Abstract
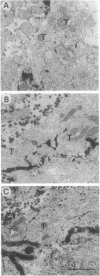
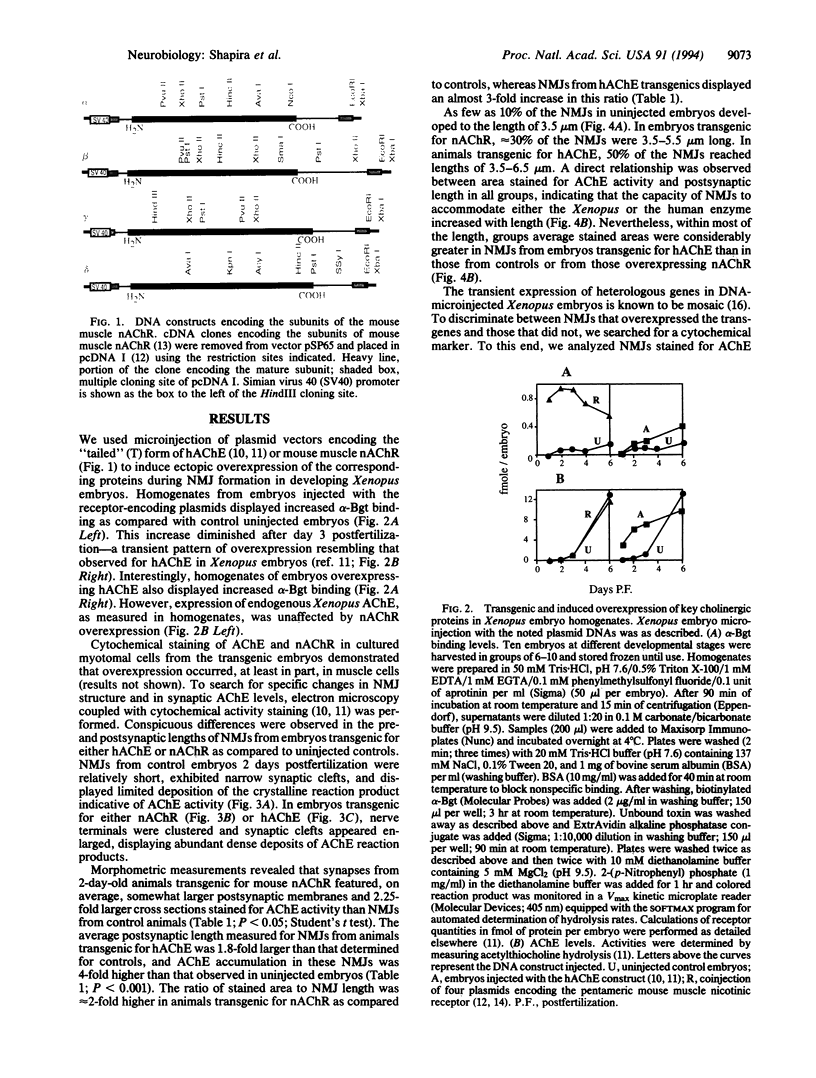
To examine the role of key cholinergic proteins in the formation of neuromuscular junctions (NMJs), we expressed DNAs encoding the mouse muscle nicotinic acetylcholine receptor (nAChR) or human brain and muscle acetylcholinesterase (hAChE) in developing Xenopus laevis embryos. Acetylthiocholine hydrolysis and alpha-bungarotoxin binding in homogenates of transgenic embryos revealed transient overexpression of the respective proteins for at least 4 days postfertilization. Moreover, hAChE injection induced an approximately 2-fold increase in endogenous Xenopus nAChR. Electron microscopy coupled with cytochemical staining for AChE activity revealed that AChE-stained areas, which reached 0.17 microns2 in NMJs of control embryos raised at 21 degrees C, increased up to 0.53 and 0.60 microns2 in nAChR and hAChE transgenics, respectively. These increases coincided with the appearance of a class of large NMJs with average postsynaptic lengths up to 1.8-fold greater than controls. As much as 57% and 34% of the NMJs in animals transgenic for nAChR and hAChE, respectively, displayed AChE activity in nerve terminals in addition to muscle labeling, as compared with 10% nerve-labeled NMJs in control animals. Moreover, area, but not length values, were > 2-fold larger in hAChE-expressing NMJs labeled in their nerve terminals than in those labeled in muscle alone, reflecting a hAChE-induced increase in synaptic cleft width. These findings indicate that modulation of cholinergic neurotransmission in NMJs modifies the features of nerve-muscle connections.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alfonso A., Grundahl K., Duerr J. S., Han H. P., Rand J. B. The Caenorhabditis elegans unc-17 gene: a putative vesicular acetylcholine transporter. Science. 1993 Jul 30;261(5121):617–619. doi: 10.1126/science.8342028. [DOI] [PubMed] [Google Scholar]
- Anglister L., McMahan U. J. Basal lamina directs acetylcholinesterase accumulation at synaptic sites in regenerating muscle. J Cell Biol. 1985 Sep;101(3):735–743. doi: 10.1083/jcb.101.3.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anglister L., Stiles J. R., Salpeter M. M. Acetylcholinesterase density and turnover number at frog neuromuscular junctions, with modeling of their role in synaptic function. Neuron. 1994 Apr;12(4):783–794. doi: 10.1016/0896-6273(94)90331-x. [DOI] [PubMed] [Google Scholar]
