Abstract
Atypical antipsychotic (AA) drugs cause significant metabolic side effects, and clinical data are emerging that demonstrate increased fracture risk and bone loss after treatment with the AA, risperidone (RIS). The pharmacology underlying the adverse effects on bone is unknown. However, RIS action in the central nervous system could be responsible because the sympathetic nervous system (SNS) is known to uncouple bone remodeling. RIS treatment in mice significantly lowered trabecular bone volume fraction (bone volume/total volume), owing to increased osteoclast-mediated erosion and reduced osteoblast-mediated bone formation. Daytime energy expenditure was also increased and was temporally associated with the plasma concentration of RIS. Even a single dose of RIS transiently elevated expression of brown adipose tissue markers of SNS activity and thermogenesis, Pgc1a and Ucp1. Rankl, an osteoclast recruitment factor regulated by the SNS, was also increased 1 hour after a single dose of RIS. Thus, we inferred that bone loss from RIS was regulated, at least in part, by the SNS. To test this, we administered RIS or vehicle to mice that were also receiving the nonselective β-blocker propranolol. Strikingly, RIS did not cause any changes in trabecular bone volume/total volume, erosion, or formation while propranolol was present. Furthermore, β2-adrenergic receptor null (Adrb2−/−) mice were also protected from RIS-induced bone loss. This is the first report to demonstrate SNS-mediated bone loss from any AA. Because AA medications are widely prescribed, especially to young adults, clinical studies are needed to assess whether β-blockers will prevent bone loss in this vulnerable population.
It is well documented that atypical antipsychotic (AA) drugs are associated with significant side effects, including obesity, hyperglycemia, and dyslipidemia (1–3), and all drugs in this class carry a black box warning label because of the severity of metabolic side effects (4). In fact, a single dose (intravenous or intracerebroventricular) of certain AAs (olanzapine, clozapine, and, to a lesser degree, risperidone [RIS]) can dramatically reduce insulin sensitivity in the liver. This effect continues after multiple doses, before drug-induced weight gain (5). The mechanism of metabolic changes is in part due to centrally mediated hepatic insulin resistance; however, the specific receptor pharmacology causing the enhanced metabolic liability of certain AAs has not yet been elucidated (6).
More recent clinical evidence has identified the skeleton as vulnerable to the actions of AA medications. Schizophrenic patients are more at risk for fractures than the general population; medication-induced disease may account for a portion of this increased risk (7). Bone mineral density is reduced, and fracture risk is increased in some patients treated with RIS and other AAs (8–16). Because of the strong relationship between the regulation of bone and energy metabolism (17–25, 49), we developed a model to study the effects of RIS treatment on bone in mice. Consistent with the clinical presentation, we found low trabecular bone volume fraction after administration of RIS, due to both increased resorption and reduced bone formation (26). However, the bone loss in mice occurred in the absence of overt metabolic dysfunction (as evidenced by no changes in body mass, fat mass, blood glucose, insulin tolerance, and glucose tolerance) (26). Although underlying metabolic dysfunction is probably still present based on clamp studies performed on rats exposed to AAs, we concluded that bone loss was not due to obesity or diabetes in RIS-treated mice and that other mechanisms must be operative (5, 26).
The dopamine receptor antagonist activity of RIS can induce clinical hyperprolactinemia, which leads to hypothalamic hypogonadism and could explain bone loss (9, 10, 13, 27). However, less than half of the patients using RIS develop hyperprolactinemia, and this is usually temporary (9, 28). In addition, in our previous, 4-week study, female mice administered RIS did not present with uterine atrophy, which is inconsistent with hypogonadism (26). AA drugs bind to multiple targets, including serotonin, histamine, and dopamine receptors, and therefore can affect additional organ systems that have a direct or indirect impact on skeletal remodeling.
Serotonin (5-hydroxytryptamine) is a crucial intermediate molecule in the regulation of bone mass by the sympathetic nervous system (SNS) (21, 29). Leptin modulates bone metabolism indirectly through the hypothalamus by activating the SNS, thereby suppressing bone formation via β-adrenergic receptors on osteoblasts (22, 25, 30). Interestingly, the consequences of leptin deficiency on bone can be reversed by suppressing serotonin production in the brainstem (22). Mice deficient in brain-derived production of serotonin (Tph2r) exhibit a phenotype opposite to that of leptin-deficient mice. Notably, Tph2−/− mice have decreased bone mass associated with low bone formation and increased bone resorption, albeit with normal levels of leptin (22). Because RIS acts as a serotonin receptor antagonist, it is plausible that SNS activity may be elevated during RIS administration and that this may contribute to the effects of RIS on bone remodeling.
In this study, we hypothesized that RIS causes SNS-mediated bone loss (26, 31). In brief, we found high energy expenditure (EE) and markers of SNS activity in RIS-treated mice, consistent with elevated SNS tone. The β-blocker propranolol (PRO) attenuated the effects of RIS on trabecular bone, suggesting that RIS works through SNS-mediated mechanisms. This was confirmed by similar studies in which RIS was administered to mice lacking the β2-adrenergic receptor (Adrb2−/−). Thus, we conclude that the effects of RIS on bone in mice are largely due to elevated SNS activity.
Materials and Methods
The experimental protocols were approved by the Institutional Animal Care and Use Committees of the Maine Medical Center Research Institute and University of New England. We uniformly used 8-week-old, female C57BL/6J mice that were maintained on 12:12-hour light/dark cycles and had standard chow and water ad libitum.
Mice: RIS and RIS + PRO studies
Female C57BL/6J (B6) mice were obtained from The Jackson Laboratory at 6 weeks of age. Female mice were chosen to compare with previous studies from our laboratory and because female rodents are more likely to have metabolic abnormalities with antipsychotic treatment (26, 32). At 8 weeks of age, mice were administered either vehicle (0.1% acetic acid) or 0.75 mg/kg RIS (in 0.1% acetic acid; Sigma-Aldrich) by oral gavage daily (between 11:00 am and 2:00 pm) for 4 weeks. The dose of RIS was chosen based on preliminary pharmacokinetic studies that evaluated plasma drug concentrations (total drug) after administration of various doses of RIS by oral gavage (K.J.M., D.B., K.L.H., unpublished data). The dose of 0.75 mg/kg produced peak serum levels of RIS and the active metabolite, paliperidone (PAL), most similar to that achieved in patients (33). A subset of mice were treated with vehicle or RIS as above for 2 weeks and then placed in metabolic cages. While in metabolic cages, RIS and vehicle administration continued daily at 10:00 am. Finally, an additional cohort of mice was administered only a single dose of RIS or vehicle (10:00 am) and killed at time 0, 1, 6 or 24 hours after dose.
