Abstract
Mouse models of disease and injury have been invaluable in investigations of the functional role of γδ T cells. They show that γδ T cells engage in immune responses both early and late, that they can function both polyclonally and as peripherally selected clones, and that they can be effector cells and immune regulators. They also suggest that functional development of γδ T cells occurs step-wise in thymus and periphery, and that it is governed by γδ TCR-signaling and other signals. Finally, they indicate that γδ T cell functions often segregate with TCR-defined subsets, in contrast to conventional T cells. From the functional studies in mice and other animal models, γδ T cells emerge as a distinct lymphocyte population with a unique and broad functional repertoire, and with important roles in Ab responses, inflammation and tissue repair. They also are revealed as a potentially useful target for immune intervention.
Unlike B cells and αβ T cells, γδ T cells were discovered through molecular means instead of by way of their functions. Key questions remain concerning possible functional differences between αβ T cells and γδ T cells, the significance of the organization of γδ T cells into a system of specialized subsets, and the function of the γδ TCR. Explanations are needed for why γδ T cells often respond faster than αβ T cells and how sometimes in very small numbers they exert powerful effects on inflammatory responses and tissue physiologies. Answers to these questions could revise conventional immunological concepts, and they might open new avenues for immunotherapy. In this brief review, we examine in vivo γδ T cell functions as revealed in mouse models.
Development of γδ T cell function
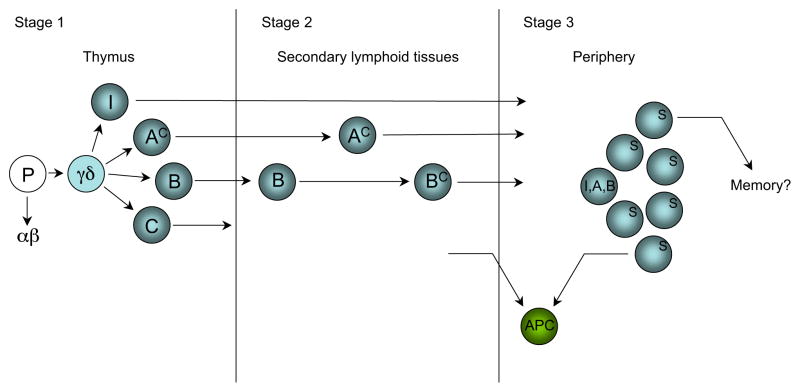
From the studies in mouse models, it appears that the development of γδ T cell function begins in the thymus and continues in the periphery, where environmental influences modulate developmental pathways. Fig. 1 shows a speculative scenario of the development of γδ T cell function in three major stages.
FIGURE 1.
Functional development of γδ T cells in stages—a speculative scenario. Stage 1 represents intrathymic differentiation under the influence of the thymic environment into subset-segregated functionally committed γδ thymocytes (I, A, B, and C); at least some cells reach functional competence already at this stage (AC); cells expressing invariant γδ TCRs (I) may follow an abbreviated path of differentiation. Stage 2 represents differentiation of some subsets within secondary lymphoid tissues, induced by other cell types (e.g., activated DCs). At this stage, functionally competent cells (I, AC, and BC) are capable of mounting polyclonal, subset-specific responses. Stage 3 represents TCR-dependent peripheral selection of clones (S) within subsets. Activated stages 2 and 3 γδ T cells might transiently convert to APCs, and especially stage 3 γδ T cells might be able acquire characteristics of memory cells.
Early functional commitment in the thymus (stage 1)
The γδ T lymphocytes arise from a common thymocyte progenitor for αβ and γδ T cells during development in the thymus. Lineage commitment and potential appear to be influenced by several factors (1, 2), and commitment may occur at more than one developmental stage (3). Although the TCR type per se does not determine the lineage decision, TCR signal strength appears to determine lineage fate and developmental stage of lineage choice (4, 5). TCR signals and their different strengths may continue to be important in subset-specific functional differentiation and commitment (Ref. 6 and below). Studies with γδ TCR-expressing thymocytes indicated early on that certain subsets already acquire functional competence while in the thymus. Thus, thymocytes expressing TCR-γδ and resembling NKT cells were found to produce IL-4 (7), and a subset of adult γδ TCR+ thymocytes that expressed Thy-1 at low levels could be induced to secrete IL-3, IL-4, IL-10, and IFN-γ, in contrast to Thy-1hi cells that only secreted IFN-γ (8). The Thy-1lo thymocytes were barely detectable in newborn mice and increased during the first 2 wk after birth (8), but a later study suggested that most originated from fetal precursors (9). Vγ1+ γδ T cells represent one of the major subsets in the secondary lymphoid organs and circulation of mice (10), and they are associated with distinct functional roles (see below). In keeping with the idea of subset-specific differentiation, the IL-4–producing thymocytes all express TCRs encoded by Vγ1 (8, 9). While investigating γδ TCR+ thymocytes for their ability to regulate allergic airway hyperresponsiveness (AHR), we found that in vivo-transferred Vγ1+ thymocytes enhanced AHR, whereas Vγ4+ thymocytes had no effect (11), pre-empting at least in part the functional pattern we had noted for peripheral γδ T cells (12). Interestingly, the γδ lineage-derived AHR enhancers are not those that produce IL-4 and IL-13 (11), unlike the above-mentioned NKT-like γδ T cell population. This indicates that the Vγ1+ thymocyte population contains more than functionally committed cell type.
Many γδ TCR+ thymocytes are already capable of producing TNF-α and IFN-γ upon activation in vitro (11), a bias that is maintained in the periphery (13). Thymocytes expressing γδ TCRs with a known specificity for the T10/T22 nonclassical class I molecules may express different Vγs and Vδs and only share a common Dδ motif (14, 15). These cells were shown to differentiate into IFN-γ rather than IL-17 producers dependent on TCR-ligand interactions in the thymus (16). However, development of IL-17–producing γδ T cells in the thymus has its own particular requirements (17). Finally, functional differentiation of thymocytes expressing the invariant Vγ5Vδ1 TCR of dendritic epidermal T cells (DETCs) in mice depends on thymic expression of Skint 1, an Ig-like molecule expressed on epithelial cells (18), which might be a ligand of this TCR.
These data in mice show that many, if not all, thymic precursors of peripheral γδ T cells leave the thymus with a defined and limited functional potential.
Polyclonal functional induction in periphery (stage 2)
Many peripheral γδ T cells in mice appear to be “resting yet activated” (19) (i.e., they acquire an intermediate state of activation from whence polyclonal responses might be elicited with little further stimulation). Perhaps this intermediate state depends on tonic signaling by cross-reactive γδ TCRs, as envisaged some time ago for certain αβ T cells (20). The unexplained “spontaneous” reactivity of hybridomas expressing Vγ1+ γδ TCRs described many years ago might be an in vitro correlate, at least for this subset (21). Peripheral γδ T cells, which leave the thymus as functionally committed precursors, are either already functionally competent or can be induced to become competent in short order. With the exception of two related populations in mice that express invariant TCRs and directly colonize peripheral epithelia and mucosae (22), most peripheral γδ T cells seem to recirculate and temporarily reside in the lymphoid tissues.
