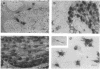
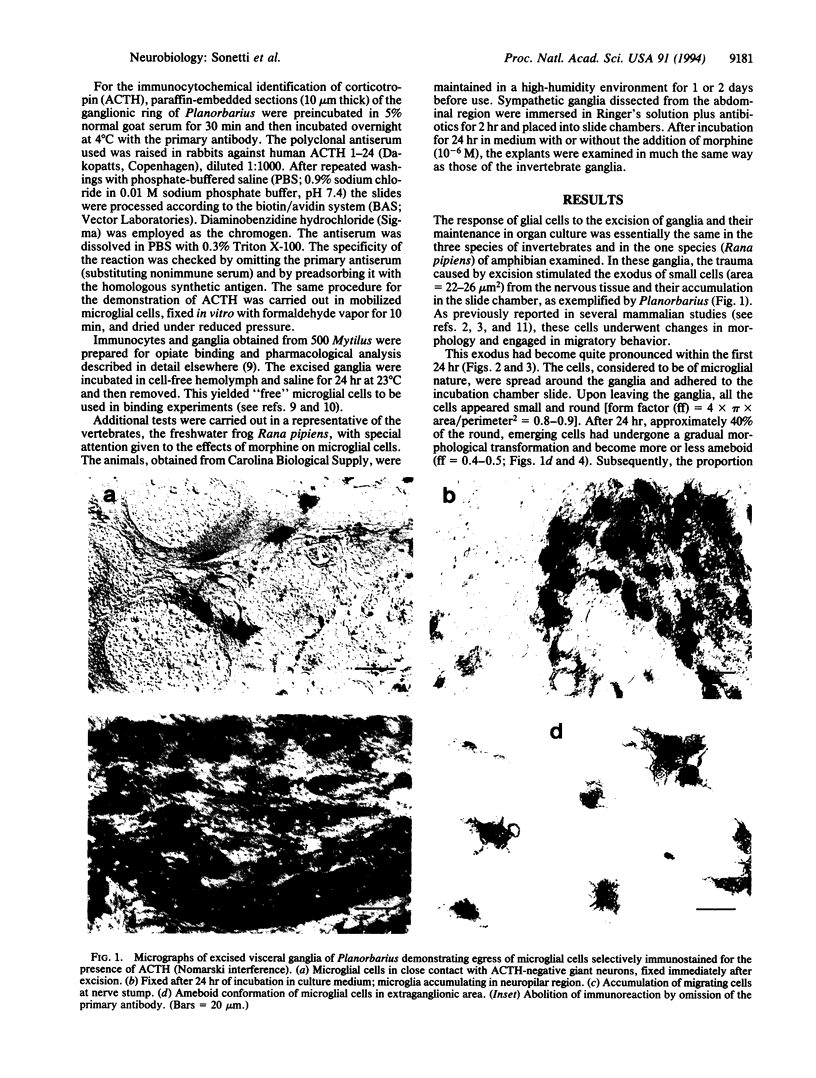
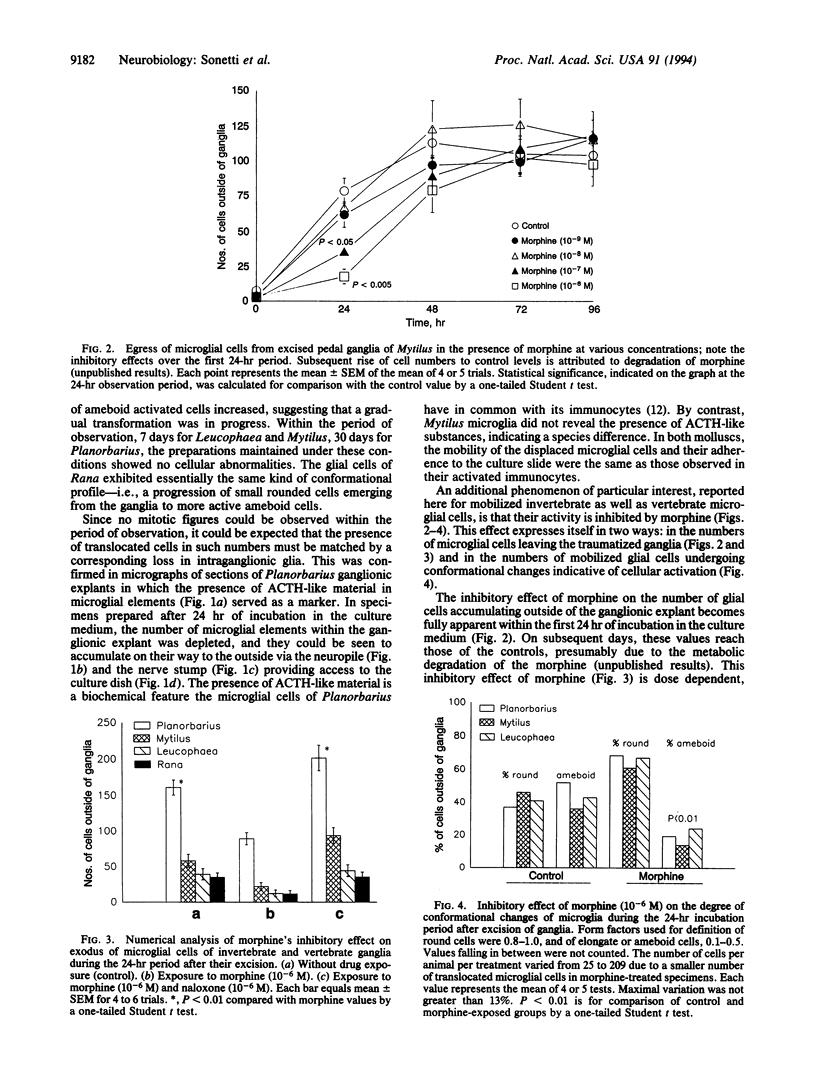
Abstract
The results of this study lend strong support to the concept of the existence in insects and molluscs of a distinctive class of neuroglial cells comparable to vertebrate microglia. The evidence presented is as valid as that used in reference to the separate status of vertebrate microglia--i.e., the demonstration of a close structural and functional relationship of these cells with cells of the immune system. As in vertebrates, the excision of ganglia from three invertebrate species (the molluscs Planorbarius corneus and Mytilus edulis and the insect Leucophaea maderae) and their maintenance in incubation media led to an exodus of small cells and their accumulation in the culture dish. During this process, they underwent conformational changes from stellate to rounded, and then to more or less ameboid, comparable to those indicative of the process of activation in the animals' immunocytes. Functional characteristics which these translocated microglia-like cells share with immunocytes are motility, phagocytotic activity, and adherence to the culture dish. Furthermore, the two cells have certain biochemical features in common--e.g., the presence of certain cytokines and (at least in Planorbarius) that of corticotropin. An additional phenomenon of particular interest for the classification of microglial elements is their response to morphine. At 10(-6) M, this drug decreases not only the number of cells emerging from the excised ganglia but also the degree of their transformation to the "active" ameboid form. This dose-dependent and naloxone-sensitive effect of morphine on microglial cells parallels that on activated immunocytes of the same species. Corresponding results demonstrating an inhibitory effect of morphine on mobilized microglial cells of the frog Rana pipiens indicate that this relationship between the two cell types under consideration also exists in vertebrates. Binding and displacement experiments with membrane homogenates of microglial cells as well as immunocytes of Mytilus have shown that the effects of morphine on both cell types are mediated by the same special opiate receptor (mu 3).
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banati R. B., Gehrmann J., Schubert P., Kreutzberg G. W. Cytotoxicity of microglia. Glia. 1993 Jan;7(1):111–118. doi: 10.1002/glia.440070117. [DOI] [PubMed] [Google Scholar]
- Bittner G. D., Mann D. W. Differential survival of isolated portions of crayfish axons. Cell Tissue Res. 1976 Jun 28;169(3):301–311. doi: 10.1007/BF00219603. [DOI] [PubMed] [Google Scholar]
- Dickson D. W., Mattiace L. A., Kure K., Hutchins K., Lyman W. D., Brosnan C. F. Microglia in human disease, with an emphasis on acquired immune deficiency syndrome. Lab Invest. 1991 Feb;64(2):135–156. [PubMed] [Google Scholar]
