Abstract
Perlecan, a large heparan sulfate proteoglycan, is a component of the basement membrane and other extracellular matrices and has been implicated in multiple biological functions. Mutations in the perlecan gene (HSPG2) cause two classes of skeletal disorders: the relatively mild Schwartz-Jampel syndrome (SJS) and severe neonatal lethal dyssegmental dysplasia, Silverman-Handmaker type (DDSH). SJS is an autosomal recessive skeletal dysplasia characterized by varying degrees of myotonia and chondrodysplasia, and patients with SJS survive. The molecular mechanism underlying the chondrodystrophic myotonia phenotype of SJS is unknown. In the present report, we identify five different mutations that resulted in various forms of perlecan in three unrelated patients with SJS. Heterozygous mutations in two patients with SJS either produced truncated perlecan that lacked domain V or significantly reduced levels of wild-type perlecan. The third patient had a homozygous 7-kb deletion that resulted in reduced amounts of nearly full-length perlecan. Unlike DDSH, the SJS mutations result in different forms of perlecan in reduced levels that are secreted to the extracellular matrix and are likely partially functional. These findings suggest that perlecan has an important role in neuromuscular function and cartilage formation, and they define the molecular basis involved in the difference in the phenotypic severity between DDSH and SJS.
Schwartz-Jampel syndrome (SJS [MIM 255800]) is a rare autosomal recessive skeletal dysplasia associated with myotonia (Aberfeld et al. 1965; Aberfeld et al. 1970). This disorder is characterized by short stature, osteochondrodysplasia, myotonia, and a characteristic face with a “fixed” facial expression, blepharophimosis, pursed lips, and, sometimes, low-set ears and myopia. Skeletal abnormalities include kyphoscoliosis, platyspondyly with coronal clefts in the vertebrae, metaphyseal and epiphyseal dysplasias, and joint contractures. Electromyography (EMG) shows persistent spontaneous activity, particularly in the face and thigh muscles, which tends to diminish at rest (Taylor et al. 1972; Jablecki and Schultz 1982). This spontaneous activity decreases with applications of curare in some patients, suggesting that the disorder is of neurogenic origin (Taylor et al. 1972; Fowler et al. 1974). Muscle biopsies show nonspecific myopathy (Fowler et al. 1974; Pascuzzi 1991). The clinical phenotype of SJS varies (Giedion et al. 1997). Mildly affected patients develop a moderate bone dysplasia in childhood. In more severely affected patients, a more obvious bone dysplasia, reminiscent of Kniest dysplasia, is present from infancy. Through linkage mapping and a positional candidate approach, Nicole et al. (2000) recently identified homozygous mutations in HSPG2 in two families with SJS. The mutations involved missense and splicing in HSPG2, but predicted mutant perlecan molecules have not been characterized.
Perlecan is a multifunctional proteoglycan present in all basement membranes and in cartilage (Iozzo et al. 1994; SundarRaj et al. 1995; French et al. 1999). The protein core is ∼400 kD, with three covalently attached heparan sulfate chains at the N-terminus and another chain at the C-terminus (fig. 1D). Perlecan is implicated in cell growth and differentiation, through interactions with growth factors, cell surface receptors, and extracellular matrix molecules (Noonan et al. 1991; Aviezer et al. 1994; Dolan et al. 1997; Olsen 1999). The N-terminal domain, domain I, contains attachment sites for heparan sulfate chains and binds to growth factors (Iozzo 1994) and acetylcholinesterase (Peng et al. 1999). Domain IV binds basement membrane molecules, such as nidogen/entactin and type IV collagen (Hopf et al. 1999). Domain V binds cell surface receptors such as α-dystroglycan and integrin β1 (Brown et al. 1997; Friedrich et al. 1999).
Figure 1.
