Abstract
Alu repetitive elements are known to be major contributors to genome instability by generating Alu-mediated copy-number variants (CNVs). Most of the reported Alu-mediated CNVs are simple deletions and duplications, and the mechanism underlying Alu–Alu-mediated rearrangement has been attributed to non-allelic homologous recombination (NAHR). Chromosome 17 at the p13.3 genomic region lacks extensive low-copy repeat architecture; however, it is highly enriched for Alu repetitive elements, with a fraction of 30% of total sequence annotated in the human reference genome, compared with the 10% genome-wide and 18% on chromosome 17. We conducted mechanistic studies of the 17p13.3 CNVs by performing high-density oligonucleotide array comparative genomic hybridization, specifically interrogating the 17p13.3 region with ∼150 bp per probe density; CNV breakpoint junctions were mapped to nucleotide resolution by polymerase chain reaction and Sanger sequencing. Studied rearrangements include 5 interstitial deletions, 14 tandem duplications, 7 terminal deletions and 13 complex genomic rearrangements (CGRs). Within the 17p13.3 region, Alu–Alu-mediated rearrangements were identified in 80% of the interstitial deletions, 46% of the tandem duplications and 50% of the CGRs, indicating that this mechanism was a major contributor for formation of breakpoint junctions. Our studies suggest that Alu repetitive elements facilitate formation of non-recurrent CNVs, CGRs and other structural aberrations of chromosome 17 at p13.3. The common observation of Alu-mediated rearrangement in CGRs and breakpoint junction sequences analysis further demonstrates that this type of mechanism is unlikely attributed to NAHR, but rather may be due to a recombination-coupled DNA replicative repair process.
Introduction
Primate-specific Alu repetitive elements, although short in length (∼300 bp), represent one of the most successful transposable elements in the human genome. As a member of the retrotransposon family, Alu elements use a reverse-transcribed RNA intermediate to insert into different positions in the genome through a ‘copy-and-paste’ mechanism (1). Overall, these elements comprise about 10% of the human genome with more than 1 million copies (2).
Through two different approaches, Alu elements are major contributors to genomic diversity and/or genome instability. The insertional activity of Alu elements can lead to disruption of a gene either in its coding or regulatory regions, causing various types of diseases (3,4). In addition to insertional mutagenesis, the sequence homology between Alu elements (average 71%) (5) provides substrates to potentially facilitate unequal crossover between genomic segments and generates Alu–Alu-mediated copy-number variants (CNVs). Alu dimorphism within a personal genome, the presence of only one Alu at an allelic position of a diploid genome, is the most frequently observed deletion in the allelic spectrum of CNV < 1000 bp in size (6). The resulting gain or loss of a certain genomic region due to rearrangements can lead to many human disease states (7).
Alu-mediated CNVs were found in both germ-line genomic disorders (8–12) and somatic mutations in malignancies (13,14). This mode of mutagenesis was estimated to account for 0.3% of human genetic diseases, but this incidence was believed to be underestimated (7). Among the observed Alu-mediated germ-line CNVs, the majority of those characterized were deletions, with some duplications presented. Although, for some cases, there may be additional complexities at the breakpoint(s) (15,16), almost all these CNVs appeared to be simple interstitial deletions or tandem duplications. The Alu–Alu mechanism for generation of CNVs has seldom been described in association with complex genomic rearrangements (CGRs) having multiple copy-number transitions. Only a few examples were described, including one Alu-mediated breakpoint junction in a patient with triplication at 17p11.2 (17), one of the two breakpoint junctions in a patient with deletion–normal–duplication at 17p12 (18), one identical breakpoint junction shared in three patients with duplication–normal–duplication at Xp22 (19) and one junction mapped in a patient with duplication–normal–duplication–normal–duplication at Xq26 (20).
The mechanism underlying Alu–Alu-mediated rearrangement, which is defined by the formation of a new chimeric Alu hybrid, has been attributed to non-allelic homologous recombination (NAHR) (21,22). However, the low degree of similarity between Alu pairs mediating rearrangements is inconsistent with the hypothesis that this is purely mediated by homologous recombination (16). Replicative mechanisms, such as fork stalling and template switching (FoSTeS)/microhomology-mediated break-induced replication (MMBIR), that are mediated by short stretches of microhomology could potentially explain Alu–Alu-mediated rearrangements (23,24). Likewise, FoSTeS/MMBIR also provides a parsimonious explanation for template switching, contributing to one or multiple iterations thereof, and to Alu-induced CGRs other than simple deletions and tandem duplications (17,18).
The gene-rich 17p13.3 region has been associated with various deletion and duplication CNV syndromes or genomic disorders, including Miller–Dieker syndrome (MDS; MIM #247200), 17p13.3 duplication syndrome (MIM #613215), 17p13.3 microdeletion syndrome (25,26) and split-hand/foot malformation with long bone deficiency 3 (SHFLD3; MIM #612576). The majority of previously reported 17p13.3 CNVs were interstitial deletions/duplications and terminal deletions, with only five CGRs (25,27,28). Moreover, CNVs with breakpoint junctions mapped to nucleotide resolution were primarily deletions (10,26).
We conducted comprehensive breakpoint mapping and junctional analyses to gain insights into mutational mechanisms in the 17p13.3 region. We investigated 17p13.3 CNVs identified in 39 unrelated individuals from 33 800 patient samples that were sent for clinical diagnostic testing at the Medical Genetics Laboratories of Baylor College of Medicine. Human chromosome 17 at p13.3 is highly enriched for Alu repetitive elements and indeed, breakpoint sequencing of these CNVs revealed that a remarkable proportion of the junctions were mediated by an Alu–Alu mechanism, including 50% of the breakpoint junctions in CGRs with evidence suggesting multiple template switches. Our studies reveal a prominent role for Alu–Alu-mediated genomic rearrangement events and further support the contention that replicative mechanisms play an important role in formation of CGRs.
Results
Interstitial deletions and duplications of 17p13.3 vary in size and genomic position
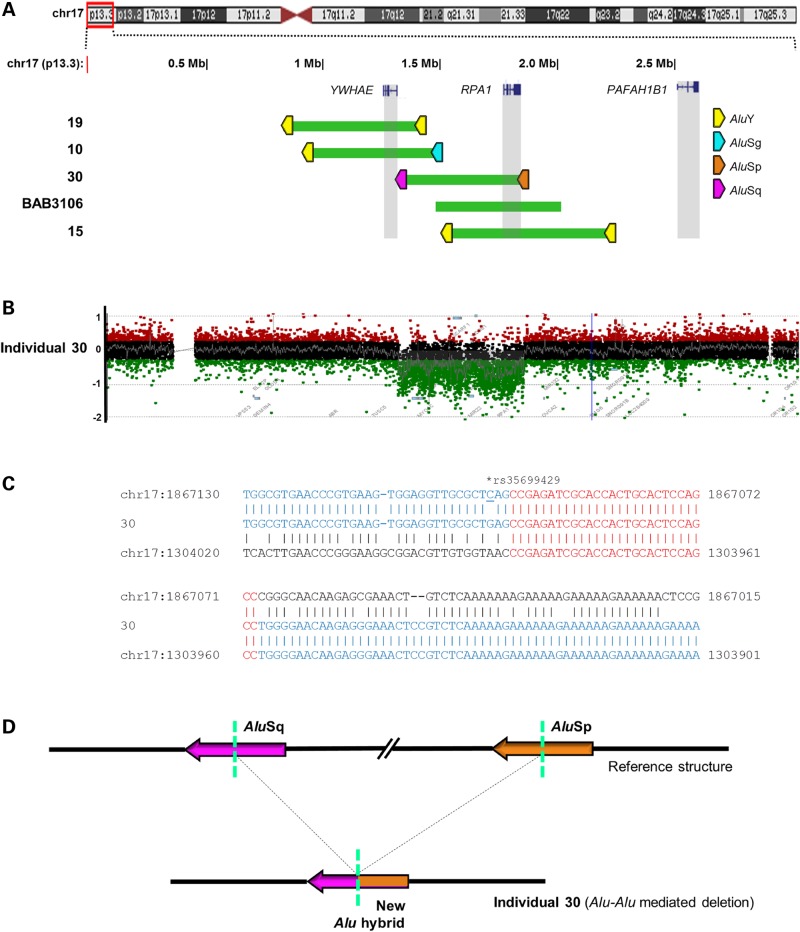
Five of the 39 rearrangements interrogated from individual personal genomes had simple interstitial deletions, ranging in size from 0.545 to 0.746 Mb (Fig. 1, Supplementary Material, Fig. S1 and Table 1). Deletions in two individuals (19 and 10) encompassed YWHAE. Four of the five deletions (in individuals 19, 10, 30 and 15) had breakpoint junctions localized to directly oriented Alu pairs with a chimeric Alu hybrid being formed (Fig. 1, Supplementary Material, Fig. S1 and Table 1). The remaining deletion in individual BAB3106 had 6 bp of microhomology between the proximal and distal reference sequences at the breakpoint junction (Supplementary Material, Fig. S1C).
Figure 1.
Interstitial deletions of 17p13.3. (A) Deletions were plotted by exact genomic coordinates according to breakpoint junction sequencing results using the UCSC genome browser custom track function (GRCh37/hg19 assembly). Green blocks represent deletions. Upper panel shows an ideogram of human chromosome 17 with the 17p13.3 region involved highlighted in the red box. Three critical genes in 17p13.3 deletion and duplication syndromes, YWHAE, RPA1 and PAFAH1B1, are highlighted in gray vertical boxes. Alu pairs that mediated the deletions are denoted by left-facing (− strand) pentagons. Whether an Alu element presents on the plus or minus strand is defined according to the UCSC genome browser (GRCh37/hg19 assembly). Pentagons filled with different colors indicate different Alu families. (B, C) Representative aCGH log2 plot and breakpoint junction sequence of individual 30 with an interstitial deletion. Breakpoint junction sequence is aligned to the proximal and distal genomic references and color-matched. Microhomology at the breakpoint is indicated in red. (D) Proposed rearrangement of Alu–Alu-mediated deletion in individual 30. Array plots and breakpoint sequences of remaining interstitial deletions are shown in Supplementary Material, Figure S1.
Table 1.