A detailed comparison of murine and human γδ T cell populations is still lacking. We examined the mouse Vγ1+ and Vγ4+ γδ T cells, because these subsets resemble the much-studied recirculating γδ T cells present in human peripheral blood. Both reside in the murine spleen, and both depend for their functional development on CD8+ dendritic cells (DCs) (11, 23), a cell type present in thymus and secondary lymphoid organs including the spleen, but not in peripheral nonlymphoid tissues (24). Our data suggest that although both of these γδ T cell types are already functionally committed, they differ in their requirement for peripheral functional induction (12, 25).
When transferred into secondary recipients, splenic Vγ1+ γδ T cells can exert dramatic functional effects in various mouse models of disease (see Table I). In models of allergic AHR and in the primary IgE response to OVA/alum (a model of adjuvant-supported vaccination), where they have a response-enhancing effect, these cells do not need to be induced in any way (12, 42). In fact, even as HSAhi thymocytes, they already have the ability to enhance AHR, following transfer into γδ T cell-deficient recipients (11). Whereas the IgE-enhancing γδ T cells may be contained within the NKT-like fraction ofVγ1+ γδTcells (39), the AHR-enhancing cells are not (see below) (42). Nevertheless, the AHR-enhancing cells also do not require induction. However, AHR-enhancing γδ T cells are functionally incompetent in mice lacking certain cytokines and receptors. In these mice, they can be induced to become fully functional by peripheral stimulation with OVA/alum (11), revealing some flexibility in their developmental pathway. It thus appears that several types of Vγ1+ γδ T cells, if not all, experience early intrathymic programming/functional induction.
Table I.
Contribution of murine γδ T cells to pathogenesis and pathology
| Model | γδ T Cells (Net Effect) | Vγ1+ | Vγ4+ | Vγ5Vδ1+(Invariant) | Vγ6Vδ1+ (Invariant) | References |
|---|---|---|---|---|---|---|
| Skin wound | Protective | Protective (promote wound healing) | 26, 27 | |||
| L. monocytogenes bacterial infection | Protective | Pathogenic (decrease resistance) | No effect | 28 | ||
| West Nile virus infection | Protective | Protective | Pathogenic (promote encephalitis) | 29 | ||
| Respiratory syncytial virus infection | Pathogenic | Pathogenic (proinflammatory) | 30 | |||
| Bacillus subtilis exposure; hypersensitivity pneumonitis | Protective | Protective, prevent lung fibrosis | 31 | |||
| Coxsackievirus B3-induced myocarditis | Protective | Protective (anti-inflammatory) | Pathogenic (proinflammatory) | 32 | ||
| Experimental autoimmune uveitis | Pathogenic | 33 | ||||
| Collagen-induced arthritis | Variable | No effect | Pathogenic (proinflammatory) | 34 | ||
| Heymann’s nephritis; adriamycin-induced nephritis | Protective | Protective (anti-inflammatory) | 35, 36 | |||
| Allergic AHR | Variable | Pathogenic (proinflammatory) | Protective (anti-inflammatory) | 12, 37 | ||
| Primary IgE response to OVA/alum (vaccination) | Variable | Enhancing | Inhibitory | 38, 39 | ||
| Subcutaneous melanoma | Protective | No effect | Protective | 40; Z. Yin, personal communication | ||
| Environmental exposure (ozone)-induced AHR | Pathogenic | Pathogenic (mediate AHR) | No effect | 41 |
Vγ4+ γδ T cells also affect disease outcome in various mouse models, but unlike theVγ1+ cells, they require peripheral signals to become functional. In contrast toVγ1+ cells, we observed that Vγ4+ cells suppress AHR and the primary IgE response to OVA/alum but require immunization or repeated challenge with Ag to develop (25, 39, 43). Thus, Vγ4+ cells might bypass some of the intrathymic programming that defines the Vγ1+ cells. However, their functional dependence on CD8+ DCs in donor mice suggests that they receive developmental signals as well, whether in thymus or periphery (23). Moreover, although Vγ4+ cells can be peripherally induced to suppress AHR and IgE, Vγ1+ cells cannot. Such suppressor-inducing conditions do not seem to have any effect on the Vγ1+ AHR enhancers but rendered Vγ1+ IgE enhancers nonfunctional, although they did not turn them into IgE suppressors (39). Thus, although there is some functional plasticity within either subset, the two do not functionally overlap. Both Vγ1+ and inducible Vγ4+ cell types are highly effective regulators of AHR and IgE in vivo (38, 39, 42) but appear to exert their functional effects as polyclonal unselected populations because they express diverse TCRs, and the treatments used to induce function do not lead to substantial changes in their TCR repertoires (25, 39).
Peripheral induction of Ag-presenting functions
Studies with ovine, human, and murine cells established that peripheral γδ T cells can be induced to acquire Ag-presenting functions (44–46), and a recent report characterized human γδ T cells as professional phagocytes (47). Because all of these studies were done in vitro, it remains to be seen whether Ag-presenting γδ T cells develop in vivo and are capable of influencing Ag-specific immune responses.
In contrast to human γδ T cells, which express MHC class II, in vitro expression of MHC class II molecules by murine cells γδ T cells has been detected but is limited to recently activated cells (46). When highly enriched γδ T cells were restimulated in vitro with plate-bound anti-CD3ε and anti-CD28 mAbs, activated γδ T cells lost surface γδ TCR expression while gaining MHC class II. Because of the absence of surface TCR, these cells became essentially “invisible” but could be identified as γδTcells by intracellular staining for TCR-δ (46). It is thus conceivable that MHC class II+ γδ T cells in vivo have escaped investigator scrutiny because of a loss of TCR expression. Taken together with MHC class II, the in vitro-restimulated γδ T cells expressed CD40 and CD80, and they were capable of presenting peptide Ags to MHC class II-restricted αβ T cells with specificities for the uveitogenic peptide IRBP1–20 (48) and the encephalitogenic peptide MOG35–55 (49). These data suggest that stimulation of peripheral γδ T cells via the TCR can induce an alternative functional program that converts them (transiently) to APCs. However, activation with cytokines might induce the conversion also (46), and the relative importance of these mechanisms remains to be determined.
Clonal selection in the periphery (stage 3)
Studies in humans (50, 51) and mice (34, 52, 53) suggest that functionally committed γδ T cells can be peripherally selected via TCR-ligand interactions. The putative TCR ligands are still unknown. This peripheral selection appears to be a slow process, perhaps largely limited to chronic disease conditions, and less effective than clonal selection of Ag-specific αβ T cells.
A recent study in the collagen-induced arthritis model in DBA/1 mice illustrates this process (34). In these mice, γδ T cells responded to injections of collagen in CFA, Vγ4+ cells in particular became activated, expanded, infiltrated the joints, and were shown to exacerbate the disease. Intracellular staining showed that the infiltrating Vγ4+ γδ T cells expressed IL-17, a cytokine associated with T cell pathogenicity in several models of autoimmune disease. In the course of this response, the TCR repertoire within the Vγ4+ subset became much more focused, suggesting that the oligoclonal response was driven by a specific ligand (34). The putative ligand driving this peripheral response does not appear to be collagen, but it might be contained within CFA or be induced through inflammation (34).