In addition to RIS or vehicle, a subset of mice was administered 0.5 mg/mL PRO (Roxane Laboratories) in the drinking water (changed 3× per week) for the duration of the RIS administration. The PRO dose was based on previous publications that examined the bone effects of PRO in mice (34, 35). Mice were administered their last dose of RIS or vehicle 1 hour before killing.
Mice: β2-adrenergic receptor knockout (Adrb2−/−) study
Adrb2−/− breeder pairs were obtained from Drs Brad Lowell and Mary Bouxsein (Beth Israel Deaconess Medical Center) and rederived at Maine Medical Center Research Institute. Experimental mice (Adrb2+/+ and Adrb2−/−) were bred from heterozygous matings. Female mice were uniformly used, and daily RIS and vehicle gavage was performed as described above from 8 to 12 weeks of age.
Indirect calorimetry
Indirect calorimetry measurements were performed using the Promethion metabolic cage system (Sable Systems) located in the Physiology Core Department of Maine Medical Center Research Institute as described in detail previously (35, 36). Data acquisition and instrument control were performed using MetaScreen version 1.7.2.3, and the raw data obtained were processed with ExpeData version 1.5.4 (Sable Systems) using an analysis script detailing all aspects of data transformation. Linear regression analyses were performed between nighttime EE and lean mass and wheel activity to determine whether these variables could explain changes in EE (37–39).
Analysis of drug concentrations in plasma
Concentrations of RIS and its primary active metabolite, 9-hydroxyrisperidone (PAL) were determined by liquid chromatography-tandem mass spectrometry analysis. See Supplemental Methods for a detailed description of this procedure.
Dual-energy X-ray absorptiometry
All mice were measured at baseline (8 weeks) and at the time of killing for fat-free mass, fat mass, and bone mineral density using a PIXImus dual-energy X-ray densitometer (GE Lunar). The PIXImus was calibrated daily with a mouse phantom provided by the manufacturer. Mice were placed ventral side down with each limb and tail positioned away from the body. Full-body scans were obtained, and X-ray absorptiometry data were gathered and processed with the manufacturer's supplied software (Lunar PIXImus 2, version 2.1). The head was specifically excluded from all analyses because of concentrated mineral in skull and teeth.
Serum leptin assay
Leptin levels were measured using a commercially available kit from ALPCO Diagnostics, according to the manufacturer's instructions. The assay sensitivity was 10 pg/mL. The interassay and intra-assay variation coefficients were <4.7% and 4.4%, respectively.
Microcomputed tomography (μCT)
The microarchitecture of the distal trabecular bone and midshaft cortical bone of the femur was analyzed by μCT as described previously (resolution 10 μm, vivaCT-40; Scanco Medical AG) (26). Measurements included bone volume/total volume (BV/TV), trabecular number (Tb.N), trabecular thickness (Tb.Th), trabecular separation (Tb.Sp), and connectivity density (Conn.D). Scans for the cortical region were measured at the midpoint of each femur, with an isotropic pixel size of 21 μm and slice thickness of 21 μm, and used to calculate the average bone area, total cross-sectional area, bone area/total area, and cortical thickness. All scans were analyzed using the manufacturer's software (version 4.05; Scanco Medical AG).
Histology and quantitative histomorphometry
Qualitative histological analysis and quantitative static and dynamic histomorphometry were performed as described previously (40). To examine bone formation rates, calcein label (20 mg/kg) and demeclocycline label (20 mg/kg) were injected intraperitoneally at 9 and 2 days before killing, respectively. Immediately after killing, tibias for histomorphometry were placed in 70% ethanol and maintained in the dark at 4°C. Histomorphometric measurements were performed on the secondary spongiosa of the proximal tibia metaphysis using an OsteoMeasure morphometry system (Osteometrics). For dynamic histomorphometry, mineralizing surface per bone surface (percentage) and mineral apposition rate (micrometers per day) were measured in unstained sections under UV light and used to calculate the bone formation rate with a surface referent (cubic micrometers per square micrometers per year), volume referent (percentage per year), and tissue referent (percentage per year). Static measurements included BV/TV (percentage), Tb.Th (micrometers), Tb.N (per millimeter), Tb.Sp (micrometers), eroded surface per bone surface (percentage), osteoid surface per bone surface (percentage), osteoid volume per tissue volume (percentage), osteoid thickness (micrometers), osteoblast surface per bone surface (percentage), osteoclast surface per bone surface (percentage), osteoblast number per bone perimeter (per millimeter), osteoblast number per tissue area (per square millimeter), osteoclast number per bone perimeter (per millimeter), osteoclast number per tissue area (per square millimeter), and adipocytes per total area (per square millimeter). Terminology and units followed the recommendations of the Histomorphometry Nomenclature Committee of the American Society for Bone and Mineral Research (41).
Real-time PCR
Whole tibia or brown fat was crushed under liquid nitrogen conditions. Total RNA was prepared using the standard TRIzol (Sigma-Aldrich) method for tissues. cDNA was generated using the High Capacity cDNA Reverse Transcriptase Kit (Applied Biosystems) according to the manufacturer's instructions. mRNA expression analysis was carried out using an iQ SYBR Green Supermix with an iQ5 thermal cycler and detection system (Bio-Rad Laboratories). Hprt was used as an internal standard control gene for all quantification (42). Primers were designed and tested to be 95% to 100% efficient by PrimerDesign. Dmp1 and Mepe primers were obtained from Integrated DNA Technologies. All primer sequences are listed in Supplemental Table 1.
Statistical analysis
All data are expressed as means ± SEM unless otherwise noted. Results were analyzed for statistically significant differences using the Student t test or two-way ANOVA followed by the Sidak multiple comparison post hoc test where appropriate. Linear regression analysis was used where indicated. All statistics were performed with Prism 6 statistical software (GraphPad Software, Inc). Statistical significance was set at P < .05.
