Abstract
Purpose
Little is known about the suitability of three commonly-used body mass index (BMI) classification system for Indigenous children. This study aims to estimate overweight and obesity prevalence among school-aged Nunavik Inuit children according to International Obesity Task Force (IOTF), Centers for Disease Control and Prevention (CDC), and World Health Organization (WHO) BMI classification systems, to measure agreement between those classification systems, and to investigate whether BMI status as defined by these classification systems is associated with levels of metabolic and inflammatory biomarkers.
Methods
Data were collected on 290 school-aged children (8–14 years; 50.7% girls) from the Nunavik Child Development Study (NCDS) with data collected in 2005–2010. Anthropometric parameters were measured and blood sampled. Participants were classified as normal weight, overweight and obese according to BMI classification systems. Weighted Kappa (kw) statistics assessed agreement between different BMI classification systems and multivariate analysis of variance ascertained their relationship with metabolic and inflammatory biomarkers.
Results
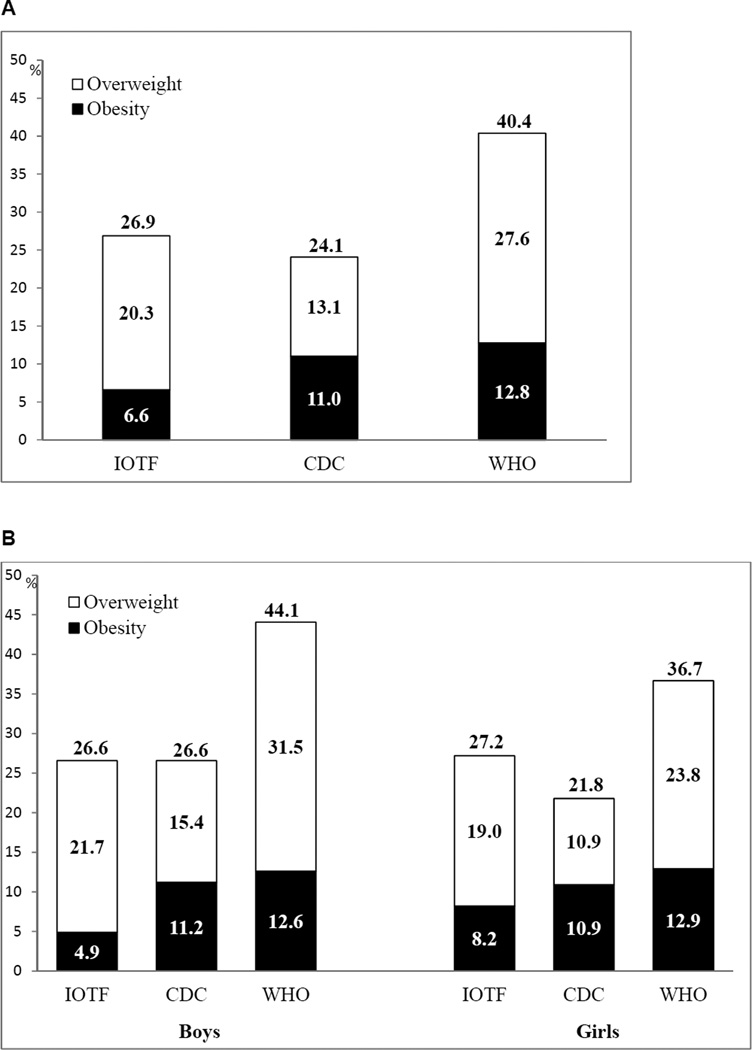
The combined prevalence rate of overweight/obesity was 26.9% (with 6.6% obesity) with IOTF, 24.1% (11.0%) with CDC, and 40.4% (12.8%) with WHO classification systems. Agreement was the highest between IOTF and CDC (kw=0.87) classifications, and substantial for IOTF and WHO (kw=0.69), and CDC and WHO (kw=0.73). Insulin and high-sensitivity C-reactive protein plasma levels were significantly higher from normal weight to obesity, regardless of classification system. Among obese subjects, higher insulin level was observed with IOTF.
Conclusion
Compared with other systems, IOTF classification appears to be more specific to identify overweight and obesity in Inuit children.
Keywords: Body mass index, Inuit, children, excess weight, overweight, obese, IOTF, CDC, WHO
Excess weight (overweight/obesity) in children is a significant public health concern because of its association with adverse obesity-related diseases [1, 2], poor psychological outcomes [3], and the heightened risk of becoming obese adults.[4] Moreover, obesity and related diseases increase the burden on healthcare systems [5], creating unhealthy and less productive societies. Early recognition of excess weight onset during childhood may identify groups at greatest risk and may spur programs to slow their excess growth.