Features of the CNVs studied in 39 subjects with 17p13.3 rearrangements
| Individuala | CNV type | CNV size (Mb) | Distal repeat | Proximal repeat | Microhomology (bp)b | Notesc |
|---|---|---|---|---|---|---|
| 10 | DEL | 0.595 | AluSg/− | AluYk4/− | 17 | Alu–Alu (82.2%) |
| 15 | DEL | 0.746 | AluYk4/− | AluY/− | 43 | Alu–Alu (90.6%) |
| 19 | DEL | 0.611 | AluY/− | AluYb8/ − | 32 | Alu–Alu (88.6%) |
| 30 | DEL | 0.563 | AluSq/− | AluSp/− | 27 | Alu–Alu (86.24%) |
| BAB3106 | DEL | 0.545 | – | – | 6 | NHEJ/MMEJ |
| 1 | DUP | 0.512 | AluSp/+ | AluSp/+ | 52 | Alu–Alu (89.94%) |
| 3 | DUP | 0.742 | – | L2/+ | 4 | MMEJ/FoSTeS/MMBIR |
| 5 | DUP | 15.161 | L1PA5/− | L1PA5/− | 17 | LINE/LINE |
| 7 | DUP | 0.37 | AluSx1/+ | – | 6 | MMEJ/FoSTeS/MMBIR |
| 8 | DUP | 0.455 | AluJr/+ | AluSx1/+ | 6 | Alu–Alu (78.2%) |
| 9 | DUP | 0.206 | – | – | N | MMEJ/FoSTeS/MMBIR |
| 14 | DUP | 0.921 | AluSx/− | AluSz/− | 3 | Alu–Alu (84.1%) |
| 21 | DUP | 0.146 | AluSx/+ | – | 2 | MMEJ/FoSTeS/MMBIR |
| 22 | DUP | 0.341 | AluY/− | AluYk4/− | 46 | Alu–Alu (90.16%) |
| 26 | DUP | 0.018 | AluSq/+ | AluY/+ | 33 | Alu–Alu (87.37%) |
| 29 | DUP | 0.879 | AluSq2/− | AluSx3/+ | 2 | MMEJ/FoSTeS/MMBIR |
| 31 | DUP | 0.028 | AluSq2/+ | LIMB4/+ | 2 | MMEJ/FoSTeS/MMBIR |
| 36 | DUP | 0.413 | – | AluSx/+ | 3 | MMEJ/FoSTeS/MMBIR |
| BAB3054 | DUP | 0.092 | AluSg/− | AluY/− | 19 | Alu–Alu (84.21%) |
| 2_1 | DUP–NML–DUP | Complex | AluY/+ | AluSc8/+ | 29 | Alu–Alu (91.5%) |
| 2_2 | DUP–NML–DUP | Complex | AluSc/+ | AluSc/+ | 9 | Alu–Alu (91.5%) |
| 4_1 | DUP–NML–INV/DUP | Complex | AluY/− | AluSx/+ | 6 | FoSTeS/MMBIR |
| 4_2 | DUP–NML–INV/DUP | Complex | AluSg/− | AluSc/+ | 34 | Alu–Alu (89.13%) |
| 6_1 | DUP–NML–INV/DUP | Complex | AluY/− | AluY/+ | 39 | Alu–Alu (89.6%) |
| 6_2 | DUP–NML–INV/DUP | Complex | AluY/− | L2b/+ | N | FoSTeS/MMBIR |
| BAB3886_1 | DUP–NML–INV/DUP | Complex | AluY/− | AluSc5/+ | 29 | Alu–Alu (87.91%) |
| BAB3886_2 | DUP–NML–INV/DUP | Complex | – | L2/− | N | FoSTeS/MMBIR |
| 27_1 | DUP-TRP/INV-DUP | Complex | AluJb/+ | – | N | FoSTeS/MMBIR |
| 27_2 | DUP-TRP/INV-DUP | Complex | LTR8/+ | L1MC2/+ | 2 | FoSTeS/MMBIR |
| 28_1 | DUP-TRP/INV-DUP | Complex | – | – | 2 | FoSTeS/MMBIR |
| 28_2 | DUP-TRP/INV-DUP | Complex | AluSg/− | AluSg/+ | 21 | Alu–Alu (84.69%) |
| 33_1 | DUP-TRP/INV-DUP | Complex | AluSx3/− | AluSz/+ | 25 | Alu–Alu (85.14%) |
| 33_2 | DUP-TRP/INV-DUP | Complex | – | (TGTG)n/+ | 2 | FoSTeS/MMBIR |
| K2_1 | DUP-TRP-DUP | Complex | AluSz/− | AluY/− | 5 | Alu–Alu (76.32%) |
| K2_2 | DUP-TRP-DUP | Complex | AluSx/ − | AluSq/− | 25 | Alu–Alu (83.16%) |
| 18 | TRP | 0.011 | AluSq/− | AluSg/− | 30 | Alu–Alu (82.01%) |
| 20 | TRP | 0.811 | AluSx1/+ | – | 1 | FoSTeS/MMBIR |
| 23_1 | Complex | Complex | MER103C/+ | AluSg4/− | N | FoSTeS/MMBIR |
| 23_2 | Complex | Complex | – | – | 2 | FoSTeS/MMBIR |
| 23_3 | Complex | Complex | – | LIMC4a/− | N | FoSTeS/MMBIR |
| 23_4 | Complex | Complex | – | – | N | FoSTeS/MMBIR |
| 23_5 | Complex | Complex | AluSx/+ | LIPREC2/+ | N | FoSTeS/MMBIR |
| 23_6 | Complex | Complex | MARNA/+ | LIME4a/+ | N | FoSTeS/MMBIR |
| 24 | Complex | Complex | AluY/− | AluSq2/− | 4 | Parental pericentric inversion |
| 17_1 | DEL-NML-DEL | 0.545 | – | – | 6 | FoSTeS/MMBIR |
| 17_2 | DEL-NML-DEL | 0.007 | AluY/− | – | N | FoSTeS/MMBIR |
| 11 | Terminal DEL | 2.92 | N/A | L1MA4 | 0 | Telomeric healing |
| 12 | Terminal DEL | 5.06 | N/A | AluSc/− | 3 | Telomeric healing |
| 25d | Terminal DEL | 2.574 | N/A | N/A | N/A | |
| 35 | Terminal DEL | 1.964 | N/A | – | 4 | Telomeric healing |
| 38 | Terminal DEL | 1.379 | N/A | – | 2 | Telomeric healing |
| BAB3291 | Terminal DEL | 2.46 | N/A | AluJo/+ | 2 | Telomeric healing |
| K1 | Terminal DEL | 2.46 | N/A | AluSp/+ | 1 | Telomeric healing |
Abbreviations: /+, repeat locates on plus strand; /−, repeat locates on negative strand; Alu–Alu, Alu–Alu-mediated rearrangement; DEL, deletion; DUP, duplication; MMBIR, microhomology-mediated break-induced replication; FoSTeS, fork stalling and template switching; NHEJ, non-homologous end joining; MMEJ, microhomology-mediated end joining; N/A, not applicable.
aFor individuals with complex CNVs, there may be more than one breakpoint junctions aligned.
bNumber of bp of microhomology sequence not shown due to multiple template switches (indicated by N). For detailed breakpoint junction sequences alignment, see supplementary figures.
cAlu–Alu, Alu–Alu-mediated rearrangement as defined by the generation of a chimeric Alu hybrid; Similarity of the sequence between Alu pairs that generate the chimeric Alu is also listed (also see Fig. 7).
dWe could not map the breakpoint of individual 25 with a terminal deletion, and therefore its breakpoint is unknown, as indicated by N/A.
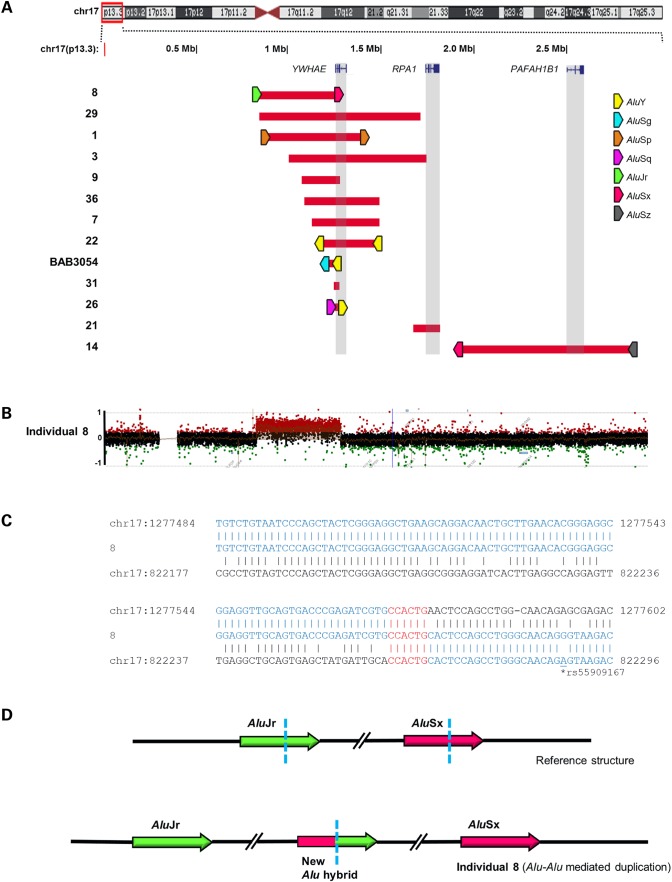
Within the 17p13.3 region, 13 of the CNVs were tandem duplications, ranging from 0.018 to 0.921 Mb in size (Fig. 2, Supplementary Material, Fig. S2 and Table 1). In addition to variation in size, these duplications that varied in both genomic position and gene content demonstrated no evidence for recurrent events. The majority of the duplications (11 out of 13) involved YWHAE only, whereas one duplication (in individual 14) encompassed PAFAH1B1. Two duplications (in individuals 3 and 21) involved RPA1, increased dosage of which may result in the increased genomic instability associated with 17p13.3 duplication syndrome (29). Six of the 13 tandem duplications (in individuals 8, 1, 22, BAB3054, 26 and 14) had breakpoint junctions located in the directly oriented pairs of Alu repetitive elements, generating chimeric Alu upon tandem duplication (Fig. 2, Supplementary Material, Fig. S2 and Table 1). The remaining duplications (in individuals 29, 3, 9, 36, 7, 31 and 21) had 2–6 bp microhomology at breakpoint junctions. Notably, duplication in individual 9 may involve a replication slippage during the rearrangement process (Supplementary Material, Fig. S2A). In contrast to Alu–Alu-mediated CNVs, the two Alu repeats located at each side of the breakpoint junction of DNA from individual 29 lie in opposite orientation in the haploid human reference (Table 1), which is unlikely to result in an Alu–Alu-mediated tandem duplication (Supplementary Material, Fig. S2D). A large interstitial duplication case was found in individual 5, spanning 15.16 Mb from 17p12 to 17p13.3. This rearrangement appeared to be generated by LINE–LINE-mediated recombination (LINE, long interspersed nuclear element. Supplementary Material, Fig. S2M) (22,30).
Figure 2.
Interstitial duplications of 17p13.3. (A) Duplications were plotted by exact genomic coordinates according to breakpoint junction sequencing results using the UCSC genome browser custom track function. Red blocks represent duplications. Upper panel shows an ideogram of human chromosome 17 with the 17p13.3 region involved highlighted in the red box. Three dosage-sensitive genes in 17p13.3 duplication syndromes, YWHAE, RPA1 and PAFAH1B1, are highlighted in gray vertical boxes. Alu pairs that mediated the duplications are denoted by right-facing (+ strand) or left-facing (− strand) pentagons. Pentagons filled with different colors indicate different Alu families. (B, C) Representative aCGH log2 plot and breakpoint junction sequence of individual 8 with tandem duplication. Breakpoint junction sequence is aligned to the proximal and distal genomic references and color-matched. Microhomology at the breakpoint is indicated in red. (D) Proposed rearrangement of Alu–Alu-mediated duplication in individual 8. Array plots and breakpoint sequences of remaining tandem duplications are shown in Supplementary Material, Figure S2. Note that individual 5 with a tandem duplication spanning 15.16 Mb from 17p12 to 17p13.3 is not listed here due to its much larger size and one breakpoint mapping in a different cytogenetic and genomic region. Array plot and breakpoint sequences of individual 5 are also shown in Supplementary Material, Figure S2.
