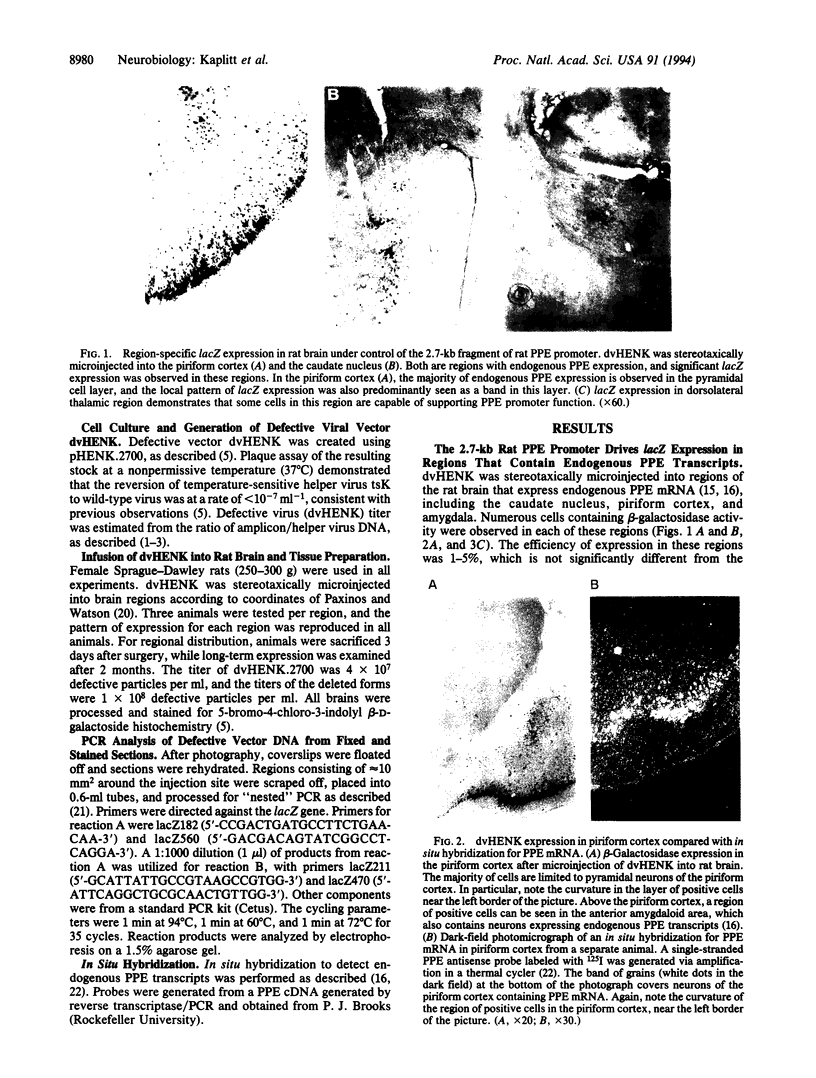
Abstract
We have previously used a defective herpes simplex virus vector to express a foreign gene in the adult rat brain. One application of this technology would be the in vivo analysis of promoter function in brain after de novo transfer, which would allow the rapid generation of vectors with localized application in a broad range of mammalian species while avoiding influences of other nearby promoters. A 2.7-kb fragment of the rat preproenkephalin promoter was placed upstream of the bacterial lacZ gene in our herpes simplex virus amplicon. A restricted pattern of lacZ expression was observed in vivo, which follows previously observed patterns of endogenous preproenkephalin expression. These results, from the direct gene transfer into an adult animal brain for in vivo promoter analysis, demonstrate that sequence information that influences restricted expression of preproenkephalin is located within 2.7 kb upstream of transcriptional initiation. lacZ expression was also observed in rat brain for 2 months after direct transfer, and PCR analysis confirmed the continued presence of amplicon DNA in lacZ-positive sections. Restricted and long-term expression observed with an endogenous promoter has important implications for gene therapy using viral vectors.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alam J., Cook J. L. Reporter genes: application to the study of mammalian gene transcription. Anal Biochem. 1990 Aug 1;188(2):245–254. doi: 10.1016/0003-2697(90)90601-5. [DOI] [PubMed] [Google Scholar]
- Brooks P. J., Kaplitt M. G., Kleopoulos S. P., Funabashi T., Mobbs C. V., Pfaff D. W. Detection of messenger RNA and low-abundance heteronuclear RNA with single-stranded DNA probes produced by amplified primer extension labeling. J Histochem Cytochem. 1993 Dec;41(12):1761–1766. doi: 10.1177/41.12.8245424. [DOI] [PubMed] [Google Scholar]
- Clayton D. F., Harrelson A. L., Darnell J. E., Jr Dependence of liver-specific transcription on tissue organization. Mol Cell Biol. 1985 Oct;5(10):2623–2632. doi: 10.1128/mcb.5.10.2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton D. F., Weiss M., Darnell J. E., Jr Liver-specific RNA metabolism in hepatoma cells: variations in transcription rates and mRNA levels. Mol Cell Biol. 1985 Oct;5(10):2633–2641. doi: 10.1128/mcb.5.10.2633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donovan D. M., Takemura M., O'Hara B. F., Brannock M. T., Uhl G. R. Preproenkephalin promoter "cassette" confers brain expression and synaptic regulation in transgenic mice. Proc Natl Acad Sci U S A. 1992 Mar 15;89(6):2345–2349. doi: 10.1073/pnas.89.6.2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Federoff H. J., Geschwind M. D., Geller A. I., Kessler J. A. Expression of nerve growth factor in vivo from a defective herpes simplex virus 1 vector prevents effects of axotomy on sympathetic ganglia. Proc Natl Acad Sci U S A. 1992 Mar 1;89(5):1636–1640. doi: 10.1073/pnas.89.5.1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geller A. I., Breakefield X. O. A defective HSV-1 vector expresses Escherichia coli beta-galactosidase in cultured peripheral neurons. Science. 