Abstract
Biodemography is a promising scientific approach based on using demographic data and methods for getting insights into biological mechanisms of observed processes. Recently new important developments happened in biodemographic studies of aging and longevity that call into question conventional aging theories and open up new research directions.
New studies found that exponential increase of mortality risk with age (the famous Gompertz law) continues even at extreme old ages in humans, rats and mice, thus challenging traditional views about old-age mortality deceleration, mortality leveling-off, and late-life mortality plateaus. This new finding represents a challenge to many aging theories, including the evolutionary theory that explains senescence by declining force of natural selection with age. New ideas are needed to explain why exactly the same exponential pattern of mortality growth is observed not only at reproductive ages, but also at very old post-reproductive ages (up to 106 years), long after the force of natural selection becomes negligible (when there is no room for its further decline).
Another important new development is a discovery of long-term ‘memory’ for early-life experiences in longevity determination. Siblings born to young mothers have significantly higher chances to live 100, and this new finding, confirmed by two independent research groups, calls for its explanation. Even the place and season of birth matters for human longevity, as new studies found. Beneficial longevity effects of young maternal age are observed only when children of the same parents are compared, while the maternal age effect often could not be detected in across-families’ studies, presumably being masked by between-family variation. It was also found that male gender of centenarian has significant positive effect on survival of adult male biological relatives (brothers and fathers) but not female relatives. Finally, large sex differences are found in longevity determinants for males and females, suggesting higher importance of occupation history for male centenarians, and higher importance of home environment history for female centenarians.
Keywords: biodemography, longevity, mortality trajectories, maternal age, centenarians
Introduction
New important developments happened recently in biodemographic studies of aging and longevity that call into question conventional aging theories and open up new research directions. In this mini-review we cover new developments in two broad areas of biodemographic research:
Studies of mortality laws in humans and laboratory animals at extreme old ages
Studies on predictors of exceptional human longevity.
1. Biodemography of old-age mortality
Attempts to develop a fundamental quantitative theory of aging, mortality, and lifespan have deep historical roots. In 1825, the British actuary Benjamin Gompertz discovered a law of mortality (see reviews in [1-4]). Specifically, he found that the force of mortality increases in geometrical progression with the age of adult humans. According to the Gompertz law, human mortality rates double over about every eight years of adult age. An exponential (Gompertzian) increase in death rates with age is observed for many biological species including fruit flies Drosophila melanogaster, nematodes, mosquitoes, human lice Pediculus humanus, flour beetles Tribolium confusum, mice, rats, dogs, horses, mountain sheep, and baboons (see review in [1, 2].
It was also believed that exponential growth of mortality with age (Gompertz law) is followed by a period of deceleration, with slower rates of mortality increase at extreme old ages [1, 5]. This mortality deceleration eventually produces the “late-life mortality leveling-off” and “late-life mortality plateaus” at extreme old ages. Greenwood and Irwin [5] provided a detailed description of this phenomenon in humans and even made the first estimates for the asymptotic value of the upper limit to human mortality. The same phenomenon of “almost non-aging” survival dynamics at extreme old ages was described for other biological species, and in some species like medfly and housefly, the mortality plateau can occupy a sizable part of their life [2, 6].
According to some researchers, the late-life mortality plateau represents a distinct phase of life when the aging slows down or stops. Evolutionary biologists believe that aging is a result of declining forces of natural selection with age. When these forces eventually bottom-up at extreme old ages, then the cessation of aging is expected according to this paradigm [7]. Population heterogeneity hypothesis is another, even more popular, explanation of mortality deceleration, which was proposed by British actuary Eric Beard in 1959 [8]. As George Sacher explained “... sub-populations with the higher injury levels die out more rapidly, resulting in progressive selection for vigour in the surviving populations” [9]. Another explanation of this phenomenon comes from the reliability theory of aging, which explains mortality leveling-off by an exhaustion of organism's redundancy (reserves) at extremely old ages, so that every additional random hit of damage results in death [1, 2]. There is also an opinion that lower (than predicted) risks of death for older people may be due to their less risky behavior [5].
The existence of mortality plateaus is well described for a number of lower organisms, including medfly, house fly Musca domestica, fruit flies Anastrepha ludens, Anastrepha obliqua, Anastrepha serpentine, parasitoid wasp Diachasmimorpha longiacaudtis, and bruchid beetle Callosobruchus maculates (see review in [2]). In the case of mammals, however, data are much more controversial. Some researchers reported short-term periods of mortality deceleration in mice at advanced ages and even used the ‘mortality deceleration’ Perks formula in their analyses [9]. However, Austad later argued that rodents do not demonstrate mortality deceleration even in the case of very large samples allowing to study data at very advanced old ages [10]. Study of baboons found no mortality deceleration at older ages [11]. Longitudinal study of mortality among seven wild primate species failed to find mortality deceleration at older ages [12]. The authors came to a conclusion that “none of the age-specific mortality relationships in our non-human primate analyses demonstrated the type of leveling off that has been shown in human and fly data sets” [12]. Thus, we may suggest that mortality deceleration is observed for many invertebrate species, but the evidence for mammals is controversial.
