Abstract
Background
Differences in carcinogen exposure from different cigarette products could contribute to differences in smoking-associated cancer incidence among Chinese compared with US smokers.
Methods
Urine concentrations of metabolites of nicotine, the tobacco-specific nitrosamine (TSNA) 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol (NNAL), and polycyclic aromatic hydrocarbon metabolites (PAHs) were compared in 238 Chinese and 203 US daily smokers.
Results
Comparing Chinese versus US smokers, daily nicotine intake and nicotine intake per cigarette smoked were found to be similar. When normalised for cigarettes per day, urine NNAL excretion was fourfold higher in US smokers, while the excretion of urine metabolites of the PAHs fluorene, phenanthrene and pyrene metabolites was 50% to fourfold higher in Chinese smokers (all, p<0.0001). Similar results were seen when NNAL and PAHs excretion was normalised for daily nicotine intake.
Conclusions
Patterns of carcinogen exposure differ, with lower exposure to TSNA and higher exposure to PAHs in Chinese compared with US smokers. These results most likely reflect country differences in cigarette tobacco blends and manufacturing processes, as well as different environmental exposures.
INTRODUCTION
China is the world's largest producer and consumer of tobacco products.1 In 2010, the adult smoking prevalence was 28%, including 53% of men aged 15 years or older.2 Cancer accounts for a large fraction of tobacco-caused deaths, with lung cancer being the most common cancer. While lung cancer is a major health problem in Chinese smokers, the relative risk (RR) of lung cancer is lower for Chinese compared with US smokers (RR 2.5 and 25, respectively).1,3 Three widely cited explanations for this difference are (1) that Chinese smokers smoke fewer cigarettes per day, (2) Chinese smokers start smoking at a later age and (3) genetic differences make Chinese smokers less susceptible to smoking-induced lung cancer than US smokers.1,4,5 Another explanation that should be considered is a difference in cigarette products that results in different profiles of exposure to tobacco smoke carcinogens.
Cigarettes expose smokers to more than 70 carcinogens. Of particular concern with respect to lung cancer are: tobacco-specific nitrosamines (TSNAs, such as 4-(methylnitrosamino)-1-(3-pyridyl)-1-buta-none (NNK) and N’-nitrosonornicotine) and polycyclic aromatic hydrocarbons (PAHs).6,7 Biomarker levels of TSNAs and PAHs independently correlate with the risk of lung cancer,8–10 and are the focus of our analysis.
Cigarettes sold in China contain and generate by machine testing lower levels of TSNAs compared with US cigarettes, even within the same global brands such as Marlboro or Camel.11–13 Consistent with this observation, biomarkers of NNK exposure are on average lower in Chinese compared with US smokers.10 An important factor in cigarette design that determines TSNA levels is nitrate content; high nitrate content results in greater nitrosation of nicotine and more generation of TSNAs during curing and smoking.14 High nitrate content of tobacco is also inversely correlated with the pyrosynthesis of PAHs when cigarettes are smoked.15 Different types of tobacco have different nitrate content and generate different levels of TSNAs and PAHs in mainstream smoke.16
To better understand possible reasons for differences in cancer risk among Chinese and US smokers, we measured biomarkers of TSNA and PAH exposure in smokers from the two countries. Since smokers smoke to obtain desired levels of nicotine, we also assessed a biomarker of daily nicotine intake so that we could normalise carcinogen exposure for nicotine intake.
METHODS
Subjects
As described previously,17 the Chinese participants for the present analysis were taxi drivers who were smoking Chinese brand cigarettes and were recruited in driver physical examination centres in Shanghai, China between January and April 2006. Two hundred and thirty eight participants were selected randomly from a total of 543 smokers in the original study group for analysis in the present study. The participants were healthy men between the ages of 18 and 65 who reported smoking five or more cigarettes per day. Each participant provided a smoking history and a single void spot urine sample. The US smokers came from two studies conducted in San Francisco in which 203 participants provided a detailed smoking history and urine sample: one of the smokers who attended a research clinic18 and other in a clinical trial of reduced nicotine content cigarettes (baseline evaluation).19
The studies were approved by the University of California San Francisco Committee on Human Research and the Shanghai Center for Disease Control and Prevention Committee on Human Subjects. Participants provided written consent.
Analytical chemistry
Urine samples from China were frozen and shipped to San Francisco General Hospital for analysis. Urine total (free +conjugated) concentrations of nicotine, cotinine and trans-3’-hydroxycotinine were measured by liquid chromatography-tandem mass spectrometry (LC-MS/MS) as described previously.20 Urine concentrations of total 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol (NNAL) were measured by LC-MS/MS as described previously.21 Several PAH metabolites: 2-naphthol (2-Nap), 1-hydroxyfluorene, 2-hydroxyfluorene, 3-hydroxy-fluorene, 1-hydroxyphenanthrene, 2-hydroxyphenanthrene, 3+4-hydroxyphenanthrene and 1-hydroxypyrene (1-HP) were determined by LC-MS/MS as described previously.22 Details on limits of quantitation and quality control measures for various assays are provided in the assay methodology papers cited above. Urine creatinine was measured in the San Francisco General Hospital clinical laboratory using a colorimetric assay.
