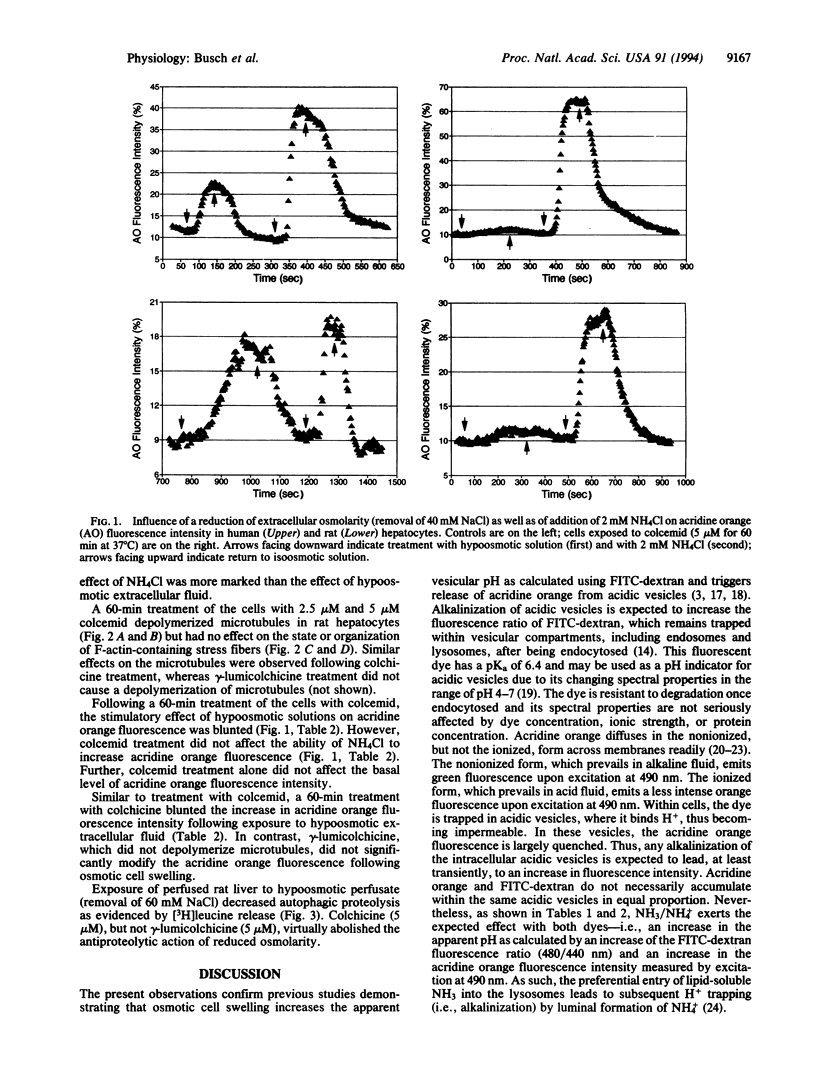
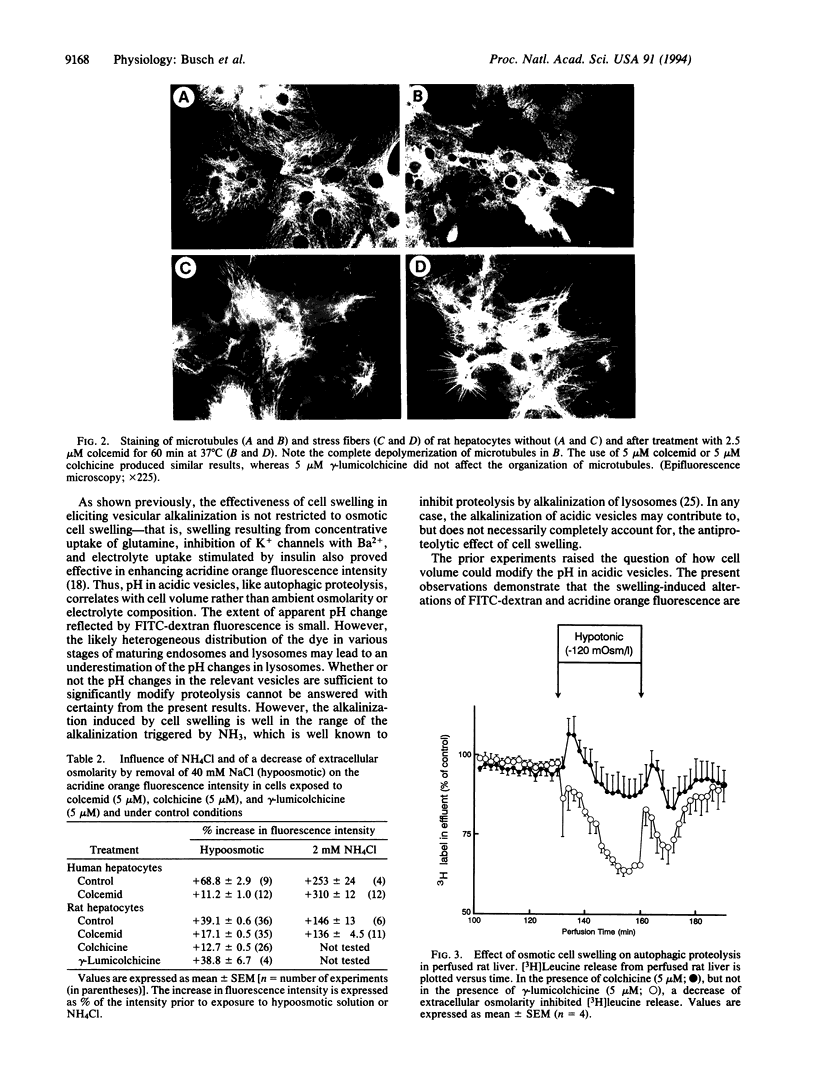
Abstract
Cell swelling is shown to induce an increase in acridine orange fluorescence intensity, an effect pointing to the alkalinization of acidic vesicles. Since autophagic hepatic proteolysis is accomplished by pH-sensitive proteinases within acidic lysosomes, this effect may contribute to the well-known inhibitory effect of cell swelling on proteolysis. In the present study, the role of microtubules in volume-dependent alterations of pH in acidic vesicles of rat and human hepatocytes was studied. Colcemid and colchicine were used to depolymerize microtubules and vesicular pH was monitored using two different fluorescent dyes, fluorescein isothiocyanate conjugated-dextran and acridine orange. Colcemid and colchicine, but not the inactive stereoisomer gamma-lumicolchicine, blunted the increase of pH during osmotic cell swelling. The alkalinization of acidic vesicles by NH4Cl was not significantly modified by colcemid or colchicine, indicating that the vesicles were still sensitive to alkalinizing procedures other than cell swelling. Further, colchicine, but not gamma-lumicolchicine, inhibited the antiproteolytic action of osmotic cell swelling. The present observations point to an involvement of the microtubule network in the link of cell volume, lysosomal pH, and proteolysis.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berry M. N., Friend D. S. High-yield preparation of isolated rat liver parenchymal cells: a biochemical and fine structural study. J Cell Biol. 1969 Dec;43(3):506–520. doi: 10.1083/jcb.43.3.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinkley B. R., Fistel S. H., Marcum J. M., Pardue R. L. Microtubules in cultured cells; indirect immunofluorescent staining with tubulin antibody. Int Rev Cytol. 1980;63:59–95. doi: 10.1016/s0074-7696(08)61757-x. [DOI] [PubMed] [Google Scholar]
- Hansen C. A., Mah S., Williamson J. R. Formation and metabolism of inositol 1,3,4,5-tetrakisphosphate in liver. J Biol Chem. 1986 Jun 25;261(18):8100–8103. [PubMed] [Google Scholar]
- Häussinger D., Hallbrucker C., vom Dahl S., Lang F., Gerok W. Cell swelling inhibits proteolysis in perfused rat liver. Biochem J. 1990 Nov 15;272(1):239–242. doi: 10.1042/bj2720239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Häussinger D., Lang F. Cell volume in the regulation of hepatic function: a mechanism for metabolic control. Biochim Biophys Acta. 1991 Dec 12;1071(4):331–350. doi: 10.1016/0304-4157(91)90001-d. [DOI] [PubMed] [Google Scholar]
