Abstract
Insulin detemir (DET) reduces glycemia comparably to other long-acting insulin formulations but causes less weight gain. Insulin signaling in the brain is catabolic, reducing food intake. We hypothesized that DET reduces weight gain, relative to other insulins, owing to increased transport into the central nervous system and/or increased catabolic action within the brain. Transport of DET and NPH insulin into the cerebrospinal fluid (CSF) was compared over several hours and after the administration of different doses peripherally in rats. DET and NPH had comparable saturable, receptor-mediated transport into the CSF. CSF insulin remained elevated significantly longer after intraperitoneal DET than after NPH. When administered acutely into the 3rd cerebral ventricle, both DET and NPH insulin reduced food intake and body weight at 24 h, and both food intake and body weight remained lower after DET than after NPH after 48 h. In direct comparison with another long-acting insulin, insulin glargine (GLAR), DET led to more prolonged increases in CSF insulin despite a shorter plasma half-life in both rats and mice. Additionally, peripheral DET administration reduced weight gain and increased CSF insulin compared with saline or GLAR in mice. Overall, these data support the hypothesis that DET has distinct effects on energy balance through enhanced and prolonged centrally mediated reduction of food intake.
Introduction
Insulin is secreted almost exclusively from pancreatic β-cells in response to increases in ambient glucose, circulating incretin hormones, and parasympathetic nervous system signaling (1). Insulin mediates a range of physiologic actions through an endocrine mechanism, and the insulin receptor (IR) is widely distributed throughout peripheral tissues and brain (2,3). Given that insulin is a large peptide, active transport is required for it to cross the blood-brain barrier (BBB) in significant quantities (4). Once transported into brain interstitial fluid, insulin acts upon IR in diverse brain regions including the hypothalamus and hippocampus (5–7). The current model of insulin transport to the central nervous system (CNS) posits that insulin enters brain interstitial fluid from the plasma via receptor-mediated transport across brain endothelial cells where it can access IR on neurons and glial cells; from the brain interstitial fluid, insulin is collected into the cerebrospinal fluid (CSF), where it is cleared by IR back into the plasma. Hence, the normal movement of insulin is plasma-to-brain interstitial fluid to CSF (8,9).
Insulin detemir (DET) has a covalently attached fatty acid that promotes binding to albumin, extending the plasma half-life from a few minutes to 5–7 h (10). A common problem with insulin treatment is weight gain; patients having insulin doses titrated to achieve glycemic targets often gain from 2 to 10 kg. The reason is unclear but is likely some combination of reduced wasting of calories from glycosuria and anabolic actions of insulin on adipose tissue (11). Several clinical trials have indicated that use of DET as a basal insulin replacement leads to less weight gain than other long-acting formulations (12–14). In fact, some trials have reported weight loss in patients switching to DET from other long-acting insulin analogs (15,16).
It has been proposed that the differential effects on weight loss of DET compared with other insulin formulations are due to an increase of insulin action in the CNS (17). Insulin signaling in the brain is catabolic, causing reduced food intake and increased energy expenditure (18–20). However, the degree to which DET penetrates the BBB is controversial, with one report indicating that DET transport is comparable with that of regular insulin (21) and another suggesting that transport of DET through the BBB does not occur (22). The current studies were designed to assess DET transport into the CNS by measuring its appearance in the CSF, compare its entry relative to other formulations of insulin, and compare the effect of acute central administration and chronic peripheral administration of DET with other formulations of insulin on parameters of energy homeostasis.
Research Design and Methods
Animals and Housing
Adult male Wistar rats (400–550g) were bred at the Metabolic Diseases Institute of the University of Cincinnati, and adult male C57Bl6/J mice (22–25 g) were purchased from Charles River Laboratories (Wilmington, MA). All animals were maintained in Association for Assessment and Accreditation of Laboratory Animal Care–accredited animal facilities and maintained on 12:12-h light:dark schedule. Rats were housed in tub cages, with those animals used for dose-response and time course studies being pair housed and those used for food intake and central infusion studies being single housed. Mice were single housed in standard Static Micro Isolator cages. Unless otherwise specified, animals had ad libitum access to water and pelleted chow diet (LM-485; Harlan Teklad, Madison, WI). All protocols were approved by the University of Cincinnati Institutional Animal Care and Use Committee.
