Abstract
To understand better the role of the creatine kinase (CK)/phosphocreatine system in muscle bioenergetics, a series of mouse mutants with subnormal muscle CK (M-CK) expression has been generated. Here we compare the phenotypes of mice deficient in M-CK (M-CK-/-) and M-CK leaky-mutant mice, which carry a targeted insertion of a hygromycin B-poly(A) resistance cassette in the second M-CK intron. Mice homozygous for this M-CK allele (M-CKI/I) have a 3-fold reduction of dimeric muscle CK enzyme activity, whereas compound heterozygotes with the null M-CK allele (M-CKI/-) display a 6-fold reduction. Unlike M-CK-/- mice, these mutants have no increased glycogen content or glycogen consumption in their fast fibers. The intermyofibrillar mitochondrial volume of these fibers is also normal, suggesting that energy transport via the CK/phosphocreatine system may function at low myofibrillar M-band CK levels. Conversely, the flux of energy through the CK reaction is still not visible by means of 31P NMR spectroscopy, indicating that relatively high levels of M-CK expression (> 34% of normal) are required to generate CK fluxes detectable by this technique. The ability of muscles to perform burst activity is also subnormal and closely correlates with the level of M-CK expression.
Full text
PDF
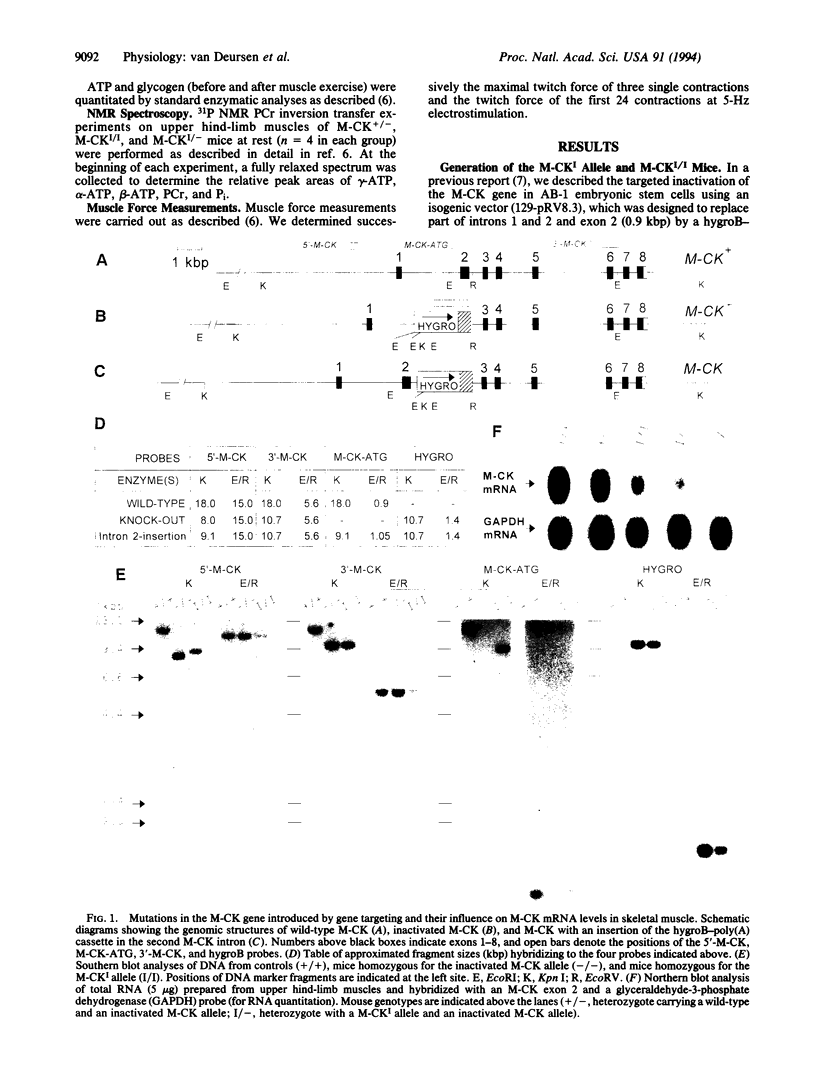


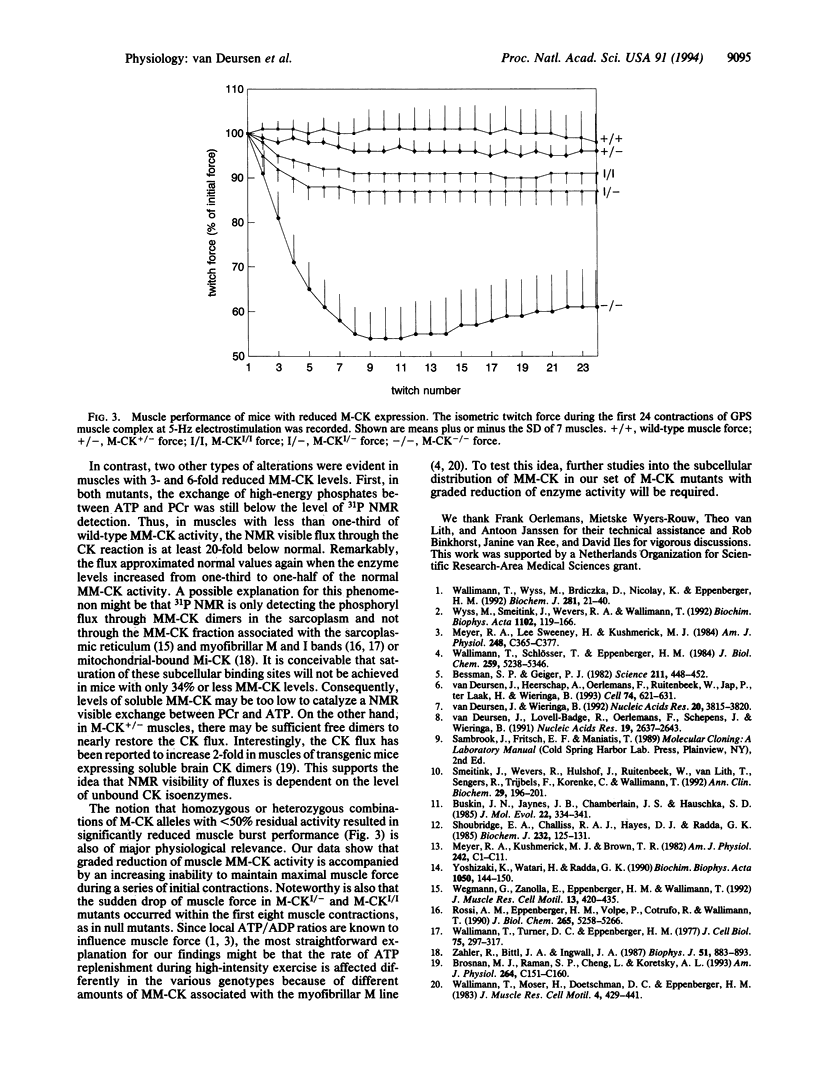
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bessman S. P., Geiger P. J. Transport of energy in muscle: the phosphorylcreatine shuttle. Science. 1981 Jan 30;211(4481):448–452. doi: 10.1126/science.6450446. [DOI] [PubMed] [Google Scholar]
- Brosnan M. J., Raman S. P., Chen L., Koretsky A. P. Altering creatine kinase isoenzymes in transgenic mouse muscle by overexpression of the B subunit. Am J Physiol. 1993 Jan;264(1 Pt 1):C151–C160. doi: 10.1152/ajpcell.1993.264.1.C151. [DOI] [PubMed] [Google Scholar]
- Buskin J. N., Jaynes J. B., Chamberlain J. S., Hauschka S. D. The mouse muscle creatine kinase cDNA and deduced amino acid sequences: comparison to evolutionarily related enzymes. J Mol Evol. 1985;22(4):334–341. doi: 10.1007/BF02115689. [DOI] [PubMed] [Google Scholar]