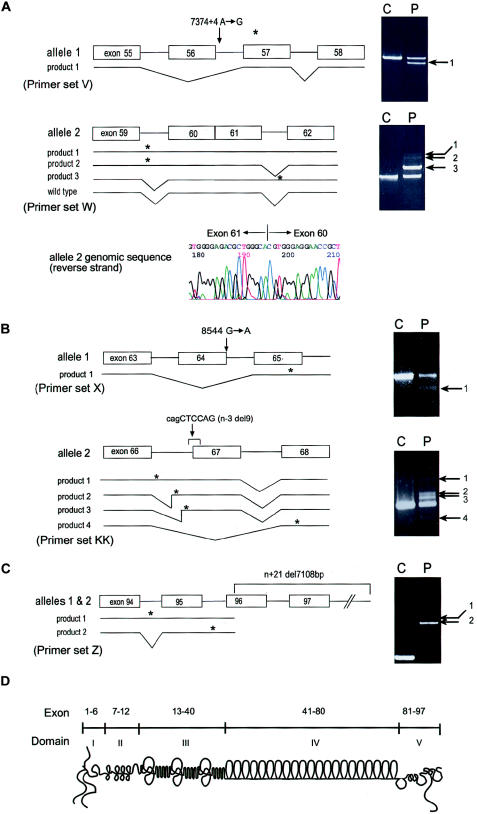
Mutation analysis and schematic diagram of perlecan. A, Results of RT-PCR, which was performed with RNA from patient 1 fibroblasts, using primer sets V and W and analyzed on 1% agarose gels as described elsewhere (Arikawa-Hirasawa et al. 2001). Sequencing revealed that product 1 from allele 1 was missing the entire exon 56 sequence and that products 1–3 from allele 2 retained intron sequences. Product 3 of allele 2 contains the sequence of intron 61, whereas products 1 and 2 contain either both introns 59 and 61 or intron 59, respectively. Exon 56 skipping in allele 1 causes frameshift and is predicted to produce a premature termination codon in exon 57. The intron 59 retention of products 1 and 2 from allele 2 is predicted to produce a premature termination codon within intron 59, and the intron 61 retention of product 3 is predicted to produce a termination codon within intron 61. Genomic sequencing identified heterozygous mutations in an A→G transition at the +4 position (donor site) of intron 56 (7374+4 A→G) in allele 1. Allele 2 showed a complete loss of intron 60, resulting in fusion of exons 60 and 61 in the perlecan gene. The exon-fusion mutation created aberrant transcripts, products 1–3, but is also predicted to produce the wild-type product. Since primer set W does not distinguish the wild-type product derived from alleles 1 or 2, we used a primer set from exons 56 and 62 to exclude a wild-type transcript from allele 1, in which exon 56 is missing and confirmed the presence of the wild-type transcript together with aberrant splicing transcripts produced by allele 2 (data not shown). B, Patient 2. RT-PCR was performed with RNA from skeletal muscle tissues of patient 2, using primer sets X and KK. Sequencing of product 1 with primers X revealed exon 64 skipping, and products 1, 2, and 3 with primers KK contained total or partial retention of intron 66. Product 4 with primers KK showed exon 67 skipping. Genomic sequencing revealed a heterozygous mutation in a G→A transition at the donor site of exon 64 (8564 G→A) in allele 1 and a 9-bp deletion (cagCTCCAG) at the acceptor junction of intron 66 and exon 67 in allele 2 (n−3 del9). C, Patient 3. RT-PCR was performed with RNA from patient 3 fibroblasts using primer set Z. Product 1 contained sequences including introns 94 and 95. Product 2, a major product, contained intron 95. Sequencing of the genomic PCR product revealed a 7,108-bp deletion beginning at the 5′ portion of exon 96 and extending to the 3′ flanking sequence of HSPG2. The deletion results in aberrant splicing, including exon skipping and intron retention. Asterisk (*) indicates a premature termination codon. Arrows indicate abnormally sized RT-PCR products. D,Schematic diagram of perlecan and its exon and domain structure. Primer sequences for RT-PCR are as follows: V (forward, ATCACGGTCACAGTAACTGGGACC; reverse, CCTGCACCGTTACTGACGTG); KK (forward, ATGGCACAAGCGTGGAGGAAACC; reverse, AGGCTTCTTGCTCAGGGCCTGG); W (forward, ATCCAGCAGCGCCTTAGTGG; reverse, CATGCCCATCAGAATTGAGTCAT); X (forward, CTCAACAACATCG ATGCCCTGGAG; reverse, CTCCAGCCCAGGACCCATTCCT); and Z (forward, AGGCAAGGACTTCATCAGCCTCGGG; reverse, TCGACTTGGATGGAACCTCTGCGG).
