1. Myelinating glia; Schwann cells and oligodendrocytes
Myelin consists of a membrane sheath that wraps around an axon, speeding the conduction of action potentials to provide efficient impulse propagation in large size animals [1]. Since it was first described by Ehrenberg in 1833, the concept of myelin has evolved from being viewed as a static component surrounding the axons to a current understanding of a complex and dynamic process of cell-cell interaction [2] [3] that supports axonal integrity and survival [4], and can be modified by functional experience [5].
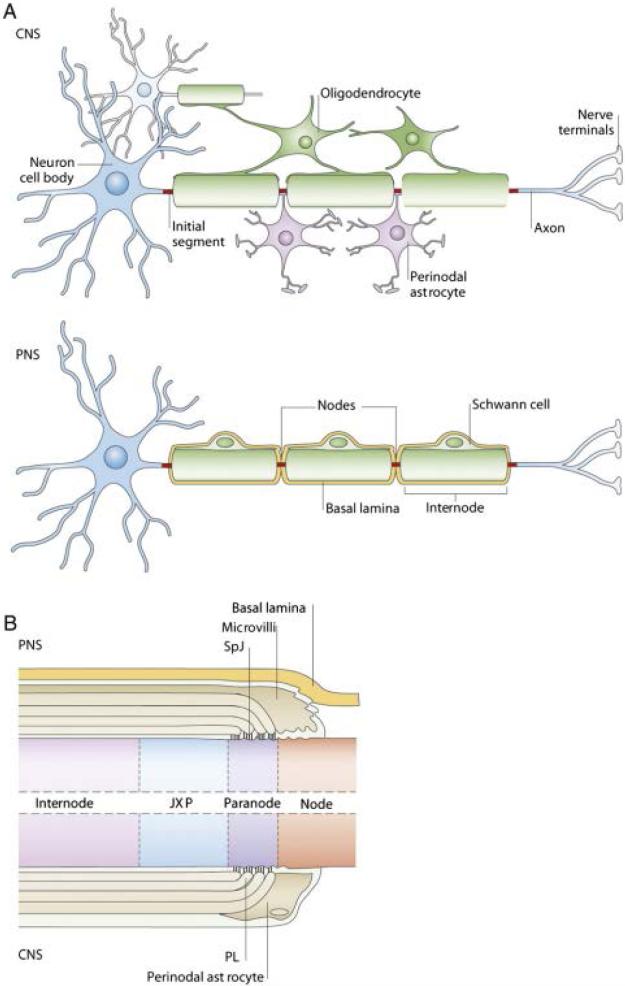
The structure of myelinated fibers is similar both in the peripheral nervous system (PNS) and the central nervous system (CNS). Myelinated segments of nerve fibers known as internodes are delimited by areas of naked axons, the nodes of Ranvier [6], where action potentials are generated. The portion of myelin adjacent to the nodes is the paranodal region, which is where the terminal lamellae of non-compact myelin contact the axon (See Fig.1).
Figure 1.
Illustration modified from Poliak and Peles et al. [11] showing the differences between myelinated fibers in the Peripheral Nervous System (PNS) and the Central Nervous System (CNS). A. Oligodendrocytes myelinate the CNS wrapping their processes around multiple axonal segments. Schwann cells are the myelinating cell type in the PNS and contact single axonal segments. Discontinuities of the myelin sheath along the axon known as nodes of Ranvier are contacted by perinodal astrocytes in the case of the CNS, whereas in the PNS Schwann cells extend microvilli to the node that is surrounded by basal lamina, highlighting the multifunctional capacity of these cells. B. Representation of a longitudinal cut of the myelinated fiber surrounding a node of Ranvier in the PNS (top) and CNS (bottom). The paranode, adjacent to the nodes, is formed by non-compact myelin and contains reflexive gap junctions establishing a communication compartment across the membranous myelin layers. The juxtaparanode (JXP) is composed of compact myelin and divides the paranodal region of the internodal region.
Two different lineages of glial cells, Schwann cells and oligodendrocytes, are responsible for myelinating the PNS and the CNS respectively. Schwann cells originate from the neural crest and develop into Schwann cell precursors and immature Schwann cells before reaching their mature state [7]. Oligodendrocytes originate from oligodendrocyte precursor cells (OPC) which are generated at the ventral neuroepithelium of the neural tube during embryogenesis or dorsal spinal cord and hindbrain in early post-natal life [8]. Both types of myelinating glial cells contact the axons they are going to myelinate early in development, however, each myelinating Schwann cell associates with a single short axonal segment, whereas a single multipolar oligodendrocyte can interact with up to 40 segments on multiple axons [2]. The initial events in myelination by oligodendrocytes are stimulated by electrical activity in axons. This suggests that electrically active axons will be preferentially myelinated, leading to the possibility that environmental experience may modulate neural development and the functional properties of neural circuits as a result of the increased conduction velocity in myelinated axons [9]. In the CNS, oligodendrocytes are coupled through gap junctions to astrocytes, which are bushy shaped glial cells that participate in brain homeostasis by removing excess neurotransmitter from the synaptic cleft. Astrocytes are also involved in synapse formation and modulation [10]. In the PNS, Schwann cells must exert all the functions of both kinds of glia in the CNS, which indicates a remarkable plasticity of these cells. This heterogeneity of functions is accompanied by changes in gap junction expression and intercellular contacts. Schwann cells express a basal lamina of extracellular matrix that surrounds the node of Ranvier; whereas in the CNS, node structure does not include basal lamina and it is instead contacted by astrocytic processes [11] (See Fig.1).
Gap junctions in myelinating glia are involved in many physiological processes beyond cell-to-cell communication, including growth control, regulation of cell permeability and calcium signalling. Moreover, in the CNS, gap junctions are hypothesised to play an important role in brain homeostasis by facilitating restoration of membrane potential after axonal activity via electrical coupling and re-distribution of potassium ions [12]. Coupling between myelinating glia and astrocytes constitutes a glial network, known as a panglial syncytium [13] that promotes the intracellular diffusion of potassium released from axons firing action potentials[14, 15].
2. Gap Junctions
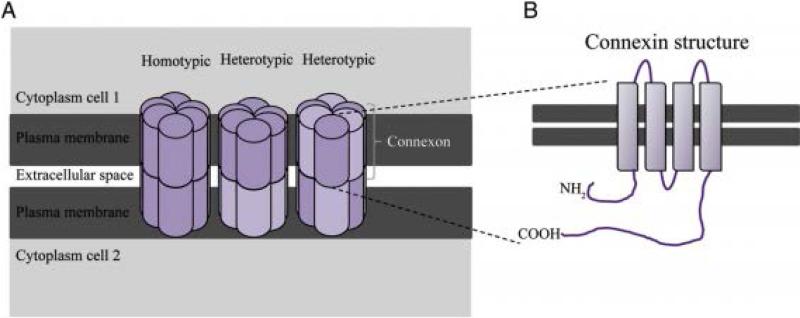
Gap junctions are formed by members of the connexin family of transmembrane proteins which converge evolutionary from innexins, the protein channels responsible for gap junction communication in invertebrates [16]. In addition, three members of a protein channel subtype homologous to innexins, the pannexins, have been identified in the CNS of vertebrates. It remains unclear whether pannexins can form gap junctions in vivo or instead, they serve as hemichannel conduits through the plasma membrane to allow release of ATP [17], a neurotransmitter and important cell-cell signalling molecule [18]. Multiple gap junction channels cluster in the cell membrane to form gap junction plaques. Each gap junction results from the docking of two hemichannels or connexons of adjacent cells, which in turn are composed of six connexins. Each connexin contains four transmembrane domains linked by two extracellular loops and one intracellular loop. Single gap junction channels can be made of similar (homotypic) or different (heterotypic) subtypes of connexins [19] ( See Fig.2). Any two compatible connexins can theoretically be coupled, but functional and biochemical experiments have shown that in general, not all connexin pairs are compatible and only connexins that are closely related to each other can form functional heterotypic channels [20]. Heterotypic channels often exhibit distinct electrophysiological and ion selective properties from those found in homotypic channels [21].
Figure 2.
A. Schematic representation of hemichannels or connexons from neighboring cells docking to form functional gap junctions that enable communication between the cytoplasm of cell 1 and the cytoplasm of cell 2. Connexons can be assembled from the same subset of connexins (homomeric) or different subsets of connexins (heteromeric). Moreover, gap junctions can present either identical connexon composition of connexin subtypes (homotypic) or different connexon composition of connexin subtypes (heterotypic). B. Connexins are transmembrane proteins composed of four transmembrane domains with alpha helix, two extracellular loops and an intracellular loop. Both N- and C-terminals are intracellular. The two extracellular loops contain three highly conserved cysteine residues responsible for the selectivity of hemichannel interactions.