Body mass index (BMI) [6] is the most widely used anthropometric measure of weight status [7]. Because BMI varies with age and gender during childhood and adolescence, its cut-off values – established for adults – could generate overweight and obesity misclassifications among children. At present, three growth references are commonly considered when assessing weight status in children: International Obesity Task Force (IOTF) [8], Centers for Disease Control and Prevention (CDC) [9], and 2007 World Health Organization (WHO) [10]. However, prevalence rates of overweight and obesity differ according to these BMI systems [11]. Among 5–17-year-old Canadians who participated in the 2009–2011 Canadian Health Measures Survey (CHMS) [12], the combined prevalence of overweight/obesity was 31.5% (with 11.7% obesity) according to WHO and 24.8% (with 8.4% obesity) according to IOTF. Among 6–19-year-old Americans who participated in the 2009–2010 National Health and Nutrition Examination Survey (NHANES) [13], combined overweight/obesity prevalence was 33.2% (including 18.2% obese) according to CDC [9]. The choice of BMI system is, therefore, critical in assessing excess childhood weight at a population level.
IOTF system (widely used internationally) is based on data from six large, nationally-representative, cross sectional surveys on child growth – in Brazil, Great Britain, Hong Kong, the Netherlands, Singapore, and the United States [8]. In contrast, the CDC system was developed from five nationally representative surveys of American children – from the National Health Examination Survey and NHANES [9]. Lastly, 2007 WHO system were derived from a combination of United States National Centre for Health Statistics 1977 pooled child growth data, and the WHO Multicentre Growth Reference Study in six countries (Brazil, Ghana, Norway, India, Oman, and the United States) [10]. Little is known about the suitability of these BMI classification systems for Indigenous populations of children likely to exhibit anthropometric parameters different from those upon which classifications are based. Since Canadian aboriginal children are not included in any of the above-mentioned datasets, studies are needed to compare and contrast prevalence estimates of overweight and obesity according to these different BMI classification systems. To our knowledge, no study has compared the three most widely-used criteria for BMI classification among school-aged Canadian Inuit children. To address this deficiency, overweight and obesity prevalence rates were estimated among Nunavik Inuit children with the IOTF, CDC, and WHO BMI classification systems, and agreement between different BMI criteria was assessed. In addition, metabolic and inflammatory parameters – associated with weight status – were investigated as concomitant validity measurements and to evaluate specificity of these classification systems.
METHODS
Study population and setting
The data in the present study were sourced from the Nunavik Child Development Study (NCDS, September 2005 to February 2010). The NCDS design has been described elsewhere [14]. Briefly, participants were school-aged Inuit children living in Nunavik, a region north of the 55th parallel in Arctic Quebec (Canada). The NCDS is a 11-year follow-up of school-aged children recruited before birth when their mothers participated in one of two cohort studies: the 1993–1998 Cord Blood Monitoring Program [15] and the 1996–2000 Environmental Contaminants and Child Development Study [16]. Inclusion criteria were age between 8.5 and 14.5 years, birth weight ≥2.5 kg, gestation duration ≥35 weeks, no major birth defects, and no major neurological or chronic health problems affecting growth. The NCDS was based on interviewer-administered questionnaires administered to participants’ caregivers (biological mothers in 67.6% of cases). The caregivers were met and interviewed to obtain information on socio-demographic background, food insecurity, obstetrical and child medical history as well as maternal lifestyle habits, including alcohol and drug use during pregnancy. Anthropometric parameters were recorded by two research nurses trained in standard measurement procedures. Weight was quantified on a digital balance, and height was recorded by stadiometer. Two measurements were taken for each parameter, and a third was obtained if a discrepancy occurred between them for weight (>500 g) and height (>0.5 cm). Final growth parameter values were based on the average of the two closest measurements. BMI was calculated as the ratio of weight (kg) to squared height (m2). Each child provided a non-fasting venous blood sample (20 mL) which was frozen in Nunavik at −80°C, transported by plane to the Centre de Toxicologie du Québec for biological analysis, or sent on dry ice to other laboratories, if necessary. Of the 294 initial participants, 4 were excluded because of missing data on height, which left 290 participants for the present analysis.
Ethical approval
Participation was voluntary and subject to written informed consent provided by each participant’s parents with oral assent given by each child. Consent and assent forms were approved by the Nunavik Nutrition and Health Committee and the Research Ethics Review Boards of Université Laval and Wayne State University.
BMI classification systems
Weight status of participants was defined according to the IOTF, CDC, and WHO BMI classification systems. 2005 IOTF cut-off values are extrapolations of adult BMI cut-off points for overweight (25 kg/m2) and obesity (30 kg/m2) at age 18 years [8]. The CDC defines overweight as 85th ≤BMI< 95th percentiles, and obesity as BMI ≥95th percentile of 2000 CDC gender-specific BMI-for-age growth charts [9]. The 2007 WHO classification system defines BMI-for-age >+1 standard deviation (SD) of the WHO growth standard median as overweight (equivalent to BMI=25 kg/m2 at 19 years) and >+2 SD as obesity (equivalent to BMI=30 kg/m2 at 19 years) [10]. Normal weight described participants who were neither overweight nor obese.
Biological parameters
Biological parameter concentrations of insulin, glucose, adiponectin, ferritin, high-sensitivity C-reactive protein (hs-CRP), interleukin-6 (IL-6), and tumor necrosis factor-alpha (TNFα) were quantified at Hôpital Laval. IL-6, TNFα, insulin, adiponectin and hs-CRP) were measured with Milliplex kits (Millipore, Billerica, MA, USA) in participants’ plasma by Luminex reader (Bio-Rad Lab, Hercules, CA, USA). Glucose concentration was analyzed by Amplex-Red Glucose assay kit, according to the manufacturer's instructions (Life Technologies).
