Abstract
Aneuploidy in human eggs is the leading cause of pregnancy loss and several genetic disorders such as Down’s syndrome. Most aneuploidy results from chromosome segregation errors during the meiotic divisions of an oocyte, the egg’s progenitor cell. The basis for particularly error-prone chromosome segregation in human oocytes is not known. Here we analyzed meiosis in over 100 live human oocytes and identified an error-prone chromosome-mediated spindle assembly mechanism as major contributor to chromosome segregation defects. Human oocytes assembled a meiotic spindle independently of either centrosomes or other microtubule organizing centers. Instead, spindle assembly was mediated by chromosomes and the small GTPase Ran in a process requiring ~16 hours. This unusually long spindle assembly period was marked by intrinsic spindle instability and abnormal kinetochore-microtubule attachments, which favor chromosome segregation errors and provide a possible explanation for high rates of aneuploidy in human eggs.
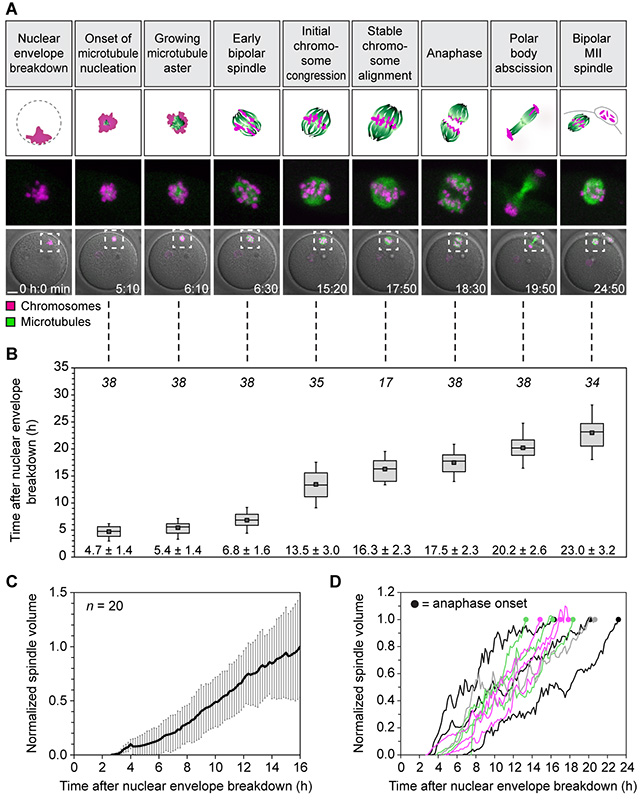
Meiosis in human oocytes is more prone to chromosome segregation errors than mitosis (1, 2), meiosis during spermatogenesis (3, 4) and female meiosis in other organisms (3, 5). Despite its importance for fertility and human development, meiosis in human eggs has hardly been studied. Human oocytes are only available in small numbers, warranting single-cell assays capable of extracting maximal information. While high resolution-live cell microscopy is an ideal method, oocyte development in the ovary poses challenges to direct imaging. We therefore established an experimental system (6) for ex vivo high resolution fluorescence microscopy of human oocytes freshly harvested from women undergoing gonadotropin-stimulated in vitro fertilization cycles. To establish the major stages of meiosis in this system, we simultaneously monitored microtubules and chromosomes for ~24-48 hours (Fig. 1 and movie S1). Similar to the situation in situ (7), human oocytes matured into fertilizable eggs over this time course as judged by the formation of a polar body. The morphologically identifiable stages (Fig. 1A) at characteristic times after nuclear envelope breakdown (NEBD, set to 0 hours) provided a time-resolved framework for human oocyte meiosis (Fig. 1B). This reference timeline post NEBD is used throughout this paper.
Fig. 1. Stages of meiosis in live human oocytes.