Connexin hemichannel opening is highly regulated in several different manners, including gating by transmembrane voltage [22], phosphorylation [23], and extracellular calcium concentration. For example, conduction through hemichannels is suppressed by the millimolar concentrations of calcium in the extracellular fluid [24]. Nogo-66 receptor NgR1 is expressed in astrocytes and it can regulate gap junction activity by modifying downstream signals that affect phosphorylation of connexins [25].
The opening of gap junction channels allows communication between neighboring cells by facilitating the exchange of small molecules and metabolites. In vertebrates, gap junctions are generally permeable to molecules smaller than 1 kDa, including cyclic nucleotides, vitamins and amino acids, as well as ions [26]. The permeability of channels formed by different connexins can exhibit some chemical selectivity beyond exclusion simply by molecular weight, indicating some chemical or charge-specific effects on permeability of different types of molecules [27] [28]. Metabolic coupling between cells and transfer of cell signaling molecules is an important function of gap junctions in general, but this has not been studied extensively in myelinating glia.
Gap junctions also enable formation of ensembles of cells coupled together into communication compartments that are jointly regulated by the concentration of a second messenger or metabolite. For example, in the neocortex gap junctions are believed to coordinate the activity of inhibitory neurons [29].
3. Gap junction communication in the myelinating glia
3.1 Panglial syncytium
Clearance of potassium and other ions after axon excitation is crucial to ensure the normal resting potential in axons, which is essential for electrical excitation and neuronal viability. Accumulation of potassium would lead to an osmotically driven water gradient resulting in pathological axonal swelling [12]. This becomes more critical when axons are tightly wrapped by myelin; highlighting the importance of gap junction communication across myelinating glia to provide a diffusion pathway from the axon out to the extracellular space. This is the main function of the gap junctions formed between neighboring oligodendrocytes, between astrocytes and oligodendrocytes, and between the layers of myelin membrane in compact myelin formed by oligodendrocytes [30] and Schwann cells [31].
Gap junctions are relatively common between oligodendrocytes and astrocytes (O/A) [32] [33], but the coupling between these cell types is weak. Some evidence suggests directional coupling, with dye flowing more freely from astrocytes into oligodendrocytes than in the reverse direction [33] [34]. This is consistent with the heterotypic coupling between different gap junction hemichannels in each cell, as astrocytes and oligodendrocytes do not share any of the same connexins. Dye coupling between adjacent oligodendrocytes varies widely in different parts of the brain and under different conditions, and when dye-coupling is observed, only a relatively small number of cells are coupled. Oligodendrocytes can exchange metabolites, ions and other gap-junction permeable molecules among themselves much easier than with astrocytes.
Coupling between oligodendrocytes (O/O) occurs through adjacent cell bodies [35]. Little if any gap junction coupling between oligodendrocytes had been observed in white matter of the corpus callosum previously [36] and spinal cord [37], but more recent evidence supports O/O communication through gap junctions (see below). 20% of oligodendrocytes in spinal cord gray matter are dye coupled [37]. Electrical coupling is seen in 3/4 of oligodendrocyte cell pairs tested in cell culture [38].
3.2 Reflexive gap junction across the myelin sheath
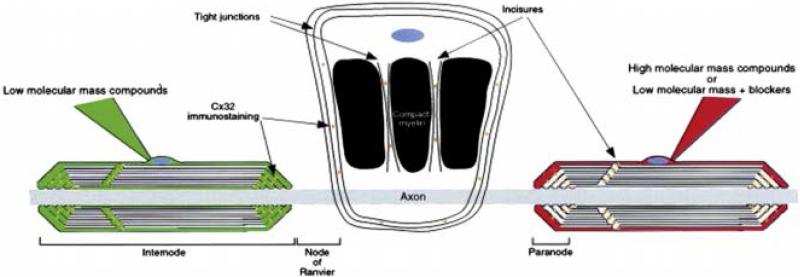
Reflexive gap junctions are gap junctions formed between different processes of the same cell. Such coupling is particularly important in myelin because of the unique topology presented by the multilaminar spiral wrapping of compacted membrane around axons. In the PNS, connexin 32 (Cx32) is expressed at the paranodes together with myelin-associated glycoprotein (MAG) and E-cadherin. The functionality of this particular location of Cx32 was explored by injecting intracellular dyes into living myelinating cells. This experiment revealed that only low molecular mass dyes, such as 5, 6-carboxyfluorescein (not high molecular mass dyes) diffuse across the myelin sheath through the paranodes and the periodic expansions in compact myelin, also known as Schmidt-Lanterman incisures (See Fig.3). This was the first functional evidence that gap junctions mediate a radial pathway of diffusion across the myelin layers, which provides a shortcut to diffusion that is one million times faster than the circumferential pathway [26]. However, this gap junction pathway is still present in Cx32 KO mice, suggesting that other connexins must be present at these locations as well. Nevertheless, these other connexins are not sufficient to preserve the functional or structural integrity of myelin, as Cx32 KO mice develop peripheral demyelinating neuropathy.
Figure 3.
Schematic representation from Balice-Gordon et al. [25] showing the diffusion of low (green) and high (red) molecular mass compounds (or low molecular mass compounds in the presence of gap junction blockers) across the PNS myelin sheath following perinuclear dye injection. Low molecular mass dyes as 5, 6-carboxyfluorescein diffuse across the myelin sheath through the paranodes and the periodic expansions in compact myelin, also known as Schmidt–Lanterman incisures. High molecular mass dyes are not able not cross the myelin layers and accumulate outside the myelin sheath. This study provided the first evidence of gap junctions mediating a radial pathway across the myelin sheath in the PNS which is one million times faster than the circumferential pathway. A Schwann cell has been unwrapped in the middle of the figure showing the location of Cx32 in the regions of non-compact myelin (Schmidt–Lanterman incisures and paranodes).
In the CNS, Cx32 is localized to the paranodes where it most likely forms reflexive channels (Cx32/Cx32). However, it is not known if they form reflexive channels in any other regions as is the case in peripheral myelin [39].
4. Types of gap junctions expressed in myelinating glia
Schwann cell protein expression of connexin subtypes Cx32, Cx43, Cx29 and Cx46 is regulated during development. Nonmyelinating Schwann cells are dye-coupled, but this abates when the cells begin to myelinate [40]. Schwann cells express Cx46 early in development while they are still proliferating and re-express it again after nerve injury [41]. Cx46 expression seen during the proliferating phase ceases when the cells undergo myelination [42]. Moreover, Cx29 is not expressed in neural crest cells, but later it is expressed in Schwann cell precursors both in vivo and in vitro, and as such, is used as a marker in Schwann cell lineage progression in mice [43].
Immunofluorescence studies show positive staining for Cx32 in Schwann cells at postnatal stages coinciding with the onset of myelination [43]. Cx32 enhances the proliferative response of Schwann cells to neuregulin-1 (NRG1), highlighting its role in primary myelinating and remyelinating events [44].
Oligodendrocytes and astrocytes express distinct sets of connexin proteins. Oligodendrocytes express Cx47 [45] [46] [47], Cx32 [48] [49], and Cx29 [50, 51]. Astrocytes express Cx43 and 30 [52] [53], and possibly Cx26 [54] [52] [55]. Oligodendrocytes couple not only to astrocytes and other oligodendrocytes, but also to OPCs [56].
Cx47 is expressed in oligodendrocytes in early embryonic periods and shows local and temporal restriction in the corpus callosum, the striatum, the cerebellum, and the spinal cord in adult animals [47]. Cx29 and Cx32 expression levels are detectable at the beginning of myelination and expression increases in adult brain [47]. However, Cx29 has not been shown to form hemichannels in the adaxonal membrane [14] [57].
It was believed that oligodendrocytes were only coupled to astrocytes [58] [59] using Cx47/Cx43 and Cx32/Cx30 heterotypic channels [50]. However, Wasseff and Scherer (2001) [60] report that astrocytes can also couple to each other (A/A) in the corpus callosum, a finding corroborated by Maglione et al, 2010 [56]. These recent findings suggest that the earlier failure to find O/A or O/O coupling in the corpus callosum [36] [61] [37] [32] may be explained by the low pH in the pipette solution (7.2) that was used, and by the inability of Lucifer Yellow to cross Cx32/Cx30 channels [62]. This is consistent with other research showing that biocytin did not label oligodendrocytes [63] when injected into astrocytes. Whether O/O coupling is mediated primarily by Cx32/Cx32 or by Cx47/Cx47 homotypic channels is not yet established, but evidence suggests that CX47, but not Cx32, is required for O/A coupling [60].