CSF Sampling
CSF samples were taken from rats after an overnight fast or mice after a 4-h fast. Animals were placed in the chamber of an anesthetic isoflurane machine (VetEquip, Pleasanton, CA). After anesthetization, the area from between the shoulders to below the skull was shaved. The animal was positioned securely into a stereotaxic instrument using the ear bars and an isoflurane nose cone. The incisor bar was positioned so that the head was ventroflexed. CSF sampling was performed using our previously published methods for rats and mice (23,24). Briefly, in rats a semiblunted 25-gauge (1 1/2-inch) needle was connected to microrenathane tubing and mounted on an electrode holder that was then positioned perpendicular to the ear bars in the horizontal plane. A 1.0-mL syringe containing 0.9% saline was attached, using a 25-gauge needle, to the distal end of the microrenathane tubing. The tubing was flushed with saline, and slight negative pressure was applied, creating a small air pocket in the needle and tubing. The incision site was swabbed with ethanol, and a mid-sagittal incision of the skin (∼20 mm) was made inferior to the occipital crest. Muscle was separated by blunt dissection using forceps to expose the atlanto-occipital membrane, and the region was cleaned with sterile saline-soaked cotton swabs. The tip of the needle was advanced horizontally toward the membrane using the controls of the sterotaxic instrument until it penetrated the dura mater and entered the cisternum magnum. Clear CSF flow was spontaneously initiated with very slight negative pressure and when the air bubble entered the syringe, the needle was removed, and CSF was collected via gravity flow into a 500-μL tube on ice. On average, ∼150 µL CSF was collected within 2 min and immediately frozen on dry ice in rats, and ∼15 µL CSF was obtained in mice using a glass capillary tube. Animals were immediately killed by decapitation, and blood was collected in chilled EDTA-coated tubes and stored on ice prior to centrifugation. The CSF and plasma samples were stored at −80°C prior to analysis. We have previously observed that <6% of the samples are contaminated with blood using this method, and any contaminated samples were discarded (23).
Identification of DET in CSF After Peripheral Injection
Dose-Response Experiment
Thirty minutes prior to CSF sampling, rats were injected with insulin (0, 0.1, 0.3, 1, 3, or 10 units/kg i.p. NPH insulin or DET) and returned to their home cage. Animals (n = 8–10/dose) were anesthetized after 25 min, and CSF was sampled precisely at 30 min postinjection.
Time Course Experiment
Rats (n = 6–8/time point) were administered NPH insulin or DET (0.5 units/kg i.p.) and returned to their home cage and had CSF sampled 5, 10, 20, 40, 80, 160, or 320 min postinjection.
Comparison of the Appearance of Long-Acting Formulations of Insulin, DET, and Glargine in Blood and CSF Over Time in Rats and Mice
Rats (n = 7–8/group) were injected with glargine (GLAR) (0.5 units/kg s.c.) or DET (0.5 units/kg s.c.). The change in dosing route was required, as GLAR is only long-acting when injected subcutaneously. After the injection, the rats were returned to their home cage, and CSF was sampled at baseline (0) and 6, 12, 24, 48, or 72 h postinjection. Mice (n = 7–8/group) were injected with GLAR (1 unit/kg s.c.) or DET (1 unit/kg s.c.). After the injection, mice were returned to their home cage, and CSF was sampled at baseline (0) and 6, 12, 24, or 48 h postinjection.