Analytical analysis
The data are reported as arithmetic means (95% confidence interval [CI]) or geometric means (95% CI) for continuous variables with skewed distribution. Characteristics of participants by gender were compared by 2-sided t-tests. Prevalence estimates of weight status according to the three BMI classification systems were presented graphically, and differences (in percentage points) between boys and girls were compared by 2-sided t-tests. Agreements between BMI systems were assessed by kappa (k) coefficients. According to Landis and Koch’s guiding principles, k coefficients between 0.21 and 0.40 are considered as fair, between 0.41 and 0.60 as moderate, between 0.61 and 0.80 as substantial, and between 0.81 and 1 as almost perfect [17]. Whether metabolic and inflammatory biomarker levels were different between the BMI classification systems was investigated by multivariate analysis of variance. All statistical analyses were performed with SAS software (version 9.3, SAS Institute Inc., Cary, NC, USA) and 2-sided p<0.05 values were considered to be statistically significant.
RESULTS
Participants’ characteristics are presented by sex in Table 1. They were aged between 8 and 14 years, 51% were girls, and average BMI was 19.5 kg/m2. All characteristic variables were similar between boys and girls.
Table 1.
Characteristics of study participants aged 8 to 14 years, Nunavik, Canada, 2005–2010
| Total (n=290) | Boys (n=143) | Girls (n=147) | P* | |
|---|---|---|---|---|
| Age (years) | 10.8± 0.8 | 10.7 ± 0.9 | 10.8 ± 0.8 | 0.40 |
| Anthropometric data | ||||
| Height, cm | 141 ± 7.4 | 141 ± 7.3 | 141 ± 7.6 | 0.80 |
| Weight, kg | 40.1 ± 9.9 | 39.5 ± 9.0 | 40.7 ± 10.7 | 0.31 |
| BMI, kg/m2 | 19.8 ± 3.2 | 19.6 ± 2.7 | 20.1 ± 3.6 | 0.17 |
| BMI z-score | 0.64 ± 0.71 | 0.67 ± 0.69 | 0.61 ± 0.73 | 0.49 |
| Metabolic biomarkers | ||||
| Non-fasting plasma insulin, pmol/L | 133 ± 138 | 117 ± 126 | 148 ± 147 | 0.06 |
| Non-fasting plasma glucose, mmol/L | 6.30 ± 1.6 | 6.37 ± 1.7 | 6.25 ± 1.5 | 0.53 |
| Adiponectin, µg/ml | 11.4 ± 5.8 | 10.8 ± 5.9 | 11.9 ± 5.6 | 0.13 |
| Inflammatory biomarkers | ||||
| Ferritin, µg/L | 24.1 ± 24 | 22.2 ± 14 | 26.0 ± 30 | 0.18 |
| hs-CRP, mg/L† | 0.95 (0.76 to 1.18) | 0.87 (0.65 to 1.15) | 1.03 (0.74 to 1.42) | 0.44 |
| IL-6, pg/ml | 2.22 ± 3.2 | 2.00 ± 2.3 | 2.42 ± 3.8 | 0.29 |
| TNFα, pg/ml | 4.22 ± 2.1 | 4.31± 2.1 | 4.15 ± 2.1 | 0.54 |
Notes: Values are presented as arithmetic means ± SD, unless indicated otherwise
P-values were obtained using 2-sided t-tests
Geometric means (95% CI)
Abbreviations: BMI, body mass index; hs-CRP, high-sensitivity C-reactive protein; IL-6, interleukin-6; TNFα, tumor necrosis factor-alpha.
Information was missing for 24 participants on plasma insulin (15 boys vs. 9 girls); 28 participants on plasma glucose (18 boys vs.10 girls); 23 participants on adiponectin (15 boys vs. 8 girls); 05 participants on ferritin (2 boys vs. 3 girls); 25 participants on hs-CRP (16 boys vs. 9 girls); 24 participants on IL-6 (15 boys and 9 girls); 24 participants on TNF-α (15 boys vs. 9 girls)
Prevalence estimations of weight status categories (overweight and obesity) for each BMI classification criterion are provided for overall participants (Fig. 1A) and by gender (Fig. 1B). Regardless of BMI classification system, overweight prevalences appeared to be higher in boys than in girls. However, these differences were not statistically significant (2.7 percentage points for IOTF, 4.5 for CDC, and 7.7 for WHO). Obesity prevalence was similar between boys and girls according to CDC and WHO. In contrast, obesity prevalence was 1.7 times higher in girls than in boys according to IOTF, but the 3.3 percentage points difference was not statistically significant.
Figure 1.