Results
Trabecular bone mass and remodeling after RIS treatment
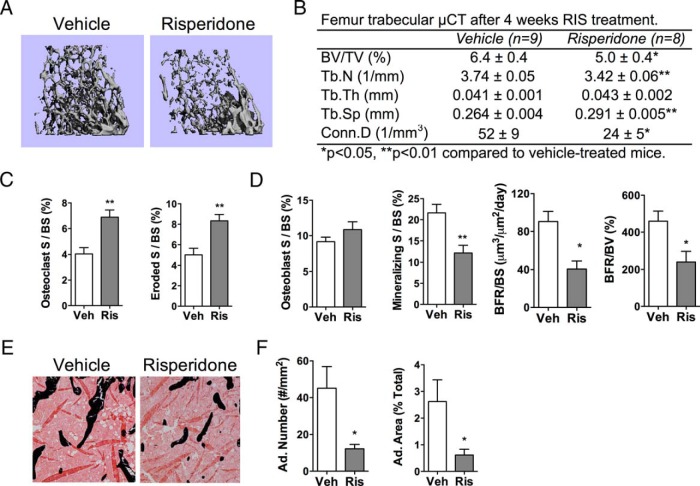
Female mice treated with RIS daily for 4 weeks had low trabecular bone volume fractions (BV/TV) compared with those for vehicle-treated controls (Figure 1, A and B). This was associated with reduced Tb.N and Conn.D, as well as increased Tb.Sp (Figure 1B). Histological findings revealed increased osteoclast parameters and reduced mineralizing surface and bone formation rate after RIS treatment (Figure 1, C and D). Measures of marrow adiposity were also significantly suppressed by RIS treatment (Figure 1, E and F). Additional μCT and histological parameters are found in Supplemental Table 2.
Figure 1.
RIS caused trabecular bone loss. RIS or vehicle (Veh) was administered to mice for 4 weeks, and analyses were performed at 12 weeks of age. A and B, Representative images (A) and quantitative parameters from μCT (B) of the trabecular bone of the distal femur. BV/TV, Tb.N, Tb.Th, Tb.Sp, and Conn.D were measured at 10-μm resolution. C and D, Quantitative osteoclast (C) and osteoblast (D) parameters from histomorphometric analyses of the trabecular bone of the proximal tibia (S, surface; BS, bone surface; BFR, bone formation rate; BV, bone volume). E, Representative images from histomorphometric analyses in the proximal tibia, where black stain is mineral (Von Kossa) and adipocytes ghosts are white. F, Quantitative adipocyte (Ad.) measurements from the proximal tibia. n = 5 to 9. *, P < .05; **, P < .01.
Body composition and EE
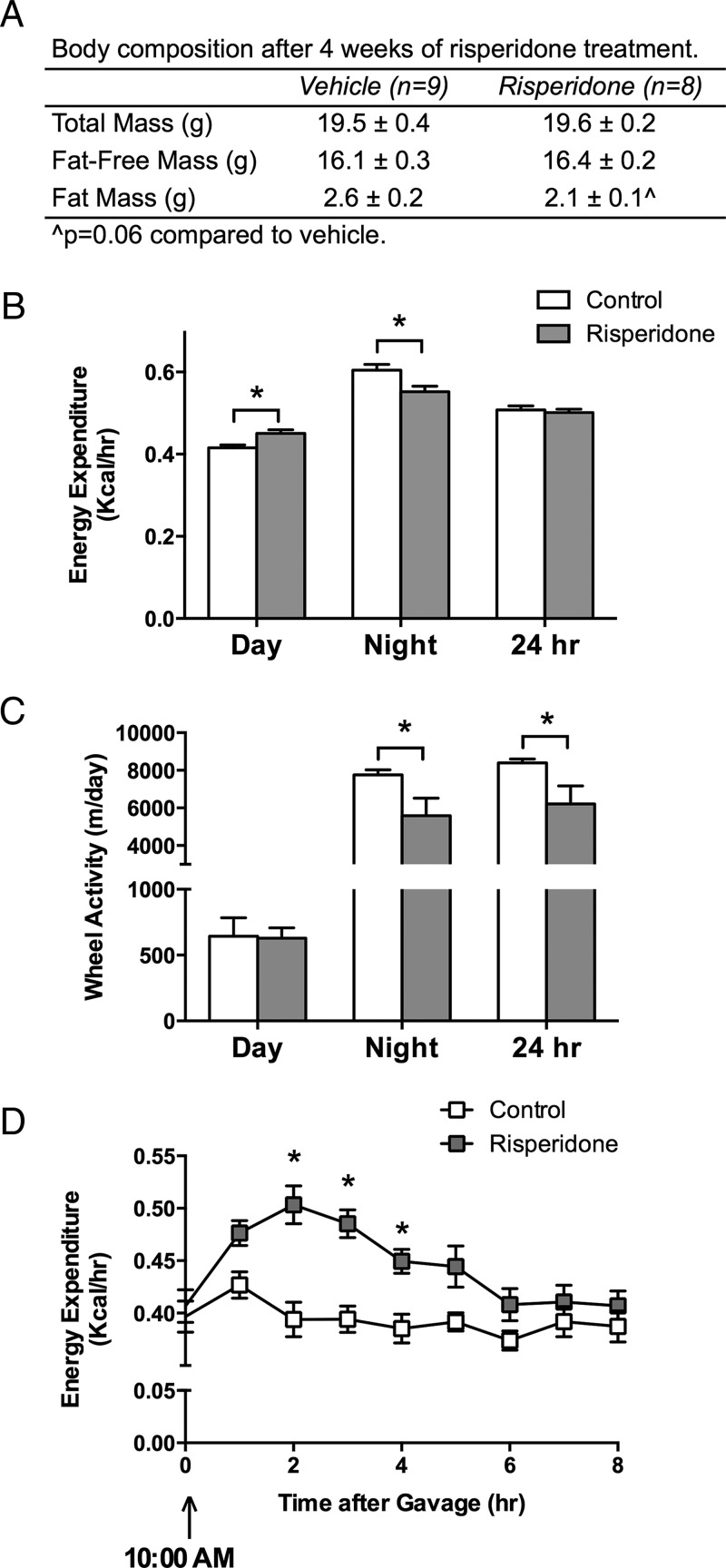
RIS did not significantly alter total mass or fat-free mass, but there was a trend toward reduced fat mass in RIS-treated mice (P = .06 compared to vehicle) (Figure 2A). Daytime EE was significantly elevated in RIS mice, but nighttime EE was reduced, resulting in no change in total 24-hour EE between RIS- and vehicle-treated mice (Figure 2B). Wheel activity was lower in RIS-treated mice during the night but was not changed during the day (Figure 2C). Other activity parameters were not significantly changed during the day, so we examined temporal changes in EE and activity to determine whether they were associated with time of dosing. EE was significantly elevated after RIS gavage, peaked at 2 hours after gavage and returned to baseline by 6 hours after gavage (Figure 2D). Oxygen consumption and carbon dioxide production were similarly elevated after RIS dosing, and the respiratory quotient was unchanged by RIS (data not shown). There were no associations between wheel meters, beam breaks, or walking distance and the temporal changes in EE after gavage (not shown). Food and water intake were unchanged between the groups at any interval (not shown).