Third-Ventricular Cannulation
Each rat was anesthetized with ketamine (70 mg/kg i.p.) and xylazine (6 mg/kg i.p.), its head was shaved, and the skull was positioned in a stereotaxic instrument as previously described (25). Surgery was performed using sterile techniques. A small hole was drilled through the skull at 2.2 mm posterior to bregma on the midline. The sagittal sinus was then displaced laterally, and a stainless-steel guide cannula (Plastics One, Roanoke, VA) was lowered ventrally into the 3rd ventricle, 7.5 mm ventral to the dura. The cannula placement was fixed with dental acrylic that was anchored to the skull with three to four screws. An obturator was inserted that extended 0.5 mm below the cannula. At least 5 days prior to food intake experiments, cannula placement was confirmed by injecting 10 ng angiotensin II (American Peptides, Sunnyvale, CA) in 1 μL saline. Rats that consumed >5 mL water within 30 min were deemed to have a viable cannula. Four rats were excluded based on this criterion.
Food Intake and Body Weight After Third-Ventricular Cannulation of Insulin in Rats
Measurements of food intake and body weight were based on an established paradigm (26). Briefly, on each of the 4 days prior to an experiment, each animal (n = 16) was individually acclimated to handling for 4–5-min/day and had food removed for the final 4 h of light. All animals underwent this protocol on three occasions, 1 week apart, in a randomized crossover manner to receive DET (8 mU), NPH insulin (8 mU), or 2 μL vehicle (sterile 0.9% saline) via third-ventricular cannulation (i3vt) on the experimental days. Injections occurred 1 h before dark onset. Food hoppers were returned at the onset of dark, and intake and body weight were assessed after 24 and 48 h.
Food Intake and Body Composition of Mice Chronically Treated With Long-Acting Formulations of Insulin
Prior to commencement of treatment, mice were grouped based on fat mass as assessed by nuclear magnetic resonance (NMR) in conscious animals as previously described (27) (EchoMRI, Waco, TX). Mice (n = 12/group) then received daily subcutaneous injections of normal saline, DET (1 unit/kg), or GLAR (1 unit/kg). Animals were weighed, and food intake was monitored weekly for 6 weeks, after which time body composition was reassessed. In a follow-up experiment, mice (n = 7–9/group) were injected subcutaneously with normal saline, GLAR (1 unit/kg), or DET (1 unit/kg) daily for 6 weeks. Twenty-four hours after their final injection, animals had CSF and plasma sampled.
Insulin ELISA and Glucose Measurement
Insulin levels in CSF and plasma were measured using insulin ELISA (Crystal Chem, Downers Grove, IL). Briefly, a sample of CSF, plasma, or insulin standard (5 µL/sample of plasma, 10 μL/sample of CSF) was added to an antibody-coated microplate. After incubation for 2 h at 4°C, 100 μL anti-insulin enzyme conjugate was dispensed into each well and incubated at room temperature for 30 min. The plate was washed, and 100 μL enzyme substrate solution was added to each well and incubated for 40 min. The stop solution was then added, and insulin concentrations were determined by subtracting the absorbance at 630 nm from the absorbance at 450 nm. Owing to differential detection of different insulin isoforms with the antibody, standard curves were produced using NPH insulin, DET, and GLAR diluted from the injected solutions. Glucose was measured in plasma in duplicate using glucometers (Accuchek; Roche Diagnostics, Indianapolis, IN) (27).
Statistical Analyses
Plasma insulin, glucose, and CSF insulin were analyzed using factorial ANOVA. Food intake, body weight, and NMR data were analyzed using repeated-measures ANOVA. Post hoc Tukey tests were performed after observations of significant interaction effects (Statistica 7.1; Statsoft). Significance was accepted at P < 0.05, with data reported as means ± SEM.
Results
Appearance of DET and NPH Insulin in Plasma and CSF After Intraperitoneal Administration
Intraperitoneal NPH insulin produced a dose-dependent increase in plasma insulin after 30 min, with a significant increase first occurring at 0.3 units/kg (P < 0.05) (Fig. 1A). Likewise, there was a dose-dependent increase of CSF insulin 30 min after the injections of NPH insulin, with apparent saturation of the CSF insulin levels occurring by the 3 unit/kg dose (Fig. 1B). There was a dose-dependent decrease in plasma glucose reflecting the increased plasma insulin (Fig. 1C).