The urine total nicotine equivalents (TNE) was determined as the molar sum of nicotine, cotinine, trans 3’-hydroxycotinine and their respective glucuronides, normalised for creatinine concentration. When measured at steady state, the sum of these metabolites accounts for 80–90% of a daily dose of nicotine.23,24 Nicotine equivalents measured in this way are highly correlated with daily intake of nicotine validated by the administration of labelled nicotine in steady state conditions.25 Since it represents the sum of nicotine metabolites generated by various pathways at steady state, urine TNE are expected to be unaffected by racial differences in rates and pathways of nicotine metabolism, as are known to occur in people of Chinese, African and Caucasian ancestry. We assessed fluorene and phenanthrene exposure as the molar sum of their several metabolites measured in urine.26
Statistical analysis
Comparisons of demographic characteristics and urine bio-marker levels of Chinese versus US smoker groups were performed using t test to compare means (age, tar level and cigarettes per day, which were normally distributed) and the Mann-Whitney rank sum test to compare medians (urine biomarkers, which were not normally distributed). Since all Chinese smokers were men but US smokers included men and women, we performed analyses including and excluding women. Spearman rank correlations between cigarettes per day and various biomarkers among Chinese and US smokers were computed.
RESULTS
Demographic data
Demographic data for our participants are provided in table 1. The US population included 134 white and 69 black smokers. The average age was significantly higher for Chinese compared with US smokers. Chinese smokers were all men, whereas about 60% of US smokers were men. The nominal tar delivery was significantly lower for cigarettes used by Chinese compared with US smokers (12.2 vs 13.6 mg, p<0.0001). Chinese smokers smoked an average of 18 cigarettes per day compared with 19.5 for US smokers, but the difference was not statistically significant.
Table 1.
Demographics and urine biomarkers in Chinese and US smokers (values are medians and IQR, unless otherwise indicated)
| Chinese smokers (n=238) | US smokers (n=203) | p Value* | US men (n=118) | p Value* | |
|---|---|---|---|---|---|
| Age | 44.3 (43.0–45.5)† | 38.3 (36.7–39.8)† | <0.000‡ | 39–8 (3.77–41.8)† | 0.0001‡ |
| Male (%) | 238 (100%) | 118 (58%) | <0.0001 | 118 (100%) | |
| Tar | 12.2 (11.9–12.6)† | 13.6 (13.0–14.2)† | <0.0001‡ | 14.2 (13.4–14.9)† | <0.0001‡ |
| CPD | 18.0 (16.7–19.3)† | 19.5 (18.2–20.7)† | NS‡ | 20.6 (18.9–22.4)† | 0.02‡ |
| Nicotine Eq (nmol/mg creatinine) | 53.4 (32.8–78.9) | 54.8 (42.7–81.6) | NS | 54.6 (38.2–78.6) | NS |
| NNAL (pmol/mg creatinine) | 0.29 (0.18–0.47) | 1.19 (0.63–2.06) | <0.0001 | 1.12 (0.62–1.81) | <0.0001 |
| 1-HP (pmol/mg creatinine) | 3.64 (2.64–5.48) | 1.17 (0.75–1.79) | <0.0001 | 1.10 (0.70–1.52) | <0.0001 |
| 2-Nap (pmol/mg creatinine) | 103 (65.3–149) | 98.3 (59.7–149) | NS | 88.7 (52.0–130.3) | 0.03 |
| Sum of fluorenes (pmol/mg creatinine) | 23.5 (15.8–32.9) | 16.6 (10.8–24.5) | <0.0001 | 16.0 (10.6–22.4) | <0.0001 |
| Sum of phenanthrenes (pmol/mg creatinine) | 9.52 (6.56–13.5) | 3.54 (2.44–5.07) | <0.0001 | 3.53 (2.52–4.90) | <0.0001 |
| Sum of PAHs (pmol/mg creatinine) | 145 (95.8–201) | 123 (78.5–184) | 0.0082 | 110 (79–158) | 0.0001 |
| Nicotine Eq/CPD (nmol/mg creatinine) | 3.16 (1.76–5.23) | 3.13 (2.07–5.06) | NS | 2.87 (1.88–4.02) | NS |
| NNAL/CPD (fmol/mg creatinine) | 18.9 (11.3–32.7) | 73.4 (37.4–112) | <0.0001 | 62.1 (28.8–99.3) | <0.0001 |
| 1-HP/CPD (pmol/mg creatinine) | 0.24 (0.15–0.40) | 0.06 (0.04–0.10) | <0.0001 | 0.05 (0.04–0.08) | <0.0001 |
| 2-Nap/CPD (pmol/mg creatinine) | 6.04 (3.69–9.75) | 5.53 (3.54–8.37) | NS | 4.68 (3.10–6.52) | 0.0001 |
| Sum of fluorenes/CPD (pmol/mg creatinine) | 1.47 (0.93–2.21) | 0.98 (0.56–1.42) | <0.0001 | 0.82 (0.53–1.25) | <0.0001 |
| Sum of phenanthrenes/CPD (pmol/mg creatinine) | 0.60 (0.39–0.93) | 0.19 (0.13–0.29) | <0.0001 | 018 (0.11–0.29) | <0.0001 |
| Sum of PAHs/CPD (pmol/mg creatinine) | 8.41 (5.56–12.9) | 7.00 (4.55–10.3) | 0.0002 | 5.66 (4.02–8.25) | <0.0001 |
| NNAL/nicotine Eq×106 | 5.55 (3.74–11.8) | 22.0 (14.2–31.8) | <0.0001 | 21.5 (12.9–31.4) | <0.0001 |
| 1-HP/ nicotine Eq×103 | 0.07 (0.05–0.13) | 0.02 (0.01–0.03) | <0.0001 | 0.02 (0.01–0.03) | <0.0001 |
| 2-Nap/nicotine Eq ×103 | 1.91 (1.50–2.61) | 1.54 (1.13–2.42) | <0.0001 | 1.46 (1.05–2.36) | <0.0001 |
| Sum of fluorene/nicotine Eq×103 | 0.43 (0.33–0.61) | 0.29 (0.21–0.38) | <0.0001 | 0.30 (0.23–0.37) | <0.0001 |
| <0.0001 | |||||
| Sum of phenanthrene/nicotine Eq×103 | 0.16 (0.11–0.33) | 0.06 (0.04–0.08) | <0.0001 | 0.06 (0.04–0.08) | <0.0001 |
| Sum of PAHs/nicotine Eq×103 | 2.65 (2.08–3.68) | 1.99 (1.44–3.00) | <0.0001 | 1.81 (1.40–2.73) | <0.0001 |
Mann-Whitney rank sum test unless otherwise noted.
