Abstract
Cereal crops and cereal consumption have had a vital role in Mankind’s history. In the recent years gluten ingestion has been linked with a range of clinical disorders. Gluten-related disorders have gradually emerged as an epidemiologically relevant phenomenon with an estimated global prevalence around 5%. Celiac disease, wheat allergy and non-celiac gluten sensitivity represent different gluten-related disorders. Similar clinical manifestations can be observed in these disorders, yet there are peculiar pathogenetic pathways involved in their development. Celiac disease and wheat allergy have been extensively studied, while non-celiac gluten sensitivity is a relatively novel clinical entity, believed to be closely related to other gastrointestinal functional syndromes. The diagnosis of celiac disease and wheat allergy is based on a combination of findings from the patient’s clinical history and specific tests, including serology and duodenal biopsies in case of celiac disease, or laboratory and functional assays for wheat allergy. On the other hand, non-celiac gluten sensitivity is still mainly a diagnosis of exclusion, in the absence of clear-cut diagnostic criteria. A multimodal pragmatic approach combining findings from the clinical history, symptoms, serological and histological tests is required in order to reach an accurate diagnosis. A thorough knowledge of the differences and overlap in clinical presentation among gluten-related disorders, and between them and other gastrointestinal disorders, will help clinicians in the process of differential diagnosis.
Keywords: Celiac disease, Gluten sensitivity, Wheat sensitivity, Allergy, Gluten-free diet
Core tip: Gluten-related disorders (celiac disease, wheat allergy and non-celiac gluten sensitivity) have emerged as an epidemiologically relevant phenomenon with an estimated global prevalence close to 5%. Although they are characterised by peculiar pathogenetic pathways involved in their development, they share similar clinical manifestations making their differential diagnosis challenging. A multimodal pragmatic approach combining findings from the patient’s clinical history, symptoms, serological and histological tests, as described in the present manuscript, is required for an accurate diagnosis.
INTRODUCTION
Cereal crops and cereal consumption have had a vital role in the history of Mankind but in the recent years gluten ingestion has been found linked with a wide range of clinical disorders. Gluten-containing cereals, such as wheat, rye and barley, have always been a main component of people’s diet in Western countries, but their consumption is increasing in Eastern countries as well, owing to the progressive adoption of Western lifestyles. In this context, gluten-related disorders have gradually emerged as an epidemiologically relevant phenomenon with a global prevalence that is estimated around 5%[1-4], drawing the attention of the scientific community. Surveys conducted among the general population confirm that increasing numbers of consumers worldwide avoid gluten-containing food, irrespective of the presence of a known illness or allergy[5,6]. Switching to a gluten-free diet (GFD) is often viewed as a lifestyle change rather than a proper dietary treatment. Accordingly, the market for gluten-free products is constantly growing, with the latest European reports estimating a compound annual growth rate of 10.4% between 2014 to 2019[7].
Different mechanisms are involved in the pathogenesis of gluten-related disorders. Gluten is the main structural protein of wheat, composed of two main fractions depending on their solubility in aqueous alcohols: the monomeric soluble gliadins (classified according to their primary structures into alpha/beta, gamma and omega types) and the poorly soluble glutenins, which are divided into high-molecular-weight (HMW) and low-molecular-weight (LMW) subunits[8]. Homologous proteins have been found in rye and barley. In celiac disease (CD) a T-cell mediated autoimmune reaction is triggered by gluten-derived peptides[9]. The autoimmune inflammatory cascade is localised in the small bowel, where it leads to the classical enteropathy and malabsorption syndrome. Among the gluten-related disorders CD is the best known one to date: patients’ genetic predisposition, the association with other autoimmune disorders, CD complications have all been extensively studied.
Wheat allergy (WA) represents another type of adverse immunologic reaction to proteins contained in wheat and related grains, with different clinical presentations depending on the route of exposure. In this setting, Immunoglobulin E (IgE) antibodies mediate the inflammatory response to several allergenic proteins [alpha-amylase/trypsin inhibitor, non-specific lipid transfer protein (nsLTP), gliadins, HMW glutenins][10].
A third type of symptomatic responses to gluten ingestion is the so-called Non-Celiac Gluten Sensitivity (NCGS). Patients affected by NCGS usually report a wide range of intestinal and extra-intestinal symptoms arising shortly after the ingestion of gluten-containing food in the absence of CD or WA[1,10]. Although the pathogenetic mechanisms leading to the onset of NGCS are far from being clearly understood, the current opinion is that there is a non-autoimmune non-allergic process[11]. To date, a complete definitive diagnostic flowchart for gluten-related disorders has yet to be established.
GLUTEN RELATED DISORDERS IN ADULTS
Celiac disease
Epidemiological studies estimate a worldwide prevalence of CD of approximately 1:100 individuals, with a considerable proportion of patients remaining undiagnosed and untreated[12,13]. The ingestion of gluten in genetically predisposed individuals carrying HLA type II DQ2/DQ8 alleles can arouse a T-cell mediated immune reaction against tissue transglutaminase, an enzyme of the extracellular matrix, leading to mucosal damage and eventually to intestinal villous atrophy[14,15]. Gliadins are supposed to be the active fractions of gluten; in fact, they content the immunogenic peptides (especially the 33mer) and are able to exert a direct cytotoxic effect on the cell[16,17]. The clinical manifestations of CD are heterogeneous and range from the so-called “classical” syndrome with diarrhea, weight loss and malnutrition, to selective malabsorption of micronutrients (iron, vitamin B12, calcium). Non-classical features include irritable-bowel-type symptoms, hypertransaminasemia, cerebellar ataxia and peripheral neuropathy[18]. In the past, most patients diagnosed with CD were children with severe organic manifestations, but in more recent years there has been an increase in diagnosis of adults and pauci-symptomatic patients[10]. CD can be associated with other disorders, such as autoimmune diseases in 25% of CD patients (type-I diabetes in 3% of cases, autoimmune thyroiditis in 10%, autoimmune hepatitis in less than 1% etc.), less frequently infertility and dermatitis herpetiformis[9]. The history of CD can be rarely complicated by refractory celiac disease or malignancies including lymphoproliferative disorders and carcinoma of the small bowel[19-24].
To date, the only available therapy for CD is a life-long GFD[18,25]. The adherence to a restrictive GFD leads to the resolution of symptoms and to the gradual healing of histological abnormalities[26] even if the complete recovery of the intestinal mucosa is rare and low-grade mucosal inflammation seems to persist in many treated celiac patients as shown by follow-up duodenal biopsies[27]. However, there is strong interest among both patients and physicians in the development of alternative treatments for CD, with the aim of achieving mucosal healing and symptoms resolution in those patients with incomplete response or inadequate compliance to the GFD[28]. Many options are currently under investigation through clinical trials, including oral proteases, zonulin-inhibitors, gluten-binding agents and desensitization strategies[17,29].