The majority of mice with a homozygous null mutation in the perlecan gene (Hspg2) develop severe chondrodysplasia and die, just after birth, of respiratory failure (Arikawa-Hirasawa et al. 1999; Costell et al. 1999). Through comparison of skeletal abnormalities of human patients to the perlecan-null mice, we recently identified mutations in HSPG2 in a lethal autosomal recessive disorder termed “dyssegmental dysplasia, Silverman-Handmaker type” (DDSH [MIM 224410] )(Arikawa-Hirasawa et al. 2001). In DDSH, we found that truncated perlecan molecules were not secreted into the extracellular matrix, indicating that functional null mutations of the perlecan gene cause DDSH.
In the present report, we have identified, among three patients with SJS, five different mutations in HSPG2 (GenBank accession number M852890), which result in different levels and forms of perlecan molecules. SJS perlecan molecules are secreted and partially functional, albeit in markedly reduced concentrations. Phenotypic differences in DDSH and SJS appear to reflect differences in the amount of functional perlecan in the matrix.
HSPG2 mutations in three patients with SJS were analyzed by RT-PCR and genomic sequencing (fig. 1). Patient 1 was an 8-year-old boy, born normally to healthy parents. At the age of 3 years, dysmorphic features were noted that became gradually prominent and consisted of tonic contraction of the facial muscles, micrognathia, low-set ears with folded helices, medial displacement of the outer canthi, narrow palpebral fissure, blepharophimosis, microstomia and pursing of the lips, high arched palate, cervical kyphosis, pes planus and valgus ankle formation, bowing of the leg bones, kyphoscoliosis, and lumbar lordosis. He had hypertrophic muscles and mild weakness of the quadriceps muscles. The creatinine kinase level was elevated (896 IU/liter); the EMG showed occasional myotonic discharges. Patient 2 was noted as having short stature and micromelia and was admitted to the hospital at 4 mo of age. Radiographs revealed square, flared ilia and short, bowed long bones. At 3 years of age, he showed thigh muscle hypertrophy and gastrocnemius muscle atrophy. Micrognathia, pursed lips, saddle nose, orbital hypertelorism, low-set ears, and high arched palate were noted. He showed a waddling gait, mild weakness of the quadriceps muscle, and percussion myotonia. EMG revealed myotonic and myopathic discharge. Necrotic and regenerating fibers were also found. Patient 3 was first reported as having micromelic chondrodysplasia (Stevenson 1982) but was reevaluated as having SJS in 2000 (Spranger et al. 2000). Micrognathia, a prominent philtrum, and bowed long bones were noted at age 3 mo. Radiographs revealed coronal clefts in the lumbar vertebrae, deficient ossification in the dorsal vertebrae, and squared, flared ilia. All long bones showed expansion of the metaphyses. At 4 years of age, facial movement became difficult because of stiffness, and the mouth opening was restricted. EMG showed pseudomyotonic discharge and rapid firing of single units in the brachioradial, deltoid, and medial gastrocnemius muscles.
Patient 1 had heterozygous mutations. Allele 1 had an A→G transition at the +4 position (donor site) of intron 56 (7374+4 A→G) that resulted in exon skipping of 56, whereas allele 2 of HSPG2 contained a fusion of exons 60 and 61 that resulted in retention of intron 59 or intron 61 or in retention of both in the mutant transcripts. The exon fusion occurred at the precise acceptor and donor sites, probably by retrotransposition. The exon fusion mutation created aberrant transcripts. It also predicts the production of the wild-type transcript. A different primer set from exons 56 and 62, which was used to exclude a wild-type transcript from allele 1, confirmed the presence of the wild-type transcript, together with aberrant splicing transcripts produced by allele 2 (data not shown). The aberrant splicing from the two alleles is predicted to create premature termination codons. Patient 2 had a G→A transition at the last nucleotide of exon 64 (8544 G→A) in allele 1. This transition mutation did not change an amino acid but resulted in skipping of exon 64. Allele 2 has a 9-bp deletion (cagCTCCAG) at the acceptor junction of intron 66 and exon 67 in allele 2 (n−3 del9) that created abnormal splicing, including total or partial retention of intron 66 or skipping of exon 67. All aberrant splicing products are predicted to create premature termination codons. Patient 3 had a 7,108-bp homozygous deletion beginning at the 5′ portion of exon 96 (n+21 del7108) and extending well beyond the 3′ flanking sequence of HSPG2. This deletion created two aberrant transcripts; product 2 (derived from intron 95 retention) and product 1 (resulting from failure of splicing of both introns 94 and 95) are predicted to produce truncated proteins missing ∼35 and ∼64 amino acids from the C-terminal, respectively.