(A) Stages of meiosis in human oocytes determined from live human oocytes expressing EGFP-MAP4 (microtubules) and H2B-mRFP1 (chromosomes). A schematic representation of each stage (scheme; microtubules in green; chromosomes in magenta) and stage-specific time-lapse images (z-projections, 4 sections, every 5 μm) merged with differential interference contrast [DIC] are shown (bottom row). Outlined regions are magnified above (middle row). Scale bar, 20 μm. Time displayed in hours: minutes.
(B) Quantification of timing of meiotic progression from live oocytes expressing EGFP-MAP4 (microtubules) and H2B-mRFP1 (chromosomes) as shown in (A). The box plot shows median (line), mean (small square), and 25th and 75th (boxes), 5th and 95th percentile (whiskers) of time after NEBD. The number of oocytes is specified in italics. Only oocytes in which the whole maturation process was recorded (from before NEBD to bipolar MII spindle formation) were included.
(C and D) The spindle volume was quantified in live human oocytes expressing EGFP-MAP4 (microtubules) as shown in (A). Averaged data from 20 oocytes (C) and examples of individual curves up until anaphase onset (D) are shown.
Before NEBD, chromosomes were highly condensed and clustered around the nucleolus. Instead of rapidly nucleating microtubules upon NEBD, human oocytes first formed a chromosome aggregate that was largely devoid of microtubules (Fig. 1A, movie S1 and fig. S1, A and B). Microtubules were first observed at ~5 hours, when they started to form a small aster within the chromosome aggregate. As the microtubule aster grew, the chromosomes became individualized and oriented on the surface of the aster with their kinetochores facing inwards. The microtubule aster then extended into an early bipolar spindle that carried the chromosomes on its surface (Fig. 1A, movie S1 and fig. S1, C to E). The chromosomes then entered the spindle but remained distributed throughout the entire spindle volume. Chromosomes first congressed in the spindle center at ~13 hours but continued to oscillate around the spindle equator. Stable chromosome alignment was typically only achieved close to anaphase onset (Fig. 1A, movie S1). Unexpectedly, the spindle volume increased over the entire course of meiosis, up until anaphase onset (Fig. 1, C and D). The barrel-shaped spindle formed in this process consisted of loosely clustered bundles of microtubules and lacked astral microtubules (movie S2 and fig. S2). At ~17 hours, the oocytes progressed into anaphase and eliminated half of the homologous chromosomes in a polar body. Nearly a day after NEBD, the oocytes had formed a bipolar metaphase II spindle and matured into a fertilizable egg. The stages and timing of meiosis were highly reproducible among oocytes (Fig. 1, A and B) and could also be observed in fixed oocytes (fig. S1, A to I). Importantly, 78.95% of imaged human oocytes extruded a polar body. This indicates that the imaging assays as well as the methods by which the oocytes were obtained and processed did not have a prominent effect on meiotic progression.
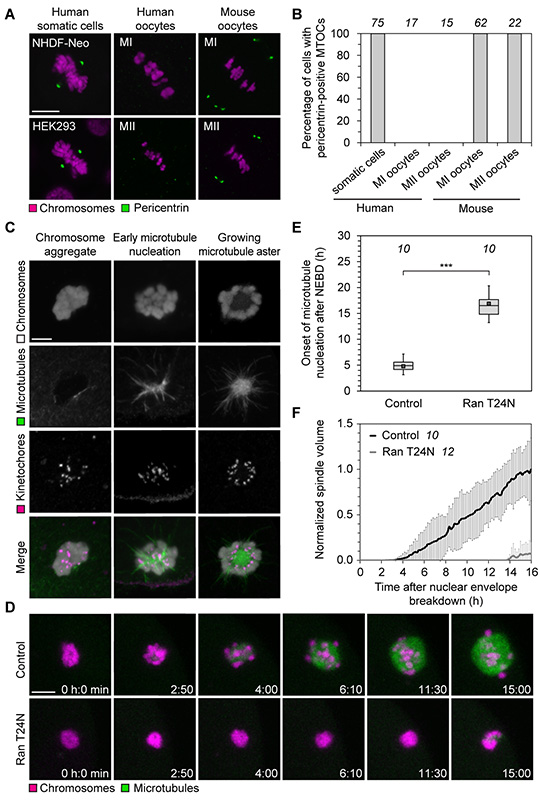
The surprisingly slow and gradual build-up of the spindle over 16 hours (Fig. 1, C and D) is in stark contrast to mitosis, where spindle assembly takes only around 30 minutes (8), or meiosis in mouse oocytes, where it takes 3-5 hours (9-11). During mitosis, two centrosomes ensure the rapid assembly of a spindle. In oocytes of many species, centrosomes are absent, but functionally replaced by microtubule organizing centers (MTOCs) that lack centrioles (9, 12). Human oocytes also lack centrosomes (13-15), but whether acentriolar MTOCs participate in spindle assembly is unclear (16-19). We consistently detected pericentrin- and γ-tubulin-positive MTOCs at the spindle poles of mitotic cells and metaphase I and II (MI and MII) mouse oocytes, but never at MI or MII spindles in human oocytes (Fig. 2, A and B, fig. S3). Thus, meiotic spindles in human oocytes lack detectable MTOCs.