Maglione et al., (2010) [56] report that the number of oligodendrocytes coupled to other oligodendrocytes in white matter is reduced 80% in Cx47 KO mice. Moreover, no O/A coupling remains after Cx47 ablation. In contrast, deleting Cx32 or Cx29, had no significant effect on O/O coupling and loss of Cx32 did not affect the expression or localization of other gap junctions [64]. Intercellular coupling was absent in Cx32/Cx47 dKO mice and the loss of oligodendrocyte gap junctions in Cx47 and Cx32 KO results in an increase in the oligodendrocytic input resistance [56]. O/A coupling was almost absent in Cx43/Cx30 dKO mice [65, 66], but some O/A coupling remained in Cx43 deficient animals even though no coupling from oligodendrocytes to OPCs was observed (See Table 1) [56].
Table 1.
Coupling characteristics in KO and dKO mice for the major oligodendrocytic and astrocytic gap junctions that participate in glial networks. A. There are some discrepancies about the major connexin subtype mediating O/O coupling. Maglione et al. [64] found a pronounced reduction in O/O coupling in Cx47 deficient mice but it was unaffected in Cx32 deficient mice while Wasseff and Scherer [67] results suggest the opposite. The later authors discuss that discrepancies can be due to methodological aspects as the dye used in the permeability assays was different in both studies as well as the genetic background of the mice used as control. However, both investigations agree in the finding that no O/O coupling remains in either the corpus callosum or neocortex of Cx47/Cx32 dKO mice. Maglione et al. [64] further inspected O/O coupling in Cx30/Cx43 dKO which was found to be reduced. The lack of Cx43 alone diminished the coupling of oligodendrocytes to immature oligodendrocyte subpopulation suggesting a role for astrocytic Cx43 in precursor population progression. B. Studies from Maglione et al. [64] and Wasseff and Scherer [67] failed to find robust O/A coupling in the corpus callosum in contraposition to the traditional view that oligodendrocytes were only coupled to astrocytes. However, the weak O/A coupling observed in control mice was reduced in Cx47 deficient mice and totally abolished in Cx47/Cx32 dKO and Cx30/Cx43 dKO. C. Conditional deletion of Cx43 in astrocytes results in 50% decay [74] of the A/A coupling in the hippocampus while loss of Cx30 causes 20% reduction [71] on the astroglial hippocampal coupling. However, A/A coupling is completely impaired by the deletion of both astrocytic connexins [72].
| Connexins | (A) O/O-coupling | (B) O/A coupling | (C) A/A coupling |
|---|---|---|---|
| Cx47 KO | Pronounced reduction [64] but other studies did not find differences [67] | Reduced [64] | Not determined |
| Cx32 KO | Present [64] but other studies found partial disruption [67] | Present [64] | Loss of Cx30 in gray matter astrocytes [71] |
| Cx29 KO | Present [64] | Present [64] | Not determined |
| Cx43 (fl/fl): Hgfap-Cre | Coupling of oligodendrocytes to immature oligodendrocyte subpopulation impaired [64] | Present [64] | 50% reduced in hippocampus |
| Cx30 upregulated partially compensate the loss of Cx43[74] | |||
| Cx30 KO | Not determined | Not determined | 20% reduced in hippocampus hippocampal slices [75] |
| Cx32/Cx47 dKO | No coupling remains in the neocortex [67] and the corpus callosum [64] | No coupling remains in the neocortex [67] and the corpus callosum [64] | |
| Cx30/Cx43 dKO | Reduced [64] | Almost abolished [64] | Absent [72] |
Thus, the latest findings suggest that potassium buffering among oligodendrocytes through gap junction coupling is more important for myelin maintenance than O/A coupling [66] [65]. This panglial syncytium is mediated predominantly by oligodendrocytes in white matter coupled among each other through Cx47 and Cx32 [56]. The formation of a panglial syncytium in the corpus callosum and also in the neocortex [29], reflects the importance of distributing metabolites intercellularly through gap junctions during myelin formation and development.
5. Hemichannel permeability to ATP in physiology and disease
During the biogenesis of gap junctions, connexons reach the plasma membrane and find their appropriate locations by an unknown mechanism. However it is a matter of discussion if these individual connexons or hemichannels are present in the plasma membrane as transient structures or they play a physiological role [67].
The work done by Geoffrey Burnstock unveiled the role of ATP as a neurotransmitter when released or co-released after synaptic vesicle exocytosis [68]. The action of ATP and other adenine derivatives not only play a role in neuronal communication, but also in glial activity. In this regard, calcium waves recorded in cultured astrocytes are triggered by ATP-induced ATP release, generating an extracellular propagation wave of ATP that, in turn, activates the intracellular calcium wave [69]. There is growing evidence that the activation of astrocytes is related to synaptic plasticity, and ATP-dependent activation of astrocytes modulates distant synaptic activity [70]. Much astrocytic release of ATP does not fit with an exocytotic source, and it was suggested that ATP would reach the extracellular medium by crossing the hemichannels built up by Cx43, a subtype of connexin present in different cells and organs throughout the body and expressed at very high levels in the central nervous system, specifically in astrocytes. Single channel recording in combination with luciferin-luciferase assay provided direct evidence that the large single channel conductance of Cx43 was accompanied with an increase of luminescence due to ATP crossing the hemichannel [71]. HeLa cells transfected with Cx43 mimicked the astrocytic intracellular calcium waves mediated by extracellular ATP, indicating that ATP crossed the plasma membrane using the intramolecular tunnel of Cx43 [72]. HeLa cells also release ATP under low extracellular calcium concentrations that induce an increase of permeability of Cx43 [73]. In C6 glioma cell line, the release of ATP is strongly decreased when Cx43 is knocked down with siRNA [74].
Interestingly, astrocytes isolated from Cx43 null mice do not release ATP when stimulated by Benzoyl-ATP (BzATP) [75], instead the results obtained by these investigators showed that Pannexin1 (Panx1) was implicated in that release. However, other authors have not obtained similar results when transfecting with Panx1 [74]. Pannexins are a group of membrane proteins that differ from connexins in aminoacid sequence, but they have similar organization within the lipidic membrane. They have four transmembrane regions, two extracellular loops, one intracellular loop and intracellular N and C termini [76]. Panx1 is ubiquitous in tissues and organs, and, has been implicated in the controlled ATP release in many cell and tissues including erythrocytes, which lack secretory granules, and astrocytes [77] [78] [79] [80] [81]. It has been suggested that Panx1 is always and exclusively forming hemichannels “in vivo”. The work of Bruzzone et al., (2003) [82] pointed out that when expressed in paired Xenopus laevis oocytes, they form a large conductance connection. Because of the high conductance of Panx1 and the purinergic receptor P2X7, it was suggested that some kind of direct interactions could explain how P2X7 receptors may support low and high conductance open states; but as a matter of fact, experimental results favored the view that they correspond to two independent structures [83] [84] [85]. However, activation of Panx1 delivers ATP to different kinds of purinergic receptors [86] and it seems that among other physiological roles, Panx1 may be involved in cell apoptosis by controlling ATP release.
The relationship between different types of connexins and ATP release has been proved. In Xenopus laevis oocytes, activation of the endogenous Cx38 with low extracellular divalent concentration triggers the release of ATP [87]. Cx26, a CO2 dependent connexin found in astrocytes from the respiratory centers of the medulla oblonga, is permeable to ATP under CO2 conditions [88] [89]. In colonic epithelial cells, Cx26 becomes permeable to ATP when interacting with Shigella [90]. In organotypic cultures of mouse cochlea, ATP release is linked to the activation of Cx26 and also with Cx30 [91]. The adhesion of macrophages to endothelial cells is mediated by a release of ATP by means of Cx37 [92].
Results obtained in Cx32 transfected C6 glioma cell line are in accordance with the view that ATP reaches the extracellular space crossing connexin hemichannels, which in turn are activated by an increase of cytoplasmatic calcium concentration [93]. There is an open question about the presence of Cx32 hemichannels at the node or Ranvier and their hypothetical permeability to ATP. It would be of interest to know if Cx32 mutations causing the peripheral demyelinating X-linked form of Charcot-Marie-Tooth disease (CMTX) show differences in conducting ATP. In this regard, repetitive electrical field stimulation of isolated sciatic nerve provokes the release of ATP; glutamate also triggers the release of ATP [94]. Cultured Schwann cells also release important amounts of ATP under UTP stimulation [95], which mimics the release of ATP from astrocytes, suggesting that glial cells from CNS and PNS may share some mechanisms for releasing ATP and activating a pathway of purinergic signaling.