Figure 1.
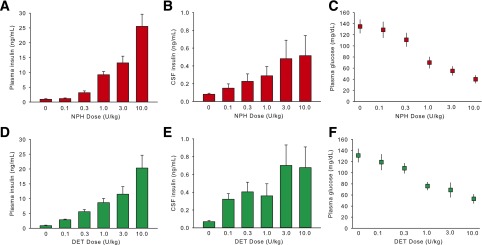
Mean ± SEM concentration of insulin in plasma and CSF and glucose in plasma 30 min after the intraperitoneal administration of DET or NPH insulin at different doses in rats. Intraperitoneal NPH insulin and DET produced similar levels as a function of dose in plasma (A and D). Likewise, there were dose-dependent increases of CSF insulin with both NPH insulin and DET, with apparent saturation of the CSF insulin levels occurring by the 3 units/kg dose (B and E). Plasma glucose decreased in a dose-dependent manner after intraperitoneal NPH insulin or DET (C and F). Significant differences are described in the text.
Plasma insulin was increased at all doses of DET relative to the 0 units/kg group (P < 0.05) (Fig. 1D). The magnitude was dose dependent, with the 10 units/kg DET group having significantly elevated plasma insulin relative to all other groups (P < 0.05). CSF insulin was also elevated after all doses relative to the saline group in a dose-dependent manner (P < 0.05). Apparent saturation of CSF insulin occurred by the 3 units/kg dose, with no difference between 3 and 10 units/kg (Fig. 1E). There was a dose-dependent decrease in plasma glucose after DET similar to that observed after NPH insulin (Fig. 1F). No significant interaction between NPH and DET treatment was observed; i.e., the two insulin formulations resulted in comparable plasma and CSF insulin profiles.
Appearance of DET and NPH Insulin in Plasma and CSF at Different Time Points After Intraperitoneal Administration
Injection of 0.5 units/kg i.p. NPH insulin produced an immediate spike in plasma insulin that peaked by the 5-min time point (P < 0.05) and then decreased. By 160 min, plasma levels were no longer elevated relative to baseline and remained at this level at 320 min (Fig. 2A). CSF insulin levels also rose rapidly after intraperitoneal NPH insulin, with the increase apparent at 5 min (P < 0.05). Peak CSF insulin occurred at approximately the 40-min time point, and the level was subsequently significantly reduced from the peak at 80 and 160 min and reached baseline at 320 min (Fig. 2B). Plasma glucose levels closely tracked plasma insulin levels, with an immediate reduction at 5 min and a return to baseline by 80 min (Fig. 2C) (P < 0.05).
Figure 2.
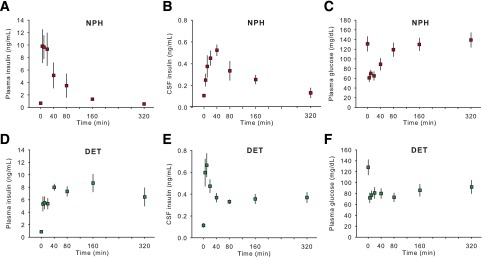
Mean ± SEM concentration of insulin in plasma and CSF and glucose in plasma at different time points after 0.5 units/kg i.p. DET or NPH insulin in rats. Plasma insulin rose rapidly after the intraperitoneal injection of NPH insulin, peaking at 5 min and then decreasing (A). CSF insulin levels also rose rapidly after intraperitoneal NPH insulin, the peak occurring at around 40 min and levels returning to baseline by 320 min (B). Plasma glucose dropped sharply after intraperitoneal NPH insulin, returning to baseline by 80 min (C). Intraperitoneal DET also increased plasma insulin after 5 min, and it remained elevated at a relatively constant level throughout the 320 min (D). CSF insulin peaked rapidly after intraperitoneal DET at the 5- and 10-min time points before reducing to a stable but elevated level between 40 and 320 min (E). Plasma glucose was reduced after DET to a constant level for the duration of the experiment (F). Significant differences are described in the text.