- Ben Aziz-Aloya R., Seidman S., Timberg R., Sternfeld M., Zakut H., Soreq H. Expression of a human acetylcholinesterase promoter-reporter construct in developing neuromuscular junctions of Xenopus embryos. Proc Natl Acad Sci U S A. 1993 Mar 15;90(6):2471–2475. doi: 10.1073/pnas.90.6.2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Changeux J. P. Compartmentalized transcription of acetylcholine receptor genes during motor endplate epigenesis. New Biol. 1991 May;3(5):413–429. [PubMed] [Google Scholar]
- Cohen M. W. Development of an amphibian neuromuscular junction in vivo and in culture. J Exp Biol. 1980 Dec;89:43–56. doi: 10.1242/jeb.89.1.43. [DOI] [PubMed] [Google Scholar]
- Duval N., Massoulié J., Bon S. H and T subunits of acetylcholinesterase from Torpedo, expressed in COS cells, generate all types of globular forms. J Cell Biol. 1992 Aug;118(3):641–653. doi: 10.1083/jcb.118.3.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall Z. W., Sanes J. R. Synaptic structure and development: the neuromuscular junction. Cell. 1993 Jan;72 (Suppl):99–121. doi: 10.1016/s0092-8674(05)80031-5. [DOI] [PubMed] [Google Scholar]
- Han H. Q., Nichols R. A., Rubin M. R., Bähler M., Greengard P. Induction of formation of presynaptic terminals in neuroblastoma cells by synapsin IIb. Nature. 1991 Feb 21;349(6311):697–700. doi: 10.1038/349697a0. [DOI] [PubMed] [Google Scholar]
- Jennekens F. G., Hesselmans L. F., Veldman H., Jansen E. N., Spaans F., Molenaar P. C. Deficiency of acetylcholine receptors in a case of end-plate acetylcholinesterase deficiency: a histochemical investigation. Muscle Nerve. 1992 Jan;15(1):63–72. doi: 10.1002/mus.880150112. [DOI] [PubMed] [Google Scholar]
- Jennings C. G., Burden S. J. Development of the neuromuscular synapse. Curr Opin Neurobiol. 1993 Feb;3(1):75–81. doi: 10.1016/0959-4388(93)90038-z. [DOI] [PubMed] [Google Scholar]
- Lauder J. M. Neurotransmitters as growth regulatory signals: role of receptors and second messengers. Trends Neurosci. 1993 Jun;16(6):233–240. doi: 10.1016/0166-2236(93)90162-f. [DOI] [PubMed] [Google Scholar]
- Laufer R., Changeux J. P. Activity-dependent regulation of gene expression in muscle and neuronal cells. Mol Neurobiol. 1989 Spring-Summer;3(1-2):1–53. doi: 10.1007/BF02935587. [DOI] [PubMed] [Google Scholar]
- Liang P., Pardee A. B. Differential display of eukaryotic messenger RNA by means of the polymerase chain reaction. Science. 1992 Aug 14;257(5072):967–971. doi: 10.1126/science.1354393. [DOI] [PubMed] [Google Scholar]
- McMahan U. J. The agrin hypothesis. Cold Spring Harb Symp Quant Biol. 1990;55:407–418. doi: 10.1101/sqb.1990.055.01.041. [DOI] [PubMed] [Google Scholar]