6. Mutations in gap junctions lead to demyelinating neuropathy in both PNS and CNS
Further understanding of the functional importance of gap junctional coupling in myelination and axonal survival comes from diseases resulting from mutations in genes encoding for Cx47 (GJC2) and Cx32 (GJB1), which are the cause of Pelizaeus-Merzbarcher-like disease (PMLD) and CMTX respectively.
6.1 Pelizaeus-Merzbarcher-like disease (PMLD)
PMLD is a recessive inherited severe leukoencephalopathy in humans caused by mutations in the gene GJC2 encoding for Cx47. Patients affected share many clinical features with Pelizaeus-Merzbarcher disease (PMD) patients, including nystagmus, progressive spasticity, ataxia and hypomyelination on MRI imaging. PMD is an X-linked disease caused by mutations in the major membrane protein of the CNS myelin, Proteolipid Protein 1 (PLP1). However, it should be emphasized that Cx47 is not altered in PMD patients [96].
Twenty-four mutations compromising different parts of Cx47 protein have been described, but despite this genetic heterogeneity, the degree of impairment shown by the patients is the same. Mutations can cause the protein to be retained in intracellular compartments such as the endoplasmic reticulum, or can impair the docking of hemichannels thus impeding the passage of molecules between cells and leading to loss of function [97].
A mutation that disrupts the SOX10 transcriptional activation site in the GJC2 promoter region has been described in a family with a mild PMLD phenotype. The fact that another mutation in the binding site of SOX10 in GJB1 is linked to CMTX suggests that transcriptional regulation of GJC2 and GJB1 genes may be critical in myelination of both the CNS and the PNS, respectively [98].
The generation of Cx47 KO mice showed no significant alterations in the CNS apart from minor ultrastructural changes, such as vacuolation of the myelinated fibers in the optic nerve [46]. The generation of a mouse expressing the Cx47 M282T mutation showed impaired motor function, reduced myelin basic protein (MBP) expression, and astrogliosis in the cerebellum of juvenile mice, a phenotype that was completely restored in three-month-old mice [99]. However, Cx32/Cx47 dKO or Cx32 KO mice expressing M282T mutation exhibit a severe phenotype with tremors and tonic seizures as a result of devastating broad demyelination of the CNS that causes death by the sixth postnatal week [45, 99]. These observations lead to the conclusion that Cx47 and Cx32 play a key role in myelination of the CNS and display redundant functionality in the mice CNS, which would not happen in humans considering the affection of PMLD patients. The phenotype exhibited by dKO animals also suggests that the main role of these connexins is to ensure homeostasis of CNS tissue by coupling oligodendrocytes and astrocytes into a network for K+ clearance after nervous activity.
6.2 X-linked Charcot-Marie-Tooth disease (CMTX)
CMTX is a dominant inherited sensory and motor peripheral neuropathy caused by mutations in the gene GJB1 encoding for Cx32 linked to the X chromosome. This is the second most common form of demyelinating Charcot-Marie-Tooth disease type 1 (CMT1), representing 10-15% of all cases. CMTX is characterised by progressive weakness and atrophy of the distal limb muscles that can result in severe deformities like feet drop [100]. Males are uniformly affected but female carriers show variable clinical features due to random X-chromosome inactivation [101]. More than 300 mutations for the gene GJB1 have been described (http://www.molgen.ua.ac.be/CMTMutations/default.cfm; Inherited Peripheral Neuropathies Mutation Database) leading to impaired Cx32 trafficking [102], voltage gating defects [103, 104] and inability to form functional gap junctions across the myelin sheath once inserted into the plasma membrane [105].
Patients affected by CMTX do not show severe CNS symptoms suggesting that Cx47 can compensate for the loss of Cx32 function. However, some studies show subtle central alterations and few mutations have been suggested to involve CNS dysfunction [106, 107]. There are a few mutations related to CMTX that do not directly affect the GJB1 gene but instead affect the binding of the transcription factor Sox10 [108] or EGR2/Knox20 to the P2 promoter that regulates Cx32 expression in Schwann cells [109].
A useful tool for the study of CMTX came from the generation of Cx32 KO which show a late-onset demyelinating neuropathy that resembles human CMTX [101]. During the first months of life these mice show only dysfunctions in the liver where Cx32 is abundantly expressed [110, 111]. The progressive peripheral demyelination starts at 3 months of age and it is characterized by unusually thin myelin sheaths, cellular onion-bulb formations, increased Schwann cell proliferation and enlarged periaxonal collars. Motor fibers are more severely affected than sensory fibres [101], but conduction velocity is only slightly decreased [112].
Strong evidence that CMTX is caused by mutations of Cx32 in Schwann cells and not in other cell types was provided by Scherer et al., (2005) [113] by expressing human Cx32 in Cx32 KO under the myelin protein zero (MPZ) promoter specific for Schwann cells, and rescuing the pathologic phenotype observed in peripheral nerves, but not in liver or spinal cord of the Cx32 KO mice [113]. Further characterization of Cx32 KO revealed new features, including alterations in the distribution of proteins such as potassium channels Kv1.1 [114], increased expression of GFAP [115], and increased number of oligodendrocyte precursor cells [116].
How the lack of Cx32 leads to disease is not fully understood. The main function attributed to Cx32 is to form reflexive gap junctions across the peripheral myelin sheath mediating a faster pathway of diffusion to the adaxonal cytoplasm. However, analysis of the diffusion rate in Cx32 KO was not slower than in the wild-type, suggesting that other gap junctions may mediate this pathway [26]. Moreover, the fact that reflexive channels exhibit different permeabilities to molecules such as cAMP suggests that gap junctions may regulate or sustain signaling cascades that favor the survival and myelination of the axons. For example, gap junctions together with ATP, mediate calcium signaling between the network of branched Schwann cells covering the lancelolate ending of the rat hair follicles [117].
It might be interesting to further explore the consequences of changes in permeability or block of signaling cascades induced by the lack or deficiencies in the channels formed by mutated proteins. Previous studies have shown that ionophoresis or changes in transjunctional voltage in Cx43 and Cx45 can change the permeability of these gap junctions to intracellular injected dyes [118]. Therefore it would be important to determine if defects on the voltage gating of Cx32 mutants induce new selective properties in the channels that can lead to disease.
7. Conclusions
Gap junction communication in myelinating glia is crucial for myelination and axonal survival in both the PNS and the CNS. There are many open questions about the signaling pathways and functions sustained by gap junctions in myelinating glia. Connexin expression changes during differentiation of Schwann cells, indicating the diverse roles that these junctions play in glial cell biology. Reflexive coupling via gap junctions solves a unique problem presented by the diffusion barriers in compact myelin, but many other important roles of gap junctions in myelinating glia are not well understood. This is evidenced by the failure to understand the pathophysiological basis for many myelin disorders associated with genetic mutations in connexins in myelinating glia.
The fundamental facts about gap junction coupling among myelinating glia in the CNS are only now emerging. Coupling among oligodendrocytes and between oligodendrocytes and astrocytes has not been studied extensively, considering the critical importance of communication among these cells and with axons in maintaining normal physiological function. Beyond potassium buffering, gap junction coupling among oligodendrocytes could be important in maintaining axonal excitability, providing nutritional support, transfer of intercellular signaling molecules necessary for maintenance of myelin, and remodeling myelin under appropriate circumstances, as could coupling between astrocytes and oligodendrocytes. Why OPCs are coupled through gap junctions to oligodendrocytes is unclear, but these progenitor cells are highly responsive to neural injury, suggesting a possible role in intercellular communication during nervous system repair or remyelination. In addition to the functional significance of gap junction coupling among myelinating glia, the physiological regulation of these channels is not well explored. Future research to elucidate the function and regulation of gap junctions in myelinating glia could lead to development of new therapeutic treatments for CMTX and PMLD and other myelin disorders, while deepening understanding of the means by which myelinating glia contribute to nervous system function and plasticity.
