Abstract
Siglecs are immunoglobulin lectin group proteins that recognize the sialic acid moiety. We previously reported that the expression of Siglec-9 on the macrophage cell line RAW264 markedly enhanced Toll-like receptor (TLR)-induced interleukin (IL)-10 production and inhibited the production of proinflammatory cytokines. In this study, we examined the lectin-dependent anti-inflammatory activities of Siglec-9. IL-10 production was modestly reduced by a mutation that disrupted the lectin activity of Siglec-9, while the reduction in tumor necrosis factor-α was not affected. Membrane fractionation experiments revealed that a part of Siglec-9 resided in the detergent-insoluble microdomain, the so-called lipid raft fraction. The amount of Siglec-9 in the lipid raft fraction rapidly increased following TLR2 stimulation by peptidoglycan and peaked after 3–10 min. This time course was similar to that of TLR2. The double tyrosine mutant in immunoreceptor tyrosine-based inhibitory motifs moved to lipid rafts in a similar manner, while lectin-defective Siglec-9 was not detected in the lipid raft fraction. The production of IL-10 was partially reduced by cholesterol oxidase that disturbed lipid raft organization. Taken together, these results suggest that Siglecs exhibit lectin-dependent changes in cellular localization, which may be partly linked to its control mechanism that increases the production of IL-10.
Keywords: Siglec, Lipid raft, Lectin, IL-10, Inflammation
Introduction
Sialic acids, which cover the cell surface as the terminal of glycosylation, play various roles in the regulation of immune responses (Pilatte et al. 1993). Although their roles are thought to be mainly mediated by electric repulsion due to a negative charge, cell surface lectins that recognize the sialic acid moiety are also involved in immune regulation. Siglecs are sialic acid-recognizing Ig-superfamily lectins prominently expressed in immune cells (Crocker et al. 2012; Pillai et al. 2012). The members of CD33-related Siglecs were previously shown to downregulate both innate and acquired immune responses possibly via cytosolic immunoreceptor tyrosine-based inhibitory motifs (ITIMs) (Crocker et al. 2012; Pillai et al. 2012). These ITIMs recruit protein tyrosine phosphatases, which contain the Src homology region 2 domain. The phosphatase activities of Src homology region 2 domain-containing tyrosine phosphatase (SHP)-1 and SHP-2 have been shown to suppress the activation pathways mediated by immunoreceptor tyrosine-based activating motifs (ITAMs) (Ravetch and Lanier 2000).
Innate immunity functions as a pathogen sensor and contributes to the eradication of pathogens and establishment of adaptive immunity. A group of transmembrane proteins, Toll-like receptors (TLRs), recognize a range of chemicals produced by bacteria, viruses, fungi, and protozoa to initiate the first line of defense against pathogens (Kondo et al. 2012; West et al. 2006). Each TLR recognizes specific structures as a pattern-recognition receptor. The importance of TLRs in pattern-recognition has been demonstrated in gene-targeted mice: the lack of a single TLR sometimes leads to the complete abrogation of an immune response against several types of pathogens.
Lipid rafts are microdomains of the plasma membrane that contain high concentrations of cholesterol and glycosphingolipids. Historically, lipid rafts have been defined functionally by their low density and insolubility in cold 1 % Triton X-100. It is generally accepted that lipid rafts can be both positive and negative regulators, possibly by controlling the physical recruitment or sequestration of proteins involved in activating and suppressing signaling. For example, ligand-specific TLR4 clustering and signaling in lipid rafts following lipopolysaccharide (LPS) stimulation of monocytes were reported previously (Triantafilou et al. 2002). Although the translocation of TLRs to the lipid rafts is partial and transient, the critical importance of lipid rafts in the regulation of TLR-induced inflammation had been demonstrated (Fessler and Parks 2011; Yvan-Charvet et al. 2008; Zhu et al. 2008, 2010). Recent studies reported that an increase in the cholesterol content in the plasma membrane led to enlarged lipid rafts that contained TLRs (Zhu et al. 2008, 2010). These phenotypes were associated with the hyper-responsivity of TLR4, 2, 7, and 9 (Yvan-Charvet et al. 2008; Zhu et al. 2008).
We previously demonstrated that the expression of Siglec-9 modulated inflammatory and anti-inflammatory responses mediated by TLRs in RAW264 cells (Ando et al. 2008). In the present study, we examined the requirement of the lectin activity of Siglec-9 for this modulation, and found that the enhanced production of interleukin (IL)-10 partly depended on its lectin activity, which may be associated with the localization of Siglec to the lipid rafts.
Materials and methods
Reagents and antibodies
Peptidoglycan (PGN) from Staphylococcus aureus, LPS from Escherichia coli 0111:B4, and cholesterol oxidase form Cellulomonas sp. were purchased from Sigma Aldrich (St. Louis, MO, USA). Sialidase derived from Streptococcus sp. was from Seikagaku Corporation (Tokyo, Japan). The anti-human Siglec-9 antibody (goat polyclonal antibody; AF1139) was purchased from R&D Systems (Minneapolis, MN, USA). The polyclonal antibodies toward flotillin-1 (H-104), CD46 (H-294), SHP-1 (C-19), SHP-2 (C-18), and TLR2 (H-175) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Biotin-labeled B subunit of cholera toxin (CTxB-biotin), which binds GM1, was purchased from Wako Pure Chemicals (Osaka, Japan). The OptEIA enzyme linked immunosorbent assay (ELISA) sets for tumor necrosis factor (TNF)-α and IL-10 were from BD PharMingen (San Jose, CA, USA).
