Abstract
The prefrontal cortex plays an important role in shaping cognition and behavior. Many studies have shown that medial prefrontal cortex (mPFC) plays a key role in seeking, extinction, and reinstatement of cocaine seeking in rodent models of relapse. Subregions of mPFC appear to play distinct roles in these behaviors, such that the prelimbic cortex (PL) is proposed to drive cocaine seeking and the infralimbic cortex (IL) is proposed to suppress cocaine seeking after extinction. This dichotomy of mPFC function may be a general attribute, as similar dorsal-ventral distinctions exist for expression vs. extinction of fear conditioning. However, other results indicate that the role of mPFC neurons in reward processing is more complex than a simple PL-seek vs. IL-extinguish dichotomy. Both PL and IL have been shown to drive and inhibit drug seeking (and other types of behaviors) depending on a range of factors including the behavioral context, the drug-history of the animal, and the type of drug investigated. This heterogeneity of findings may reflect multiple subcircuits within each of these PFC areas supporting unique functions. It may also reflect the fact that the mPFC plays a multifaceted role in shaping cognition and behavior, including those overlapping with cocaine seeking and extinction. Here we discuss research leading to the hypothesis that dorsal and ventral mPFC differentially control drug seeking and extinction. We also present recent results calling the absolute nature of a PL vs. IL dichotomy into question. Finally, we consider alternate functions for mPFC that correspond less to response execution and inhibition and instead incorporate the complex cognitive behavior for which the mPFC is broadly appreciated.
Keywords: prefrontal, frontal, prelimbic, infralimbic, cortex, addiction, cocaine, drugs, cognition, networks
Introduction
The prefrontal cortex (PFC) includes a collection of brain regions intimately associated with the regulation of cognitive, emotional, and motivational processes. Included among these functions are those related to control of behavior: attention, response inhibition, planning, and decision-making (Balleine and Dickinson, 1998; Dalley et al., 2004; Euston et al., 2012; Miller and Cohen, 2001). Dysregulaton of these functions is at the core of addiction, and, ultimately related to the balance between execution and inhibition of behavior (Goldstein and Volkow, 2011). Although considerable research has focused on the role of motivational processes in addiction, it is ultimately a disorder of the balance between motivation and self-regulation, where self-regulation is underpinned by behavioral control functions described above. Given the primacy that deficits in self-control play in driving compulsive drug use and addiction, it is no surprise that there is substantial interest in understanding how PFC both moderates reward or drug seeking behaviors as well as in how its dysfunction can result in diseases such as addiction.
There have been a number of reviews relating PFC function to reward seeking-related behaviors and its dysfunction to addiction (Goldstein and Volkow, 2011; Kalivas, 2008; Peters et al., 2013). The goals of the current review are to (1) summarize the preclinical findings associating the PFC with drug seeking, particularly seeking of cocaine and related psychostimulants, (2) discuss models relating PFC subdivision function to drug seeking and addiction, and (3) provide a discussion of future directions necessary to provide a comprehensive understanding of how best to study the PFC in relation to drug seeking and addiction. Given the widespread use and powerfully predictive outcomes of rodent models (Bentzley et al., 2013; Crombag et al., 2008; Epstein et al., 2006; Mahler et al., 2012), we will focus on these for the majority of the review. However, we do so knowing that there are significant differences between rodent and primate (including human) PFC (Kesner and Churchwell, 2011; Uylings et al., 2003; Wise, 2008) and that there have been substantial advances in understanding how the human PFC functions in relation to drug abuse and addiction as described in a number of comprehensive reviews (Goldstein and Volkow, 2011; Moeller and Goldstein, 2014). We first briefly discuss the role of the human PFC in drug abuse and addiction before turning to a more comprehensive discussion of rodent models.
PFC in drug abuse and addiction in humans
There are a number of reasons why a consideration of human PFC function in drug abuse and addiction is worth briefly discussing in this review that focuses on rodent models of drug seeking. First, addiction is exclusively a human disease. Second, the prefrontal cortex plays an important role in the regulation of behaviors disrupted in addiction such as impulse control, response inhibition and executive function, and this area is massively expanded in human and non-human primates relative to rodents (Wise, 2008). Although it is difficult to make precise relationships between human and rodent PFC, it is clear that these overlapping areas are critically important as potential targets for understanding how prolonged drug use results in a loss of control over behavior.
Response to direct effects of drug
Intravenous cocaine delivery in short-term abstinent cocaine-addicted patients is associated with positive blood oxygen level-dependent (BOLD) responses in multiple prefrontal cortical regions, including dorsolateral prefrontal cortex (dlPFC), anterior cingulate, anterior orbital gyrus, orbitofrontal cortex (OFC), medial orbital gyrus, and frontopolar cortex (FPC) (Breiter et al., 1997; Kufahl et al., 2008; Kufahl et al., 2005; Risinger et al., 2005). These prefrontal responses may be enhanced in cocaine addicts, as intravenous methylphenidate (which cocaine addicts report as being similar to cocaine) increases metabolic responses in right medial orbital prefrontal cortex in addicted subjects, but decreases metabolism in control subjects (Volkow et al., 2005). Prefrontal responses to cocaine appear to be mediated, at least in part, by expectation of drug, as BOLD signals are significantly enhanced in lateral OFC, frontopolar cortex and anterior cingulate after expected versus unexpected cocaine delivery (Kufahl et al., 2008). In contrast, expectation has little effect on the activation of subcortical regions, with responses in these regions mainly associated with the pharmacological effects of cocaine (Goldstein and Volkow, 2011; Kufahl et al., 2008). Prefrontal activity also appears to be associated with the perceived pleasurable effects of intravenous cocaine delivery, as BOLD signals in most regions are positively correlated with ‘rush’ ratings (Breiter et al., 1997). These data indicate that the effects of cocaine on PFC function in humans are related to both the liking as well as the wanting of delivered cocaine, perhaps differentiated by subregional activation.
Effects of drug-associated cues
Studies investigating patterns of activation in response to drug-associated stimuli typically involve exposing participants to either video or pictures of people using drugs or handling drug-associated paraphernalia. These studies have generally demonstrated that relative to controls, cocaine-addicted individuals exhibit enhanced activation in prefrontal regions following exposure to drug-associated stimuli, particularly in cingulate cortex (Kilts et al., 2004; Kuhn and Gallinat, 2011; Marhe et al., 2013; Wexler et al., 2001), left lateral OFC and right dorsolateral PFC (Bonson et al., 2002). Activation of prefrontal regions following exposure to drug cues has also been reported in nicotine (Brody et al., 2002; Brody et al., 2007; Kober et al., 2010b; Yalachkov et al., 2009), alcohol (Grusser et al., 2004; Heinz et al., 2007) and heroin (Li et al., 2012; Li et al., 2013; Li et al., 2014; Xiao et al., 2006) addicted individuals. Importantly, measures of PFC activity following cue exposure appear to have some clinical relevance (Goldstein and Volkow, 2011). For example, cue-induced PFC activity is generally positively correlated with self-reported craving in cocaine, nicotine and alcohol addicts (Bonson et al., 2002; Heinz et al., 2004; Yalachkov et al., 2009). Anterior cingulate activity during a cocaine Stroop task can predict cocaine use three months later (Marhe et al., 2013). Similarly, PFC responses to alcohol related imagery can predict drinking behavior at a three-month follow up (Grusser et al., 2004).
Interestingly, one study compared activation patterns in cocaine addicts that were exposed to videos of both drug use and sexually-explicit content (Garavan et al., 2000). Both videos produced activation of various prefrontal regions, although, only anterior cingulate showed significantly greater activation during the cocaine film than the sex film, suggesting that this region may play a unique role in regulating craving elicited by drug-associated stimuli.
An important role for PFC has also been shown in behavioral modulation of cue reactivity. In a study by Volkow et al. (Volkow et al., 2010), cocaine addicts were asked to inhibit craving induced by videos of cocaine-related imagery. Consistent with previous studies, control participants (not asked to inhibit their craving) exhibited increased glucose metabolism in medial PFC, and this activation was correlated with craving levels. In contrast, participants that inhibited their craving exhibited significantly reduced activity in right medial OFC (Volkow et al., 2010) and activation of right inferior frontal gyrus, a region known to be involved in inhibitory control (Goldstein and Volkow, 2011). In a similar study involving treatment-seeking cigarette smokers, instruction to resist craving while viewing smoking-related videos was associated with increased activity in ACC and dorsal lateral PFC (Brody et al., 2007). Further, when cigarette smokers were asked to consider the long-term consequences of cigarette use, increased activity was observed in regions involved in cognitive control, including dorsal lateral OFC and inferior frontal gyrus, whereas activity was decreased in PFC regions involved in craving, including medial OFC and anterior cingulate (Kober et al., 2010a).
Together, these data provide two main conclusions. First, the PFC is significantly implicated in multiple aspects of drug abuse and addiction including both expression of motivation and craving as well as exertion of restraint to suppress craving. Second, the human PFC is heterogeneous, with different subregions playing selective roles in regulating each of these components of drug use or abstinence. As discussed later in this review, there are similar differences in PFC subregions in rodent models of drug abuse and addiction, strengthening the connection between basic/translational animal models and clinical research in humans.
Despite the comprehensive results associating human PFC and drug abuse and addition, there are significant limitations of human studies. Foremost among these is the inability to disentangle cause and effect – e.g., it is not possible to manipulate the degree of addiction in human populations, and studies investigating effects of drug taking, while present and informative, are limited in number (Risinger et al., 2005). Additionally, the results of these studies are often difficult to interpret, as subjects typically express comorbid psychiatric disorders known to be associated with deficits in prefrontal functioning, including depression (Suh et al., 2009). Further, and perhaps more importantly, studies in humans are largely limited to non-invasive investigations. Although pharmacological, genetic, behavioral, and functional imaging investigations have provided substantial insight into the relationship between PFC function and addiction, these studies are inherently limited in what we see as a critically important dimension: they are unable to disentangle neuronal/circuit-related heterogeneity. Put simply, it is highly unlikely that all neurons of a given area (e.g., the PFC) function in a similar manner and are equally disrupted in drug addiction. Given this point, an understanding of how neural circuits are disrupted at the cellular level in addiction is absolutely essential in a comprehensive characterization of addiction, and in potentially developing treatments that target diseased systems. There have been fewer investigations of non-human primate PFC in addiction models, although these studies have been informative and influential (Baeg et al., 2009; Bradberry and Rubino, 2004). Given the wealth of information provided by rodent models of addiction, and the potential for dissecting specific cellular networks associated with compulsive drug use in these models, we focus here on the rodent PFC – specifically the medial PFC (mPFC) - in drug abuse and addiction.
Anatomy of the rodent medial prefrontal cortex
The mPFC is a key element of the mesocorticolimbic system that is thought to regulate drug-taking behavior (Kalivas, 2008; Kalivas, 2009). Rat mPFC receives dopaminergic input from fibers originating from the A10 group within the ventral tegmental area (Swanson, 1982), as well as from various limbic and sensory regions, including amygdala and hippocampus (Hoover and Vertes, 2007). Together, these inputs allow mPFC to evaluate the salience and motivational significance of drug associated contexts and stimuli (Kalivas, 2009; Lasseter et al., 2010). mPFC can then exert executive control over the selection and initiation of drug-seeking via its projections to the nucleus accumbens (NAc), which in turn engages motor structures such as dorsal striatum and ventral pallidum (Kalivas, 2009; Lasseter et al., 2010; Vertes, 2004).
In rat, mPFC is typically referred to as the structures located along the medial wall of PFC (Dalley et al., 2004; Groenewegen et al., 1997; Heidbreder and Groenewegen, 2003; Kesner and Churchwell, 2011). These structures are often grouped into a dorsal mPFC subregion, which includes precentral cortex (PrC) and anterior cingulate cortex (ACC), and a ventral subregion, which includes prelimbic (PL), infralimbic (IL) and ventral orbital (VO) cortices. Despite their lateral location, the dorsal agranular insular (AID) and dorsolateral orbitofrontal cortex (DLO) are often included as mPFC structures, given their interconnectedness with both PL and IL (Conde et al., 1995; Kesner and Churchwell, 2011; Vertes, 2004). In behavioral studies such as those described below, dorsal vs. ventral mPFC is frequently divided at the PL/IL border, with PL and ACC encompassing dorsal mPFC and IL and, to some degree, VO encompassing ventral mPFC. This detail underscores the important point that terminology is often imprecise in defining mPFC subregions and that efforts must be made to align function with specific areas.
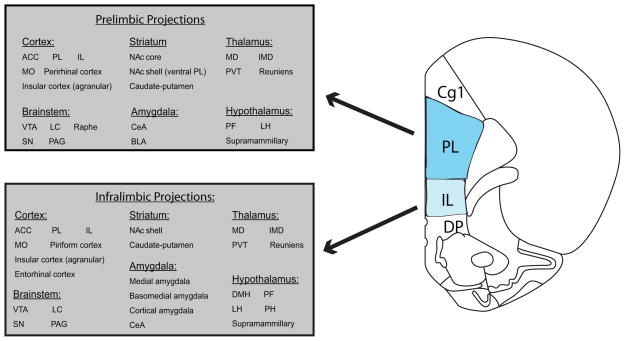
Significant attention has been given to the delineation of PL and IL, with this boundary largely defined by dissociable anatomical connectivity. With a few exceptions, the projection patterns of PL and IL are vastly different (Vertes, 2004; Vertes, 2006), particularly with respect to the density of their projections to other reward-related brain regions. Differences in the afferent connections of PL versus IL with reward-related structures are summarized in Figure 1. For example, PL projects predominantly to NAc core, whereas IL projects almost exclusively to NAc shell (Berendse et al., 1992; Sesack et al., 1989; Vertes, 2004; Vertes, 2006). Similarly, PL fibers distribute primarily to the capsular central amygdala and basolateral amygdala. In contrast, IL projects widely throughout the amygdala, including to medial, basomedial, cortical and central amygdala nuclei (Beckstead, 1979; Hurley et al., 1991; Vertes, 2004; Vertes, 2006). Finally, whilst PL lightly innervates posterior and lateral hypothalamus en route to the brainstem, IL sends significant projections to dorsomedial hypothalamus, perifornical region, posterior and supramammillary nuclei (Hurley et al., 1991; Sesack et al., 1989; Vertes, 2004; Vertes, 2006). For a more complete overview of connectivities of PL and IL, we direct the reader to several excellent papers (Heidbreder and Groenewegen, 2003; Vertes, 2004; Vertes, 2006).