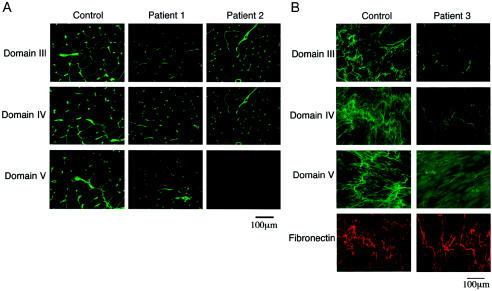
We next examined the expression of perlecan in muscles from patients 1 and 2 by immunostaining with domain-specific antiperlecan antibodies (fig. 2). Perlecan was localized in the control tissue in the basal lamina of the muscle fibers and in the capillaries. Similar but weaker staining for domains III–V was observed in the muscles of patient 1 (fig. 2A), consistent with the presence of the wild-type transcript. We observed a similar immunostaining of fibroblasts from patient 1 (data not shown). In patient 2, domains III and IV were present in the muscle at a reduced level compared with the control specimen, but domain V was missing, suggesting a defect in domain V (fig. 1). The aberrant splicing transcripts from both alleles of patient 2 are predicted to produce truncated perlecan molecules that lack the C-terminal part of domain IV and all of domain V. Thus, the immunostaining results agree with the predicted truncated proteins. We examined immunostaining of cultured fibroblasts from patient 3, because no tissue from a muscle biopsy of this patient was available. In patient 3 fibroblasts, domains III–V were present in the matrix but with significantly less staining than in the control specimen (fig. 2B). Because a 7-kb homozygous deletion in HSPG2 of patient 3 eliminates only 35–64 amino acids in the C-terminal domain V, these results are consistent with the genetic analysis.
Figure 2.
Immunostaining of perlecan in muscle tissues and cultured fibroblasts. A, Muscle tissue from patients 1 and 2 and from an unaffected control subject, stained with domain-specific anti-perlecan antibodies as described elsewhere (Arikawa-Hirasawa et al. 2001, 2002). In patient 1, antibodies to domains III–V stained the basal lamina of the muscle, whereas, in patient 2, domain V staining was absent. The staining in muscle tissue of patient 1 is significantly reduced. B, Cultured fibroblasts from patient 3 stained with domain-specific anti-perlecan antibodies and with anti-fibronectin polyclonal antibodies. Domains III–V stained the extracellular matrix at significantly reduced levels compared to control fibroblasts, whereas fibronectin stained strongly in both.
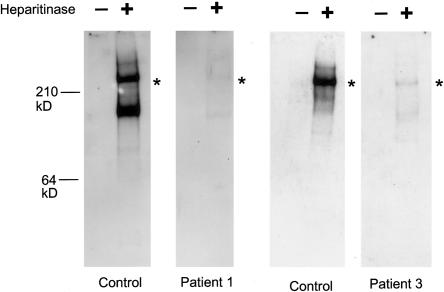
Perlecan secretion from fibroblasts of patients 1 and 3 was next examined using western blot analysis (fig. 3). After removal of heparan sulfate chains by heparitinase treatment, conditioned media from normal fibroblasts showed a protein core of perlecan that was ∼400-kD. Patient 1 fibroblasts secreted a 400-kD protein core in significantly reduced amounts compared with the wild-type protein core, supporting the presence of the wild-type transcript. The fibroblasts also secreted a smaller immunostainable band of ∼150 kD, which may be a degraded product as is seen in the control fibroblasts. Conditioned media from patient 3 fibroblasts contained a 400-kD protein, which is predicted to lack ∼35–64 C-terminal amino acids. As with patient 1, the amount of perlecan secreted was significantly reduced compared with that of the control.
Figure 3.