Several studies of old-age mortality in humans came to a conclusion that mortality deceleration does exist and starts after age 80 [13, 14]. It should be noted, however, that analysis of old-age mortality in humans encounters certain methodological problems related to data aggregation and age misreporting among very old. More homogeneous single-year birth cohorts in many countries with good vital statistics have very small numbers of survivors to age 100 that makes estimates of mortality at advanced ages unreliable. On the other hand, aggregation of data for several birth cohorts in order to increase the sample size creates a mixture of different populations. The problem of age misreporting by older people is another important problem affecting estimates of mortality at advanced ages. It was found that mortality deceleration is more expressed in the case of data with poor quality compared to data with better quality [15].
Recently the new developments happened in this research area thanks to the use of more detailed and more accurate data. In particular, the U.S. Social Security Administration Death Master File (DMF) was used for accurate estimation of hazard rates at extremely old ages in extinct birth cohorts. Availability of month of birth and month of death information in this data source provides a unique opportunity to obtain more accurate hazard rate estimates for every month of age. The study of twenty single-year extinct birth cohorts with good data quality found that mortality deceleration at advanced ages is negligible up to the advanced age of 106 years[15]. This finding was further supported by additional studies of mortality in 22 single-year U.S. birth cohorts based on data from the Human Mortality Database and data on mortality of 1681 siblings of centenarians [16, 17]. The same conclusion was made after analysis of mortality trajectories in 8 cohorts of laboratory mice, and 10 cohorts of laboratory rats [17]. Thus, for all three mammalian species, the Gompertz model fits mortality data significantly better than the ‘mortality deceleration’ Kannisto model (according to the Akaike's information criterion as the goodness-of-fit measure) [17].
There are several reasons why earlier studies [13, 14], including our own research [1, 2], reported mortality deceleration and mortality leveling-off at advanced ages. First, mortality deceleration may be caused by age misreporting in death data for older individuals [18, 19]. Studies conducted more than 10 years ago used data for older birth cohorts when age reporting was not particularly accurate. In the United States, this may have impaired the accuracy of mortality rate estimates in the past.
Second, mortality deceleration may be a consequence of data aggregation. Most developed countries have much smaller populations compared to the United States and hence studies of mortality at advanced ages for these countries have to combine many single-year birth cohorts, thereby increasing the heterogeneity of the sample.
Some researchers used inappropriate estimates of the instantaneous mortality rate (hazard rate). At the most advanced ages, the rates of death are so high it is impossible to assume the number of dying is distributed uniformly within the studied one-year age intervals. As a result, the estimates of mortality rates (or central death rates) are biased downward at advanced ages. Many studies analyze age-specific probability of death rather than hazard rate, which is biased downward at old ages [20, 21]. It is not surprising that probability of death has a tendency of deceleration at advanced ages when mortality is high, taking into account that this mortality indicator has a theoretical upper limit equal to one. For example, a study of mortality among supercentenarians demonstrated that probability of death for this group does not increase with age [21].
These results suggest that mortality deceleration at advanced ages is not a universal phenomenon, and survival of mammalian species follows the Gompertz law up to very old ages.
This new finding represents a challenge to many aging theories, including the evolutionary theory that explains senescence by declining force of natural selection with age. New ideas are needed to explain why exactly the same exponential pattern of mortality growth is observed not only at reproductive ages, but also at very old post-reproductive ages (up to 106 years), long after the force of natural selection becomes negligible (when there is no room for its further decline).
A new finding on wide applicability of the Gompertz law to all adult ages leads to another burning research question: How is it possible for different diseases and causes of death to ‘negotiate’ with each other in order to produce a simple exponential function for all-cause mortality (given that contribution of different causes of death in all-cause mortality changes dramatically with age)?
2. Biodemography of exceptional longevity
Search for longevity predictors is another promising and rapidly developing direction of biodemographic studies. Centenarians (persons living to age 100 and over) represent a population, which could be useful in identifying factors leading to long life and avoidance of fatal diseases. Even if some early-life or middle-life factors have a moderate effect on risk of death, persons with this trait/condition will be accumulated among long-lived individuals. Thus, study of centenarians may be a sensitive way to find genetic, familial, environmental, and life-course factors associated with lower mortality and better survival.