Prevalence of normal weight, overweight, and obesity (A: whole study sample; B: by gender) according to IOTF, CDC, and WHO reference criteria among Inuit children, Nunavik, Canada, 2005–2010
Abbreviation: CDC, Centres for Disease Control and Prevention; WHO, World Health Organization; IOTF, International Obesity Task Force
Table 2 depicts the levels of agreement between different BMI criteria. The k statistic indicated almost perfect agreement between IOTF and CDC (k=0.87, 95% CI: 0.81–0.92; with 92.75% for % agreement). However, substantial agreement was noted between IOTF and WHO (k=0.69, 95% CI: 0.62–0.76; with 80.35% for % agreement), and between CDC and WHO (k=0.73, 95% CI: 0.66–0.80; with 82.07% for % agreement). Mean metabolic and inflammatory biomarker concentrations according to weight status derived from the three BMI classification systems are reported in Table 3. Mean non-fasting plasma insulin (pmol/L) and hs-CRP (mg/L) concentrations were significantly higher from normal weight to obesity regardless of BMI reference criteria (Ptrend<0.05). In addition, only WHO showed a significant trend for adiponectin (µg/ml) (Ptrend =0.03). Among obese participants, the higher mean concentrations of non-fasting plasma insulin were observed for IOTF (312, 95% CI: 252–371), followed by CDC (243, 95% CI: 196–289) and WHO (232, 95% CI: 188–275). In a sub-analysis, we compared plasma insulin levels among children who were considered obese by CDC or WHO classifications, but not IOTF classification (obese-discordant). Compared with IOTF, both CDC- and WHO-discordant obese children showed significantly lower plasma insulin concentrations: the difference was 164 pmol/L (95% CI: 26–302; p=0.02) between IOTF and CDC-discordant, and 160 pmol/L (95% CI: 22–298; p=0.03) between IOTF and WHO-discordant obese children. Therefore, children who were considered obese by CDC or WHO classifications, but not IOTF classification, exhibit a less severe clinical obesity.
Table 2.
Agreements between weight status based on IOTF, CDC and WHO classification
| IOTF | CDC | Total | ||
| Normal weight | Overweight | Obese | ||
| Normal weight | 212 (73.1%) | 0 | 0 | 212 |
| Overweight | 8 (2.76%) | 38 (13.1%) | 13 (4.48%) | 59 |
| Obese | 0 | 0 | 19 (6.55%) | 19 |
| Total | 220 | 38 | 32 | 290 |
| % agreement: 92.75%; kw=0.87 (95% CI, 0.81−0.92), P<0.001 | ||||
| IOTF | WHO | Total | ||
| Normal weight | Overweight | Obese | ||
| Normal weight | 173 (59.66%) | 39 (13.45%) | 0 | 212 |
| Overweight | 0 | 41 (14.14%) | 18 (6.21%) | 59 |
| Obese | 0 | 0 | 19 (6.55%) | 19 |
| Total | 173 | 80 | 37 | 290 |
| % agreement: 80.35%; kw=0.69 (95% CI, 0.62−0.76), P<0.001 | ||||
| CDC | WHO | Total | ||
| Normal weight | Overweight | Obese | ||
| Normal weight | 173 (59.66%) | 47 (16.21%) | 0 | 220 |
| Overweight | 0 | 33 (11.38%) | 5 (1.72%) | 38 |
| Obese | 0 | 0 | 32 (11.0%) | 32 |
| Total | 173 | 80 | 37 | 290 |
| % agreement: 82.07%; kw=0.73 (95% CI, 0.66−0.80), P<0.001 | ||||
Note: % agreement was calculated by adding the concordant percentages
Abbreviation: kw, weighted Kappa
Table 3.
Mean metabolic and inflammatory biomarker concentrations according to weight status classifications, Nunavik, Canada, 2005–2010
| Metabolic/inflammatory biomarkers | Normal weight | Overweight | Obesity | Ptrend |
|---|---|---|---|---|
| IOTF | ||||
| Non-fasting plasma insulin, pmol/L | 1111 (92 to 129) | 1542 (120 to 188) | 3123 (252 to 371) | <0.001 |
| Non-fasting plasma glucose, mmol/L | 6.2 (6.0 to 6.4) | 6.5 (6.1 to 6.9) | 6.7 (6.0 to 7.5) | 0.12 |
| Adiponectin, µg/ml | 11.6 (10.8 to 12.5) | 11.1 (9.5 to 12.6) | 9.9 (7.2 to 12.6) | 0.19 |
| Ferritin, µg/L | 23.0 (19.8 to 26.2) | 27.3 (21.2 to 33.4) | 26.9 (16.3 to 37.6) | 0.22 |
| hs-CRP, mg/L† | 0.811 (0.63 to 1.04) | 1.141,2 (0.71 to 1.83) | 2.462 (1.09 to 5.56) | 0.01 |
| IL-6, pg/ml | 2.1 (1.7 to 2.6) | 2.0 (1.2 to 2.9) | 3.6 (2.1 to 5.1) | 0.21 |
| TNFα, pg/ml | 4.2 (3.9 to 4.5) | 4.4 (3.9 to 5.0) | 4.4 (3.4 to 5.4) | 0.35 |
