-Ala-(pNH2)Phe-Glu-Ile-Ile-Gly-NH2 |
Figure 2.
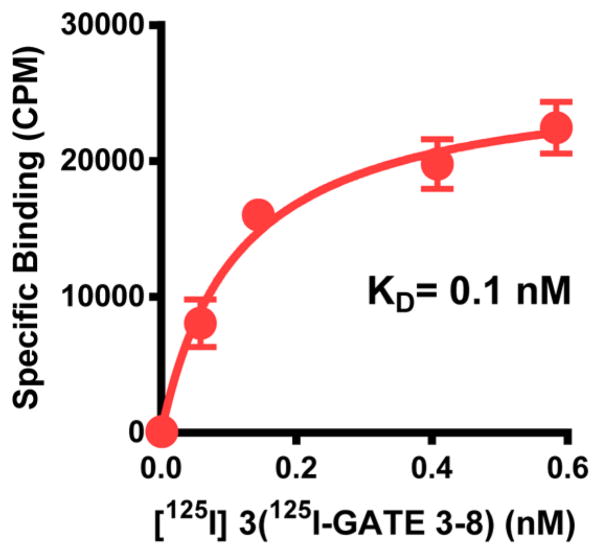
Saturation curve of [125I]-3 (125I-GATE3-8) in DOR-CHO membranes.
Table 2.
HPLC Data of Tyr-D-Ala-(pX)Phe-Glu-Ile-Ile-Gly-NH2 Peptides Synthesizeda
| peptide | (pX)Phe | HPLC k′ (solvent 1) | HPLC k′ (solvent 2) | M + 1 (calcd) | mass spectral analysis (M + 1) | purity |
|---|---|---|---|---|---|---|
| 1 | (pF)Phe | 5.20 | 13.64 | 828.94 | 829.93 | >98% |
| 2 | (pCl)Phe | 5.72 | 13.86 | 845.39 | 844.94 | >95% |
| 3 | (pI)Phe | 6.65 | 14.73 | 936.85 | 936.36 | >95% |
| 4 | (pBr)Phe | 5.87 | 14.24 | 889.85 | 890.10 | >98% |
| 5 | (pNH2)Phe | 3.35 | 7.99 | 825.96 | 825.68 | >98% |
HPLC k′ = [peptide retention time − solvent retention time]/solvent retention time in solvent 1 (10–90% acetonitrile in 0.01% TFA/water over 15 min) or solvent 2 (10–90% methanol in 0.01% TFA/water over 15 min) with a flow rate of 1.5 mL/min. HPLC analysis was performed using a PerkinElmer HPLC system with a Phenomenex Kinetex C18 column (2.6 μm, 4.6 × 100 mm) at a wavelength of 214 nm. Mass spectral analysis took place at CEM Corporation (Matthews, NC) using a Thermoscientific LCQ Advantage system with an Atlantis dC18 analytical column (2.1 × 150 mm). Peptide purity was determined by comparing the area under the main peptide peak to that of the other peaks present.