It has been shown that many centenarians have been healthy or well functioning throughout most of their lives; thus, centenarians represent a useful model for delayed aging [22]. Contemporary populations are subjected to rapid population aging; so identifying the pathways to healthy longevity are of particular importance. These are important issues not only for demographic forecasts of mortality and population aging. They have policy implications for health care and pension expenditures, and are important for improving our understanding of the fundamental mechanisms of human aging and longevity.
Many studies of centenarians are focused on the search for longevity genes, while other important predictors of exceptional longevity are often overlooked. Of particular interest are factors of early-life developmental programming as an important determinant of health and survival in later life, and the corollary hypothesis that infectious diseases in childhood have long-lasting effects on subsequent survival at advanced [23].
In our most recent studies we used computerized family histories as a primary source of information on long-lived individuals (centenarians). Family histories (genealogies) have proven to be a useful source of information for studies in historical demography and biodemography Making use of a substantial body of information available in online historical sources allowed us to test a number of hypotheses regarding links between early-life conditions, middle-age experience, and exceptional longevity.
Why centenarians are different from their shorter-lived peers?
The prevalence of centenarians in modern populations is very low, about 1 per 10,000 [24], and therefore, traditional methods of population sampling are difficult and not feasible for obtaining large samples of centenarians. A case-control design has proven to be the most appropriate and cost-effective approach for studies of rare conditions, and is extremely useful for centenarian studies. The control selection strategy in this study was to avoid bias arising from non-comparability between cases and controls. In order to minimize this bias, controls are selected to be a representative sample of the population, which produced the cases (i.e., are selected from the same pool of computerized family histories).
The availability of data on U.S. centenarians and their shorter-lived peers (died at age 65 years) born in 1890-1891 allowed us to test several hypotheses on early-life conditions and longevity in a straightforward manner. This approach confirmed that parental longevity is a strong independent predictor of survival to age 100, so this variable cannot be ignored in gerontological studies (see Table 1). At the same time, early exposure to infections as estimated indirectly from child mortality in families of cases and controls had no effect on longevity. Overall, childhood conditions reported in the 1900 census were not predictive for exceptional longevity for either men or women [25]. On the other hand, some early-life characteristics (birth in North East region of the United States and birth in the second half of the year) turned out to be significant predictors of exceptional longevity (for men but not women). This study also found a strong positive effect of farmer occupation at middle age on attaining exceptional longevity for men. Husband's farmer occupation had no effect on longevity of women. This finding agrees with the results of other studies including our earlier study of centenarians based on population-based sample of survivors to age 100 from the 1887 birth cohort [26]. Only a few factors were related to exceptional longevity of women: parental longevity and, surprisingly, the availability of radio in household in 1930 [25]. Effects of radio as a proxy for household wealth might potentially explain the latter finding. However, more direct characteristics of household wealth (whether it was owned or rented property) demonstrated no association with exceptional longevity. Earlier studies found that radio listening increased quality of life and decreased depression [27]. This study demonstrated that only few selected factors turned out to be significant predictors of survival after age 65 years while many other early-life and midlife living conditions do not significantly affect mortality at this age period. It also revealed significant gender differences in the spectrum of predictors of exceptional longevity.
Table 1.
Predictors of survival to age 100: effects of parental longevity, early-life and midlife conditions. Results of multivariate logistic regression.
| Variable | Men, N=711 | Women, N=744 | ||||
|---|---|---|---|---|---|---|
| Odds ratio | 95% CI | p-value | Odds ratio | 95% CI | p-value | |
| Father lived 80+ | 1.87 | 1.37-2.57 | <0.001 | 1.99 | 1.44-2.74 | <0.001 |
| Mother lived 80+ | 1.94 | 1.41-2.65 | <0.001 | 2.33 | 1.71-3.17 | <0.001 |
| Farmer in 1930* | 1.79 | 1.21-2.31 | <0.001 | 1.21 | 0.87-1.66 | 0.253 |
| Born in the North-East region | 1.86 | 1.14-3.03 | 0.013 | 0.98 | 0.60-1.59 | 0.931 |
| Born in the second half of year | 1.46 | 1.08-2.00 | 0.050 | 1.21 | 0.90-1.64 | 0.211 |
| Radio in household, 1930 | 0.96 | 0.70-1.33 | 0.810 | 1.64 | 1.18-2.27 | 0.003 |
Husband or head of household farmer in 1930 for women.