| CDC | ||||
| Non-fasting plasma insulin, pmol/L | 1111 (93 to 130) | 1622 (117 to 206) | 2433 (196 to 289) | <0.001 |
| Non-fasting plasma glucose, mmol/L | 6.2 (6.0 to 6.4) | 6.4 (5.9 to 7.0) | 6.7 (6.1 to 7.3) | 0.09 |
| Adiponectin, µg/ml | 11.7 (10.9 to 12.5) | 11.2 (9.3 to 13.2) | 9.5 (7.5 to 11.6) | 0.06 |
| Ferritin, µg/L | 22.9 (19.7 to 26.0) | 27.6 (20.0 to 35.3) | 28.7 (20.5 to 36.9) | 0.12 |
| hs-CRP, mg/L† | 0.821 (0.64 to 1.04) | 1.111,2 (0.61 to 2.02) | 1.962 (1.04 to 3.70) | 0.01 |
| IL-6, pg/ml | 2.1 (1.7 to 2.6) | 2.2 (1.1 to 3.2) | 2.7 (1.6 to 3.9) | 0.40 |
| TNFα, pg/ml | 4.2 (3.9 to 4.5) | 4.5 (3.8 to 5.2) | 4.1 (3.4 to 4.9) | 0.91 |
| WHO | ||||
| Non-fasting plasma insulin, pmol/L | 1071 (86 to 127) | 1421 (111 to 173) | 2322 (188 to 275) | <0.001 |
| Non-fasting plasma glucose, mmol/L | 6.2 (6.0 to 6.5) | 6.2 (5.8 to 6.6) | 6.8 (8.2 to 7.3) | 0.13 |
| Adiponectin, µg/ml | 11.81 (10.9 to 12.7) | 11.71 (10.3 to 13.0) | 9.02 (7.1 to 10.9) | 0.02 |
| Ferritin, µg/L | 23.5 (19.9 to 27.1) | 23.9 (18.6 to 29.1) | 27.5 (19.8 to 35.2) | 0.42 |
| hs-CRP, mg/L† | 0.781 (0.59 to 1.02) | 1.011.2 (0.67 to 1.53) | 1.892 (1.06 to 3.39) | 0.01 |
| IL-6, pg/ml | 2.1 (1.6 to 2.6) | 2.2 (1.5 to 3.0) | 2.6 (1.6 to 3.7) | 0.38 |
| TNFα, pg/ml | 4.2 (3.9 to 4.5) | 4.3 (3.8 to 4.8) | 4.1 (3.4 to 4.8) | 0.98 |
Note: Values with different superscript numbers are statistically different (P<0.05)
Geometric means (95% CI)
Abbreviations: CDC, Centres for Disease Control and Prevention; hs-CRP, high-sensitivity C-reactive protein; IL-6, interleukin-6; WHO: World Health Organization; IOTF: International Obesity Task Force; TNFα, tumor necrosis factor-alpha.
Information was missing for 24 participants on plasma insulin; 28 participants on plasma glucose ; 23 participants on adiponectin; 5 participants on ferritin; 25 participants on hs-CRP; 24 participants on IL-6; 24 participants on TNF-α.
DISCUSSION
Three prior selections should be considered when classifying children’s weight status: an anthropometric indicator, a reference population for comparison, and cut-off points that best identify individuals and populations at risk of overweight- and obesity-related morbidity and mortality [18]. To our knowledge, this is the first comparison-based approach attempting to assess three sets of commonly-used BMI reference criteria among Nunavik Inuit school-aged children. The results showed that BMI references produce different prevalence estimates for overweight and obesity. IOTF and CDC generated lower obesity prevalence compared to WHO criteria, which consistently reported higher prevalence of overweight and obesity in overall participants and both genders. Despite the observed concordance between the different references, some inconsistencies in our results suggest a precautionary approach with reference criteria to assess the prevalence of overweight or obesity among Nunavik children, particularly when they could be considered in planning preventive care services and evaluating the impact of policy initiatives.
Differences in prevalence according to BMI classification criteria were also noted among non-indigenous children worldwide [19–34]. In contrast to studies that showed the lowest estimates of both overweight and obesity with IOTF compared to CDC among non-indigenous children [21, 24, 34], our results indicate almost two times lower prevalence of obesity, but 1½ times higher prevalence of overweight with IOTF compared to CDC. Moreover, our results by gender, according to IOTF and CDC, are in line with those of Lobstein and Jackson-Leach [28] who compared their own results with IOTF to Odgen et al. [35] with CDC in the US population. Among 5–17 year-old Canadians who participated in the 2009–2011 CHMS (residents of Indian Reserves and some remote areas were excluded) [12], IOTF-estimated prevalence was 16.4% for overweight and 8.4% for obesity, whereas with WHO, prevalence was 19.8% for overweight and 11.7% for obesity.