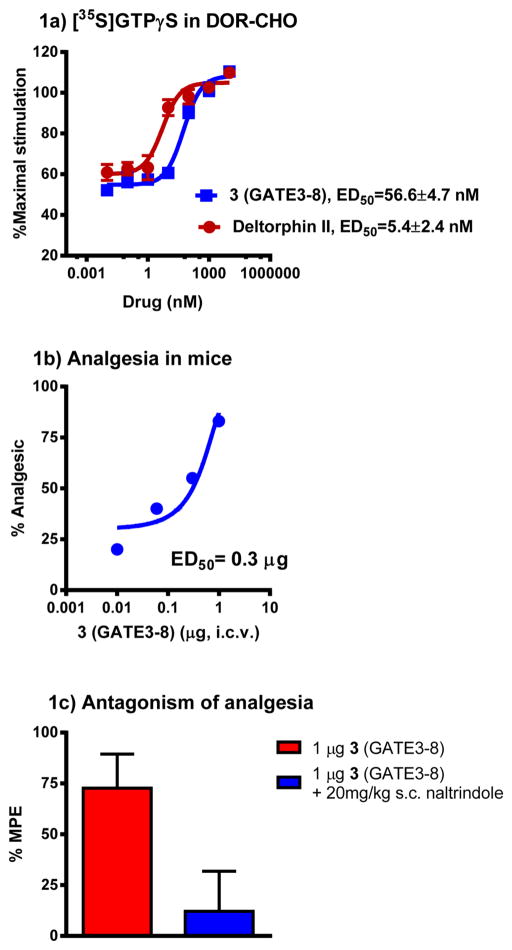
In radioligand binding assays versus [125I]BNtxA in opioid-transfected CHO membranes, all analogues showed selective binding for DOR over MOR, KOR, and 6TM/E11 sites. As the size of the halogen substituent increased, the affinity of the peptide for DOR increased, raising the possibility of the peptide forming weak halogen bonds in the protein pocket. There was also no significant difference between the affinity of 3 (Ki = 0.76 nM) and 4 (Ki = 0.74 nM) (Table 3). 3 was further characterized in [35S]GTPγS functional assays in DOR-transfected CHO cells. 3 was a full agonist, similar to the prototypic DOR agonist DPDPE, although it was about 18-fold less potent (ED50 = 57 nM) than DPDPE (EC50 = 3 nM) and 10-fold less potent than deltorphin II (Figure 1a). In in vivo tail flick analgesia assays, 3 was as potent (ED50 = 0.3 μg) as DPDPE (ED50 = 0.4 μg) when given supraspinally (Figure 1b). The analgesia was also antagonized by the DOR-selective opioid antagonist naltrindole (NTI), confirming that analgesia was exhibited through the activation of DOR (Figure 1c).
Table 3.
Competition Assays against 125IBNtxA by Tyr-D-Ala-(pX)Phe-Glu-Ile-Ile-Gly-NH2a
| peptide | (pX)Phe | MOR (nM) | DOR (nM) | KOR (nM) | 6TM/E11 (nM) |
|---|---|---|---|---|---|
| [D-Ala2]deltorphin II | >1000 | 0.34 ± 0.1 | >1000 | >1000 | |
| 1 | (pF)Phe | >1000 | 6 ± 2.47 | >1000 | >1000 |
| 2 | (pCl)Phe | >1000 | 2.44 ± 0.08 | >1000 | >1000 |
| 3 | (pI)Phe | >1000 | 0.76 ± 0.45 | >1000 | >1000 |
| 4 | (pBr)Phe | >1000 | 0.74 ± 0.36 | >1000 | >1000 |
| 5 | (pNH2)Phe | >1000 | 26 ± 4.16 | >1000 | >1000 |
Competition studies were performed with the indicated compounds against [125I]BNtxA (0.1 nM) in membranes from CHO cells stably expressing the indicated cloned opioid receptor or in mouse brain membranes for 6TM/E11 sites with blockers to prevent binding to traditional mu, kappa1, and delta receptors, as described in the Methods section. Ki values were calculated from the IC50 values and represent the mean ± SEM of at least three independent replications. KD’s used for calculating Ki’s were 0.11, 0.03, 0.24, and 0.16 nM for MOR-1, KOR-1, DOR-1, and 6TM/E11, respectively.
Figure 1.
Pharmacology of Tyr-D-Ala-(pI)Phe-Glu-Ile-Ile-Gly-NH2 3 (GATE3-8). (a) GTPγS stimulation. Efficacy data were obtained using agonist-induced stimulation of [35S]GTPγS binding. Efficacy is represented as EC50 (nM) and percent maximal stimulation relative to standard agonist DPDPE (DOR) at 100 nM. All values are expressed as the mean ± SEM of three separate assays performed in triplicate. (b) Analgesia. Groups of mice (n = 10) received 3 (i.c.v.) at the indicated doses and were tested 15 min later at peak effect to generate the analgesic dose–response curve. ED50 values (and 95% confidence limits) were 0.3 μg (0.1, 0.47) in CD1 mice using the radiant heat tail flick assay. (c) Sensitivity of 3 to opioid antagonists. Groups of mice (n = 10) received a fixed dose of 3 (GATE3-8, 1 μg, i.c.v.) alone or with NTI (20 mg/kg, s.c.) given 15 min before 3. Tail flick analgesia was measured 15 min after 3 was administered. Similar results were observed in two independent replications. Analgesia of 3 was antagonized by NTI (ANOVA followed by Bonferroni multiple comparison test; p < 0.05).