Cells and cultures
Mouse RAW264 macrophage cell line was obtained from the Riken BioResource Center (Tsukuba, Japan) and maintained in RPMI1640 (Nissui Pharmaceutical, Tokyo, Japan) containing 10 % heat-inactivated fetal calf serum (PAA Laboratories, Pasching, Austria), 0.03 % l-glutamine (Wako Pure Chemical Industries, Osaka, Japan), 5 × 10−5 M 2-mercaptoethanol (Wako Pure Chemical Industries), 100 U/ml penicillin G (Wako Pure Chemical Industries), and 100 μg/ml streptomycin (Wako Pure Chemical Industries). RAW264 cells expressing human Siglec-9 and Siglec-9YFYF were described previously (Ando et al. 2008). Mock-transfected RAW264 cells were used as control cells.
Regarding the TLR stimulation, RAW264 cells (4 × 104) were cultured in microtiter plates in 100 μl of serum-free RPMI1640 with sialidase or cholesterol oxidase for 30 min, followed by the stimulation with LPS or PGN in serum-containing medium. The amount of TNF-α or IL-10 in the culture supernatant was measured by ELISA. The data are shown as the mean ± standard deviation.
Isolation of lipid rafts
RAW264 cells (2 × 108) were lysed in 1.5 ml of lysis buffer (TNE buffer (25 mM Tris–HCl, pH 7.5, 150 mM NaCl, 5 mM EDTA) containing 1 % Triton X100 (Calbiochem, La Jolla, CA, USA), 20 μg/ml aprotinin (Wako Pure Chemical Industries), 100 μg/ml phenylmethylsulfonylfluoride (Wako Pure Chemical Industries), 1 mM Na3VO4 (Wako Pure Chemical Industries), 10 μM Na2MoO4 (Wako Pure Chemical Industries), and 10 mM NaF (Wako Pure Chemical Industries)) for 1 h on ice. The membrane fraction was collected after centrifugation at 9,200×g for 10 min. The total volume of the sample was adjusted to 1.75 ml by TNE buffer and mixed with an equal volume of 85 % sucrose in TNE buffer. The sample was placed in the bottom of a centrifuge tube, and overlaid with 6 ml of 30 % sucrose and 3.5 ml of 5 % sucrose in TNE buffer. The sample was centrifuged at 35,000 rpm for 18 h at 4 °C (RPS40T rotor, Hitachi Koki, Tokyo, Japan) and fractions of 1 ml each were gently collected from the top of the gradient to the bottom. A pooled raft sample, corresponding to fractions 3–5 based on the GM1 enrichment, was concentrated by centrifugation using Amicon Ultra (Millipore, Billerica, MA, USA). Cells were incubated with 50 μg/ml of PGN for 3–30 min at 37 °C before cell lysis and fractionation for the stimulation.
Western blotting
Samples were separated by SDS-PAGE and transferred onto a polyvinlydifluoride membrane, followed by incubation with 1.5 % skimmed milk for blocking. The membrane was proven with appropriate dilution of the primary antibody for 2 h, followed by incubation with the peroxidase-conjugated second antibody for 1 h at room temperature. The proteins were visualized with Western Lightning Chemiluminescence Reagent Plus (Perkin Elmer Life Sciences, Boston, MA, USA) according to the manufacturer’s instructions, and analyzed with a Luminescent image analyzer LAS-3000 mini (Fujifilm, Tokyo, Japan). GM1 was detected by CTxB-biotin followed by peroxidase-conjugated avidin.
Immunostaining and confocal microscopy
For GM1-crosslink, cells were incubated with CTxB-biotin for 5 min followed by incubation with fluorescence isothiocyanate (FITC)-conjugated avidin (Zymed, South San Francisco, CA, USA) for 10 min at 37 °C. Cells were fixed by 1 % formaldehyde for 10 min at room temperature, and sequentially incubated with anti-Siglec-9 and rhodamine B isothiocyanate-conjugated anti-goat IgG antibodies. For un-crosslinked control, cells were fixed, then stained with CTxB and anti-Siglec-9 antibody. Images were captured using Fluoview FV300 (Olympus, Tokyo, Japan).
Results
Function of Siglec-9 was partly dependent on its lectin activity
RAW264 macrophage cells have been widely used to study TLR signaling; however, the expression level of mouse CD33-related Siglecs was very low. Thus, we used cells that expressed human Siglecs at a level similar to that of circulating macrophages and demonstrated that Siglec-9 inhibited the production of proinflammatory cytokines and enhanced that of the anti-inflammatory cytokine IL-10 upon TLR stimulation. These activities of Siglec-9 required cytoplasmic tyrosine residues (Ando et al. 2008). To assess the possible role of lectin activity in the above function of Siglec-9, we established cells that stably expressed mutated Siglec-9 lacking lectin activity (Siglec-9RA) by replacing a critical arginine residue conserved within CD33-related Siglecs (arginine 120 in Siglec-9) to alanine. We confirmed the lack of lectin activity and similar levels of expression between wild-type and mutated Siglec-9 by flow cytometric analysis (Shoji et al. unpublished results).
