Abstract
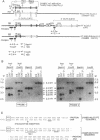
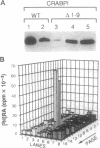
The cellular retinoic acid binding proteins I and II (CRABPI and CRABPII) bind retinoic acid with high affinity, exhibit distinct patterns of expression during embryonic development, and are thought to play important roles in the RA signaling pathway. We have generated a targeted mutation of the CRABPI gene using the "hit-and-run" strategy and shown that it prevents the production of a functional CRABPI protein. Homozygous mutant mice were normal, indicating that CRABPI does not play a crucial role in the RA signaling pathway.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boylan J. F., Gudas L. J. The level of CRABP-I expression influences the amounts and types of all-trans-retinoic acid metabolites in F9 teratocarcinoma stem cells. J Biol Chem. 1992 Oct 25;267(30):21486–21491. [PubMed] [Google Scholar]
- Chambon P. The retinoid signaling pathway: molecular and genetic analyses. Semin Cell Biol. 1994 Apr;5(2):115–125. doi: 10.1006/scel.1994.1015. [DOI] [PubMed] [Google Scholar]
- Dencker L., Annerwall E., Busch C., Eriksson U. Localization of specific retinoid-binding sites and expression of cellular retinoic-acid-binding protein (CRABP) in the early mouse embryo. Development. 1990 Oct;110(2):343–352. doi: 10.1242/dev.110.2.343. [DOI] [PubMed] [Google Scholar]
- Fiorella P. D., Napoli J. L. Expression of cellular retinoic acid binding protein (CRABP) in Escherichia coli. Characterization and evidence that holo-CRABP is a substrate in retinoic acid metabolism. J Biol Chem. 1991 Sep 5;266(25):16572–16579. [PubMed] [Google Scholar]
- Gaub M. P., Lutz Y., Ruberte E., Petkovich M., Brand N., Chambon P. Antibodies specific to the retinoic acid human nuclear receptors alpha and beta. Proc Natl Acad Sci U S A. 1989 May;86(9):3089–3093. doi: 10.1073/pnas.86.9.3089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giguère V. Retinoic acid receptors and cellular retinoid binding proteins: complex interplay in retinoid signaling. Endocr Rev. 1994 Feb;15(1):61–79. doi: 10.1210/edrv-15-1-61. [DOI] [PubMed] [Google Scholar]
- Gossler A., Doetschman T., Korn R., Serfling E., Kemler R. Transgenesis by means of blastocyst-derived embryonic stem cell lines. Proc Natl Acad Sci U S A. 1986 Dec;83(23):9065–9069. doi: 10.1073/pnas.83.23.9065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafson A. L., Dencker L., Eriksson U. Non-overlapping expression of CRBP I and CRABP I during pattern formation of limbs and craniofacial structures in the early mouse embryo. Development. 1993 Feb;117(2):451–460. doi: 10.1242/dev.117.2.451. [DOI] [PubMed] [Google Scholar]
- Hasty P., Ramírez-Solis R., Krumlauf R., Bradley A. Introduction of a subtle mutation into the Hox-2.6 locus in embryonic stem cells. Nature. 1991 Mar 21;350(6315):243–246. doi: 10.1038/350243a0. [DOI] [PubMed] [Google Scholar]
- Kessel M., Gruss P. Homeotic transformations of murine vertebrae and concomitant alteration of Hox codes induced by retinoic acid. Cell. 1991 Oct 4;67(1):89–104. doi: 10.1016/0092-8674(91)90574-i. [DOI] [PubMed] [Google Scholar]
- Leid M., Kastner P., Chambon P. Multiplicity generates diversity in the retinoic acid signalling pathways. Trends Biochem Sci. 1992 Oct;17(10):427–433. doi: 10.1016/0968-0004(92)90014-z. [DOI] [PubMed] [Google Scholar]
- Leid M., Kastner P., Lyons R., Nakshatri H., Saunders M., Zacharewski T., Chen J. Y., Staub A., Garnier J. M., Mader S. Purification, cloning, and RXR identity of the HeLa cell factor with which RAR or TR heterodimerizes to bind target sequences efficiently. Cell. 1992 Jan 24;68(2):377–395. doi: 10.1016/0092-8674(92)90478-u. [DOI] [PubMed] [Google Scholar]