Fig. 2. Chromosomes mediate spindle assembly in human oocytes.
(A) Immunofluorescence staining of pericentrin and chromosomes (Hoechst) in somatic cells, mouse and human MI and MII oocytes. Scale bars, 10 μm.
(B) Spindles of somatic cells as well as metaphase I (MI) and metaphase II (MII) spindles in mouse and human oocytes as shown in (A) were scored for the presence of pericentrin-positive MTOCs. The number of cells is specified in italics.
(C) Immunofluorescence staining (z-projections of 6 sections, every 0.3 μm) of kinetochores (CREST), microtubules (α-tubulin) and chromosomes (Hoechst) in human oocytes fixed at different times shortly after NEBD. Scale bar, 10 μm.
(D) Live human oocytes expressing H2B-mRFP1 (chromosomes) and EGFP-MAP4 (microtubules) upon microinjection with Ran T24N (lower panel) or BSA (top panel). z-projections of 4 sections, every 5 μm. Scale bar, 10 μm.
(E) Onset of microtubule nucleation in live human oocytes expressing EGFP-MAP4 upon microinjection with either Ran T24N or BSA. Box plot as in Fig. 1B. The number of oocytes is specified in italic. ***P<10−14 (t-test). Two Ran T24N-injected oocytes never nucleated microtubules.
(F) The spindle volume was quantified in live human oocytes expressing EGFP-MAP4 upon microinjection with either Ran T24N or BSA. The number of oocytes is specified in italics.
In Xenopus egg extracts, chromosomes can serve as sites of microtubule nucleation if centrosomes are absent (20). Also human oocytes initiated microtubule nucleation in the region of the chromosome aggregate (78/78 live human oocytes). High-resolution imaging of fixed human oocytes confirmed that microtubules were first nucleated on chromosomes, emanating primarily from kinetochores (Fig. 2C, movie S3 and fig. S4). MTOC-nucleated cytoplasmic asters, such as those seen in chromosomal proximity upon NEBD in mouse oocytes (9), could not be detected. Thus, chromosomes, not MTOCs, serve as major sites of microtubule nucleation in human oocytes.
Chromatin-mediated spindle assembly is driven by the small GTPase Ran. GTP-bound Ran is replenished around chromosomes by its chromatin-bound GTP exchange factor RCC1 and locally releases spindle assembly factors from inhibitory binding to importins (21-23). To test if Ran-GTP is required for spindle assembly in human oocytes, we blocked its function with the GDP-locked mutant Ran T24N, which acts as a dominant-negative variant of Ran (24, 25). Ran T24N severely delayed the onset of microtubule nucleation and impaired spindle assembly (Fig. 2, D to F, and movie S4). In mouse and Drosophila oocytes, spindles have defects but still assemble if Ran is inhibited (9, 26, 27). Thus, our data suggest that spindle assembly in human oocytes is independent of MTOCs, but mediated by chromosomes and dependent on Ran-GTP.