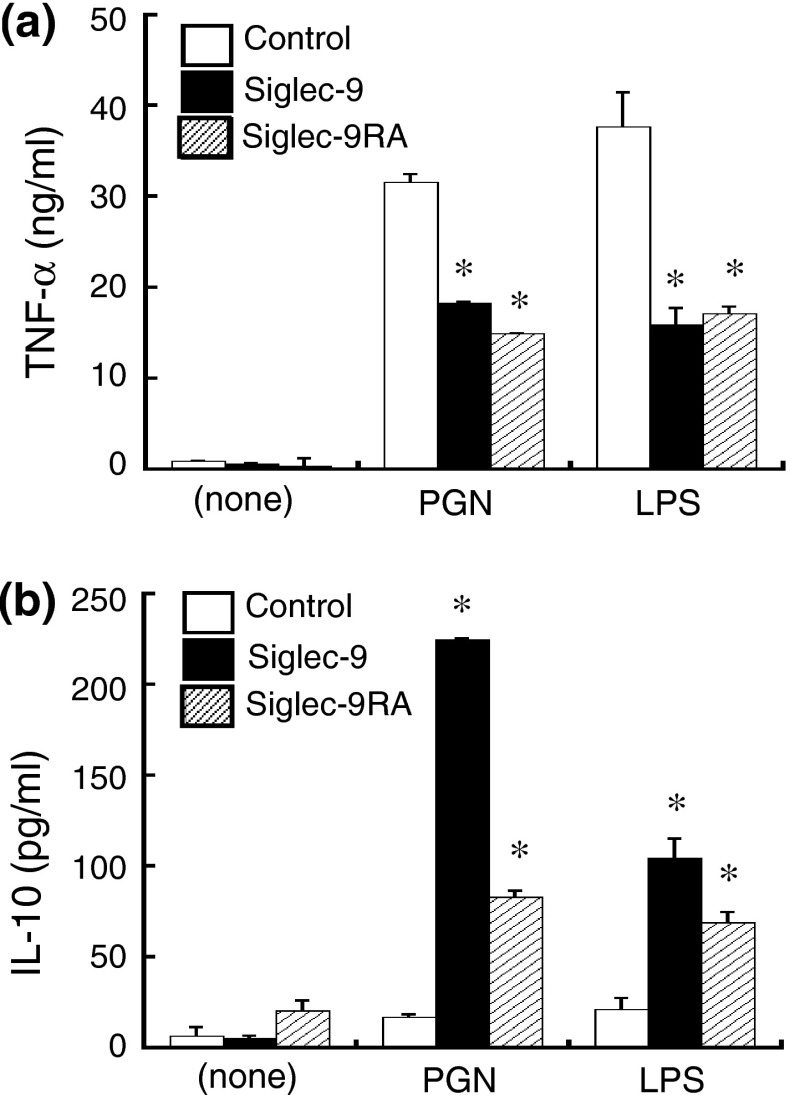
When cells were stimulated with either LPS or PGN, the amount of TNF-α in the supernatant of Siglec-9-expressing cells was almost half that of the control cells (Fig. 1a), as reported previously (Ando et al. 2008). The levels of TNF-α produced by cells expressing wild-type Siglec-9 and its lectin mutant were similar. On the other hand, the level of IL-10 produced by Siglec-9RA-expressing cells was half or one third that by Siglec-9-expressing cells, but was still higher than that by control cells (Fig. 1b). These results suggest that the enhanced production of IL-10 by Siglec-9 is partly dependent on its lectin activity, whereas the inhibition of TNF-α was not.
Fig. 1.