Mean and 95% CI.
t test.
1-HP, 1-hydroxypyrene; 2-Nap, 2-naphthol; CPD, cigarettes per day; Eq, equivalents; NNAL, 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol; NS, not significant; PAH, polycyclic aromatic hydrocarbon.
Among US smokers, men were significantly older (39.7 vs 36.1 years, p=0.02), tar yield was higher (14.2 vs 12.8, p=0.03) and the number of cigarettes smoked per day was higher (20.6 vs 17.8, p=0.02) compared with women. Among US smokers, comparing black versus white smokers, there were significant differences in age (42 vs 36.3 years), tar yield (16.2 vs 12.3 mg) and cigarettes smoked per day (17.5 vs 20.5). There were no significant black versus white differences in TNE, NNAL or metabolites of 1-HP, fluorene or phenanthrenes per cigarette smoked. 2-Nap excretion per cigarette was significantly lower in black versus white smokers (medians 4.49 vs 5.79, p=0.008).
Nicotine and carcinogen exposure
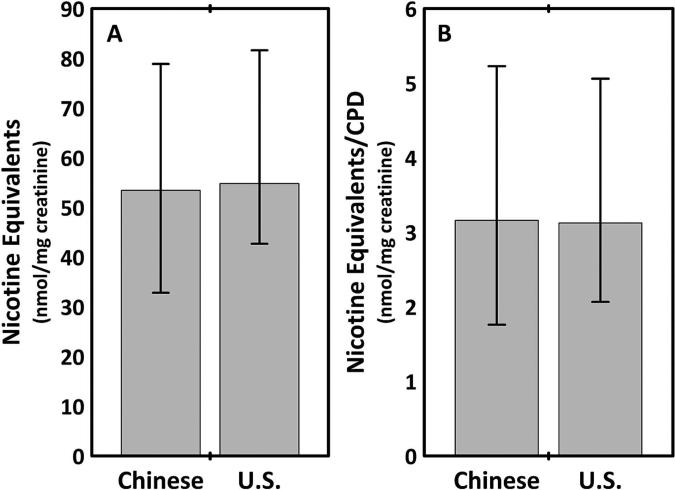
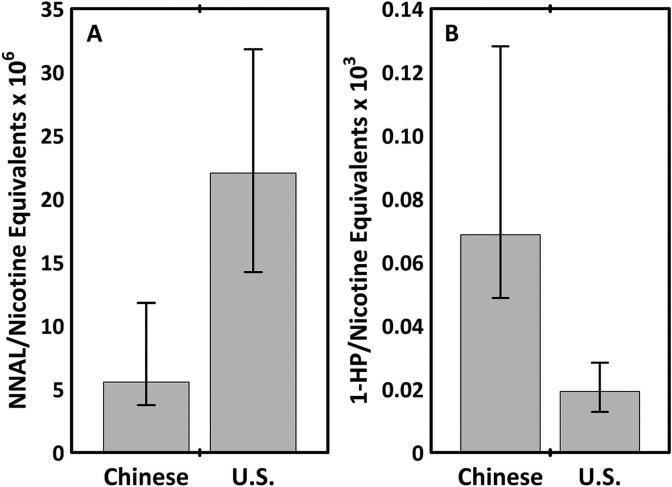
Daily nicotine intake, assessed as urinary TNE per milligram creatinine or TNE per cigarette smoked per day, was not significantly different in Chinese compared with US smokers (table 1, figure 1). Urine NNAL levels, expressed either as absolute values, normalised for the number of cigarettes smoked per day or normalised for urine TNE were approximately fourfold higher in US compared with Chinese smokers (figure 2). Urine concentrations of three PAH metabolites (1-HP, sum of fluorene and sum of phenanthrene metabolites) as well as the sum of all PAH metabolites were significantly higher in Chinese compared with US smokers, for absolute values or when normalised for cigarettes per day or for nicotine equivalents. The excretion of 1-HP and phenanthrene metabolites was approximately threefold higher in Chinese compared with US smokers. The excretion of the PAH metabolite 2-Nap, expressed as absolute values or normalised for cigarettes per day, was not significantly different in Chinese versus US smokers, but when normalised for urine TNE 2-Nap excretion it was significantly higher in Chinese smokers. Differences between Chinese and US smokers were similar when women were included or excluded from the analysis.