Injection of 0.5 units/kg i.p. DET also resulted in increased plasma insulin after 5 min relative to baseline (P < 0.05), and levels remained similar at 10 and 20 min. Unlike what occurred after NPH insulin, plasma insulin levels remained elevated 320 min after DET treatment (Fig. 2D) (P < 0.05). There was a rapid peak in CSF insulin levels apparent at the 5- and 10-min time points (P < 0.05). CSF insulin became stable by the 40-min time point and remained at that level through to the 320-min time point, with all points remaining elevated relative to baseline (P < 0.05) (Fig. 2E). Plasma glucose levels were reduced by DET at a relatively constant level for the duration of the experiment (Fig. 2F) (P < 0.05).
Planned comparisons between NPH and DET treatment revealed a more rapid and higher increase of insulin in plasma in NPH relative to DET-treated animals at 5, 10, and 20 min (Ps < 0.05) and a more prolonged elevation of insulin in both plasma (160 and 320 min, Ps < 0.05) and CSF (320 min, P < 0.05) after DET. DET also produced a sustained hypoglycemia relative to NPH (80, 160, and 320 min, Ps < 0.05) (Fig. 2).
i3vt DET and NPH Insulin Reduce Food Intake and Body Weight
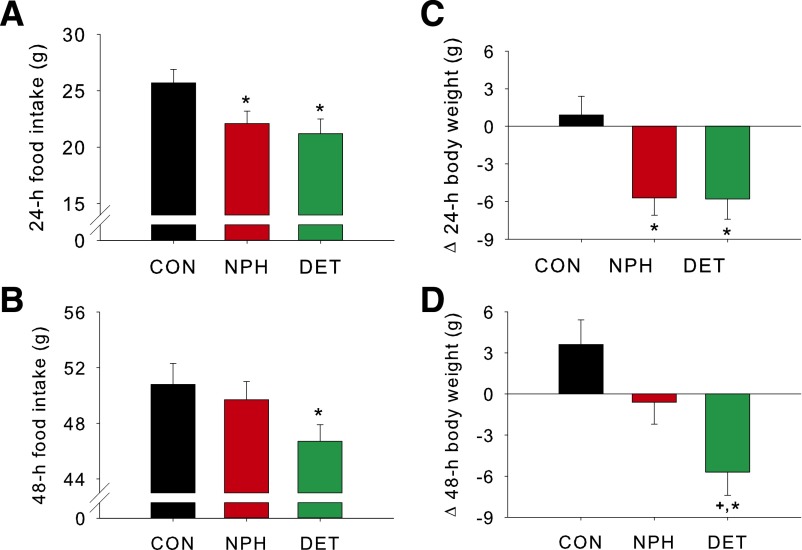
After i3vt injection of either DET or NPH insulin, rats ate significantly less food over the subsequent 24 h than when saline was administered (P < 0.05). There was no difference in the 24-h intake between DET or NPH insulin treatment (Fig. 3A). After 48 h, intake by the DET-treated animals remained significantly reduced (P < 0.05), whereas it had returned to saline-control levels in the NPH insulin group (Fig. 3B). Body weight loss was consistent with the food intake data, with both NPH insulin and DET producing significant weight loss after 24 h (P < 0.05) and with only DET rats maintaining this weight loss after 48 h (P < 0.05) (Fig. 3C and D).
Figure 3.
i3vt DET and NPH insulin reduce food intake and body weight in rats. i3vt DET and NPH insulin each reduced food intake over a 24-h period (A) relative to control (CON). After 48 h, intake by the DET group remained reduced, whereas that of the NPH group had returned to CON levels (B). Both NPH insulin and DET rats lost weight in the 24-h postinfusion (C); however, only the DET group maintained the weight loss after 48 h (D). Data are means ± SEM. *P < 0.05 vs. control, +P < 0.05 vs. NPH insulin.