Direct iodination of opioids (tyrosine in position 1 in this case) presents a problem since incorporation of iodine usually leads to a loss of affinity. Thereby, the precursor to [125I]-3, 5, was radioiodinated using a modified Sandmeyer reaction (Scheme 1). In saturation binding assays to CHO-DOR cell membranes, the KD was 0.1 ± 0.02 nM and the Bmax was 196.97 ± 12.44 fmol/mg (Figure 2). We performed competition assays to pharmacologically validate that the site is DOR. The prototypic DOR agonists DPDPE (Ki = 4.5 ± 0.6 nM) and SNC80 (Ki = 9.8 ± 0.7 nM) bound the site with high affinity, and DOR antagonists such as naltrindole (Ki = 0.79 ± 0.16 nM) and naltriben (Ki = 0.14 ± 0.04 nM) also displayed high affinities, consistent with literature values (Table 4).
Scheme 1. Radioiodination of 5 to 125I-3 (125I-GATE3-8)a.
aReagents and conditions: (a) NaNO2, 2 N H2SO4, H3NSO3, 0°C, 5 min; (b) 18-Crown-6, H2O, Na125I, Cu(I)(CH3CN)4BF4, 3 h.
Table 4.
Competition Assays against [125I]-3 (125I-GATE3-8) in DOR-CHOa
| Compound | DOR-CHO Ki (nM) |
|---|---|
| DPDPE | 4.5 ± 0.6 |
| SNC80 | 9.8 ± 0.7 |
| Naltrindole | 0.79 ± 0.16 |
| Naltriben | 0.14 ± 0.04 |
Competition studies were performed with the indicated compounds against [125I]-3 (0.2 nM) in membranes from CHO cells stably expressing DOR. Ki values were calculated from the IC50 values17 and represent the mean ± SEM of at least three independent replications. The KD used for calculating Ki values was 0.1 nM.
CONCLUSIONS
We synthesized halogenated analogues of [D-Ala2]deltorphin II, and all analogues maintained selectivity for DOR over MOR and KOR. As the size of the halogen substituent increased, the affinity of the peptide for the δ-opioid receptor increased. Tyr-D-Ala-(p-I)Phe-Glu-Ile-Ile-Gly-NH2 3, a [D-Ala2]deltorphin II analogue, was found to be highly DOR-selective in in vitro radioligand binding assays and was a full agonist in [35S]GTPγS functional assays. 3 was analgesic in mice when given supraspinally. Analgesia was blocked by the selective DOR antagonist naltrindole, indicating that the analgesia is mediated by DOR. Peptide 5 was readily radioiodinated using a modified Sandmeyer reaction and bound DOR with high affinity. Competition assays confirmed that the site is DOR, as prototypical DOR agonists and antagonists competed [125I]-3 (125I-GATE3-8) with affinities consistent with literature values. Together, our findings suggest that the compound may be useful to elucidate the molecular mechanisms of DOR-mediated analgesia in the brain using radioimaging and in vivo behavioral assays.