Perlecan secreted by cultured fibroblasts in patients 1 and 3. Media from cultured normal or patient fibroblasts were concentrated, were then adjusted to contain 0.35g CsCl/g of 4M guanidine, and were centrifuged for 72 h at 40,000 RPM in a 50-Ti rotor at 12°C. A proteoglycan-containing fraction was pooled, concentrated, and digested with 0.1 mU heparitinase and 0.25 mU chondroitinase ABC (Seikagaku). Digested and undigested samples were electrophoresed in 3%–8% polyacrylamide tris-acetate gels, were transferred to nitrocellulose, and were blotted with polyclonal antibodies to the perlecan protein core. In patient 1, a perlecan protein core of ∼400 kD was detected after heparitinase digestion, but the amount was reduced significantly, correlating with the reduced amount of the wild-type perlecan transcript. Heparitinase treatment is necessary for the blotting, because perlecan-containing heparan sulfate chains do not transfer well onto a membrane because of the negatively charged heparan sulfate chains. As with patient 1, patient 3 fibroblasts secreted the 400-kD perlecan core protein in much more reduced amounts than did the control cells. The truncated perlecan in patient 3 is missing only ∼35–64 amino acids of the most C-terminal part of domain V, suggesting the mutant perlecan to be almost the same size as the wild-type perlecan. Asterisks (*) indicate positions of the ∼400-kD protein core.
In the patients we studied for the present report, partial functional mutations in HSPG2 caused SJS, which is characterized by myotonia associated with a chondrodysplasia phenotype. Five mutations were identified in three patients with SJS, with one homozygous and four heterozygous mutations. These mutations produce different forms of the perlecan molecule. In patient 1, the staining of domain V in muscles was present at levels similar to those of other domains, and western blot analysis showed no obviously truncated molecules. These results suggest that, in patient 1, only the normal protein is secreted in the matrix, although in a reduced amount. The predicted truncated molecules were likely unstable because of proteolytic degradation or instability of the mutant transcripts. In patient 2, mutant perlecan lacking domain V was produced with reduced levels, compared with the control. In patient 3, a 7-kb homozygous deletion near the end of HSPG2 produces a nearly full-length perlecan molecule at reduced levels.
In patients with DDSH, we did not detect perlecan in the cartilage matrix or in the matrix from cultured fibroblasts (Arikawa-Hirasawa et al. 2001); however, in contrast to results in patients with DDSH, we found that mutant perlecan molecules or reduced amounts of wild-type perlecan are secreted and localized in the matrix of tissues from patients with SJS. In DDSH, there is mucoid degeneration of the resting cartilage, as well as abnormal chondrocyte columnar structures and defective mineralization of the growth plate. Although only limited information is available about the histology of cartilage from patients with SJS, there is a report that indicates poor chondrocyte columnar organization in epiphyseal cartilage of one patient with SJS (Aberfeld et al. 1965). The SJS perlecan molecules in the matrix are partially functional, which results in a milder chondrodysplasia phenotype than that of DDSH. In normal growth-plate cartilage, perlecan likely strengthens the extracellular matrix structure by interacting with other molecules. Impaired cartilage matrix resulting from partially functional perlecan may contribute to the skeletal abnormalities in patients with SJS. Patients 2 and 3 showed obvious micromelia in their early infancy, but patient 1 showed a chondrodysplasia phenotype that was not obvious until the patient was 3–4 years of age. This difference may result from the fact that only patient 1 produces the normal perlecan molecule. Because the truncated perlecan molecules of patients 2 and 3 are defective in domain V, the C-terminal domain V may be responsible for the early onset of skeletal abnormalities. It is conceivable that domain V plays a role in matrix-cell interaction through its binding to a chondrocyte receptor, such as integrin, providing stable formation of the cartilage matrix structure. Some of the skeletal abnormalities, such as scoliosis and hip degeneration, in patients with SJS could be caused, in part, by the myotonia in SJS.