- Feinberg I. Schizophrenia: caused by a fault in programmed synaptic elimination during adolescence? J Psychiatr Res. 1982;17(4):319–334. doi: 10.1016/0022-3956(82)90038-3. [DOI] [PubMed] [Google Scholar]
- Fontana A., Kristensen F., Dubs R., Gemsa D., Weber E. Production of prostaglandin E and an interleukin-1 like factor by cultured astrocytes and C6 glioma cells. J Immunol. 1982 Dec;129(6):2413–2419. [PubMed] [Google Scholar]
- Howes E. A., Armett-Kibel C., Smith P. J. A blood-derived attachment factor enhances the in vitro growth of two glial cell types from adult cockroach. Glia. 1993 May;8(1):33–41. doi: 10.1002/glia.440080105. [DOI] [PubMed] [Google Scholar]
- Morgese V. J., Elliott E. J., Muller K. J. Microglial movement to sites of nerve lesion in the leech CNS. Brain Res. 1983 Aug 1;272(1):166–170. doi: 10.1016/0006-8993(83)90375-x. [DOI] [PubMed] [Google Scholar]
- Ottaviani E., Caselgrandi E., Franchini A., Franceschi C. CRF provokes the release of norepinephrine by hemocytes of Viviparus ater (Gastropoda, Prosobranchia): further evidence in favour of the evolutionary hypothesis of the mobile immune-brain. Biochem Biophys Res Commun. 1993 May 28;193(1):446–452. doi: 10.1006/bbrc.1993.1644. [DOI] [PubMed] [Google Scholar]
- Ottaviani E., Caselgrandi E., Petraglia F., Franceschi C. Stress response in the freshwater snail Planorbarius corneus (L.) (Gastropoda, Pulmonata): interaction between CRF, ACTH, and biogenic amines. Gen Comp Endocrinol. 1992 Sep;87(3):354–360. doi: 10.1016/0016-6480(92)90041-h. [DOI] [PubMed] [Google Scholar]
- Ottaviani E., Cossarizza A., Ortolani C., Monti D., Franceschi C. ACTH-like molecules in gastropod molluscs: a possible role in ancestral immune response and stress. Proc Biol Sci. 1991 Sep 23;245(1314):215–218. doi: 10.1098/rspb.1991.0112. [DOI] [PubMed] [Google Scholar]
- Ottaviani E., Petraglia F., Montagnani G., Cossarizza A., Monti D., Franceschi C. Presence of ACTH and beta-endorphin immunoreactive molecules in the freshwater snail Planorbarius corneus (L.) (Gastropoda, Pulmonata) and their possible role in phagocytosis. Regul Pept. 1990 Jan;27(1):1–9. doi: 10.1016/0167-0115(90)90200-g. [DOI] [PubMed] [Google Scholar]
- PIPA R. L. Studies on the hexapod nervous system. III. Histology and histochemistry of cockroach neuroglia. J Comp Neurol. 1961 Feb;116:15–26. doi: 10.1002/cne.901160103. [DOI] [PubMed] [Google Scholar]
- Paemen L. R., Porchet-Hennere E., Masson M., Leung M. K., Hughes T. K., Jr, Stefano G. B. Glial localization of interleukin-1 alpha in invertebrate ganglia. Cell Mol Neurobiol. 1992 Oct;12(5):463–472. doi: 10.1007/BF00711547. [DOI] [PubMed] [Google Scholar]