METHODS
Chemistry
Peptide Synthesis
Peptide synthesis was performed according to standard Fmoc methodology11 using a Discover SPS microwave system and Accent cleavage system (CEM Corporation, Matthews, NC). Fmoc-Gly-CLEAR-Amide resin was purchased from Peptides International (Louisville, KY). Solvents N,N-dimethylformamide (DMF), dicloromethane (DCM), acetonitrile, and trifluoroacetic acid (TFA) were purchased from Fisher Scientific (Fair Lawn, NJ). Reagents diisopropylcarbodiimide (DIC), phenol, and piperidine were purchased from Sigma-Aldrich (St. Louis, MO), and Oxyma pure was purchased from Advanced ChemTech (Louisville, KY). Amino acids Fmoc-D-Ala-OH·H2O, Fmoc-Glu(OtBu)-OH·H2O, and Fmoc-Ile-OH were purchased from Peptides International (Louisville, KY). Amino acids Fmoc-Tyr(tBu)-OH and Fmoc-Phe(4-Cl)-OH were purchased from Novabiochem (Billerica, MA). Amino acid Fmoc-Phe(4-F)-OH was purchased from Advanced ChemTech (Louisville, KY). Amino acids Fmoc-(4-Br)-Phe-OH and Fmoc-(4-NHBoc)-Phe-OH were purchased from Chem-Impex (Wood Dale, IL). Amino acid Fmoc-(4-I)-Phe-OH was purchased from Anaspec (Fremont, CA). All reagents were ACS grade or better and used without further purification.
Synthesis was performed on a solid support in a Discover SPS microwave synthesizer. Approximately 250 mg (0.1 mmol) of Fmoc-Gly-CLEAR-Amide resin was placed in a 50 mL fritted plastic reaction vessel, and the resin was swelled in 50% DMF and 50% DCM for 1 h. The initial deprotection was performed with about 6 mL of 20% piperidine in DMF for 30 s at 70 °C followed by 30 s at 75 °C. The resin was washed with DMF, and a Kaiser test was performed to confirm the presence of a primary amine. A 3-fold excess of the first amino acid was dissolved in DMF along with a 5-fold excess of Oxyma pure. A 5-fold excess of DIC was added to the amino acid mixture, and then the entire volume was added to the resin. Coupling was performed for 5 min at 75 °C. Another Kaiser test was then performed to confirm completion of the coupling reaction. The deprotection and coupling cycle was repeated as necessary until all amino acids were added to the growing peptide chain. After addition of the last amino acid, the deprotection was repeated one last time and the resin was washed with DCM. Cleavage of the peptide was performed using the Discover SPS microwave in addition to the Accent cleavage system. Approximately 6 mL of cleavage cocktail (90% TFA/5% water/5% phenol) was added to the dried resin, and cleavage was performed for 30 min at 38 °C. Cleaved peptide was collected by filtration and ether precipitation followed by centrifugation for 30 min at 4 °C. The peptide was dissolved in water and lyophilized to a powder. HPLC analysis revealed that the crude peptide’s purity was greater than 95%, as demonstrated by area under the curve. Peptides were analyzed for purity and molecular weight by LC-MS. Purity was greater than 95% for all peptides with an M + 1 mass spectral analysis within equipment error
Iodination
5 was iodinated using a modified Sandmeyer reaction.8,12 18-Crown-6 (303 mM) and tetrakis copper(I)-tetrafluoroborate (254 nM) were dissolved in 40 μL of water and incubated with 4 mCi Na125I on ice. A second reaction vial containing 8 mM 5 was incubated with 2 N sulfuric acid on ice. 50 mM sodium nitrite was added and incubated on ice for 5 min. The reaction was quenched with 290 mM sulfamic acid. The reactions were combined and placed on ice. Following a 3 h incubation, the reaction was centrifuged and the supernatant was injected onto a reverse-phase HPLC C18 column (Thermo Scientific, 150 × 4.6 mm, 5 μm). The gradient started at 5% acetonitrile containing 0.1% TFA (1 mL/min). During the first 5 min, the gradient was increased to 30%, held at 30% for 5 min, increased to 50% during the following 10 min, and finally increased to 95% during the final 5 min. The desired product eluted at 15 min and was obtained with an unoptimized radiochemical yield of 1.3%. The product was confirmed by running 3 as a standard (Figure S1). The yields obtained were lower than expected; we have used the Sandmeyer reaction on a different peptide, alpha-neoendorphin, with yields of around ~29%.8 Efforts are underway to improve the radiochemical yields so that this protocol can be used on a variety of peptides.