The diagnosis of CD is classically based on a combination of findings from a patient’s clinical history, serologic testing and gastroscopy by means of duodenal biopsies. Even in the absence of clinical symptoms, the screening for CD should be considered among the first-degree relatives of celiac patients, patients with type-I diabetes mellitus and patients with Down’s syndrome, given the high prevalence of CD in these and other at risk groups[12,18,30].
Due to the low costs and high reproducibility, the preferred serologic test for the detection of CD in subjects above 2 years of age is the anti-transglutaminase IgA antibody (TTG), which shows a specificity and sensibility of around 95%[31]. TTG test has a wide diffusion as enzyme linked immunosorbent assay or radioimmunoassay commercial kits and gained an important efficiency since the introduction of human recombinant substrates. The anti-endomysium antibody (EMA) has a higher specificity (around 99%) and can be used as a confirmatory test in cases of uncertain diagnosis in high-risk populations[32]. However, EMA test is expensive (monkey esophagus or human umbilical cord are used as substrates) and operator dependent due to the interpretation of immunofluorescent pattern[32]. Deamidated gliadin peptide (DGP) IgA and IgG, substituting the anti gliadin antibodies, are used in combination with TTG IgA in children who are less than 2 years old[33]. Multiple biopsies of the duodenum (at least four) are recommended as a critical component of the diagnostic evaluation and represent the gold standard in adults. CD damage in the duodenal mucosa is not uniformly distributed so multiple biopsies are necessary to reduce the probability of false negative results[34]. The signs of gluten-related enteropathy out of duodenal biopsy range from an increase in the intraepithelial lymphocytes to villous atrophy, as staged by Marsh et al[14] and successively by Oberhuber et al[35]. However, the lymphocytic infiltration of the intestinal epithelium in the absence of villous atrophy (Marsh 1) is considered a non-specific finding, warranting further investigations[36].
In view of its high negative predictive value, the genetic testing for HLA DQ2/DQ8 is suggested in order to rule out CD in select clinical situations, as in the cases of patients at high risk of CD but already on GFD (as mentioned above), equivocal histologic findings in seronegative patients, or discrepancies between histology and serology[18]. Given that around 95% celiac patients carry the HLA-DQ2 heterodimer and the remaining 5% are HLA DQ8 carriers, a negative HLA genotyping can effectively exclude the presence of CD[37-39].
In fact the diagnosis of CD is not always clear-cut: the available international guidelines suggest some strategies to achieve the correct diagnosis in particular cases. It is recommended to rule out IgA deficiency, a condition which is present in up to 2% celiac patients and leads to false negative results; in those cases TTG IgG should be tested[40]. Moreover, other causes of villous atrophy, such as common variable immunodeficiency, autoimmune and chronic inflammatory disorders, drugs and neoplasia, Giardiasis have to be excluded in all patients with particular attention in case of negative serology[41,42].
It is recommended to assess serology and duodenal histology while the patient is still on a gluten-containing diet[18]. Patients with suspected but unproven CD who are already on a GFD at the time of referral, may not show histologic changes or antibody titers consistent with CD due to the improvement of the standard diagnostic tests caused by the GFD itself[43]. In order to diagnose CD accurately, such individuals should be tested for the presence of HLA DQ2/DQ8 and, if positive, gluten should be re-introduced under medical supervision via the so-called “gluten challenge” before planning any serologic testing and duodenal biopsies[43]. Still unclear are: what daily intake of gluten is adequate and how long the gluten challenge should last in order to achieve a correct diagnosis. For a long time, the guidelines have recommended to prescribe 10 grams of gluten per day for a duration of 6-8 wk[44]. However, some recent studies have showed that lower doses of gluten over shorter periods (3 g per day for 2 wk) determine diagnostic changes in histology and/or serology in up to 90% subjects[43]. The new proposed low-dose 14 d long gluten challenge has shown higher compliance and tolerability. The added diagnostic sensitivity of extending the low-dose challenge from 2 to 8 wk is yet to be known.
Wheat allergy
Depending on the route of allergen exposure, WA is classified into occupational asthma (baker’s asthma) and rhinitis; food allergy (FA), affecting the skin, the gastrointestinal tract or the respiratory tract; wheat-dependent exercise-induced anaphylaxis (WDEIA) and contact urticaria. Ingested wheat can cause IgE-mediated wheat allergies in both children and adults.
Although the sensitization to wheat assessed by serum IgE is more prevalent in adults, WA shows greater prevalence in children[45,46]. Immediate wheat allergy is mainly seen in children who commonly outgrow it by school-age, the same as with egg or milk allergy[47,48]. The majority of wheat allergic children suffer from moderate-to-severe atopic dermatitis and wheat ingestion may elicit typical IgE mediated reactions, including urticaria, angioedema, bronchial obstruction, nausea and abdominal pain, or in severe cases systemic anaphylaxis[47]. In adults FA to ingested wheat is infrequent: the most common variant in adults is the WDEIA, where symptoms result from the combination of causative food intake and physical exercise (as well as non-steroidal anti-inflammatory drugs or alcohol). In adults, FA gastrointestinal symptoms could be mild and difficult to recognize, the most common are diarrhea and bloating.
Known since the times of the Roman Empire, baker’s asthma and rhinitis are well-characterized allergic responses to the inhalation of wheat flours, affecting up to 10%-15% bakers, millers and pastry factory workers[49]. Some patients may develop symptoms also after eating meals contaminated by uncooked wheat flour, otherwise no problems are usually reported after the ingestion of cooked wheat[50].
Many allergenic proteins are involved in WA[51] and the latest updated version of the WHO/IUIS Allergen Nomenclature Database describes 21 different well-classified wheat allergens. Although some allergens seem mainly associated with respiratory symptoms [alpha-amylase/trypsin inhibitor), FA (non-specific lipid transfer protein (nsLTP), gliadins], WDEIA (omega-5 gliadin), or contact urticaria (HMW glutenins), there is a clear overlap between the ranges of proteins responsible for different clinical conditions[2,52].