Figure 1.
(A) Urine total nicotine equivalents (TNE); (B) TNE/cigarettes per day (CPD) ratio in Chinese compared with US smokers. Data shown as medians and IQ intervals.
Figure 2.
(A) Urine 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol/urine total nicotine equivalents ratio (NNAL/TNE); (B) urine 1-hydroxypyrene (1-HP)/TNE ratio in Chinese compared with US smokers. Data shown as medians and IQ intervals.
DISCUSSION
We present novel data on urine biomarkers of nicotine and carcinogen exposure among Chinese compared with US smokers. We found that the daily intake of nicotine, assessed by urinary TNE, as well as nicotine intake per cigarette, was similar in Chinese compared with US smokers. Measured either as absolute concentrations or as concentrations normalised for cigarettes smoked per day, exposure to the TSNA NNK, assessed by urinary total NNAL, was fourfold higher in US compared with Chinese smokers. However, exposure to three PAHs was significantly higher among Chinese compared with US smokers. On average, pyrene and phenanthrene exposures were 3–4-fold higher and fluorene exposure was 50% higher in Chinese compared with US smokers. Differences in NNAL and PAH exposure were similar after normalisation by daily nicotine intake, as assessed by urine TNE. These findings may have implications in understanding differences in tobacco-related disease risks among smokers in the two countries.
Although not directly compared with US smokers and not normalised for nicotine intake, urine NNAL levels in Chinese smokers as reported by Yuan et al10 were lower than those typically found in US smokers.27 Other authors have reported higher levels of r-1, t-2, 3, c-4-tertrahydroxy-1, 2, 3, 4-tetrahydrophenanthrene (Phe-T), a metabolite of the PAH phenanthrene in non-smokers from Shanghai compared with non-smokers in the USA, but are unaware of a direct comparison of multiple PAH metabolites in Chinese versus US smokers, or of prior studies in which PAH exposure was normalised for nicotine intake (a marker of total smoke exposure).28
TSNAs and PAHs are two major classes of tobacco carcinogens.29 The nitrosamine NNK has been implicated in causing oral cancer, lung cancer and pancreatic cancer.30 TSNA levels in cigarette tobacco vary widely among cigarettes and across countries.11,12 TSNA levels in the mainstream smoke of one popular Chinese cigarette brand were found to be almost 40-fold lower than those of two popular US cigarette brands.12 TSNAs are formed during the curing, processing and fermenting of cigarette tobacco, and may also form during cigarette combustion.29,31 The level of TSNAs depends on the type of tobacco used, the nitrate content of the tobacco and the curing process. Chinese cigarettes tend to be made of bright tobacco which is flue-cured and is relatively low in TSNA content. In contrast, US cigarettes are typically made of blends of tobacco that contain considerable amounts of burley and reconstituted tobacco that have much higher TSNA levels.14,15 Thus, country difference in the type of tobacco used and the way tobacco is processed most likely underlie our observed country differences in NNK exposure.
PAHs are a diverse group of carcinogens formed during the combustion of tobacco and other organic materials. PAHs are found in tobacco smoke, broiled foods and polluted environments. Several of the higher molecular weight PAHs such as benzo(a)pyrene are highly carcinogenic in animals, including induction of lung tumours.31 PAHs form adducts with DNA, which are found in p53 mutations and have been associated with increased risk of human lung cancer.32,33 Akplan et al34 reported that the PAH levels in mainstream smoke from Chinese cigarettes were higher than those of European cigarettes, although in that study the nicotine and tar levels were also higher in Chinese cigarettes.
To the best of our knowledge, our study is the first to describe the excretion of multiple tobacco smoke-derived PAH metabolites in Chinese smokers. We measured several PAH metabolites because different sources of combustion may result in different patterns of PAH generation and exposure. For example, we recently reported different patterns of PAH expousure comparing smokers of cigarettes versus water pipers.35 We observed that country differences were greater for some PAHs (pyrene and phenanthrene) than for others (fluorene and napthalene). As mentioned previously, phe-T, a metabolite of phenanthrene, has been found to be higher in Shanghai non-smokers compared with US non-smokers. PAH generation from cigarettes appears to be related to the nitrate content and the type of tobacco.15,36 PAH generation is inversely related to the nitrate content, believed to be a result of nitrogen oxides formed during tobacco combustion scavenging carbon and hydrogen radicals that are major precursors for the pyrosynthesis of PAHs.15 PAH yields are higher from bright tobacco, the primary type of tobacco in Chinese cigarettes; and PAH yields are lower in reconstituted and burley tobacco, as found in US blends. Thus, our findings of country differences in urinary PAH excretion are consistent with expectations from differences in the type of tobacco used in Chinese vs US cigarettes. In general, factors that increase TSNA levels decrease PAHs generation and vice versa. In this study, machine-determined tar yields were lower on average for cigarettes smoked by Chinese compared with US smokers. Such differences are not likely to explain our results because tar emissions by machine testing are not meaningful measures of smoke exposure.37 Since smokers smoke to obtain desired levels of nicotine, it is important to consider carcinogen exposure in relation to nicotine intake.38 We found that large country differences in nitrosamine and PAH exposure remained after normalising for each individual's daily intake of nicotine.