Comparison of the Appearance of Insulin in Plasma and CSF After Administration of Different Long-Acting Formulations of Insulin, DET, and GLAR
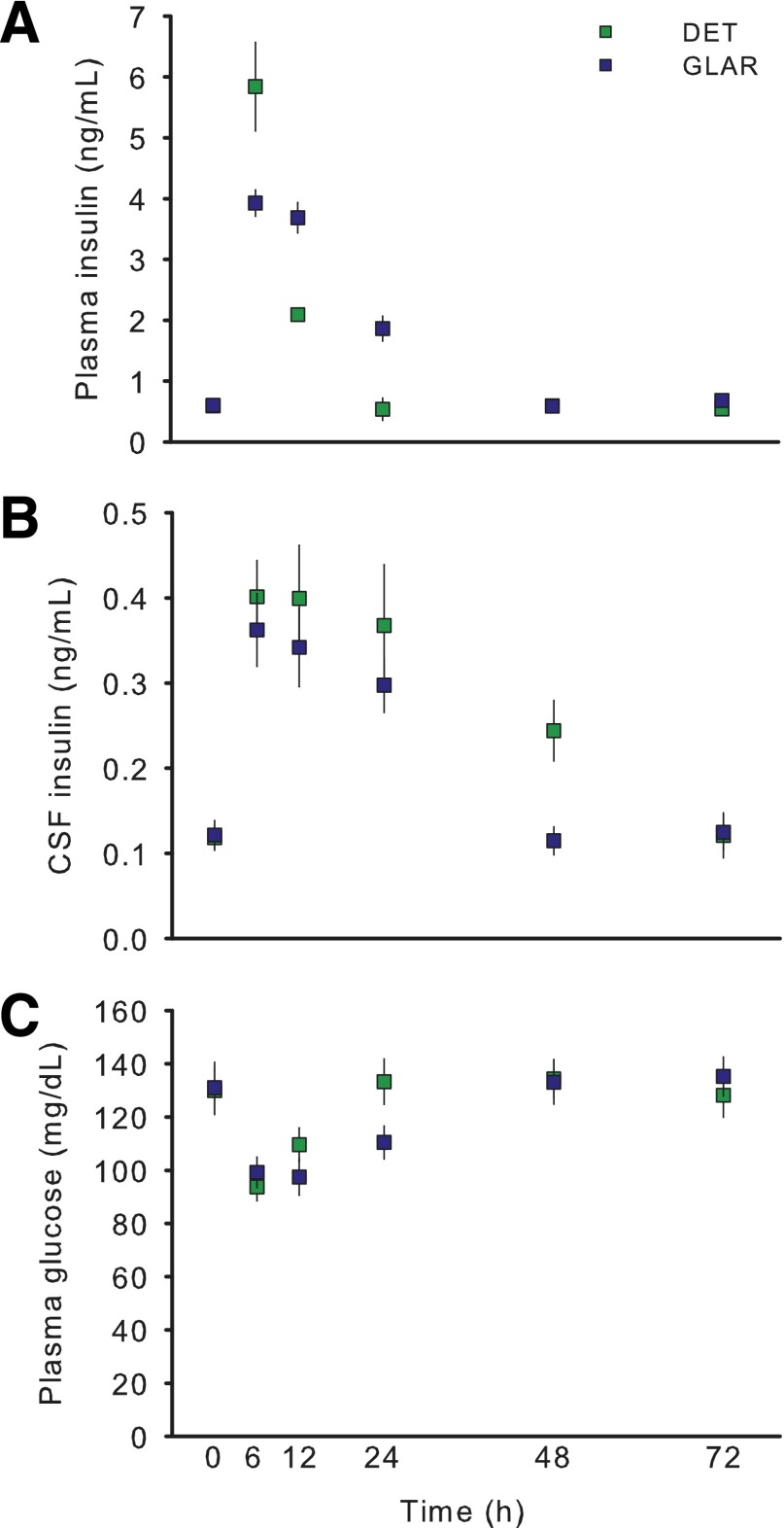
Injection of either DET or GLAR (0.5 units/kg s.c.) led to an increase in plasma insulin. At the first measured time point after baseline (6 h), plasma insulin was elevated more in DET- than in GLAR-treated animals (Fig. 4A) (P < 0.05), and by 12 h this effect was reversed (P < 0.05), with GLAR-treated animals having higher plasma insulin. By 24 h through to 72 h, plasma insulin had returned to baseline levels in DET-treated animals. Plasma insulin of GLAR-treated animals remained slightly elevated at 24 h (P < 0.05) but returned to baseline by 48 h. CSF levels of insulin were comparably increased in both groups at 6, 12, and 24 h (Fig. 4B) (Ps < 0.05), but at 48 h DET-treated animals continued to have elevated CSF insulin (P < 0.05), whereas levels in GLAR-treated animals had returned to baseline. Plasma glucose levels were reduced at both 6 h and 12 h by both DET and GLAR (Fig. 4C) (Ps < 0.05) but, consistent with plasma insulin, remained lower at 24 h only in GLAR-treated animals.