The diagnosis of WA is classically based on skin prick tests (SPT), in vitro specific Immunoglobulin E (sIgE) assays and functional assays. SPTs and sIgE in vitro assays are the first-level diagnostics for WA. However, they are affected by a low predictive value. In particular, their low sensitivity can be explained by the fact that the commercial test reagents are mixtures of water/salt-soluble wheat proteins that lack allergens from the insoluble gluten fraction. In addition, some authors have showed that commercial wheat flour SPT solutions differ in protein content showing that the improvement and standardization of SPT for wheat is highly recommended[53]. SIgE in vitro assays are more sensitive (about 75%-80%) than SPT but less specific (about 60%), mainly due to the cross-reactivity with grass pollens[54]. The identification of molecular allergens for laboratory methods has profoundly changed the diagnostic approach to allergic diseases in the recent years. Molecular-based allergy (MA) diagnostics could overcome some limitations of sIgE in vitro assays using wheat flour extracts. Unfortunately, until now only omega-5 gliadin (Tri a 19) and nsLTP (Tri a 14) are available in the ImmunoCAP™ assay, whereas the alpha-amylase/trypsin inhibitor (Tri a aA/TI) is available only in the microarray ISAC™ assay. The sIgE to omega-5 gliadin assay is highly reliable and now widely used to identify the patients with WDEIA[55]. However, the test is estimated to miss approximately 20% cases[55]. On the other hand, the sIgE to alpha-amylase/trypsin and sIgE to nsLTP inhibitors have resulted useful for both the diagnosis of FA and baker’s asthma[56]. If SPT and the sIgE assays with flour extracts or molecular allergens are inconclusive, functional assays are required. They are considered the gold standard for the diagnosis but are accompanied by a risk of severe induced reactions and are impractical in busy practice settings. Functional tests include a bronchial challenge test in baker’s asthma and a double-blind placebo-controlled food challenge or an open oral food challenge in FA. In the recent years a flow cytometry-assisted basophil activation test (BAT) has been introduced as an in vitro functional test for the diagnosis of immediate-type allergy, and it seems a good alternative for those patients at risk of severe anaphylactic reactions or with contradictory test results. Some authors have recently showed the usefulness of BAT for the diagnosis of WA and in particular its ability to discriminate tolerant vs. allergic subjects among hypersensitized people[57,58]. Although BAT is more expensive and technically challenging compared to conventional in vitro tests, its use has been gradually increasing in clinical practice.
In conclusion, WA is not easily diagnosed with conventional SPT or sIgE assays using wheat flour extracts, since their diagnostic predictivity is unsatisfactory. Challenge tests remain the gold standard for WA diagnosis, but they are cumbersome and potentially dangerous. MA diagnostics and BAT represent new useful tools for the in vitro diagnosis of WA and in some cases may effectively replace the in-vivo functional tests.
Non celiac gluten sensitivity
“Non-celiac gluten sensitivity” is the proposed definition for the condition in which gastrointestinal and extra-intestinal symptoms are triggered by gluten consumption, in the absence of celiac-specific antibodies and villous atrophy as well as of any allergy related processes[1]. The earliest descriptions of sensitivity to gluten appeared in the literature during the 1980’s[59]. Nowadays, the overall prevalence of the condition is difficult to estimate. According to a study performed by the National Health and Nutrition Examination Survey in the United States, the prevalence of self-prescribed GFD in an unselected population of subjects aged 6 years or older was 0.5%[60]. The self-reported prevalence of NCGS was 13% in a United Kingdom population questionnaire-based survey, with less than 1% subjects having a medical diagnosis of the condition[5]. When explored in a selected setting of patients affected by irritable bowel syndrome (IBS), the prevalence of GS, as proved through a double-blind placebo-controlled challenge, was up to 28%[61]. Even in the absence of identified risk factors, the condition seems to be associated with the female gender and young/middle age[3,5,60].
The clinical presentation of NCGS includes gastrointestinal symptoms, such as abdominal pain, bloating and altered bowel habit, and systemic symptoms, such as fatigue, headache, bone or joint pain, mood disorders and skin manifestations (e.g. eczema or rash)[1,3,62]. Symptoms usually closely follow the consumption of gluten and disappear after gluten withdrawal.
Contrary to CD and wheat allergy, there are no clear serologic or histopathologic criteria for clinicians to confirm the diagnosis of NCGS. The accurately CD detecting antibodies, namely TTG and EMA IgA, are constantly negative in NCGS. However, the presence of antigliadin IgG has been reported in up to 50% patients, while antigliadin IgA antibodies rarely occur (7%)[3,63,64]. As far as any intestinal damage is concerned, an increase in the number of lymphocytes infiltrating the epithelium has been described in a subset of NCGS patients[64]. The frequency of NCGS is higher in first-degree relatives of celiac patients and HLA-DQ2 and DQ8 genotypes are observed in 50% NCGS patients, which finding is mildly elevated in comparison with the general population[62].
To date, NCGS is mainly a “diagnosis of exclusion” made after other wheat-related and non-wheat-related disorders have been ruled out. In fact, NCGS has often been described as an IBS-like entity, given the apparently functional nature of both syndromes and the evident overlap of symptoms[65]. Moreover, it has been observed that both patients with self-reported NCGS and IBS improve after the dietary reduction of FODMAPs (fermentable, oligo-, di-, monosaccharides, and polyols)[66] and that IBS patients, especially those with the IBS-D (diarrhea) subtype, benefit from a GFD[67]. The recent evidence about the efficacy of a low-FODMAPs diet in this subsets of patients suggests the hypothesis that some components of wheat other than gluten may be responsible for triggering symptoms[68,69]. In fact, oligosaccharides like fructans, contained in wheat and related grains, have been proven able to exert an osmotic effect in the intestinal lumen and increase gas production from bacterial fermentation[70,71]. Other plant proteins contained in wheat, such as lectins, agglutinins and amylase-trypsin inhibitors, may have a role in the development of symptoms after the ingestion of cereals by triggering the innate immune response[72-74]. For these reasons, and given the scattered data regarding the pathogenesis of NCGS, it has been suggested that the “non-celiac wheat sensitivity” definition may be more appropriate[75,76].
In a recent study by Biesiekierski et al[77] the concept of NCGS as a syndrome has been questioned. In that study, patients with self-reported NCGS on a GFD showed further improvement when placed on a low FODMAP diet and blinded gluten re-introduction led to no specific or dose-dependent effect. However, in those patients the reintroduction of both gluten and whey protein probably had a nocebo effect similar in all groups, which might have concealed the true effect of gluten/wheat re-introduction.
A more appropriate standard for the confirmation of NCGS would be an elimination diet followed by double-blind placebo-controlled gluten challenge[62]. This method can be particularly useful in order to differentiate NCGS from IBS[61].
Recently Kabbani et al[78] have proposed a diagnostic algorithm based on the combination of absence or presence of various clinical, serologic and histological markers with the purpose of identifying NCGS and distinguishing it from CD. This algorithm may prove a useful tool in clinical practice because it provides suggestions for the effective evaluation of patients with gluten-related symptoms already undertaking a GFD, for whom the exclusion of CD and confirmation of NCGS may be a cumbersome task[78].
According to this algorithm, a negative HLA-DQ2 and DQ8 genotype can be useful to rule out CD even in the absence of serology[5], although this would only exclude half the patients with NCGS: in such a case a gluten challenge followed by serology and duodenal biopsies is warranted[78]. In patients with suspected NCGS, a short-term low-dose gluten challenge as discussed above can turn out to be a more suitable pragmatic approach than the traditional 8 wk long approach[43]. It has been calculated that the application of this diagnostic approach in secondary-care gastrointestinal practice would result in the identification of CD in 7% patients referred for suspected NCGS, while the remaining 93% would receive the confirmation of NCGS[5].
Nowadays, due to the absence of any reliable biomarker for NCGS diagnosis, a double-blind placebo-controlled gluten challenge could be considered a possible gold standard to compare other algorithms or markers.