1988 Sep 23;241(4873):1667–1669. doi: 10.1126/science.241.4873.1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlan R. E., Shivers B. D., Romano G. J., Howells R. D., Pfaff D. W. Localization of preproenkephalin mRNA in the rat brain and spinal cord by in situ hybridization. J Comp Neurol. 1987 Apr 8;258(2):159–184. doi: 10.1002/cne.902580202. [DOI] [PubMed] [Google Scholar]
- Howells R. D., Kilpatrick D. L., Bailey L. C., Noe M., Udenfriend S. Proenkephalin mRNA in rat heart. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1960–1963. doi: 10.1073/pnas.83.6.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iadarola M. J., Naranjo J. R., Duchemin A. M., Quach T. T. Expression of cholecystokinin and enkephalin mRNA in discrete brain regions. Peptides. 1989 May-Jun;10(3):687–692. doi: 10.1016/0196-9781(89)90160-5. [DOI] [PubMed] [Google Scholar]
- Joshi J., Sabol S. L. Proenkephalin gene expression in C6 rat glioma cells: potentiation of cyclic adenosine 3',5'-monophosphate-dependent transcription by glucocorticoids. Mol Endocrinol. 1991 Aug;5(8):1069–1080. doi: 10.1210/mend-5-8-1069. [DOI] [PubMed] [Google Scholar]
- Khachaturian H., Lewis M. E., Hollt V., Watson S. J. Telencephalic enkephalinergic systems in the rat brain. J Neurosci. 1983 Apr;3(4):844–855. doi: 10.1523/JNEUROSCI.03-04-00844.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilpatrick D. L., Howells R. D., Noe M., Bailey L. C., Udenfriend S. Expression of preproenkephalin-like mRNA and its peptide products in mammalian testis and ovary. Proc Natl Acad Sci U S A. 1985 Nov;82(21):7467–7469. doi: 10.1073/pnas.82.21.7467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilpatrick D. L., Zinn S. A., Fitzgerald M., Higuchi H., Sabol S. L., Meyerhardt J. Transcription of the rat and mouse proenkephalin genes is initiated at distinct sites in spermatogenic and somatic cells. Mol Cell Biol. 1990 Jul;10(7):3717–3726. doi: 10.1128/mcb.10.7.3717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraner S. D., Chong J. A., Tsay H. J., Mandel G. Silencing the type II sodium channel gene: a model for neural-specific gene regulation. Neuron. 1992 Jul;9(1):37–44. doi: 10.1016/0896-6273(92)90218-3. [DOI] [PubMed] [Google Scholar]
- Kwong A. D., Frenkel N. Herpes simplex virus amplicon: effect of size on replication of constructed defective genomes containing eucaryotic DNA sequences. J Virol. 1984 Sep;51(3):595–603. doi: 10.1128/jvi.51.3.595-603.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Gamma E. F., Agarwal B. L., DeCristofaro J. D. Regulation of adrenomedullary preproenkephalin mRNA: effects of hypoglycemia during development. Brain Res Mol Brain Res. 1992 Apr;13(3):189–197. doi: 10.1016/0169-328x(92)90026-8. [DOI] [PubMed] [Google Scholar]