In the absence of information on obesity-related outcomes, the plasma concentrations of several metabolic and inflammatory biomarkers – such as insulin, glucose, adiponectin, ferritin, hs-CRP, IL-6, and TNFα – served as surrogates to investigate the accuracy of BMI classification criteria. Plasma insulin and CRP concentrations were significantly higher from normal weight to obesity regardless of BMI reference criteria. Only WHO criteria yielded significantly elevated adiponectin, which might have been due to augmented statistical power – i.e. a larger number of obesity cases (n=37 for WHO, n=32 for CDC, and n=19 for IOTF). The highest mean of plasma insulin levels were observed in the obese category defined by IOTF (Table 3). In addition, children who were considered obese by CDC or WHO classifications, but not IOTF classification, exhibited less severe clinical obesity – characterized by lower levels of insulin. The presence of false positive obese in CDC or WHO classifications could explain the lower insulin levels observed. Because total overweight and obese children identified with CDC or IOTF classification were quite similar, the lower rate of obese identified with IOTF is also reflected in a higher classification in the overweight category. In other words, the false positive obese identified with CDC were adequately classified as overweight by IOTF.
In our context, IOTF classification system allowed us to identify children with obesity and higher insulin disturbances compared to those recognized by CDC and WHO criteria. According to WHO classification, 52% of obesity cases would be classified as obesity with IOTF and 86% with CDC. Several studies suggest that obesity prevalence with IOTF criteria was lower than with CDC [21, 34, 36, 37]. Among preschoolers Nunavik Inuit (3–5 years of age), Galloway et al. [38] also reported 2 ½ times lower prevalence of obesity with IOTF (12.6%) compared to CDC (32.2%). This indicates that IOTF criteria might be more specific and less sensitive in identifying obesity in children than CDC and WHO. Having a tool that would reduce the likelihood of false positives is essential for monitoring BMI in children – especially in the period of rapid growth around puberty, during which self-esteem is paramount. Being improperly classified as obese can physically and emotionally harm children.
In the absence of diagnostic accuracy analysis (sensitivity, specificity, and positive and negative likelihood) – which would be best determined with longitudinal BMI values in adulthood – to judge the performance of these BMI classification systems, the present study suggests that the IOTF system has better ability than CDC and WHO to discriminate individuals with obesity.
Our study has limitations, and the results should therefore be interpreted with caution. First, it is limited to 8–14-year-old participants, and we cannot comment on whether our findings are generalizable to those aged more than 14 years. Moreover, because participants were Inuit children, generalization of the results to other populations is limited. Secondly, the absence of information on a gold standard, such as body fat percentage and fat distribution, does not allow us to complete our comparative approach by diagnostic accuracy analysis to rigorously assess the performance of each BMI classification criterion among Nunavik Inuit children. Third, the cross-sectional design of our study does not provide longitudinal follow-up of weight status. Thus, BMI tracking analysis is not feasible, and we cannot comment on the behavior of each BMI criterion over time. Nonetheless, the strengths of this study include relatively large sample size, direct measurement of weight and height instead of self-reported data, which may underestimate the prevalence of overweight and obesity, and the consideration of metabolic and inflammatory biomarkers.
Identification of excess weight among children is of considerable clinical and public health relevance. International BMI classification systems are useful to compare excess weight between studies and countries and for monitoring global trends, but there is no conclusive evidence of validity in populations different from those for which they were developed. Our results indicate that obesity prevalence with the IOTF system was lower than those obtained with other classification systems, and associated with elevated non-fasting plasma insulin levels among obese individuals. Interpretation of this study’s data leads to the conclusion that the IOTF system seems to be more suitable for assessing overweight and obesity among Inuit children. Further research in this regard is mandatory in representative samples of children – from Nunavik and other Inuit populations – to clarify the present findings.
IMPLICATIONS AND CONTRIBUTION.
Identification of excess weight among children is of considerable clinical and public health relevance. Little is known about the suitability of the 3 commonly-used BMI reference systems for Indigenous children. This study indicates that IOTF system appears to be more specific in assessing obesity among Inuit children than other classification system.
Acknowledgements
The authors are grateful to the Nunavik population, particularly the parents and children who participated in this study.
Funding Sources: This research was supported by grants from the National Institutes of Health/National Institute of Environmental Health Sciences (R01-ES007902); Northern Contaminants Program, Indian and Northern Affairs Canada; Health Canada; and Hydro-Québec (Environmental Child Health Initiative); Joseph Young, Sr., Fund, State of Michigan. The funding sources were not involved in data collection, data analysis, manuscript writing, and publication.
Abbreviations
- CDC
Centres for Disease Control and Prevention
- hs-CRP
high-sensitivity C-reactive protein
- IL-6
interleukin-6
- WHO
World Health Organization
- IOTF
International Obesity Task Force
- TNFα
tumor necrosis factor-alpha
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributors: GM obtained funding and was investigator of the NCDS; TCMM, PA, ASJ, SM, CR, GM, and ML created the concept for the current analysis, and provided statistical expertise. TCMM, PA, GM, and ML analyzed the data. TCMM wrote the first draft of the manuscript. All authors contributed to the interpretation of the results and to critical revision of the manuscript for important intellectual content and approved the final version of the manuscript.
Competing interests: None.