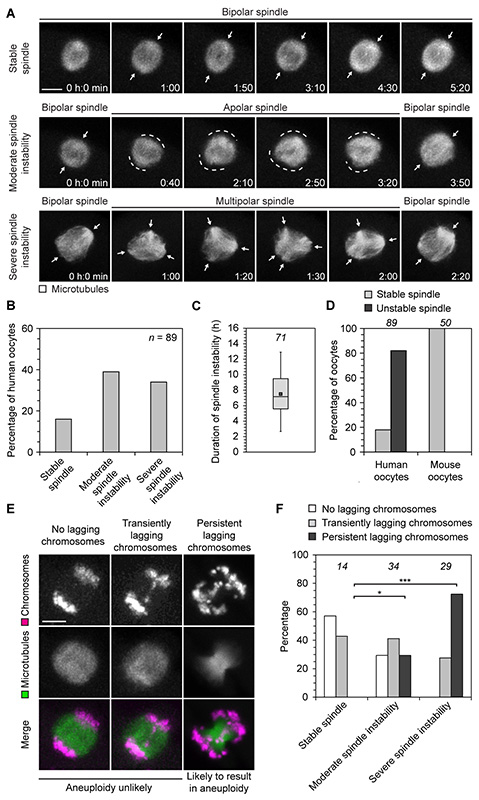
The period between chromosome-mediated microtubule nucleation (~5 hours) and establishment of a bipolar spindle with aligned chromosomes (~16 hours) displayed significant spindle instability. Although the microtubule aster transformed into a bipolar spindle at ~7 hours, this spindle often failed to maintain bipolarity (Fig. 3A and movie S5). In 44% of oocytes, the spindles rounded up and became apolar, which we classified as moderate spindle instability. In 38% of oocytes, the spindles even progressed through a prolonged multipolar stage, which we classified as severe spindle instability (Fig. 3, A and B). Multipolar and apolar spindles were also observed in fixed oocytes that had not been imaged (fig. S6). Although spindle instability lasted for an average of 7.5 ± 3.1 hours (Fig. 3C), the vast majority of oocytes progressed into anaphase with bipolar spindles and extruded a polar body (fig. S5). The apparent instability of the spindle in human oocytes is in stark contrast to mitotic spindles and meiotic spindles in other species such as mouse oocytes, which rarely become unstable upon establishment of a bipolar spindle (Fig. 3D).
Fig. 3. Spindle instability correlates with chromosome segregation errors.
(A) Live human oocytes expressing EGFP-MAP4 (microtubules). z-projections of 4-5 sections, every 3-4 μm. Arrows highlight defined spindle poles; dashed lines mark undefined spindle poles. Scale bar, 10 μm. Polar body extrusion in these cells is shown in fig. S5B.
(B) Live human oocytes expressing EGFP-MAP4 as shown in (A) were scored for the presence and degree of spindle instability. n specifies number of oocytes.
(C) The duration of spindle instability was measured in live human oocytes expressing EGFP-MAP4 as shown in (A). Box plot as in Fig. 1B. The number of oocytes is specified in italics.
(D) Live human and mouse oocytes expressing EGFP-MAP4 (microtubules) were scored for the presence of spindle instability. The number of oocytes is specified in italic.
(E) Illustration of classes of lagging chromosomes in live human oocytes expressing H2B-mRFP1 (chromosomes) and EGFP-MAP4 (microtubules). z-projections of 3-5 sections, every 3-5 μm. Scale bar, 10 μm.
(F) Live human oocytes expressing H2B-mRFP1 (chromosomes) and EGFP-MAP4 (microtubules) as shown in (E) were scored for the presence of transiently lagging or persistent lagging chromosomes. The number of oocytes is specified in italics. *P<0.05, ***P<10−6 (Fisher’s exact test).