- Le Gal La Salle G., Robert J. J., Berrard S., Ridoux V., Stratford-Perricaudet L. D., Perricaudet M., Mallet J. An adenovirus vector for gene transfer into neurons and glia in the brain. Science. 1993 Feb 12;259(5097):988–990. doi: 10.1126/science.8382374. [DOI] [PubMed] [Google Scholar]
- Miller A. D. Human gene therapy comes of age. Nature. 1992 Jun 11;357(6378):455–460. doi: 10.1038/357455a0. [DOI] [PubMed] [Google Scholar]
- Mori N., Stein R., Sigmund O., Anderson D. J. A cell type-preferred silencer element that controls the neural-specific expression of the SCG10 gene. Neuron. 1990 Apr;4(4):583–594. doi: 10.1016/0896-6273(90)90116-w. [DOI] [PubMed] [Google Scholar]
- Romano G. J., Mobbs C. V., Howells R. D., Pfaff D. W. Estrogen regulation of proenkephalin gene expression in the ventromedial hypothalamus of the rat: temporal qualities and synergism with progesterone. Brain Res Mol Brain Res. 1989 Jan;5(1):51–58. doi: 10.1016/0169-328x(89)90017-x. [DOI] [PubMed] [Google Scholar]
- Rosen H., Douglass J., Herbert E. Isolation and characterization of the rat proenkephalin gene. J Biol Chem. 1984 Nov 25;259(22):14309–14313. [PubMed] [Google Scholar]
- Rusconi S. Transgenic regulation in laboratory animals. Experientia. 1991 Sep 15;47(9):866–877. doi: 10.1007/BF01929876. [DOI] [PubMed] [Google Scholar]
- Scharfmann R., Axelrod J. H., Verma I. M. Long-term in vivo expression of retrovirus-mediated gene transfer in mouse fibroblast implants. Proc Natl Acad Sci U S A. 1991 Jun 1;88(11):4626–4630. doi: 10.1073/pnas.88.11.4626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder E. Y., Deitcher D. L., Walsh C., Arnold-Aldea S., Hartwieg E. A., Cepko C. L. Multipotent neural cell lines can engraft and participate in development of mouse cerebellum. Cell. 1992 Jan 10;68(1):33–51. doi: 10.1016/0092-8674(92)90204-p. [DOI] [PubMed] [Google Scholar]
- Spaete R. R., Frenkel N. The herpes simplex virus amplicon: a new eucaryotic defective-virus cloning-amplifying vector. Cell. 1982 Aug;30(1):295–304. doi: 10.1016/0092-8674(82)90035-6. [DOI] [PubMed] [Google Scholar]
- Spaete R. R., Frenkel N. The herpes simplex virus amplicon: analyses of cis-acting replication functions. Proc Natl Acad Sci U S A. 1985 Feb;82(3):694–698. doi: 10.1073/pnas.82.3.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshikawa K., Williams C., Sabol S. L. Rat brain preproenkephalin mRNA. cDNA cloning, primary structure, and distribution in the central nervous system. J Biol Chem. 1984 Nov 25;259(22):14301–14308. [PubMed] [Google Scholar]
- Zinn S. A., Ebert K. M., Mehta N. D., Joshi J., Kilpatrick D. L. Selective transcription of rat proenkephalin fusion genes from the spermatogenic cell-specific promoter in testis of transgenic mice. J Biol Chem. 1991 Dec 15;266(35):23850–23855. [PubMed] [Google Scholar]