Effect of Centenarian Gender on Survival of Centenarian Relatives
Numerous studies demonstrate that biological relatives of centenarians have substantial survival advantage compared to relatives of shorter-lived individuals. At the same time little is known about the role of centenarian gender in these effects. We explored effects of centenarian gender on survival of their biological (parents and siblings) and non-biological (spouses and siblings-in-law) relatives. We used a database of 1,945 validated U.S. centenarians born in 1880-1895 as well as their siblings, spouses and siblings-in-law.
Comparison of mean lifespan for adult relatives (those who survived to age 50) reveals a survival advantage of brothers and sisters of centenarians compared to the same-sex siblings-in-law: brothers lived 2.6 years longer, and sisters lived 2.9 years longer on average compared to siblings-in-law of the same sex (Table 2) with differences in lifespan being statistically significant (p<0.001). Although fathers of centenarians are born about 30 years earlier than brothers-in-law of centenarians they still have higher lifespan conditional on survival to age 50 than later-born non-biological relatives such as siblings-in-law (p<0.001) and husbands of centenarians (p=0.04). On the other hand, mothers of centenarians (who survived to age 50) have the shortest lifespan among all relatives – 77.2 years on average. Relatively low lifespan of mothers of centenarians is most likely related to physiological burden of childbearing in these large families, which was shown to increase mortality of mothers having many children [28]. Overall, siblings-in-law have the lowest lifespan compared to biological relatives and spouses born in a similar time period. The latter result is not surprising taking into account that siblings-in-law do not have genetic or environmental advantages of other relatives of centenarians. Interestingly, lifespan of siblings-in-law is still higher than mean lifespan of the general population (1900 U.S. birth cohort). This difference is particularly high for men (1.7 years, p<0.001), while for women this difference is not statistically significant (Table 2). This finding indicates that comparing survival of siblings or other biological relatives of centenarians to the general population may overstate their survival advantage and hence overestimate the genetic contribution to lifespan. Thus, a proper control group should be siblings-in law, rather than a general population.
Table 2.
Mean life span conditional on survival to age 50 (LS50) with 95% confidence intervals (95% CI) for relatives of centenarians compared to the 1900 US birth cohort).
| Relatives of centenarians | Male relatives | Female relatives | ||
|---|---|---|---|---|
| Sample size, N | LS50 (95% CI), years | Sample size, N | LS50 (95% CI), years | |
| Parents | 1590 | 76.2 (75.7-76.8) | 1557 | 77.2 (76.7-77.8) |
| All Siblings | 5324 | 77.6 (77.3-77.9) | 4877 | 82.4 (82.0-82.7) |
| Married Siblings | 3221 | 77.7 (77.3-78.1) | 3028 | 82.2 (81.8-82.6) |
| Spouses | 876 | 75.4 (74.6-76.1) | 283 | 81.4 (80.1-82.7) |
| Siblings-in-law | 2349 | 75.0 (74.6-75.5) | 2407 | 79.5 (79.0-79.9) |
| 1900 US birth cohort | 73.3 | 79.4 | ||
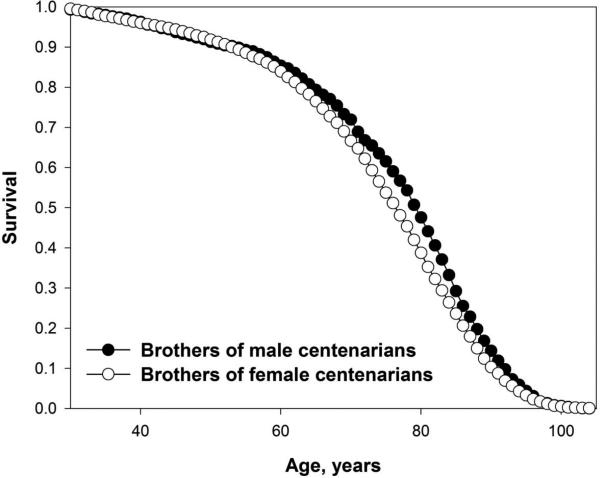
It was found that male gender of centenarian has significant positive effect on survival of adult male relatives (brothers and fathers) but not female relatives[25]. Comparison of married siblings and siblings-in-law of centenarians found a strong positive effect of centenarian male gender on survival of brothers and a weak positive effect of female gender on survival of centenarian sisters compared to the same-sex siblings-in-law. Figure 1 shows survival curves after age 30 for male siblings of centenarians depending on centenarian gender. Note that brothers of male centenarians have substantially better survival than brothers of female centenarians and this difference in survival is highly statistically significant (p<0.001) according to generalized Wilcoxon test. This survival advantage is particularly strong after age 65 years while before the age 50 years the differences in survival are minimal. Thus, having a centenarian brother is associated with better late-life survival for males. Taking into account that female gender of centenarian has a much weaker effect on survival of sisters compared to the effect of male gender of centenarian on the survival of brothers, we may hypothesize that male centenarians and their brothers share living conditions and lifestyle favorable for men. This favorable lifestyle could come from their fathers, whose businesses and occupation were often inherited by sons in the past [29]. For example, farming is beneficial for longevity of males [26]. Thus, it is reasonable to suggest that brothers of male centenarians shared farming occupation of their fathers and long-lived brothers, which contributed to their survival advantage. On the other hand, female centenarians have the same likelihood to live on a farm or be married to a farmer as shorter-lived female controls [25]. Thus, we may suggest that brothers of female centenarians have the same likelihood to be a farmer as brothers of shorter lived controls and hence do not have additional survival advantage related to farming. This explanation is also consistent with our earlier findings as well as results of other studies, which found positive effects of farming and farm background on late-life survival [30, 31]. Finally, wives of male centenarians had a significantly better survival compared to wives of brothers of centenarians indicating importance of within-family living conditions and lifestyle in longevity determination. As expected, centenarian gender had no effect on survival of centenarian siblings-in-law. This study suggests that intra-familial environmental conditions and lifestyle may play more significant role in exceptional longevity than it was thought before.