Figure 1.
Schematic summarizing the projections of PL and IL to reward-related regions. Note that although there is overlap in the targets of PL and IL fibers, in many cases the density of these projections differ greatly. For further information regarding the relative densities of PL versus IL inputs to cortical and subcortical regions, we direct the reader to several excellent papers (eg. Vertes, 2004, 2006; Heidbreder & Groenewegen, 2003). Coronal section adapted from (Paxinos and Watson, 1997) ACC, anterior cingulate cortex; BLA, basolateral amygdala; CeA, central amygdala; IL, infralimbic cortex; IMD, intermediodorsal thalamus; LC, locus coeruleus; LH; lateral hypothalamus; MD, mediodorsal thalamus; MO, medial orbital cortex; NA, nucleus accumbens; PAG, periaqueductal gray; PF, perifornical area of the hypothalamus; PH, posterior hypothalamus; PL, prelimbic cortex; PVT, paraventricular thalamus; SN, substantia nigra; VTA, ventral tegmental area.
Finally, it is important to note that segregating PL vs IL based on anatomical connectivity alone is a somewhat imperfect approach, as mPFC projections typically follow a dorsal-ventral gradient. For example, ventral regions of PL tend to innervate both core and shell regions of NAc (Heidbreder and Groenewegen, 2003). Whilst a precise boundary between these two regions is unlikely to exist, there is significant functional evidence to suggest that PL and IL have dissociable roles in various behaviors, including drug seeking (outlined below).
A prominent role of mPFC in animal models of drug-seeking
Animal models of drug use and abuse: a micro-primer
Rodent models of drug use and addiction are a critical component of our understanding the neuroscience of addiction, down to the molecular level, as addressed in the introduction. There are a number of behavioral paradigms associated with modeling drug use and abuse in animals. The benefits and drawbacks of different behavioral models are described elsewhere (Ahmed, 2012; Bentzley et al., 2013; Crombag et al., 2008; Epstein et al., 2006; Mahler et al., 2012; Marchant et al., 2013; Martin-Fardon and Weiss, 2013). We briefly note some of the major techniques frequently used and discussed here. In sensitization studies, animals are given repeated injections of drug. Locomotor responses to injections, which are enhanced over time, are measured as an influence of the sensitizing aspect of the drug on reward/motivation-related circuitry. In conditioned place preference studies, drugs injections are paired with a particular environment while saline or another neutral stimulus is associated with a second environment. The degree of preference that an animal exhibits for the drug-associated environment is considered to be a measure of preference for (or in some cases aversion to) a drug. In self-administration studies, animals self-deliver (often intravenous) infusions of drug by responding on an operandum – typically a lever-press or nose-poke. An animal’s preference and motivation for drug can be measured by recording responses. During extinction training, animals that previously self-administered drugs are presented with the same operanda which no longer provide drugs. Thus, as an animal learns to extinguish its behavior, investigators are able to study the development of response inhibition based on an absence of reinforcement. Finally, during reinstatement testing, extinguished animals (or sometimes those who have simply gone through a period of abstinence) are provided with cues, contexts, or drug “primes”, all of which trigger a resumption of drug seeking. This process is considered to reflect motivation for drugs even in their absence and is a valuable model of relapse in humans (Crombag et al., 2008; Epstein et al., 2006). There are, of course, a large number of other behavioral models and variants on these models which are commonly used, and the reader is directed to more comprehensive reviews to acquire a more nuanced description of those mentioned here and others (Ahmed, 2012; Crombag et al., 2008; Epstein et al., 2006; Mahler et al., 2012; Martin-Fardon and Weiss, 2013). However, these models will be referred to throughout the remainder of this review, so a passing familiarity may be helpful.
The rodent mPFC – relationship to drug use and abuse
Using the behavioral techniques described above, studies have begun to characterize the role of rodent mPFC in both drug seeking as well as in drug-induced disruptions in behavioral function. In both humans and rodents the mPFC is involved in high-level cognitive processes such as learning and decision-making, which are often disrupted following prolonged drug abuse. Chronic cocaine leads to mPFC-dependent cognitive decline in both humans and animals (Bolla et al., 2003; Briand et al., 2008; Cunha et al., 2010). These cognitive impairments lead to the vicious cycle of drug taking, withdrawal, and relapse—a marked characteristic of drug addicts. As mentioned above, mPFC is also a prominent region in the canonical mesocorticolimbic “reward” circuit (McGlinchey et al., In Preparation; Moorman and Aston-Jones, Submitted-a; Tzschentke and Schmidt, 1998; Tzschentke and Schmidt, 2000), and thus many preclinical studies have focused on the mPFC in various behavioral paradigms of reward and drug use and abuse.
In the early 1970’s, it was shown that animals will self-administer electrical stimulation of the mPFC, and then later that such stimulation of mPFC neurons produces a conditioned place preference (Duvauchelle and Ettenberg, 1991; Mora and Cobo, 1990; Phillips and Fibiger, 1978; Robertson and Laferriere, 1989; Routtenberg and Sloan, 1972). Rats also self-administer cocaine directly in mPFC (Goeders and Smith, 1983; Goeders and Smith, 1984; Goeders et al., 1986; Goeders and Smith, 1986; Goeders and Smith, 1993) and cocaine facilitates intracranial electrical self-stimulation in this region (Corbett, 1991; McGregor et al., 1992; Moody and Frank, 1990). Infusion of dopamine or cocaine into mPFC can also increase cocaine-seeking behaviors (McFarland and Kalivas, 2001; Park et al., 2002). Likewise, lesion or inactivation manipulations of mPFC reduce cocaine-induced sensitization and reliably attenuate cocaine seeking, as measured in various reinstatement paradigms and cocaine-induced place preference (Capriles et al., 2003; Fuchs et al., 2005; McFarland and Kalivas, 2001; McLaughlin and See, 2003; Paxinos and Watson, 1997; Stefanik et al., 2013; Tzschentke and Schmidt, 1998); however mPFC lesions have also shown to expedite the acquisition of cocaine-taking (Weissenborn et al., 1997).
Additionally, mPFC exhibits cellular-level plasticity during and following cocaine use or relapse behavior. Arc and c-fos mRNA and their respective proteins (markers of neuronal activation) show increased expression in mPFC regions during acquisition of drug self-administration and cocaine reinstatement paradigms (Ciccocioppo et al., 2001; Fumagalli et al., 2009; Neisewander et al., 2000). Recordings of single mPFC neuron activity during cocaine and heroin self-administration reveal a strong behavioral association: a substantial number of mPFC neurons are modulated (excited or inhibited) at different phases during drug seeking behaviors (Chang et al., 1997a; Chang et al., 1997b; Chang et al., 1998; Chang et al., 2000; Moorman and Aston-Jones, Submitted-b; Rebec and Sun, 2005; Sun and Rebec, 2006; West et al., 2014).
Despite a clear influence of mPFC on drug-seeking behavior, this structure is not a unified entity (nor even dichotomous, as reviewed below). There are anatomical subdivisions, most notably along the dorsoventral axis, that appear to correspond to functional differences, including in the effects of mPFC manipulation on drug seeking (Berendse et al., 1992; Groenewegen et al., 1997; Hoover and Vertes, 2007; Kolb, 1984; Van Eden and Buijs, 2000), and see “Anatomy of the rodent medial prefrontal cortex,” above). These effects vary depending on the type of drug, form of withdrawal (extinction training vs. abstinence), and behavioral context studied. A focus on these subregional differences indicates that the mPFC as a whole is not a single neural system. Instead, regions within mPFC play strikingly different roles in shaping motivated behaviors, including those related to drug abuse and addiction.
Dichotomous functions for dorsal (PL) and ventral (IL) mPFC in drug seeking: Going vs. Stopping?
Most research investigating the functional differences in mPFC subregion has focused on distinguishing differences between the dorsal prelimbic (PL) and ventral infralimbic (IL) cortices. Research in this vein has revealed a substantial number of interesting differences that bear relevance on the regulation of drug seeking. In particular, the dorsal regions of the mPFC (typically the PL, and sometimes including the anterior cingulate, ACC, although see note in “Anatomy of the rodent medial prefrontal cortex,” above) have been demonstrated to play an important role in executing behaviors, whereas IL appears to be more involved in response inhibition (Gass and Chandler, 2013; Peters et al., 2009; Van den Oever et al., 2010). The specifics of these differences are still under investigation and are not necessarily carved in stone, as is discussed below. However, there has been sufficient progress, both in the field of drug abuse/addiction models, and in other areas of research, to warrant consideration of how dorsal and ventral mPFC differences might map onto the control of drug seeking and taking.
PL vs. IL involvement in fear expression vs. extinction
Early studies characterizing differential function for dorsal and ventral mPFC were centered on learning, expression, and extinction of fear conditioning. It has been well-established that PL plays an important role for expression of fear-related behaviors, whereas IL plays an important role in inhibition of fear-related behavior, most notably during extinction of fear-associated stimuli (Maren and Quirk, 2004; Nieuwenhuis and Takashima, 2011; Peters et al., 2009; Sotres-Bayon and Quirk, 2010a). PL neurons fire during expression of conditioned fear responses, and fear conditioning increases the excitability of PL neurons (Burgos-Robles et al., 2009). Activation of PL neurons drives or enhances fear-related freezing behavior whereas inhibition or lesions of PL diminishes or blocks fear-related freezing (Maren and Quirk, 2004; Morgan and LeDoux, 1995; Peters et al., 2009; Sierra-Mercado et al., 2011; Sotres-Bayon and Quirk, 2010a; Vidal-Gonzalez et al., 2006). Conversely, IL neurons fire when animals are presented with extinguished cues that once predicted footshock, most notably when animals no longer freeze to the cues (Chang et al., 2010; Milad and Quirk, 2002). In addition, activation of IL facilitates whereas inhibition of IL inhibits extinction of fear conditioning, respectively (Maren and Quirk, 2004; Morgan and LeDoux, 1995). Importantly manipulations of PL have little impact on fear-extinction related behaviors and PL neurons are minimally activated during presentation of extinguished fear-related stimuli. Conversely, manipulations of IL do not strongly influence fear expression, and neural activity in IL appears to correlate most strongly with strength of extinction. A recent study described Fos expression which was higher in the PL during the presentation of fear conditioned cues and higher in the IL in response to extinguished cues (Orsini et al., 2011). These general results have been repeatedly demonstrated by a number of laboratories, lending strong support to the general hypothesis that PL is critical for expression and IL is critical for inhibition of fear-related behaviors. This framework is the subject of a number of reviews focused specifically on mPFC regulation of fear behaviors, to which we direct the interested reader (Gilmartin et al., 2014; Maren and Quirk, 2004; Peters et al., 2009; Sotres-Bayon and Quirk, 2010b)
PL drives cocaine seeking: effects of PL manipulation
Not long after mPFC subregions began to be explored with respect to fear expression and extinction, studies from the drug-abuse field began demonstrating that mPFC subregions differentially regulated expression of drug seeking. This work notably described an essential function for PL in promoting drug seeking, particularly that of cocaine. This research has gained momentum in recent years and has been demonstrated using a number of different techniques.
Lesions or pharmacological inactivations of PL using, e.g., baclofen/muscimol or lidocaine, have pronounced effects of cocaine-related behaviors, generally decreasing cocaine seeking or conditioning. PL inactivation during maintenance reduced drug seeking (Di Pietro et al., 2006). PL inhibition blocks reinstatement induced by stress (Capriles et al., 2003; McFarland et al., 2004), cocaine-prime (Capriles et al., 2003; Di Pietro et al., 2006; McFarland and Kalivas, 2001; Vassoler et al., 2013), context (Fuchs et al., 2005), and cues (Di Pietro et al., 2006; Gipson et al., 2013; McLaughlin and See, 2003). PL and BLA disconnection decreased cocaine reinstatement (Mashhoon et al., 2010). PL inactivation also blocks spontaneous reinstatement induced by inactivation of IL, a phenomenon described further below (Peters et al., 2008a). PL lesioned rats showed decreased reinstatement after abstinence (Pelloux et al., 2013). This influence of PL on reinstatement is due to projections to other reward-circuit brain areas, most prominently the NAc. Stress- and cocaine prime-induced reinstatement increased NAc core glutamate which was blocked by PL inactivation (Baker et al., 2003; McFarland et al., 2003; McFarland et al., 2004). Optogenetic inhibition of PL-NAc projections decreases cocaine and cocaine+cue-induced reinstatement (Stefanik et al., 2013). PL is also involved in drug-related behaviors outside self-administration: dmPFC lesions blocked cocaine sensitization and glutamate increases in NAc (Pierce et al., 1998), and muscimol infusions into PL decreased cocaine-induced hyperactivity (Franklin and Druhan, 2000a).
Other forms of PL manipulation support a role in cocaine seeking. Both cocaine and dopamine infusions in PL produce reinstatement of drug seeking (McFarland and Kalivas, 2001; Park et al., 2002). PL dopamine antagonist treatment decreases reinstatement induced by shock (Capriles et al., 2003; McFarland et al., 2004) or cocaine priming injection (McFarland and Kalivas, 2001; McFarland et al., 2003; Park et al., 2002; Sun and Rebec, 2005). PL dopamine antagonist infusions also decrease stress- and prime-induced reinstatement of conditioned place preference (Sanchez et al., 2003). BDNF infusion in PL decreases abstinence, cue, and cocaine reinstatement (Berglind et al., 2007; Berglind et al., 2009; Whitfield et al., 2011), and serotonin 2C receptor antagonists administered to PL decreased cue and prime-induced reinstatement (Pentkowski et al., 2010).