Two TCR-defined γδ T cell subsets in mice, the DETCs in the skin expressing invariant Vγ5Vδ1 TCRs and the γδ T cells expressing the related invariant Vγ6Vδ1 TCRs, which colonize the mucosae of the female reproductive tract and the lung, resemble clonally expanded γδ T cell populations (“I” in Fig. 1), although their invariant TCR appears to be selected in the thymus. These cells are activated by inducible autologous ligands (54, 55), and they exert relatively uniform functions. The activated DETCs promote epithelial repair and wound healing (26, 56). Vγ6Vδ1+ γδ T cells seem to respond to inflammation (55, 57), might play an immune-regulatory role during pregnancy (58), exert antibacterial activities in the lung, and inhibit the development of pulmonary fibrosis (31). The evolutionary conservation of these pseudoclonal γδ T cell populations in rodents—there is no human equivalent—might reflect a particular need for their rapid responses early in development.
Evidence that the TCR remains involved in all stages of functional development
At the onset of development, TCR-signaling supports the lineage decision between αβ and γδ T cells, with strong signals through the γδ TCR favoring γδ T cell development (3–5). Subsequently, innate TCR interactions with autologous ligands seem to play a role in the functional commitment of γδ T cells (6, 16). It is not yet clear whether such TCR signals determine subset differences in functional commitment (19). However, although still in the thymus, cells expressing different γδ TCRs already exhibit different functional potentials in vitro and in vivo (11). Autologous ligands and their in situ expression patterns in the thymus might be detectable using soluble versions of the γδ TCRs in question, as has been demonstrated in principle in vitro (59, 60).
Polyclonal activation and functional induction of committed cells in the peripheral lymphoid organs do not necessarily involve the TCR and might be accomplished with cytokines alone. However, γδ T cells at this stage can be activated via the TCR, with subsequent changes in function. Most obviously perhaps, the polyclonal conversion to Ag-presenting functions in vitro can be induced by TCR engagement (46). A role for poly- or oligoclonal TCR engagement is also suggested by data linking the expression of certain VγVδ pairs with distinct functions. Thus, only Vγ1Vδ5+ cells promote AHR in mice hyper-sensitized to OVA, and Vγ1Vδ6+ γδ T cells express a unique cytokine profile when compared with other Vγ1+ cells (8, 9). The ability of polyclonal Vγ1+ hybridomas to secrete cytokines “spontaneously” suggests that their TCRs might detect self-determinants (21, 61–64).
Finally, clonal expansion in the periphery implicitly involves TCR-ligand interactions, but putative ligands remain to be identified.
Terms of functional engagement
Early and very rapid responses of γδ T cells were found both in models of infection and exposure to injury. Thus, the influence of γδ T cells can be detected within a few hours of exposure to ozone, in this case mediating nonspecific AHR (41). In this short time frame, expansion of γδ T cells is not expected to play a role in their function. In contrast, γδ T cells can undergo large expansions, and the largest expansions were found relatively late in infectious and chronic inflammation, with several 100-fold increases of local γδ T cell populations (31, 34). During inflammation, γδ T cells often assume a regulatory role, as was first suggested in a model of pulmonary infection with influenza virus (65), and later demonstrated in mice infected with Listeria monocytogenes (66). In Listeria infection of the liver, γδ T cells were found to be required for the resolution of neutrophilic inflammation and the subsequent infiltration of macrophages (67). However, the same γδ T cell types that respond in infectious inflammation can also respond in sterile inflammation (55). Elegant studies with γδ T cells in the skin expressing an invariant TCR revealed that these cells respond locally, and very rapidly, to tissue injury (27). Histological analyses of recent skin wounds showed activated γδ T cells proximal to the wound edge, and further studies determined that the γδ T cells must be able to recognize keratinocytes (68) and induce them to produce hyaluronan, which in turn attracts macrophages that are instrumental in the repair process (26, 69).
However, the influence of γδ T cells is also evident in the absence of injury and inflammation, most notably perhaps in B cell development. That γδ T cells can provide B cell help and support Ab production in immunized or infected mice has been known for some time (70, 71). There is also evidence that they might regulate peripheral levels of specific Abs (38, 72). More recently, it has come to light that they even support and regulate Abs that develop without intentional immunization (39, 73, 74).
Functional balance
Comparisons of the roles of Vγ1+ andVγ4+ γδ T cells now have been made in a number of mouse models of disease (Table I), and the functional contributions of these subsets often appear to be opposed. Thus, when cells contained within one subset exacerbate disease pathology, cells within the other diminish it (12, 28, 29, 32, 33, 37, 40) (Z. Yin, unpublished results). Functional equilibrium may be reached at different levels, depending on external influences. For example, we found that airway allergen challenge strengthens the IgE-suppressive capability of Vγ4+ cells while at the same time diminishing the IgE-enhancing capability of Vγ1+ cells (39). It is still unclear whether the example of Vγ1+ and Vγ4+ γδ T cells can be generalized. Perhaps functional equilibrium is also achievable between cell types of less closely related lineages (e.g., γδ T cells and αβ T cells). In any case, one lesson from these studies is that depleting or reconstituting total γδ T cells might reveal little in terms of functional effects when functions are balanced, and the full regulatory range of these populations can easily be missed (38, 39, 42, 75).
When and where γδ T cell functions are needed
Because γδ T cells are the first T lymphocytes to arise in ontogeny (76, 77), it seems likely that they play an important role in immune protection early in development. Studies in mouse models and with human cells support this notion. Specifically, in young mice infected with Eimeria vermiformis, an intestinal parasite responsible for coccidiosis, γδ T cells were shown to be required for host resistance against this pathogen (78). In contrast, in adult mice, αβ T cells are both necessary and sufficient for protection. The relative ineffectiveness of αβ T cells in young mice might be due to an early Th2 bias of these cells so that γδ T cells come to function as a Th1 substitute (79). Human neonates harbor highly active γδ T cells. By comparison with αβ T cells, these cells exhibit stronger, pleiotropic functional responsiveness, and they lack deficits in IFN-γ production present in neonatal αβ T cells (80). The γδ T cells in infants respond strongly to immunization with bacillus Calmette-Guérin vaccine (81), and environmental factors are likely to shape the neonatal repertoire of γδ T cells (82), with possible long-term consequences for immune functions. Even prior to birth, γδ T cells might protect the developing organism. The reproductive tract in female mice harbors a distinct population of γδ T cells (83). During pregnancy in mice, γδ T cells increase vastly in numbers as the placenta develops (58). At least some of these cells appear to be capable of recognizing determinants on trophoblasts (84). Whether such cells play an immune-regulatory role or perhaps protect the fetus against infections remains to be determined (85, 86).