Figure 1.
Survival curves for male siblings (brothers) of centenarians, by centenarian gender.
Why centenarians are different from their shorter-lived siblings?
Siblings share early childhood conditions including parental socio-economic status, genetic background and geographical location while spouses share common adulthood environment. It was shown that longevity has a significant familial component suggesting the need to control for this important factor. Comparison of centenarians and their shorter-lived siblings is conducted using a within-family analysis by the method of conditional logistic regression, which allows researchers to control for unobserved shared childhood or adulthood environment and common genetic background. We have developed and analyzed a computerized database of 1,711 validated centenarians born in the United States between 1880 and 1895, as well as their shorter-lived siblings [25] .
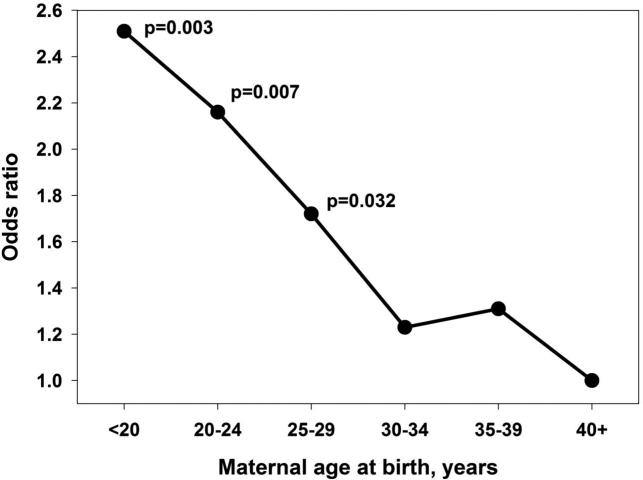
The first study explored the effects of parental age at a person's birth on chances of survival to age 100. We found significant beneficial effects of a young maternal age at a person's birth on survival to age 100 with particularly strong positive influence at a maternal age of 20-24 years [25, 26, 32]. The effect of a young mother was particularly prominent in smaller families [25], which is pertinent today because of the smaller average family size in contemporary population (Figure 2). Thus, the within-family analysis of the paternal-and maternal-age effects on human longevity demonstrated that a young age of the mother increases the chances of children to reach longevity. The finding of a beneficial effect of young maternal age on offspring survival to age 100 in humans is also reported for laboratory animals [33, 34], and hence may have biological explanation. There is an empirical evidence that the quality of female eggs in human beings rapidly declines with age and this deterioration starts rather early—before age 30 [35].
Figure 2.
Effects of maternal age at person's birth on odds to live to 100 (conditional on survival to age 50). The within-family study of 2,153 centenarians and their siblings who survived to age 50. Data on smaller families with less than nine children. Calculated using Stata 11 statistical package (procedure clogit).