GLUTEN RELATED DISORDERS IN CHILDHOOD
Celiac disease
The diagnostic pathway of CD in children has been recently modified by the Guidelines of the European Society of Pediatric Gastroenterology and Nutrition[33]. The clinical suspicion of CD should be raised in children with unexplained chronic gastrointestinal symptoms, as well as extraintestinal manifestations such as growth retardation, iron deficiency anemia, weight loss, chronic fatigue, delayed puberty, amenorrhea, recurring bone fractures or alterations of liver function tests. Children and adolescents with Down’s syndrome, Turner‘s syndrome, Williams syndrome, IgA deficiency, autoimmune thyroiditis, type-1 diabetes, or autoimmune disorders of the liver, first-degree relatives of celiac patients, should also be given blood tests for the diagnosis of CD[18,33].
The serological tests used in children older than 2 years are TTG and EMA[33]. The combination of TTG and DGP, however, shows a better performance in younger children[79]. Only in the case of primary or secondary IgA deficiency, additional testing with IgG antibodies is recommended (TTG IgG, DGP IgG, EMA IgG)[80]. In the case of positive serology, the current guidelines suggest to follow two different paths for the diagnostic confirmation. In the presence of clinical symptoms, TTG antibody levels at least 10 times higher than the cut-off and positive EMA, the diagnosis of CD can be established by the documentation of HLA compatibility and clinical remission after 6 mo on GFD. If these criteria are not met, similarly as in adult patients, an upper endoscopy with duodenal biopsies should be performed to confirm the presence of histological signs of enteropathy[33].
Non celiac gluten sensitivity
The literature on NCGS in children is scarce. Two studies, both published in 2012 by Tanpowpong et al[81] deal with gluten avoidance in children in New Zealand[6,81]. In the first study, the prevalence of gluten avoidance in a cohort of children was reported to be five time higher than that of actual CD (5% vs 1%)[6]. In the second one, several clinical features such as irritability, poor temper, diarrhea, weight issues, pervasive developmental disorder and family history of CD were found to be independent predictors of gluten avoidance[81]. The relationship between autism and gluten has also been broadly studied, with a single double-blind cross-over study not demonstrating any GFD benefit in autistic children who were not affected by CD[82]. To date, the only paper dealing with the subject of NCGS in children advises to evaluate symptomatic children, tested negative for CD and WA, for NCGS[83]. The suggested approach is similar to the adults, recommending a gluten challenge after at least 8 wk on GFD[83]. Due to the lack of evidence, however, no guidelines are available on NCGS in children.
Wheat allergy
Epidemiological studies report a prevalence of WA in children of around 0.4%[84]. A United States study estimated the resolution rates of pediatric wheat allergy at: 29% by the age of 4 years, 56% by the age of 8% and 65% by the age of 12[48]. The guidelines published in 2010 by the American National Institute of Allergy and Infectious Disease recommend that food allergies including WA should be considered in those individuals presenting with anaphylaxis or a combination of clinical symptoms occurring within minutes to hours after ingesting food, especially in young children or if it is the second episode after the ingestion of specific food. A SPT enables the identification of food categories responsible for triggering IgE-mediated allergic reactions, but a positive SPT alone cannot be considered diagnostic[85]. Similarly, the sole presence of allergen-specific IgE in the serum is not sufficient to confirm the diagnosis of WA. The reference standard for the diagnosis of food allergies is the double-blind placebo-controlled food challenge (as for NCGS). However, a single-blind or an open-food challenge may be considered diagnostic if the challenge elicits objective symptoms, according to the patient’s medical history and with support from laboratory tests[85].
CONCLUSION
Gluten-related disorders are emerging as a relevant clinical entity along with the increasing popularity of the GFD. An expanding body of evidence sustains the on-going debate on the appropriateness of gluten elimination from the diet in the absence of CD or WA. A thorough knowledge of the differences and overlap in clinical presentations among gluten-related disorders, and between them and other gastrointestinal disorders, can help clinicians in the process of differential diagnosis following a correct flowchart (Figure 1). A multimodal pragmatic approach combining the findings from the clinical history, symptoms, serological and histological tests (Table 1) is strongly required in order to reach an accurate diagnosis.
Figure 1.
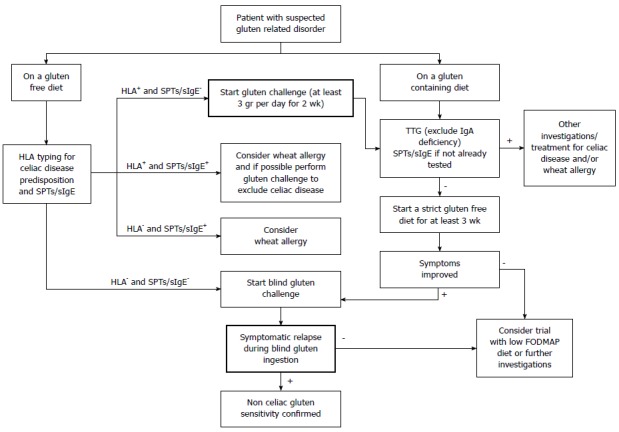
Diagnostic flowchart in case of suspected gluten related disorder. HLA: Human leukocyte antigen; SPTs/sIgE: Skin prick tests/specific Immunoglobulin E.
Table 1.