Next, we investigated if spindle instability correlates with chromosome segregation errors. Normal chromosome segregation is characterized by simultaneous separation of all homologous chromosomes. Chromosomes that lag behind during anaphase increase the possibility of aneuploidy due to inappropriate partitioning of chromosomes upon cytokinesis. We thus scored our imaging dataset for the presence and degree of lagging chromosomes and spindle instability. Oocytes with prominent chromosome bridges or chromosomes that remained in the center of the spindle during anaphase were classified as having persistent lagging chromosomes. Oocytes with a few chromosomes that segregated more slowly than the rest of the chromosomes but did not remain in the center of the spindle were classified as having transiently lagging chromosomes (Fig. 3E). Strikingly, 72% of oocytes with severe spindle instability went on to have persistent lagging chromosomes during anaphase (Fig. 3F). In contrast, oocytes with a stable spindle were never observed to have persistent lagging chromosomes. Oocytes with unstable spindles were also significantly more likely to have chromosome alignment defects (fig. S7).
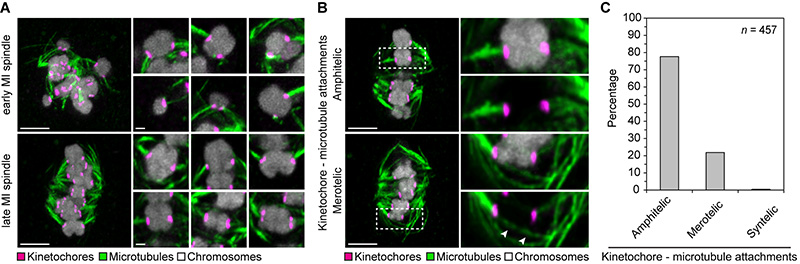
The chromosome segregation defects could be due to progression into anaphase with abnormal kinetochore-microtubule attachments. To test this hypothesis, we fixed oocytes using a cold treatment assay that preferentially preserves kinetochore-associated microtubules. During early spindle assembly, the kinetochores were already associated with microtubules, but a prominent spindle axis was absent. The kinetochore fibres were instead randomly oriented relative to each other and only partially focused into several small poles distributed throughout the chromosome-microtubule assembly. Oocytes fixed close to anaphase onset had formed a bipolar spindle, in which most bivalent chromosomes were bi-oriented (Fig. 4A, and movie S6). But unexpectedly, only around 80% of kinetochores were correctly attached to microtubules, being linked to a single spindle pole (amphitelic attachment). By contrast, 20% of kinetochores remained attached to both spindle poles (merotelic attachment) (Fig. 4, B and C, fig. S8). These data suggest that human oocytes are less efficient in correcting kinetochore-microtubule attachments than mitotic cells (28) and mouse oocytes (29). Our observation that persistent lagging chromosomes are most likely to occur in oocytes with severe spindle instability suggests that these oocytes are particularly likely to progress into anaphase with abnormal kinetochore-microtubule attachments.
Fig. 4. Correction of kinetochore-microtubule attachments is incomplete close to anaphase.
(A) Immunofluorescence staining (z-projections of 13 sections, every 0.3 μm) of kinetochores (CREST) and microtubules (α-tubulin) in cold-treated human oocytes fixed during early or late spindle assembly. Chromosomes labelled with Hoechst. Scale bar overview, 5 μm. High resolution images of 6 individual chromosome bivalents from overview are shown on the right (z-projections of 2 sections, every 0.3 μm). Scale bar details, 1 μm.
(B) Illustration of amphitelic and merotelic kinetochore-microtubule attachments. Immunofluorescence staining (z-projections of 3 sections, every 0.3 μm) of kinetochores (CREST) and microtubules (α-tubulin) in cold-treated human oocytes fixed close to anaphase onset. Chromosomes labelled with Hoechst. The outlined regions are magnified on the right. Arrowheads highlight merotelically attached microtubules. Scale bar, 5 μm. (A and B) All images were deconvolved. Background signal outside of the spindle area was masked in kinetochore channel (magenta).