Pharmacology
Receptor-Binding Assays
Competition-binding assays in CHO cells stably expressing MOR, DOR, or KOR were performed at 25 °C in potassium phosphate buffer (50 mM, pH 7.4), with the inclusion of MgSO4 (5 mM) in the MOR assays. Competition assays were carried out using [125I]BNtxA6a as described. Specific binding was defined as the difference between total binding and nonspecific binding, determined in the presence of levallorphan (8 μM). Protein concentration was determined as described by Lowry et al.,13 using bovine serum albumin as the standard. Protein concentrations of MOR-CHO, DOR-CHO, and KOR-CHO membranes were between 20 and 90 μg/mL, and incubation times were 90 min. [125I]3 (125I-GATE 3-8) assays (0.25 mL; 0.045 mg protein) were performed in the presence of a protease inhibitor containing 2 μg/mL each of leupeptin, pepstatin, aprotinin, and bestatin and 0.2 mM PMSF.
6TM/E11 competition binding assays6b using [125I]BNtxA (1; 0.15 nM) were carried out in whole brain membrane homogenates (0.5 mL; 0.5 mg protein) at 25 °C in potassium phosphate buffer (50 mM, pH 7.4) with magnesium sulfate (5 mM) for 90 min in the presence of CTAP, U50488H, and DPDPE, all at 200 nM, to block traditional opioid binding sites.
Tail Flick Analgesia Assays
Male CD1 mice (25–35 g; Charles River Breeding Laboratories, Wilmington, MA) were maintained on a 12 h light/dark cycle with Purina rodent chow and water available ad libitum. Mice were housed in groups of five until testing. All animal experiments were reviewed and approved by the Institutional Animal Care and Use Committee of Memorial Sloan-Kettering Cancer Center. Analgesia was determined using the radiant heat tail flick technique14 using a machine (Ugo Basile; model no. 37360). The intensity was set to achieve a baseline between 2 and 3 s. The latency to withdraw the tail from a focused light stimulus was measured electronically using a photocell. Baseline latencies (2.0 to 3.0 s) were determined before experimental treatments for all animals. Post-treatment tail flick latencies were determined as indicated for each experiment, and a maximal latency of 10 s for tail flick was used to minimize tissue damage. Naltrindole was given subcutaneously, and cumulative dose–response experiments were carried out with two independent assays with each group (n = 10). 3 (GATE3-8) was delivered intracerebroventricularly (i.c.v.) as previously described.15 Briefly, the mice were anesthetized by isoflurane. A small incision was made, and 3 (2 μL/mouse) was injected using a 10 μL Hamilton syringe fitted with a 27 gauge needle. Injections were made into the right lateral ventricle at the following coordinates: 2 mm caudal to bregma, 2 mm lateral to sagittal suture, and 2 mm in depth. Mice were tested for analgesia 15 min postinjection. The combined results are presented as the ED50 with 95% confidence limits (n = 20). Analgesia was defined quantally as a doubling, or greater, of the baseline latency. Similar results were obtained by analyzing the data in a graded response manner. Analgesic ED50’s and confidence limits were determined using nonlinear regression analysis (Prism, GraphPad Software, La Jolla, CA).
[35S]GTPγS-Binding Assay
[35S]GTPγS binding was performed on membranes (60 μg; 1 mL) prepared from transfected cells in the presence or absence of the indicated opioid for 60 min at 30 °C in assay buffer (50 mM Tris-HCl, pH 7.4, 3 mM MgCl2, 0.2 mM EGTA, and 10 mM NaCl) containing 0.05 nM [35S]GTPγS; 2 μg/mL each of leupeptin, pepstatin, aprotinin, and bestatin; and 30 μMGDP, as previously reported.16 After the incubation, the reaction was filtered through glass-fiber filters (Whatman Schleicher & Schuell, Keene, NH) and washed three times with 3 mL of ice-cold 50 mM Tris-HCl, pH 7.4, on a semiautomatic cell harvester. Filters were transferred into vials with 5 mL of Liquiscent (National Diagnostics, Atlanta, GA), and the radioactivity in vials was determined by scintillation spectroscopy in a Tri-Carb 2900TR counter (PerkinElmer Life and Analytical Sciences). Basal binding was determined in the presence of GDP and the absence of drug.