Because γδ T cell subsets are locally segregated, it seems likely that they also have specific functions related to the tissues in which they reside. Perhaps the most impressive example of this is the DETCs in rodents, which selectively colonize the epidermis and play a distinctive role in wound healing (26, 27, 56). Apparently, this function is so important that it justifies the existence of an entire γδ T cell subset expressing an invariant γδ TCR (Vγ5/Vδ1) (87). Whether these cells are also functionally homogeneous remains to be tested rigorously (88). A second type of γδ T cell in mice and rats expressing an invariant TCR (Vγ6/Vδ1) also has local functions in the tissues. In contrast to the DETCs, however, these cells form smaller steady-state populations, which expand to a much larger size only when their functions are required (57). Cells expressing Vγ6/Vδ1 invariant γδ TCRs in the lung (89) expand during chronic exposure with live bacteria (31). Importantly, cells within this subset help preventing pulmonary fibrosis (90). Vγ6/Vδ1 TCR+ cells also expand in nephritis of mice and rats (35, 36, 91) and in testicular inflammation in mice (55), in either tissue with a protective effect. The heterogeneous γδ T cells in the lymphoid tissues likely have multiple functions. For example, Vγ1+ cells seem to play a role in driving and regulating background levels of B cell differentiation (39, 73, 74), without apparent need for stimulation. When the immune system is challenged through vaccination or during infections (70, 71), they continue to support B cell differentiation, but now the help from αβ T cells becomes dominant (39). The γδ T cells, which can be found in the lymphoid organs, reappear in the peripheral nonlymphoid tissues where they can be induced to engage functionally (Table I). Vγ4+ cells, for example, contain inducible regulators of lung function, capable of inhibiting bronchoconstriction (43). They also contain inducible proinflammatory effectors, as demonstrated in mouse models of coxsackievirus B3 infection (32, 92), of respiratory syncytial virus infection of the lung (30), and of collagen-induced arthritis (34). Vγ1+ cells in the lymphoid tissues tend to oppose the effects of Vγ4+ cells in the tissues (12, 32, 39), though not always. Besides Vγ4+ and Vγ1+ γδ T cells, there are other γδ T cells in the lymphoid tissues (e.g., up to 50% of splenic γδ T cells), whose functions have not yet been studied separately. Investigations of such cells in mice likely will broaden the perspective of distinct γδ T cell functions. In contrast, the main experimental source of human γδ T cells continues to be peripheral blood, which contains a more limited spectrum of γδ T cells (93).
Is participation in the stress response a distinguishing function of γδ T cells?
The idea that γδ T cells play a key role in immune surveillance of stress in the tissues can be traced back to early days in this field of research (94), and it continues to stimulate speculation. A comprehensive analysis of this idea has been recently published by A. Hayday (19), who concluded that γδ T cells indeed play a significant role in the immune responses to stress. However, γδ T cells are by no means the only lymphocytes capable of recognizing and responding to cellular stress. Furthermore, there are constitutive γδ T cell functions that are difficult to reconcile with a focus on cellular stress, because they occur under steady-state conditions. An example is the already mentioned role of γδ T cells in B cell differentiation and the development of Abs. Although earlier studies showed an involvement of γδ T cells in induced Ab responses (38, 70, 71, 95, 96), recent observations suggest a far more critical influence of γδ T cells on the development of polyclonal noninduced Abs (39, 73, 74), with potential downstream effects on immune competence (97). Moreover, assuming that the immune responses to stress are immediate and polyclonal, the slow peripheral selection of oligo- or monoclonal γδ T cells (34, 98) does not fit well either. However, the response to stress likely is one of several natural capabilities of γδ T cells.
Why γδ T cell functions are a potential target for immune intervention
The association of distinct functions with TCR-defined subsets of γδ T cells invites the targeting of these subsets with Abs for immune intervention. Ab targeting of the TCR is nothing new, but γδ T cells represent a special case. In this study, because the targeting can be limited to small subsets with distinct functions, undesirable side effects of the Ab treatment (“cytokine storm,” immune deficiency) are expected to be minimal, because relatively few cells are involved. Maximal regulatory effects might be achievable by inactivating one subset while stimulating another, when γδ T cell subset functions are balanced. Furthermore, because distinct functions are associated with subsets instead of clones, the hurdle of developing clonotypic Abs does not exist, and a single Ab with a given subset specificity is likely to be useful in different diseases and many patients.
Conclusions
Animal models and especially mouse models of disease provide access to γδ T cells and their functions in a manner that is not achievable with human beings. Studies in mice already have generated a far more comprehensive picture of γδ T cell functions than could be obtained with human cell culture, and they are likely to provide first-hand information in the future. Because differences between γδ T cells in primates and rodents exist, any finding in mice must be validated in humans, but mice and other animal models remain irreplaceable as discovery tools.
Acknowledgments
This work was supported by National Institutes of Health Grants AI40611 and HL65410 (to W.K.B.), AI44920 and AI063400 (to R.L.O.), Grant 2007CB914801 from the National Basic Research Program of China, and National Outstanding Young Scientist Award 30725015 from the National Science Foundation of China (to Z.Y.).
Abbreviations used in this paper
- AHR
airway hyperresponsiveness
- DC
dendritic cell
- DETC
dendritic epidermal T cell
Footnotes
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Melichar HJ, Narayan K, Der SD, Hiraoka Y, Gardiol N, Jeannet G, Held W, Chambers CA, Kang J. Regulation of γδ versus αβ T lymphocyte differentiation by the transcription factor SOX13. Science. 2007;315:230–233. doi: 10.1126/science.1135344. [DOI] [PubMed] [Google Scholar]