(C) 10 cold-treated oocytes fixed close to anaphase onset (as shown in B) were scored for amphitelic, merotelic or syntelic kinetochore-microtubule attachments. In one case, the bivalent’s kinetochores were attached to the same instead of opposite poles (syntelic attachment).
This study allows us to draw several conclusions with implications for the causes of egg aneuploidy. The single most striking feature of human oocyte meiosis is an unusually dynamic and slowly assembling meiotic spindle. This feature could be the consequence of absent centrosomes or other MTOCs, either of which could more rapidly generate a bipolar spindle. Instead, chromosomes and Ran-GTP are employed for spindle assembly. The spindles assembled by this mechanism display a high proportion of abnormal kinetochore-microtubule attachments. The spindles are also intrinsically unstable, and the degree of spindle instability correlates with the degree of chromosome segregation errors. Spindle instability could hinder the establishment of accurate kinetochore-microtubule attachments and thereby promote chromosome segregation errors. Alternatively, spindle instability may reflect attempts of the chromosomes to establish stable bipolar microtubule attachments, which could be more challenging in human oocytes, possibly due structural features of their chromosomes. Progression into anaphase with these abnormal attachments would put the oocyte at risk of chromosome segregation errors (fig. S9), providing at least one mechanism for the relatively frequent aneuploidy of eggs, even in young women (3, 30). Our findings may also explain why human oocytes are more prone to aneuploidy than oocytes from mouse or other organisms, where the presence of MTOCs may render spindle assembly and chromosome segregation more efficient.
Supplementary Material
Acknowledgments
We are grateful to the clinicians at Bourn Hall Clinic for their support of this study, the nursing team who were instrumental in recruiting patients and obtaining their informed consent, and also to the Embryology team for identifying and preparing the test oocytes and for their enthusiastic support of this project from the outset.
We thank T. Izzard for help in obtaining approval of this study by the UK’s National Research Ethics Service; S. Munro, R. Hegde and D. Clift for comments on the manuscript. The research leading to these results has received financial support from the European Research Council under grant agreement no. 337415 and from the Lister Institute of Preventive Medicine.
Footnotes
References and Notes
- 1.Nagaoka SI, Hassold TJ, Hunt PA. Human aneuploidy: mechanisms and new insights into an age-old problem. Nat Rev Genet. 2012;13:493–504. doi: 10.1038/nrg3245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Knouse KA, Wu J, Whittaker CA, Amon A. Single cell sequencing reveals low levels of aneuploidy across mammalian tissues. Proc Natl Acad Sci U S A. 2014;111:13409–13414. doi: 10.1073/pnas.1415287111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pacchierotti F, Adler ID, Eichenlaub-Ritter U, Mailhes JB. Gender effects on the incidence of aneuploidy in mammalian germ cells. Environ Res. 2007;104:46–69. doi: 10.1016/j.envres.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 4.Templado C, Vidal F, Estop A. Aneuploidy in human spermatozoa. Cytogenet Genome Res. 2011;133:91–99. doi: 10.1159/000323795. [DOI] [PubMed] [Google Scholar]
- 5.Danylevska A, Kovacovicova K, Awadova T, Anger M. The frequency of precocious segregation of sister chromatids in mouse female meiosis I is affected by genetic background. Chromosome Res. 2014;22:365–373. doi: 10.1007/s10577-014-9428-6. [DOI] [PubMed] [Google Scholar]
- 6.Materials and methods are available as supplementary materials on Science Online.