Diagnostic tests for gluten related disorders
| Serology | Genetic | Histology | Skin tests | Challenges | |
| Celiac disease | Anti transglutaminase IgA plus IgA dosage are used as screening test Anti endomysium as confirmatory test | HLA typing is useful to exclude celiac disease | Presence of duodenal atrophy is considered the gold standard in adulthood | Not used | Gluten challenge is used in case of genetically predisposed patients following a gluten free diet |
| Wheat allergy | The research of IgE against the suspected allergens is sensitive | Not used | Not strictly indicated | Skin reactions against allergens have a low sensitivity especially in case of wheat due to the absence of specific components in commercial reagents | Challenges with the suspected allergens are still considered the gold standard although potentially dangerous for the patient |
| Non celiac gluten sensitivity | Anti gliadin IgG positive in 50% of cases | Not used | Not strictly indicated; a mild duodenal intraepithelial lymphocytosis is possible in up to 50% of suspected cases | Not used | Double blind challenge with gluten could be considered the gold standard for diagnosis |
Footnotes
Conflict-of-interest: Luca Elli has received fees as board member of Dr Schaer Institute. Other authors have no conflict of interest.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: January 29, 2015
First decision: March 26, 2015
Article in press: May 4, 2015
P- Reviewer: Boros M, Bohr J, Gassler N, Miheller P S- Editor: Qi Y L- Editor: A E- Editor: Liu XM
References
- 1.Catassi C, Bai JC, Bonaz B, Bouma G, Calabrò A, Carroccio A, Castillejo G, Ciacci C, Cristofori F, Dolinsek J, et al. Non-Celiac Gluten sensitivity: the new frontier of gluten related disorders. Nutrients. 2013;5:3839–3853. doi: 10.3390/nu5103839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Inomata N. Wheat allergy. Curr Opin Allergy Clin Immunol. 2009;9:238–243. doi: 10.1097/ACI.0b013e32832aa5bc. [DOI] [PubMed] [Google Scholar]
- 3.Volta U, Bardella MT, Calabrò A, Troncone R, Corazza GR. An Italian prospective multicenter survey on patients suspected of having non-celiac gluten sensitivity. BMC Med. 2014;12:85. doi: 10.1186/1741-7015-12-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buscarini E, Conte D, Cannizzaro R, Bazzoli F, De Boni M, Delle Fave G, Farinati F, Ravelli P, Testoni PA, Lisiero M, et al. White paper of Italian Gastroenterology: delivery of services for digestive diseases in Italy: weaknesses and strengths. Dig Liver Dis. 2014;46:579–589. doi: 10.1016/j.dld.2014.02.019. [DOI] [PubMed] [Google Scholar]
- 5.Aziz I, Lewis NR, Hadjivassiliou M, Winfield SN, Rugg N, Kelsall A, Newrick L, Sanders DS. A UK study assessing the population prevalence of self-reported gluten sensitivity and referral characteristics to secondary care. Eur J Gastroenterol Hepatol. 2014;26:33–39. doi: 10.1097/01.meg.0000435546.87251.f7. [DOI] [PubMed] [Google Scholar]
- 6.Tanpowpong P, Ingham TR, Lampshire PK, Kirchberg FF, Epton MJ, Crane J, Camargo CA. Coeliac disease and gluten avoidance in New Zealand children. Arch Dis Child. 2012;97:12–16. doi: 10.1136/archdischild-2011-300248. [DOI] [PubMed] [Google Scholar]
- 7.Gluten-Free Products Market worth $6839.9 Million by 2019. Available from: http://www.marketsandmarkets.com/PressReleases/gluten-free-products.asp.
- 8.Shewry PR, Halford NG, Belton PS, Tatham AS. The structure and properties of gluten: an elastic protein from wheat grain. Philos Trans R Soc Lond B Biol Sci. 2002;357:133–142. doi: 10.1098/rstb.2001.1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rubio-Tapia A, Murray JA. Celiac disease. Curr Opin Gastroenterol. 2010;26:116–122. doi: 10.1097/MOG.0b013e3283365263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ludvigsson JF, Leffler DA, Bai JC, Biagi F, Fasano A, Green PH, Hadjivassiliou M, Kaukinen K, Kelly CP, Leonard JN, et al. The Oslo definitions for coeliac disease and related terms. Gut. 2013;62:43–52. doi: 10.1136/gutjnl-2011-301346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aziz I, Hadjivassiliou M, Sanders DS. Self-reported gluten sensitivity: an international concept in need of consensus? Am J Gastroenterol. 2014;109:1498–1499. doi: 10.1038/ajg.2014.205. [DOI] [PubMed] [Google Scholar]
- 12.Fasano A, Berti I, Gerarduzzi T, Not T, Colletti RB, Drago S, Elitsur Y, Green PH, Guandalini S, Hill ID, et al. Prevalence of celiac disease in at-risk and not-at-risk groups in the United States: a large multicenter study. Arch Intern Med. 2003;163:286–292. doi: 10.1001/archinte.163.3.286. [DOI] [PubMed] [Google Scholar]
- 13.Volta U, Bellentani S, Bianchi FB, Brandi G, De Franceschi L, Miglioli L, Granito A, Balli F, Tiribelli C. High prevalence of celiac disease in Italian general population. Dig Dis Sci. 2001;46:1500–1505. doi: 10.1023/a:1010648122797. [DOI] [PubMed] [Google Scholar]
- 14.Marsh MN. Gluten, major histocompatibility complex, and the small intestine. A molecular and immunobiologic approach to the spectrum of gluten sensitivity (‘celiac sprue’) Gastroenterology. 1992;102:330–354. [PubMed] [Google Scholar]
- 15.Sollid LM, Markussen G, Ek J, Gjerde H, Vartdal F, Thorsby E. Evidence for a primary association of celiac disease to a particular HLA-DQ alpha/beta heterodimer. J Exp Med. 1989;169:345–350. doi: 10.1084/jem.169.1.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Elli L, Dolfini E, Bardella MT. Gliadin cytotoxicity and in vitro cell cultures. Toxicol Lett. 2003;146:1–8. doi: 10.1016/j.toxlet.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 17.Schuppan D, Junker Y, Barisani D. Celiac disease: from pathogenesis to novel therapies. Gastroenterology. 2009;137:1912–1933. doi: 10.1053/j.gastro.2009.09.008. [DOI] [PubMed] [Google Scholar]
- 18.Rubio-Tapia A, Hill ID, Kelly CP, Calderwood AH, Murray JA. ACG clinical guidelines: diagnosis and management of celiac disease. Am J Gastroenterol. 2013;108:656–676; quiz 677. doi: 10.1038/ajg.2013.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Freeman HJ. Lymphoproliferative and intestinal malignancies in 214 patients with biopsy-defined celiac disease. J Clin Gastroenterol. 2004;38:429–434. doi: 10.1097/00004836-200405000-00008. [DOI] [PubMed] [Google Scholar]