Complex rearrangements of 17p13.3: duplication–normal–duplication
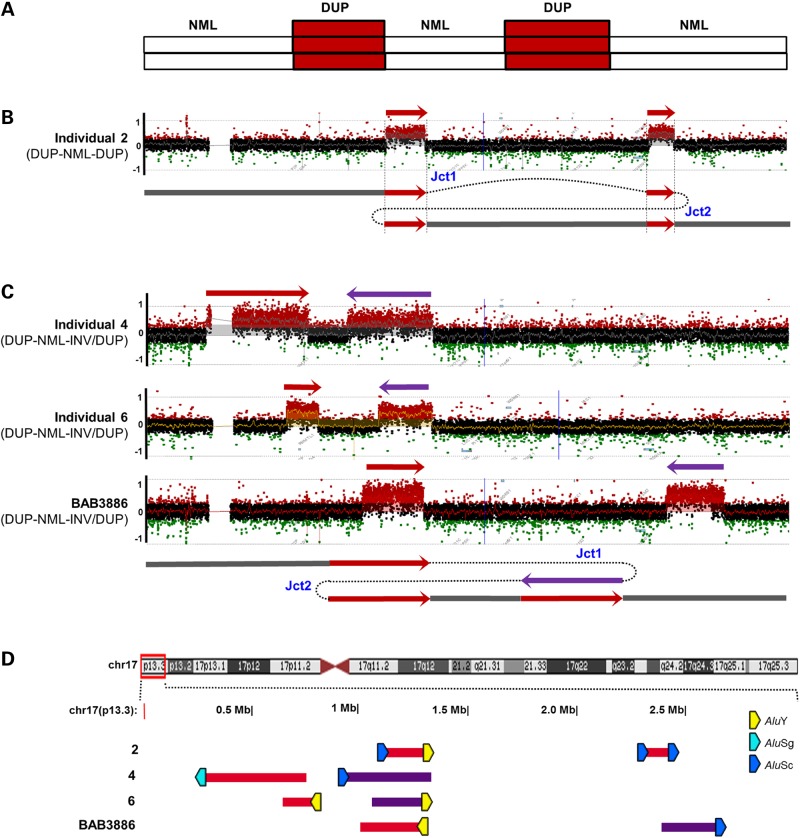
Among the 13 individuals with complex rearrangements (individuals 2, 4, 6, 17, 18, 20, 23, 24, 27, 28, 33, K2 and BAB3886; see Table 1), 4 had array comparative genomic hybridization (aCGH) results consistent with two duplications separated by a copy-number neutral region (individuals 2, 4, 6 and BAB3886; Fig. 3A). Breakpoint junction analysis further revealed that DNA from individual 2 had a duplication–normal–duplication (DUP–NML–DUP) pattern, whereas the remaining three had one inverted duplication (DUP–NML–INV/DUP) (Fig. 3).
Figure 3.
Complex rearrangements distinguished by a DUP–NML–DUP pattern of 17p13.3. (A) Illustration of the DUP–NML–DUP rearrangement pattern from aCGH results. (B) Array CGH log2 plot and proposed generation mechanism of DUP–NML–DUP rearrangement in individual 2. (C) Array CGH log2 plot and proposed generation mechanism of DUP–NML–INV/DUP rearrangement in individuals 4, 6 and BAB3886. (D) CNVs were plotted by exact genomic coordinates according to breakpoint junction sequencing results using the UCSC genome browser custom track function. Red blocks, duplication; Purple blocks, inverted duplication. Upper panel shows an ideogram of human chromosome 17 with the 17p13.3 region involved highlighted in the red box. Alu pairs that mediated the complex rearrangements are denoted by right-facing (+ strand) or left-facing (− strand) pentagons. Pentagons filled with different colors indicate different Alu families. Breakpoint sequences of all the DUP–NML–DUP individuals are shown in Supplementary Material, Figure S3. Jct1 and Jct2, junction 1 and junction 2.
There are two potential explanations for the CNVs in individual 2 based on the breakpoint junction analysis. The first mechanism requires a combination of two alleles: the first allele is a tandem duplication of chromosome 17, whereas the second is a deletion of the homologous chromosome spanning the gap between the segments with increased copy number (Supplementary Material, Fig. S3A). The duplication or deletion could be inherited, however this hypothesis cannot be tested due to the absence of parental DNA. A second and simpler explanation of the mechanism requires only one allele generated by two template switches (Fig. 3B). Both the breakpoint junctions in individual 2 were situated in directly oriented pairs of Alu repetitive elements, generating chimeric Alu structures (Supplementary Material, Fig. S3A and Table 1).
Breakpoint junction analysis demonstrated a DUP–NML–INV/DUP pattern in individuals 4, 6 and BAB3886 (Fig. 3C, Supplementary Material, Figs S3 and S4). Based on the breakpoint junctions, the centromeric end of the first duplication was connected to that of the second duplication (junction 1 in Fig. 3C and Supplementary Material, Fig. S4A), whereas the telomeric ends of the two duplications were also connected (junction 2 in Fig. 3C and Supplementary Material, Fig. S4A), resulting in one of the duplications being inverted (Supplementary Material, Figs S3 and S4) with respect to the genomic reference. There are two possible mechanisms to generate this type of DUP–NML–INV/DUP rearrangement. The first mechanism is a one-step event and requires two iterative template switches that occur on the reference genome haplotype (Fig. 3C). During replication, a template switch occurs to the opposite strand, creating breakpoint junction 1 and an inversion, followed by a switch back to the original strand to avoid the generation of a potential acentric chromosome (Supplementary Material, Fig. S4A) (31,32). The second potential mechanism for DUP–NML–INV/DUP requires the postulation of a tandem duplication on an inversion haplotype and reflects a potential ‘artifact’ of the appearance of a DUP–NML–DUP structure from aCGH given the haploid human genome reference and the absence of its incorporation of copy-number neutral structural variations (SVs), specifically inversion haplotypes (17). This latter model necessitates that the subject inherits an inversion haplotype and then acquires a simple tandem duplication of part of the inverted segment (Supplementary Material, Fig. S4A). According to this mechanism, one of the two parents would also harbor the inversion and breakpoint junction 1 (Supplementary Material, Fig. S4A). However, long-range polymerase chain reaction (PCR) analysis of BAB3887 and BAB3888 (the mother and father of BAB3886, respectively) failed to detect such a postulated junction (Supplementary Material, Fig. S4B). Additionally, if such an inversion were to have occurred, a third potential breakpoint junction would be formed (breakpoint junction 3 in Supplementary Material, Fig. S4A). Multiple PCR reactions failed to amplify such a breakpoint junction in any of the DUP–NML–INV/DUP individuals (data not shown). Therefore, the first one-step mechanism is the most likely explanation for the DUP–NML–INV/DUP pattern of rearrangement, however other potential rearrangement mechanisms may also be possible. Breakpoint junction analysis demonstrated that breakpoint junction 2 in individual 4 and breakpoint junction 1 in individuals 6 and BAB3886 appeared to be Alu–Alu-mediated. The remaining breakpoints showed microhomology (6 bp microhomology of breakpoint junction 1 in individual 4) or templated insertion from nearby sequences (breakpoint junctions 2 in individuals 6 and BAB3886) (Fig. 3D, Supplementary Material, Fig. S3B–D and Table 1).
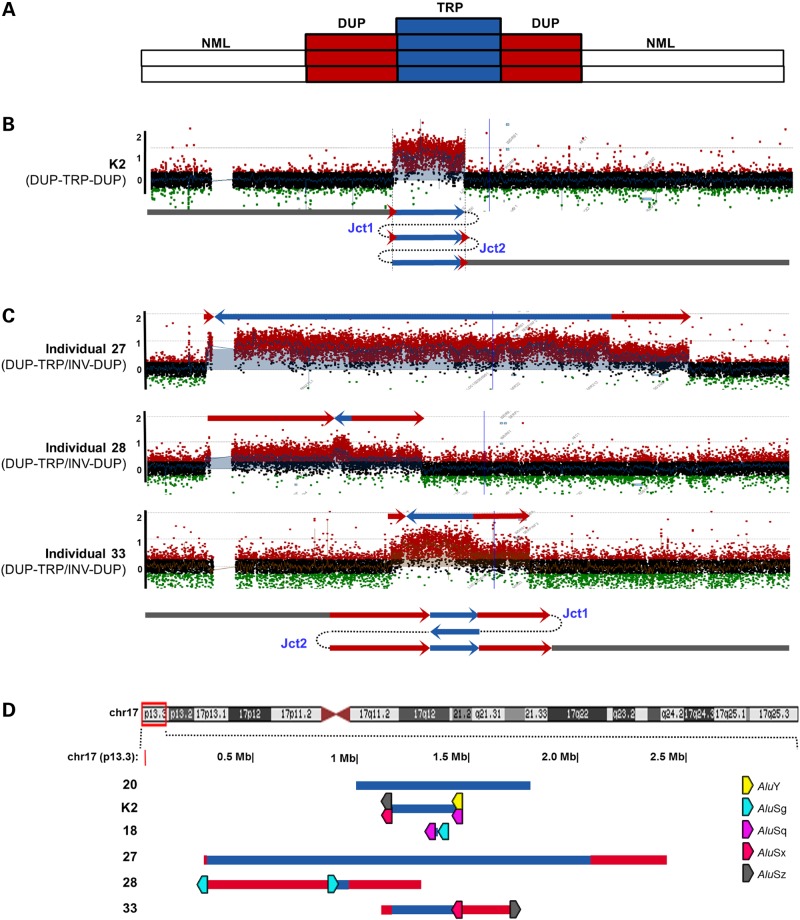
Triplication generated through Alu–Alu-mediated events
Among the 13 individuals with complex rearrangements, three had simple triplications (individuals 20, K2 and 18), whereas three showed DUP–TRP–DUP patterns (individuals 27, 28 and 33) by aCGH (Fig. 4A and Supplementary Material, Fig. S5). The largest triplication found in individual 20 (0.81 Mb) involved YWHAE and part of RPA1. The triplication in K2 (0.32 Mb) involved only YWHAE, and the smallest triplication (in individual 18, 11 kb) harbored sequence of neither of the two genes critical for causing MDS. As generation of a triplication potentially requires two breakpoint junctions, both the breakpoint junctions of K2 were mapped to nucleotide resolution and both appeared to be Alu–Alu-mediated with directly oriented Alu pairs (Fig. 4B and Supplementary Material, Fig. S5B). Upon careful inspection of the two breakpoints in K2, both sides of the triplicated region contained short duplicated sequences (2413 bp on the distal side and 792 bp on the proximal side), therefore it was further classified as a DUP–TRP–DUP CGR (Supplementary Material, Fig. S5B). For the remaining two individuals with triplications, we were able to resolve one out of the two predicted breakpoint junctions for each. The mapped breakpoint junction in individual 18 indicated an Alu–Alu-mediated event due to the generation of a new Alu hybrid, whereas the mapped breakpoint junction in individual 20 showed 1 bp microhomology (Supplementary Material, Fig. S5A and C). Similar to K2 junctional sequences, the mapped breakpoints in individuals 20 and 18 involved distal and proximal references on the same strand, suggesting that triplicated segments were in the same orientation embedded in duplicated segments.