- 7.Gemzell CA. Induction of ovulation with human pituitary gonadotrophins. Fertil Steril. 1962;13:153–168. doi: 10.1016/s0015-0282(16)34445-4. [DOI] [PubMed] [Google Scholar]
- 8.Held M, Schmitz MH, Fischer B, Walter T, Neumann B, et al. CellCognition: time-resolved phenotype annotation in high-throughput live cell imaging. Nat Methods. 2010;7:747–754. doi: 10.1038/nmeth.1486. [DOI] [PubMed] [Google Scholar]
- 9.Schuh M, Ellenberg J. Self-organization of MTOCs replaces centrosome function during acentrosomal spindle assembly in live mouse oocytes. Cell. 2007;130:484–498. doi: 10.1016/j.cell.2007.06.025. [DOI] [PubMed] [Google Scholar]
- 10.Holubcova Z, Howard G, Schuh M. Vesicles modulate an actin network for asymmetric spindle positioning. Nat Cell Biol. 2013;15:937–947. doi: 10.1038/ncb2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kolano A, Brunet S, Silk AD, Cleveland DW, Verlhac MH. Error-prone mammalian female meiosis from silencing the spindle assembly checkpoint without normal interkinetochore tension. Proc Natl Acad Sci U S A. 2012;109:E1858–1867. doi: 10.1073/pnas.1204686109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Manandhar G, Schatten H, Sutovsky P. Centrosome reduction during gametogenesis and its significance. Biol Reprod. 2005;72:2–13. doi: 10.1095/biolreprod.104.031245. [DOI] [PubMed] [Google Scholar]
- 13.Szollosi D, Mandelbaum J, Plachot M, Salat-Baroux J, Cohen J. Ultrastructure of the human preovulatory oocyte. J In Vitro Fert Embryo Transf. 1986;3:232–242. doi: 10.1007/BF01132810. [DOI] [PubMed] [Google Scholar]
- 14.Sathananthan AH, Selvaraj K, Girijashankar ML, Ganesh V, Selvaraj P, et al. From oogonia to mature oocytes: inactivation of the maternal centrosome in humans. Microsc Res Tech. 2006;69:396–407. doi: 10.1002/jemt.20299. [DOI] [PubMed] [Google Scholar]
- 15.Sathananthan AH. Ultrastructure of human gametes, fertilization and embryos in assisted reproduction: a personal survey. Micron. 2013;44:1–20. doi: 10.1016/j.micron.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 16.Pickering SJ, Johnson MH, Braude PR, Houliston E. Cytoskeletal organization in fresh, aged and spontaneously activated human oocytes. Hum Reprod. 1988;3:978–989. doi: 10.1093/oxfordjournals.humrep.a136828. [DOI] [PubMed] [Google Scholar]
- 17.Battaglia DE, Goodwin P, Klein NA, Soules MR. Influence of maternal age on meiotic spindle assembly in oocytes from naturally cycling women. Hum Reprod. 1996;11:2217–2222. doi: 10.1093/oxfordjournals.humrep.a019080. [DOI] [PubMed] [Google Scholar]
- 18.Battaglia DE, Klein NA, Soules MR. Changes in centrosomal domains during meiotic maturation in the human oocyte. Mol Hum Reprod. 1996;2:845–851. doi: 10.1093/molehr/2.11.845. [DOI] [PubMed] [Google Scholar]
- 19.George MA, Pickering SJ, Braude PR, Johnson MH. The distribution of alpha- and gamma-tubulin in fresh and aged human and mouse oocytes exposed to cryoprotectant. Mol Hum Reprod. 1996;2:445–456. doi: 10.1093/molehr/2.6.445. [DOI] [PubMed] [Google Scholar]
- 20.Heald R, Tournebize R, Blank T, Sandaltzopoulos R, Becker P, et al. Self-organization of microtubules into bipolar spindles around artificial chromosomes in Xenopus egg extracts. Nature. 1996;382:420–425. doi: 10.1038/382420a0. [DOI] [PubMed] [Google Scholar]
- 21.Carazo-Salas RE, Guarguaglini G, Gruss OJ, Segref A, Karsenti E, et al. Generation of GTP-bound Ran by RCC1 is required for chromatin-induced mitotic spindle formation. Nature. 1999;400:178–181. doi: 10.1038/22133. [DOI] [PubMed] [Google Scholar]