Non-standard abbreviations
- A
astrocyte
- O
oligodendrocyte
- KO
Knockout
- dKO
double knockout
References
- 1.Zalc B, Goujet D, Colman D. The origin of the myelination program in vertebrates. Curr Biol. 2008;18:R511–512. doi: 10.1016/j.cub.2008.04.010. [DOI] [PubMed] [Google Scholar]
- 2.Simons M, Trotter J. Wrapping it up: the cell biology of myelination. Curr Opin Neurobiol. 2007;17:533–540. doi: 10.1016/j.conb.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 3.Rosenbluth J. A brief history of myelinated nerve fibers: one hundred and fifty years of controversy. J Neurocytol. 1999;28:251–262. doi: 10.1023/a:1007083409850. [DOI] [PubMed] [Google Scholar]
- 4.Nave KA. Myelination and support of axonal integrity by glia. Nature. 2010;468:244–252. doi: 10.1038/nature09614. [DOI] [PubMed] [Google Scholar]
- 5.Fields RD. Neuroscience. Change in the brain's white matter. Science. 2010;330:768–769. doi: 10.1126/science.1199139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Salzer JL. Clustering sodium channels at the node of Ranvier: close encounters of the axon-glia kind. Neuron. 1997;18:843–846. doi: 10.1016/s0896-6273(00)80323-2. [DOI] [PubMed] [Google Scholar]
- 7.Jessen KR, Mirsky R. The origin and development of glial cells in peripheral nerves. Nature reviews. 2005;6:671–682. doi: 10.1038/nrn1746. [DOI] [PubMed] [Google Scholar]
- 8.Miron VE, Kuhlmann T, Antel JP. Cells of the oligodendroglial lineage, myelination, and remyelination. Biochimica et biophysica acta. 2011;1812:184–193. doi: 10.1016/j.bbadis.2010.09.010. [DOI] [PubMed] [Google Scholar]
- 9.Wake H, Lee PR, Fields RD. Control of Local Protein Synthesis and Initial Events in Myelination by Action Potentials. Science. 2011;333:1647–1651. doi: 10.1126/science.1206998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Allen NJ, Barres BA. Neuroscience: Glia - more than just brain glue. Nature. 2009;457:675–677. doi: 10.1038/457675a. [DOI] [PubMed] [Google Scholar]
- 11.Poliak S, Peles E. The local differentiation of myelinated axons at nodes of Ranvier. Nature Reviews. 2003;4:968–980. doi: 10.1038/nrn1253. [DOI] [PubMed] [Google Scholar]
- 12.Rash JE. Molecular disruptions of the panglial syncytium block potassium siphoning and axonal saltatory conduction: pertinence to neuromyelitis optica and other demyelinating diseases of the central nervous system. Neuroscience. 168:982–1008. doi: 10.1016/j.neuroscience.2009.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rash JE, Duffy HS, Dudek FE, Bilhartz BL, Whalen LR, Yasumura T. Grid- mapped freeze-fracture analysis of gap junctions in gray and white matter of adult rat central nervous system, with evidence for a “panglial syncytium” that is not coupled to neurons. The Journal of comparative neurology. 1997;388:265–292. doi: 10.1002/(sici)1096-9861(19971117)388:2<265::aid-cne6>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 14.Kamasawa N, Sik A, Morita M, Yasumura T, Davidson KG, Nagy JI, Rash JE. Connexin-47 and connexin-32 in gap junctions of oligodendrocyte somata, myelin sheaths, paranodal loops and Schmidt-Lanterman incisures: implications for ionic homeostasis and potassium siphoning. Neuroscience. 2005;136:65–86. doi: 10.1016/j.neuroscience.2005.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Theis M, Sohl G, Eiberger J, Willecke K. Emerging complexities in identity and function of glial connexins. Trends Neurosci. 2005;28:188–195. doi: 10.1016/j.tins.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 16.Alexopoulos H, Bottger A, Fischer S, Levin A, Wolf A, Fujisawa T, Hayakawa S, Gojobori T, Davies JA, David CN, Bacon JP. Evolution of gap junctions: the missing link? Curr Biol. 2004;14:R879–880. doi: 10.1016/j.cub.2004.09.067. [DOI] [PubMed] [Google Scholar]
- 17.Dahl G, Locovei S. Pannexin: to gap or not to gap, is that a question? IUBMB life. 2006;58:409–419. doi: 10.1080/15216540600794526. [DOI] [PubMed] [Google Scholar]
- 18.Fields RD, Burnstock G. Purinergic signalling in neuron-glia interactions. Nature reviews Neuroscience. 2006;7:423–436. doi: 10.1038/nrn1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Willecke K, Eiberger J, Degen J, Eckardt D, Romualdi A, Guldenagel M, Deutsch U, Sohl G. Structural and functional diversity of connexin genes in the mouse and human genome. Biological chemistry. 2002;383:725–737. doi: 10.1515/BC.2002.076. [DOI] [PubMed] [Google Scholar]
- 20.Bruzzone R, White TW, Paul DL. Connections with connexins: the molecular basis of direct intercellular signaling. Eur J Biochem. 1996;238:1–27. doi: 10.1111/j.1432-1033.1996.0001q.x. [DOI] [PubMed] [Google Scholar]
- 21.Barrio LC, Suchyna T, Bargiello T, Xu LX, Roginski RS, Bennett MV, Nicholson BJ. Gap junctions formed by connexins 26 and 32 alone and in combination are differently affected by applied voltage. Proceedings of the National Academy of Sciences of the United States of America. 1991;88:8410–8414. doi: 10.1073/pnas.88.19.8410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goodenough DA, Paul DL. Gap junctions. Cold Spring Harbor perspectives in biology. 2009;1:a002576. doi: 10.1101/cshperspect.a002576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lampe PD, Lau AF. Regulation of gap junctions by phosphorylation of connexins. Archives of biochemistry and biophysics. 2000;384:205–215. doi: 10.1006/abbi.2000.2131. [DOI] [PubMed] [Google Scholar]
- 24.Gomez-Hernandez JM, de Miguel M, Larrosa B, Gonzalez D, Barrio LC. Molecular basis of calcium regulation in connexin-32 hemichannels. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:16030–16035. doi: 10.1073/pnas.2530348100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang Y, Wu Y, Liu M, Wang J, Ju G. Nogo-66 inhibits the dye-coupling of astrocytic gap junctions in vitro. Neurochemical research. 2011;36:1129–1134. doi: 10.1007/s11064-011-0460-z. [DOI] [PubMed] [Google Scholar]
- 26.Balice-Gordon RJ, Bone LJ, Scherer SS. Functional gap junctions in the schwann cell myelin sheath. The Journal of cell biology. 1998;142:1095–1104. doi: 10.1083/jcb.142.4.1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harris AL. Emerging issues of connexin channels: biophysics fills the gap. Quarterly reviews of biophysics. 2001;34:325–472. doi: 10.1017/s0033583501003705. [DOI] [PubMed] [Google Scholar]
- 28.Bennett MV, Barrio LC, Bargiello TA, Spray DC, Hertzberg E, Saez JC. Gap junctions: new tools, new answers, new questions. Neuron. 1991;6:305–320. doi: 10.1016/0896-6273(91)90241-q. [DOI] [PubMed] [Google Scholar]
- 29.Sutor B, Hagerty T. Involvement of gap junctions in the development of the neocortex. Biochimica et biophysica acta. 2005;1719:59–68. doi: 10.1016/j.bbamem.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 30.Magnotti LM, Goodenough DA, Paul DL. Functional heterotypic interactions between astrocyte and oligodendrocyte connexins. Glia. 2011;59:26–34. doi: 10.1002/glia.21073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Scherer SS. Nodes, paranodes, and incisures: from form to function. Annals of the New York Academy of Sciences. 1999;883:131–142. [PubMed] [Google Scholar]
- 32.Ransom BR, Kettenmann H. Electrical coupling, without dye coupling, between mammalian astrocytes and oligodendrocytes in cell culture. Glia. 1990;3:258–266. doi: 10.1002/glia.440030405. [DOI] [PubMed] [Google Scholar]
- 33.Robinson SR, Hampson EC, Munro MN, Vaney DI. Unidirectional coupling of gap junctions between neuroglia. Science. 1993;262:1072–1074. doi: 10.1126/science.8093125. [DOI] [PubMed] [Google Scholar]
- 34.Zahs KR. Heterotypic coupling between glial cells of the mammalian central nervous system. Glia. 1998;24:85–96. [PubMed] [Google Scholar]
- 35.Butt AM, Ransom BR. Morphology of astrocytes and oligodendrocytes during development in the intact rat optic nerve. The Journal of comparative neurology. 1993;338:141–158. doi: 10.1002/cne.903380110. [DOI] [PubMed] [Google Scholar]