- 20.Catassi C, Bearzi I, Holmes GK. Association of celiac disease and intestinal lymphomas and other cancers. Gastroenterology. 2005;128:S79–S86. doi: 10.1053/j.gastro.2005.02.027. [DOI] [PubMed] [Google Scholar]
- 21.Bardella MT, Elli L, De Matteis S, Floriani I, Torri V, Piodi L. Autoimmune disorders in patients affected by celiac sprue and inflammatory bowel disease. Ann Med. 2009;41:139–143. doi: 10.1080/07853890802378817. [DOI] [PubMed] [Google Scholar]
- 22.Elli L, Bonura A, Garavaglia D, Rulli E, Floriani I, Tagliabue G, Contiero P, Bardella MT. Immunological comorbity in coeliac disease: associations, risk factors and clinical implications. J Clin Immunol. 2012;32:984–990. doi: 10.1007/s10875-012-9693-0. [DOI] [PubMed] [Google Scholar]
- 23.Rostami K, Steegers EA, Wong WY, Braat DD, Steegers-Theunissen RP. Coeliac disease and reproductive disorders: a neglected association. Eur J Obstet Gynecol Reprod Biol. 2001;96:146–149. doi: 10.1016/s0301-2115(00)00457-7. [DOI] [PubMed] [Google Scholar]
- 24.Elli L, Contiero P, Tagliabue G, Tomba C, Bardella MT. Risk of intestinal lymphoma in undiagnosed coeliac disease: results from a registered population with different coeliac disease prevalence. Dig Liver Dis. 2012;44:743–747. doi: 10.1016/j.dld.2012.04.020. [DOI] [PubMed] [Google Scholar]
- 25.Elli L, Discepolo V, Bardella MT, Guandalini S. Does gluten intake influence the development of celiac disease-associated complications? J Clin Gastroenterol. 2014;48:13–20. doi: 10.1097/MCG.0b013e3182a9f898. [DOI] [PubMed] [Google Scholar]
- 26.Lee SK, Lo W, Memeo L, Rotterdam H, Green PH. Duodenal histology in patients with celiac disease after treatment with a gluten-free diet. Gastrointest Endosc. 2003;57:187–191. doi: 10.1067/mge.2003.54. [DOI] [PubMed] [Google Scholar]
- 27.Lanzini A, Lanzarotto F, Villanacci V, Mora A, Bertolazzi S, Turini D, Carella G, Malagoli A, Ferrante G, Cesana BM, et al. Complete recovery of intestinal mucosa occurs very rarely in adult coeliac patients despite adherence to gluten-free diet. Aliment Pharmacol Ther. 2009;29:1299–1308. doi: 10.1111/j.1365-2036.2009.03992.x. [DOI] [PubMed] [Google Scholar]
- 28.Aziz I, Evans KE, Papageorgiou V, Sanders DS. Are patients with coeliac disease seeking alternative therapies to a gluten-free diet? J Gastrointestin Liver Dis. 2011;20:27–31. [PubMed] [Google Scholar]
- 29.Lähdeaho ML, Kaukinen K, Laurila K, Vuotikka P, Koivurova OP, Kärjä-Lahdensuu T, Marcantonio A, Adelman DC, Mäki M. Glutenase ALV003 attenuates gluten-induced mucosal injury in patients with celiac disease. Gastroenterology. 2014;146:1649–1658. doi: 10.1053/j.gastro.2014.02.031. [DOI] [PubMed] [Google Scholar]
- 30.Bardella MT, Elli L, Velio P, Fredella C, Prampolini L, Cesana B. Silent celiac disease is frequent in the siblings of newly diagnosed celiac patients. Digestion. 2007;75:182–187. doi: 10.1159/000107979. [DOI] [PubMed] [Google Scholar]
- 31.van der Windt DA, Jellema P, Mulder CJ, Kneepkens CM, van der Horst HE. Diagnostic testing for celiac disease among patients with abdominal symptoms: a systematic review. JAMA. 2010;303:1738–1746. doi: 10.1001/jama.2010.549. [DOI] [PubMed] [Google Scholar]
- 32.Leffler DA, Schuppan D. Update on serologic testing in celiac disease. Am J Gastroenterol. 2010;105:2520–2524. doi: 10.1038/ajg.2010.276. [DOI] [PubMed] [Google Scholar]
- 33.Husby S, Koletzko S, Korponay-Szabó IR, Mearin ML, Phillips A, Shamir R, Troncone R, Giersiepen K, Branski D, Catassi C, et al. European Society for Pediatric Gastroenterology, Hepatology, and Nutrition guidelines for the diagnosis of coeliac disease. J Pediatr Gastroenterol Nutr. 2012;54:136–160. doi: 10.1097/MPG.0b013e31821a23d0. [DOI] [PubMed] [Google Scholar]
- 34.Ravelli A, Villanacci V, Monfredini C, Martinazzi S, Grassi V, Manenti S. How patchy is patchy villous atrophy?: distribution pattern of histological lesions in the duodenum of children with celiac disease. Am J Gastroenterol. 2010;105:2103–2110. doi: 10.1038/ajg.2010.153. [DOI] [PubMed] [Google Scholar]
- 35.Oberhuber G, Granditsch G, Vogelsang H. The histopathology of coeliac disease: time for a standardized report scheme for pathologists. Eur J Gastroenterol Hepatol. 1999;11:1185–1194. doi: 10.1097/00042737-199910000-00019. [DOI] [PubMed] [Google Scholar]
- 36.Aziz I, Evans KE, Hopper AD, Smillie DM, Sanders DS. A prospective study into the aetiology of lymphocytic duodenosis. Aliment Pharmacol Ther. 2010;32:1392–1397. doi: 10.1111/j.1365-2036.2010.04477.x. [DOI] [PubMed] [Google Scholar]
- 37.Megiorni F, Mora B, Bonamico M, Barbato M, Montuori M, Viola F, Trabace S, Mazzilli MC. HLA-DQ and susceptibility to celiac disease: evidence for gender differences and parent-of-origin effects. Am J Gastroenterol. 2008;103:997–1003. doi: 10.1111/j.1572-0241.2007.01716.x. [DOI] [PubMed] [Google Scholar]
- 38.Thomas HJ, Ahmad T, Rajaguru C, Barnardo M, Warren BF, Jewell DP. Contribution of histological, serological, and genetic factors to the clinical heterogeneity of adult-onset coeliac disease. Scand J Gastroenterol. 2009;44:1076–1083. doi: 10.1080/00365520903100473. [DOI] [PubMed] [Google Scholar]
- 39.Kaukinen K, Partanen J, Mäki M, Collin P. HLA-DQ typing in the diagnosis of celiac disease. Am J Gastroenterol. 2002;97:695–699. doi: 10.1111/j.1572-0241.2002.05471.x. [DOI] [PubMed] [Google Scholar]
- 40.Villalta D, Alessio MG, Tampoia M, Tonutti E, Brusca I, Bagnasco M, Pesce G, Bizzaro N. Diagnostic accuracy of IgA anti-tissue transglutaminase antibody assays in celiac disease patients with selective IgA deficiency. Ann N Y Acad Sci. 2007;1109:212–220. doi: 10.1196/annals.1398.025. [DOI] [PubMed] [Google Scholar]
- 41.Pallav K, Leffler DA, Tariq S, Kabbani T, Hansen J, Peer A, Bhansali A, Najarian R, Kelly CP. Noncoeliac enteropathy: the differential diagnosis of villous atrophy in contemporary clinical practice. Aliment Pharmacol Ther. 2012;35:380–390. doi: 10.1111/j.1365-2036.2011.04938.x. [DOI] [PubMed] [Google Scholar]