PAHs are important environmental pollutants formed by the incomplete combustion of organic materials. Major sources are motor vehicle exhaust, coal and oil fed power plants and cooking. Since industrial pollution may be higher in Shanghai than in San Francisco, one must consider the contribution of environmental sources for the higher PAHs observed in Chinese smokers, which has been observed for phenanthrene and pyrene in Chinese non-smokers exposed to industrial pollution.28,39 Also, there may be greater exposure to fried foods, another source of PAHs, among Chinese smokers. An argument against environmental sources as the sole explanation is that in Chinese as well as US smokers there were similarly strong correlations between various urine PAH metabolite levels and level of cigarette smoke exposure, evidenced either by cigarettes per day or urine TNE (table 2). We reasoned that if the PAHs metabolites in Chinese smokers were derived primarily from environmental pollution rather than tobacco smoke, there would be weaker correlations between PAH metabolites and tobacco smoke exposure among Chinese compared with US smokers. That the correlations were similarly strong suggests that differences in environmental exposures do not fully explain country differences in PAH exposure. We cannot, however, exclude the possibility that among Chinese smokers there is a correlation between cigarette smoking and exposure to environmental pollutants, which could contribute to the positive correlations between PAH exposure and cigarettes per day or TNE.
Table 2.
Correlation between biomarkers within groups by country (Chinese vs US smokers)
| CPD | Nicotine Eq | NNAL | 1-HP | 2-Nap | Sum of fluorene | |
|---|---|---|---|---|---|---|
| Nicotine Eq | 0.23* vs 0.25* | |||||
| NNAL | 0.28* vs 0.27* | 0.49* vs 0.65* | ||||
| 1-HP | 0.20* vs 0.19 | 0.39* vs 0.39* | 0.32* vs 0.39* | |||
| 2-Nap | 0.19* vs 0.37* | 0.76* vs 0.62* | 0.48* vs 0.47* | 0.48* vs 0.44* | ||
| Sum of fluorene | 0.26* vs 0.23* | 0.73* vs 0.66* | 0.53* vs 0.54* | 0.54* vs 0.72* | 0.75* vs 0.65* | |
| Sum of phenanthrenes | 0.18* vs 0.13 | 0.22* vs 0.41* | 0.29* vs 0.35* | 0.70* vs 0.79* | 0.32* vs 0.43* | 0.56* vs 0.78* |
Spearman rank coefficients.
Significant correlation, p<0.05.
1-HP, 1-hydroxypyrene; 2-Nap, 2-naphthol; CPD, cigarettes per day; Eq, equivalents; NNAL, 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol.
A limitation of our study is that we do not have a non-smoker control group for PAH exposure in China. In addition, our Chinese smokers came primarily from one city in China, Shanghai, and US smokers came from one city in the USA, San Francisco. This raises questions about generalisability. In support of generalisability among the San Francisco smokers is that biomarker data of this population are similar to that reported in NHANES, which is a representative US population.40,41 We know of no similar biomarker data collected from the general Chinese population. However, since all Chinese cigarette brands are sold nationally, one would expect that similar products are used in Shanghai compared with other parts of China, and we can expect biomarkers of carcinogen exposure to be similar to national values. The Chinese smokers worked as taxi drivers, which may have exposed them to higher levels of air pollution than the US smokers who were volunteer research participants with various occupations. Another generalisability concern arises from the heterogeneity of sex and race in US smokers. Sex does not appear to be an explanation in that differences in biomarkers of exposure between Chinese and US smokers were similar with or without inclusion of women in the analysis. A comparison of differences in biomarkers in US African-American versus Caucasian smokers indicated few differences, so we do not believe that racial heterogeneity explains the observed country differences.
Both TSNAs and PAHs are tumorigenic and urine metabolite levels have been independently associated with the risk of lung cancer among smokers.8,9,10,42 Our data demonstrate that the ratio of NNK/PAH exposure is much higher in US compared with Chinese smokers. The ratio of NNK/PAH exposure among US smokers has changed from the 1950s to the present, during which time PAH deliveries have declined while NNK levels have increased. Among US smokers, the incidence of lung cancer has increased during this period of time, with a substantial increase in the proportion of lung cancers that are adenocarcinoma compared with squamous cell carcinoma.43 In that Chinese smokers have a markedly lower NNK exposure, lung cancer risk would be expected to decrease, but as they have higher PAH exposure, their lung cancer risk would be expected to increase compared with US smokers. The net effect of this oppositional change in carcinogen exposure is unknown, but based on historical trends in exposure and lung cancer risk in US smokers, one must consider the possibility that differences in toxicant exposure contribute to country differences in lung cancer risk. Since the Chinese smoker toxicant profile resembles the US smoker profile of many years ago, a lower overall risk of lung cancer and a greater proportion of squamous cell carcinoma might be expected. We are unaware of any data on the histological types of lung cancer among Chinese smokers. It would be of interest to determine whether the proportion of squamous cell carcinoma versus adenocarcinoma is higher in Chinese smokers, resembling that of US smokers in the 1950s.