- 36.Berger T, Schnitzer J, Kettenmann H. Developmental changes in the membrane current pattern, K+ buffer capacity, and morphology of glial cells in the corpus callosum slice. J Neurosci. 1991;11:3008–3024. doi: 10.1523/JNEUROSCI.11-10-03008.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pastor A, Kremer M, Moller T, Kettenmann H, Dermietzel R. Dye coupling between spinal cord oligodendrocytes: differences in coupling efficiency between gray and white matter. Glia. 1998;24:108–120. [PubMed] [Google Scholar]
- 38.Kettenmann H, Ransom BR. Electrical coupling between astrocytes and between oligodendrocytes studied in mammalian cell cultures. Glia. 1988;1:64–73. doi: 10.1002/glia.440010108. [DOI] [PubMed] [Google Scholar]
- 39.Meier C, Dermietzel R, Davidson KG, Yasumura T, Rash JE. Connexin32- containing gap junctions in Schwann cells at the internodal zone of partial myelin compaction and in Schmidt-Lanterman incisures. J Neurosci. 2004;24:3186–3198. doi: 10.1523/JNEUROSCI.5146-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Konishi T. Dye coupling between mouse Schwann cells. Brain research. 1990;508:85–92. doi: 10.1016/0006-8993(90)91121-v. [DOI] [PubMed] [Google Scholar]
- 41.Chandross KJ, Kessler JA, Cohen RI, Simburger E, Spray DC, Bieri P, Dermietzel R. Altered connexin expression after peripheral nerve injury. Molecular and cellular neurosciences. 1996;7:501–518. doi: 10.1006/mcne.1996.0036. [DOI] [PubMed] [Google Scholar]
- 42.Chandross KJ, Spray DC, Cohen RI, Kumar NM, Kremer M, Dermietzel R, Kessler JA. TNF alpha inhibits Schwann cell proliferation, connexin46 expression, and gap junctional communication. Molecular and cellular neurosciences. 1996;7:479–500. doi: 10.1006/mcne.1996.0035. [DOI] [PubMed] [Google Scholar]
- 43.Li J, Habbes HW, Eiberger J, Willecke K, Dermietzel R, Meier C. Analysis of connexin expression during mouse Schwann cell development identifies connexin29 as a novel marker for the transition of neural crest to precursor cells. Glia. 2007;55:93–103. doi: 10.1002/glia.20427. [DOI] [PubMed] [Google Scholar]
- 44.Freidin M, Asche S, Bargiello TA, Bennett MV, Abrams CK. Connexin 32 increases the proliferative response of Schwann cells to neuregulin-1 (Nrg1) Proceedings of the National Academy of Sciences of the United States of America. 2009;106:3567–3572. doi: 10.1073/pnas.0813413106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Menichella DM, Goodenough DA, Sirkowski E, Scherer SS, Paul DL. Connexins are critical for normal myelination in the CNS. J Neurosci. 2003;23:5963–5973. doi: 10.1523/JNEUROSCI.23-13-05963.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Odermatt B, Wellershaus K, Wallraff A, Seifert G, Degen J, Euwens C, Fuss B, Bussow H, Schilling K, Steinhauser C, Willecke K. Connexin 47 (Cx47)-deficient mice with enhanced green fluorescent protein reporter gene reveal predominant oligodendrocytic expression of Cx47 and display vacuolized myelin in the CNS. J Neurosci. 2003;23:4549–4559. doi: 10.1523/JNEUROSCI.23-11-04549.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Parenti R, Cicirata F, Zappala A, Catania A, La Delia F, Cicirata V, Tress O, Willecke K. Dynamic expression of Cx47 in mouse brain development and in the cuprizone model of myelin plasticity. Glia. 2010;58:1594–1609. doi: 10.1002/glia.21032. [DOI] [PubMed] [Google Scholar]
- 48.Dermietzel R, Traub O, Hwang TK, Beyer E, Bennett MV, Spray DC, Willecke K. Differential expression of three gap junction proteins in developing and mature brain tissues. Proceedings of the National Academy of Sciences of the United States of America. 1989;86:10148–10152. doi: 10.1073/pnas.86.24.10148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Scherer SS, Deschenes SM, Xu YT, Grinspan JB, Fischbeck KH, Paul DL. Connexin32 is a myelin-related protein in the PNS and CNS. J Neurosci. 1995;15:8281–8294. doi: 10.1523/JNEUROSCI.15-12-08281.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Altevogt BM, Paul DL. Four classes of intercellular channels between glial cells in the CNS. J Neurosci. 2004;24:4313–4323. doi: 10.1523/JNEUROSCI.3303-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Altevogt BM, Kleopa KA, Postma FR, Scherer SS, Paul DL. Connexin29 is uniquely distributed within myelinating glial cells of the central and peripheral nervous systems. J Neurosci. 2002;22:6458–6470. doi: 10.1523/JNEUROSCI.22-15-06458.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nagy JI, Li X, Rempel J, Stelmack G, Patel D, Staines WA, Yasumura T, Rash JE. Connexin26 in adult rodent central nervous system: demonstration at astrocytic gap junctions and colocalization with connexin30 and connexin43. The Journal of comparative neurology. 2001;441:302–323. doi: 10.1002/cne.1414. [DOI] [PubMed] [Google Scholar]
- 53.Sohl G, Odermatt B, Maxeiner S, Degen J, Willecke K. New insights into the expression and function of neural connexins with transgenic mouse mutants. Brain Res Brain Res Rev. 2004;47:245–259. doi: 10.1016/j.brainresrev.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 54.Mercier F, Hatton GI. Connexin 26 and basic fibroblast growth factor are expressed primarily in the subpial and subependymal layers in adult brain parenchyma: roles in stem cell proliferation and morphological plasticity? The Journal of comparative neurology. 2001;431:88–104. doi: 10.1002/1096-9861(20010226)431:1<88::aid-cne1057>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 55.Filippov V, Kronenberg G, Pivneva T, Reuter K, Steiner B, Wang LP, Yamaguchi M, Kettenmann H, Kempermann G. Subpopulation of nestin-expressing progenitor cells in the adult murine hippocampus shows electrophysiological and morphological characteristics of astrocytes. Molecular and cellular neurosciences. 2003;23:373–382. doi: 10.1016/s1044-7431(03)00060-5. [DOI] [PubMed] [Google Scholar]
- 56.Maglione M, Tress O, Haas B, Karram K, Trotter J, Willecke K, Kettenmann H. Oligodendrocytes in mouse corpus callosum are coupled via gap junction channels formed by connexin47 and connexin32. Glia. 2010;58:1104–1117. doi: 10.1002/glia.20991. [DOI] [PubMed] [Google Scholar]
- 57.Nagy JI, Ionescu AV, Lynn BD, Rash JE. Connexin29 and connexin32 at oligodendrocyte and astrocyte gap junctions and in myelin of the mouse central nervous system. The Journal of comparative neurology. 2003;464:356–370. doi: 10.1002/cne.10797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rash JE, Yasumura T, Dudek FE, Nagy JI. Cell-specific expression of connexins and evidence of restricted gap junctional coupling between glial cells and between neurons. J Neurosci. 2001;21:1983–2000. doi: 10.1523/JNEUROSCI.21-06-01983.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rash JE, Yasumura T, Davidson KG, Furman CS, Dudek FE, Nagy JI. Identification of cells expressing Cx43, Cx30, Cx26, Cx32 and Cx36 in gap junctions of rat brain and spinal cord. Cell communication & adhesion. 2001;8:315–320. doi: 10.3109/15419060109080745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wasseff SK, Scherer SS. Cx32 and Cx47 mediate oligodendrocyte:astrocyte and oligodendrocyte:oligodendrocyte gap junction coupling. Neurobiology of disease. 2010;42:506–513. doi: 10.1016/j.nbd.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Butt AM, Ransom BR. Visualization of oligodendrocytes and astrocytes in the intact rat optic nerve by intracellular injection of lucifer yellow and horseradish peroxidase. Glia. 1989;2:470–475. doi: 10.1002/glia.440020609. [DOI] [PubMed] [Google Scholar]