- 42.Edling L, Rathsman S, Eriksson S, Bohr J. Celiac disease and giardiasis: a case report. Eur J Gastroenterol Hepatol. 2012;24:984–987. doi: 10.1097/MEG.0b013e328354f3f5. [DOI] [PubMed] [Google Scholar]
- 43.Leffler D, Schuppan D, Pallav K, Najarian R, Goldsmith JD, Hansen J, Kabbani T, Dennis M, Kelly CP. Kinetics of the histological, serological and symptomatic responses to gluten challenge in adults with coeliac disease. Gut. 2013;62:996–1004. doi: 10.1136/gutjnl-2012-302196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rostom A, Murray JA, Kagnoff MF. American Gastroenterological Association (AGA) Institute technical review on the diagnosis and management of celiac disease. Gastroenterology. 2006;131:1981–2002. doi: 10.1053/j.gastro.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 45.Vierk KA, Koehler KM, Fein SB, Street DA. Prevalence of self-reported food allergy in American adults and use of food labels. J Allergy Clin Immunol. 2007;119:1504–1510. doi: 10.1016/j.jaci.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 46.Matricardi PM, Bockelbrink A, Beyer K, Keil T, Niggemann B, Grüber C, Wahn U, Lau S. Primary versus secondary immunoglobulin E sensitization to soy and wheat in the Multi-Centre Allergy Study cohort. Clin Exp Allergy. 2008;38:493–500. doi: 10.1111/j.1365-2222.2007.02912.x. [DOI] [PubMed] [Google Scholar]
- 47.Ramesh S. Food allergy overview in children. Clin Rev Allergy Immunol. 2008;34:217–230. doi: 10.1007/s12016-007-8034-1. [DOI] [PubMed] [Google Scholar]
- 48.Keet CA, Matsui EC, Dhillon G, Lenehan P, Paterakis M, Wood RA. The natural history of wheat allergy. Ann Allergy Asthma Immunol. 2009;102:410–415. doi: 10.1016/S1081-1206(10)60513-3. [DOI] [PubMed] [Google Scholar]
- 49.Salvatori N, Reccardini F, Convento M, Purinan A, Colle R, De Carli S, Garzoni M, Lafiandra D, De Carli M. Asthma induced by inhalation of flour in adults with food allergy to wheat. Clin Exp Allergy. 2008;38:1349–1356. doi: 10.1111/j.1365-2222.2008.03023.x. [DOI] [PubMed] [Google Scholar]
- 50.Brisman J. Baker’s asthma. Occup Environ Med. 2002;59:498–502; quiz 502, 426. doi: 10.1136/oem.59.7.498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tatham AS, Shewry PR. Allergens to wheat and related cereals. Clin Exp Allergy. 2008;38:1712–1726. doi: 10.1111/j.1365-2222.2008.03101.x. [DOI] [PubMed] [Google Scholar]
- 52.Salcedo G, Quirce S, Diaz-Perales A. Wheat allergens associated with Baker’s asthma. J Investig Allergol Clin Immunol. 2011;21:81–92; quiz 94. [PubMed] [Google Scholar]
- 53.Sander I, Merget R, Degens PO, Goldscheid N, Brüning T, Raulf-Heimsoth M. Comparison of wheat and rye flour skin prick test solutions for diagnosis of baker’s asthma. Allergy. 2004;59:95–98. doi: 10.1046/j.1398-9995.2003.00349.x. [DOI] [PubMed] [Google Scholar]
- 54.Sander I, Raulf-Heimsoth M, Düser M, Flagge A, Czuppon AB, Baur X. Differentiation between cosensitization and cross-reactivity in wheat flour and grass pollen-sensitized subjects. Int Arch Allergy Immunol. 1997;112:378–385. doi: 10.1159/000237483. [DOI] [PubMed] [Google Scholar]
- 55.Matsuo H, Dahlström J, Tanaka A, Kohno K, Takahashi H, Furumura M, Morita E. Sensitivity and specificity of recombinant omega-5 gliadin-specific IgE measurement for the diagnosis of wheat-dependent exercise-induced anaphylaxis. Allergy. 2008;63:233–236. doi: 10.1111/j.1398-9995.2007.01504.x. [DOI] [PubMed] [Google Scholar]
- 56.Quirce S, Diaz-Perales A. Diagnosis and management of grain-induced asthma. Allergy Asthma Immunol Res. 2013;5:348–356. doi: 10.4168/aair.2013.5.6.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chinuki Y, Kaneko S, Dekio I, Takahashi H, Tokuda R, Nagao M, Fujisawa T, Morita E. CD203c expression-based basophil activation test for diagnosis of wheat-dependent exercise-induced anaphylaxis. J Allergy Clin Immunol. 2012;129:1404–1406. doi: 10.1016/j.jaci.2012.02.049. [DOI] [PubMed] [Google Scholar]
- 58.Tokuda R, Nagao M, Hiraguchi Y, Hosoki K, Matsuda T, Kouno K, Morita E, Fujisawa T. Antigen-induced expression of CD203c on basophils predicts IgE-mediated wheat allergy. Allergol Int. 2009;58:193–199. doi: 10.2332/allergolint.08-OA-0023. [DOI] [PubMed] [Google Scholar]
- 59.Cooper BT, Holmes GK, Ferguson R, Thompson RA, Allan RN, Cooke WT. “Gluten-sensitive diarrhea without evidence of celiac disease”. Gastroenterology. 1981;81:192–194. [PubMed] [Google Scholar]
- 60.DiGiacomo DV, Tennyson CA, Green PH, Demmer RT. Prevalence of gluten-free diet adherence among individuals without celiac disease in the USA: results from the Continuous National Health and Nutrition Examination Survey 2009-2010. Scand J Gastroenterol. 2013;48:921–925. doi: 10.3109/00365521.2013.809598. [DOI] [PubMed] [Google Scholar]
- 61.Biesiekierski JR, Newnham ED, Irving PM, Barrett JS, Haines M, Doecke JD, Shepherd SJ, Muir JG, Gibson PR. Gluten causes gastrointestinal symptoms in subjects without celiac disease: a double-blind randomized placebo-controlled trial. Am J Gastroenterol. 2011;106:508–514; quiz 515. doi: 10.1038/ajg.2010.487. [DOI] [PubMed] [Google Scholar]
- 62.Sapone A, Bai JC, Ciacci C, Dolinsek J, Green PH, Hadjivassiliou M, Kaukinen K, Rostami K, Sanders DS, Schumann M, et al. Spectrum of gluten-related disorders: consensus on new nomenclature and classification. BMC Med. 2012;10:13. doi: 10.1186/1741-7015-10-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Volta U, Tovoli F, Cicola R, Parisi C, Fabbri A, Piscaglia M, Fiorini E, Caio G. Serological tests in gluten sensitivity (nonceliac gluten intolerance) J Clin Gastroenterol. 2012;46:680–685. doi: 10.1097/MCG.0b013e3182372541. [DOI] [PubMed] [Google Scholar]
- 64.Carroccio A, Mansueto P, Iacono G, Soresi M, D’Alcamo A, Cavataio F, Brusca I, Florena AM, Ambrosiano G, Seidita A, et al. Non-celiac wheat sensitivity diagnosed by double-blind placebo-controlled challenge: exploring a new clinical entity. Am J Gastroenterol. 2012;107:1898–1906; quiz 1907. doi: 10.1038/ajg.2012.236. [DOI] [PubMed] [Google Scholar]