[125I]-3 saturation studies were carried out on DOR-CHO membranes. Results are from a representative experiment, and only specific binding is reported. Ratios of total to nonspecific binding were 2.5:1. Experiments were replicated at least three times. KD and Bmax were determined by nonlinear regression analysis, and the mean ± SEM of the replicates was determined. The KD value was best fit with a single site, KD = 0.11 ± 0.02 nM, Bmax = 196.97 ± 12.44 fmol/mg.
Supplementary Material
Acknowledgments
Funding
This work was supported, in part, by research grants from the National Institute on Drug Abuse (DA034106) to S.M. and (DA06241) to G.W.P., a Core Grant from the National Cancer Institute to MSKCC (CA08748), and the National Science Foundation Graduate Research Fellowship under grant no. DGE-1257284 to G.F.M.
The authors thank Dr. Andras Varadi for feedback and assistance with drafting the manuscript.
ABBREVIATIONS
- 6TM/E11
six transmembrane exon 11
- MOR
mu opioid receptor
- KOR
kappa1 opioid receptor
- DOR
delta opioid receptor
- IBNtxA
3′-iodobenzoylnaltrexamide
- s.c
subcutaneous
- CHO
Chinese hamster ovary cells
- DPDPE
[D-Pen2,D-Pen5]Enkephalin
- K2CO3
potassium carbonate
- DMF
N,N-dimethylformamide
- DCM
dicloromethane
- TFA
trifluoroacetic acid
- Tyr
tyrosine
- D-Ala
D-Alanine
- Phe
phenylalanine
- (pF)Phe
para-fluorophenylalanine
- (pCl)Phe
para-chlorophenylalanine
- (pBr)Phe
para-bromo-phenylalanine
- (pI)Phe
para-iodo-phenylalanine
- (pNH2)Phe
para-amino-phenylalanine
- Glu
glutamic acid
- Ile
isoleucine
- Gly
glycine
- HPLC
high-pressure liquid chromatography
- DIC
diisopropylcarbodiimide
Footnotes
Notes
The authors declare no competing financial interest.
Figure S1: UV of HPLC-purified 3, UV of HPLC-purified 125I-iodinated 5 to produce [125I]-3, radiometric detection of 125I-iodinated 5 to produce [125I]-3, and radiometric detection of purified [125I]-3. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.(a) Pasternak GW. Pharmacological mechanisms of opioid analgesics. Clin Neuropharmacol. 1993;16:1–18. doi: 10.1097/00002826-199302000-00001. [DOI] [PubMed] [Google Scholar]; (b) Pasternak GW. The pharmacology of mu analgesics: from patients to genes. Neuroscientist. 2001;7:220–31. doi: 10.1177/107385840100700307. [DOI] [PubMed] [Google Scholar]
- 2.(a) Clark JA, Liu L, Price M, Hersh B, Edelson M, Pasternak GW. Kappa opiate receptor multiplicity: evidence for two U50,488-sensitive kappa 1 subtypes and a novel kappa 3 subtype. J Pharmacol Exp Ther. 1989;251:461–8. [PubMed] [Google Scholar]; (b) Jiang Q, Takemori AE, Sultana M, Portoghese PS, Bowen WD, Mosberg HI, Porreca F. Differential antagonism of opioid delta antinociception by [D-Ala2,Leu5,Cys6]enkephalin and naltrindole 5′-isothiocyanate: evidence for delta receptor subtypes. J Pharmacol Exp Ther. 1991;257:1069–75. [PubMed] [Google Scholar]; (c) Pasternak GW. Multiple opiate receptors: deja vu all over again. Neuropharmacology. 2004;47:312–23. doi: 10.1016/j.neuropharm.2004.07.004. [DOI] [PubMed] [Google Scholar]; (d) Pasternak GW. Molecular insights into μ opioid pharmacology: from the clinic to the bench. Clin J Pain. 2010;26:S3–S9. doi: 10.1097/AJP.0b013e3181c49d2e. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Rothman RB, Bykov V, de Costa BR, Jacobson AE, Rice KC, Brady LS. Interaction of endogenous opioid peptides and other drugs with four kappa opioid binding sites in guinea pig brain. Peptides. 1990;11:311–31. doi: 10.1016/0196-9781(90)90088-m. [DOI] [PubMed] [Google Scholar]; (f) Takemori AE, Portoghese PS. Enkephalin antinociception in mice is mediated by delta 1- and delta 2-opioid receptors in the brain and spinal cord, respectively. Eur J Pharmacol. 1993;242:145–50. doi: 10.1016/0014-2999(93)90074-r. [DOI] [PubMed] [Google Scholar]; (g) Wolozin BL, Pasternak GW. Classification of multiple morphine and enkephalin binding sites in the central nervous system. Proc Natl Acad Sci USA. 1981;78:6181–5. doi: 10.1073/pnas.78.10.6181. [DOI] [PMC free article] [PubMed] [Google Scholar]; (h) Zukin RS, Eghbali M, Olive D, Unterwald EM, Tempel A. Characterization and visualization of rat and guinea pig brain kappa opioid receptors: evidence for kappa 1 and kappa 2 opioid receptors. Proc Natl Acad Sci USA. 1988;85:4061–5. doi: 10.1073/pnas.85.11.4061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Holden NE. CRC Handbook of Chemistry and Physics. CRC Press; Boca Raton, FL: 2013. [Google Scholar]
- 4.Zhu Y, King MA, Schuller AG, Nitsche JF, Reidl M, Elde RP, Unterwald E, Pasternak GW, Pintar JE. Retention of supraspinal delta-like analgesia and loss of morphine tolerance in delta opioid receptor knockout mice. Neuron. 1999;24:243–52. doi: 10.1016/s0896-6273(00)80836-3. [DOI] [PubMed] [Google Scholar]
- 5.(a) Adam MJ, Wilbur DS. Radiohalogens for imaging and therapy. Chem Soc Rev. 2005;34:153–63. doi: 10.1039/b313872k. [DOI] [PubMed] [Google Scholar]; (b) Seevers RH, Counsell RE. Radioiodination techniques for small organic molecules. Chem Rev. 1982;82:575–90. [Google Scholar]; (c) Volkert WA, Hoffman TJ. Therapeutic radiopharmaceuticals. Chem Rev. 1999;99:2269–92. doi: 10.1021/cr9804386. [DOI] [PubMed] [Google Scholar]
- 6.(a) Majumdar S, Burgman M, Haselton N, Grinnell S, Ocampo J, Pasternak AR, Pasternak GW. Generation of novel radiolabeled opiates through site-selective iodination. Bioorg Med Chem Lett. 2011;21:4001–4. doi: 10.1016/j.bmcl.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Majumdar S, Grinnell S, Le Rouzic V, Burgman M, Polikar L, Ansonoff M, Pintar J, Pan YX, Pasternak GW. Truncated G protein-coupled mu opioid receptor MOR-1 splice variants are targets for highly potent opioid analgesics lacking side effects. Proc Natl Acad Sci USA. 2011;108:19778–83. doi: 10.1073/pnas.1115231108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Erspamer V, Melchiorri P, Falconieri-Erspamer G, Negri L, Corsi R, Severini C, Barra D, Simmaco M, Kreil G. Deltorphins: a family of naturally occurring peptides with high affinity and selectivity for delta opioid binding sites. Proc Natl Acad Sci USA. 1989;86:5188–92. doi: 10.1073/pnas.86.13.5188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.(a) Pickett JE, Nagakura K, Pasternak AR, Grinnell SG, Majumdar S, Lewis JS, Pasternak GW. Sandmeyer reaction repurposed for the site-selective, non-oxidizing radioiodination of fully-deprotected peptides: studies on the endogenous opioid peptide α-neoendorphin. Bioorg Med Chem Lett. 2013;23:4347–50. doi: 10.1016/j.bmcl.2013.05.090. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Sharma SD, Toth G, Hruby VJ. A simple general method for (radio)iodination of a phenylalanine residue in peptides: preparation of [cyclic][D-Pen2,4′-125I-Phe4,D-Pen5]enkephalin, a peptide with extraordinary selectivity for δ-opioid receptors. J Org Chem. 1991;56:4981–3. [Google Scholar]