- 62.Orthmann-Murphy JL, Freidin M, Fischer E, Scherer SS, Abrams CK. Two distinct heterotypic channels mediate gap junction coupling between astrocyte and oligodendrocyte connexins. J Neurosci. 2007;27:13949–13957. doi: 10.1523/JNEUROSCI.3395-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Haas B, Schipke CG, Peters O, Sohl G, Willecke K, Kettenmann H. Activity- dependent ATP-waves in the mouse neocortex are independent from astrocytic calcium waves. Cereb Cortex. 2006;16:237–246. doi: 10.1093/cercor/bhi101. [DOI] [PubMed] [Google Scholar]
- 64.Sargiannidou I, Vavlitou N, Aristodemou S, Hadjisavvas A, Kyriacou K, Scherer SS, Kleopa KA. Connexin32 mutations cause loss of function in Schwann cells and oligodendrocytes leading to PNS and CNS myelination defects. J Neurosci. 2009;29:4736–4749. doi: 10.1523/JNEUROSCI.0325-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wallraff A, Kohling R, Heinemann U, Theis M, Willecke K, Steinhauser C. The impact of astrocytic gap junctional coupling on potassium buffering in the hippocampus. J Neurosci. 2006;26:5438–5447. doi: 10.1523/JNEUROSCI.0037-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lutz SE, Zhao Y, Gulinello M, Lee SC, Raine CS, Brosnan CF. Deletion of astrocyte connexins 43 and 30 leads to a dysmyelinating phenotype and hippocampal CA1 vacuolation. J Neurosci. 2009;29:7743–7752. doi: 10.1523/JNEUROSCI.0341-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Thompson RJ, Macvicar BA. Connexin and pannexin hemichannels of neurons and astrocytes. Channels. 2008:81–86. doi: 10.4161/chan.2.2.6003. [DOI] [PubMed] [Google Scholar]
- 68.Burnstock G, Fredholm BB, Verkhratsky A. Adenosine and ATP receptors in the brain. Current topics in medicinal chemistry. 2011;11:973–1011. doi: 10.2174/156802611795347627. [DOI] [PubMed] [Google Scholar]
- 69.Cotrina ML, Lin JH, Alves-Rodrigues A, Liu S, Li J, Azmi-Ghadimi H, Kang J, Naus CC, Nedergaard M. Connexins regulate calcium signaling by controlling ATP release. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:15735–15740. doi: 10.1073/pnas.95.26.15735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pascual O, Casper KB, Kubera C, Zhang J, Revilla-Sanchez R, Sul JY, Takano H, Moss SJ, McCarthy K, Haydon PG. Astrocytic purinergic signaling coordinates synaptic networks. Science. 2005;310:113–116. doi: 10.1126/science.1116916. [DOI] [PubMed] [Google Scholar]
- 71.Kang J, Kang N, Lovatt D, Torres A, Zhao Z, Lin J, Nedergaard M. Connexin 43 hemichannels are permeable to ATP. J Neurosci. 2008;28:4702–4711. doi: 10.1523/JNEUROSCI.5048-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Paemeleire K, Martin PE, Coleman SL, Fogarty KE, Carrington WA, Leybaert L, Tuft RA, Evans WH, Sanderson MJ. Intercellular calcium waves in HeLa cells expressing GFP-labeled connexin 43, 32, or 26. Molecular biology of the cell. 2000;11:1815–1827. doi: 10.1091/mbc.11.5.1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Braet K, Aspeslagh S, Vandamme W, Willecke K, Martin PE, Evans WH, Leybaert L. Pharmacological sensitivity of ATP release triggered by photoliberation of inositol-1,4,5-trisphosphate and zero extracellular calcium in brain endothelial cells. Journal of cellular physiology. 2003;197:205–213. doi: 10.1002/jcp.10365. [DOI] [PubMed] [Google Scholar]
- 74.De Vuyst E, Wang N, Decrock E, De Bock M, Vinken M, Van Moorhem M, Lai C, Culot M, Rogiers V, Cecchelli R, Naus CC, Evans WH, Leybaert L. Ca(2+) regulation of connexin 43 hemichannels in C6 glioma and glial cells. Cell calcium. 2009;46:176–187. doi: 10.1016/j.ceca.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 75.Iglesias R, Dahl G, Qiu F, Spray DC, Scemes E. Pannexin 1: the molecular substrate of astrocyte “hemichannels”. J Neurosci. 2009;29:7092–7097. doi: 10.1523/JNEUROSCI.6062-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Baranova A, Ivanov D, Petrash N, Pestova A, Skoblov M, Kelmanson I, Shagin D, Nazarenko S, Geraymovych E, Litvin O, Tiunova A, Born TL, Usman N, Staroverov D, Lukyanov S, Panchin Y. The mammalian pannexin family is homologous to the invertebrate innexin gap junction proteins. Genomics. 2004;83:706–716. doi: 10.1016/j.ygeno.2003.09.025. [DOI] [PubMed] [Google Scholar]
- 77.Silverman WR, de Rivero Vaccari JP, Locovei S, Qiu F, Carlsson SK, Scemes E, Keane RW, Dahl G. The pannexin 1 channel activates the inflammasome in neurons and astrocytes. The Journal of biological chemistry. 2009;284:18143–18151. doi: 10.1074/jbc.M109.004804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Silverman W, Locovei S, Dahl G. Probenecid, a gout remedy, inhibits pannexin 1 channels. American journal of physiology. 2008;295:C761–767. doi: 10.1152/ajpcell.00227.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ransford GA, Fregien N, Qiu F, Dahl G, Conner GE, Salathe M. Pannexin 1 contributes to ATP release in airway epithelia. American journal of respiratory cell and molecular biology. 2009;41:525–534. doi: 10.1165/rcmb.2008-0367OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Locovei S, Bao L, Dahl G. Pannexin 1 in erythrocytes: function without a gap. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:7655–7659. doi: 10.1073/pnas.0601037103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bao L, Locovei S, Dahl G. Pannexin membrane channels are mechanosensitive conduits for ATP. FEBS letters. 2004;572:65–68. doi: 10.1016/j.febslet.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 82.Bruzzone R, Hormuzdi SG, Barbe MT, Herb A, Monyer H. Pannexins, a family of gap junction proteins expressed in brain. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:13644–13649. doi: 10.1073/pnas.2233464100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pelegrin P, Barroso-Gutierrez C, Surprenant A. P2X7 receptor differentially couples to distinct release pathways for IL-1beta in mouse macrophage. J Immunol. 2008;180:7147–7157. doi: 10.4049/jimmunol.180.11.7147. [DOI] [PubMed] [Google Scholar]
- 84.Iglesias R, Locovei S, Roque A, Alberto AP, Dahl G, Spray DC, Scemes E. P2X7 receptor-Pannexin1 complex: pharmacology and signaling. American journal of physiology. 2008;295:C752–760. doi: 10.1152/ajpcell.00228.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yan Z, Khadra A, Sherman A, Stojilkovic SS. Calcium-dependent block of P2X7 receptor channel function is allosteric. The Journal of general physiology. 2011;138:437–452. doi: 10.1085/jgp.201110647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Li S, Tomic M, Stojilkovic SS. Characterization of novel Pannexin 1 isoforms from rat pituitary cells and their association with ATP-gated P2X channels. General and comparative endocrinology. 2011;174:202–210. doi: 10.1016/j.ygcen.2011.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bahima L, Aleu J, Elias M, Martin-Satue M, Muhaisen A, Blasi J, Marsal J, Solsona C. Endogenous hemichannels play a role in the release of ATP from Xenopus oocytes. Journal of cellular physiology. 2006;206:95–102. doi: 10.1002/jcp.20440. [DOI] [PubMed] [Google Scholar]
- 88.Huckstepp RT, Eason R, Sachdev A, Dale N. CO2-dependent opening of connexin 26 and related beta connexins. The Journal of physiology. 2010;588:3921–3931. doi: 10.1113/jphysiol.2010.192096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Huckstepp RT, id Bihi R, Eason R, Spyer KM, Dicke N, Willecke K, Marina N, Gourine AV, Dale N. Connexin hemichannel-mediated CO2-dependent release of ATP in the medulla oblongata contributes to central respiratory chemosensitivity. The Journal of physiology. 2010;588:3901–3920. doi: 10.1113/jphysiol.2010.192088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tran Van Nhieu G, Clair C, Bruzzone R, Mesnil M, Sansonetti P, Combettes L. Connexin-dependent inter-cellular communication increases invasion and dissemination of Shigella in epithelial cells. Nature cell biology. 2003;5:720–726. doi: 10.1038/ncb1021. [DOI] [PubMed] [Google Scholar]