Figure 2.
RIS dosing transiently increased EE. RIS or vehicle (Veh) was administered to mice for 2 weeks; then mice were placed in metabolic cages, and daily dosing was continued during the duration of the time the mice were in the cages. Therefore, the metabolic analyses were performed on mice at 11 weeks of age and body composition upon removal from the cages at 12 weeks of age. A, Body composition was determined by dual-energy X-ray absorptiometry. B, EE (kilocalories per hour) was measured with metabolic cages and averaged during the day and night and over a 24-hour period. C, Wheel activity (meters per day) was measured and totaled during the day and night and over a 24-hour period. D, EE (kilocalories per hour) was averaged over 1-hour periods after dosing with vehicle or RIS. n = 8 to 9. *, P < .05.
Plasma RIS exposure
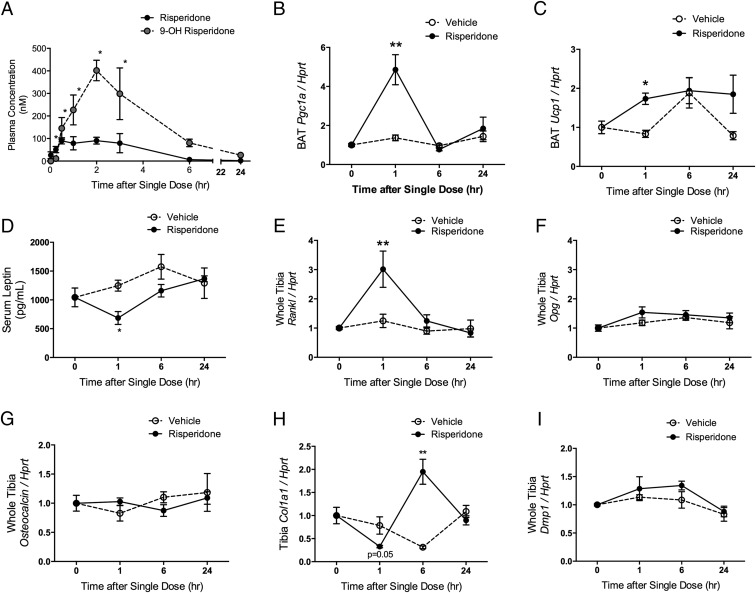
Total (bound and free) concentrations of RIS and its active metabolite PAL were detectable within 2 minutes after dose in plasma, and PAL concentrations in plasma peaked at 2 hours after gavage (Figure 3A).
Figure 3.
Changes in gene expression in BAT and bone occur during peak drug levels of RIS and PAL in the plasma. A, A single dose of RIS was administered to 8-week-old female mice. RIS and its active metabolite PAL (9-hydroxyrisperidone) were measured in the plasma of n = 3 mice per group and time point. *, P < .05, compared with baseline. B–I, A single dose of RIS was administered to 8-week-old female mice (n = 4 per group and time point). BAT mRNA levels of Pgc1a (B) and Ucp1 (C) were measured at baseline (time 0) and 1, 6, and 24 hours after a single dose of RIS and normalized to a housekeeping gene, Hprt. D, Serum leptin levels were measured with an ELISA. Whole crushed tibia mRNA levels (bone and marrow) of Rankl (E), Opg (F), osteocalcin (G), Col1a1 (H), and Dmp1 (I) were measured at baseline (time 0) and 1, 6, and 24 hours after a single dose of RIS and normalized to a housekeeping gene, Hprt. *,P < .05, compared with vehicle-treated controls at the same time point.
Effects of a single dose of RIS on thermogenesis and bone remodeling
RIS-mediated changes in EE have previously been attributed to induction of thermogenesis in rodents. A single dose of RIS caused increased Pgc1a and Ucp1 expression in brown adipose tissue (BAT) 1 hour after gavage, which normalized by 6 hours (Figure 3, B and C). Conversely, serum leptin levels were significantly reduced 1 hour after gavage and normalized by 6 hours (Figure 3D). Similarly, RIS induced an acute increase in expression of the osteoclast recruitment factor Rankl in bone at 1 hour, which returned to baseline by 6 hours (Figure 3E). The expression levels of the receptor activator of nuclear factor κB ligand (RANKL) decoy receptor, osteoprotegerin (Opg), were unchanged during RIS treatment (Figure 3F). Osteoblast parameters osteocalcin, osteoglycin, and osteomodulin and osteocyte parameters Dmp1, Mepe, and sclerostin were unchanged by acute RIS treatment (Figure 3, G and I, and not shown). However, Col1a1 was differentially regulated by RIS, with a significant increase in expression at 6 hours after the dose (Figure 3H).
Effect of PRO on RIS-mediated bone loss
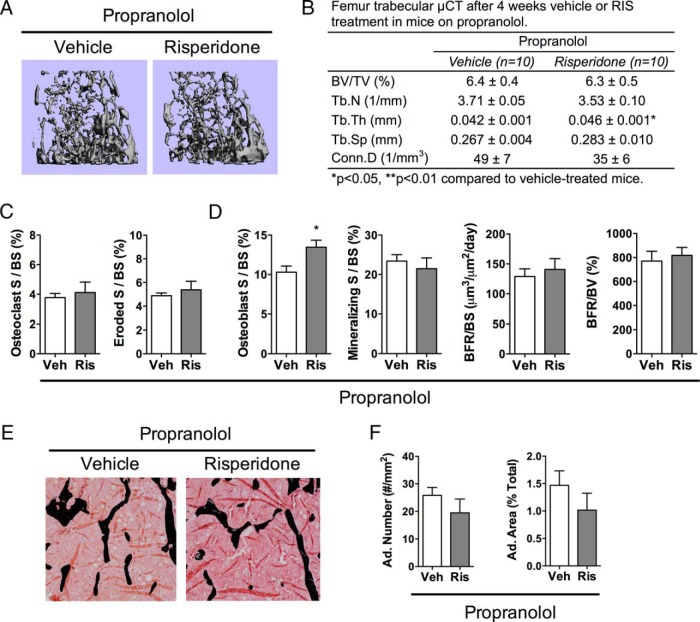
A subset of vehicle- and RIS-treated mice were also administered the nonselective β-adrenergic receptor antagonist, PRO. RIS did not cause any negative microarchitectural changes in the presence of PRO (Figure 4, A and B). However, RIS did increase Tb.Th compared with that in vehicle-treated mice with PRO present. Histological analyses indicated that RIS did not change osteoclast parameters or mineralizing surface with PRO present; however, RIS did increase osteoblast surface in the presence of PRO (Figure 4, C and D). Measures of marrow adiposity not changed by RIS treatment in the presence of PRO (Figure 4, E and F). Additional μCT and histological parameters are found in Supplemental Table 3.