Higher PAH and lower NNK exposure among Chinese smokers could be associated with different risks of tobacco-associated cancers other than lung cancer. In addition, urinary PAH metabolite excretion, independent of cigarette smoking, has been associated with inflammatory biomarkers that are predictive of cardiovascular disease risk.44 In any case, country differences in cigarette composition and related differences in exposure to tobacco smoke toxicants should be considered in comparative disease epidemiology studies.
In summary, Chinese and US smokers have strikingly different profiles of carcinogen exposure, with or without normalisation for cigarettes smoked or intake of nicotine per day. Lower nitrosamine exposure among Chinese smokers could explain, at least in part, the lower lung cancer rates in Chinese compared with US smokers. Higher PAH exposure among Chinese smokers from cigarette smoking and environmental pollution could result in increases in other types of cancer, and this possibility warrants further exploration. Country-specific differences in tobacco carcinogen exposure should be considered in assessing international differences in smoking-related disease epidemiology.
What this paper adds
Patterns of carcinogen exposure differ, with lower exposure to tobacco-specific nitrosamines and higher exposure to polycyclic aromatic hydrocarbons, in Chinese compared with US smokers. Most likely, this reflects country differences in cigarette tobacco blends and manufacturing processes, and possibly environmental exposures. Country differences in cigarette composition and exposure to tobacco smoke toxicants should be considered in comparative smoking and health epidemiology studies.
Acknowledgements
The authors thank Yao Haihong, Miao Sun, Wang Yuheng and Chen Yisheng for their help in conducting the survey. They are grateful to Lisa Yu and Trisha Mao for performing the nicotine metabolite analyses, to Olivia Yturralde for the PAH metabolite analyses and to Christopher Havel for analytical chemistry advice and for performing the NNAL analyses, and Scott Rostler for editorial assistance. Funding for the laboratory infrastructure in the Division of Clinical Pharmacology at UCSF was provided by the National Institutes of Health, P30 DA012393.
Funding National Cancer Institute Training Grant CA-113710, the William Cahan Endowment and the UCSF Bland Lane Center of Excellence on Secondhand Smoke, both funded by the Flight Attendants Medical Research Institute, USPHS Grant DA01293 from National Institute on Drug Abuse and the UCSF Helen Diller Family Comprehensive Cancer Center, and the China CDC.
Footnotes
Trial registration number NCT00264342.
To cite: Benowitz NL, Gan Q, Goniewicz ML, et al. Tob Control Published Online First: [please include Day Month Year] doi:10.1136/tobaccocontrol-2014-051945
NLB serves as a paid consultant to pharmaceutical companies that are developing or that market smoking cessation medications. He has also been a paid expert witness in litigation against tobacco companies, including on issues related to light cigarettes. None of the other authors have any competing interests to declare.
Patient consent Obtained.
Ethics approval This study was conducted with the approval of the UCSF and Shanghai CDC.
Provenance and peer review Not commissioned; externally peer reviewed.
Data sharing statement Permission for data sharing was not obtained when consent for study participation was requested.
Contributors NLB, QG and SG contributed to the planning, conduct and reporting of the study; MLG, WL, JX, XL and PJ contributed to the conduct and reporting of the study. NLB is the guarantor.
REFERENCES
- 1.Gu D, Kelly TN, Wu X, et al. Mortality attributable to smoking in China. N Engl J Med. 2009;360:150–9. doi: 10.1056/NEJMsa0802902. [DOI] [PubMed] [Google Scholar]
- 2.Zhang J, Ou JX, Bai CX. Tobacco smoking in China: prevalence, disease burden, challenges and future strategies. Respirology. 2011;16:1165–72. doi: 10.1111/j.1440-1843.2011.02062.x. [DOI] [PubMed] [Google Scholar]
- 3.Thun MJ, Carter BD, Feskanich D, et al. 50-year trends in smoking-related mortality in the United States. N Engl J Med. 2013;368:351–64. doi: 10.1056/NEJMsa1211127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fujieda M, Yamazaki H, Saito T, et al. Evaluation of CYP2A6 genetic polymorphisms as determinants of smoking behavior and tobacco-related lung cancer risk in male Japanese smokers. Carcinogenesis. 2004;25:2451–8. doi: 10.1093/carcin/bgh258. [DOI] [PubMed] [Google Scholar]
- 5.Liu T, Xie CB, Ma WJ, et al. Association between CYP2A6 genetic polymorphisms and lung cancer: a meta-analysis of case-control studies. Environ Mol Mutagen. 2013;54:133–40. doi: 10.1002/em.21751. [DOI] [PubMed] [Google Scholar]