- 91.Majumder P, Crispino G, Rodriguez L, Ciubotaru CD, Anselmi F, Piazza V, Bortolozzi M, Mammano F. ATP-mediated cell-cell signaling in the organ of Corti: the role of connexin channels. Purinergic signalling. 2010;6:167–187. doi: 10.1007/s11302-010-9192-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wong CW, Christen T, Roth I, Chadjichristos CE, Derouette JP, Foglia BF, Chanson M, Goodenough DA, Kwak BR. Connexin37 protects against atherosclerosis by regulating monocyte adhesion. Nature medicine. 2006;12:950–954. doi: 10.1038/nm1441. [DOI] [PubMed] [Google Scholar]
- 93.De Vuyst E, Decrock E, Cabooter L, Dubyak GR, Naus CC, Evans WH, Leybaert L. Intracellular calcium changes trigger connexin 32 hemichannel opening. The EMBO journal. 2006;25:34–44. doi: 10.1038/sj.emboj.7600908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Liu GJ, Bennett MR. ATP secretion from nerve trunks and Schwann cells mediated by glutamate. Neuroreport. 2003;14:2079–2083. doi: 10.1097/00001756-200311140-00014. [DOI] [PubMed] [Google Scholar]
- 95.Liu GJ, Werry EL, Bennett MR. Secretion of ATP from Schwann cells in response to uridine triphosphate. The European journal of neuroscience. 2005;21:151–160. doi: 10.1111/j.1460-9568.2004.03831.x. [DOI] [PubMed] [Google Scholar]
- 96.Uhlenberg B, Schuelke M, Ruschendorf F, Ruf N, Kaindl AM, Henneke M, Thiele H, Stoltenburg-Didinger G, Aksu F, Topaloglu H, Nurnberg P, Hubner C, Weschke B, Gartner J. Mutations in the gene encoding gap junction protein alpha 12 (connexin 46.6) cause Pelizaeus-Merzbacher-like disease. American journal of human genetics. 2004;75:251–260. doi: 10.1086/422763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Orthmann-Murphy JL, Enriquez AD, Abrams CK, Scherer SS. Loss-of-function GJA12/Connexin47 mutations cause Pelizaeus-Merzbacher-like disease. Molecular and cellular neurosciences. 2007;34:629–641. doi: 10.1016/j.mcn.2007.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Osaka H, Hamanoue H, Yamamoto R, Nezu A, Sasaki M, Saitsu H, Kurosawa K, Shimbo H, Matsumoto N, Inoue K. Disrupted SOX10 regulation of GJC2 transcription causes Pelizaeus-Merzbacher-like disease. Annals of neurology. 2010;68:250–254. doi: 10.1002/ana.22022. [DOI] [PubMed] [Google Scholar]
- 99.Tress O, Maglione M, Zlomuzica A, May D, Dicke N, Degen J, Dere E, Kettenmann H, Hartmann D, Willecke K. Pathologic and phenotypic alterations in a mouse expressing a connexin47 missense mutation that causes Pelizaeus-Merzbacher-like disease in humans. PLoS genetics. 2011;7:e1002146. doi: 10.1371/journal.pgen.1002146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Young P, Suter U. The causes of Charcot-Marie-Tooth disease. Cell Mol Life Sci. 2003;60:2547–2560. doi: 10.1007/s00018-003-3133-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Scherer SS, Xu YT, Nelles E, Fischbeck K, Willecke K, Bone LJ. Connexin32-null mice develop demyelinating peripheral neuropathy. Glia. 1998;24:8–20. doi: 10.1002/(sici)1098-1136(199809)24:1<8::aid-glia2>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 102.Yum SW, Kleopa KA, Shumas S, Scherer SS. Diverse trafficking abnormalities of connexin32 mutants causing CMTX. Neurobiology of disease. 2002;11:43–52. doi: 10.1006/nbdi.2002.0545. [DOI] [PubMed] [Google Scholar]
- 103.Abrams CK, Bennett MV, Verselis VK, Bargiello TA. Voltage opens unopposed gap junction hemichannels formed by a connexin 32 mutant associated with X-linked Charcot-Marie-Tooth disease. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:3980–3984. doi: 10.1073/pnas.261713499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Oh S, Ri Y, Bennett MV, Trexler EB, Verselis VK, Bargiello TA. Changes in permeability caused by connexin 32 mutations underlie X-linked Charcot-Marie-Tooth disease. Neuron. 1997;19:927–938. doi: 10.1016/s0896-6273(00)80973-3. [DOI] [PubMed] [Google Scholar]
- 105.Bruzzone R, White TW, Scherer SS, Fischbeck KH, Paul DL. Null mutations of connexin32 in patients with X-linked Charcot-Marie-Tooth disease. Neuron. 1994;13:1253–1260. doi: 10.1016/0896-6273(94)90063-9. [DOI] [PubMed] [Google Scholar]
- 106.Kleopa KA, Yum SW, Scherer SS. Cellular mechanisms of connexin32 mutations associated with CNS manifestations. Journal of neuroscience research. 2002;68:522–534. doi: 10.1002/jnr.10255. [DOI] [PubMed] [Google Scholar]
- 107.Murru MR, Vannelli A, Marrosu G, Cocco E, Corongiu D, Tranquilli S, Cherchi MV, Mura M, Barberini L, Mallarini G, Marrosu MG. A novel Cx32 mutation causes X-linked Charcot-Marie-Tooth disease with brainstem involvement and brain magnetic resonance spectroscopy abnormalities. Neurol Sci. 2006;27:18–23. doi: 10.1007/s10072-006-0560-8. [DOI] [PubMed] [Google Scholar]
- 108.Bondurand N, Girard M, Pingault V, Lemort N, Dubourg O, Goossens M. Human Connexin 32, a gap junction protein altered in the X-linked form of Charcot-Marie-Tooth disease, is directly regulated by the transcription factor SOX10. Human molecular genetics. 2001;10:2783–2795. doi: 10.1093/hmg/10.24.2783. [DOI] [PubMed] [Google Scholar]
- 109.Houlden H, Girard M, Cockerell C, Ingram D, Wood NW, Goossens M, Walker RW, Reilly MM. Connexin 32 promoter P2 mutations: a mechanism of peripheral nerve dysfunction. Annals of neurology. 2004;56:730–734. doi: 10.1002/ana.20267. [DOI] [PubMed] [Google Scholar]
- 110.Stumpel F, Ott T, Willecke K, Jungermann K. Connexin 32 gap junctions enhance stimulation of glucose output by glucagon and noradrenaline in mouse liver. Hepatology. 1998;28:1616–1620. doi: 10.1002/hep.510280622. [DOI] [PubMed] [Google Scholar]
- 111.Temme A, Buchmann A, Gabriel HD, Nelles E, Schwarz M, Willecke K. High incidence of spontaneous and chemically induced liver tumors in mice deficient for connexin32. Curr Biol. 1997;7:713–716. doi: 10.1016/s0960-9822(06)00302-2. [DOI] [PubMed] [Google Scholar]
- 112.Anzini P, Neuberg DH, Schachner M, Nelles E, Willecke K, Zielasek J, Toyka KV, Suter U, Martini R. Structural abnormalities and deficient maintenance of peripheral nerve myelin in mice lacking the gap junction protein connexin 32. J Neurosci. 1997;17:4545–4551. doi: 10.1523/JNEUROSCI.17-12-04545.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Scherer SS, Xu YT, Messing A, Willecke K, Fischbeck KH, Jeng LJ. Transgenic expression of human connexin32 in myelinating Schwann cells prevents demyelination in connexin32-null mice. J Neurosci. 2005;25:1550–1559. doi: 10.1523/JNEUROSCI.3082-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Neuberg DH, Sancho S, Suter U. Altered molecular architecture of peripheral nerves in mice lacking the peripheral myelin protein 22 or connexin32. Journal of neuroscience research. 1999;58:612–623. doi: 10.1002/(sici)1097-4547(19991201)58:5<612::aid-jnr2>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 115.Nicholson SM, Gomes D, de Nechaud B, Bruzzone R. Altered gene expression in Schwann cells of connexin32 knockout animals. Journal of neuroscience research. 2001;66:23–36. doi: 10.1002/jnr.1194. [DOI] [PubMed] [Google Scholar]
- 116.Melanson-Drapeau L, Beyko S, Dave S, Hebb AL, Franks DJ, Sellitto C, Paul DL, Bennett SA. Oligodendrocyte progenitor enrichment in the connexin32 null-mutant mouse. J Neurosci. 2003;23:1759–1768. doi: 10.1523/JNEUROSCI.23-05-01759.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Takahashi-Iwanaga H, Nio-Kobayashi J, Habara Y, Furuya K. A dual system of intercellular calcium signaling in glial nets associated with lanceolate sensory endings in rat vibrissae. The Journal of comparative neurology. 2008;510:68–78. doi: 10.1002/cne.21756. [DOI] [PubMed] [Google Scholar]
- 118.Palacios-Prado N, Bukauskas FF. Modulation of metabolic communication through gap junction channels by transjunctional voltage; synergistic and antagonistic effects of gating and ionophoresis. Biochimica et biophysica acta. 2011 doi: 10.1016/j.bbamem.2011.09.001. doi:10.1016/j.bbamem.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]