Figure 4.
Triplications of 17p13.3. (A) Illustration of the DUP–TRP–DUP rearrangement pattern from aCGH results. (B) Array CGH log2 plot and proposed generation mechanism of triplication in individual K2. Note that both sides of the triplicated region contained short duplicated sequences (2413 bp in the distal side and 792 bp in the proximal side) as indicated by small red arrows. (C). Array CGH log2 plot and proposed generation mechanism of DUP-TRP/INV-DUP pattern rearrangement in individuals 27, 28 and 33. (D) CNVs were plotted by exact genomic coordinates according to breakpoint junction sequencing results using the UCSC genome browser custom track function. Red blocks, duplication; Blue blocks, triplication. Upper panel shows an ideogram of human chromosome 17 with the 17p13.3 region involved highlighted in the red box. Alu pairs that mediated the complex rearrangements are denoted by right-facing (+ strand) or left-facing (− strand) pentagons. Pentagons filled with different colors indicate different Alu families. Note that the triplication in individual K2 was generated by two breakpoints that both were Alu–Alu-mediated, and the two pairs of Alu repetitive elements are AluSz/AluY and AluSx/AluSq. Array plots and breakpoint sequences of additional cases with triplication are shown in Supplementary Material, Figure S5. Jct1 and Jct2, junction 1 and junction 2.
Based on the aCGH results, DNA from individuals 27, 28 and 33 contained triplication flanked by duplications (Fig. 4C). Breakpoint junction analysis revealed a consistent pattern for all three rearrangements—breakpoint junction 1 connects the centromeric ends of both the large duplication rearrangement and the triplication, whereas junction 2 joins the telomeric ends of these two, indicating that the triplicated region in the middle was inverted, resulting in a DUP-TRP/INV-DUP pattern (33). Breakpoint junction 2 in individual 28 and breakpoint 1 in individual 33 appeared to be mediated by opposite-oriented Alu repeats (or inverted repeats) that led to the inverted triplication (Supplementary Material, Fig. S5D–F). A 2 bp microhomology was found at breakpoint junction 2 in individual 27, breakpoint junction 1 in individual 28 and breakpoint junction 2 in individual 33 (Supplementary Material, Fig. S5D–F).
Additional complex rearrangement patterns
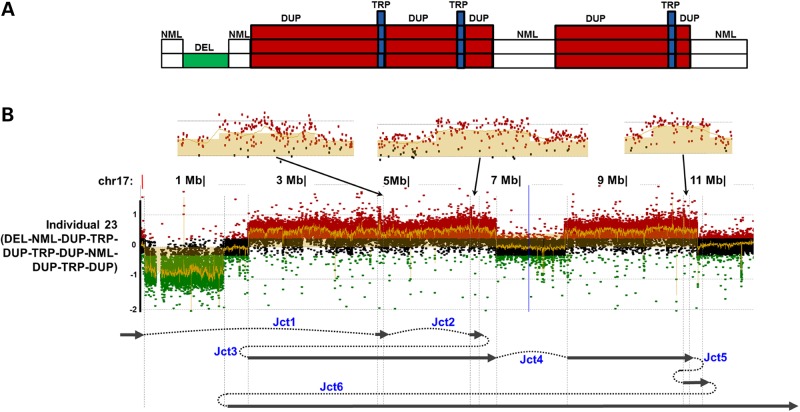
The most complex rearrangement in this study was observed in individual 23. The rearrangement exhibited a ‘DEL–NML–DUP–TRP–DUP–TRP–DUP–NML–DUP–TRP–DUP’ pattern encompassing a significant portion of the short arm of chromosome 17 from 17p13.1 to 17p13.3 with multiple copy-number transition states and potentially six breakpoint junctions (Fig. 5 and Supplementary Material, Fig. S6A). We successfully mapped these breakpoint junctions to nucleotide resolution. Notably, although the copy-number changes were in 17p13.3, none of the breakpoint junctions had both distal and proximal reference sequences present at 17p13.3, but rather at 17p13.1 or 17p13.2. In addition, none of the six breakpoint junctions appeared to be Alu–Alu-mediated, potentially indicating a reduced probability to form Alu–Alu-mediated CNVs outside the 17p13.3 region. Microhomology at breakpoint junction 2 (2 bp) and small templated insertions at breakpoint junction 1 (47 bp), junction 3 (15 bp), junction 4 (22 bp, mapped to another locus of chromosome 17), junction 5 (1 bp) and junction 6 (9 bp) were observed (Supplementary Material, Fig. S6A). The complexity of the rearrangement pattern and the small templated insertions at the breakpoints indicated a possible chromoanasynthesis/chromothripsis event (32,34) that was likely mediated by multiple template switches during the replication process (35).
Figure 5.
Individual 23 with complex rearrangement. (A) Illustration of the DEL–NML–DUP–TRP–DUP–TRP–DUP–NML–DUP–TRP–DUP rearrangement pattern in individual 23 from aCGH results. (B) Array CGH log2 plot and proposed generation mechanism of the complex rearrangement in individual 23. Enlarged array plots of the three small triplicated regions are shown on the top.
Individual 17 had an interstitial 0.55 Mb deletion. Upon careful inspection, a 7 kb region with normal copy number was found within and near the distal end of the deletion (Supplementary Material, Fig. S6B). Breakpoint analysis revealed a joining of the distal and proximal normal regions of the 0.55 Mb deletion (junction 1 of individual 17) with microhomology (6 bp) observed. Analysis of the other breakpoint (junction 2) indicated that a deletion occurred on one homolog of the chromosome, whereas a tandem duplication of 7 kb was present on the other homolog, resulting in an overall DEL–NML–DEL pattern from the array.
Individual 24 exhibited a 4.1 Mb duplication at 17pter and a 0.69 Mb deletion at 17qter. Breakpoint junction analysis indicated that the duplicated 17pter region inverted and joined to the truncated chromosome 17 long arm (Supplementary Material, Fig. S6C). Similar rearrangements have previously been observed on chromosome 17, and a pericentric inversion in one of the parents likely underlies the rearrangement (36).
Telomeric healing of terminal deletions
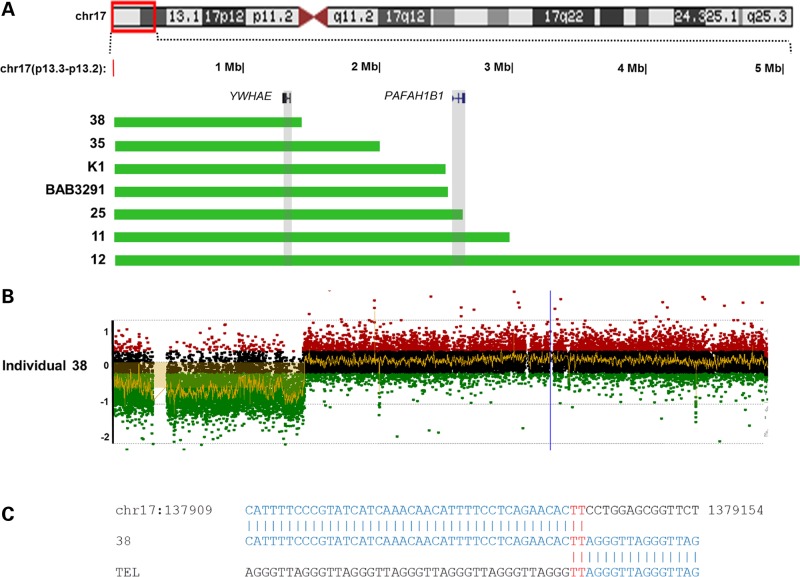
Seven individuals in this study harbored terminal deletions of 17p13.3, ranging in size from 1.38 to 5.06 Mb (individuals 11, 12, 38, 25, 35, BAB3291 and K1, Fig. 6 and Supplementary Material, Fig. S7). All seven deletions contained YWHAE, and the deletions in individuals 25, 11 and 12 also involved PAFAH1B1. Individual 12 contained a larger 5.06 Mb terminal deletion spanning from 17p13.2 to 17p13.3 (Fig. 6 and Supplementary Material, Fig. S7F). Breakpoint junctions showed addition of telomere repeats (TTAGGG)n to the truncated chromosome 17, indicating telomerase-mediated healing events in all except individual 25 in whom we could not amplify the junction fragment (26,37).
Figure 6.
Terminal 17p deletion cases. (A) CNVs were plotted by exact genomic coordinates according to breakpoint junction sequencing results using the UCSC genome browser custom track function (except for individual 25 without breakpoint junctions mapped to nucleotide resolution, which were plotted according to aCGH result, with resolution higher than 200 bp per probe). Green blocks represent deletion. Upper panel shows an ideogram of human chromosome 17 with the 17p13.2–17p13.3 region involved highlighted in the red box. Two critical genes in 17p13.3 deletion syndromes, YWHAE and PAFAH1B1, are highlighted in gray boxes. (B, C) Representative array CGH log2 plot and breakpoint junction sequence of individual 38 with terminal deletion. Array plots and breakpoint sequences of additional cases with terminal deletion are shown in Supplementary Material, Figure S7.
Alu–Alu-mediated rearrangements
The mutational signatures present at each of the breakpoints are shown in Table 1. Strikingly, 47% of the breakpoints within the 17p13.3 region (with both distal and proximal reference sequence present within 17p13.3) were mediated by Alu elements as defined by the generation of a chimeric Alu hybrid. If one restricts the analysis to interstitial rearrangements, 53% of breakpoint junctions were Alu–Alu-mediated. Using the reference human genome and RepeatMasker annotations, we found that 30.0% of the 17p13.3 region is comprised of Alu elements; this is markedly higher than the 9.95% observed on a genome-wide scale and even higher than the 18.4% elsewhere on chromosome 17.