Figure 4.
PRO prevented trabecular bone loss from RIS. RIS or vehicle (Veh) was administered to mice that were also receiving PRO treatment for 4 weeks, and analyses were performed at 12 weeks of age. A and B, Representative images (A) and quantitative parameters from μCT (B) of the trabecular bone of the distal femur. BV/TV, Tb.N, Tb.Th, Tb.Sp, and Conn.D were measured at 10-μm resolution. C and D, Quantitative osteoclast (C) and osteoblast (D) parameters from histomorphometric analyses of the trabecular bone of the proximal tibia (S, surface; BS, bone surface; BFR, bone formation rate; BV, bone volume). E, Representative images from histomorphometric analyses in the proximal tibia, where black stain is mineral (Von Kossa) and adipocytes ghosts are white. F, Quantitative adipocyte (Ad.) measurements from the proximal tibia. n = 5 to 9. *, P < .05.
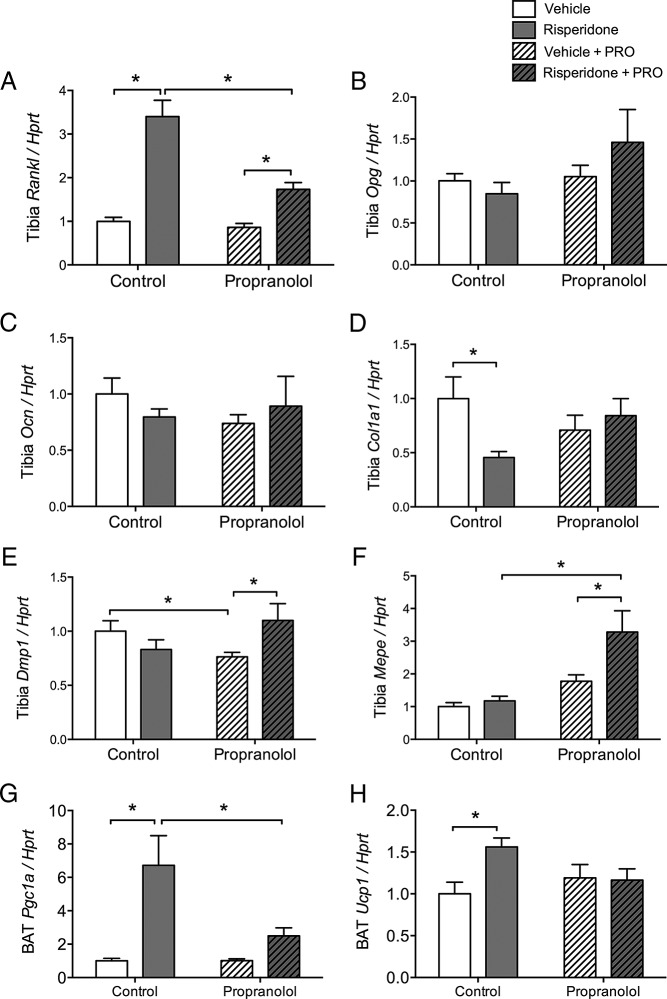
Effect of PRO on RIS-induced bone and BAT gene expression
Tibia mRNA was examined for markers of remodeling in mice treated for 4 weeks with RIS and PRO. Rankl was significantly increased by RIS treatment alone, and this was attenuated in the PRO-treated mice. Opg was not changed by either treatment (Figure 5, A and B). Osteoblast parameters osteocalcin, osteoglycin, and osteomodulin were not changed by RIS treatment alone or with PRO (Figure 5C and not shown). Col1a1 was significantly suppressed by RIS alone, and this was prevented by PRO treatment (Figure 5D). Osteocyte parameters Dmp1 and Mepe were elevated by combined RIS and PRO treatment compared with those for PRO alone, whereas sclerostin was unchanged (Figure 5, E and F, and not shown). After 4 weeks of RIS treatment, BAT Pgc1a and Ucp1 remained high and PRO significantly attenuated this increase (Figure 5, G and H).
Figure 5.
Propranolol attenuated up-regulation of Rankl and down-regulation of Col1a1 in tibia of RIS-treated mice. Female, 8-week-old B6 mice were treated for 4 weeks with vehicle (no fill) or RIS (filled) treatment, either without or with propranolol present (diagonal lines). A–F, Gene expression analyses were performed at 12 weeks of age. mRNA levels of Rankl (A), Opg (B), osteocalcin (C), Col1a1 (D), Dmp1 (E), and Mepe (F) were measured in the crushed whole tibia and normalized to the housekeeping gene Hprt. G and H, mRNA levels of Pgc1a (G) and Ucp1 (H) were measured in BAT and normalized to Hprt. *, P < .05. n = 5 to 9.
Effect of Adrb2 deletion on RIS-induced bone loss
RIS caused significant loss of Tb.N and Conn.D and an increase in trabecular separation in Adrb2+/+ mice (Table 1). Trabecular microarchitecture from vehicle-treated Adrb2+/+ mice was not different from that of vehicle-treated Adrb2−/− mice. However, trabecular bone changes from RIS were significantly attenuated in the absence of Adrb2 such that Tb.N and Tb.Sp were not different between vehicle- and RIS-treated Adrb2−/− mice (Table 1). Cortical microarchitecture was unchanged by either Adrb2 deletion or RIS treatment (not shown).
Table 1.