- Häussinger D., Saha N., Hallbrucker C., Lang F., Gerok W. Involvement of microtubules in the swelling-induced stimulation of transcellular taurocholate transport in perfused rat liver. Biochem J. 1993 Apr 15;291(Pt 2):355–360. doi: 10.1042/bj2910355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Häussinger D., Stoll B., vom Dahl S., Theodoropoulos P. A., Markogiannakis E., Gravanis A., Lang F., Stournaras C. Effect of hepatocyte swelling on microtubule stability and tubulin mRNA levels. Biochem Cell Biol. 1994 Jan-Feb;72(1-2):12–19. doi: 10.1139/o94-003. [DOI] [PubMed] [Google Scholar]
- Lake J. R., Van Dyke R. W., Scharschmidt B. F. Acidic vesicles in cultured rat hepatocytes. Identification and characterization of their relationship to lysosomes and other storage vesicles. Gastroenterology. 1987 May;92(5 Pt 1):1251–1261. [PubMed] [Google Scholar]
- Matteoni R., Kreis T. E. Translocation and clustering of endosomes and lysosomes depends on microtubules. J Cell Biol. 1987 Sep;105(3):1253–1265. doi: 10.1083/jcb.105.3.1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meijer A. J., Gimpel J. A., Deleeuw G. A., Tager J. M., Williamson J. R. Role of anion translocation across the mitochondrial membrane in the regulation of urea synthesis from ammonia by isolated rat hepatocytes. J Biol Chem. 1975 Oct 10;250(19):7728–7738. [PubMed] [Google Scholar]
- Mithieux G., Rousset B. Identification of a lysosome membrane protein which could mediate ATP-dependent stable association of lysosomes to microtubules. J Biol Chem. 1989 Mar 15;264(8):4664–4668. [PubMed] [Google Scholar]
- Mortimore G. E., Pösö A. R. Intracellular protein catabolism and its control during nutrient deprivation and supply. Annu Rev Nutr. 1987;7:539–564. doi: 10.1146/annurev.nu.07.070187.002543. [DOI] [PubMed] [Google Scholar]
- Ohkuma S., Poole B. Fluorescence probe measurement of the intralysosomal pH in living cells and the perturbation of pH by various agents. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3327–3331. doi: 10.1073/pnas.75.7.3327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olmsted J. B., Borisy G. G. Microtubules. Annu Rev Biochem. 1973;42:507–540. doi: 10.1146/annurev.bi.42.070173.002451. [DOI] [PubMed] [Google Scholar]