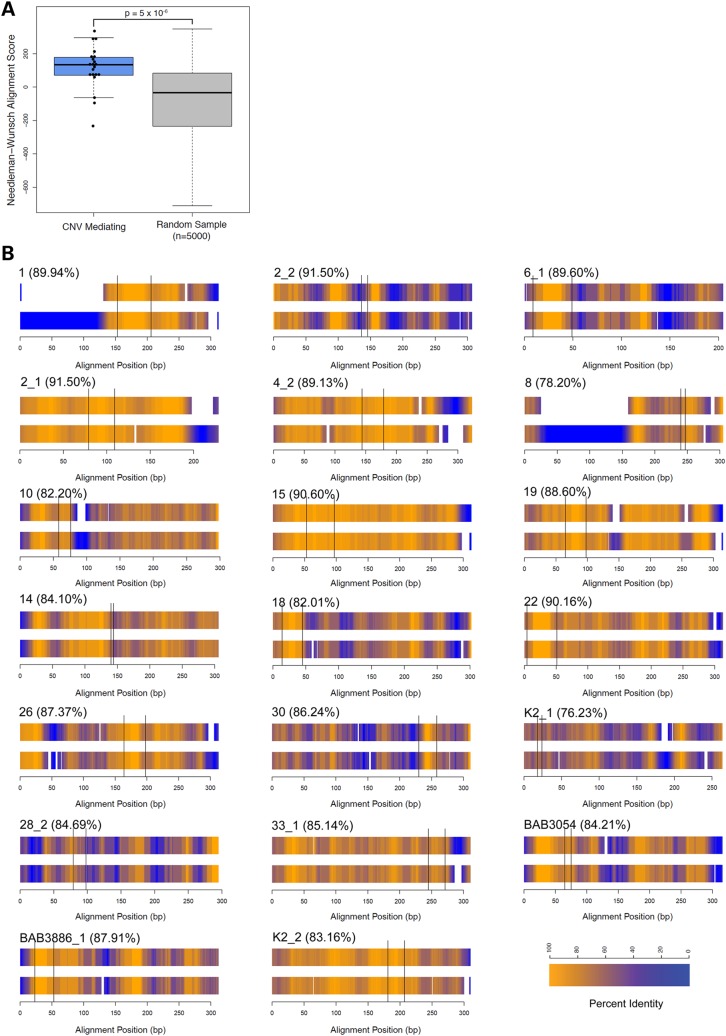
We hypothesized that particular Alu elements in the region are providing sequence similarity to serve as a substrate for a template switch or alternatively for a homologous or homeologous recombination. To investigate this, we performed Smith–Waterman alignments of the pairs of Alu actually mediating the CNVs identified in this study and compared their alignment scores to randomly selected Alu pairs in the region. We found that CNV-mediating pairs align significantly better than randomly selected pairs (Wilcoxon signed rank test, P = 5 × 10−6, Fig. 7A). Sequence alignment of the Alu pairs revealed microhomology/crossover locations throughout the Alu structures (Fig. 7B). Notably, the Alu elements that mediated the 17p13.3 CNVs more frequently utilized different families of Alu elements, within a given Alu–Alu substrate pair (13 out of 20 [65%]). The recombination join point or breakpoint junction often occurred in a region of higher identity, although some breakpoint junctions (e.g. K2_1) localize to homeologous regions with a number of mismatches.
Figure 7.
Analysis of Alu–Alu-mediated breakpoints. (A) Smith–Waterman alignment scores of Alu pairs that mediate CNVs are significantly higher than randomly selected pairs in the region (P = 5 × 10−6, Wilcoxon signed rank test). (B) Alu pairs aligned for each Alu-mediated breakpoint. The telomeric Alu is on top in each pairing. Heat map shading depicts a 20 bp sliding window of identity. Microhomology/crossover spots are flanked by black lines, which occupy various positions within the Alu pairs. The percent identity for each aligned pair, excluding gaps, is also presented.
Discussion
Although the first discovery of the connection between MDS and 17p13.3 deletion occurred three decades ago (38), the mechanisms responsible for the generation of the CNVs within the 17p13.3 region have remained poorly understood. Much of the literature regarding CNVs in this region is focused on genotype–phenotype correlations. Studies that attempted to explore mechanisms for CNV formation have mainly focused on deletions (10,26), whereas investigations of duplications and CGRs have rarely been reported (27).
Here, we gain mechanistic insights by exploring the patterns of copy-number changes, as evidenced by deviations from the normal diploid state and determining the breakpoint junction sequences in 39 individuals, 5 with interstitial deletion, 14 with tandem duplication, 7 with terminal deletion and 13 with CGRs. Among the 13 CGRs, we observed diverse patterns of copy-number changes: four DUP–NML–DUP structures, six CGRs with triplicated segments (of which four were flanked by duplications), one inverted duplication/translocation, one DEL–NML–DEL and one with an extremely complex ‘DEL–NML–DUP–TRP–DUP–TRP–DUP–NML–DUP–TRP–DUP’ pattern. In all but three individuals, we mapped the breakpoint junctions to nucleotide resolution by PCR and Sanger sequencing. The information obtained from these breakpoint junction sequences allowed us to surmise potential rearrangement mechanisms involved in 17p13.3.
Subtelomeric regions exhibit increased genome instability and are associated with various pathogenic CNVs that can cause autism, intellectual disability, neurocognitive deficits and multiple congenital anomalies. Subtelomeric rearrangements have been identified on every chromosome (39,40), including some regions with well-described genotype–phenotype correlations and mutational mechanisms, most notably, deletions on 1p (MIM #607872) (41,42), 9q (MIM #158170) (43,44) and 22q (MIM #606232) (45). Subtelomeric CNVs are typically non-recurrent and have unique breakpoints that are generated through mechanisms other than NAHR (44,46). The 17p13.3 CNVs analyzed in this study reproduced these characteristics—the CNVs varied in size and none of them shared identical breakpoint junctions and exhibited the hallmarks of various mutational mechanisms (Table 1). Due to the non-recurrent nature and variable size of 17p13.3 CNVs, it is understandable that diverse phenotypes have been observed, as different rearrangements involve different dosage-sensitive genes (26,27,47).
Unlike some subtelomeric genomic regions that have abundant segmental duplications (48), the 17p13.3 region contains few of these repeats, but rather consists of gene-rich sequences. Although lacking long repeat sequences (low-copy repeats or LCRs), 17p13.3 is highly enriched for Alu repetitive elements. This region contains ∼30% Alu sequences in the human genome reference (hg19/GRCh37), compared with ∼18% for chromosome 17 and ∼10% at the genome-wide scale. This places the 17p13.3 region in the 98.8th percentile of Alu repeats in a single region when compared with equally-sized regions genome-wide.
The abundance of Alu repetitive elements provides a potential substrate for the generation of CNVs. Among the breakpoint junctions mapped to nucleotide resolution within 17p13.3, breakpoint junctions in 80% of the interstitial deletions (4 out of 5), 46% of the tandem duplications (6 out of 13) and 50% of the CGRs (10 out of 20) were mediated by Alu–Alu rearrangement. In total, 47% of the 17p13.3 breakpoint junctions mapped to nucleotide resolution appeared to be Alu–Alu-mediated (Table 2). In addition, at the 17p13.3 region, 30 of 35 (86%) subjects (36 of 43 sequenced breakpoints, 84%) had at least one Alu or other transposable element involved in facilitating rearrangement formation (Table 1). Previous studies of randomly selected non-recurrent CNVs identified in subjects referred for clinical diagnostic testing but not ascertained for a particular phenotype have shown Alu–Alu-mediated rearrangements in ∼7–29.2% of CNVs (49,50). Thus, rearrangements of the 17p13.3 region appear to be significantly enriched in Alu–Alu-mediated CNVs (Fisher's exact test, P < 0.005). Therefore, we speculate that the 17p13.3 region favors Alu–Alu-mediated rearrangement mechanisms, in turn leading to non-recurrent CNVs resulting in phenotypically diverse syndromes.
Table 2.
Mutational signatures of the 17p13.3 breakpoints for CNVs characterized in this studya
| Signature | CGR | Simple DEL or DUP | Percentage |
|---|---|---|---|
| Alu–Alu | 10b | 10c | 46.5% (20 out of 43) |
| FoSTeS/MMBIR | 10d | 8e | 41.9% (18 out of 43)f |
| NHEJ or MMEJ | 0 | ||
| Telomeric healing | N/A | 5g | 11.6% (5 out of 43) |
| Total | 20 (46.5%) | 23 (53.5%) | 100% (43 out of 43) |
Abbreviations: DEL, deletion; DUP, duplication; CGR, complex genomic rearrangement; Alu–Alu, Alu–Alu-mediated rearrangement; MMBIR, microhomology-mediated break-induced replication; FoSTeS, fork stalling and template switching; NHEJ, non-homologous end joining; MMEJ, microhomology-mediated end joining; N/A, not applicable.
aOnly breakpoint junctions mapped within the 17p13.3 region and at nucleotide resolution are shown (e.g. breakpoint junctions in individuals 5, 12, 23 and 24 are not considered due to either or both proximal and distal sequences of their breakpoints mapping outside the 17p13.3 region).
bThese 10 breakpoint junctions include: 2_1, 2_2, 4_2, 6_1, BAB3886_1, 28_2, 33_1, K2_1, K2_2 and 18 as shown in Table 1.
cThese 10 breakpoint junctions include: 10, 15, 19, 30, 1, 8, 14, 22, 26 and BAB3054 as shown in Table 1.
dThese 10 breakpoint junctions include: 4_1, 6_2, BAB3886_2, 27_1, 27_2, 28_1, 33_2, 20, 17_1 and 17_2 as shown in Table 1.
eThese eight breakpoint junctions include: BAB3106, 3, 7, 9, 21, 29, 31 and 36 as shown in Table 1. These eight breakpoint junctions in simple deletions or duplications may be generated through FoSTeS/MMBIR, NHEJ or MMEJ. Refer to the ‘Results’ section for detailed descriptions of each breakpoint.
fPercentage of these types of mutational signatures, FoSTeS/MMBIR, NHEJ and MMEJ are calculated together.
gThese five breakpoint junctions include: 11, 35, 38, BAB3291 and K1 as shown in Table 1.
Breakpoint junctions in simple deletions and duplications that occurred without the formation of a new Alu hybrid but showed 2–6 bp microhomology (in BAB3106 with an interstitial deletion and in individuals 29, 3, 9, 36, 7, 31 and 21 with tandem duplications) may be generated through various mechanisms (Table 2). Non-homologous end joining (NHEJ) (51,52) or microhomology-mediated end joining (MMEJ) (53,54) could potentially explain the simple deletion in BAB3106. Although NHEJ has been proposed to generate duplications (55), due to a gain of genomic material, MMEJ or replicative mechanisms, e.g. FoSTeS/MMBIR, may more readily explain the increased copy number that can accompany a replicative process (23,24). Other mechanisms with the capacity to generate deletions or duplications, e.g. single-strand annealing (SSA) and synthesis-dependent strand annealing, require long uninterrupted repeat sequences for strand invasion, which was absent from the breakpoint junctions we observed in this study (56).
The molecular mechanism of Alu–Alu-mediated rearrangement has long been proposed to be due to NAHR (9,57). This was partially because simple deletions and duplications were thought to be much more frequently observed with Alu–Alu events, and complex rearrangements were rarely described (7). However, in this study, we observed that 50% of the breakpoints of CGRs (10 out of 20) within 17p13.3 were Alu–Alu-mediated events. Moreover, the orientation of the Alu pairs—whether directly oriented or in opposite-orientation—determined the overall rearrangement pattern when chimeric Alus were generated. For example, although all individuals 2, 4, 6 and BAB3886 exhibited a ‘DUP–NML–DUP’ pattern of rearrangement from the aCGH results, DNA from individual 2 had both breakpoint junctions mediated by directly oriented Alu pairs, therefore resulting in duplications without inversion. In contrast, in individuals 4, 6 and BAB3886, one of the two breakpoint junctions was mediated by Alu pairs in opposite orientation, resulting in one inverted duplication (Fig. 3 and Supplementary Material, Fig. S3). Similar observations of CNVs with ‘DUP–NML–INV/DUP’ pattern mediated by Alu–Alu rearrangement have been previously reported in other genomic regions (19,32).
Rearrangement patterns consistent with Alu pair orientations were also observed in individuals with triplication. Both breakpoint junctions of the triplication in individual K2 were mediated by directly oriented Alu pairs, whereas in individuals 28 and 33, one of the breakpoint junctions was mediated by Alu pairs in opposite orientation. As a result, the triplication in the former was not inverted, whereas the latter two had an inverted triplicated region (Fig. 4 and Supplementary Material, Fig. S5). The ‘DUP-TRP/INV-DUP’ CNV pattern was originally described at Xq28 (MECP2), and these rearrangements were found to be mostly mediated by inverted long repeats (856 bp–11.3 kb) with at least 98% sequence identity (33). Here, we showed that inverted small repetitive elements like Alus (∼300 bp in length) with ∼85% sequence identity could also generate this type of complex rearrangement with inverted triplication embedded in a duplication (Table 1 and Fig. 7). Additionally, for CGR in individual 27, neither of the breakpoint junctions appeared to be mediated by repetitive elements (Table 1), indicating that the absence of inverted repeats can also generate this type of DUP-TRP/INV-DUP rearrangement product structure.