REFERENCES
- 1.Cali AM, Caprio S. Obesity in children and adolescents. J Clin Endocrinol Metab. 2008;93:S31–S36. doi: 10.1210/jc.2008-1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Craig LC, Love J, Ratcliffe B, et al. Overweight and cardiovascular risk factors in 4- to 18-year-olds. Obes Facts. 2008;1:237–242. doi: 10.1159/000156720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gouveia MJ, Frontini R, Canavarro MC, et al. Quality of life and psychological functioning in pediatric obesity: the role of body image dissatisfaction between girls and boys of different ages. Quality of life research : an international journal of quality of life aspects of treatment, care and rehabilitation. 2014 doi: 10.1007/s11136-014-0711-y. [DOI] [PubMed] [Google Scholar]
- 4.Whitaker RC, Wright JA, Pepe MS, et al. Predicting obesity in young adulthood from childhood and parental obesity. N Engl J Med. 1997;337:869–873. doi: 10.1056/NEJM199709253371301. [DOI] [PubMed] [Google Scholar]
- 5.Hampl SE, Carroll CA, Simon SD, et al. Resource utilization and expenditures for overweight and obese children. Archives of pediatrics & adolescent medicine. 2007;161:11–14. doi: 10.1001/archpedi.161.1.11. [DOI] [PubMed] [Google Scholar]
- 6.Keys A, Fidanza F, Karvonen MJ, et al. Indices of relative weight and obesity. International journal of epidemiology. 2014;43:655–665. doi: 10.1093/ije/dyu058. [DOI] [PubMed] [Google Scholar]
- 7.Katzmarzyk PT. Anthropometric Indicators in Relation to the Gold Standards. In: Bray GA, Bouchard C, editors. Handbook of obesity — Epidemiology, etiology, and physiopathology. Third edition. Boca Raton: Taylor & Francis Group; 2014. pp. 37–46. [Google Scholar]
- 8.Cole TJ, Bellizzi MC, Flegal KM, et al. Establishing a standard definition for child overweight and obesity worldwide: international survey. BMJ. 2000;320:1240–1243. doi: 10.1136/bmj.320.7244.1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kuczmarski RJ, Ogden CL, Guo SS, et al. 2000 CDC Growth Charts for the United States: methods and development. Vital Health Stat. 2002;11:1–190. [PubMed] [Google Scholar]
- 10.de Onis M, Onyango AW, Borghi E, et al. Development of a WHO growth reference for school-aged children and adolescents. Bull World Health Organ. 2007;85:660–667. doi: 10.2471/BLT.07.043497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Flegal KM. Defining obesity in children and adolescents: epidemiologic approaches. Critical reviews in food science and nutrition. 1993;33:307–312. doi: 10.1080/10408399309527625. [DOI] [PubMed] [Google Scholar]
- 12.Roberts KC, Shields M, de Groh M, et al. Overweight and obesity in children and adolescents: results from the 2009 to 2011 Canadian Health Measures Survey. Health reports. 2012;23:37–41. [PubMed] [Google Scholar]
- 13.Ogden CL, Carroll MD, Kit BK, et al. Prevalence of obesity and trends in body mass index among US children and adolescents, 1999–2010. JAMA. 2012;307:483–490. doi: 10.1001/jama.2012.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pirkle CM, Lucas M, Dallaire R, et al. Food insecurity and nutritional biomarkers in relation to stature in Inuit children from Nunavik. Canadian journal of public health = Revue canadienne de sante publique. 2014;105:e233–e238. doi: 10.17269/cjph.105.4520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dewailly E, Bruneau S, Ayotte P, et al. Health status at birth of inuit newborn prenatally exposed to organochlorines. Chemosphere. 1993;27:359–366. [Google Scholar]
- 16.Muckle G, Ayotte P, Dewailly EE, et al. Prenatal exposure of the northern Quebec Inuit infants to environmental contaminants. Environmental health perspectives. 2001;109:1291–1299. doi: 10.1289/ehp.011091291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–174. [PubMed] [Google Scholar]
- 18.de Onis M, Lobstein T. Defining obesity risk status in the general childhood population: which cut-offs should we use? International journal of pediatric obesity : IJPO : an official journal of the International Association for the Study of Obesity. 2010;5:458–460. doi: 10.3109/17477161003615583. [DOI] [PubMed] [Google Scholar]
- 19.Baya Botti A, Perez-Cueto FJ, Vasquez Monllor PA, et al. International BMI-for-age references underestimate thinness and overestimate overweight and obesity in Bolivian adolescents. Nutricion hospitalaria. 2010;25:428–436. [PubMed] [Google Scholar]
- 20.El-Ghaziri M, Boodai S, Young D, et al. Impact of using national v. international definitions of underweight, overweight and obesity: an example from Kuwait. Public health nutrition. 2011;14:2074–2078. doi: 10.1017/S1368980011001285. [DOI] [PubMed] [Google Scholar]
- 21.Flegal KM, Ogden CL, Wei R, et al. Prevalence of overweight in US children: comparison of US growth charts from the Centers for Disease Control and Prevention with other reference values for body mass index. The American journal of clinical nutrition. 2001;73:1086–1093. doi: 10.1093/ajcn/73.6.1086. [DOI] [PubMed] [Google Scholar]