- 6.Hecht SS. Lung carcinogenesis by tobacco smoke. Int J Cancer. 2012;131:2724–32. doi: 10.1002/ijc.27816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yuan JM, Butler LM, Stepanov I, et al. Urinary tobacco smoke-constituent biomarkers for assessing risk of lung cancer. Cancer Res. 2014;74:401–11. doi: 10.1158/0008-5472.CAN-13-3178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Church TR, Anderson KE, Caporaso NE, et al. A prospectively measured serum biomarker for a tobacco-specific carcinogen and lung cancer in smokers. Cancer Epidemiol Biomarkers Prev. 2009;18:260–6. doi: 10.1158/1055-9965.EPI-08-0718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hecht SS, Murphy SE, Stepanov I, et al. Tobacco smoke biomarkers and cancer risk among male smokers in the Shanghai Cohort Study. Cancer Lett. 2013;334:34–8. doi: 10.1016/j.canlet.2012.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yuan JM, Gao YT, Murphy SE, et al. Urinary levels of cigarette smoke constituent metabolites are prospectively associated with lung cancer development in smokers. Cancer Res. 2011;71:6749–57. doi: 10.1158/0008-5472.CAN-11-0209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ashley DL, Beeson MD, Johnson DR, et al. Tobacco-specific nitrosamines in tobacco from U.S. brand and non-US. brand cigarettes. Nicotine Tob Res. 2003;5:323–31. doi: 10.1080/1462220031000095311. [DOI] [PubMed] [Google Scholar]
- 12.Wu W, Zhang L, Jain RB, et al. Determination of carcinogenic tobacco-specific nitrosamines in mainstream smoke from U.S.-brand and non-U.S.-brand cigarettes from 14 countries. Nicotine Tob Res. 2005;7:443–51. doi: 10.1080/14622200500125898. [DOI] [PubMed] [Google Scholar]
- 13.Gray N, Zaridze D, Robertson C, et al. Variation within global cigarette brands in tar, nicotine, and certain nitrosamines: analytic study. Tob Control. 2000;9:351. doi: 10.1136/tc.9.3.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fischer S, Spiegelhalder B, Preussmann R. Preformed tobacco-specific nitrosamines in tobacco—role of nitrate and influence of tobacco type. Carcinogenesis. 1989;10:1511–17. doi: 10.1093/carcin/10.8.1511. [DOI] [PubMed] [Google Scholar]
- 15.Hoffmann D, Hoffmann I. The changing cigarette, 1950–1995. J Toxicol Environ Health. 1997;50:307–64. doi: 10.1080/009841097160393. [DOI] [PubMed] [Google Scholar]
- 16.Adams JD, Lee SJ, Hoffmann D. Carcinogenic agents in cigarette smoke and the influence of nitrate on their formation. Carcinogenesis. 1984;5:221–3. doi: 10.1093/carcin/5.2.221. [DOI] [PubMed] [Google Scholar]
- 17.Gan Q, Lu W, Xu J, et al. Chinese ‘low-tar’ cigarettes do not deliver lower levels of nicotine and carcinogens. Tob Control. 2010;19:374–9. doi: 10.1136/tc.2009.033092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Benowitz NL, Dains KM, Dempsey D, et al. Racial differences in the relationship between number of cigarettes smoked and nicotine and carcinogen exposure. Nicotine Tob Res. 2011;13:772–83. doi: 10.1093/ntr/ntr072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Benowitz NL, Dains KM, Hall SM, et al. Smoking behavior and exposure to tobacco toxicants during 6 months of smoking progressively reduced nicotine content cigarettes. Cancer Epidemiol Biomarkers Prev. 2012;21:761–9. doi: 10.1158/1055-9965.EPI-11-0644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dempsey D, Tutka P, Jacob P, III, et al. Nicotine metabolite ratio as an index of cytochrome P450 2A6 metabolic activity. Clin Pharmacol Ther. 2004;76:64–72. doi: 10.1016/j.clpt.2004.02.011. [DOI] [PubMed] [Google Scholar]
- 21.Jacob P, III, Havel C, Lee DH, et al. Subpicogram per milliliter determination of the tobacco-specific carcinogen metabolite 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol in human urine using liquid chromatography-tandem mass spectrometry. Anal Chem. 2008;80:8115–21. doi: 10.1021/ac8009005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jacob P, III, Wilson M, Benowitz NL. Determination of Phenolic Metabolites of Polycyclic Aromatic Hydrocarbons in Human Urine as Their Pentafluorobenzyl Ether Derivatives Using Liquid Chromatography-Tandem Mass Spectrometry. Anal Chem. 2007;79:587–98. doi: 10.1021/ac060920l. [DOI] [PubMed] [Google Scholar]
- 23.Benowitz NL, Jacob P, III, Fong I, et al. Nicotine metabolic profile in man: comparison of cigarette smoking and transdermal nicotine. J Pharmacol Exp Ther. 1994;268:296–303. [PubMed] [Google Scholar]
- 24.Feng S, Kapur S, Sarkar M, et al. Respiratory retention of nicotine and urinary excretion of nicotine and its five major metabolites in adult male smokers. Toxicol Lett. 2007;173:101–6. doi: 10.1016/j.toxlet.2007.06.016. [DOI] [PubMed] [Google Scholar]