Recent studies of rearrangement breakpoint junctions determined in samples from subjects with spastic paraplegia 4 (SPG4, MIM #182601) caused by pathogenic deletions or duplications of the SPAST gene demonstrated that 70% (38 out of 54) of CNVs were mediated by directly oriented Alu pairs (15,16). The Alu pairs that mediate the 17p13.3 CNVs in this study have similar characteristics with the Alu pairs that mediate CNVs involving SPAST. The average percent identity for each Alu pair in our study is 86.0% (range 76.2–91.5%; Fig. 7) compared with 82.7% (range 75.8–90.7%) involving SPAST. Notably, the Alu elements that mediated the CNVs in both scenarios more frequently utilized different Alu families within a pair, a finding inconsistent with an NAHR-mediated event (33 out of 39, 85% in the SPG4 study versus 13 out of 20, [65%] in this study) (Table 1). The average Alu–Alu-mediated CNV breakpoint microhomology also had a wider range in our study (mean 26 bp, range 3–52 bp, Table 1) compared with that evaluating CNVs in SPAST (mean 17 bp, range 5–38 bp).
Several mechanisms were proposed to potentially generate Alu-mediated CNVs other than NAHR in the SPG4 study, including MMEJ, SSA, FoSTeS/MMBIR and homeologous recombination. These newly proposed mechanisms were based primarily on the observation that: (i) many of the simple deletions and duplications were Alu–Alu events, but the overwhelming majority formed a recombinant hybrid from substrate constituents representing different Alu family members and (ii) the percent identity of the involved Alu was <90%, inconsistent with an homologous recombination process, but rather consistent with an homeologous and/or microhomology-mediated process (16). In addition, a previous smaller study at the SPAST locus, which investigated the mechanism for deletion of the same exon in multiple unrelated subjects, revealed: (i) different-sized genomic deletions underlying the same exonic loss, (ii) the utilization of different Alu sequence pairs as substrates for the recombinant/hybrid Alu formed and (iii) evidence for potential multiple template switches (15). All the Alu-mediated CNVs in the larger SPG4 study appeared to be simple deletions or duplications generated through directly oriented Alu pairs. The complex nature of the Alu-mediated CGRs with multiple template switches, as we observed in the current study, especially mediated by opposite-oriented Alu pairs, are more consistent with the features we observe in replicative mechanisms. In addition, even apparently simple deletions with breakpoint junctions revealing evidence for Alu–Alu-mediated events can potentially arise from multiple iterative Alu–Alu template switches (15).
One such mechanism is classical break-induced replication (BIR). BIR is initiated by invasion of a single-ended double-strand break into homologous sequence followed by extensive DNA synthesis. BIR has been proposed to be one of the mechanisms responsible for generating non-recurrent CNVs in humans (33,58). Notably, BIR is dependent on the strand invasion and homology search protein Rad51 (59). Previous studies in humans suggested that the minimal efficient processing segment substrates of Rad51 were 300–500 bp with uninterrupted homology (60), which would preclude the use of Alu elements. More recent studies of Rad51− or apparently Rad51-dependent processes, however, questioned these strict limits (61,62). Thus, Rad51 or another of its paralogs in humans (63) may indeed potentially mediate BIR using Alu elements as substrates, although less efficiently, also explaining the rarity of identical Alu–Alu-mediated CNVs. Alternatively, in the MMBIR mechanism, which is proposed to be independent of Rad51, single-stranded DNA could potentially switch to any template with 2–15 bp of shared microhomology (23,64). Whether homeology (such as provided by the Alu elements) helps guide strand invasion during template switching in recombination coupled DNA synthesis is unclear at this time.
Recent studies in the model organism Saccharomyces cerevisiae further support the above hypothesis. Ty elements in yeast contain a large central region flanked by two long terminal repeats (LTRs, or δ elements) (65). These LTRs, either within a Ty element or present as solo LTRs as the product of excision of the central region of Ty elements (66), are analogous to Alu elements in humans, which are present in many copies in the yeast genome and share high sequence homology (67). Ty-mediated BIR followed by DNA double-strand break (DSB) leading to deletions, duplications and translocations are well documented and studied (67–69). By inducing low levels of replicative DNA polymerase and therefore introducing more deletions and duplications in yeast, it was observed that most of the breakpoints of these CNVs were located at Ty elements (70). In budding yeast, it was recently demonstrated that Ty-mediated template switching during BIR/MMBIR was a prominent mechanism causing complex chromosome rearrangements (71). Ty and δ element-mediated multiple template switches resulted in CGRs with multiple copy-number transitions, which was similar to the Alu-mediated CGRs we observed in humans in this study (71).
Overall, our results show that Alu–Alu-mediated rearrangement is a common cause of the non-recurrent CNVs of chromosome 17 at p13.3 that mediate intricate phenotypes causing genomic disorders in humans. Our data suggest that the high concentration of Alu elements in the region serve as a substrate with increased homology/homeology for formation of CNVs. Moreover, we provide evidence that Alu–Alu-mediated mechanisms contribute considerably to the formation of complex CNVs and that the orientation of the Alu pair may determine whether an inversion of a genomic segment is involved, and these mechanisms are unlikely attributed to NAHR. Additional studies of genome-wide Alu elements may identify additional loci that are particularly susceptible to such structural variations.
Materials and Methods
Subjects
Individuals with CNVs in the 17p13.3 region were identified by chromosomal microarray analysis (CMA) clinical testing at Baylor College of Medicine (BCM) Medical Genetics Laboratories. Of the 33 800 individuals tested from 27 May 2008 to 23 December 2013, a total of 39 unrelated subjects were identified with copy-number changes involving known disease genes including PAFAH1B1, YWHAE and bHLHA9, or disease candidate gene RPA1 within the 17p13.3 region. Individuals with interstitial deletions involving PAFAH1B1, but no other disease genes, those with unbalanced translocations or likely benign copy-number gains at the one end of a disease gene, were not included in this study. This study was approved by the Institutional Review Board for Human Subject Research at BCM (IRB No. H-22231 or H-25466). Informed consent was obtained prior to collecting identifiable DNA samples (BAB3054, BAB3106, BAB3291, BAB3886, BAB3887, BAB3888, K1 and K2). The remaining DNA samples were de-identified for breakpoint and mechanistic studies (named individual 1, individual 2, individual 3, etc.).
Clinical CMA
Custom-designed BCM OLIGO V6.5, V7, V8 or V9 oligonucleotide arrays were performed as previously described (72,73). Arrays were designed to specifically interrogate clinically significant regions with an average resolution of 30 kb between probes.
High-density aCGH
To further characterize the CNVs identified by CMA in the 17p13.3 region, two custom, high-density Agilent arrays were designed. A 4 × 180 K array with ∼200 bp per probe coverage spanning 17pter to 17p13.2 and ∼2000 bp per probe coverage spanning the rest of chromosome 17 was used to study individuals 5, 23 and 24. For the remaining individuals, an 8 × 60 K array with ∼150 bp per probe covering the first 6 Mb of chromosome 17p (17pter to 17p13.2) and ∼5000 bp per probe covering the remaining chromosome 17 was used. Hybridization controls were gender-matched (HapMap individual NA10851 as male control and HapMap individual NA15510 as female control). For the anonymous individuals with unknown gender, the male control DNA (HapMap individual NA10851) was used. Scanned array images were processed using Agilent Feature Extraction software (version 10) and extracted files were analyzed using Agilent Genomic Workbench (version 7.0.4.0). Array designs and sequence alignment for breakpoint analysis were based on the February 2009 genome build (GRCh37/hg19 assembly).
Breakpoint junction mapping
To further confirm the CNVs identified by high-density arrays and map the breakpoint junctions, primers flanking the predicted breakpoints were designed, and long-range PCRs were conducted using TaKaRa LA Taq according to the manufacturer's protocol (TaKaRa Bio Company, Cat. No. RR002). Generally, 200 ng of genomic DNA and 0.5 μl of each primer (10 μm) were put into a 25 μl scale PCR reaction mixture containing 2.5 μl 10× LA Taq buffer, 4 μl dNTP mixture (2.5 mm each) and 0.25 μl LA Taq enzyme. Annealing temperature was set at 68°C and extension time was set for 1 min per kb. PCR products were prepared for sequencing using ExoSAP-IT (Affymetrix, Cat. No. 78201) according to the manufacturer's protocol or gel extracted and purified with the Zymoclean Gel DNA Recovery Kit (Zymo Research, Cat. No. D4001). Purified PCR products were then sequenced by Sanger dideoxynucleotide sequencing (BCM Sequencing Core, Houston, TX, USA).
Alu pair analysis
We obtained the annotations for each Alu element in the 17p13.3 region from the RepeatMasker track of the UCSC genome browser (74). We obtained the sequence encoding each Alu (the + or – reference sequence depending on the orientation of the element) using the element annotation and the BSgenome package implemented in the R Statistical Programing Language. We subsequently aligned the Alu sequences to each other using the R Biostrings package and computed the identity as the fraction of identical positions over the total number of aligned positions excluding gaps.
Statistical analysis
To assess how the 17p13.3 region's Alu content compares with the rest of the genome, we adopted a Monte Carlo approach. We randomly selected windows across the genome of the same size as the 17p13.3 region. We rejected windows that overlapped any gaps in the hg19 assembly using the UCSC gaps track, including centromeres and telomeres. We subsequently determined fractional Alu content using the RepeatMasker track. This process was repeated 10 000 times. We next determined the empirical cumulative distribution function of the simulated data and evaluated it at the fractional content of 17p13.3. To assess enrichment in Alu–Alu-mediated CNVs at 17p13.3, we assumed a null hypothesis that the CNVs from previous studies (49,50,75) and those we detected in the current study were sampled from the same distribution. We performed a Fisher's exact test with the pooled data from the previous studies versus our observations.
Authors' Contributions
S.G., W.B. and J.R.L. designed the study. S.G. conducted the experiments. S.G., B.Y., C.M.B.C. and J.R.L. analyzed the data. I.M.C. performed the bioinformatics analysis. S.G. and J.R.L. wrote the manuscript. B.Y., I.M.C., C.R.B., C.M.B.C., S.C.S.N. and C.A.B. revised the manuscript. A.P., C.A.B., S.C.S.N., A.E., C.A.S., P.S., S.W.C. and W.B. identified the cases through clinical diagnosis. All authors read and approved the final manuscript.