Effect of Adrb2 Deletion on RIS-Induced Trabecular Bone Loss
|
Adrb2+/+ |
Adrb2−/− |
|||
|---|---|---|---|---|
| Vehicle (n = 10) | Risperidone (n = 10) | Vehicle (n = 11) | Risperidone (n = 11) | |
| BV/TV, % | 6.7 ± 0.5 | 5.6 ± 0.5 | 6.3 ± 0.4 | 5.7 ± 0.5 |
| Tb.N, 1/mm | 3.46 ± 0.08 | 3.06 ± 0.06a | 3.37 ± 0.06 | 3.21 ± 0.10 |
| Tb.Th, mm | 0.045 ± 0.001 | 0.046 ± 0.002 | 0.043 ± 0.001 | 0.045 ± 0.002 |
| Tb.Sp, mm | 0.288 ± 0.008 | 0.328 ± 0.007a | 0.295 ± 0.006 | 0.313 ± 0.010 |
| Conn.D, 1/mm3 | 59 ± 8 | 35 ± 4b | 63 ± 5 | 43 ± 5b |
P < .01, comparing vehicle vs risperidone within each genotype.
P < .05, comparing vehicle vs risperidone within each genotype.
Discussion
The present study provides the first evidence that RIS causes SNS-mediated bone loss. Using multiple methodologies, we demonstrate that RIS-induced bone loss was prevented both by treatment with the β-adrenergic receptor antagonist PRO and by deletion of Adrb2. These findings confirm that RIS causes bone loss through up-regulation of SNS activity. Although we did not measure SNS outflow directly, we did observe elevated expression of BAT markers of thermogenesis and elevated EE, which were both transient in nature and associated with blood levels of the drug. Finally, there was also a transient activation of expression of the osteoclast recruitment factor, Rankl, which has previously been shown to be up-regulated after a SNS stimulus (20). However, leptin was suppressed a single dose of RIS (Figure 3) but after chronic dosing (not shown), the suppression of leptin is probably a consequence of increased β-adrenergic signaling (43).
The low trabecular bone mass from RIS treatment was due to a significant increase in bone resorption and decrease in bone formation. All bone resorption parameters, ie, number of osteoclasts, osteoclastic surface, and eroded surface, were elevated, probably due to elevated Rankl expression (Figures 1, 3, and 5). Adding to the negative bone balance, the dynamic bone formation parameters were lower in the RIS-treated group (Figure 1D), although static parameters were unchanged, indicating normal osteoblast recruitment/numbers, but impaired mineralization (Supplemental Table 2). Strikingly, not only were all of the negative effects of RIS on bone resorption and trabecular homeostasis rescued by treatment with PRO but also the percentage of the trabecular surface covered by osteoblasts was actually increased with PRO present (Figure 4). The RIS-induced increase in Rankl was also significantly attenuated by PRO (Figure 5). Some insight into the mechanism of the protective effects of PRO on osteoblasts was provided by gene expression analyses (Figure 5). PRO prevented the RIS-induced suppression of Col1a1 and stimulated RIS-induced Dmp1 and Mepe, which are elevated in late osteoblasts and osteocytes (44). The ability of RIS to promote osteoblast number and gene expression in the presence of PRO suggests that RIS may have some positive effect on osteoblasts, but these are outweighed by the negative effect of SNS stimulus in the absence of a β-blocker. Indeed, RIS binds several receptors present on osteoblasts, and this is the subject of further investigation in our laboratory.
Another area of interest is the differential effect of RIS on body composition in humans vs rodents. A recent meta-analysis demonstrated that nearly all antipsychotics cause weight gain clinically (45). In addition to hyperphagia, reduced EE can contribute to antipsychotic-induced weight gain in patients, with many studies showing reduced or unchanged EE (46). However, the results vary from study to study, and it is unclear whether alterations in EE are due to changes in fat-free mass or physical activity. In rodents, however, EE changes have been demonstrated. Cope et al (47) found that 4 mg/kg RIS treatment (in 2 daily peanut butter pills) in 12-week-old female C57BL/6 mice increased BAT Ucp1 expression and core body temperature during the light, but not the dark, phase. This was in addition to hyperphagia and weight gain. Recently, Li et al (48) showed that resting EE was higher in similarly dosed mice. Similar to our finding of reduced wheel activity (Figure 2), the authors found fewer x,y beam breaks over their 3-week study and elevated Ucp1 expression in the BAT of their mice (48). We did not observe weight gain or hyperphagia in our mice, which could in part be due to the lower dose used in our study (0.75 mg/kg once daily by oral gavage) compared to that above (47, 48).
An important aspect of our strategy was to evaluate the effects of RIS dosing that mimics clinically relevant drug exposure in patients. The 0.75 mg/kg dose was based on preliminary pharmacokinetic studies that evaluated plasma drug concentrations (total drug) after administration of various doses of RIS (Figure 3 and our unpublished data). The dose of 0.75 mg/kg produced peak serum levels of RIS and the active metabolite, PAL, most similar to those achieved in patients (33). The issue of dose selection/optimization remains a problem with rodent models for testing for off-target antipsychotic complications due to the inability of lower doses to induce weight gain. However, because we observe trabecular bone changes from RIS, this dose is appropriate to examine bone mass changes independent of potential weight gain and hyperphagia. It is most likely that the combination of increased daytime EE, reduced nighttime energy expenditure, and euphagia between control and RIS mice all contribute to the absence of weight change (Figure 2). In future studies with RIS and other AAs, it will be important to determine whether SNS-mediated effects on bone remain in play when the dose is high enough to induce weight gain.
The increased EE we observed could be due to elevated thermogenesis in response to RIS, but we have not tested this directly. In our study, we found increased markers of thermogenesis in BAT (Figures 3 and 5) and increased EE (Figure 2), which are both consistent with increased SNS outflow. Sympathetic output due to thermogenic demand has been associated with trabecular bone loss in another model with impaired brown fat function and increased browning of white adipose tissue (35). The mechanism of RIS-induced thermogenesis and the consequence of this for body weight should be further examined to better understand AA-induced weight gain in humans. It is plausible that patients may respond differently to AAs, and that those without weight gain may be more comparable to rodent models.
In conclusion, female C57BL/6J mice exhibit SNS-mediated bone loss from RIS treatment, which is accompanied by transient changes in BAT thermogenesis markers and EE after dosing. Although this study was performed in females, we do not have any reason to suspect that males have bone loss through a different mode of action. Potential sex differences and other mechanisms through which RIS affects bone (including via prolactin-induced hypogonadism and direct effects bone cells themselves) are currently under investigation. Although the mechanisms whereby RIS activates SNS outflow to bone remain unclear, the role of the SNS in mediating bone loss in humans treated with AA medications should be prospectively examined, particularly because some individuals are concomitantly treated with β-blockers (31). Future clinical studies will provide insight into the pathophysiologic consequences of AA treatment and help to inform clinical decision-making about this widely prescribed class of medications.