Another characteristic of the SJS phenotype involves abnormal neuromuscular functions. A unique set of molecules—including acetylcholine esterase (AChE), acetylcholine receptor (AChR), and perlecan—cluster at sites of nerve-muscle contact to form the neuromuscular junction (NMJ), where muscle contraction is initiated. The collagen-tailed form of AChE is highly expressed in innervated regions of skeletal muscle fibers and is attached to the synaptic basal lamina (Massoulie et al. 1993). In vertebrate skeletal muscle, ACh acts as a neurotransmitter, and AChE is responsible for rapid termination of neurotransmission, by hydrolyzing ACh at the synapse. In vitro studies show that perlecan binds to AChE, suggesting that perlecan is an acceptor site for AChE at the NMJ (Peng et al. 1999). How do the perlecan defects cause the myotonic phenotype of SJS? Unlike the mutations in perlecan-null mice and patients with DDSH, mutations in the patients with SJS produce partially functional perlecan molecules, which are present in the basal lamina of the muscle. We recently demonstrated, using perlecan-null mice, that clustering molecules, such as AChR and agrin, were present at the NMJ but that AChE was completely absent, indicating that perlecan is a key molecule for localizing AChE at the synapse (Arikawa-Hirasawa et al. 2002). The reduced amounts of either truncated or normal perlecan molecules may result in reduced clustering of AChE at the SJS NMJ, which would likely cause a greater concentration of ACh. This would stimulate AChR activity and thus cause myotonia. This mechanism is different from that of other hereditary myotonias, which are caused by defects in muscle membrane proteins involved in the sodium channel. Mutations in the gene COLQ for a collagenlike subunit of AChE were reported to cause end-plate AChE deficiency (EAD), which is characterized by a congenital myasthenic syndrome associated with weakness and fatigability on exertion (Donger et al. 1998; Ohno et al. 1998). In this disorder, AChE cannot form a heteromeric asymmetric complex with COLQ, and it is therefore inactive for enzymatic activity. The difference in the phenotype of SJS and EAD may result from the difference of AChE activity. In SJS, normal AChE is produced and clustered at the NMJ—although in reduced amounts—whereas, in EAD, there is no active AChE. Without any AChE activity, there is no recycling of ACh, which may lead to excessive depletion of ACh that, in turn, causes a myasthenic syndrome. Curare, which blocks transmission, can abolish persistent spontaneous activity of the electromyography in patients with SJS but not in other myotonias that result from defects in muscle membrane proteins (Taylor et al. 1972). This curare effect is in concordance with our proposed mechanism by which defects in perlecan cause SJS myotonia. However, in some patients with SJS, curare does not arrest the spontaneous activity (Spaans et al. 1990). This may result from a secondary defect in muscle membranes caused by mutations in the perlecan gene. Chronic impairment of NMJ activity due to increased levels of ACh is known to cause degeneration of the folds of the NMJ (Engel et al. 1977; Salpeter et al. 1979). Less stable synaptic basal lamina structures may be formed because of the significant reduction of wild-type perlecan or because of mutant perlecan defective in domain V, which leads to reduced expression of clustering molecules.
Recently, Spranger et al. reported that several other skeletal disorders previously identified as chondrodysplasias, such as kyphomelic chondrodysplasia, micromelic chondrodysplasia, and Burton disease, can be reclassified, on the basis of clinical examination, as SJS (Spranger et al. 2000). One of the patients with SJS in the present report was originally classified as having micromelic chondrodysplasia. Thus, the clinical phenotype of SJS encompasses a broad spectrum. Molecular and biochemical analysis of perlecan from these patients should provide more insight into phenotype-genotype correlations and functional domains of perlecan.
Acknowledgments
We thank Drs. C. McDonald, W. Fowler, E. Hoffman, C. Schwartz, and W. Wilcox, for helpful discussions, and Drs. R. Rotundo and H. Kleinman, for comments on the manuscript. We also thank H. Grant, for editorial help, and R. Granger, for technical help. This work was supported by National Institutes of Health intramural funds to Y.Y., C.F., and M.D. and by grants to J.R.H. from the Shriners of North America. A.H.L. is a Howard Hughes Medical Institute–National Institutes Research Fellow.