PL drives cocaine seeking: cellular correlates
Observation of PL activation and plasticity after cocaine self-administration or reinstatement confirm a strong relationship between PL and drug seeking. Cocaine conditioning increased Fos activation in PL (Franklin and Druhan, 2000b). Fos in PL neurons is also increased on the last day of cocaine self-administration (Zavala et al., 2007), the first day of extinction (when seeking levels were high) (Nic Dhonnchadha et al., 2012), and during cocaine-associated context discriminative or discrete cues that produced reinstatement (Ciccocioppo et al., 2001; Zhou et al., 2014). PL ARC mRNA is also elevated in cue-induced reinstatement (Zavala et al., 2008). After a period of cocaine abstinence, which has the impact of incubating, or upregulating craving for cocaine (Grimm et al., 2001), c-fos, zif268, and ARC mRNA are all elevated in PL (Hearing et al., 2008). A recent study investigating low- vs. high-frequency cocaine use models demonstrated that high-frequency cocaine self-administration increased Fos in PL, and that optogenetic inhibition of PL decreased cocaine prime-induced reinstatement in this population of animals (Martin-Garcia et al., 2014). Cocaine self-administration also increases spines and dendritic bulbs in PL (Robinson et al., 2001). Silent synapses in projections from PL to NAc core emerge after cocaine self-administration, and electrophysiological induction of long-term depression at these synapses both reverses the expression of these synapses and decreases cocaine seeking elicited by 45 days withdrawal (Ma et al., 2014). Recordings of PL neurons demonstrated these cells fire during cocaine self-administration and reinstatement, and the proportion of modulated neurons is enhanced after 1 month abstinence or in cocaine-induced reinstatement (Chang et al., 1997a; Moorman and Aston-Jones, Submitted-b; Rebec and Sun, 2005; Sun and Rebec, 2006; West et al., 2014).
PL drives seeking of other drugs of abuse and natural rewards
PL also plays a role in the seeking/conditioning of other drugs of abuse, exhibiting a largely overlapping profile of influence over seeking of cocaine and other psychostimulants. PL inactivation decreases cue-induced and meth-induced methamphetamine reinstatement (Hiranita et al., 2006; Rocha and Kalivas, 2010) and cue-induced reinstatement of MDMA (Ball and Slane, 2012), and amphetamine self-administration increases spines and dendritic bulbs in PL (Crombag et al., 2005). PL inactivation blocks cue- and heroin prime-induced reinstatement of heroin seeking (LaLumiere and Kalivas, 2008; Rogers et al., 2008). Zif268 was elevated during abstinence-induced heroin reinstatement (Schmidt et al., 2005), and heroin prime-induced reinstatement increases Fos (Shalev et al., 2003). Increased Fos activation was also observed in PL after conditioning for morphine, nicotine, and chocolate conditioned place preference (Schroeder et al., 2000; Schroeder et al., 2001). Discriminative-stimulus induced reinstatement of alcohol seeking increased PL Fos (Dayas et al., 2007), and optogenetic inhibition of PL reduced conflict (quinine or shock)-resistant alcohol seeking (Seif et al., 2013).
PL also plays a role in seeking of natural, typically food-based, rewards. PL inactivation decreases responding to the rewarded lever in a discriminative-stimulus sucrose seeking task (Ishikawa et al., 2008a; Ishikawa et al., 2008b). PL inactivation also decreased food pellet seeking and had no effect on expression of extinction of food seeking (Sangha et al., 2014). Optogenetic inactivation of dorsal mPFC decreased stress-induced reinstatement of food seeking (Calu et al., 2013), and optogenetic activation of PL parvalbumin GABAergic interneurons (which presumably inhibit PL output) enhanced reward extinction learning, but did not influence consumption during conditioning or expression (Sparta et al., 2014). As would be expected from the results described above, PL neurons are activated during natural reward seeking (Burgos-Robles et al., 2013; Moorman and Aston-Jones, Submitted-a).
In sum, there is a plethora of evidence indicating a role for the PL/dorsal mPFC in promoting the seeking of cocaine and other drugs and natural rewards. Combined, these data support the hypothesis that PL is associated with expression of behavior and is in line with fear conditioning studies.
IL suppresses cocaine seeking: effects of IL manipulation and cellular correlates
In the same way that PL promotes both expression of fear conditioning and reward related, a number of findings have implicated IL in the suppression or restraint of fear- (as described above) and reward-seeking behaviors. This has typically been exhibited through studies of extinction learning, in which animals learn to withhold responding based on the absence of a previously conditioned outcome (footshock or drug reward).
Unlike PL, many studies have shown that IL does not appear to be involved in driving drug-seeking. vmPFC lesions did not block cocaine sensitization and the associated glutamate increases in NAc (Pierce et al., 1998), nor does it block cue, context, stress, or cocaine prime-induced reinstatement (Capriles et al., 2003; Fuchs et al., 2005; McFarland and Kalivas, 2001; McFarland et al., 2004; McLaughlin and See, 2003). IL inactivation did not decrease cue or prime induced reinstatement of methamphetamine (Hiranita et al., 2006) or cue-induced reinstatement of MDMA (Ball and Slane, 2012). Zif268 was decreased in IL during both heroin and sucrose seeking (Schmidt et al., 2005).
Beyond simply not being involved, data have shown that IL plays an active role in suppressing cocaine seeking. IL inactivation induces enhanced lever pressing in late extinction of cocaine self-administration (Peters et al., 2008a) and increased spontaneous recovery after 28 days abstinence (Peters et al., 2008b). Conversely, IL activation decreases cocaine-induced reinstatement (Peters et al., 2008a). Similarly, IL PEPA (a positive allosteric modulator of AMPA receptors) decreased cue-induced reinstatement for cocaine (LaLumiere et al., 2012). Inactivation of IL immediately following each extinction session for 5 days enhanced cocaine seeking on subsequent days 6–12 whereas daily post-extinction session PEPA in IL decreased extinction responding (LaLumiere et al., 2010). A noradrenergic component to IL regulation of cocaine extinction learning was demonstrated by showing that beta agonists and antagonists in IL pre-training increased and decreased extinction, respectively (LaLumiere et al., 2010). Opposite to the findings in PL, silent synapses from IL projections to NAc shell emerge after withdrawal from cocaine self-administration. Further, using LTD to reverse them increased, as opposed to decreased cocaine seeking elicited by 45 days withdrawal (Ma et al., 2014). Optogenetic activation of IL facilitated late-stage extinction for Pavlovian-associated cocaine (Van den Oever et al., 2013). Finally, learned behavioral inhibition of cocaine seeking using a conditioned inhibitory “stop signal” cue increased Fos in IL (Navailles et al., 2014), although this effect was minor compared to the activation of PL (described below).
IL suppresses seeking of other drugs of abuse and natural rewards
IL activation appears to be involved in suppressing behaviors related to other drugs besides cocaine as well as natural rewards. IL inactivation produced spontaneous renewal of heroin conditioned place preference after extinction (Ovari and Leri, 2008). PKMZ inhibition in IL disrupted extinction for morphine conditioned place preference and morphine withdrawal conditioned place aversion (He et al., 2011). Blocking GluR2 endocytosis in IL decreases cue-induced heroin seeking (Van den Oever et al., 2008). IL Fos activation was higher in extinction of beer seeking vs. contextual renewal of beer seeking in some studies (Marchant et al., 2010), though not in others (Marchant et al., 2009). IL inactivation increases responding to the non-rewarded lever as well as nonspecific lever responding in a discriminative stimulus-driven sucrose-seeking task (Ghazizadeh et al., 2012; Ishikawa et al., 2008a; Ishikawa et al., 2008b), and in a Pavlovian natural reward conditioning task, IL lesions enhance renewal of extinguished conditioning (Rhodes and Killcross, 2004; Rhodes and Killcross, 2007). In addition, IL inactivation or lesions increase premature responding in a five-choice serial reaction time task (Chudasama et al., 2003; Murphy et al., 2005; Murphy et al., 2012).
Evidence for an imperfect mapping between PL/IL and Go/Stop
The results presented thus far have supported the idea of a push-pull relationship between PL and IL in both fear and reward/drug seeking contexts (Gass and Chandler, 2013; Ma et al., 2014; Peters et al., 2009; Van den Oever et al., 2010). Although this model has been important in developing experiments to probe the function of the PFC in drug abuse and addiction, this go/stop dichotomy likely represents an overly simplistic framework. The prefrontal cortex is a massively complex collection of brain regions, all of which perform a wide variety of cognitive functions (Bissonette et al., 2013; Cassaday et al., 2014; Dalley et al., 2004; De Bruin et al., 2000; Kesner and Churchwell, 2011; Miller and Cohen, 2001; St Onge and Floresco, 2010). Consequently, there have been a growing number of studies that do not support a PL/IL dichotomy along the lines of behavioral expression/inhibition. Understanding the limits of this model is essential for a characterizing prefrontal function and has the potential to refine our ability to identify treatments for psychiatric disorders such as drug and alcohol abuse and addiction.
There are at least two lines of evidence rejecting the conceptualization that the PL plays a selective role in driving drug or reward seeking. First is evidence of an absence of seeking-related function or evidence of a very selective, non-generalized role. Second is the growing evidence that PL activation actually plays an important role in suppressing reward and drug-seeking behaviors in certain contexts.
Evidence that PL does not exclusively drive cocaine seeking
Evidence of a complex role for PL in cocaine seeking dates back at least to 1997 when it was observed that lesions of PL did not diminish and, in some cases enhanced, cocaine self-administration (Weissenborn et al., 1997). PL lesions do not decrease acquisition of cocaine conditioned place-preference (Zavala et al., 2003). PL and BLA disconnection did not decrease maintenance of cocaine self-administration (Mashhoon et al., 2010). No increase in zif268 was observed in PL following presentation of a cocaine-paired cue (Thomas et al., 2003) and, in fact, a decrease in γ-PKC was seen (Thomas and Everitt, 2001). Intriguingly, PL glutamate projection neurons exhibited decreased Fos, whereas PL GABA neurons exhibited increased Fos during cocaine conditioned place preference testing (Miller and Marshall, 2004), underscoring the need to identify potential unique contributions of different phenotypes of prefrontal neurons influencing drug-seeking behavior, described in “Future directions”, below. During electrophysiological recording in self-administration paradigms, PL activity is less related to the execution of the lever press and more related to the associated cues: conditioned cues drive PL activity in the absence of levers (West et al., 2014), and removal of the conditioned cue decreased PL activation (Rebec and Sun, 2005).
Evidence that PL does not play a general role in driving reward seeking
Interestingly, many of the early studies of PL function during drug self-administration failed to see a more general role of PL in reward-seeking. Inactivation or DA blockade in PL had no effect on lever pressing for sucrose or food (Capriles et al., 2003). Inactivation of PL also did not block food reinstatement (McFarland and Kalivas, 2001; McFarland et al., 2003). PL inactivation did not decrease sucrose seeking and actually enhanced conditioned heroin seeking (Schmidt et al., 2005). Food self-administration does not increase spines and dendritic bulbs in PL (Robinson et al., 2001), and Zif268 enhancement was not observed in PL resulting from sucrose reinstatement (Schmidt et al., 2005). Optogenetic inhibition of dorsal mPFC did not decrease food-induced reinstatement or food-reinforced food-seeking (Calu et al., 2013). In the absence of conflict, PL inactivation, either pharmacological (Willcocks and McNally, 2013) or optogenetic (Seif et al., 2013) did not reduce alcohol seeking. PL inactivation decreased learning, but not maintenance, of responding for a cocaine-paired conditioned reinforcer (Di Ciano et al., 2007).
Evidence that PL plays an active role in suppressing seeking of cocaine and other reinforcers
Perhaps more importantly than the absence of influence listed above are recent reports that PL in fact serves an inhibitory role in regulating drug- and reward-seeking behaviors. PL inhibition increases natural reward-seeking, both lever presses and well entries (Jonkman et al., 2009). PL inhibition increases unrewarded and non-specific lever-presses in a discriminative-stimulus sucrose-seeking task (Ishikawa et al., 2008a; Ishikawa et al., 2008b). Response inhibition training, but not immediate reward training enhanced excitability of PL neurons (Hayton et al., 2010; Hayton et al., 2011).
More recently, investigators have found a particularly important role for PL in inhibiting cocaine and sucrose seeking when restraint is the optimal behavior. Studies in which cocaine seeking was paired with a “stop signal” demonstrated that correct inhibition of seeking increased Fos in PL (Mihindou et al., 2013; Navailles et al., 2014), and that pharmacological inactivation of PL decreased the ability to inhibit cocaine seeking (Mihindou et al., 2013). Similarly, a recent report demonstrated the PL inhibition decreased the ability of cocaine- or sucrose-paired footshock to inhibit responding (Limpens et al., 2014). Optogenetic inhibition of the PL in high-frequency self-administering rats actually increased cocaine self-administration (Martin-Garcia et al., 2014). Finally, using a model of punishment-resistant cocaine seeking, Chen and colleagues found that optogenetic inhibition increased and stimulation decreased cocaine seeking respectively in punishment-resistant cocaine-seeking rats (Chen et al., 2013), suggesting a role for PL in contextually-appropriate inhibition of drug seeking.
Outside of the context of self-administration, a number of studies have implicated dorsal mPFC (ACC and PL) in response inhibition as well. Inactivation/lesions of PL impair inhibitory control in stop-signal and waiting tasks (Bari et al., 2011; Broersen and Uylings, 1999; Narayanan et al., 2006; Narayanan and Laubach, 2006; Risterucci et al., 2003), and dorsal mPFC neurons fire during waiting periods (Narayanan and Laubach, 2009). In contrast, ventral mPFC (IL) manipulations are largely without effect in the studies above (though see (Chudasama et al., 2003; Murphy et al., 2005; Murphy et al., 2012)). These results, combined with recent findings in cocaine seeking inhibition paradigms, indicate an important role for PL in response inhibition, perhaps in parallel with its role in response execution demonstrated in so many previous reports.
Thus, recent research challenges both the generality of the PL influence over drug or reward seeking and, in many cases, indicates that opposing roles may be played by this area. Exactly what determines which role is played when is unknown, and may relate to contextual behavioral influences and/or unique contributions of specific neuronal populations within the PL.