Acknowledgments
We thank Drs Lowell and Bouxsein for the Adrb2−/− mice, Dr Xuehui Yang, Phuong Le, and Terry Henderson for technical assistance, and Dr Anyonya Guntur and Casey Doucette for critical review of the article.
This work was supported by National Institutes of Health Grants DK095143 to K.L.H., AR061932 to K.J.M., AG040217 to C.J.R., GM103392 to Dr Robert Friesel (Maine Medical Center Research Institute [MMCRI]) and GM106391 to Dr Don Wojchowski (MMCRI) and institutional funds from the University of New England and the Maine Medical Center.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AA
- atypical antipsychotic
- BAT
- brown adipose tissue
- BV/TV
- bone volume/total volume
- Conn.D
- connectivity density
- μCT
- micro-computed tomography
- EE
- energy expenditure
- PAL
- paliperidone
- PRO
- propranolol
- RIS
- risperidone
- SNS
- sympathetic nervous system
- Tb.N
- trabecular number
- Tb.Th
- trabecular thickness
- Tb.Sp
- trabecular separation.
References
- 1. Calarge CA, Acion L, Kuperman S, Tansey M, Schlechte JA. Weight gain and metabolic abnormalities during extended risperidone treatment in children and adolescents. J Child Adolesc Psychopharmacol. 2009;19:101–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Komossa K, Rummel-Kluge C, Schwarz S, et al. Risperidone versus other atypical antipsychotics for schizophrenia. Cochrane Database Syst Rev. 2011;1:CD006626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Patel JK, Buckley PF, Woolson S, et al. Metabolic profiles of second-generation antipsychotics in early psychosis: findings from the CAFE study. Schizophr Res. 2009;111:9–16. [DOI] [PubMed] [Google Scholar]
- 4. American Diabetes Association, American Psychiatric Association, American Association of Clinical Endocrinologists, North American Association for the Study of Obesity. Consensus development conference on antipsychotic drugs and obesity and diabetes. Diabetes Care. 2004;27:596–601. [DOI] [PubMed] [Google Scholar]
- 5. Houseknecht KL, Robertson AS, Zavadoski W, Gibbs EM, Johnson DE, Rollema H. Acute effects of atypical antipsychotics on whole-body insulin resistance in rats: implications for adverse metabolic effects. Neuropsychopharmacology. 2007;32:289–297. [DOI] [PubMed] [Google Scholar]
- 6. Martins PJ, Haas M, Obici S. Central nervous system delivery of the antipsychotic olanzapine induces hepatic insulin resistance. Diabetes. 2010;59:2418–2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tsai KY, Lee CC, Chou YM, et al. The risks of major osteoporotic fractures in patients with schizophrenia: a population-based 10-year follow-up study. Schizophr Res. 2014;159:322–328. [DOI] [PubMed] [Google Scholar]
- 8. Halbreich U. Osteoporosis, schizophrenia and antipsychotics: the need for a comprehensive multifactorial evaluation. CNS Drugs. 2007;21:641–657. [DOI] [PubMed] [Google Scholar]
- 9. Calarge CA, Zimmerman B, Xie D, Kuperman S, Schlechte JA. A cross-sectional evaluation of the effect of risperidone and selective serotonin reuptake inhibitors on bone mineral density in boys. J Clin Psychiatry. 2010;71:338–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Becker D, Liver O, Mester R, Rapoport M, Weizman A, Weiss M. Risperidone, but not olanzapine, decreases bone mineral density in female premenopausal schizophrenia patients. J Clin Psychiatry. 2003;64:761–766. [DOI] [PubMed] [Google Scholar]
- 11. Liperoti R, Onder G, Lapane KL, et al. Conventional or atypical antipsychotics and the risk of femur fracture among elderly patients: results of a case-control study. J Clin Psychiatry. 2007;68:929–934. [DOI] [PubMed] [Google Scholar]
- 12. Dore DD, Trivedi AN, Mor V, Friedman JH, Lapane KL. Atypical antipsychotic use and risk of fracture in persons with Parkinsonism. Mov Disord. 2009;24:1941–1948. [DOI] [PubMed] [Google Scholar]
- 13. Meaney AM, O'Keane V. Bone mineral density changes over a year in young females with schizophrenia: relationship to medication and endocrine variables. Schizophr Res. 2007;93:136–143. [DOI] [PubMed] [Google Scholar]
- 14. Kishimoto T, Watanabe K, Shimada N, Makita K, Yagi G, Kashima H. Antipsychotic-induced hyperprolactinemia inhibits the hypothalamo-pituitary-gonadal axis and reduces bone mineral density in male patients with schizophrenia. J Clin Psychiatry. 2008;69:385–391. [DOI] [PubMed] [Google Scholar]
- 15. Rigler SK, Shireman TI, Cook-Wiens GJ, et al. Fracture risk in nursing home residents initiating antipsychotic medications. J Am Geriatr Soc. 2013;61:715–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fraser LA, Liu K, Naylor KL, et al. Falls and fractures with atypical antipsychotic medication use: a population-based cohort study. JAMA Intern Med. 2015;175:450–452. [DOI] [PubMed] [Google Scholar]
- 17. Ducy P, Amling M, Takeda S, et al. Leptin inhibits bone formation through a hypothalamic relay: a central control of bone mass. Cell. 2000;100:197–207. [DOI] [PubMed] [Google Scholar]
- 18. Fulzele K, Riddle RC, DiGirolamo DJ, et al. Insulin receptor signaling in osteoblasts regulates postnatal bone acquisition and body composition. Cell. 2010;142:309–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ferron M, Wei J, Yoshizawa T, et al. Insulin signaling in osteoblasts integrates bone remodeling and energy metabolism. Cell. 2010;142:296–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Elefteriou F, Ahn JD, Takeda S, et al. Leptin regulation of bone resorption by the sympathetic nervous system and CART. Nature. 2005;434:514–520. [DOI] [PubMed] [Google Scholar]
- 21. Yadav VK, Oury F, Tanaka KF, et al. Leptin-dependent serotonin control of appetite: temporal specificity, transcriptional regulation, and therapeutic implications. J Exp Med. 2011;208:41–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yadav VK, Oury F, Suda N, et al. A serotonin-dependent mechanism explains the leptin regulation of bone mass, appetite, and energy expenditure. Cell. 2009;138:976–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hinoi E, Gao N, Jung DY, et al. An osteoblast-dependent mechanism contributes to the leptin regulation of insulin secretion. Ann NY Acad Sci. 2009;1173(suppl. 1):E20–E30. [DOI] [PubMed] [Google Scholar]