The anti-inflammatory activities of Siglec-9 were partly dependent on its lectin activity. a The reduction in TNF-α production by Sigec-9 was independent of its lectin activity. Cells were stimulated with either PGN (25 μg/ml) or LPS (500 ng/ml) for 4 h and the amount of TNF-α in the culture supernatant was measured by ELISA. b The enhanced production of IL-10 by Siglec-9 was partly dependent on its lectin activity. Cells were stimulated for 8 h. Mock-transfected RAW264 cells were used as controls. Data are representative of five independent experiments. *P < 0.05 versus control by the Student’s t test
A part of Siglec-9 localized to lipid rafts depending on its lectin activity
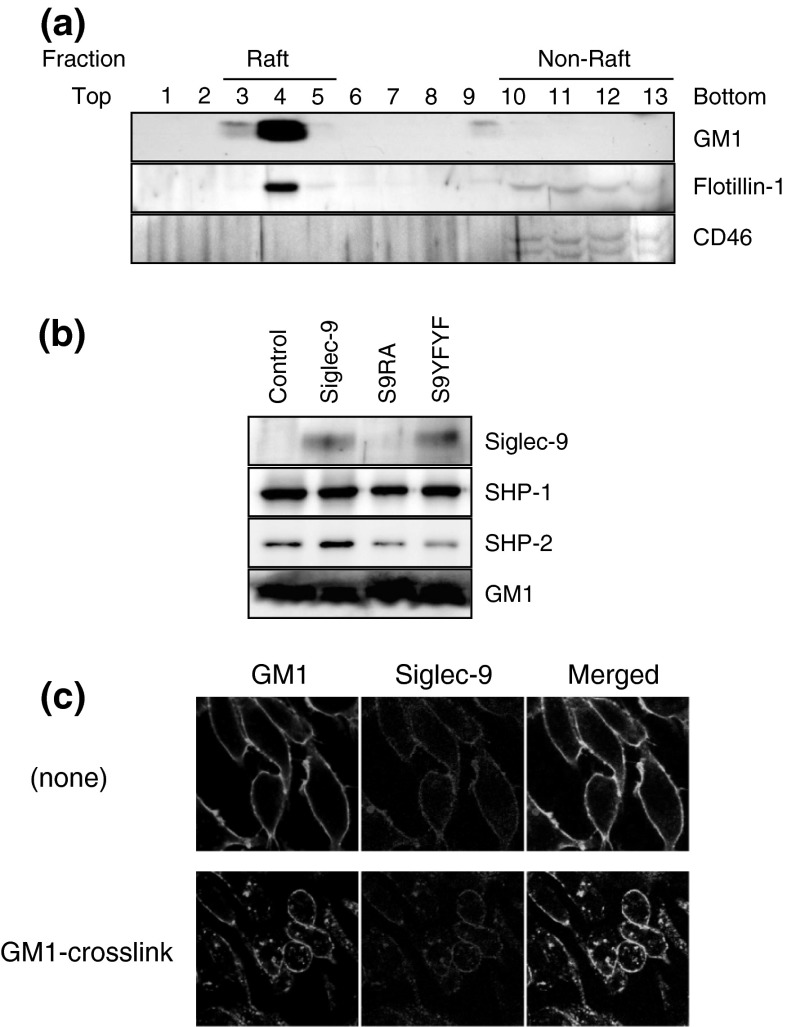
Lipid rafts are an important region of the cellular membrane in which various signaling proteins and receptors form clusters. Since TLR was shown to exist in the raft fractions and because lipid rafts are important for TLR-induced inflammatory responses (Triantafilou et al. 2002; Zhu et al. 2010), we examined whether Siglec-9 also localized to lipid rafts. After non-stimulated cell lysates were fractionated by a sucrose gradient, fractions were collected from the top to the bottom, and numbered 1–13. Figure 2a shows the successful separation of lipid raft and non-raft fractions. The lipid raft markers GM1 and flottilin-1 were almost specifically detected in fractions 3–5, while CD46 as a non-raft marker was detected in fractions 10–13. Siglec-9 was clearly detected in the pooled raft fraction (Fig. 2b). Siglec-9YFYF, in which two tyrosine residues in the ITIM motifs were mutated to phenylalanine, was detected in rafts with similar intensities. On the other hand, the lectin mutant of Siglec-9 (Siglec-9RA) was not detected in the rafts. These results showed that a part of Siglec-9 was localized to the lipid rafts, and that this was dependent on lectin activity, but not cytosolic tyrosine residues. Both SHP-1 and SHP-2 were detected in the pooled raft fraction of unstimulated RAW264 cells. The amount of SHP-2, but not SHP-1, slightly increased in cells expressing Siglec-9. A similar distribution pattern was not observed with Siglec-9YFYF, which lacked the tyrosine residues required for SHP-binding, or Siglec-9RA, which did not localize to the lipid rafts.
Fig. 2.
Siglec-9 localized to lipid rafts in RAW264 via its lectin activity. a Confirmation of lipid raft isolation. Each fraction in sucrose gradient centrifugation was probed with CTxB-biotin to detect GM1 and anti-flotillin-1 antibody as a lipid-raft marker, and anti-CD46 antibody as a non-raft marker. b Localization of Siglec-9 to the lipid rafts in unstimulated cells. Since the protein concentration was very low, lipid raft fractions (fractions 3–5) were pooled and concentrated. S9RA, Siglec-9RA; S9YFYF, Siglec-9YFYF. c Colocalization of Siglec-9 with GM1 in GM1-crosslinked cells. Siglec-9 and GM1 were stained with the anti-Siglec-9 antibody and CTxB, respectively. Data are representative of 2–5 independent experiments
Confocal microscopy analysis was performed to further confirm the association between Siglec-9 and the lipid rafts. GM1 was crosslinked in cells by CTxB-biotin plus avidin-FITC to induce artificial reorganization of cell membrane by GM1 aggregation, and the changes in localization of GM1 and Siglec-9 were examined. Under non-crosslinked conditions (none), Siglec-9 and GM1 were evenly distributed in the cell membrane (Fig. 2c), whereas GM1-containing dots were observed after crosslinking, and Siglec-9 was enriched in the dot. These results are consistent with Siglec-9 being localized to GM1-enriched lipid rafts.