- 2.Ueda-Hayakawa I, Mahlios J, Zhuang Y. Id3 restricts the developmental potential of γ δ lineage during thymopoiesis. J Immunol. 2009;182:5306–5316. doi: 10.4049/jimmunol.0804249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kreslavsky T, Garbe AI, Krueger A, von Boehmer H. T cell receptor-instructed αβ versus γδ lineage commitment revealed by single-cell analysis. J Exp Med. 2008;205:1173–1186. doi: 10.1084/jem.20072425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hayes SM, Li L, Love PE. TCR signal strength influences αβ/γδ lineage fate. Immunity. 2005;22:583–593. doi: 10.1016/j.immuni.2005.03.014. [DOI] [PubMed] [Google Scholar]
- 5.Lauritsen JP, Wong GW, Lee SY, Lefebvre JM, Ciofani M, Rhodes M, Kappes DJ, Zúñiga-Pflücker JC, Wiest DL. Marked induction of the helix-loop-helix protein Id3 promotes the γδ T cell fate and renders their functional maturation Notch independent. Immunity. 2009;31:565–575. doi: 10.1016/j.immuni.2009.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jensen KDC, Chien YH. Thymic maturation determines γδ T cell function, but not their antigen specificities. Curr Opin Immunol. 2009;21:140–145. doi: 10.1016/j.coi.2009.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vicari AP, Mocci S, Openshaw P, O’Garra A, Zlotnik A. Mouse γ δ TCR+NK1.1+ thymocytes specifically produce interleukin-4, are major histocompatibility complex class I independent, and are developmentally related to α β TCR+NK1.1+ thymocytes. Eur J Immunol. 1996;26:1424–1429. doi: 10.1002/eji.1830260704. [DOI] [PubMed] [Google Scholar]
- 8.Azuara V, Levraud JP, Lembezat MP, Pereira P. A novel subset of adult γ δ thymocytes that secretes a distinct pattern of cytokines and expresses a very restricted T cell receptor repertoire. Eur J Immunol. 1997;27:544–553. doi: 10.1002/eji.1830270228. [DOI] [PubMed] [Google Scholar]
- 9.Grigoriadou K, Boucontet L, Pereira P. Most IL-4–producing γ δ thymocytes of adult mice originate from fetal precursors. J Immunol. 2003;171:2413–2420. doi: 10.4049/jimmunol.171.5.2413. [DOI] [PubMed] [Google Scholar]
- 10.Pereira P, Gerber D, Huang SY, Tonegawa S. Ontogenic development and tissue distribution of V γ 1-expressing γ/δ T lymphocytes in normal mice. J Exp Med. 1995;182:1921–1930. doi: 10.1084/jem.182.6.1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jin N, Roark CL, Miyahara N, Taube C, Aydintug MK, Wands JM, Huang Y, Hahn YS, Gelfand EW, O’Brien RL, Born WK. Allergic airway hyperresponsiveness-enhancing γδ T cells develop in normal untreated mice and fail to produce IL-4/13, unlike Th2 and NKT cells. J Immunol. 2009;182:2002–2010. doi: 10.4049/jimmunol.0803280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hahn YS, Taube C, Jin N, Sharp L, Wands JM, Aydintug MK, Lahn M, Huber SA, O’Brien RL, Gelfand EW, Born WK. Different potentials of γ δ T cell subsets in regulating airway responsiveness: V γ 1+ cells, but not V γ 4+ cells, promote airway hyperreactivity, Th2 cytokines, and airway inflammation. J Immunol. 2004;172:2894–2902. doi: 10.4049/jimmunol.172.5.2894. [DOI] [PubMed] [Google Scholar]
- 13.Yin Z, Chen C, Szabo SJ, Glimcher LH, Ray A, Craft J. T-Bet expression and failure of GATA-3 cross-regulation lead to default production of IFN-γ by γδ T cells. J Immunol. 2002;168:1566–1571. doi: 10.4049/jimmunol.168.4.1566. [DOI] [PubMed] [Google Scholar]
- 14.Shin S, El-Diwany R, Schaffert S, Adams EJ, Garcia KC, Pereira P, Chien Y-H. Antigen recognition determinants of γδ T cell receptors. Science. 2005;308:252–255. doi: 10.1126/science.1106480. [DOI] [PubMed] [Google Scholar]
- 15.Adams EJ, Strop P, Shin S, Chien YH, Garcia KC. An autonomous CDR3δ is sufficient for recognition of the nonclassical MHC class I molecules T10 and T22 by γδ T cells. Nat Immunol. 2008;9:777–784. doi: 10.1038/ni.1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jensen KDC, Su X, Shin S, Li L, Youssef S, Yamasaki S, Steinman L, Saito T, Locksley RM, Davis MM, et al. Thymic selection determines γδ T cell effector fate: antigen-naive cells make interleukin-17 and antigen-experienced cells make interferon γ. Immunity. 2008;29:90–100. doi: 10.1016/j.immuni.2008.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Do JS, Fink PJ, Li L, Spolski R, Robinson J, Leonard WJ, Letterio JJ, Min B. Cutting edge: spontaneous development of IL-17–producing γδ T cells in the thymus occurs via a TGF-β1–dependent mechanism. J Immunol. 2010;184:1675–1679. doi: 10.4049/jimmunol.0903539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boyden LM, Lewis JM, Barbee SD, Bas A, Girardi M, Hayday AC, Tigelaar RE, Lifton RP. Skint1, the prototype of a newly identified immunoglobulin superfamily gene cluster, positively selects epidermal γδ T cells. Nat Genet. 2008;40:656–662. doi: 10.1038/ng.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hayday AC. γδ T cells and the lymphoid stress-surveillance response. Immunity. 2009;31:184–196. doi: 10.1016/j.immuni.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 20.Mason D. A very high level of crossreactivity is an essential feature of the T-cell receptor. Immunol Today. 1998;19:395–404. doi: 10.1016/s0167-5699(98)01299-7. [DOI] [PubMed] [Google Scholar]
- 21.O’Brien RL, Happ MP, Dallas A, Palmer E, Kubo R, Born WK. Stimulation of a major subset of lymphocytes expressing T cell receptor γ δ by an antigen derived from Mycobacterium tuberculosis. Cell. 1989;57:667–674. doi: 10.1016/0092-8674(89)90135-9. [DOI] [PubMed] [Google Scholar]
- 22.Heyborne K, Fu YX, Kalataradi H, Reardon C, Roark C, Eyster C, Vollmer M, Born W, O’Brien R. Evidence that murine V γ 5 and V γ 6 γ δ-TCR+ lymphocytes are derived from a common distinct lineage. J Immunol. 1993;151:4523–4527. [PubMed] [Google Scholar]
- 23.Cook L, Miyahara N, Jin N, Wands JM, Taube C, Roark CL, Potter TA, Gelfand EW, O’Brien RL, Born WK. Evidence that CD8+ dendritic cells enable the development of γδ T cells that modulate airway hyperresponsiveness. J Immunol. 2008;181:309–319. doi: 10.4049/jimmunol.181.1.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Naik SH, Metcalf D, van Nieuwenhuijze A, Wicks I, Wu L, O’Keefe M, Shortman K. Intrasplenic steady-state dendritic cell precursors that are distinct from monocytes. Nat Immunol. 2006;7:663–671. doi: 10.1038/ni1340. [DOI] [PubMed] [Google Scholar]