Evidence that IL plays a limited role in suppressing cocaine seeking
Along the same lines as findings that PL does not exclusively drive drug seeking, recent results have demonstrated that IL is not selectively/uniquely responsible for suppression of behavior. This has been observed in both cocaine-seeking studies as well as in those related to other drugs of abuse and natural rewards. For example, although PL-lesioned rats showed decreased reinstatement after abstinence, IL-lesioned rats also show a decrease in responding (Pelloux et al., 2013). Similarly, inactivation of IL decreased cocaine prime-induced reinstatement (Vassoler et al., 2013). IL inactivation also decreased learning and maintenance of responding for a cocaine-paired conditioned reinforcer (Di Ciano et al., 2007). Infusion of a serotonin 2C receptor antagonist in IL decreased cued and prime-induced reinstatement of cocaine seeking (Pentkowski et al., 2010). Unlike PL, inhibition of IL did not decrease ability to inhibit cocaine seeking in a stop-signal drug-seeking paradigm, and Fos was not activated in IL, during cocaine seeking inhibition or extinction (McGlinchey et al., In Preparation; Mihindou et al., 2013), though it was observed in a second, but to a lesser degree than that observed for PL (Navailles et al., 2014). Inhibition with baclofen/muscimol in IL decreased pressing in a cue-induced reinstatement following 30 days of incubation after cocaine self-administration, whereas IL activation with bicuculine/saclofen actually increased reinstatement pressing (Koya et al., 2009).
IL neurons are also engaged during cocaine seeking and reinstatement. IL ARC was increased during cue-induced reinstatement (Zavala et al., 2008). IL ERK is upregulated after 30 days of incubation followed by cue-induced reinstatement of cocaine seeking (Koya et al., 2009). IL Fos is increased in context-induced reinstatement (Hamlin et al., 2008), after cocaine conditioning (Franklin and Druhan, 2000b), and on last day of cocaine self-administration (Zavala et al., 2007) as well as on the first (Nic Dhonnchadha et al., 2012), but not last (Zavala et al., 2007) day of extinction. Similar to PL, high-frequency cocaine self-administration increased Fos in IL (Martin-Garcia et al., 2014). In two studies specifically characterizing the activity of PL and IL neurons during cocaine seeking, IL neurons demonstrated robust responses during both cocaine self-administration and reinstatement (Moorman and Aston-Jones, Submitted-b; West et al., 2014), whereas very few IL neurons were active after prolonged extinction (Moorman and Aston-Jones, Submitted-b).
Evidence that IL plays a role in driving seeking of other drugs of abuse and natural rewards
The lack of influence of IL on suppressing behavior has become strikingly clear outside the context of cocaine seeking where, in fact, IL actually drives heroin seeking. IL neurons are Fos-activated in context-induced heroin reinstatement and inactivation of IL decreases heroin reinstatement (Bossert et al., 2011; Bossert et al., 2012). Increased zif268 is observed in IL after cue-induced reinstatement of heroin seeking (Koya et al., 2006), and inactivation of IL decreased cue and heroin prime reinstatement of heroin seeking (Rogers et al., 2008), as did infusion of CB1 antagonists in IL (Alvarez-Jaimes et al., 2008). IL inactivation also decreases cue-induced but not methamphetamine prime-induced reinstatement (Rocha and Kalivas, 2010). IL Fos is increased for discriminative-stimulus induced reinstatement for alcohol (Dayas et al., 2007), and IL inactivation did not influence extinction for alcohol and actually slowed responses during extinction (Willcocks and McNally, 2013).
With respect to natural rewards, there appears to be no clear exclusively inhibitory role for IL neurons. IL inactivation decreases responding to the rewarded lever in a discriminative stimulus task (Ishikawa et al., 2008a). Blocking GluR2 endocytosis in IL does not decrease cue-induced sucrose seeking (Van den Oever et al., 2008). IL inactivation decreased food pellet seeking and had no effect on expression of extinction (Sangha et al., 2014). IL neurons are activated during reward seeking, though the pattern of activity differs from that observed in PL (Burgos-Robles et al., 2013; Moorman and Aston-Jones, Submitted-a). In contrast to PL, immediate reward training, but not response inhibition training, enhanced excitability of IL neurons in vitro (Hayton et al., 2010; Hayton et al., 2011).
Summary: Diverse roles for PL and IL in execution and inhibition of drug- and reward-seeking
The results presented above clearly indicate that a basic dichotomy between execution and inhibition of behavior is not the optimal way to conceptualize the relative contributions of PL and IL to reward/drug seeking. This “Go/Stop” model (Gass and Chandler, 2013; Peters et al., 2009; Van den Oever et al., 2010) has been highly influential as a means to approach the question of what role these prefrontal regions play in expression and restraint of reward seeking. Recent results, however, both in the drug and natural reward contexts clearly indicate that more complex frameworks need to be considered. Although there are certainly elements of neural function related to driving and suppressing reward seeking, these functions do not appear to belong clearly to one prefrontal region or another. An additional factor that comes to bear on these functions is that of context. Thus, PL appears to play a very different role in cocaine seeking during traditional self-administration and reinstatement paradigms (where it facilitates behavior) as opposed to during response-conflict paradigms (where is suppresses behavior). Similarly, IL has repeatedly been shown to play a role in extinction of conditioned fear-predicting stimuli but plays an important role in driving heroin seeking. By using the Go/Stop model as a jumping-off point we are beginning to address advanced ways of considering the functions of these subregions in regulating behavior. In order to integrate these results with other lines of research, it is worth briefly considering other proposed models of differential PL/IL function that have been proposed outside the context of drug-seeking.
Alternate models of PL/IL dichotomous function
The mPFC has been demonstrated to play a role in a wide range of cognitive and behavioral functions including attention, learning and memory, response planning and inhibition, sequence representation, flexible behavior, and decision-making (Bissonette et al., 2013; Cassaday et al., 2014; Dalley et al., 2004; De Bruin et al., 2000; Kesner and Churchwell, 2011; Miller and Cohen, 2001; St Onge and Floresco, 2010). Given the complexity and diversity of mPFC function, it is unsurprising that a number of hypotheses have been put forward to explain the divergent roles of mPFC subregions in all of these behaviors.
One conceptualization of a PL/IL dichotomy that has been demonstrated in multiple studies is the distinction between goal-directed vs. habitual behavior (Balleine and Dickinson, 1998; Corbit and Balleine, 2003; Coutureau and Killcross, 2003; Haddon and Killcross, 2007; Killcross and Coutureau, 2003; Smith et al., 2012; Smith and Graybiel, 2013). This hypothesis has been tested using a range of behavioral paradigms with some of the clearest effects being seen by devaluing a reward (using, e.g., satiation or illness) and assessing whether behavior persists (habitual) or degrades (goal-directed). In these cases, PL appears most influential during goal-directed behavior: PL neurons are activated and PL manipulation influences flexible behavior. As behaviors become more habitual (with, e.g., repetition), IL plays a more predominant role: PL neurons fire less frequently whereas IL neurons fire more, and although PL manipulations have less of an effect, IL inactivation can produce a return to goal-directed behavior. Data supporting this model has been collected from a number of labs using a variety of techniques, indicating that it may be a useful framework to apply to regulation of drug seeking, such as those described above. In many ways, the recent observation of the role of PL in context-guided suppression of drug-seeking (Chen et al., 2013; Mihindou et al., 2013), is well-aligned with this framework. However, the goal/habit model also likely suffers from the same problems of oversimplification as the go/stop model. Both hypothetical frameworks, although valuable for testing predictions, shoehorn diverse mPFC encoding into two basic dichotomous functions. As we note below, a more refined conceptualization of mPFC function, integrated with these models, is likely a valuable future direction.
Other investigators propose dividing mPFC along cognitive and emotional boundaries. A recent review proposed that dorsal mPFC (ACC) plays a more important role in attention whereas ventral mPFC (IL) influences executive function (Cassaday et al., 2014), although the authors suggest that a gradation of function across the dorsal-ventral axis may be more appropriate conceptualization than absolute boundaries. Other proposed differences include dorsal mPFC encoding of actions or spatial locations vs. ventral mPFC encoding of emotional or autonomic responses (Euston et al., 2012). This conceptualization is based on a number of studies, including pharmacological (e.g., (Ashwell and Ito, 2014)), physiological (e.g., (Sul et al., 2010)) and anatomical (e.g., (Vertes, 2006)) investigations. Anatomically speaking, salient parallels have been drawn between dorsal mPFC in rodents and dorsolateral PFC in primates vs. ventral mPFC in rodents and ventral/orbital mPFC in primates (Vertes, 2006), which may facilitate additional parallels between human and primate prefrontal function.
Future directions
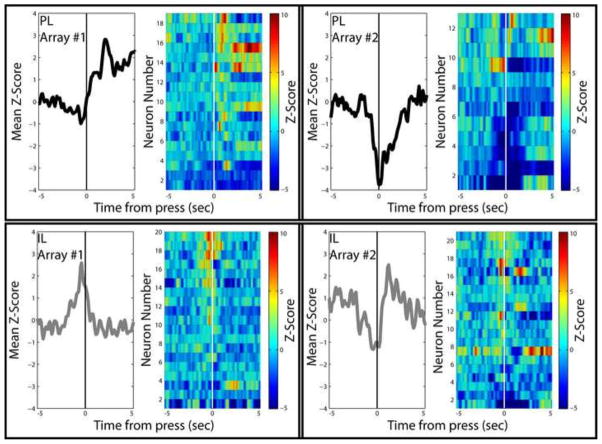
One perspective that seems to emerge from a consideration of studies investigating dorsal and ventral mPFC is that there is no simple functional demarcation that respects this anatomical boundary. In certain behavioral frameworks, notably fear conditioning and extinction, a basic dichotomy of response execution vs. inhibition appears consistent (Maren and Quirk, 2004; Quirk and Mueller, 2008). However, these boundaries quickly break down upon consideration of the function of these brain areas in further tasks. Future studies will need to disentangle the influence of multiple factors on PFC function. For example, there appears to be a difference in mPFC contributions to cocaine seeking after extinction vs. abstinence (Fuchs et al., 2006; Lasseter et al., 2010). Similarly, short- vs. long-access to cocaine, passive vs. active receipt of cocaine, extent of exposure, and whether seeking is challenged all have been shown to differentially engage mPFC function. These points underscore the fact that both PL and IL are highly heterogeneous areas that play diverse roles in many behaviors, and ascribing a unified function to such complex brain areas may be overly simplistic. In this regard, it is worth considering the results of neuronal recording studies during cocaine seeking. In almost all studies where PL or IL neurons have been recorded during cocaine self-administration, extinction, or reinstatement, neurons from the same region exhibit different response properties, (Moorman and Aston-Jones, Submitted-b; West et al., 2014). In many cases, this heterogeneous response profile can be seen from neuron populations recorded on the same electrode array in the same rat implanted in the same brain region (Figure 2). Thus, the idea that an entire area brain area, particularly one as diverse as the mPFC, should conform to an individual basic function seems untenable.
Figure 2.
Example recordings from PL and IL neurons during cocaine self-administration. Each box shows the activity of all neurons recorded from a single array implanted in either PL (top) or IL (bottom) neurons. Each array was implanted in a different rat. Within each box, mean z-scores (left) represent the average of all recorded neurons. Pseudocolor heat plots (right) show the average activity of each neuron. All figures are aligned on cocaine-reinforced lever press. The left two boxes show arrays in PL and IL from which neurons were recorded which were primarily excited at lever press. The right two boxes show arrays in PL and IL from which neurons were recorded which were primarily inhibited at lever press. This figure demonstrates two important details regarding the role of PL and IL neurons in cocaine seeking. First, both PL and IL neurons exhibit both excitatory and inhibitory responses during cocaine self-administration. Second, even within each array, neurons exhibit a variety of responses – some excited, some inhibited, and some nonresponsive – over a wide range of timeframes. These and other data argue against a selective encoding of cocaine seeking in either PL or IL but, instead, indicate that networks within both regions are likely involved (Moorman and Aston-Jones, Submitted-b).
There is, however, almost certainly some degree of functional coherence within these complex neural systems. We are not purporting that every neuron performs a different function and that all of the mPFC plays any and every role in behavior. The major challenge going forward is to understand where, when and how specific functions are represented in these complex areas. One possibility might be an anatomical arrangement that has not yet been considered. A strong candidate for this new framework is afferent and efferent connectivity. Neurons in PL and IL also project to a wide variety of other brain regions involved in reward and drug seeking, as described above. For example, mPFC neurons activated during cue reinstatement of cocaine seeking do not project to VTA (Mahler and Aston-Jones, 2012), but instead project to other regions such as the ventral striatum (McGlinchey et al., In Preparation) (Figure 3). Optogenetic activation of mPFC projections to dorsal raphe nucleus promoted escape behavior in a forced swim test, whereas activation of mPFC projections to lateral habenula inhibited escape behavior (Warden et al., 2012). These and other results indicate that some of the complexity surrounding the mPFC relates to distinct mPFC neuronal populations whose function varies with projection targets. Membership in these specific projection networks may circumscribe function, reflecting developmental schema and allowing an organized representation of multiple behaviors within a single brain region.
Figure 3.
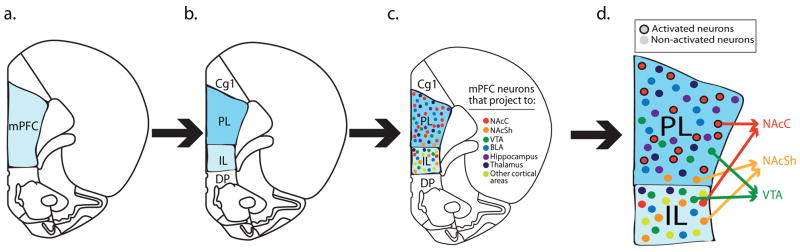
Progressive conceptualizations of mPFC differentiation. a) mPFC as a unified structure. b) Structural distinctions within the mPFC emerge, e.g., prelimbic (PL) and infralimbic (IL) cortices. c) Only specific neuronal ensembles within PL and IL based on their projection targets are necessary for particular behaviors, not the region as a whole. d) Data from our lab indicate that PL neurons projecting to nucleus accumbens core (NAcC), but not to nucleus accumbens shell (NAcSh) or ventral tegmental area (VTA), activate during cue-induced cocaine seeking (Mahler and Aston-Jones, 2012; McGlinchey et al., In Preparation).