- 25.Benowitz NL, Dains KM, Dempsey D, et al. Estimation of nicotine dose after low-level exposure using plasma and urine nicotine metabolites. Cancer Epidemiol Biomarkers Prev. 2010;19:1160–6. doi: 10.1158/1055-9965.EPI-09-1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Benowitz NL, Hukkanen J, Jacob P., III Nicotine chemistry, metabolism, kinetics and biomarkers. Handb Exp Pharmacol. 2009:29–60. doi: 10.1007/978-3-540-69248-5_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xia Y, Bernert JT, Jain RB, et al. Tobacco-specific nitrosamine 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol (NNAL) in smokers in the United States: NHANES 2007– 2008. Biomarkers. 2011;16:112–19. doi: 10.3109/1354750X.2010.533288. [DOI] [PubMed] [Google Scholar]
- 28.Yuan JM, Butler LM, Gao YT, et al. Urinary metabolites of a polycyclic aromatic hydrocarbon and volatile organic compounds in relation to lung cancer development in lifelong never smokers in the Shanghai Cohort Study. Carcinogenesis. 2014;35:339–45. doi: 10.1093/carcin/bgt352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hecht SS. Tobacco carcinogens, their biomarkers and tobacco-induced cancer. Nat Rev Cancer. 2003;3:733–44. doi: 10.1038/nrc1190. [DOI] [PubMed] [Google Scholar]
- 30.Gray N, Boyle P. The case of the disappearing nitrosamines: a potentially global phenomenon. Tob Control. 2004;13:13–16. doi: 10.1136/tc.2003.005579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.IARC Working Group on the Evaluation of Carcinogenic Risks to Humans Tobacco smoke and involuntary smoking. IARC Monogr Eval Carcinog Risks Hum. 2004;83:1–1438. [PMC free article] [PubMed] [Google Scholar]
- 32.Smith LE, Denissenko MF, Bennett WP, et al. Targeting of lung cancer mutational hotspots by polycyclic aromatic hydrocarbons. J Natl Cancer Inst. 2000;92:803–11. doi: 10.1093/jnci/92.10.803. [DOI] [PubMed] [Google Scholar]
- 33.HHS . How tobacco smoke causes disease: the biology and behavioral basis for smoking-attributable disease: a report of the surgeon general. US. Department of Health and Human Services, Centers for Disease Control and Prevention, Coordinating Center for Health Promotion, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; Atlanta, GA: 2010. [PubMed] [Google Scholar]
- 34.Akpan V, Huang S, Lodovici M, et al. High levels of carcinogenic polycyclic aromatic hydrocarbons (PAH) in 20 brands of Chinese cigarettes. J Appl Toxicol. 2006;26:480–3. doi: 10.1002/jat.1165. [DOI] [PubMed] [Google Scholar]
- 35.Jacob P, III, Abu Raddaha AH, Dempsey D, et al. Comparison of nicotine and carcinogen exposure with water pipe and cigarette smoking. Cancer Epidemiol Biomarkers Prev. 2013;22:765–72. doi: 10.1158/1055-9965.EPI-12-1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ding YS, Zhang L, Jain RB, et al. Levels of tobacco-specific nitrosamines and polycyclic aromatic hydrocarbons in mainstream smoke from different tobacco varieties. Cancer Epidemiol Biomarkers Prev. 2008;17:3366–71. doi: 10.1158/1055-9965.EPI-08-0320. [DOI] [PubMed] [Google Scholar]
- 37.Benowitz NL. Compensatory smoking of low-yield cigarettes. Risks associated with smoking cigarettes with low machine yields of tar and nicotine Smoking and Tobacco Control Monograph No 13. Vol. 13. National Cancer Institute; 2001. pp. 39–63. [Google Scholar]
- 38.Burns DM, Dybing E, Gray N, et al. Mandated lowering of toxicants in cigarette smoke: a description of the World Health Organization TobReg proposal. Tob Control. 2008;17:132–41. doi: 10.1136/tc.2007.024158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wu MT, Simpson CD, Christiani DC, et al. Relationship of exposure to coke-oven emissions and urinary metabolites of benzo(a)pyrene and pyrene in coke-oven workers. Cancer Epidemiol Biomarkers Prev. 2002;11:311–14. [PubMed] [Google Scholar]
- 40.Bernert JT, Pirkle JL, Xia Y, et al. Urine concentrations of a tobacco-specific nitrosamine carcinogen in the US. population from secondhand smoke exposure. Cancer Epidemiol Biomarkers Prev. 2010;19:2969–77. doi: 10.1158/1055-9965.EPI-10-0711. [DOI] [PubMed] [Google Scholar]
- 41.Suwan-ampai P, Navas-Acien A, Strickland PT, et al. Involuntary tobacco smoke exposure and urinary levels of polycyclic aromatic hydrocarbons in the United States, 1999 to 2002. Cancer Epidemiol Biomarkers Prev. 2009;18:884–93. doi: 10.1158/1055-9965.EPI-08-0939. [DOI] [PubMed] [Google Scholar]
- 42.Yuan JM, Gao YT, Wang R, et al. Urinary levels of volatile organic carcinogen and toxicant biomarkers in relation to lung cancer development in smokers. Carcinogenesis. 2012;33:804–9. doi: 10.1093/carcin/bgs026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.HHS . The health consequences of smoking—50 years of progress. A report of the surgeon general. US. Department of Health and Human Services, Centers for Disease Control and Prevention, Coordinating Center for Health Promotion, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; Atlanta, GA: 2014. [PubMed] [Google Scholar]
- 44.Alshaarawy O, Zhu M, Ducatman A, et al. Polycyclic aromatic hydrocarbon biomarkers and serum markers of inflammation. A positive association that is more evident in men. Environ Res. 2013;126:98–104. doi: 10.1016/j.envres.2013.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]