- 25.Jin N, Taube C, Sharp L, Hahn YS, Yin X, Wands JM, Roark CL, O’Brien RL, Gelfand EW, Born WK. Mismatched antigen prepares γ δ T cells for suppression of airway hyperresponsiveness. J Immunol. 2005;174:2671–2679. doi: 10.4049/jimmunol.174.5.2671. [DOI] [PubMed] [Google Scholar]
- 26.Jameson J, Havran WL. Skin γδ T-cell functions in homeostasis and wound healing. Immunol Rev. 2007;215:114–122. doi: 10.1111/j.1600-065X.2006.00483.x. [DOI] [PubMed] [Google Scholar]
- 27.Jameson J, Ugarte K, Chen N, Yachi P, Fuchs E, Boismenu R, Havran WL. A role for skin γδ T cells in wound repair. Science. 2002;296:747–749. doi: 10.1126/science.1069639. [DOI] [PubMed] [Google Scholar]
- 28.O’Brien RL, Yin X, Huber SA, Ikuta K, Born WK. Depletion of a γ δ T cell subset can increase host resistance to a bacterial infection. J Immunol. 2000;165:6472–6479. doi: 10.4049/jimmunol.165.11.6472. [DOI] [PubMed] [Google Scholar]
- 29.Welte T, Lamb J, Anderson JF, Born WK, O’Brien RL, Wang T. Role of two distinct γδ T cell subsets during West Nile virus infection. FEMS Immunol Med Microbiol. 2008;53:275–283. doi: 10.1111/j.1574-695X.2008.00430.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dodd J, Riffault S, Kodituwakku JS, Hayday AC, Openshaw PJ. Pulmonary V γ 4+ γ δ T cells have proinflammatory and antiviral effects in viral lung disease. J Immunol. 2009;182:1174–1181. doi: 10.4049/jimmunol.182.2.1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Simonian PL, Roark CL, Diaz del Valle F, Palmer BE, Douglas IS, Ikuta K, Born WK, O’Brien RL, Fontenot AP. Regulatory role of γδ T cells in the recruitment of CD4+ and CD8+ T cells to lung and subsequent pulmonary fibrosis. J Immunol. 2006;177:4436–4443. doi: 10.4049/jimmunol.177.7.4436. [DOI] [PubMed] [Google Scholar]
- 32.Huber SA, Graveline D, Newell MK, Born WK, O’Brien RL. V γ 1+ T cells suppress and V γ 4+ T cells promote susceptibility to coxsackievirus B3-induced myocarditis in mice. J Immunol. 2000;165:4174–4181. doi: 10.4049/jimmunol.165.8.4174. [DOI] [PubMed] [Google Scholar]
- 33.Cui Y, Shao H, Lan C, Nian H, O’Brien RL, Born WK, Kaplan HJ, Sun D. Major role of γδ T cells in the generation of IL-17+ uveitogenic T cells. J Immunol. 2009;183:560–567. doi: 10.4049/jimmunol.0900241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roark CL, French JD, Taylor MA, Bendele AM, Born WK, O’Brien RL. Exacerbation of collagen-induced arthritis by oligoclonal, IL-17–producing γ δ T cells. J Immunol. 2007;179:5576–5583. doi: 10.4049/jimmunol.179.8.5576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wu H, Knight JF, Alexander SI. Regulatory γ δ T cells in Heymann nephritis express an invariant Vγ6/Vδ1 with a canonical CDR3 sequence. Eur J Immunol. 2004;34:2322–2330. doi: 10.1002/eji.200324780. [DOI] [PubMed] [Google Scholar]
- 36.Wu H, Wang YM, Wang Y, Hu M, Zhang GY, Knight JF, Harris DC, Alexander SI. Depletion of γδ T cells exacerbates murine adriamycin nephropathy. J Am Soc Nephrol. 2007;18:1180–1189. doi: 10.1681/ASN.2006060622. [DOI] [PubMed] [Google Scholar]
- 37.Lahn M, Kanehiro A, Takeda K, Joetham A, Schwarze J, Köhler G, O’Brien R, Gelfand EW, Born W. Negative regulation of airway responsiveness that is dependent on γδ T cells and independent of αβ T cells. [Published erratum appears in 2000 Nat. Med. 6: 229.] Nat Med. 1999;5:1150–1156. doi: 10.1038/13476. [DOI] [PubMed] [Google Scholar]
- 38.McMenamin C, Pimm C, McKersey M, Holt PG. Regulation of IgE responses to inhaled antigen in mice by antigen-specific γ δ T cells. Science. 1994;265:1869–1871. doi: 10.1126/science.7916481. [DOI] [PubMed] [Google Scholar]
- 39.Huang Y, Jin N, Roark CL, Aydintug MK, Wands JM, Huang H, O’Brien RL, Born WK. The influence of IgE-enhancing and IgE-suppressive γδ T cells changes with exposure to inhaled ovalbumin. J Immunol. 2009;183:849–855. doi: 10.4049/jimmunol.0804104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gao Y, Yang W, Pan M, Scully E, Girardi M, Augenlicht LH, Craft J, Yin Z. γδ T cells provide an early source of interferon γ in tumor immunity. J Exp Med. 2003;198:433–442. doi: 10.1084/jem.20030584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Matsubara S, Takeda K, Jin N, Okamoto M, Matsuda H, Shiraishi Y, Park JW, McConville G, Joetham A, O’Brien RL, et al. Vγ1+ T cells and tumor necrosis factor-α in ozone-induced airway hyperresponsiveness. Am J Respir Cell Mol Biol. 2009;40:454–463. doi: 10.1165/rcmb.2008-0346OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jin N, Miyahara N, Roark CL, French JD, Aydintug MK, Matsuda JL, Gapin L, O’Brien RL, Gelfand EW, Born WK. Airway hyperresponsiveness through synergy of γδ T cells and NKT cells. J Immunol. 2007;179:2961–2968. doi: 10.4049/jimmunol.179.5.2961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hahn YS, Taube C, Jin N, Takeda K, Park JW, Wands JM, Aydintug MK, Roark CL, Lahn M, O’Brien RL, et al. V γ 4+ γ δ T cells regulate airway hyperreactivity to methacholine in ovalbumin-sensitized and challenged mice. J Immunol. 2003;171:3170–3178. doi: 10.4049/jimmunol.171.6.3170. [DOI] [PubMed] [Google Scholar]
- 44.Collins RA, Werling D, Duggan SE, Bland AP, Parsons KR, Howard CJ. γδ T cells present antigen to CD4+ αβ T cells. J Leukoc Biol. 1998;63:707–714. doi: 10.1002/jlb.63.6.707. [DOI] [PubMed] [Google Scholar]
- 45.Brandes M, Willimann K, Moser B. Professional antigen-presentation function by human γδ T Cells. Science. 2005;309:264–268. doi: 10.1126/science.1110267. [DOI] [PubMed] [Google Scholar]
- 46.Cheng L, Cui Y, Shao H, Han G, Zhu L, Huang Y, O’Brien RL, Born WK, Kaplan HJ, Sun D. Mouse γδT cells are capable of expressing MHC class II molecules, and of functioning as antigen-presenting cells. J Neuroimmunol. 2008;203:3– 11. doi: 10.1016/j.jneuroim.2008.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wu Y, Wu W, Wong WM, Ward E, Thrasher AJ, Goldblatt D, Osman M, Digard P, Canaday DH, Gustafsson K. Human γ δ T cells: a lymphoid lineage cell capable of professional phagocytosis. J Immunol. 2009;183:5622–5629. doi: 10.4049/jimmunol.0901772. [DOI] [PubMed] [Google Scholar]