- 65.Verdu EF, Armstrong D, Murray JA. Between celiac disease and irritable bowel syndrome: the “no man’s land” of gluten sensitivity. Am J Gastroenterol. 2009;104:1587–1594. doi: 10.1038/ajg.2009.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shepherd SJ, Halmos E, Glance S. The role of FODMAPs in irritable bowel syndrome. Curr Opin Clin Nutr Metab Care. 2014;17:605–609. doi: 10.1097/MCO.0000000000000116. [DOI] [PubMed] [Google Scholar]
- 67.Vazquez-Roque MI, Camilleri M, Smyrk T, Murray JA, Marietta E, O’Neill J, Carlson P, Lamsam J, Janzow D, Eckert D, et al. A controlled trial of gluten-free diet in patients with irritable bowel syndrome-diarrhea: effects on bowel frequency and intestinal function. Gastroenterology. 2013;144:903–911.e3. doi: 10.1053/j.gastro.2013.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shepherd SJ, Parker FC, Muir JG, Gibson PR. Dietary triggers of abdominal symptoms in patients with irritable bowel syndrome: randomized placebo-controlled evidence. Clin Gastroenterol Hepatol. 2008;6:765–771. doi: 10.1016/j.cgh.2008.02.058. [DOI] [PubMed] [Google Scholar]
- 69.Gibson PR, Shepherd SJ. Evidence-based dietary management of functional gastrointestinal symptoms: The FODMAP approach. J Gastroenterol Hepatol. 2010;25:252–258. doi: 10.1111/j.1440-1746.2009.06149.x. [DOI] [PubMed] [Google Scholar]
- 70.Gibson PR, Shepherd SJ. Food choice as a key management strategy for functional gastrointestinal symptoms. Am J Gastroenterol. 2012;107:657–666; quiz 667. doi: 10.1038/ajg.2012.49. [DOI] [PubMed] [Google Scholar]
- 71.Murray K, Wilkinson-Smith V, Hoad C, Costigan C, Cox E, Lam C, Marciani L, Gowland P, Spiller RC. Differential effects of FODMAPs (fermentable oligo-, di-, mono-saccharides and polyols) on small and large intestinal contents in healthy subjects shown by MRI. Am J Gastroenterol. 2014;109:110–119. doi: 10.1038/ajg.2013.386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Junker Y, Zeissig S, Kim SJ, Barisani D, Wieser H, Leffler DA, Zevallos V, Libermann TA, Dillon S, Freitag TL, et al. Wheat amylase trypsin inhibitors drive intestinal inflammation via activation of toll-like receptor 4. J Exp Med. 2012;209:2395–2408. doi: 10.1084/jem.20102660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Haas H, Falcone FH, Schramm G, Haisch K, Gibbs BF, Klaucke J, Pöppelmann M, Becker WM, Gabius HJ, Schlaak M. Dietary lectins can induce in vitro release of IL-4 and IL-13 from human basophils. Eur J Immunol. 1999;29:918–927. doi: 10.1002/(SICI)1521-4141(199903)29:03<918::AID-IMMU918>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 74.Dalla Pellegrina C, Perbellini O, Scupoli MT, Tomelleri C, Zanetti C, Zoccatelli G, Fusi M, Peruffo A, Rizzi C, Chignola R. Effects of wheat germ agglutinin on human gastrointestinal epithelium: insights from an experimental model of immune/epithelial cell interaction. Toxicol Appl Pharmacol. 2009;237:146–153. doi: 10.1016/j.taap.2009.03.012. [DOI] [PubMed] [Google Scholar]
- 75.Carroccio A, Rini G, Mansueto P. Non-celiac wheat sensitivity is a more appropriate label than non-celiac gluten sensitivity. Gastroenterology. 2014;146:320–321. doi: 10.1053/j.gastro.2013.08.061. [DOI] [PubMed] [Google Scholar]
- 76.Sanders DS, Aziz I. Non-celiac wheat sensitivity: separating the wheat from the chat! Am J Gastroenterol. 2012;107:1908–1912. doi: 10.1038/ajg.2012.344. [DOI] [PubMed] [Google Scholar]
- 77.Biesiekierski JR, Peters SL, Newnham ED, Rosella O, Muir JG, Gibson PR. No effects of gluten in patients with self-reported non-celiac gluten sensitivity after dietary reduction of fermentable, poorly absorbed, short-chain carbohydrates. Gastroenterology. 2013;145:320–8.e1-320-8.e3. doi: 10.1053/j.gastro.2013.04.051. [DOI] [PubMed] [Google Scholar]
- 78.Kabbani TA, Vanga RR, Leffler DA, Villafuerte-Galvez J, Pallav K, Hansen J, Mukherjee R, Dennis M, Kelly CP. Celiac disease or non-celiac gluten sensitivity? An approach to clinical differential diagnosis. Am J Gastroenterol. 2014;109:741–746; quiz 747. doi: 10.1038/ajg.2014.41. [DOI] [PubMed] [Google Scholar]
- 79.Aberg AK, Olcén P. Serologic screening for celiac disease in children: a comparison between established assays and tests with deamidated gliadin-derived peptides plus conjugates for both IgA and IgG antibodies. APMIS. 2009;117:808–813. doi: 10.1111/j.1600-0463.2009.02541.x. [DOI] [PubMed] [Google Scholar]
- 80.McGowan KE, Lyon ME, Butzner JD. Celiac disease and IgA deficiency: complications of serological testing approaches encountered in the clinic. Clin Chem. 2008;54:1203–1209. doi: 10.1373/clinchem.2008.103606. [DOI] [PubMed] [Google Scholar]
- 81.Tanpowpong P, Broder-Fingert S, Katz AJ, Camargo CA. Predictors of gluten avoidance and implementation of a gluten-free diet in children and adolescents without confirmed celiac disease. J Pediatr. 2012;161:471–475. doi: 10.1016/j.jpeds.2012.02.049. [DOI] [PubMed] [Google Scholar]
- 82.Elder JH, Shankar M, Shuster J, Theriaque D, Burns S, Sherrill L. The gluten-free, casein-free diet in autism: results of a preliminary double blind clinical trial. J Autism Dev Disord. 2006;36:413–420. doi: 10.1007/s10803-006-0079-0. [DOI] [PubMed] [Google Scholar]
- 83.Francavilla R, Cristofori F, Castellaneta S, Polloni C, Albano V, Dellatte S, Indrio F, Cavallo L, Catassi C. Clinical, serologic, and histologic features of gluten sensitivity in children. J Pediatr. 2014;164:463–467.e1. doi: 10.1016/j.jpeds.2013.10.007. [DOI] [PubMed] [Google Scholar]
- 84.Zuidmeer L, Goldhahn K, Rona RJ, Gislason D, Madsen C, Summers C, Sodergren E, Dahlstrom J, Lindner T, Sigurdardottir ST, et al. The prevalence of plant food allergies: a systematic review. J Allergy Clin Immunol. 2008;121:1210–1218.e4. doi: 10.1016/j.jaci.2008.02.019. [DOI] [PubMed] [Google Scholar]
- 85.Boyce JA, Assa’ad A, Burks AW, Jones SM, Sampson HA, Wood RA, Plaut M, Cooper SF, Fenton MJ, Arshad SH, et al. Guidelines for the diagnosis and management of food allergy in the United States: report of the NIAID-sponsored expert panel. J Allergy Clin Immunol. 2010;126:S1–S58. doi: 10.1016/j.jaci.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]