- Neville L. F., Gnatt A., Loewenstein Y., Seidman S., Ehrlich G., Soreq H. Intramolecular relationships in cholinesterases revealed by oocyte expression of site-directed and natural variants of human BCHE. EMBO J. 1992 Apr;11(4):1641–1649. doi: 10.1002/j.1460-2075.1992.tb05210.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrick J., Boulter J., Goldman D., Gardner P., Heinemann S. Molecular biology of nicotinic acetylcholine receptors. Ann N Y Acad Sci. 1987;505:194–207. doi: 10.1111/j.1749-6632.1987.tb51292.x. [DOI] [PubMed] [Google Scholar]
- Quinn A., Harrison R., Jehanli A. M., Lunt G. G., Walsh S. An ELISA for the detection of anti-acetylcholine receptor antibodies using biotinylated alpha-bungarotoxin. J Immunol Methods. 1988 Mar 16;107(2):197–203. doi: 10.1016/0022-1759(88)90218-9. [DOI] [PubMed] [Google Scholar]
- Salpeter M. M., Loring R. H. Nicotinic acetylcholine receptors in vertebrate muscle: properties, distribution and neural control. Prog Neurobiol. 1985;25(4):297–325. doi: 10.1016/0301-0082(85)90018-8. [DOI] [PubMed] [Google Scholar]
- Seed B. An LFA-3 cDNA encodes a phospholipid-linked membrane protein homologous to its receptor CD2. 1987 Oct 29-Nov 4Nature. 329(6142):840–842. doi: 10.1038/329840a0. [DOI] [PubMed] [Google Scholar]
- Seidman S., Aziz-Aloya R. B., Timberg R., Loewenstein Y., Velan B., Shafferman A., Liao J., Norgaard-Pedersen B., Brodbeck U., Soreq H. Overexpressed monomeric human acetylcholinesterase induces subtle ultrastructural modifications in developing neuromuscular junctions of Xenopus laevis embryos. J Neurochem. 1994 May;62(5):1670–1681. doi: 10.1046/j.1471-4159.1994.62051670.x. [DOI] [PubMed] [Google Scholar]
- Soreq H., Seidman S. Xenopus oocyte microinjection: from gene to protein. Methods Enzymol. 1992;207:225–265. doi: 10.1016/0076-6879(92)07016-h. [DOI] [PubMed] [Google Scholar]
- Tucek S. The synthesis of acetylcholine: twenty years of progress. Prog Brain Res. 1990;84:467–477. [PubMed] [Google Scholar]
- Vize P. D., Melton D. A., Hemmati-Brivanlou A., Harland R. M. Assays for gene function in developing Xenopus embryos. Methods Cell Biol. 1991;36:367–387. doi: 10.1016/s0091-679x(08)60288-5. [DOI] [PubMed] [Google Scholar]
- Wyttenbach C. R., Thompson S. C. The effects of the organophosphate insecticide malathion on very young chick embryos: malformations detected by histological examination. Am J Anat. 1985 Oct;174(2):187–202. doi: 10.1002/aja.1001740208. [DOI] [PubMed] [Google Scholar]