Siglec-9 moved to lipid rafts following PGN stimulation
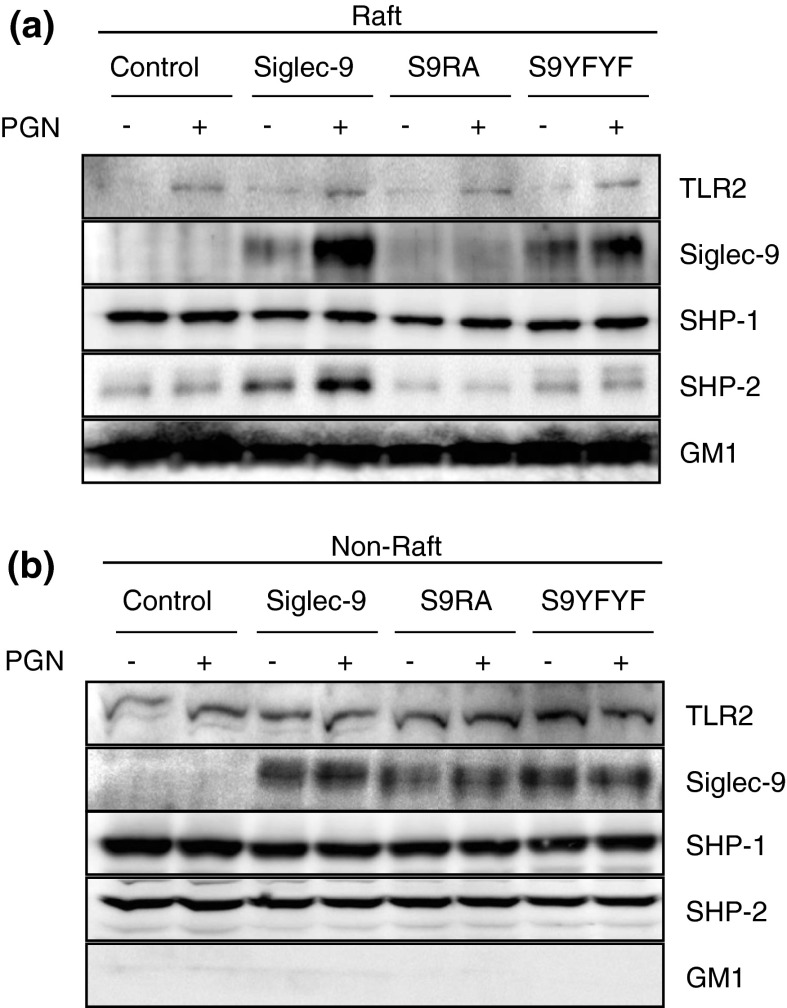
We next assessed whether the distribution of Siglec-9 was changed by the activation of macrophages (Fig. 3). Stimulation with PGN for 10 min induced the relocalization of TLR2 to the lipid rafts, as was reported previously with several TLRs (Triantafilou et al. 2002). We simultaneously observed that the amount of Siglec-9 in the pooled raft fraction was significantly increased following stimulation with PGN. This increase was also observed with Siglec-9YFYF, but not with Siglec-9RA. These results suggest that stimulation with PGN facilitated the relocalization of Siglec-9 to the lipid rafts. The amount of SHP-1 in the lipid rafts was similar between the cells and was not changed by the stimulation. On the other hand, the increase in SHP-2 in the lipid rafts may have been mediated by the accumulation of Siglec-9. A similar phenomenon was not observed with Siglec-9YFYF.
Fig. 3.
Siglec-9 relocalized in lipid rafts following PGN stimulation. a, b Wild-type or mutated Siglec-9-expressing RAW264 cells were incubated with or without 50 μg/ml of PGN for 10 min, then lysed in lysis buffer, and separated by a sucrose density gradient. Pooled lipid raft fraction was analyzed after concentration (a), while pooled non-raft fractions were analyzed without concentration (b). Data are representative of 2–5 independent experiments
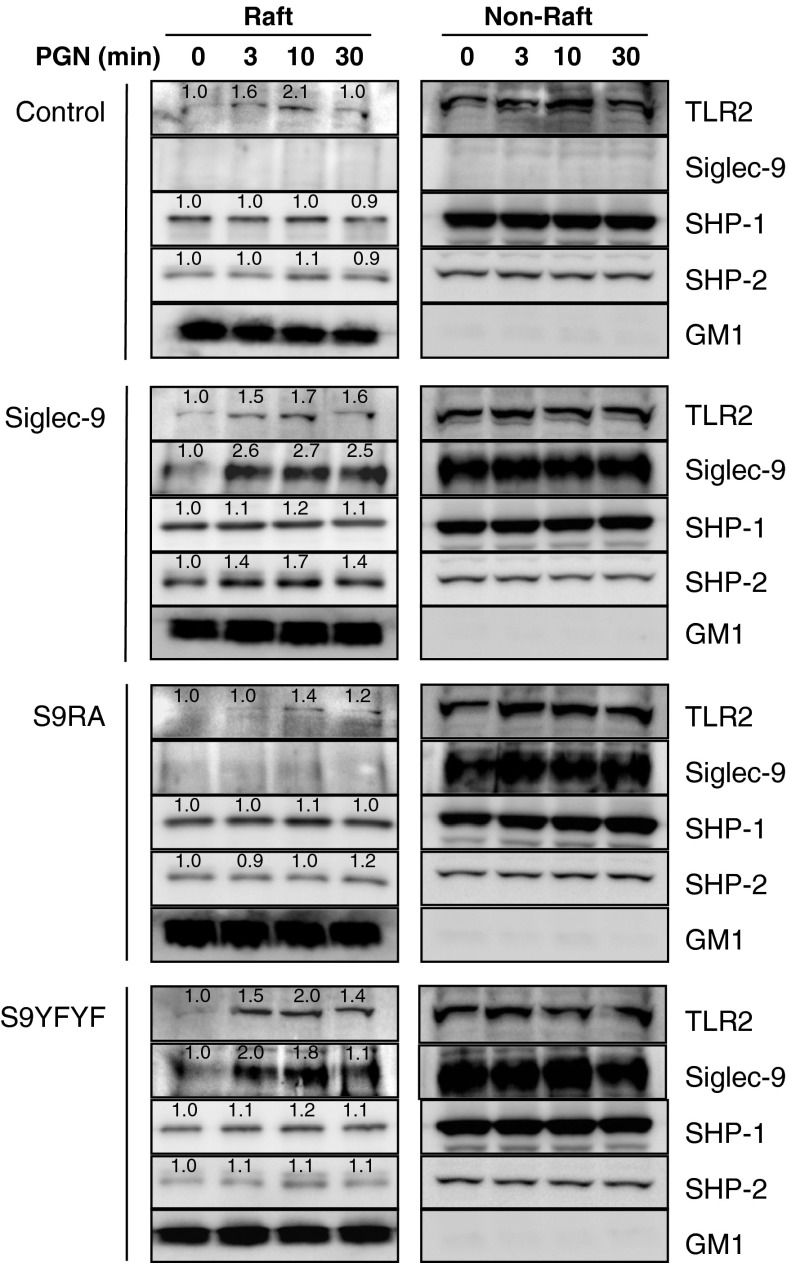