- 22.Fu WP, Lee HC, Ng CJ, et al. Screening for childhood obesity: international vs population-specific definitions. Which is more appropriate? International journal of obesity and related metabolic disorders : journal of the International Association for the Study of Obesity. 2003;27:1121–1126. doi: 10.1038/sj.ijo.0802385. [DOI] [PubMed] [Google Scholar]
- 23.Goon DT, Toriola AL, Shaw BS. Screening for body-weight disorders in Nigerian children using contrasting definitions. Obesity reviews : an official journal of the International Association for the Study of Obesity. 2010;11:508–515. doi: 10.1111/j.1467-789X.2009.00682.x. [DOI] [PubMed] [Google Scholar]
- 24.Kain J, Uauy R, Vio F, et al. Trends in overweight and obesity prevalence in Chilean children: comparison of three definitions. European journal of clinical nutrition. 2002;56:200–204. doi: 10.1038/sj.ejcn.1601301. [DOI] [PubMed] [Google Scholar]
- 25.Khang YH, Park MJ. Trends in obesity among Korean children using four different criteria. International journal of pediatric obesity : IJPO : an official journal of the International Association for the Study of Obesity. 2011;6:206–214. doi: 10.3109/17477166.2010.490270. [DOI] [PubMed] [Google Scholar]
- 26.Khasnutdinova SL, Grjibovski AM. Prevalence of stunting, underweight, overweight and obesity in adolescents in Velsk district, north-west Russia: a cross-sectional study using both international and Russian growth references. Public health. 2010;124:392–397. doi: 10.1016/j.puhe.2010.03.017. [DOI] [PubMed] [Google Scholar]
- 27.Kovalskys I, Rausch Herscovici C, De Gregorio MJ. Nutritional status of school-aged children of Buenos Aires, Argentina: data using three references. Journal of public health. 2011;33:403–411. doi: 10.1093/pubmed/fdq079. [DOI] [PubMed] [Google Scholar]
- 28.Lobstein T, Jackson-Leach R. Child overweight and obesity in the USA: prevalence rates according to IOTF definitions. International journal of pediatric obesity : IJPO : an official journal of the International Association for the Study of Obesity. 2007;2:62–64. doi: 10.1080/17477160601103948. [DOI] [PubMed] [Google Scholar]
- 29.Maiti S, De D, Ali KM, et al. Overweight and obesity among early adolescent school girls in urban area of west bengal, India: prevalence assessment using different reference standards. International journal of preventive medicine. 2013;4:1070–1074. [PMC free article] [PubMed] [Google Scholar]
- 30.Meyer E, Carrillo R, Roman EM, et al. Prevalence of overweight and obesity in students from different altitudinal zones of Jujuy according to three international references (IOTF, CDC and WHO) Archivos argentinos de pediatria. 2013;111:516–522. doi: 10.5546/aap.2013.eng.516. [DOI] [PubMed] [Google Scholar]
- 31.Misra A, Shah P, Goel K, et al. The high burden of obesity and abdominal obesity in urban Indian schoolchildren: a multicentric study of 38,296 children. Annals of nutrition & metabolism. 2011;58:203–211. doi: 10.1159/000329431. [DOI] [PubMed] [Google Scholar]
- 32.Shields M, Tremblay MS. Canadian childhood obesity estimates based on WHO, IOTF and CDC cut-points. International journal of pediatric obesity : IJPO : an official journal of the International Association for the Study of Obesity. 2010;5:265–273. doi: 10.3109/17477160903268282. [DOI] [PubMed] [Google Scholar]
- 33.Tuan NT, Nicklas TA. Age, sex and ethnic differences in the prevalence of underweight and overweight, defined by using the CDC and IOTF cut points in Asian children. European journal of clinical nutrition. 2009;63:1305–1312. doi: 10.1038/ejcn.2009.90. [DOI] [PubMed] [Google Scholar]
- 34.Zimmermann MB, Gubeli C, Puntener C, et al. Overweight and obesity in 6–12 year old children in Switzerland. Swiss Med Wkly. 2004;134:523–528. doi: 10.4414/smw.2004.10640. [DOI] [PubMed] [Google Scholar]
- 35.Ogden CL, Carroll MD, Curtin LR, et al. Prevalence of overweight and obesity in the United States, 1999–2004. JAMA. 2006;295:1549–1555. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- 36.Janssen I, Katzmarzyk PT, Srinivasan SR, et al. Utility of childhood BMI in the prediction of adulthood disease: comparison of national and international references. Obes Res. 2005;13:1106–1115. doi: 10.1038/oby.2005.129. [DOI] [PubMed] [Google Scholar]
- 37.Reilly JJ, Kelly J, Wilson DC. Accuracy of simple clinical and epidemiological definitions of childhood obesity: systematic review and evidence appraisal. Obesity reviews : an official journal of the International Association for the Study of Obesity. 2010;11:645–655. doi: 10.1111/j.1467-789X.2009.00709.x. [DOI] [PubMed] [Google Scholar]
- 38.Galloway T, Niclasen BV, Muckle G, et al. Growth measures among preschool-age Inuit children living in Canada and Greenland. Scandinavian journal of public health. 2012;40:712–717. doi: 10.1177/1403494812462495. [DOI] [PubMed] [Google Scholar]