Also, in human beings, some additional sociobehavioral mechanisms may be also involved, on top of more general biological mechanisms. One such mechanism may involve positive effects of prolonged maternal care on health and longevity or the so-called ‘mothering hypothesis’ [30]. According to this hypothesis, maternal-age effects in human beings are mediated through the length of mothering or exposure to the maternal care. Some researchers believe that the observed relationship between maternal age and mortality is most likely explained by the effects of early maternal loss among the children of old mothers rather than physiological mechanisms related to maternal aging [36]. Specifically it was found that detrimental effects of old maternal age disappear after controlling for maternal education and lifespan overlap between the child and the mother. In our study we used the within-family approach, which is more robust to the effects of parental socio-economic status and maternal education in particular. Almost all mothers in our sample were housewives and finished their education by the time of marriage. As a result, maternal education remained the same for all the siblings in the family. In addition to that, controlling for maternal longevity does not result in disappearance of the effects of maternal age on longevity. Although late-born children of long-lived mothers have a substantial exposure to maternal care (exceeding 35 years) they still demonstrate lower likelihood of survival to age 100. Thus, the maternal age effects on survival found in the within-family analysis are more likely to be related to some physiological mechanisms. Similar results were obtained in other independent studies of maternal age effects on longevity applying the within-family approach [26, 30, 37].
These results provide evidence that early-life programming influences human longevity. A recent study of Canadian centenarians [38], which applied the same within-family approach, confirmed our initial finding of a inverse relationship between maternal age and likelihood of survival to age 100 [26]. Another recent study of historical Swedish cohorts also demonstrated beneficial effects of young maternal age on lifespan of offspring [39].
In addition to maternal age, the within-family approach can be applied to the studies of season-of-birth effects onf exceptional longevity. Month of birth is a useful proxy characteristic for seasonal environmental effects acting during in-utero and early infancy development. Little information is available on the month of birth association with exceptional longevity. To analyze net effects of birth month on exceptional longevity, not confounded by possible changes in birth and infant death seasonality, childhood conditions and genetic background, we conducted a matched study using a multivariate conditional logistic regression method [40]. Month of birth of 1,574 validated centenarians born in the United States in 1880-1895 were compared to the same information obtained for 10,885 shorter-lived siblings and 1,083 spouses. This within-family analysis found that months of birth have significant long-lasting effects on survival to age 100; adult siblings born in September-November have significantly higher odds of becoming centenarians compared to siblings born in March. A similar month-of-birth pattern was found for centenarian spouses. This differential survival leads to excess of fall-born persons among centenarians [40]. These findings support the idea of early-life programming of human aging and longevity, and are in a good agreement with our earlier reports on the effects of month-of-birth on mortality in the United States [41], and are consistent with our study of centenarians and shorter-lived peers (see above). The results of our study were obtained by using a more conclusive within-family analysis, and were not confounded by between-family variation. They demonstrate that month-of-birth has a strong independent effect on human longevity. Thus, similar results were obtained using three different sources of data.
This study on exceptional longevity investigates the biological and social correlates of why some people survive to extreme old age (older than 100 years). Some results of this study may have practical implications. Specifically, we found beneficial effects of young maternal age at person's birth on person's longevity [25, 26]. This finding highlights the consequences of delaying childbearing (becoming more common in developed countries) on offspring's health. Studies on links between season of birth and human longevity [25, 40] emphasized important effects of early-life conditions on late-life survival and suggested that environmental conditions and lifestyle may be as important as genetics in determining longevity. We also found that mortality (hazard rate) after age 100 does not decelerate but follows the traditional Gompertz law [15, 17]. This finding will allow demographers and gerontologists to improve forecasts of human mortality and the size of the older population. These are all significant issues in the rapidly expanding field of biodemography of aging and longevity.
Acknowledgements
We would like to acknowledge support from the U.S. National Institute on Aging (NIA grant R01 AG028620).