We next examined the time-course of relocalization (Fig. 4). Cells were stimulated with PGN for 3–30 min, and fractionated samples were subjected to western blotting. The relocalization of TLR2 and Siglec-9 in the lipid rafts peaked 3–10 min after PGN stimulation. A similar time course was observed for Siglec-9YFYF; however, the localization of Siglec-9RA did not change. These results suggest that Siglec-9 and TLR2 showed similar time courses of movement to the lipid rafts following PGN stimulation.
Fig. 4.
Time course of Siglec-9 localization to the lipid rafts following PGN stimulation. RAW264 cells expressing wild-type or mutated Siglec-9 were stimulated with 50 μg/ml of PGN for 0, 3, 10, and 30 min, and lipid raft fractions were analyzed as in Fig. 3. Numbers indicate relative band intensity. Data are representative of 2–4 independent experiments
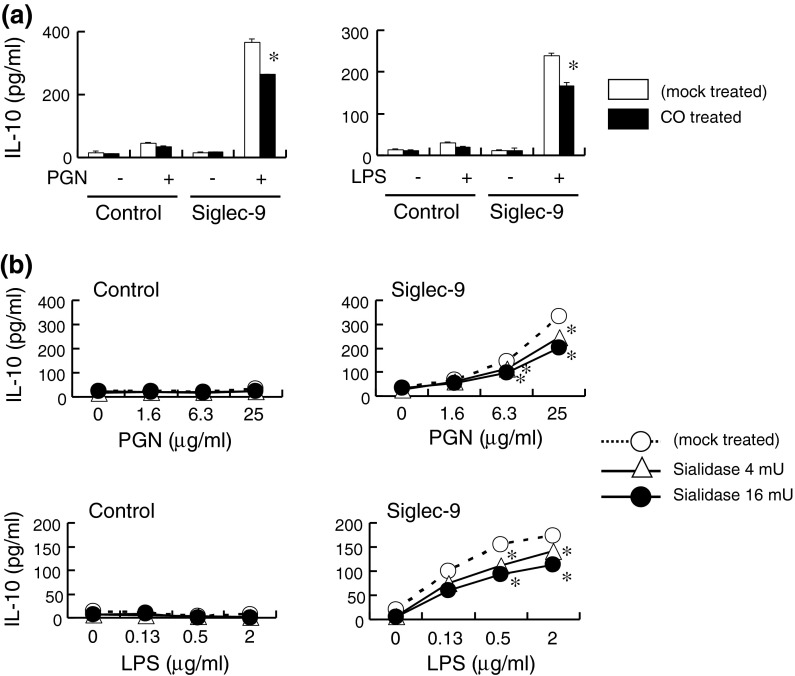
Full enhancement of IL-10 production by Siglec-9 required membrane integrity and cell surface sialic acids
The involvement of rafts in biological functions has mainly been investigated by perturbing the raft structures. We examined the effect of cholesterol oxidase, which affects raft dynamics by oxidizing cholesterol, on the production of IL-10 in Siglec-9-expressing cells. Cholesterol oxidase slightly reduced IL-10 production by Siglec-9-expressing cells stimulated with either PGN or LPS (Fig. 5a), while IL-10 was not detected in control cells. No significant difference was observed in a prolonged culture (24 h), which may have been due to the replacement of oxidized cholesterol during cultivation (data not shown). These results suggest that IL-10 production was, at least partly, dependent on the integrity of the cell membrane.