- Perry V. H. The role of macrophages in models of neurological and psychiatric disorder. Psychol Med. 1992 Aug;22(3):551–555. doi: 10.1017/s0033291700038009. [DOI] [PubMed] [Google Scholar]
- Reinecke M. The glial cells of the cerebral ganglia of Helix pomatia L. (Gastropoda, Pulmonata). II. Uptake of ferritin and 3H-glutamate. Cell Tissue Res. 1976 Jun 28;169(3):361–382. doi: 10.1007/BF00219608. [DOI] [PubMed] [Google Scholar]
- Rieske E., Graeber M. B., Tetzlaff W., Czlonkowska A., Streit W. J., Kreutzberg G. W. Microglia and microglia-derived brain macrophages in culture: generation from axotomized rat facial nuclei, identification and characterization in vitro. Brain Res. 1989 Jul 17;492(1-2):1–14. doi: 10.1016/0006-8993(89)90883-4. [DOI] [PubMed] [Google Scholar]
- Stefano G. B., Bilfinger T. V., Fricchione G. L. The immune-neuro-link and the macrophage: postcardiotomy delirium, HIV-associated dementia and psychiatry. Prog Neurobiol. 1994 Mar;42(4):475–488. doi: 10.1016/0301-0082(94)90048-5. [DOI] [PubMed] [Google Scholar]
- Stefano G. B., Cadet P., Scharrer B. Stimulatory effects of opioid neuropeptides on locomotory activity and conformational changes in invertebrate and human immunocytes: evidence for a subtype of delta receptor. Proc Natl Acad Sci U S A. 1989 Aug;86(16):6307–6311. doi: 10.1073/pnas.86.16.6307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefano G. B., Digenis A., Spector S., Leung M. K., Bilfinger T. V., Makman M. H., Scharrer B., Abumrad N. N. Opiate-like substances in an invertebrate, an opiate receptor on invertebrate and human immunocytes, and a role in immunosuppression. Proc Natl Acad Sci U S A. 1993 Dec 1;90(23):11099–11103. doi: 10.1073/pnas.90.23.11099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefano G. B. Invertebrate and vertebrate neuroimmune and autoimmunoregulatory commonalties involving opioid peptides. Cell Mol Neurobiol. 1992 Oct;12(5):357–366. doi: 10.1007/BF00711538. [DOI] [PubMed] [Google Scholar]
- Stefano G. B., Leung M. K., Zhao X. H., Scharrer B. Evidence for the involvement of opioid neuropeptides in the adherence and migration of immunocompetent invertebrate hemocytes. Proc Natl Acad Sci U S A. 1989 Jan;86(2):626–630. doi: 10.1073/pnas.86.2.626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefano G. B., Melchiorri P., Negri L., Hughes T. K., Jr, Scharrer B. [D-Ala2]deltorphin I binding and pharmacological evidence for a special subtype of delta opioid receptor on human and invertebrate immune cells. Proc Natl Acad Sci U S A. 1992 Oct 1;89(19):9316–9320. doi: 10.1073/pnas.89.19.9316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefano G. B., Scharrer B. Endogenous morphine and related opiates, a new class of chemical messengers. Adv Neuroimmunol. 1994;4(2):57–67. doi: 10.1016/s0960-5428(05)80001-4. [DOI] [PubMed] [Google Scholar]