Figure 4.
Comparison of the appearance of insulin in the plasma and CSF after the subcutaneous administration of two long-acting formulations of insulin, DET, and GLAR in rats. Injection of either DET or GLAR at 0.5 units/kg s.c. led to increased plasma insulin at 6–12 h. By 24 h, only GLAR-treated rats continued to have elevated insulin (A). In contrast, CSF insulin levels were similarly increased in both groups at 6, 12, and 24 h; but at 48 h, DET-treated animals had elevated CSF insulin levels, while CSF insulin of GLAR-treated animals had returned to baseline (B). Plasma glucose levels were reduced at both 6 and 12 h by both DET and GLAR but were lower at 24 h only in GLAR-treated animals (C). Data are means ± SEM.
Effect of Chronic Subcutaneous Administration of Long-Acting Formulations of Insulin on Body Weight and Body Composition in Mice
Daily subcutaneous injection of DET reduced weight gain compared with both saline and GLAR treatment after 3 weeks (P < 0.05), and this difference was maintained for the remainder of the 6-week treatment period (P < 0.05) (Fig. 5A). Body weight of the GLAR group was actually increased relative to that of saline-treated animals by week 6 of treatment (P < 0.05). Cumulative food intake was significantly lower in the DET group than in either the GLAR or saline groups (P < 0.05) (Fig. 5B). Similar to the effects observed on body weight, body fat was significantly lower in DET animals and higher in GLAR animals compared with the saline-control group (P < 0.05) (Fig. 5C). Lean mass did not differ among groups (Fig. 5D).
Figure 5.
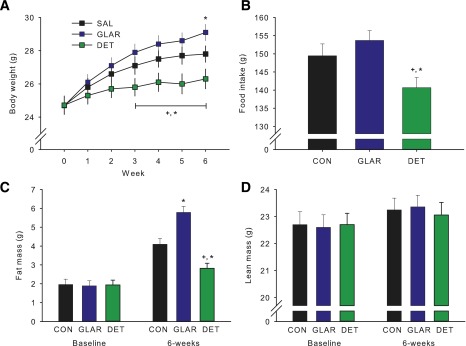
Chronic subcutaneous administration of DET reduces food intake and body weight relative to subcutaneous GLAR or vehicle in mice. DET reduced weight gain compared with both saline control (CON) and GLAR treatment by week 3. GLAR resulted in increased body weight relative to control at week 6 (A). Total food intake was reduced in the DET group compared with that in both GLAR and control (B). Fat mass was lower in DET and higher in GLAR compared with control (C). Lean mass was not altered by any treatment (D). Data are means ± SEM. *P < 0.05 vs. control, +P < 0.05 vs. GLAR.