Fig. 5.
The enhanced production of IL-10 partly required the integrity of the cellular membrane and cell surface sialic acids. a Cells were pretreated with cholesterol oxidase (1 U/ml), and stimulated with PGN (25 μg/ml) or LPS (2 μg/ml) for 8 h. CO, cholesterol oxidase. b Cells were pretreated with sialidase and stimulated as in (a). Data are representative of two independent experiments. *P < 0.05 versus mock treated cells by the Student’s t test
To examine whether sialidase treatment affected IL-10 production, cells were pretreated with sialidase, which cleaves both the α2,3- and α2,6-linkages of sialic acids. Western blotting of Siglec-9 revealed a decrease in the apparent molecular weight of Siglec-9 following the sialidase treatment, which was attributed to the removal of sialic acids (data not shown). When cells were stimulated with PGN or LPS, IL-10 production was partially impaired by the sialidase treatment of Siglec-9-expressing cells (Fig. 5b). On the other hand, control cells did not produce a detectable amount of IL-10. These results suggest that the full activity of Siglec-9 to induce IL-10 production required the presence of sialic acids on itself or on the cell surface. However, we could not exclude the possibility that the non-specific toxicity of sialidase affected the results because the production of TNF-α was slightly reduced by the sialidase treatment in both control and Siglec-9-expressing cells in some experiments (data not shown).
Discussion
The present study demonstrated that the increase in IL-10 production by stimulated Siglec-9-expressing RAW264 cells was partly dependent on the lectin activity of Siglec-9: activation of IL-10 production by Siglec-9 was higher than that of lectin-defective Siglec-9RA (Fig. 1b). This result shows that Siglec-9 exerts its activity in both lectin-dependent and lectin-independent manners. Based on this assumption, the lectin-independent mechanism may be involved in the inhibition of TNF-α production: inhibition of TNF-α production was almost similar between Siglec-9- and Siglec-9RA-expressing cells (Fig. 1a). A balance between the lectin-dependent and lectin-independent pathways has been reported previously for the non CD33-related Siglec, CD22 (Siglec-2) on B cell functions (Poe et al. 2004). Regarding the inhibition of T cell receptor-induced stimulation of Jurkat cells, it has been suggested that Siglec-9 activity partly depended on its lectin activity (Ikehara et al. 2004).
Our results clearly showed that Siglec-9 partly localized to lipid rafts in a lectin-dependent manner. To the best of our knowledge, this is the first study to describe a Siglec in a lipid raft environment on macrophages. In our assay for cytokine production, the effects of Siglec-9 on TLR2 and TLR4 stimulation are similar (Ando et al. 2008), including partial inhibition of IL-10 production by cholesterol oxidase treatment (Fig. 5). Although direct confirmation is necessary, it may be possible that Siglec-9 relocalized in lipid rafts by several TLR ligands. The mechanism of relocalization upon stimulation remains unclear; however, a simple explanation possible for the localization of Siglec-9 into the lipid raft fraction is passive movement by binding proteins that have sialic acids and reside/move in lipid rafts. In this regard, it is noteworthy that TLRs contain sialic acid; sialic acids on TLR4 regulated LPS-induced responses in macrophages (Amith et al. 2009, 2010). Lysosomal Neu1 sialidase was shown to localize to the cell surface following LPS stimulation, which removed sialic acids including those on TLR4, leading to the facilitation of MyD88/TLR4 complex formation and activation of the downstream signaling of TLR. Although the binding affinity of Siglec to sialic acids is weak, Siglec-9 may interact with α2,3-sialic acids on TLR and be located close to TLR4 as well as TLR2 in the lipid raft, which may cause the partial inhibition of TLR signaling. So far, we failed to detect direct interaction between Siglec-9 and TLR2 by co-immunoprecipitation experiments either in the presence or absence of PGN (data not shown). However, the possibility still remains that sialic acid-dependent interaction between Siglec-9 and sialic acids on TLR2 was undetectable level by immunoprecipitation assay. Since only a part of Siglec-9 relocated to the lipid rafts upon stimulation, Siglec-9 in the non-raft fraction may exert an important function especially in the lectin-independent control mechanism of cytokine production. Further analysis is necessary to deduce the lectin-dependent and lectin-independent mechanisms of Siglec-9.
Acknowledgments
The authors are grateful to Drs. Ken Kitajima and Shinji Miyata for their helpful discussions, and Takeshi Hashimoto, Yuki Nakagami, and Ikuko Yamai for their technical assistance.