Similarly, afferent connectivity likely plays an important role in behavioral function. Inputs to mPFC subregions may be excitatory as in those from hippocampus or amygdala, for example, but may also be neuromodulatory. The mPFC receives strong catecholaminergic projections, for example, and the interplay between neuromodulatory systems represents another parameter that may influence functional relationship to behavior. Another surprisingly understudied framework to consider is that of within-region neuronal phenotype. Not only are there clear functional differences between pyramidal and GABAergic interneurons and their influence over cocaine-related behaviors (Miller and Marshall, 2004), but there are diverse subtypes of GABAergic and likely glutamatergic neurons in mPFC, each of which play different functions in sculpting behavior (Kvitsiani et al., 2013; Pi et al., 2013).
As a final point, we note that we have focused specifically on mPFC, mainly PL and IL cortex. This is with the knowledge that no brain area functions alone and that there is a large number of interconnected brain regions all of which perform critical roles in reward/drug seeking. Even within the frontal cortex, we did not discuss rodent cingulate or orbitofrontal cortices, both of which are rapidly becoming appreciated to play critical roles in elements of drug seeking and addiction (Lasseter et al., 2010; Lucantonio et al., 2012; Schoenbaum and Shaham, 2008). Future research will benefit from a network view where the interaction across brain regions is acknowledged to play an essential role in controlling reward seeking, drug abuse, and addiction. Taken together, it is becoming clear that reward seeking, seeking and taking of drugs of abuse, drug abuse, and addiction are subserved by populations of neurons – prefrontal and otherwise - that are anatomically and phenotypically diverse and are differentially interconnected to brain areas which are potentially equally diverse (Figure 3). Dysfunction in any or multiple nodes in a broad, heterogeneous network may be responsible for the disease nature of compulsive drug use. As such, understanding reward- and drug-related behaviors in as broad a context as possible, while challenging, seems the most logical – and necessary – course of action in future research.
Highlights.
The prefrontal cortex is important in regulating cognition and behavior
It plays an important role in seeking and taking, extinction, and reinstatement of drugs and natural rewards
Current theories of the relationship between rodent medial prefrontal cortex (mPFC) and behavior associate specific functions with specific subregions, e.g., dorsal mPFC plays a role in driving drug and reward seeking and ventral mPFC plays a role in inhibiting reward and drug seeking
Recent results challenge this framework and suggest alternate conceptualizations of how mPFC relates to drug/reward seeking
These results and alternate hypotheses connecting mPFC to drug and reward seeking are discussed as are important future directions necessary to develop a comprehensive understanding of the relationship between mPFC, reward seeking, and addiction
Acknowledgments
Supported by grants from the National Institutes of Health to DEM (R21 DA032005), EMM (F31 DA035561), GAJ (R01 DA006214) and the National Health and Medical Research Council CJ Martin Fellowship to MHJ (1072706).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahmed SH. The science of making drug-addicted animals. Neuroscience. 2012;211:107–25. doi: 10.1016/j.neuroscience.2011.08.014. [DOI] [PubMed] [Google Scholar]
- Alvarez-Jaimes L, Polis I, Parsons LH. Attenuation of cue-induced heroin-seeking behavior by cannabinoid CB1 antagonist infusions into the nucleus accumbens core and prefrontal cortex, but not basolateral amygdala. Neuropsychopharmacology. 2008;33:2483–93. doi: 10.1038/sj.npp.1301630. [DOI] [PubMed] [Google Scholar]
- Ashwell R, Ito R. Excitotoxic lesions of the infralimbic, but not prelimbic cortex facilitate reversal of appetitive discriminative context conditioning: the role of the infralimbic cortex in context generalization. Front Behav Neurosci. 2014;8:63. doi: 10.3389/fnbeh.2014.00063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baeg EH, et al. Orbitofrontal and anterior cingulate cortex neurons selectively process cocaine-associated environmental cues in the rhesus monkey. J Neurosci. 2009;29:11619–27. doi: 10.1523/JNEUROSCI.3206-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker DA, et al. Neuroadaptations in cystine-glutamate exchange underlie cocaine relapse. Nat Neurosci. 2003;6:743–9. doi: 10.1038/nn1069. [DOI] [PubMed] [Google Scholar]
- Ball KT, Slane M. Differential involvement of prelimbic and infralimbic medial prefrontal cortex in discrete cue-induced reinstatement of 3,4-methylenedioxymethamphetamine (MDMA; ecstasy) seeking in rats. Psychopharmacology (Berl) 2012;224:377–85. doi: 10.1007/s00213-012-2762-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balleine BW, Dickinson A. Goal-directed instrumental action: contingency and incentive learning and their cortical substrates. Neuropharmacology. 1998;37:407–19. doi: 10.1016/s0028-3908(98)00033-1. [DOI] [PubMed] [Google Scholar]
- Bari A, et al. Prefrontal and monoaminergic contributions to stop-signal task performance in rats. J Neurosci. 2011;31:9254–63. doi: 10.1523/JNEUROSCI.1543-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckstead RM. An autoradiographic examination of corticocortical and subcortical projections of the mediodorsal-projection (prefrontal) cortex in the rat. J Comp Neurol. 1979;184:43–62. doi: 10.1002/cne.901840104. [DOI] [PubMed] [Google Scholar]
- Bentzley BS, Fender KM, Aston-Jones G. The behavioral economics of drug self-administration: a review and new analytical approach for within-session procedures. Psychopharmacology (Berl) 2013;226:113–25. doi: 10.1007/s00213-012-2899-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berendse HW, Galis-de Graaf Y, Groenewegen HJ. Topographical organization and relationship with ventral striatal compartments of prefrontal corticostriatal projections in the rat. J Comp Neurol. 1992;316:314–47. doi: 10.1002/cne.903160305. [DOI] [PubMed] [Google Scholar]
- Berglind WJ, et al. A BDNF infusion into the medial prefrontal cortex suppresses cocaine seeking in rats. Eur J Neurosci. 2007;26:757–66. doi: 10.1111/j.1460-9568.2007.05692.x. [DOI] [PubMed] [Google Scholar]
- Berglind WJ, et al. A single intra-PFC infusion of BDNF prevents cocaine-induced alterations in extracellular glutamate within the nucleus accumbens. J Neurosci. 2009;29:3715–9. doi: 10.1523/JNEUROSCI.5457-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissonette GB, Powell EM, Roesch MR. Neural structures underlying set-shifting: roles of medial prefrontal cortex and anterior cingulate cortex. Behav Brain Res. 2013;250:91–101. doi: 10.1016/j.bbr.2013.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolla KI, et al. Orbitofrontal cortex dysfunction in abstinent cocaine abusers performing a decision-making task. Neuroimage. 2003;19:1085–94. doi: 10.1016/s1053-8119(03)00113-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonson KR, et al. Neural systems and cue-induced cocaine craving. Neuropsychopharmacology. 2002;26:376–86. doi: 10.1016/S0893-133X(01)00371-2. [DOI] [PubMed] [Google Scholar]
- Bossert JM, et al. Ventral medial prefrontal cortex neuronal ensembles mediate context-induced relapse to heroin. Nat Neurosci. 2011;14:420–2. doi: 10.1038/nn.2758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossert JM, et al. Role of projections from ventral medial prefrontal cortex to nucleus accumbens shell in context-induced reinstatement of heroin seeking. J Neurosci. 2012;32:4982–91. doi: 10.1523/JNEUROSCI.0005-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradberry CW, Rubino SR. Phasic alterations in dopamine and serotonin release in striatum and prefrontal cortex in response to cocaine predictive cues in behaving rhesus macaques. Neuropsychopharmacology. 2004;29:676–85. doi: 10.1038/sj.npp.1300386. [DOI] [PubMed] [Google Scholar]
- Breiter HC, et al. Acute effects of cocaine on human brain activity and emotion. Neuron. 1997;19:591–611. doi: 10.1016/s0896-6273(00)80374-8. [DOI] [PubMed] [Google Scholar]
- Briand LA, et al. Persistent Alterations in Cognitive Function and Prefrontal Dopamine D2 Receptors Following Extended, but Not Limited, Access to Self-Administered Cocaine. Neuropsychopharmacology. 2008;33:2969–2980. doi: 10.1038/npp.2008.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody AL, et al. Brain metabolic changes during cigarette craving. Arch Gen Psychiatry. 2002;59:1162–72. doi: 10.1001/archpsyc.59.12.1162. [DOI] [PubMed] [Google Scholar]
- Brody AL, et al. Neural substrates of resisting craving during cigarette cue exposure. Biol Psychiatry. 2007;62:642–51. doi: 10.1016/j.biopsych.2006.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broersen LM, Uylings HB. Visual attention task performance in Wistar and Lister hooded rats: response inhibition deficits after medial prefrontal cortex lesions. Neuroscience. 1999;94:47–57. doi: 10.1016/s0306-4522(99)00312-7. [DOI] [PubMed] [Google Scholar]
- Burgos-Robles A, Vidal-Gonzalez I, Quirk GJ. Sustained conditioned responses in prelimbic prefrontal neurons are correlated with fear expression and extinction failure. J Neurosci. 2009;29:8474–82. doi: 10.1523/JNEUROSCI.0378-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgos-Robles A, Bravo-Rivera H, Quirk GJ. Prelimbic and infralimbic neurons signal distinct aspects of appetitive instrumental behavior. PLoS One. 2013;8:e57575. doi: 10.1371/journal.pone.0057575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calu DJ, et al. Optogenetic inhibition of dorsal medial prefrontal cortex attenuates stress-induced reinstatement of palatable food seeking in female rats. J Neurosci. 2013;33:214–26. doi: 10.1523/JNEUROSCI.2016-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capriles N, et al. A role for the prefrontal cortex in stress- and cocaine-induced reinstatement of cocaine seeking in rats. Psychopharmacology (Berl) 2003;168:66–74. doi: 10.1007/s00213-002-1283-z. [DOI] [PubMed] [Google Scholar]
- Cassaday HJ, Nelson AJ, Pezze MA. From attention to memory along the dorsal-ventral axis of the medial prefrontal cortex: some methodological considerations. Front Syst Neurosci. 2014;8:160. doi: 10.3389/fnsys.2014.00160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang CH, Berke JD, Maren S. Single-unit activity in the medial prefrontal cortex during immediate and delayed extinction of fear in rats. PLoS One. 2010;5:e11971. doi: 10.1371/journal.pone.0011971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang JY, et al. Single neuronal responses in medial prefrontal cortex during cocaine self-administration in freely moving rats. Synapse. 1997a;26:22–35. doi: 10.1002/(SICI)1098-2396(199705)26:1<22::AID-SYN3>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Chang JY, et al. Neuronal responses in prefrontal cortex and nucleus accumbens during heroin self-administration in freely moving rats. Brain Res. 1997b;754:12–20. doi: 10.1016/s0006-8993(97)00012-7. [DOI] [PubMed] [Google Scholar]
- Chang JY, Janak PH, Woodward DJ. Comparison of mesocorticolimbic neuronal responses during cocaine and heroin self-administration in freely moving rats. J Neurosci. 1998;18:3098–115. doi: 10.1523/JNEUROSCI.18-08-03098.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang JY, Janak PH, Woodward DJ. Neuronal and behavioral correlations in the medial prefrontal cortex and nucleus accumbens during cocaine self-administration by rats. Neuroscience. 2000;99:433–43. doi: 10.1016/s0306-4522(00)00218-9. [DOI] [PubMed] [Google Scholar]
- Chen BT, et al. Rescuing cocaine-induced prefrontal cortex hypoactivity prevents compulsive cocaine seeking. Nature. 2013;496:359–62. doi: 10.1038/nature12024. [DOI] [PubMed] [Google Scholar]
- Chudasama Y, et al. Dissociable aspects of performance on the 5-choice serial reaction time task following lesions of the dorsal anterior cingulate, infralimbic and orbitofrontal cortex in the rat: differential effects on selectivity, impulsivity and compulsivity. Behav Brain Res. 2003 doi: 10.1016/j.bbr.2003.09.020. [DOI] [PubMed] [Google Scholar]
- Ciccocioppo R, Sanna PP, Weiss F. Cocaine-predictive stimulus induces drug-seeking behavior and neural activation in limbic brain regions after multiple months of abstinence: reversal by D(1) antagonists. Proc Natl Acad Sci U S A. 2001;98:1976–81. doi: 10.1073/pnas.98.4.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conde F, et al. Afferent connections of the medial frontal cortex of the rat. II. Cortical and subcortical afferents. J Comp Neurol. 1995;352:567–93. doi: 10.1002/cne.903520407. [DOI] [PubMed] [Google Scholar]
- Corbett D. Cocaine enhances the reward value of medial prefrontal cortex self-stimulation. Neuroreport. 1991;2:805–8. doi: 10.1097/00001756-199112000-00019. [DOI] [PubMed] [Google Scholar]
- Corbit LH, Balleine BW. The role of prelimbic cortex in instrumental conditioning. Behav Brain Res. 2003;146:145–57. doi: 10.1016/j.bbr.2003.09.023. [DOI] [PubMed] [Google Scholar]
- Coutureau E, Killcross S. Inactivation of the infralimbic prefrontal cortex reinstates goal-directed responding in overtrained rats. Behav Brain Res. 2003;146:167–74. doi: 10.1016/j.bbr.2003.09.025. [DOI] [PubMed] [Google Scholar]
- Crombag HS, et al. Opposite effects of amphetamine self-administration experience on dendritic spines in the medial and orbital prefrontal cortex. Cereb Cortex. 2005;15:341–8. doi: 10.1093/cercor/bhh136. [DOI] [PubMed] [Google Scholar]