Supplementary Material
Funding
We thank the patients and their families for participating in this study. I.M.C. is a fellow of the Baylor College of Medicine Medical Scientist Training Program (T32GM007330). He was supported by a fellowship from the National Institute of Neurological Disorders and Stroke (F31 NS083159). C.R.B. is an HHMI Fellow of the Damon Runyon Cancer Research Foundation (DRG 2155-13). S.C.S.N. is a recipient of the DDCF Clinical Scientist Development Award. This work was supported in part by grants from the US National Institute of Neurological Disorders and Stroke (R01NS058529), National Institute of General Medical Sciences (R01GM106373) and National Human Genome Research Institute (NHGRI)/National Heart Lung and Blood Institute (NHLBI) (U54 HG006542) to the Baylor-Hopkins Center for Mendelian Genomics. This work was also supported by the Doris Duke Charitable Foundation (DDCF) (Grant #2013095). The project described was supported by Baylor College of Medicine IDDRC from the Eunice Kennedy Shriver National Institute of Child Health & Human Development (Grant Number 1 U54 HD083092).
Supplementary Material
Acknowledgements
The content is solely the responsibility of the authors and does not necessarily represent the official views of the Eunice Kennedy Shriver National Institute of Child Health & Human Development or the National Institutes of Health.
Conflict of Interest statement. J.R.L. has stock ownership in 23andMe, is a paid consultant for Regeneron Pharmaceuticals, has stock options in Lasergen, Inc. and is a co-inventor on multiple United States and European patents related to molecular diagnostics for inherited neuropathies, eye diseases and bacterial genomic fingerprinting. The Department of Molecular and Human Genetics at Baylor College of Medicine derives revenue from the chromosomal microarray analysis (CMA) and clinical exome sequencing offered in the Baylor Miraca Genetics Laboratory (BMGL: http://www.bmgl.com/BMGL/Default.aspx).
References
- 1.Batzer M.A., Deininger P.L. (2002) Alu repeats and human genomic diversity. Nat. Rev. Genet., 3, 370–379. [DOI] [PubMed] [Google Scholar]
- 2.Lander E.S., Linton L.M., Birren B., Nusbaum C., Zody M.C., Baldwin J., Devon K., Dewar K., Doyle M., FitzHugh W., et al. (2001) Initial sequencing and analysis of the human genome. Nature, 409, 860–921. [DOI] [PubMed] [Google Scholar]
- 3.Deininger P. (2011) Alu elements: know the SINEs. Genome Biol., 12, 236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Witherspoon D.J., Zhang Y., Xing J., Watkins W.S., Ha H., Batzer M.A., Jorde L.B. (2013) Mobile element scanning (ME-Scan) identifies thousands of novel Alu insertions in diverse human populations. Genome Res., 23, 1170–1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shen M.R., Batzer M.A., Deininger P.L. (1991) Evolution of the master Alu gene(s). J. Mol. Evol., 33, 311–320. [DOI] [PubMed] [Google Scholar]
- 6.Wheeler D.A., Srinivasan M., Egholm M., Shen Y., Chen L., McGuire A., He W., Chen Y.J., Makhijani V., Roth G.T., et al. (2008) The complete genome of an individual by massively parallel DNA sequencing. Nature, 452, 872–876. [DOI] [PubMed] [Google Scholar]
- 7.Deininger P.L., Batzer M.A. (1999) Alu repeats and human disease. Mol. Genet. Metab., 67, 183–193. [DOI] [PubMed] [Google Scholar]
- 8.Brooks E.M., Branda R.F., Nicklas J.A., O'Neill J.P. (2001) Molecular description of three macro-deletions and an Alu–Alu recombination-mediated duplication in the HPRT gene in four patients with Lesch–Nyhan disease. Mutat. Res., 476, 43–54. [DOI] [PubMed] [Google Scholar]
- 9.Sen S.K., Han K., Wang J., Lee J., Wang H., Callinan P.A., Dyer M., Cordaux R., Liang P., Batzer M.A. (2006) Human genomic deletions mediated by recombination between Alu elements. Am. J. Hum. Genet., 79, 41–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mei D., Lewis R., Parrini E., Lazarou L.P., Marini C., Pilz D.T., Guerrini R. (2008) High frequency of genomic deletions—and a duplication—in the LIS1 gene in lissencephaly: implications for molecular diagnosis. J. Med. Genet., 45, 355–361. [DOI] [PubMed] [Google Scholar]
- 11.Franke G., Bausch B., Hoffmann M.M., Cybulla M., Wilhelm C., Kohlhase J., Scherer G., Neumann H.P. (2009) Alu–Alu recombination underlies the vast majority of large VHL germline deletions: molecular characterization and genotype–phenotype correlations in VHL patients. Hum. Mutat., 30, 776–786. [DOI] [PubMed] [Google Scholar]
- 12.Shlien A., Baskin B., Achatz M.I., Stavropoulos D.J., Nichols K.E., Hudgins L., Morel C.F., Adam M.P., Zhukova N., Rotin L., et al. (2010) A common molecular mechanism underlies two phenotypically distinct 17p13.1 microdeletion syndromes. Am. J. Hum. Genet., 87, 631–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Strout M.P., Marcucci G., Bloomfield C.D., Caligiuri M.A. (1998) The partial tandem duplication of ALL1 (MLL) is consistently generated by Alu-mediated homologous recombination in acute myeloid leukemia. Proc. Natl Acad. Sci. USA, 95, 2390–2395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.O'Neil J., Tchinda J., Gutierrez A., Moreau L., Maser R.S., Wong K.K., Li W., McKenna K., Liu X.S., Feng B., et al. (2007) Alu elements mediate MYB gene tandem duplication in human T-ALL. J. Exp. Med., 204, 3059–3066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boone P.M., Liu P., Zhang F., Carvalho C.M., Towne C.F., Batish S.D., Lupski J.R. (2011) Alu-specific microhomology-mediated deletion of the final exon of SPAST in three unrelated subjects with hereditary spastic paraplegia. Genet. Med., 13, 582–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boone P.M., Yuan B., Campbell I.M., Scull J.C., Withers M.A., Baggett B.C., Beck C.R., Shaw C.J., Stankiewicz P., Moretti P., et al. (2014) The Alu-rich genomic architecture of SPAST predisposes to diverse and functionally distinct disease-associated CNV alleles. Am. J. Hum. Genet., 95, 143–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang F., Khajavi M., Connolly A.M., Towne C.F., Batish S.D., Lupski J.R. (2009) The DNA replication FoSTeS/MMBIR mechanism can generate genomic, genic and exonic complex rearrangements in humans. Nat. Genet., 41, 849–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang F., Seeman P., Liu P., Weterman M.A., Gonzaga-Jauregui C., Towne C.F., Batish S.D., De Vriendt E., De Jonghe P., Rautenstrauss B., et al. (2010) Mechanisms for nonrecurrent genomic rearrangements associated with CMT1A or HNPP: rare CNVs as a cause for missing heritability. Am. J. Hum. Genet., 86, 892–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Szafranski P., Golla S., Jin W., Fang P., Hixson P., Matalon R., Kinney D., Bock H.G., Craigen W., Smith J.L., et al. (2014) Neurodevelopmental and neurobehavioral characteristics in males and females with CDKL5 duplications. Eur. J. Hum. Genet., doi:10.1038/ejhg.2014.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carvalho C.M., Pehlivan D., Ramocki M.B., Fang P., Alleva B., Franco L.M., Belmont J.W., Hastings P.J., Lupski J.R. (2013) Replicative mechanisms for CNV formation are error prone. Nat. Genet., 45, 1319–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hedges D.J., Deininger P.L. (2007) Inviting instability: transposable elements, double-strand breaks, and the maintenance of genome integrity. Mutat. Res., 616, 46–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kidd J.M., Graves T., Newman T.L., Fulton R., Hayden H.S., Malig M., Kallicki J., Kaul R., Wilson R.K., Eichler E.E. (2010) A human genome structural variation sequencing resource reveals insights into mutational mechanisms. Cell, 143, 837–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hastings P.J., Ira G., Lupski J.R. (2009) A microhomology-mediated break-induced replication model for the origin of human copy number variation. PLoS Genet., 5, e1000327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee J.A., Carvalho C.M., Lupski J.R. (2007) A DNA replication mechanism for generating nonrecurrent rearrangements associated with genomic disorders. Cell, 131, 1235–1247. [DOI] [PubMed] [Google Scholar]
- 25.Bruno D.L., Anderlid B.M., Lindstrand A., van Ravenswaaij-Arts C., Ganesamoorthy D., Lundin J., Martin C.L., Douglas J., Nowak C., Adam M.P., et al. (2010) Further molecular and clinical delineation of co-locating 17p13.3 microdeletions and microduplications that show distinctive phenotypes. J. Med. Genet., 47, 299–311. [DOI] [PubMed] [Google Scholar]
- 26.Nagamani S.C., Zhang F., Shchelochkov O.A., Bi W., Ou Z., Scaglia F., Probst F.J., Shinawi M., Eng C., Hunter J.V., et al. (2009) Microdeletions including YWHAE in the Miller–Dieker syndrome region on chromosome 17p13.3 result in facial dysmorphisms, growth restriction, and cognitive impairment. J. Med. Genet., 46, 825–833. [DOI] [PubMed] [Google Scholar]
- 27.Bi W., Sapir T., Shchelochkov O.A., Zhang F., Withers M.A., Hunter J.V., Levy T., Shinder V., Peiffer D.A., Gunderson K.L., et al. (2009) Increased LIS1 expression affects human and mouse brain development. Nat. Genet., 41, 168–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Luk H.M., Wong V.C., Lo I.F., Chan K.Y., Lau E.T., Kan A.S., Tang M.H., Tang W.F., She W.M., Chu Y.W., et al. (2014) A prenatal case of split-hand malformation associated with 17p13.3 triplication—a dilemma in genetic counseling. Eur. J. Med. Genet., 57, 81–84. [DOI] [PubMed] [Google Scholar]
- 29.Outwin E., Carpenter G., Bi W., Withers M.A., Lupski J.R., O'Driscoll M. (2011) Increased RPA1 gene dosage affects genomic stability potentially contributing to 17p13.3 duplication syndrome. PLoS Genet., 7, e1002247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Han K., Lee J., Meyer T.J., Remedios P., Goodwin L., Batzer M.A. (2008) L1 recombination-associated deletions generate human genomic variation. Proc. Natl Acad. Sci. USA, 105, 19366–19371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Carvalho C.M., Bartnik M., Pehlivan D., Fang P., Shen J., Lupski J.R. (2012) Evidence for disease penetrance relating to CNV size: Pelizaeus–Merzbacher disease and manifesting carriers with a familial 11 Mb duplication at Xq22. Clin. Genet., 81, 532–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu P., Erez A., Nagamani S.C., Dhar S.U., Kolodziejska K.E., Dharmadhikari A.V., Cooper M.L., Wiszniewska J., Zhang F., Withers M.A., et al. (2011) Chromosome catastrophes involve replication mechanisms generating complex genomic rearrangements. Cell, 146, 889–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Carvalho C.M., Ramocki M.B., Pehlivan D., Franco L.M., Gonzaga-Jauregui C., Fang P., McCall A., Pivnick E.K., Hines-Dowell S., Seaver L.H., et al. (2011) Inverted genomic segments and complex triplication rearrangements are mediated by inverted repeats in the human genome. Nat. Genet., 43, 1074–1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stephens P.J., Greenman C.D., Fu B., Yang F., Bignell G.R., Mudie L.J., Pleasance E.D., Lau K.W., Beare D., Stebbings L.A., et al. (2011) Massive genomic rearrangement acquired in a single catastrophic event during cancer development. Cell, 144, 27–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maher C.A., Wilson R.K. (2012) Chromothripsis and human disease: piecing together the shattering process. Cell, 148, 29–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yokoyama Y., Narahara K., Teraoka M., Koyama K., Seino Y., Yagi S., Konishi T., Miyawaki T. (1997) Cryptic pericentric inversion of chromosome 17 detected by fluorescence in situ hybridization study in familial Miller–Dieker syndrome. Am. J. Med. Genet., 71, 236–237. [DOI] [PubMed] [Google Scholar]