- 9.(a) Carpino LA, Han GY. 9-Fluorenylmethoxycarbonyl function, a new base-sensitive amino-protecting group. J Am Chem Soc. 1970;92:5748–9. [Google Scholar]; (b) Carpino LA, Han GY. 9-Fluorenylmethoxycarbonyl amino-protecting group. J Org Chem. 1972;37:3404–9. [Google Scholar]
- 10.Charpentier S, Sagan S, Naim M, Delfour A, Nicolas P. Mechanism of δ-opioid receptor selection by the address domain of dermenkephalin. Eur J Pharmacol, Mol Pharmacol Sect. 1994;266:175–80. doi: 10.1016/0922-4106(94)90107-4. [DOI] [PubMed] [Google Scholar]
- 11.(a) Joseph CG, Wilson KR, Wood MS, Sorenson NB, Phan DV, Xiang Z, Witek RM, Haskell-Luevano C. The 1,4-benzodiazepine-2,5-dione small molecule template results in melanocortin receptor agonists with nanomolar potencies. J Med Chem. 2008;51:1423–31. doi: 10.1021/jm701303z. [DOI] [PubMed] [Google Scholar]; (b) Wilczynski A, Wilson KR, Scott JW, Edison AS, Haskell-Luevano C. Structure–activity relationships of the unique and potent agouti-related protein (AGRP)–melanocortin chimeric Tyr-c[β-Asp-His-DPhe-Arg-Trp-Asn-Ala-Phe-Dpr]-Tyr-NH2 peptide template. J Med Chem. 2005;48:3060–75. doi: 10.1021/jm049010r. [DOI] [PubMed] [Google Scholar]
- 12.Fang L. MS Thesis. The University of Arizona; 1991. Characterization of new iodinated peptide radioligand for delta opioid receptors. [Google Scholar]
- 13.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. J Biol Chem. 1951;193:265–75. [PubMed] [Google Scholar]
- 14.D’Amour FE, Smith DL. A method for determining loss of pain sensation. J Pharmacol Exp Ther. 1941;72:74–9. [Google Scholar]
- 15.(a) Haley TJ, McCormick WG. Pharmacological effects produced by intracerebral injection of drugs in the conscious mouse. Br J Pharmacol Chemother. 1957;12:12–15. doi: 10.1111/j.1476-5381.1957.tb01354.x. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Kest B, Lee CE, McLemore GL, Inturrisi CE. An antisense oligodeoxynucleotide to the delta opioid receptor (DOR-1) inhibits morphine tolerance and acute dependence in mice. Brain Res Bull. 1996;39:185–8. doi: 10.1016/0361-9230(95)02092-6. [DOI] [PubMed] [Google Scholar]
- 16.(a) Bolan EA, Pan YX, Pasternak GW. Functional analysis of MOR-1 splice variants of the mouse mu opioid receptor gene. Oprm Synapse. 2004;51:11–8. doi: 10.1002/syn.10277. [DOI] [PubMed] [Google Scholar]; (b) Pan YX, Xu J, Bolan E, Moskowitz HS, Xu M, Pasternak GW. Identification of four novel exon 5 splice variants of the mouse mu-opioid receptor gene: functional consequences of C-terminal splicing. Mol Pharmacol. 2005;68:866–75. doi: 10.1124/mol.105.011858. [DOI] [PubMed] [Google Scholar]
- 17.Cheng Y, Prusoff WH. Relationship between the inhibition constant (K1) and the concentration of inhibitor which causes 50% inhibition (I50) of an enzymatic reaction. Biochem Pharmacol. 1973;22:3099–108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.