- Crombag HS, et al. Review. Context-induced relapse to drug seeking: a review. Philos Trans R Soc Lond B Biol Sci. 2008;363:3233–43. doi: 10.1098/rstb.2008.0090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunha PJ, et al. The frontal assessment battery (FAB) reveals neurocognitive dysfunction in substance-dependent individuals in distinct executive domains: Abstract reasoning, motor programming, and cognitive flexibility. Addict Behav. 2010;35:875–81. doi: 10.1016/j.addbeh.2010.05.005. [DOI] [PubMed] [Google Scholar]
- Dalley JW, Cardinal RN, Robbins TW. Prefrontal executive and cognitive functions in rodents: neural and neurochemical substrates. Neurosci Biobehav Rev. 2004;28:771–84. doi: 10.1016/j.neubiorev.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Dayas CV, et al. Distinct patterns of neural activation associated with ethanol seeking: effects of naltrexone. Biol Psychiatry. 2007;61:979–89. doi: 10.1016/j.biopsych.2006.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Bruin JP, et al. Role of the prefrontal cortex of the rat in learning and decision making: effects of transient inactivation. Prog Brain Res. 2000;126:103–13. doi: 10.1016/S0079-6123(00)26010-X. [DOI] [PubMed] [Google Scholar]
- Di Ciano P, et al. Role of the prelimbic cortex in the acquisition, re-acquisition or persistence of responding for a drug-paired conditioned reinforcer. Neuroscience. 2007;150:291–8. doi: 10.1016/j.neuroscience.2007.09.016. [DOI] [PubMed] [Google Scholar]
- Di Pietro NC, Black YD, Kantak KM. Context-dependent prefrontal cortex regulation of cocaine self-administration and reinstatement behaviors in rats. Eur J Neurosci. 2006;24:3285–98. doi: 10.1111/j.1460-9568.2006.05193.x. [DOI] [PubMed] [Google Scholar]
- Duvauchelle CL, Ettenberg A. Haloperidol attenuates conditioned place preferences produced by electrical stimulation of the medial prefrontal cortex. Pharmacol Biochem Behav. 1991;38:645–50. doi: 10.1016/0091-3057(91)90027-y. [DOI] [PubMed] [Google Scholar]
- Epstein DH, et al. Toward a model of drug relapse: an assessment of the validity of the reinstatement procedure. Psychopharmacology (Berl) 2006;189:1–16. doi: 10.1007/s00213-006-0529-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Euston DR, Gruber AJ, McNaughton BL. The role of medial prefrontal cortex in memory and decision making. Neuron. 2012;76:1057–70. doi: 10.1016/j.neuron.2012.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin TR, Druhan JP. Involvement of the nucleus accumbens and medial prefrontal cortex in the expression of conditioned hyperactivity to a cocaine-associated environment in rats. Neuropsychopharmacology. 2000a;23:633–44. doi: 10.1016/S0893-133X(00)00162-7. [DOI] [PubMed] [Google Scholar]
- Franklin TR, Druhan JP. Expression of Fos-related antigens in the nucleus accumbens and associated regions following exposure to a cocaine-paired environment. Eur J Neurosci. 2000b;12:2097–106. doi: 10.1046/j.1460-9568.2000.00071.x. [DOI] [PubMed] [Google Scholar]
- Fuchs RA, et al. The role of the dorsomedial prefrontal cortex, basolateral amygdala, and dorsal hippocampus in contextual reinstatement of cocaine seeking in rats. Neuropsychopharmacology. 2005;30:296–309. doi: 10.1038/sj.npp.1300579. [DOI] [PubMed] [Google Scholar]
- Fuchs RA, Branham RK, See RE. Different neural substrates mediate cocaine seeking after abstinence versus extinction training: a critical role for the dorsolateral caudate-putamen. J Neurosci. 2006;26:3584–8. doi: 10.1523/JNEUROSCI.5146-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fumagalli F, et al. Single session of cocaine intravenous self-administration shapes goal-oriented behaviours and up-regulates Arc mRNA levels in rat medial prefrontal cortex. Int J Neuropsychopharmacol. 2009;12:423–9. doi: 10.1017/S1461145708009681. [DOI] [PubMed] [Google Scholar]
- Garavan H, et al. Cue-induced cocaine craving: neuroanatomical specificity for drug users and drug stimuli. Am J Psychiatry. 2000;157:1789–98. doi: 10.1176/appi.ajp.157.11.1789. [DOI] [PubMed] [Google Scholar]
- Gass JT, Chandler LJ. The Plasticity of Extinction: Contribution of the Prefrontal Cortex in Treating Addiction through Inhibitory Learning. Front Psychiatry. 2013;4:46. doi: 10.3389/fpsyt.2013.00046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghazizadeh A, et al. Prefrontal cortex mediates extinction of responding by two distinct neural mechanisms in accumbens shell. J Neurosci. 2012;32:726–37. doi: 10.1523/JNEUROSCI.3891-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmartin MR, Balderston NL, Helmstetter FJ. Prefrontal cortical regulation of fear learning. Trends Neurosci. 2014;37:455–64. doi: 10.1016/j.tins.2014.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gipson CD, et al. Relapse induced by cues predicting cocaine depends on rapid, transient synaptic potentiation. Neuron. 2013;77:867–72. doi: 10.1016/j.neuron.2013.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goeders NE, Smith JE. Cortical dopaminergic involvement in cocaine reinforcement. Science. 1983;221:773–5. doi: 10.1126/science.6879176. [DOI] [PubMed] [Google Scholar]
- Goeders NE, Smith JE. Parameters of intracranial self-administration of cocaine into the medial prefrontal cortex. NIDA Res Monogr. 1984;55:132–7. [PubMed] [Google Scholar]
- Goeders NE, Dworkin SI, Smith JE. Neuropharmacological assessment of cocaine self-administration into the medial prefrontal cortex. Pharmacol Biochem Behav. 1986;24:1429–40. doi: 10.1016/0091-3057(86)90206-6. [DOI] [PubMed] [Google Scholar]
- Goeders NE, Smith JE. Reinforcing properties of cocaine in the medical prefrontal cortex: primary action on presynaptic dopaminergic terminals. Pharmacol Biochem Behav. 1986;25:191–9. doi: 10.1016/0091-3057(86)90252-2. [DOI] [PubMed] [Google Scholar]
- Goeders NE, Smith JE. Intracranial cocaine self-administration into the medial prefrontal cortex increases dopamine turnover in the nucleus accumbens. J Pharmacol Exp Ther. 1993;265:592–600. [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND. Dysfunction of the prefrontal cortex in addiction: neuroimaging findings and clinical implications. Nat Rev Neurosci. 2011;12:652–69. doi: 10.1038/nrn3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm JW, et al. Neuroadaptation. Incubation of cocaine craving after withdrawal. Nature. 2001;412:141–2. doi: 10.1038/35084134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groenewegen HJ, Wright CI, Uylings HB. The anatomical relationships of the prefrontal cortex with limbic structures and the basal ganglia. J Psychopharmacol. 1997;11:99–106. doi: 10.1177/026988119701100202. [DOI] [PubMed] [Google Scholar]
- Grusser SM, et al. Cue-induced activation of the striatum and medial prefrontal cortex is associated with subsequent relapse in abstinent alcoholics. Psychopharmacology (Berl) 2004;175:296–302. doi: 10.1007/s00213-004-1828-4. [DOI] [PubMed] [Google Scholar]
- Haddon JE, Killcross S. Contextual control of choice performance: behavioral, neurobiological, and neurochemical influences. Ann N Y Acad Sci. 2007;1104:250–69. doi: 10.1196/annals.1390.000. [DOI] [PubMed] [Google Scholar]
- Hamlin AS, Clemens KJ, McNally GP. Renewal of extinguished cocaine-seeking. Neuroscience. 2008;151:659–70. doi: 10.1016/j.neuroscience.2007.11.018. [DOI] [PubMed] [Google Scholar]
- Hayton SJ, et al. Target-specific encoding of response inhibition: increased contribution of AMPA to NMDA receptors at excitatory synapses in the prefrontal cortex. J Neurosci. 2010;30:11493–500. doi: 10.1523/JNEUROSCI.1550-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayton SJ, Olmstead MC, Dumont EC. Shift in the intrinsic excitability of medial prefrontal cortex neurons following training in impulse control and cued-responding tasks. PLoS One. 2011;6:e23885. doi: 10.1371/journal.pone.0023885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He YY, et al. PKMzeta maintains drug reward and aversion memory in the basolateral amygdala and extinction memory in the infralimbic cortex. Neuropsychopharmacology. 2011;36:1972–81. doi: 10.1038/npp.2011.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hearing MC, et al. Relapse to cocaine seeking increases activity-regulated gene expression differentially in the prefrontal cortex of abstinent rats. Psychopharmacology (Berl) 2008;198:77–91. doi: 10.1007/s00213-008-1090-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidbreder CA, Groenewegen HJ. The medial prefrontal cortex in the rat: evidence for a dorso-ventral distinction based upon functional and anatomical characteristics. Neuroscience & Biobehavioral Reviews. 2003;27:555–579. doi: 10.1016/j.neubiorev.2003.09.003. [DOI] [PubMed] [Google Scholar]
- Heinz A, et al. Correlation between dopamine D(2) receptors in the ventral striatum and central processing of alcohol cues and craving. Am J Psychiatry. 2004;161:1783–9. doi: 10.1176/appi.ajp.161.10.1783. [DOI] [PubMed] [Google Scholar]
- Heinz A, et al. Brain activation elicited by affectively positive stimuli is associated with a lower risk of relapse in detoxified alcoholic subjects. Alcohol Clin Exp Res. 2007;31:1138–47. doi: 10.1111/j.1530-0277.2007.00406.x. [DOI] [PubMed] [Google Scholar]
- Hiranita T, et al. Suppression of methamphetamine-seeking behavior by nicotinic agonists. Proc Natl Acad Sci U S A. 2006;103:8523–7. doi: 10.1073/pnas.0600347103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoover WB, Vertes RP. Anatomical analysis of afferent projections to the medial prefrontal cortex in the rat. Brain Struct Funct. 2007;212:149–79. doi: 10.1007/s00429-007-0150-4. [DOI] [PubMed] [Google Scholar]
- Hurley KM, et al. Efferent projections of the infralimbic cortex of the rat. J Comp Neurol. 1991;308:249–76. doi: 10.1002/cne.903080210. [DOI] [PubMed] [Google Scholar]
- Ishikawa A, et al. Contributions of the amygdala and medial prefrontal cortex to incentive cue responding. Neuroscience. 2008a;155:573–84. doi: 10.1016/j.neuroscience.2008.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa A, et al. Dorsomedial prefrontal cortex contribution to behavioral and nucleus accumbens neuronal responses to incentive cues. J Neurosci. 2008b;28:5088–98. doi: 10.1523/JNEUROSCI.0253-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonkman S, et al. The rat prelimbic cortex mediates inhibitory response control but not the consolidation of instrumental learning. Behav Neurosci. 2009;123:875–85. doi: 10.1037/a0016330. [DOI] [PubMed] [Google Scholar]
- Kalivas PW. Addiction as a pathology in prefrontal cortical regulation of corticostriatal habit circuitry. Neurotox Res. 2008;14:185–9. doi: 10.1007/BF03033809. [DOI] [PubMed] [Google Scholar]
- Kalivas PW. The glutamate homeostasis hypothesis of addiction. Nat Rev Neurosci. 2009;10:561–72. doi: 10.1038/nrn2515. [DOI] [PubMed] [Google Scholar]
- Kesner RP, Churchwell JC. An analysis of rat prefrontal cortex in mediating executive function. Neurobiol Learn Mem. 2011;96:417–31. doi: 10.1016/j.nlm.2011.07.002. [DOI] [PubMed] [Google Scholar]
- Killcross S, Coutureau E. Coordination of actions and habits in the medial prefrontal cortex of rats. Cereb Cortex. 2003;13:400–8. doi: 10.1093/cercor/13.4.400. [DOI] [PubMed] [Google Scholar]
- Kilts CD, et al. The neural correlates of cue-induced craving in cocaine-dependent women. Am J Psychiatry. 2004;161:233–41. doi: 10.1176/appi.ajp.161.2.233. [DOI] [PubMed] [Google Scholar]
- Kober H, et al. Regulation of craving by cognitive strategies in cigarette smokers. Drug Alcohol Depend. 2010a;106:52–5. doi: 10.1016/j.drugalcdep.2009.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kober H, et al. Prefrontal-striatal pathway underlies cognitive regulation of craving. Proc Natl Acad Sci U S A. 2010b;107:14811–6. doi: 10.1073/pnas.1007779107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolb B. Functions of the frontal cortex of the rat: a comparative review. Brain Res. 1984;320:65–98. doi: 10.1016/0165-0173(84)90018-3. [DOI] [PubMed] [Google Scholar]
- Koya E, et al. Enhanced cortical and accumbal molecular reactivity associated with conditioned heroin, but not sucrose-seeking behaviour. J Neurochem. 2006;98:905–15. doi: 10.1111/j.1471-4159.2006.03917.x. [DOI] [PubMed] [Google Scholar]
- Koya E, et al. Role of ventral medial prefrontal cortex in incubation of cocaine craving. Neuropharmacology. 2009;56(Suppl 1):177–85. doi: 10.1016/j.neuropharm.2008.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kufahl P, et al. Expectation modulates human brain responses to acute cocaine: a functional magnetic resonance imaging study. Biol Psychiatry. 2008;63:222–30. doi: 10.1016/j.biopsych.2007.03.021. [DOI] [PubMed] [Google Scholar]
- Kufahl PR, et al. Neural responses to acute cocaine administration in the human brain detected by fMRI. Neuroimage. 2005;28:904–14. doi: 10.1016/j.neuroimage.2005.06.039. [DOI] [PubMed] [Google Scholar]
- Kuhn S, Gallinat J. Common biology of craving across legal and illegal drugs - a quantitative meta-analysis of cue-reactivity brain response. Eur J Neurosci. 2011;33:1318–26. doi: 10.1111/j.1460-9568.2010.07590.x. [DOI] [PubMed] [Google Scholar]