- Lufkin T., Dierich A., LeMeur M., Mark M., Chambon P. Disruption of the Hox-1.6 homeobox gene results in defects in a region corresponding to its rostral domain of expression. Cell. 1991 Sep 20;66(6):1105–1119. doi: 10.1016/0092-8674(91)90034-v. [DOI] [PubMed] [Google Scholar]
- Lufkin T., Lohnes D., Mark M., Dierich A., Gorry P., Gaub M. P., LeMeur M., Chambon P. High postnatal lethality and testis degeneration in retinoic acid receptor alpha mutant mice. Proc Natl Acad Sci U S A. 1993 Aug 1;90(15):7225–7229. doi: 10.1073/pnas.90.15.7225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyn S., Giguère V. Localization of CRABP-I and CRABP-II mRNA in the early mouse embryo by whole-mount in situ hybridization: implications for teratogenesis and neural development. Dev Dyn. 1994 Apr;199(4):280–291. doi: 10.1002/aja.1001990404. [DOI] [PubMed] [Google Scholar]
- Mark M., Lufkin T., Vonesch J. L., Ruberte E., Olivo J. C., Dollé P., Gorry P., Lumsden A., Chambon P. Two rhombomeres are altered in Hoxa-1 mutant mice. Development. 1993 Oct;119(2):319–338. doi: 10.1242/dev.119.2.319. [DOI] [PubMed] [Google Scholar]
- Merrick W. C. Mechanism and regulation of eukaryotic protein synthesis. Microbiol Rev. 1992 Jun;56(2):291–315. doi: 10.1128/mr.56.2.291-315.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morriss-Kay G. Retinoic acid and craniofacial development: molecules and morphogenesis. Bioessays. 1993 Jan;15(1):9–15. doi: 10.1002/bies.950150103. [DOI] [PubMed] [Google Scholar]
- Rochette-Egly C., Lutz Y., Saunders M., Scheuer I., Gaub M. P., Chambon P. Retinoic acid receptor gamma: specific immunodetection and phosphorylation. J Cell Biol. 1991 Oct;115(2):535–545. doi: 10.1083/jcb.115.2.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross A. C. Cellular metabolism and activation of retinoids: roles of cellular retinoid-binding proteins. FASEB J. 1993 Feb 1;7(2):317–327. doi: 10.1096/fasebj.7.2.8440409. [DOI] [PubMed] [Google Scholar]
- Ruberte E., Friederich V., Morriss-Kay G., Chambon P. Differential distribution patterns of CRABP I and CRABP II transcripts during mouse embryogenesis. Development. 1992 Aug;115(4):973–987. doi: 10.1242/dev.115.4.973. [DOI] [PubMed] [Google Scholar]
- Siegenthaler G. Gel electrophoresis of cellular retinoic acid-binding protein, cellular retinol-binding protein, and serum retinol-binding protein. Methods Enzymol. 1990;189:299–307. doi: 10.1016/0076-6879(90)89301-w. [DOI] [PubMed] [Google Scholar]
- Stoner C. M., Gudas L. J. Mouse cellular retinoic acid binding protein: cloning, complementary DNA sequence, and messenger RNA expression during the retinoic acid-induced differentiation of F9 wild type and RA-3-10 mutant teratocarcinoma cells. Cancer Res. 1989 Mar 15;49(6):1497–1504. [PubMed] [Google Scholar]
- Tabin C. J. Retinoids, homeoboxes, and growth factors: toward molecular models for limb development. Cell. 1991 Jul 26;66(2):199–217. doi: 10.1016/0092-8674(91)90612-3. [DOI] [PubMed] [Google Scholar]
- Takase S., Ong D. E., Chytil F. Transfer of retinoic acid from its complex with cellular retinoic acid-binding protein to the nucleus. Arch Biochem Biophys. 1986 Jun;247(2):328–334. doi: 10.1016/0003-9861(86)90591-6. [DOI] [PubMed] [Google Scholar]
- Wei L. N., Tsao J. L., Chu Y. S., Jeannotte L., Nguyen-Huu M. C. Molecular cloning and transcriptional mapping of the mouse cellular retinoic acid-binding protein gene. DNA Cell Biol. 1990 Sep;9(7):471–478. doi: 10.1089/dna.1990.9.471. [DOI] [PubMed] [Google Scholar]