Electronic-Database Information
Accession numbers and URLs for data in this article are as follows:
- GenBank, http://www.ncbi.nlm.nih.gov/GenBank/ (for the sequence of HSPG2 cDNA [accession number M852890] and for HSPG2-containing human chromosome 1 working draft sequence segment [accession number NT_004576])
- Online Mendelian Inheritance in Man (OMIM), http://www.ncbi..nih.gov/Omin/ (for SJS [MIM 255800] and DDSH [MIM 224410])
References
- Aberfeld DC, Hinterbuchner LP, Schneider M (1965) Myotonia, dwarfism, diffuse bone disease and unusual ocular and facial abnormalities (a new syndrome). Brain 88:313–322 [DOI] [PubMed] [Google Scholar]
- Aberfeld DC, Namba T, Vye MV, Grob D (1970) Chondrodystrophic myotonia: report of two cases—myotonia, dwarfism, diffuse bone disease, and unusual ocular and facial abnormalities. Arch Neurol 22:455–462 [DOI] [PubMed] [Google Scholar]
- Arikawa-Hirasawa E, Rossi SG, Rotundo RL, Yamada Y (2002) Absence of acetylcholinesterase at the neuromuscular junctions of perlecan-null mice. Nat Neurosci 5:119–123 [DOI] [PubMed] [Google Scholar]
- Arikawa-Hirasawa E, Watanabe H, Takami H, Hassell JR, Yamada Y (1999) Perlecan is essential for cartilage and cephalic development. Nat Genet 23:354–358 [DOI] [PubMed] [Google Scholar]
- Arikawa-Hirasawa E, Wilcox WR, Le AH, Silverman N, Govindraj P, Hassell JR, Yamada Y (2001) Dyssegmental dysplasia, Silverman-Handmaker type, is caused by functional null mutations of the perlecan gene. Nat Genet 27:431–434 [DOI] [PubMed] [Google Scholar]
- Aviezer D, Hecht D, Safran M, Eisinger M, David G, Yayon A (1994) Perlecan, basal lamina proteoglycan, promotes basic fibroblast growth factor-receptor binding, mitogenesis, and angiogenesis. Cell 79:1005–1013 [DOI] [PubMed] [Google Scholar]
- Brown JC, Sasaki T, Gohring W, Yamada Y, Timpl R (1997) The C-terminal domain V of perlecan promotes β1 integrin-mediated cell adhesion, binds heparin, nidogen and fibulin-2 and can be modified by glycosaminoglycans. Eur J Biochem 250:39–46 [DOI] [PubMed] [Google Scholar]
- Costell M, Gustafsson E, Aszodi A, Morgelin M, Bloch W, Hunziker E, Addicks K, Timpl R, Fassler R (1999) Perlecan maintains the integrity of cartilage and some basement membranes. J Cell Biol 147:1109–1122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolan M, Horchar T, Rigatti B, Hassell JR (1997) Identification of sites in domain I of perlecan that regulate heparan sulfate synthesis. J Biol Chem 272:4316–4322 [DOI] [PubMed] [Google Scholar]
- Donger C, Krejci E, Serradell AP, Eymard B, Bon S, Nicole S, Chateau D, Gary F, Fardeau M, Massoulie J, Guicheney P (1998) Mutation in the human acetylcholinesterase-associated collagen gene, COLQ, is responsible for congenital myasthenic syndrome with end-plate acetylcholinesterase deficiency (type Ic). Am J Hum Genet 63:967–975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel AG, Lambert EH, Gomez MR (1977) A new myasthenic syndrome with end-plate acetylcholinesterase deficiency, small nerve terminals, and reduced acetylcholine release. Ann Neurol 1:315–330 [DOI] [PubMed] [Google Scholar]
- Fowler WM Jr, Layzer RB, Taylor RG, Eberle ED, Sims GE, Munsat TL, Philippart M, Wilson BW (1974) The Schwartz-Jampel syndrome: its clinical, physiological and histological expressions. J Neurol Sci 22:127–146 [DOI] [PubMed] [Google Scholar]
- French MM, Smith SE, Akanbi K, Sanford T, Hecht J, Farach-Carson MC, Carson DD (1999) Expression of the heparan sulfate proteoglycan, perlecan, during mouse embryogenesis and perlecan chondrogenic activity in vitro. J Cell Biol 145:1103–1115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedrich MV, Gohring W, Morgelin M, Brancaccio A, David G, Timpl R (1999) Structural basis of glycosaminoglycan modification and of heterotypic interactions of perlecan domain V. J Mol Biol 294:259–270 [DOI] [PubMed] [Google Scholar]