- Meyer R. A., Kuchmerick M. J., Brown T. R. Application of 31P-NMR spectroscopy to the study of striated muscle metabolism. Am J Physiol. 1982 Jan;242(1):C1–11. doi: 10.1152/ajpcell.1982.242.1.C1. [DOI] [PubMed] [Google Scholar]
- Meyer R. A., Sweeney H. L., Kushmerick M. J. A simple analysis of the "phosphocreatine shuttle". Am J Physiol. 1984 May;246(5 Pt 1):C365–C377. doi: 10.1152/ajpcell.1984.246.5.C365. [DOI] [PubMed] [Google Scholar]
- Rossi A. M., Eppenberger H. M., Volpe P., Cotrufo R., Wallimann T. Muscle-type MM creatine kinase is specifically bound to sarcoplasmic reticulum and can support Ca2+ uptake and regulate local ATP/ADP ratios. J Biol Chem. 1990 Mar 25;265(9):5258–5266. [PubMed] [Google Scholar]
- Shoubridge E. A., Challiss R. A., Hayes D. J., Radda G. K. Biochemical adaptation in the skeletal muscle of rats depleted of creatine with the substrate analogue beta-guanidinopropionic acid. Biochem J. 1985 Nov 15;232(1):125–131. doi: 10.1042/bj2320125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smeitink J., Wevers R., Hulshof J., Ruitenbeek W., van Lith T., Sengers R., Trijbels F., Korenke C., Wallimann T. A method for quantitative measurement of mitochondrial creatine kinase in human skeletal muscle. Ann Clin Biochem. 1992 Mar;29(Pt 2):196–201. doi: 10.1177/000456329202900213. [DOI] [PubMed] [Google Scholar]
- Wallimann T., Moser H., Eppenberger H. M. Isoenzyme-specific localization of M-line bound creatine kinase in myogenic cells. J Muscle Res Cell Motil. 1983 Aug;4(4):429–441. doi: 10.1007/BF00711948. [DOI] [PubMed] [Google Scholar]
- Wallimann T., Schlösser T., Eppenberger H. M. Function of M-line-bound creatine kinase as intramyofibrillar ATP regenerator at the receiving end of the phosphorylcreatine shuttle in muscle. J Biol Chem. 1984 Apr 25;259(8):5238–5246. [PubMed] [Google Scholar]
- Wallimann T., Turner D. C., Eppenberger H. M. Localization of creatine kinase isoenzymes in myofibrils. I. Chicken skeletal muscle. J Cell Biol. 1977 Nov;75(2 Pt 1):297–317. doi: 10.1083/jcb.75.2.