- Parker J. C. In defense of cell volume? Am J Physiol. 1993 Nov;265(5 Pt 1):C1191–C1200. doi: 10.1152/ajpcell.1993.265.5.C1191. [DOI] [PubMed] [Google Scholar]
- Roos A., Boron W. F. Intracellular pH. Physiol Rev. 1981 Apr;61(2):296–434. doi: 10.1152/physrev.1981.61.2.296. [DOI] [PubMed] [Google Scholar]
- Sabolić I., Burckhardt G. Proton pathways in rat renal brush-border and basolateral membranes. Biochim Biophys Acta. 1983 Oct 12;734(2):210–220. doi: 10.1016/0005-2736(83)90119-0. [DOI] [PubMed] [Google Scholar]
- Seglen P. O. Inhibitor of protein degradation formed during incubation of isolated rat hepatocytes in a cell culture medium. Its identification as ammonia. Exp Cell Res. 1977 Jun;107(1):207–217. doi: 10.1016/0014-4827(77)90402-5. [DOI] [PubMed] [Google Scholar]
- Sies H., Summer K. H. Hydroperoxide-metabolizing systems in rat liver. Eur J Biochem. 1975 Sep 15;57(2):503–512. doi: 10.1111/j.1432-1033.1975.tb02325.x. [DOI] [PubMed] [Google Scholar]
- Sies H. The use of perfusion of liver and other organs for the study of microsomal electron-transport and cytochrome P-450 systems. Methods Enzymol. 1978;52:48–59. doi: 10.1016/s0076-6879(78)52005-3. [DOI] [PubMed] [Google Scholar]
- Thomas J. A., Buchsbaum R. N., Zimniak A., Racker E. Intracellular pH measurements in Ehrlich ascites tumor cells utilizing spectroscopic probes generated in situ. Biochemistry. 1979 May 29;18(11):2210–2218. doi: 10.1021/bi00578a012. [DOI] [PubMed] [Google Scholar]
- Van Dyke R. W., Hornick C. A., Belcher J., Scharschmidt B. F., Havel R. J. Identification and characterization of ATP-dependent proton transport by rat liver multivesicular bodies. J Biol Chem. 1985 Sep 15;260(20):11021–11026. [PubMed] [Google Scholar]
- Völkl H., Busch G. L., Häussinger D., Lang F. Alkalinization of acidic cellular compartments following cell swelling. FEBS Lett. 1994 Jan 24;338(1):27–30. doi: 10.1016/0014-5793(94)80110-x. [DOI] [PubMed] [Google Scholar]
- Völkl H., Friedrich F., Häussinger D., Lang F. Effect of cell volume on Acridine Orange fluorescence in hepatocytes. Biochem J. 1993 Oct 1;295(Pt 1):11–14. doi: 10.1042/bj2950011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warnock D. G., Reenstra W. W., Yee V. J. Na+/H+ antiporter of brush border vesicles: studies with acridine orange uptake. Am J Physiol. 1982 Jun;242(6):F733–F739. doi: 10.1152/ajprenal.1982.242.6.F733. [DOI] [PubMed] [Google Scholar]