- Giedion A, Boltshauser E, Briner J, Eich G, Exner G, Fendel H, Kaufmann L, Steinmann B, Spranger J, Superti-Furga A (1997) Heterogeneity in Schwartz-Jampel chondrodystrophic myotonia. Eur J Pediatr 156:214–223 [DOI] [PubMed] [Google Scholar]
- Hopf M, Gohring W, Kohfeldt E, Yamada Y, Timpl R (1999) Recombinant domain IV of perlecan binds to nidogens, laminin-nidogen complex, fibronectin, fibulin-2 and heparin. Eur J Biochem 259:917–925 [DOI] [PubMed] [Google Scholar]
- Iozzo RV (1994) Perlecan: a gem of a proteoglycan. Matrix Biol 14:203–208 [DOI] [PubMed] [Google Scholar]
- Iozzo RV, Cohen IR, Grassel S, Murdoch AD (1994) The biology of perlecan: the multifaceted heparan sulphate proteoglycan of basement membranes and pericellular matrices. Biochem J 302:625–639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jablecki C, Schultz P (1982) Single muscle fiber recordings in the Schwartz-Jampel syndrome. Muscle Nerve Suppl 5:S64–S69 [PubMed] [Google Scholar]
- Massoulie J, Pezzementi L, Bon S, Krejci E, Vallette FM (1993) Molecular and cellular biology of cholinesterases. Prog Neurobiol 41:31–91 [DOI] [PubMed] [Google Scholar]
- Nicole S, Davoine CS, Topaloglu H, Cattolico L, Barral D, Beighton P, Hamida CB, Hammouda H, Cruaud C, White PS, Samson D, Urtizberea JA, Lehmann-Horn F, Weissenbach J, Hentati F, Fontaine B (2000) Perlecan, the major proteoglycan of basement membranes, is altered in patients with Schwartz-Jampel syndrome (chondrodystrophic myotonia). Nat Genet 26:480–483 [DOI] [PubMed] [Google Scholar]
- Noonan DM, Fulle A, Valente P, Cai S, Horigan E, Sasaki M, Yamada Y, Hassell JR (1991) The complete sequence of perlecan, a basement membrane heparan sulfate proteoglycan, reveals extensive similarity with laminin A chain, low density lipoprotein-receptor, and the neural cell adhesion molecule. J Biol Chem 266:22939–22947 [PubMed] [Google Scholar]
- Ohno K, Brengman J, Tsujino A, Engel AG (1998) Human endplate acetylcholinesterase deficiency caused by mutations in the collagen-like tail subunit (ColQ) of the asymmetric enzyme. Proc Natl Acad Sci USA 95:9654–9659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen BR (1999) Life without perlecan has its problems. J Cell Biol 147:909–912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascuzzi RM (1991) Schwartz-Jampel syndrome. Semin Neurol 11:267–273 [DOI] [PubMed] [Google Scholar]
- Peng HB, Xie H, Rossi SG, Rotundo RL (1999) Acetylcholinesterase clustering at the neuromuscular junction involves perlecan and dystroglycan. J Cell Biol 145:911–921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salpeter MM, Kasprzak H, Feng H, Fertuck H (1979) Endplates after esterase inactivation in vivo: correlation between esterase concentration, functional response and fine structure. J Neurocytol 8:95–115 [DOI] [PubMed] [Google Scholar]
- Spaans F, Theunissen P, Reekers AD, Smit L, Veldman H (1990) Schwartz-Jampel syndrome. I. Clinical, electromyographic, and histologic studies. Muscle Nerve 13:516–527 [DOI] [PubMed] [Google Scholar]
- Spranger J, Hall BD, Hane B, Srivastava A, Stevenson RE (2000) Spectrum of Schwartz-Jampel syndrome includes micromelic chondrodysplasia, kyphomelic dysplasia, and Burton disease. Am J Med Genet 94:287–295 [DOI] [PubMed] [Google Scholar]
- Stevenson RE (1982) Micromelic chondrodysplasia. Proc Greenwood Genet Ctr 1:52–56 [Google Scholar]
- SundarRaj N, Fite D, Ledbetter S, Chakravarti S, Hassell JR (1995) Perlecan is a component of cartilage matrix and promotes chondrocyte attachment. J Cell Sci 108:2663–2672 [DOI] [PubMed] [Google Scholar]
- Taylor RG, Layzer RB, Davis HS, Fowler WM Jr (1972) Continuous muscle fiber activity in the Schwartz-Jampel syndrome. Electroencephalogr Clin Neurophysiol 33:497–509 [DOI] [PubMed] [Google Scholar]