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallimann T., Wyss M., Brdiczka D., Nicolay K., Eppenberger H. M. Intracellular compartmentation, structure and function of creatine kinase isoenzymes in tissues with high and fluctuating energy demands: the 'phosphocreatine circuit' for cellular energy homeostasis. Biochem J. 1992 Jan 1;281(Pt 1):21–40. doi: 10.1042/bj2810021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegmann G., Zanolla E., Eppenberger H. M., Wallimann T. In situ compartmentation of creatine kinase in intact sarcomeric muscle: the acto-myosin overlap zone as a molecular sieve. J Muscle Res Cell Motil. 1992 Aug;13(4):420–435. doi: 10.1007/BF01738037. [DOI] [PubMed] [Google Scholar]
- Wyss M., Smeitink J., Wevers R. A., Wallimann T. Mitochondrial creatine kinase: a key enzyme of aerobic energy metabolism. Biochim Biophys Acta. 1992 Sep 25;1102(2):119–166. doi: 10.1016/0005-2728(92)90096-k. [DOI] [PubMed] [Google Scholar]
- Yoshizaki K., Watari H., Radda G. K. Role of phosphocreatine in energy transport in skeletal muscle of bullfrog studied by 31P-NMR. Biochim Biophys Acta. 1990 Feb 19;1051(2):144–150. doi: 10.1016/0167-4889(90)90186-h. [DOI] [PubMed] [Google Scholar]
- Zahler R., Bittl J. A., Ingwall J. S. Analysis of compartmentation of ATP in skeletal and cardiac muscle using 31P nuclear magnetic resonance saturation transfer. Biophys J. 1987 Jun;51(6):883–893. doi: 10.1016/S0006-3495(87)83416-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Deursen J., Heerschap A., Oerlemans F., Ruitenbeek W., Jap P., ter Laak H., Wieringa B. Skeletal muscles of mice deficient in muscle creatine kinase lack burst activity. Cell. 1993 Aug 27;74(4):621–631. doi: 10.1016/0092-8674(93)90510-w. [DOI] [PubMed] [Google Scholar]
- van Deursen J., Lovell-Badge R., Oerlemans F., Schepens J., Wieringa B. Modulation of gene activity by consecutive gene targeting of one creatine kinase M allele in mouse embryonic stem cells. Nucleic Acids Res. 1991 May 25;19(10):2637–2643. doi: 10.1093/nar/19.10.2637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Deursen J., Wieringa B. Targeting of the creatine kinase M gene in embryonic stem cells using isogenic and nonisogenic vectors. Nucleic Acids Res. 1992 Aug 11;20(15):3815–3820. doi: 10.1093/nar/20.15.3815. [DOI] [PMC free article] [PubMed] [Google Scholar]