- 48.Avichezer D, Chan CC, Silver PB, Wiggert B, Caspi RR. Residues 1–20 of IRBP and whole IRBP elicit different uveitogenic and immunological responses in interferon γ deficient mice. Exp Eye Res. 2000;71:111–118. doi: 10.1006/exer.2000.0860. [DOI] [PubMed] [Google Scholar]
- 49.Sun D, Zhang Y, Wei B, Peiper SC, Shao H, Kaplan HJ. Encephalitogenic activity of truncated myelin oligodendrocyte glycoprotein (MOG) peptides and their recognition by CD8+ MOG-specific T cells on oligomeric MHC class I molecules. Int Immunol. 2003;15:261–268. doi: 10.1093/intimm/dxg023. [DOI] [PubMed] [Google Scholar]
- 50.Hohlfeld R, Engel AG, Ii K, Harper MC. Polymyositis mediated by T lymphocytes that express the γ/δ receptor. N Engl J Med. 1991;324:877–881. doi: 10.1056/NEJM199103283241303. [DOI] [PubMed] [Google Scholar]
- 51.Shimonkevitz R, Colburn C, Burnham JA, Murray RS, Kotzin BL. Clonal expansions of activated γ/δ T cells in recent-onset multiple sclerosis. Proc Natl Acad Sci USA. 1993;90:923–927. doi: 10.1073/pnas.90.3.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sim GK, Augustin A. Dominantly inherited expression of BID, an invariant undiversified T cell receptor δ chain. Cell. 1990;61:397–405. doi: 10.1016/0092-8674(90)90522-g. [DOI] [PubMed] [Google Scholar]
- 53.Sim GK, Augustin A. Extrathymic positive selection of γ δ T cells. V γ 4J γ 1 rearrangements with “GxYS” junctions. J Immunol. 1991;146:2439–2445. [PubMed] [Google Scholar]
- 54.Havran WL, Chien YH, Allison JP. Recognition of self antigens by skin-derived T cells with invariant γ δ antigen receptors. Science. 1991;252:1430–1432. doi: 10.1126/science.1828619. [DOI] [PubMed] [Google Scholar]
- 55.Mukasa A, Born WK, O’Brien RL. Inflammation alone evokes the response of a TCR-invariant mouse γδ T cell subset. J Immunol. 1999;162:4910–4913. [PubMed] [Google Scholar]
- 56.Toulon A, Breton L, Taylor KR, Tenenhaus M, Bhavsar D, Lanigan C, Rudolph R, Jameson J, Havran WL. A role for human skin-resident T cells in wound healing. J Exp Med. 2009;206:743–750. doi: 10.1084/jem.20081787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Roark CL, Aydintug MK, Lewis J, Yin X, Lahn M, Hahn YS, Born WK, Tigelaar RE, O’Brien RL. Subset-specific, uniform activation among V γ 6/V δ 1+ γ δ T cells elicited by inflammation. J Leukoc Biol. 2004;75:68–75. doi: 10.1189/jlb.0703326. [DOI] [PubMed] [Google Scholar]
- 58.Heyborne KD, Cranfill RL, Carding SR, Born WK, O’Brien RL. Characterization of γ δ T lymphocytes at the maternal-fetal interface. J Immunol. 1992;149:2872–2878. [PubMed] [Google Scholar]
- 59.Aydintug MK, Roark CL, Chain JL, Born WK, O’Brien RL. Macrophages express multiple ligands for γδ TCRs. Mol Immunol. 2008;45:3253–3263. doi: 10.1016/j.molimm.2008.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Aydintug MK, Roark CL, Yin X, Wands JM, Born WK, O’Brien RL. Detection of cell surface ligands for the γ δ TCR using soluble TCRs. J Immunol. 2004;172:4167–4175. doi: 10.4049/jimmunol.172.7.4167. [DOI] [PubMed] [Google Scholar]
- 61.O’Brien RL, Fu YX, Cranfill R, Dallas A, Ellis C, Reardon C, Lang J, Carding SR, Kubo R, Born W. Heat shock protein Hsp60-reactive γ δ cells: a large, diversified T-lymphocyte subset with highly focused specificity. Proc Natl Acad Sci USA. 1992;89:4348–4352. doi: 10.1073/pnas.89.10.4348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Happ MP, Kubo RT, Palmer E, Born WK, O’Brien RL. Limited receptor repertoire in a mycobacteria-reactive subset of γ δ T lymphocytes. Nature. 1989;342:696–698. doi: 10.1038/342696a0. [DOI] [PubMed] [Google Scholar]
- 63.Wilde DB, Roberts K, Sturmhöfel K, Kikuchi G, Coligan JE, Shevach EM. Mouse autoreactive γ/δ T cells. I. Functional properties of autoreactive T cell hybridomas. Eur J Immunol. 1992;22:483–489. doi: 10.1002/eji.1830220229. [DOI] [PubMed] [Google Scholar]
- 64.Kikuchi GE, Roberts K, Shevach EM, Coligan JE. Gene transfer demonstrates that the V γ 1.1C γ 4V δ 6C δ T cell receptor is essential for autoreactivity. J Immunol. 1992;148:1302–1307. [PubMed] [Google Scholar]
- 65.Carding SR, Allan W, Kyes S, Hayday A, Bottomly K, Doherty PC. Late dominance of the inflammatory process in murine influenza by γ/δ + T cells. J Exp Med. 1990;172:1225–1231. doi: 10.1084/jem.172.4.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mombaerts P, Arnoldi J, Russ F, Tonegawa S, Kaufmann SHE. Different roles of α β and γ δ T cells in immunity against an intracellular bacterial pathogen. Nature. 1993;365:53–56. doi: 10.1038/365053a0. [DOI] [PubMed] [Google Scholar]
- 67.Fu YX, Roark CE, Kelly K, Drevets D, Campbell P, O’Brien R, Born W. Immune protection and control of inflammatory tissue necrosis by γ δ T cells. J Immunol. 1994;153:3101–3115. [PubMed] [Google Scholar]
- 68.Jameson JM, Cauvi G, Witherden DA, Havran WL. A keratinocyte- responsive γ δ TCR is necessary for dendritic epidermal T cell activation by damaged keratinocytes and maintenance in the epidermis. J Immunol. 2004;172:3573– 3579. doi: 10.4049/jimmunol.172.6.3573. [DOI] [PubMed] [Google Scholar]
- 69.Jameson JM, Cauvi G, Sharp LL, Witherden DA, Havran WL. γδ T cell-induced hyaluronan production by epithelial cells regulates inflammation. J Exp Med. 2005;201:1269–1279. doi: 10.1084/jem.20042057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wen L, Pao W, Wong FS, Peng Q, Craft J, Zheng B, Kelsoe G, Dianda L, Owen MJ, Hayday AC. Germinal center formation, immunoglobulin class switching, and autoantibody production driven by “non α/β” T cells. J Exp Med. 1996;183:2271–2282. doi: 10.1084/jem.183.5.2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pao W, Wen L, Smith AL, Gulbranson-Judge A, Zheng B, Kelsoe G, MacLennan ICM, Owen MJ, Hayday AC. γ δ T cell help of B cells is induced by repeated parasitic infection, in the absence of other T cells. Curr Biol. 1996;6:1317–1325. doi: 10.1016/s0960-9822(02)70718-5. [DOI] [PubMed] [Google Scholar]
- 72.McMenamin C, McKersey M, Kühnlein P, Hünig T, Holt PG. γ δ T cells down-regulate primary IgE responses in rats to inhaled soluble protein antigens. J Immunol. 1995;154:4390–4394. [PubMed] [Google Scholar]