- 22.Gruss OJ, Carazo-Salas RE, Schatz CA, Guarguaglini G, Kast J, et al. Ran induces spindle assembly by reversing the inhibitory effect of importin alpha on TPX2 activity. Cell. 2001;104:83–93. doi: 10.1016/s0092-8674(01)00193-3. [DOI] [PubMed] [Google Scholar]
- 23.Karsenti E, Vernos I. The mitotic spindle: a self-made machine. Science. 2001;294:543–547. doi: 10.1126/science.1063488. [DOI] [PubMed] [Google Scholar]
- 24.Carazo-Salas RE, Gruss OJ, Mattaj IW, Karsenti E. Ran-GTP coordinates regulation of microtubule nucleation and dynamics during mitotic-spindle assembly. Nat Cell Biol. 2001;3:228–234. doi: 10.1038/35060009. [DOI] [PubMed] [Google Scholar]
- 25.Klebe C, Bischoff FR, Ponstingl H, Wittinghofer A. Interaction of the nuclear GTP-binding protein Ran with its regulatory proteins RCC1 and RanGAP1. Biochemistry. 1995;34:639–647. doi: 10.1021/bi00002a031. [DOI] [PubMed] [Google Scholar]
- 26.Dumont J, Petri S, Pellegrin F, Terret ME, Bohnsack MT, et al. A centriole- and RanGTP-independent spindle assembly pathway in meiosis I of vertebrate oocytes. J Cell Biol. 2007;176:295–305. doi: 10.1083/jcb.200605199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cesario J, McKim KS. RanGTP is required for meiotic spindle organization and the initiation of embryonic development in Drosophila. Journal of cell science. 2011;124:3797–3810. doi: 10.1242/jcs.084855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cimini D, Moree B, Canman JC, Salmon ED. Merotelic kinetochore orientation occurs frequently during early mitosis in mammalian tissue cells and error correction is achieved by two different mechanisms. Journal of cell science. 2003;116:4213–4225. doi: 10.1242/jcs.00716. [DOI] [PubMed] [Google Scholar]
- 29.Kitajima TS, Ohsugi M, Ellenberg J. Complete kinetochore tracking reveals error-prone homologous chromosome biorientation in mammalian oocytes. Cell. 2011;146:568–581. doi: 10.1016/j.cell.2011.07.031. [DOI] [PubMed] [Google Scholar]
- 30.Obradors A, Rius M, Daina G, Ramos L, Benet J, et al. Whole-chromosome aneuploidy analysis in human oocytes: focus on comparative genomic hybridization. Cytogenet Genome Res. 2011;133:119–126. doi: 10.1159/000324233. [DOI] [PubMed] [Google Scholar]
- 31.Faddy M, Gosden R, Ahuja K, Elder K. Egg sharing for assisted conception: a window on oocyte quality. Reprod Biomed Online. 2011;22:88–93. doi: 10.1016/j.rbmo.2010.08.009. [DOI] [PubMed] [Google Scholar]
- 32.Rienzi L, Martinez F, Ubaldi F, Minasi MG, Iacobelli M, et al. Polscope analysis of meiotic spindle changes in living metaphase II human oocytes during the freezing and thawing procedures. Hum Reprod. 2004;19:655–659. doi: 10.1093/humrep/deh101. [DOI] [PubMed] [Google Scholar]
- 33.Jaffe LA, Terasaki M. Quantitative microinjection of oocytes, eggs, and embryos. Methods Cell Biol. 2004;74:219–242. doi: 10.1016/s0091-679x(04)74010-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Strickland L, von Dassow G, Ellenberg J, Foe V, Lenart P, et al. Light microscopy of echinoderm embryos. Methods Cell Biol. 2004;74:371–409. doi: 10.1016/s0091-679x(04)74016-9. [DOI] [PubMed] [Google Scholar]
- 35.Otsuki J, Nagai Y, Chiba K. Lipofuscin bodies in human oocytes as an indicator of oocyte quality. J Assist Reprod Genet. 2007;24:263–270. doi: 10.1007/s10815-007-9130-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.