References
- Amith SR, Jayanth P, Franchuk S, Siddiqui S, Seyrantepe V, Gee K, Basta S, Beyaert R, Pshezhetsky AV, Szewczuk MR. Dependence of pathogen molecule-induced Toll-like receptor activation and cell function on Neu1 sialidase. Glycoconj J. 2009;26:1197–1212. doi: 10.1007/s10719-009-9239-8. [DOI] [PubMed] [Google Scholar]
- Amith SR, Jayanth P, Franchuk S, Finlay T, Seyrantepe V, Beyaert R, Pshezhetsky AV, Szewczuk MR. Neu1 desialylation of sialyl alpha-2,3-linked beta-galactosyl residues of TOLL-like receptor 4 is essential for receptor activation and cellular signaling. Cell Signal. 2010;22:314–324. doi: 10.1016/j.cellsig.2009.09.038. [DOI] [PubMed] [Google Scholar]
- Ando M, Tu W, Nishijima K, Iijima S. Siglec-9 enhances IL-10 production in macrophages via tyrosine-based motifs. Biochem Biophys Res Commun. 2008;369:878–883. doi: 10.1016/j.bbrc.2008.02.111. [DOI] [PubMed] [Google Scholar]
- Crocker PR, McMillan SJ, Richards HE. CD33-related siglecs as potential modulators of inflammatory responses. Ann N Y Acad Sci. 2012;1253:102–111. doi: 10.1111/j.1749-6632.2011.06449.x. [DOI] [PubMed] [Google Scholar]
- Fessler MB, Parks JS. Intracellular lipid flux and membrane microdomains as organizing principles in inflammatory cell signaling. J Immunol. 2011;187:1529–1535. doi: 10.4049/jimmunol.1100253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikehara Y, Ikehara SK, Paulson JC. Negative regulation of T cell receptor signaling by Siglec-7 (p70/AIRM) and Siglec-9. J Biol Chem. 2004;279:43117–43125. doi: 10.1074/jbc.M403538200. [DOI] [PubMed] [Google Scholar]
- Kondo T, Kawai T, Akira S. Dissecting negative regulation of Toll-like receptor signaling. Trends Immunol. 2012;33:449–458. doi: 10.1016/j.it.2012.05.002. [DOI] [PubMed] [Google Scholar]
- Pilatte Y, Bignon J, Lambré CR. Sialic acids as important molecules in the regulation of the immune system: pathophysiological implications of sialidases in immunity. Glycobiology. 1993;3:201–218. doi: 10.1093/glycob/3.3.201. [DOI] [PubMed] [Google Scholar]
- Pillai S, Netravali IA, Cariappa A, Mattoo H. Siglecs and immune regulation. Annu Rev Immunol. 2012;30:357–392. doi: 10.1146/annurev-immunol-020711-075018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poe JC, Fujimoto Y, Hasegawa M, Haas KM, Miller AS, Sanford IG, Bock CB, Fujimoto M, Tedder TF. CD22 regulates B lymphocyte function in vivo through both ligand-dependent and ligand-independent mechanisms. Nat Immunol. 2004;5:1078–1087. doi: 10.1038/ni1121. [DOI] [PubMed] [Google Scholar]
- Ravetch JV, Lanier LL. Immune inhibitory receptors. Science. 2000;290:84–89. doi: 10.1126/science.290.5489.84. [DOI] [PubMed] [Google Scholar]
- Triantafilou M, Miyake K, Golenbock DT, Triantafilou K. Mediators of innate immune recognition of bacteria concentrate in lipid rafts and facilitate lipopolysaccharide-induced cell activation. J Cell Sci. 2002;115:2603–2611. doi: 10.1242/jcs.115.12.2603. [DOI] [PubMed] [Google Scholar]
- West AP, Koblansky AA, Ghosh S. Recognition and signaling by toll-like receptors. Annu Rev Cell Dev Biol. 2006;22:409–437. doi: 10.1146/annurev.cellbio.21.122303.115827. [DOI] [PubMed] [Google Scholar]
- Yvan-Charvet L, Welch C, Pagler TA, Ranalletta M, Lamkanfi M, Han S, Ishibashi M, Li R, Wang N, Tall AR. Increased inflammatory gene expression in ABC transporter-deficient macrophages: free cholesterol accumulation, increased signaling via toll-like receptors, and neutrophil infiltration of atherosclerotic lesions. Circulation. 2008;118:1837–1847. doi: 10.1161/CIRCULATIONAHA.108.793869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X, Lee JY, Timmins JM, Brown JM, Boudyguina E, Mulya A, Gebre AK, Willingham MC, Hiltbold EM, Mishra N, Maeda N, Parks JS. Increased cellular free cholesterol in macrophage-specific Abca1 knock-out mice enhances pro-inflammatory response of macrophages. J Biol Chem. 2008;283:22930–22941. doi: 10.1074/jbc.M801408200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X, Owen JS, Wilson MD, Li H, Griffiths GL, Thomas MJ, Hiltbold EM, Fessler MB, Parks JS. Macrophage ABCA1 reduces MyD88-dependent Toll-like receptor trafficking to lipid rafts by reduction of lipid raft cholesterol. J Lipid Res. 2010;51:3196–3206. doi: 10.1194/jlr.M006486. [DOI] [PMC free article] [PubMed] [Google Scholar]