- Kvitsiani D, et al. Distinct behavioural and network correlates of two interneuron types in prefrontal cortex. Nature. 2013 doi: 10.1038/nature12176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaLumiere RT, Kalivas PW. Glutamate release in the nucleus accumbens core is necessary for heroin seeking. J Neurosci. 2008;28:3170–7. doi: 10.1523/JNEUROSCI.5129-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaLumiere RT, Niehoff KE, Kalivas PW. The infralimbic cortex regulates the consolidation of extinction after cocaine self-administration. Learn Mem. 2010;17:168–75. doi: 10.1101/lm.1576810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaLumiere RT, Smith KC, Kalivas PW. Neural circuit competition in cocaine-seeking: roles of the infralimbic cortex and nucleus accumbens shell. Eur J Neurosci. 2012;35:614–22. doi: 10.1111/j.1460-9568.2012.07991.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasseter HC, et al. Prefrontal cortical regulation of drug seeking in animal models of drug relapse. Curr Top Behav Neurosci. 2010;3:101–17. doi: 10.1007/7854_2009_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, et al. Craving correlates with mesolimbic responses to heroin-related cues in short-term abstinence from heroin: an event-related fMRI study. Brain Res. 2012;1469:63–72. doi: 10.1016/j.brainres.2012.06.024. [DOI] [PubMed] [Google Scholar]
- Li Q, et al. Assessing Cue-Induced Brain Response as a Function of Abstinence Duration in Heroin-Dependent Individuals: An Event-Related fMRI Study. PLoS ONE. 2013;8:e62911. doi: 10.1371/journal.pone.0062911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, et al. Predicting subsequent relapse by drug-related cue-induced brain activation in heroin addiction: an event-related functional magnetic resonance imaging study. Addict Biol. 2014 doi: 10.1111/adb.12182. [DOI] [PubMed] [Google Scholar]
- Limpens JH, et al. Using conditioned suppression to investigate compulsive drug seeking in rats. Drug Alcohol Depend. 2014;142:314–24. doi: 10.1016/j.drugalcdep.2014.06.037. [DOI] [PubMed] [Google Scholar]
- Lucantonio F, et al. The impact of orbitofrontal dysfunction on cocaine addiction. Nat Neurosci. 2012;15:358–66. doi: 10.1038/nn.3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma YY, et al. Bidirectional modulation of incubation of cocaine craving by silent synapse-based remodeling of prefrontal cortex to accumbens projections. Neuron. 2014;83:1453–67. doi: 10.1016/j.neuron.2014.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahler SV, Aston-Jones GS. Fos activation of selective afferents to ventral tegmental area during cue-induced reinstatement of cocaine seeking in rats. J Neurosci. 2012;32:13309–26. doi: 10.1523/JNEUROSCI.2277-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahler SV, et al. Multiple roles for orexin/hypocretin in addiction. Prog Brain Res. 2012;198:79–121. doi: 10.1016/B978-0-444-59489-1.00007-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchant NJ, Hamlin AS, McNally GP. Lateral hypothalamus is required for context-induced reinstatement of extinguished reward seeking. J Neurosci. 2009;29:1331–42. doi: 10.1523/JNEUROSCI.5194-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchant NJ, Furlong TM, McNally GP. Medial dorsal hypothalamus mediates the inhibition of reward seeking after extinction. J Neurosci. 2010;30:14102–15. doi: 10.1523/JNEUROSCI.4079-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchant NJ, Li X, Shaham Y. Recent developments in animal models of drug relapse. Curr Opin Neurobiol. 2013;23:675–83. doi: 10.1016/j.conb.2013.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S, Quirk GJ. Neuronal signalling of fear memory. Nat Rev Neurosci. 2004;5:844–52. doi: 10.1038/nrn1535. [DOI] [PubMed] [Google Scholar]
- Marhe R, et al. Individual differences in anterior cingulate activation associated with attentional bias predict cocaine use after treatment. Neuropsychopharmacology. 2013;38:1085–93. doi: 10.1038/npp.2013.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Fardon R, Weiss F. Modeling relapse in animals. Curr Top Behav Neurosci. 2013;13:403–32. doi: 10.1007/7854_2012_202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Garcia E, et al. Frequency of Cocaine Self-Administration Influences Drug Seeking in the Rat: Optogenetic Evidence for a Role of the Prelimbic Cortex. Neuropsychopharmacology. 2014 doi: 10.1038/npp.2014.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mashhoon Y, Wells AM, Kantak KM. Interaction of the rostral basolateral amygdala and prelimbic prefrontal cortex in regulating reinstatement of cocaine-seeking behavior. Pharmacol Biochem Behav. 2010;96:347–53. doi: 10.1016/j.pbb.2010.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland K, Kalivas PW. The circuitry mediating cocaine-induced reinstatement of drug-seeking behavior. J Neurosci. 2001;21:8655–63. doi: 10.1523/JNEUROSCI.21-21-08655.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland K, Lapish CC, Kalivas PW. Prefrontal glutamate release into the core of the nucleus accumbens mediates cocaine-induced reinstatement of drug-seeking behavior. J Neurosci. 2003;23:3531–7. doi: 10.1523/JNEUROSCI.23-08-03531.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland K, et al. Limbic and motor circuitry underlying footshock-induced reinstatement of cocaine-seeking behavior. J Neurosci. 2004;24:1551–60. doi: 10.1523/JNEUROSCI.4177-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGlinchey EM, Mahler SV, Aston-Jones G. Specific activation of prelimbic cortical neurons that project to nucleus accumbens core during conditioned cocaine seeking In Preparation. [Google Scholar]
- McGregor IS, Atrens DM, Jackson DM. Cocaine facilitation of prefrontal cortex self-stimulation: a microstructural and pharmacological analysis. Psychopharmacology (Berl) 1992;106:239–47. doi: 10.1007/BF02801979. [DOI] [PubMed] [Google Scholar]
- McLaughlin J, See RE. Selective inactivation of the dorsomedial prefrontal cortex and the basolateral amygdala attenuates conditioned-cued reinstatement of extinguished cocaine-seeking behavior in rats. Psychopharmacology (Berl) 2003;168:57–65. doi: 10.1007/s00213-002-1196-x. [DOI] [PubMed] [Google Scholar]
- Mihindou C, et al. Discriminative inhibitory control of cocaine seeking involves the prelimbic prefrontal cortex. Biol Psychiatry. 2013;73:271–9. doi: 10.1016/j.biopsych.2012.08.011. [DOI] [PubMed] [Google Scholar]
- Milad MR, Quirk GJ. Neurons in medial prefrontal cortex signal memory for fear extinction. Nature. 2002;420:70–4. doi: 10.1038/nature01138. [DOI] [PubMed] [Google Scholar]
- Miller CA, Marshall JF. Altered prelimbic cortex output during cue-elicited drug seeking. J Neurosci. 2004;24:6889–97. doi: 10.1523/JNEUROSCI.1685-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Moeller SJ, Goldstein RZ. Impaired self-awareness in human addiction: deficient attribution of personal relevance. Trends Cogn Sci. 2014 doi: 10.1016/j.tics.2014.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moody CA, Frank RA. Cocaine facilitates prefrontal cortex self-stimulation. Pharmacol Biochem Behav. 1990;35:743–6. doi: 10.1016/0091-3057(90)90318-c. [DOI] [PubMed] [Google Scholar]
- Moorman DE, Aston-Jones G. Neurons in rat medial prefrontal cortex encode context-appropriate rule execution for reward-seeking and extinction. In Preparation-a. [Google Scholar]
- Moorman DE, Aston-Jones G. Signaling by dorsal and ventral medial prefrontal neurons during cocaine seeking and extinction. In Preparation-b. [Google Scholar]
- Mora F, Cobo M. The neurobiological basis of prefrontal cortex self-stimulation: a review and an integrative hypothesis. Prog Brain Res. 1990;85:419–31. doi: 10.1016/s0079-6123(08)62693-x. [DOI] [PubMed] [Google Scholar]
- Morgan MA, LeDoux JE. Differential contribution of dorsal and ventral medial prefrontal cortex to the acquisition and extinction of conditioned fear in rats. Behav Neurosci. 1995;109:681–8. doi: 10.1037//0735-7044.109.4.681. [DOI] [PubMed] [Google Scholar]
- Murphy ER, Dalley JW, Robbins TW. Local glutamate receptor antagonism in the rat prefrontal cortex disrupts response inhibition in a visuospatial attentional task. Psychopharmacology (Berl) 2005;179:99–107. doi: 10.1007/s00213-004-2068-3. [DOI] [PubMed] [Google Scholar]
- Murphy ER, et al. Impulsive behaviour induced by both NMDA receptor antagonism and GABAA receptor activation in rat ventromedial prefrontal cortex. Psychopharmacology (Berl) 2012 Jan;219(2):401–10. doi: 10.1007/s00213-011-2572-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayanan NS, Horst NK, Laubach M. Reversible inactivations of rat medial prefrontal cortex impair the ability to wait for a stimulus. Neuroscience. 2006;139:865–76. doi: 10.1016/j.neuroscience.2005.11.072. [DOI] [PubMed] [Google Scholar]
- Narayanan NS, Laubach M. Top-down control of motor cortex ensembles by dorsomedial prefrontal cortex. Neuron. 2006;52:921–31. doi: 10.1016/j.neuron.2006.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayanan NS, Laubach M. Delay activity in rodent frontal cortex during a simple reaction time task. J Neurophysiol. 2009;101:2859–71. doi: 10.1152/jn.90615.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navailles S, et al. Coordinated Recruitment of Cortical-Subcortical Circuits and Ascending Dopamine and Serotonin Neurons During Inhibitory Control of Cocaine Seeking in Rats. Cereb Cortex. 2014 doi: 10.1093/cercor/bhu112. [DOI] [PubMed] [Google Scholar]
- Neisewander JL, et al. Fos protein expression and cocaine-seeking behavior in rats after exposure to a cocaine self-administration environment. J Neurosci. 2000;20:798–805. doi: 10.1523/JNEUROSCI.20-02-00798.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nic Dhonnchadha BA, et al. Changes in expression of c-Fos protein following cocaine-cue extinction learning. Behav Brain Res. 2012;234:100–6. doi: 10.1016/j.bbr.2012.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieuwenhuis IL, Takashima A. The role of the ventromedial prefrontal cortex in memory consolidation. Behav Brain Res. 2011;218:325–34. doi: 10.1016/j.bbr.2010.12.009. [DOI] [PubMed] [Google Scholar]
- Orsini CA, et al. Hippocampal and prefrontal projections to the basal amygdala mediate contextual regulation of fear after extinction. J Neurosci. 2011;31:17269–77. doi: 10.1523/JNEUROSCI.4095-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ovari J, Leri F. Inactivation of the ventromedial prefrontal cortex mimics re-emergence of heroin seeking caused by heroin reconditioning. Neurosci Lett. 2008;444:52–5. doi: 10.1016/j.neulet.2008.08.015. [DOI] [PubMed] [Google Scholar]
- Park WK, et al. Cocaine administered into the medial prefrontal cortex reinstates cocaine-seeking behavior by increasing AMPA receptor-mediated glutamate transmission in the nucleus accumbens. J Neurosci. 2002;22:2916–25. doi: 10.1523/JNEUROSCI.22-07-02916.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain, in stereotaxic coordinates. Academic Press; San Diego: 1997. [DOI] [PubMed] [Google Scholar]
- Pelloux Y, Murray JE, Everitt BJ. Differential roles of the prefrontal cortical subregions and basolateral amygdala in compulsive cocaine seeking and relapse after voluntary abstinence in rats. Eur J Neurosci. 2013 doi: 10.1111/ejn.12289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pentkowski NS, et al. Stimulation of medial prefrontal cortex serotonin 2C (5-HT(2C)) receptors attenuates cocaine-seeking behavior. Neuropsychopharmacology. 2010;35:2037–48. doi: 10.1038/npp.2010.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters J, LaLumiere RT, Kalivas PW. Infralimbic prefrontal cortex is responsible for inhibiting cocaine seeking in extinguished rats. J Neurosci. 2008a;28:6046–53. doi: 10.1523/JNEUROSCI.1045-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters J, et al. Opposing roles for the ventral prefrontal cortex and the basolateral amygdala on the spontaneous recovery of cocaine-seeking in rats. Psychopharmacology (Berl) 2008b;197:319–26. doi: 10.1007/s00213-007-1034-2. [DOI] [PubMed] [Google Scholar]
- Peters J, Kalivas PW, Quirk GJ. Extinction circuits for fear and addiction overlap in prefrontal cortex. Learn Mem. 2009;16:279–88. doi: 10.1101/lm.1041309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters J, Pattij T, De Vries TJ. Targeting cocaine versus heroin memories: divergent roles within ventromedial prefrontal cortex. Trends Pharmacol Sci. 2013;34:689–95. doi: 10.1016/j.tips.2013.10.004. [DOI] [PubMed] [Google Scholar]
- Phillips AG, Fibiger HC. The role of dopamine in maintaining intracranial self-stimulation in the ventral tegmentum, nucleus accumbens, and medial prefrontal cortex. Can J Psychol. 1978;32:58–66. doi: 10.1037/h0081676. [DOI] [PubMed] [Google Scholar]
- Pi HJ, et al. Cortical interneurons that specialize in disinhibitory control. Nature. 2013;503:521–4. doi: 10.1038/nature12676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce RC, et al. Ibotenic acid lesions of the dorsal prefrontal cortex disrupt the expression of behavioral sensitization to cocaine. Neuroscience. 1998;82:1103–14. doi: 10.1016/s0306-4522(97)00366-7. [DOI] [PubMed] [Google Scholar]
- Quirk GJ, Mueller D. Neural mechanisms of extinction learning and retrieval. Neuropsychopharmacology. 2008;33:56–72. doi: 10.1038/sj.npp.1301555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebec GV, Sun W. Neuronal substrates of relapse to cocaine-seeking behavior: role of prefrontal cortex. J Exp Anal Behav. 2005;84:653–66. doi: 10.1901/jeab.2005.105-04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes SE, Killcross S. Lesions of rat infralimbic cortex enhance recovery and reinstatement of an appetitive Pavlovian response. Learn Mem. 2004;11:611–6. doi: 10.1101/lm.79704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes SE, Killcross AS. Lesions of rat infralimbic cortex enhance renewal of extinguished appetitive Pavlovian responding. Eur J Neurosci. 2007;25:2498–503. doi: 10.1111/j.1460-9568.2007.05486.x. [DOI] [PubMed] [Google Scholar]