References
- 1.Gavrilov LA, Gavrilova NS. The biology of life span: A quantitative approach. Harwood Academic Publisher; New York: 1991. [Google Scholar]
- 2.Gavrilov LA, Gavrilova NS. Reliability theory of aging and longevity. In: Masoro EJ, Austad SN, editors. Handbook of the biology of aging. Academic Press; San Diego: 2006. pp. 3–42. [Google Scholar]
- 3.Carnes BA, Olshansky SJ, Grahn D. Continuing the search for a law of mortality. Popul Dev Rev. 1996;22:231–264. [Google Scholar]
- 4.Olshansky SJ. On the biodemography of aging: A review essay. Popul Dev Rev. 1998;24:381–393. [Google Scholar]
- 5.Greenwood M, Irwin JO. The biostatistics of senility. Hum Biol. 1939;11:1–23. [Google Scholar]
- 6.Carey JR, Liedo P, Orozco D, Vaupel JW. Slowing of mortality-rates at older ages in large medfly cohorts. Science. 1992;258:457–461. doi: 10.1126/science.1411540. [DOI] [PubMed] [Google Scholar]
- 7.Mueller LD, Rauser CL, Rose MR. Does aging stop? Oxford University Press; Oxford: 2011. [Google Scholar]
- 8.Beard RE. Note on some mathematical mortality models. In: Wolstenholme EW, O'Connor MO, editors. The lifespan of animals. Little, Brown.; Boston: 1959. pp. 302–311. [Google Scholar]
- 9.Sacher GA. The gompertz transformation in the study of the injury-mortality relationship: Application to late radiation effects and ageing. In: Lindop PJ, Sacher GA, editors. Radiation and aging. Taylor and Francis; London: 1966. pp. 411–441. [Google Scholar]
- 10.Austad SN. Concepts and theories of aging. In: Masoro EJ, Austad SN, editors. Handbook of the biology of aging. Academic Press; San Diego: 2001. pp. 3–22. [Google Scholar]
- 11.Bronikowski AM, Alberts SC, Altmann J, Packer C, Carey KD, Tatar M. The aging baboon: Comparative demography in a non-human primate. Proceedings of the National Academy of Sciences of the USA. 2002;99:9591–9595. doi: 10.1073/pnas.142675599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bronikowski AM, Altmann J, Brockman DK, Cords M, Fedigan LM, Pusey A, Stoinski T, Morris WF, Strier KB, Alberts SC. Aging in the natural world: Comparative data reveal similar mortality patterns across primates. Science. 2011;331:1325–1328. doi: 10.1126/science.1201571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Horiuchi S, Wilmoth JR. Deceleration in the age pattern of mortality at older ages. Demography. 1998;35:391–412. [PubMed] [Google Scholar]
- 14.Thatcher AR, Kannisto V, Vaupel JW. The force of mortality at ages 80 to 120. Odense University Press; Odense: 1998. [Google Scholar]
- 15.Gavrilov LA, Gavrilova NS. Mortality measurement at advanced ages: A study of the social security administration death master file. North American Actuarial Journal. 2011;15:432–447. doi: 10.1080/10920277.2011.10597629. PMID: 22308064. PMCID: PMC23269912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gavrilova NS, Gavrilov LA. Ageing and longevity: Mortality laws and mortality forecasts for ageing populations [in czech: Starnuti a dlouhovekost: Zakony a prognozy umrtnosti pro starnouci populace]. Demografie. 2011;53:109–128. NIHMS329960. [PMC free article] [PubMed] [Google Scholar]
- 17.Gavrilova NS, Gavrilov LA. Biodemography of old-age mortality in humans and rodents. J Gerontol A Biol Sci Med Sci. 2014 Feb 17; doi: 10.1093/gerona/glu009. [Epub ahead of print], doi: 10.1093/gerona/glu1009, PMC Journal – In Process. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Coale AJ, Kisker EE. Mortality crossovers - reality or bad data. Pop Stud-J Demog. 1986;40:389–401. [Google Scholar]
- 19.Gavrilov LA, Gavrilova NS. Mortality measurement at advanced ages: A study of the social security administration death master file. North American Actuarial Journal. 2011;15:432–447. doi: 10.1080/10920277.2011.10597629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Modig K, Drefahl S, Ahlbom A. Limitless longevity: Comment on the contribution of rectangularization to the secular increase of life expectancy. Int J Epidemiol. 2013 doi: 10.1093/ije/dyt035. doi: 10.1093/ije/dyt1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Robine JM, Vaupel JW. Supercentenarians: Slower ageing individuals or senile elderly? Exp Gerontol. 2001;36:915–930. doi: 10.1016/s0531-5565(00)00250-3. [DOI] [PubMed] [Google Scholar]
- 22.Engberg H, Oksuzyan A, Jeune B, Vaupel JW, Christensen K. Centenarians - a useful model for healthy aging? A 29-year follow-up of hospitalizations among 40 000 danes born in 1905. Aging Cell. 2009;8:270–276. doi: 10.1111/j.1474-9726.2009.00474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Finch CE, Crimmins EM. Inflammatory exposure and historical changes in human life-spans. Science. 2004;305:1736–1739. doi: 10.1126/science.1092556. [DOI] [PubMed] [Google Scholar]