- 24. Hinoi E, Gao N, Jung DY, et al. The sympathetic tone mediates leptin's inhibition of insulin secretion by modulating osteocalcin bioactivity. J Cell Biol. 2008;183:1235–1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Shi Y, Yadav VK, Suda N, et al. Dissociation of the neuronal regulation of bone mass and energy metabolism by leptin in vivo. Proc Natl Acad Sci USA. 2008;105:20529–20533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Motyl KJ, Dick-de-Paula I, Maloney AE, et al. Trabecular bone loss after administration of the second-generation antipsychotic risperidone is independent of weight gain. Bone. 2012;50:490–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bushe C, Yeomans D, Floyd T, Smith SM. Categorical prevalence and severity of hyperprolactinaemia in two UK cohorts of patients with severe mental illness during treatment with antipsychotics. J Psychopharmacol. 2008;22:56–62. [DOI] [PubMed] [Google Scholar]
- 28. Findling RL, Kusumakar V, Daneman D, Moshang T, De Smedt G, Binder C. Prolactin levels during long-term risperidone treatment in children and adolescents. J Clin Psychiatry. 2003;64:1362–1369. [DOI] [PubMed] [Google Scholar]
- 29. Kumar KK, Tung S, Iqbal J. Bone loss in anorexia nervosa: leptin, serotonin, and the sympathetic nervous system. Ann NY Acad Sci. 2010;1211:51–65. [DOI] [PubMed] [Google Scholar]
- 30. Karsenty G, Yadav VK. Regulation of bone mass by serotonin: molecular biology and therapeutic implications. Annu Rev Med. 2011;62:323–331. [DOI] [PubMed] [Google Scholar]
- 31. Calarge CA, Ivins SD, Motyl KJ, Shibli-Rahhal AA, Bliziotes MM, Schlechte JA. Possible mechanisms for the skeletal effects of antipsychotics in children and adolescents. Ther Adv Psychopharmacol. 2013;3:278–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Davey KJ, O'Mahony SM, Schellekens H, et al. Gender-dependent consequences of chronic olanzapine in the rat: effects on body weight, inflammatory, metabolic and microbiota parameters. Psychopharmacology. 2012;221:155–169. [DOI] [PubMed] [Google Scholar]
- 33. Álamo C, López-Muñoz F. The pharmacological role and clinical applications of antipsychotics' active metabolites: paliperidone versus risperidone. Clin Exp Pharmacol. 2013;3:117. [Google Scholar]
- 34. Takeda S, Elefteriou F, Levasseur R, et al. Leptin regulates bone formation via the sympathetic nervous system. Cell. 2002;111:305–317. [DOI] [PubMed] [Google Scholar]
- 35. Motyl KJ, Bishop KA, DeMambro VE, et al. Altered thermogenesis and impaired bone remodeling in Misty mice. J Bone Miner Res. 2013;28:1885–1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lighton JR, Turner RJ. The hygric hypothesis does not hold water: abolition of discontinuous gas exchange cycles does not affect water loss in the ant Camponotus vicinus. J Exp Biol. 2008;211:563–567. [DOI] [PubMed] [Google Scholar]
- 37. Kaiyala KJ, Schwartz MW. Toward a more complete (and less controversial) understanding of energy expenditure and its role in obesity pathogenesis. Diabetes. 2011;60:17–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Arch JR, Hislop D, Wang SJ, Speakman JR. Some mathematical and technical issues in the measurement and interpretation of open-circuit indirect calorimetry in small animals. Int J Obes (Lond). 2006;30:1322–1331. [DOI] [PubMed] [Google Scholar]
- 39. Kaiyala KJ, Morton GJ, Leroux BG, Ogimoto K, Wisse B, Schwartz MW. Identification of body fat mass as a major determinant of metabolic rate in mice. Diabetes. 2010;59:1657–1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. de Paula FJ, Dick-de-Paula I, Bornstein S, et al. VDR haploinsufficiency impacts body composition and skeletal acquisition in a gender-specific manner. Calcif Tissue Int. 2011;89:179–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Parfitt AM, Drezner MK, Glorieux FH, et al. Bone histomorphometry: standardization of nomenclature, symbols, and units. Report of the ASBMR Histomorphometry Nomenclature Committee. J Bone Miner Res. 1987;2:595–610. [DOI] [PubMed] [Google Scholar]
- 42. Vengellur A, LaPres JJ. The role of hypoxia inducible factor 1alpha in cobalt chloride induced cell death in mouse embryonic fibroblasts. Toxicol Sci. 2004;82:638–646. [DOI] [PubMed] [Google Scholar]
- 43. Kosaki A, Yamada K, Kuzuya H. Reduced expression of the leptin gene (ob) by catecholamine through a GS protein-coupled pathway in 3T3–L1 adipocytes. Diabetes. 1996;45:1744–1749. [DOI] [PubMed] [Google Scholar]
- 44. Woo SM, Rosser J, Dusevich V, Kalajzic I, Bonewald LF. Cell line IDG-SW3 replicates osteoblast-to-late-osteocyte differentiation in vitro and accelerates bone formation in vivo. J Bone Miner Res. 2011;26:2634–2646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Bak M, Fransen A, Janssen J, van Os J, Drukker M. Almost all antipsychotics result in weight gain: a meta-analysis. PLoS One. 2014;9:e94112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Cuerda C, Velasco C, Merchán-Naranjo J, Garcia-Peris P, Arango C. The effects of second-generation antipsychotics on food intake, resting energy expenditure and physical activity. Eur J Clin Nutr. 2014;68:146–152. [DOI] [PubMed] [Google Scholar]
- 47. Cope MB, Li X, Jumbo-Lucioni P, et al. Risperidone alters food intake, core body temperature, and locomotor activity in mice. Physiol Behav. 2009;96:457–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Li X, Johnson MS, Smith DL, Jr, et al. Effects of risperidone on energy balance in female C57BL/6J mice. Obesity. 2013;21:1850–1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Lee NK, Sowa H, Hinoi E, et al. Endocrine regulation of energy metabolism by the skeleton. Cell. 2007;130:456–469. [DOI] [PMC free article] [PubMed] [Google Scholar]