- Risinger RC, et al. Neural correlates of high and craving during cocaine self-administration using BOLD fMRI. NeuroImage. 2005;26:1097–1108. doi: 10.1016/j.neuroimage.2005.03.030. [DOI] [PubMed] [Google Scholar]
- Risterucci C, et al. Excitotoxic lesions of the prelimbic-infralimbic areas of the rodent prefrontal cortex disrupt motor preparatory processes. Eur J Neurosci. 2003;17:1498–508. doi: 10.1046/j.1460-9568.2003.02541.x. [DOI] [PubMed] [Google Scholar]
- Robertson A, Laferriere A. Disruption of the connections between the mediodorsal and sulcal prefrontal cortices alters the associability of rewarding medial cortical stimulation to place and taste stimuli in rats. Behav Neurosci. 1989;103:770–8. doi: 10.1037//0735-7044.103.4.770. [DOI] [PubMed] [Google Scholar]
- Robinson TE, et al. Cocaine self-administration alters the morphology of dendrites and dendritic spines in the nucleus accumbens and neocortex. Synapse. 2001;39:257–66. doi: 10.1002/1098-2396(20010301)39:3<257::AID-SYN1007>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Rocha A, Kalivas PW. Role of the prefrontal cortex and nucleus accumbens in reinstating methamphetamine seeking. Eur J Neurosci. 2010;31:903–9. doi: 10.1111/j.1460-9568.2010.07134.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers JL, Ghee S, See RE. The neural circuitry underlying reinstatement of heroin-seeking behavior in an animal model of relapse. Neuroscience. 2008;151:579–88. doi: 10.1016/j.neuroscience.2007.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Routtenberg A, Sloan M. Self-stimulation in the frontal cortex of Rattus norvegicus. Behav Biol. 1972;7:567–72. doi: 10.1016/s0091-6773(72)80218-9. [DOI] [PubMed] [Google Scholar]
- Sanchez CJ, et al. Manipulation of dopamine d1-like receptor activation in the rat medial prefrontal cortex alters stress- and cocaine-induced reinstatement of conditioned place preference behavior. Neuroscience. 2003;119:497–505. doi: 10.1016/s0306-4522(03)00078-2. [DOI] [PubMed] [Google Scholar]
- Sangha S, et al. Alterations in reward, fear and safety cue discrimination after inactivation of the rat prelimbic and infralimbic cortices. Neuropsychopharmacology. 2014;39:2405–13. doi: 10.1038/npp.2014.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt ED, et al. Differential involvement of the prelimbic cortex and striatum in conditioned heroin and sucrose seeking following long-term extinction. Eur J Neurosci. 2005;22:2347–56. doi: 10.1111/j.1460-9568.2005.04435.x. [DOI] [PubMed] [Google Scholar]
- Schoenbaum G, Shaham Y. The role of orbitofrontal cortex in drug addiction: a review of preclinical studies. Biol Psychiatry. 2008;63:256–62. doi: 10.1016/j.biopsych.2007.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder BE, et al. Morphine-associated environmental cues elicit conditioned gene expression. Synapse. 2000;37:146–58. doi: 10.1002/1098-2396(200008)37:2<146::AID-SYN8>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Schroeder BE, Binzak JM, Kelley AE. A common profile of prefrontal cortical activation following exposure to nicotine- or chocolate-associated contextual cues. Neuroscience. 2001;105:535–45. doi: 10.1016/s0306-4522(01)00221-4. [DOI] [PubMed] [Google Scholar]
- Seif T, et al. Cortical activation of accumbens hyperpolarization-active NMDARs mediates aversion-resistant alcohol intake. Nat Neurosci. 2013;16:1094–100. doi: 10.1038/nn.3445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sesack SR, et al. Topographical organization of the efferent projections of the medial prefrontal cortex in the rat: an anterograde tract-tracing study with Phaseolus vulgaris leucoagglutinin. J Comp Neurol. 1989;290:213–42. doi: 10.1002/cne.902900205. [DOI] [PubMed] [Google Scholar]
- Shalev U, et al. Selective induction of c-Fos immunoreactivity in the prelimbic cortex during reinstatement of heroin seeking induced by acute food deprivation in rats. Behav Brain Res. 2003;145:79–88. doi: 10.1016/s0166-4328(03)00103-7. [DOI] [PubMed] [Google Scholar]
- Sierra-Mercado D, Padilla-Coreano N, Quirk GJ. Dissociable roles of prelimbic and infralimbic cortices, ventral hippocampus, and basolateral amygdala in the expression and extinction of conditioned fear. Neuropsychopharmacology. 2011;36:529–38. doi: 10.1038/npp.2010.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KS, et al. Reversible online control of habitual behavior by optogenetic perturbation of medial prefrontal cortex. Proc Natl Acad Sci U S A. 2012;109:18932–7. doi: 10.1073/pnas.1216264109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KS, Graybiel AM. A Dual Operator View of Habitual Behavior Reflecting Cortical and Striatal Dynamics. Neuron. 2013 doi: 10.1016/j.neuron.2013.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotres-Bayon F, Quirk GJ. Prefrontal control of fear: more than just extinction. Current Opinion in Neurobiology. 2010a;20:231–235. doi: 10.1016/j.conb.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotres-Bayon F, Quirk GJ. Prefrontal control of fear: more than just extinction. Curr Opin Neurobiol. 2010b;20:231–5. doi: 10.1016/j.conb.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparta DR, et al. Activation of prefrontal cortical parvalbumin interneurons facilitates extinction of reward-seeking behavior. J Neurosci. 2014;34:3699–705. doi: 10.1523/JNEUROSCI.0235-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Onge JR, Floresco SB. Prefrontal cortical contribution to risk-based decision making. Cereb Cortex. 2010;20:1816–28. doi: 10.1093/cercor/bhp250. [DOI] [PubMed] [Google Scholar]
- Stefanik MT, et al. Optogenetic inhibition of cocaine seeking in rats. Addict Biol. 2013;18:50–3. doi: 10.1111/j.1369-1600.2012.00479.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh JJ, et al. Low prefrontal perfusion linked to depression symptoms in methadone-maintained opiate-dependent patients. Drug Alcohol Depend. 2009;99:11–7. doi: 10.1016/j.drugalcdep.2008.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sul JH, et al. Distinct roles of rodent orbitofrontal and medial prefrontal cortex in decision making. Neuron. 2010;66:449–60. doi: 10.1016/j.neuron.2010.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun W, Rebec GV. The role of prefrontal cortex D1-like and D2-like receptors in cocaine-seeking behavior in rats. Psychopharmacology (Berl) 2005;177:315–23. doi: 10.1007/s00213-004-1956-x. [DOI] [PubMed] [Google Scholar]
- Sun W, Rebec GV. Repeated cocaine self-administration alters processing of cocaine-related information in rat prefrontal cortex. J Neurosci. 2006;26:8004–8. doi: 10.1523/JNEUROSCI.1413-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson LW. The projections of the ventral tegmental area and adjacent regions: a combined fluorescent retrograde tracer and immunofluorescence study in the rat. Brain Res Bull. 1982;9:321–53. doi: 10.1016/0361-9230(82)90145-9. [DOI] [PubMed] [Google Scholar]
- Thomas KL, Everitt BJ. Limbic-cortical-ventral striatal activation during retrieval of a discrete cocaine-associated stimulus: a cellular imaging study with gamma protein kinase C expression. J Neurosci. 2001;21:2526–35. doi: 10.1523/JNEUROSCI.21-07-02526.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas KL, Arroyo M, Everitt BJ. Induction of the learning and plasticity-associated gene Zif268 following exposure to a discrete cocaine-associated stimulus. Eur J Neurosci. 2003;17:1964–72. doi: 10.1046/j.1460-9568.2003.02617.x. [DOI] [PubMed] [Google Scholar]
- Tzschentke TM, Schmidt WJ. The development of cocaine-induced behavioral sensitization is affected by discrete quinolinic acid lesions of the prelimbic medial prefrontal cortex. Brain Research. 1998;795:71–76. doi: 10.1016/s0006-8993(98)00254-6. [DOI] [PubMed] [Google Scholar]
- Tzschentke TM, Schmidt WJ. Functional relationship among medial prefrontal cortex, nucleus accumbens, and ventral tegmental area in locomotion and reward. Crit Rev Neurobiol. 2000;14:131–42. [PubMed] [Google Scholar]
- Uylings HBM, Groenewegen HJ, Kolb B. Do rats have a prefrontal cortex? Behavioural Brain Research. 2003;146:3–17. doi: 10.1016/j.bbr.2003.09.028. [DOI] [PubMed] [Google Scholar]
- Van den Oever MC, et al. Prefrontal cortex AMPA receptor plasticity is crucial for cue-induced relapse to heroin-seeking. Nat Neurosci. 2008;11:1053–8. doi: 10.1038/nn.2165. [DOI] [PubMed] [Google Scholar]
- Van den Oever MC, et al. Prefrontal cortex plasticity mechanisms in drug seeking and relapse. Neurosci Biobehav Rev. 2010;35:276–84. doi: 10.1016/j.neubiorev.2009.11.016. [DOI] [PubMed] [Google Scholar]
- Van den Oever MC, et al. Ventromedial prefrontal cortex pyramidal cells have a temporal dynamic role in recall and extinction of cocaine-associated memory. J Neurosci. 2013;33:18225–33. doi: 10.1523/JNEUROSCI.2412-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Eden CG, Buijs RM. Functional neuroanatomy of the prefrontal cortex: autonomic interactions. Prog Brain Res. 2000;126:49–62. doi: 10.1016/S0079-6123(00)26006-8. [DOI] [PubMed] [Google Scholar]
- Vassoler FM, et al. Deep brain stimulation of the nucleus accumbens shell attenuates cocaine reinstatement through local and antidromic activation. J Neurosci. 2013;33:14446–54. doi: 10.1523/JNEUROSCI.4804-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vertes RP. Differential projections of the infralimbic and prelimbic cortex in the rat. Synapse. 2004;51:32–58. doi: 10.1002/syn.10279. [DOI] [PubMed] [Google Scholar]
- Vertes RP. Interactions among the medial prefrontal cortex, hippocampus and midline thalamus in emotional and cognitive processing in the rat. Neuroscience. 2006;142:1–20. doi: 10.1016/j.neuroscience.2006.06.027. [DOI] [PubMed] [Google Scholar]
- Vidal-Gonzalez I, et al. Microstimulation reveals opposing influences of prelimbic and infralimbic cortex on the expression of conditioned fear. Learn Mem. 2006;13:728–33. doi: 10.1101/lm.306106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, et al. Activation of orbital and medial prefrontal cortex by methylphenidate in cocaine-addicted subjects but not in controls: relevance to addiction. J Neurosci. 2005;25:3932–9. doi: 10.1523/JNEUROSCI.0433-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, et al. Cognitive control of drug craving inhibits brain reward regions in cocaine abusers. Neuroimage. 2010;49:2536–43. doi: 10.1016/j.neuroimage.2009.10.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warden MR, et al. A prefrontal cortex-brainstem neuronal projection that controls response to behavioural challenge. Nature. 2012;492:428–32. doi: 10.1038/nature11617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissenborn R, Robbins TW, Everitt BJ. Effects of medial prefrontal or anterior cingulate cortex lesions on responding for cocaine under fixed-ratio and second-order schedules of reinforcement in rats. Psychopharmacology (Berl) 1997;134:242–57. doi: 10.1007/s002130050447. [DOI] [PubMed] [Google Scholar]
- West EA, et al. Prelimbic and infralimbic cortical regions differentially encode cocaine-associated stimuli and cocaine-seeking before and following abstinence. Eur J Neurosci. 2014;39:1891–902. doi: 10.1111/ejn.12578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wexler BE, et al. Functional magnetic resonance imaging of cocaine craving. Am J Psychiatry. 2001;158:86–95. doi: 10.1176/appi.ajp.158.1.86. [DOI] [PubMed] [Google Scholar]
- Whitfield TW, Jr, et al. The suppressive effect of an intra-prefrontal cortical infusion of BDNF on cocaine-seeking is Trk receptor and extracellular signal-regulated protein kinase mitogen-activated protein kinase dependent. J Neurosci. 2011;31:834–42. doi: 10.1523/JNEUROSCI.4986-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willcocks AL, McNally GP. The role of medial prefrontal cortex in extinction and reinstatement of alcohol-seeking in rats. Eur J Neurosci. 2013;37:259–68. doi: 10.1111/ejn.12031. [DOI] [PubMed] [Google Scholar]
- Wise SP. Forward frontal fields: phylogeny and fundamental function. Trends Neurosci. 2008;31:599–608. doi: 10.1016/j.tins.2008.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao Z, et al. Thirsty heroin addicts show different fMRI activations when exposed to water-related and drug-related cues. Drug and Alcohol Dependence. 2006;83:157–162. doi: 10.1016/j.drugalcdep.2005.11.012. [DOI] [PubMed] [Google Scholar]
- Yalachkov Y, Kaiser J, Naumer MJ. Brain regions related to tool use and action knowledge reflect nicotine dependence. J Neurosci. 2009;29:4922–9. doi: 10.1523/JNEUROSCI.4891-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zavala AR, et al. Role of the prelimbic subregion of the medial prefrontal cortex in acquisition, extinction, and reinstatement of cocaine-conditioned place preference. Brain Res. 2003;990:157–64. doi: 10.1016/s0006-8993(03)03452-8. [DOI] [PubMed] [Google Scholar]
- Zavala AR, et al. Fos and glutamate AMPA receptor subunit coexpression associated with cue-elicited cocaine-seeking behavior in abstinent rats. Neuroscience. 2007;145:438–52. doi: 10.1016/j.neuroscience.2006.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zavala AR, et al. Upregulation of Arc mRNA expression in the prefrontal cortex following cue-induced reinstatement of extinguished cocaine-seeking behavior. Synapse. 2008;62:421–31. doi: 10.1002/syn.20502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, et al. Fos expression induced by cocaine-conditioned cues in male and female rats. Brain Struct Funct. 2014;219:1831–40. doi: 10.1007/s00429-013-0605-8. [DOI] [PMC free article] [PubMed] [Google Scholar]