- 37.Flint J., Craddock C.F., Villegas A., Bentley D.P., Williams H.J., Galanello R., Cao A., Wood W.G., Ayyub H., Higgs D.R. (1994) Healing of broken human chromosomes by the addition of telomeric repeats. Am. J. Hum. Genet., 55, 505–512. [PMC free article] [PubMed] [Google Scholar]
- 38.Stratton R.F., Dobyns W.B., Airhart S.D., Ledbetter D.H. (1984) New chromosomal syndrome: Miller–Dieker syndrome and monosomy 17p13. Hum. Genet., 67, 193–200. [DOI] [PubMed] [Google Scholar]
- 39.Shao L., Shaw C.A., Lu X.Y., Sahoo T., Bacino C.A., Lalani S.R., Stankiewicz P., Yatsenko S.A., Li Y., Neill S., et al. (2008) Identification of chromosome abnormalities in subtelomeric regions by microarray analysis: a study of 5380 cases. Am. J. Med. Genet. A, 146A, 2242–2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ballif B.C., Sulpizio S.G., Lloyd R.M., Minier S.L., Theisen A., Bejjani B.A., Shaffer L.G. (2007) The clinical utility of enhanced subtelomeric coverage in array CGH. Am. J. Med. Genet. A, 143A, 1850–1857. [DOI] [PubMed] [Google Scholar]
- 41.Ballif B.C., Gajecka M., Shaffer L.G. (2004) Monosomy 1p36 breakpoints indicate repetitive DNA sequence elements may be involved in generating and/or stabilizing some terminal deletions. Chromosome Res., 12, 133–141. [DOI] [PubMed] [Google Scholar]
- 42.Ballif B.C., Yu W., Shaw C.A., Kashork C.D., Shaffer L.G. (2003) Monosomy 1p36 breakpoint junctions suggest pre-meiotic breakage-fusion-bridge cycles are involved in generating terminal deletions. Hum. Mol. Genet., 12, 2153–2165. [DOI] [PubMed] [Google Scholar]
- 43.Yatsenko S.A., Brundage E.K., Roney E.K., Cheung S.W., Chinault A.C., Lupski J.R. (2009) Molecular mechanisms for subtelomeric rearrangements associated with the 9q34.3 microdeletion syndrome. Hum. Mol. Genet., 18, 1924–1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yatsenko S.A., Hixson P., Roney E.K., Scott D.A., Schaaf C.P., Ng Y.T., Palmer R., Fisher R.B., Patel A., Cheung S.W., et al. (2012) Human subtelomeric copy number gains suggest a DNA replication mechanism for formation: beyond breakage-fusion-bridge for telomere stabilization. Hum. Genet., 131, 1895–1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bonaglia M.C., Giorda R., Beri S., De Agostini C., Novara F., Fichera M., Grillo L., Galesi O., Vetro A., Ciccone R., et al. (2011) Molecular mechanisms generating and stabilizing terminal 22q13 deletions in 44 subjects with Phelan/McDermid syndrome. PLoS Genet., 7, e1002173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Luo Y., Hermetz K.E., Jackson J.M., Mulle J.G., Dodd A., Tsuchiya K.D., Ballif B.C., Shaffer L.G., Cody J.D., Ledbetter D.H., et al. (2011) Diverse mutational mechanisms cause pathogenic subtelomeric rearrangements. Hum. Mol. Genet., 20, 3769–3778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Armour C.M., Bulman D.E., Jarinova O., Rogers R.C., Clarkson K.B., DuPont B.R., Dwivedi A., Bartel F.O., McDonell L., Schwartz C.E., et al. (2011) 17p13.3 microduplications are associated with split-hand/foot malformation and long-bone deficiency (SHFLD). Eur. J. Hum. Genet., 19, 1144–1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Linardopoulou E.V., Williams E.M., Fan Y., Friedman C., Young J.M., Trask B.J. (2005) Human subtelomeres are hot spots of interchromosomal recombination and segmental duplication. Nature, 437, 94–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vissers L.E., Bhatt S.S., Janssen I.M., Xia Z., Lalani S.R., Pfundt R., Derwinska K., de Vries B.B., Gilissen C., Hoischen A., et al. (2009) Rare pathogenic microdeletions and tandem duplications are microhomology-mediated and stimulated by local genomic architecture. Hum. Mol. Genet., 18, 3579–3593. [DOI] [PubMed] [Google Scholar]
- 50.Verdin H., D'Haene B., Beysen D., Novikova Y., Menten B., Sante T., Lapunzina P., Nevado J., Carvalho C.M., Lupski J.R., et al. (2013) Microhomology-mediated mechanisms underlie non-recurrent disease-causing microdeletions of the FOXL2 gene or its regulatory domain. PLoS Genet., 9, e1003358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Inoue K., Osaka H., Thurston V.C., Clarke J.T., Yoneyama A., Rosenbarker L., Bird T.D., Hodes M.E., Shaffer L.G., Lupski J.R. (2002) Genomic rearrangements resulting in PLP1 deletion occur by nonhomologous end joining and cause different dysmyelinating phenotypes in males and females. Am. J. Hum. Genet., 71, 838–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lieber M.R., Ma Y., Pannicke U., Schwarz K. (2003) Mechanism and regulation of human non-homologous DNA end-joining. Nat. Rev. Mol. Cell Biol., 4, 712–720. [DOI] [PubMed] [Google Scholar]
- 53.Kent T., Chandramouly G., McDevitt S.M., Ozdemir A.Y., Pomerantz R.T. (2015) Mechanism of microhomology-mediated end-joining promoted by human DNA polymerase theta. Nat. Struct. Mol. Biol., 22, 230–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McVey M., Lee S.E. (2008) MMEJ repair of double-strand breaks (director's cut): deleted sequences and alternative endings. Trends Genet., 24, 529–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Carvalho C.M., Zhang F., Lupski J.R. (2011) Structural variation of the human genome: mechanisms, assays, and role in male infertility. Syst. Biol. Reprod. Med., 57, 3–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Paques F., Haber J.E. (1999) Multiple pathways of recombination induced by double-strand breaks in Saccharomyces cerevisiae. Microbiol. Mol. Biol. Rev., 63, 349–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ade C., Roy-Engel A.M., Deininger P.L. (2013) Alu elements: an intrinsic source of human genome instability. Curr. Opin. Virol., 3, 639–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Malkova A., Ira G. (2013) Break-induced replication: functions and molecular mechanism. Curr. Opin. Genet. Dev., 23, 271–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Saini N., Ramakrishnan S., Elango R., Ayyar S., Zhang Y., Deem A., Ira G., Haber J.E., Lobachev K.S., Malkova A. (2013) Migrating bubble during break-induced replication drives conservative DNA synthesis. Nature, 502, 389–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Reiter L.T., Hastings P.J., Nelis E., De Jonghe P., Van Broeckhoven C., Lupski J.R. (1998) Human meiotic recombination products revealed by sequencing a hotspot for homologous strand exchange in multiple HNPP deletion patients. Am. J. Hum. Genet., 62, 1023–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mundia M.M., Desai V., Magwood A.C., Baker M.D. (2014) Nascent DNA synthesis during homologous recombination is synergistically promoted by the rad51 recombinase and DNA homology. Genetics, 197, 107–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shuvarikov A., Campbell I.M., Dittwald P., Neill N.J., Bialer M.G., Moore C., Wheeler P.G., Wallace S.E., Hannibal M.C., Murray M.F., et al. (2013) Recurrent HERV-H-mediated 3q13.2-q13.31 deletions cause a syndrome of hypotonia and motor, language, and cognitive delays. Hum. Mutat., 34, 1415–1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Park J.Y., Yoo H.W., Kim B.R., Park R., Choi S.Y., Kim Y. (2008) Identification of a novel human Rad51 variant that promotes DNA strand exchange. Nucleic Acids Res., 36, 3226–3234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hastings P.J., Lupski J.R., Rosenberg S.M., Ira G. (2009) Mechanisms of change in gene copy number. Nat. Rev. Genet., 10, 551–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cameron J.R., Loh E.Y., Davis R.W. (1979) Evidence for transposition of dispersed repetitive DNA families in yeast. Cell, 16, 739–751. [DOI] [PubMed] [Google Scholar]
- 66.Gafner J., Philippsen P. (1980) The yeast transposon Ty1 generates duplications of target DNA on insertion. Nature, 286, 414–418. [DOI] [PubMed] [Google Scholar]
- 67.Hoang M.L., Tan F.J., Lai D.C., Celniker S.E., Hoskins R.A., Dunham M.J., Zheng Y., Koshland D. (2010) Competitive repair by naturally dispersed repetitive DNA during non-allelic homologous recombination. PLoS Genet., 6, e1001228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lemoine F.J., Degtyareva N.P., Lobachev K., Petes T.D. (2005) Chromosomal translocations in yeast induced by low levels of DNA polymerase a model for chromosome fragile sites. Cell, 120, 587–598. [DOI] [PubMed] [Google Scholar]
- 69.Argueso J.L., Westmoreland J., Mieczkowski P.A., Gawel M., Petes T.D., Resnick M.A. (2008) Double-strand breaks associated with repetitive DNA can reshape the genome. Proc. Natl Acad. Sci. USA, 105, 11845–11850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Song W., Dominska M., Greenwell P.W., Petes T.D. (2014) Genome-wide high-resolution mapping of chromosome fragile sites in Saccharomyces cerevisiae. Proc. Natl Acad. Sci. USA, 111, E2210–E2218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Anand R.P., Tsaponina O., Greenwell P.W., Lee C.S., Du W., Petes T.D., Haber J.E. (2014) Chromosome rearrangements via template switching between diverged repeated sequences. Genes Dev., 28, 2394–2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Boone P.M., Bacino C.A., Shaw C.A., Eng P.A., Hixson P.M., Pursley A.N., Kang S.H., Yang Y., Wiszniewska J., Nowakowska B.A., et al. (2010) Detection of clinically relevant exonic copy-number changes by array CGH. Hum. Mutat., 31, 1326–1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ou Z., Kang S.H., Shaw C.A., Carmack C.E., White L.D., Patel A., Beaudet A.L., Cheung S.W., Chinault A.C. (2008) Bacterial artificial chromosome-emulation oligonucleotide arrays for targeted clinical array-comparative genomic hybridization analyses. Genet. Med., 10, 278–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kent W.J., Sugnet C.W., Furey T.S., Roskin K.M., Pringle T.H., Zahler A.M., Haussler D. (2002) The human genome browser at UCSC. Genome Res., 12, 996–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Campbell I.M., Yuan B., Robberecht C., Pfundt R., Szafranski P., McEntagart M.E., Nagamani S.C., Erez A., Bartnik M., Wisniowiecka-Kowalnik B., et al. (2014) Parental somatic mosaicism is underrecognized and influences recurrence risk of genomic disorders. Am. J. Hum. Genet., 95, 173–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.