- 24.Hadley EC, Rossi WK, Albert S, Bailey-Wilson J, Baron J, Cawthon R, Christian JC, Corder EH, Franceschi C, Kestenbaum B, Kruglyak L, Lauderdale DS, Lubitz J, Martin GM, McClearn GE, McGue M, Miles T, Mineau G, Ouellett G, Pedersen NL, Preston SH, Page WF, Province M, Schachter F, Schork NJ, Vaupel JW, Vijg J, Wallace R, Wang E, Wijsman EM, Wor NAGE. Genetic epidemiologic studies on age-specified traits. Am J Epidemiol. 2000;152:1003–1008. doi: 10.1093/aje/152.11.1003. [DOI] [PubMed] [Google Scholar]
- 25.Gavrilov LA, Gavrilova NS. Determinants of exceptional human longevity: New ideas and findings. Vienna Yearbook of Population Research. 2013;11:295–323. doi: 10.1553/populationyearbook2013s295. NIHMS601424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gavrilov LA, Gavrilova NS. Biodemography of exceptional longevity: Early-life and mid-life predictors of human longevity. Biodemography and Social Biology. 2012;58:14–39. doi: 10.1080/19485565.2012.666121. doi: 10.1080/19485565.19482012.19666121. PMID: 22582891 PMCID: PMC13354762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Travers C, Bartlett HP. Silver memories: Implementation and evaluation of a unique radio program for older people. Aging & Mental Health. 2011;15:169–177. doi: 10.1080/13607863.2010.508774. [DOI] [PubMed] [Google Scholar]
- 28.Gagnon A, Smith KR, Tremblay M, Vezina H, Pare PP, Desjardins B. Is there a trade-off between fertility and longevity? A comparative study of women from three large historical databases accounting for mortality selection. Am J Hum Biol. 2009;21:533–540. doi: 10.1002/ajhb.20893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ruggles S. The decline of intergenerational coresidence in the united states, 1850 to 2000. Am Sociol Rev. 2007;72:964–989. doi: 10.1177/000312240707200606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gavrilova NS, Gavrilov LA. Search for predictors of exceptional human longevity: Using computerized genealogies and internet resources for human longevity studies. North American Actuarial Journal. 2007;11:49–67. [Google Scholar]
- 31.Preston SH, Hill ME, Drevenstedt GL. Childhood conditions that predict survival to advanced ages among african-americans. Social Science & Medicine. 1998;47:1231–1246. doi: 10.1016/s0277-9536(98)00180-4. [DOI] [PubMed] [Google Scholar]
- 32.Gavrilova NS, Gavrilov LA. Search for mechanisms of exceptional human longevity. Rejuv Res. 2010;13:262–264. doi: 10.1089/rej.2009.0968. PMID: 20370503. PMCID: PMC22946054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tarin JJ, Gomez-Piquer V, Rausell F, Navarro S, Hermenegildo C, Cano A. Delayed motherhood decreases life expectancy of mouse offspring. Biology of Reproduction. 2005;72:1336–1343. doi: 10.1095/biolreprod.104.038919. [DOI] [PubMed] [Google Scholar]
- 34.Carnes BA, Riesch R, Schlupp I. The delayed impact of parental age on offspring mortality in mice. J Gerontol a-Biol. 2012;67:351–357. doi: 10.1093/gerona/glr116. [DOI] [PubMed] [Google Scholar]
- 35.Heffner LJ. Advanced maternal age--how old is too old? New Engl J Med. 2004;351:1927–1929. doi: 10.1056/NEJMp048087. [DOI] [PubMed] [Google Scholar]
- 36.Myrskyla M, Fenelon A. Maternal age and offspring adult health: Evidence from the health and retirement study. Demography. 2012;49:1231–1257. doi: 10.1007/s13524-012-0132-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jarry V, Gagnon A, Bourbeau R. To what extent is exceptional longevity associated with parental age at childbirth and birth order?: Determinants of Unusual and Differential Longevity. Vienna Institute of Demography; Vienna, Austria: 2012. [Google Scholar]
- 38.Jarry V, Gagnon A, Bourbeau R. Maternal age, birth order and other early-life factors: A family-level approach to exploring exceptional survival. Vienna Yearbook of Population Research. 2013;11:263–284. [Google Scholar]
- 39.Wilding M, Coppola G, De Icco F, Arenare L, Di Matteo L, Dale B. Maternal non-mendelian inheritance of a reduced lifespan? A hypothesis. J Assist Reprod Genet. 2014;31:637–643. doi: 10.1007/s10815-014-0222-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gavrilov LA, Gavrilova NS. Season of birth and exceptional longevity: Comparative study of american centenarians, their siblings, and spouses. Journal of aging research. 2011 doi: 10.4061/2011/104616. Article ID 104616, 104611 pages, doi:104610.104061/102011/104616. PMID: 22187646. PMCID: PMC23236478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gavrilov LA, Gavrilova NS. Mortality measurement at advanced ages: A study of the social security administration death master file; Living to 100 and beyond: Survival at advanced ages [online monograph] soa monograph m–li08–1. The Society of Actuaries; Shaumburg, IL: 2008. p. 32. [DOI] [PMC free article] [PubMed] [Google Scholar]