- 73.Felices M, Yin CC, Kosaka Y, Kang J, Berg LJ. Tec kinase Itk in γδT cells is pivotal for controlling IgE production in vivo. Proc Natl Acad Sci USA. 2009;106:8308–8313. doi: 10.1073/pnas.0808459106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Qi Q, Xia M, Hu J, Hicks E, Iyer A, Xiong N, August A. Enhanced development of CD4+ γδ T cells in the absence of Itk results in elevated IgE production. Blood. 2009;114:564–571. doi: 10.1182/blood-2008-12-196345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lahn M, Kanehiro A, Takeda K, Terry J, Hahn YS, Aydintug MK, Konowal A, Ikuta K, O’Brien RL, Gelfand EW, Born WK. MHC class I-dependent Vγ4+ pulmonary T cells regulate α β T cell-independent airway responsiveness. Proc Natl Acad Sci USA. 2002;99:8850–8855. doi: 10.1073/pnas.132519299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Havran WL, Allison JP. Developmentally ordered appearance of thymocytes expressing different T-cell antigen receptors. Nature. 1988;335:443–445. doi: 10.1038/335443a0. [DOI] [PubMed] [Google Scholar]
- 77.De Rosa SC, Andrus JP, Perfetto SP, Mantovani JJ, Herzenberg LA, Herzenberg LA, Roederer M. Ontogeny of γ δ T cells in humans. J Immunol. 2004;172:1637–1645. doi: 10.4049/jimmunol.172.3.1637. [DOI] [PubMed] [Google Scholar]
- 78.Ramsburg E, Tigelaar R, Craft J, Hayday A. Age-dependent requirement for γδ T cells in the primary but not secondary protective immune response against an intestinal parasite. J Exp Med. 2003;198:1403–1414. doi: 10.1084/jem.20030050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hayday AC, Roberts S, Ramsburg E. γδ Cells and the regulation of mucosal immune responses. Am J Respir Crit Care Med. 2000;162:S161–S163. doi: 10.1164/ajrccm.162.supplement_3.15tac4. [DOI] [PubMed] [Google Scholar]
- 80.Gibbons DL, Haque SF, Silberzahn T, Hamilton K, Langford C, Ellis P, Carr R, Hayday AC. Neonates harbour highly active γδ T cells with selective impairments in preterm infants. Eur J Immunol. 2009;39:1794–1806. doi: 10.1002/eji.200939222. [DOI] [PubMed] [Google Scholar]
- 81.Mazzola TN, Da Silva MT, Moreno YM, Lima SC, Carniel EF, Morcillo AM, Antonio MA, Zanolli ML, Netto AA, Blotta MH, et al. Robust γδ+ T cell expansion in infants immunized at birth with BCG vaccine. Vaccine. 2007;25:6313–6320. doi: 10.1016/j.vaccine.2007.06.039. [DOI] [PubMed] [Google Scholar]
- 82.Cairo C, Propp N, Auricchio G, Armstrong CL, Abimiku A, Mancino G, Colizzi V, Blattner W, Pauza CD. Altered cord blood γδ T cell repertoire in Nigeria: possible impacts of environmental factors on neonatal immunity. Mol Immunol. 2008;45:3190–3197. doi: 10.1016/j.molimm.2008.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Heilig JS, Tonegawa S. Diversity of murine γ genes and expression in fetal and adult T lymphocytes. Nature. 1986;322:836–840. doi: 10.1038/322836a0. [DOI] [PubMed] [Google Scholar]
- 84.Heyborne K, Fu YX, Nelson A, Farr A, O’Brien R, Born W. Recognition of trophoblasts by γ δ T cells. J Immunol. 1994;153:2918–2926. [PubMed] [Google Scholar]
- 85.Arck PC, Ferrick DA, Steele-Norwood D, Croitoru K, Clark DA. Murine T cell determination of pregnancy outcome. I. Effects of strain, αβ T cell receptor, γδ T cell receptor, and γδ T cell subsets. [Published erratum appears in 1997 Am. J. Reprod. Immunol. 38: 438.] Am J Reprod Immunol. 1997;37:492. doi: 10.1111/j.1600-0897.1997.tb00265.x. [DOI] [PubMed] [Google Scholar]
- 86.Arck PC, Ferrick DA, Steele-Norwood D, Egan PJ, Croitoru K, Carding SR, Dietl J, Clark DA. Murine T cell determination of pregnancy outcome. Cell Immunol. 1999;196:71–79. doi: 10.1006/cimm.1999.1535. [DOI] [PubMed] [Google Scholar]
- 87.Asarnow DM, Kuziel WA, Bonyhadi M, Tigelaar RE, Tucker PW, Allison JP. Limited diversity of γ δ antigen receptor genes of Thy-1+ dendritic epidermal cells. Cell. 1988;55:837–847. doi: 10.1016/0092-8674(88)90139-0. [DOI] [PubMed] [Google Scholar]
- 88.Boismenu R, Hobbs MV, Boullier S, Havran WL. Molecular and cellular biology of dendritic epidermal T cells. Semin Immunol. 1996;8:323–331. doi: 10.1006/smim.1996.0043. [DOI] [PubMed] [Google Scholar]
- 89.Hayes SM, Sirr A, Jacob S, Sim GK, Augustin A. Role of IL-7 in the shaping of the pulmonary γ δ T cell repertoire. J Immunol. 1996;156:2723–2729. [PubMed] [Google Scholar]
- 90.Simonian PL, Roark CL, Wehrmann F, Lanham AK, Diaz del Valle F, Born WK, O’Brien RL, Fontenot AP. Th17-polarized immune response in a murine model of hypersensitivity pneumonitis and lung fibrosis. J Immunol. 2009;182:657–665. [PMC free article] [PubMed] [Google Scholar]
- 91.Ando T, Wu H, Watson D, Hirano T, Hirakata H, Fujishima M, Knight JF. Infiltration of canonical Vγ4/Vδ1 γδ T cells in an adriamycin-induced progressive renal failure model. J Immunol. 2001;167:3740–3745. doi: 10.4049/jimmunol.167.7.3740. [DOI] [PubMed] [Google Scholar]
- 92.Huber SA, Sartini D. Roles of tumor necrosis factor α (TNF-α) and the p55 TNF receptor in CD1d induction and coxsackievirus B3-induced myocarditis. J Virol. 2005;79:2659–2665. doi: 10.1128/JVI.79.5.2659-2665.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Parker CM, Groh V, Band H, Porcelli SA, Morita C, Fabbi M, Glass D, Strominger JL, Brenner MB. Evidence for extrathymic changes in the T cell receptor γ/δ repertoire. J Exp Med. 1990;171:1597–1612. doi: 10.1084/jem.171.5.1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Janeway CA, Jr, Jones B, Hayday A. Specificity and function of T cells bearing γ δ receptors. Immunol Today. 1988;9:73–76. doi: 10.1016/0167-5699(88)91267-4. [DOI] [PubMed] [Google Scholar]
- 95.Zuany-Amorim C, Ruffié C, Hailé S, Vargaftig BB, Pereira P, Pretolani M. Requirement for γδ T cells in allergic airway inflammation. Science. 1998;280:1265–1267. doi: 10.1126/science.280.5367.1265. [DOI] [PubMed] [Google Scholar]
- 96.Svensson L, Lilliehöök B, Larsson R, Bucht A. γδ T cells contribute to the systemic immunoglobulin E response and local B-cell reactivity in allergic eosinophilic airway inflammation. Immunology. 2003;108:98–108. doi: 10.1046/j.1365-2567.2003.01561.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Born WK, Huang Y, Jin N, Huang H, O’Brien RL. Balanced approach of γδ T cells to type 2 immunity. Immunol Cell Biol. 2010 doi: 10.1038/icb.2009.105. [DOI] [PubMed] [Google Scholar]
- 98.Wiendl H, Malotka J, Holzwarth B, Weltzien HU, Wekerle H, Hohlfeld R, Dornmair K. An autoreactive γ δ TCR derived from a polymyositis lesion. J Immunol. 2002;169:515–521. doi: 10.4049/jimmunol.169.1.515. [DOI] [PubMed] [Google Scholar]