Appearance of Insulin in Plasma and CSF of Mice After Administration of 1 Unit/kg s.c. DET and GLAR
In mice, subcutaneous DET or GLAR led to an increase in plasma insulin similar to what occurred in rats. At the first measured time point after baseline (6 h), plasma insulin was elevated to a greater extent in DET- than in GLAR-treated mice (Fig. 6A) (P < 0.05), and by 12 h this effect was reversed (P < 0.05), with GLAR-treated animals having higher plasma concentrations. At 24 and 48 h, plasma insulin had returned to baseline in DET-treated animals, whereas plasma insulin of GLAR-treated animals remained elevated at 24 h (P < 0.05) and returned to baseline by 48 h. CSF insulin was comparably increased in both groups at 6 and 12 h (Ps < 0.05). After 24 and 48 h, DET-treated mice had elevated CSF insulin levels (P < 0.05), whereas levels in GLAR-treated animals were lower at 24 h and had returned to baseline by 48 h (Fig. 6B). After 6 weeks of treatment with DET or GLAR, with sampling occurring 24 h after the final injection, animals treated with GLAR but not DET continued to have elevated plasma insulin relative to controls (Fig. 6C) (P < 0.05). However, the reverse was observed in the CSF, where DET-treated, but not GLAR-treated, mice had increased insulin (Fig. 6D) (P < 0.05).
Figure 6.
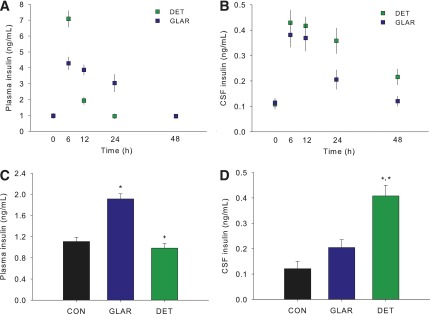
Appearance of insulin in plasma and CSF at different times after the administration of subcutaneous DET and GLAR in mice and the effect of chronic DET and GLAR treatment on CSF insulin. Injection of either DET or GLAR (1 unit/kg s.c.) led to increased plasma insulin at 6–12 h. By 24 h, only GLAR-treated animals continued to have elevated plasma insulin (A). In contrast, CSF insulin levels were increased in both groups at 6, 12, and 24 h; but at 48 h, DET-treated animals had elevated CSF insulin levels, whereas CSF insulin of GLAR-treated animals had returned to baseline (B). Twenty-four hours after cessation of chronic treatment with DET or GLAR, mice treated with GLAR continued to have elevated plasma insulin (C), but the reverse occurred in CSF, where DET-treated, but not GLAR-treated, mice had elevated insulin. Data are means ± SEM. *P < 0.05 vs. control, +P < 0.05 vs. GLAR. CON, control.
Discussion
Long-acting insulin formulations are an important part of a treatment regimen for diabetic patients because of the importance of basal insulin to control fasting and intermeal glycemia (12,28). A common clinical side effect of treatment with insulin is weight gain (29). Based on recent data from clinical trials, this side effect seems to be reduced with DET (12–14), which is less anabolic than other long-acting insulins (15,16). Importantly, these effects appear to be independent of glycemic control (16). We hypothesized that the weight reduction observed with DET treatment is due to increased presence or activity of insulin in the CNS, where it reduces food intake and has a net catabolic effect. In the experiments reported here, insulin was detected in the CSF after presumably passing through the brain neuropil. Overall, the CSF appearance of insulin after DET and NPH was comparable, and this was true in rats and mice. The appearance of insulin in CSF was dose dependent and saturable at higher doses—pharmacologic characteristics previously reported for regular insulin (30). Based on these findings, we propose that insulin appearance in the CSF after DET administration peripherally is the result of active transport from the blood, similar to what occurs for other insulin formulations. The observation that the appearance of insulin in the CSF after NPH and DET was comparable implies that the altered physicochemical properties of DET, such as a higher binding to albumin in the plasma and the presence of an attached fatty acid, do not interfere with its transport into the brain; the data are consistent, however, with IR-mediated transport. Interestingly, CSF insulin levels were elevated longer in DET than GLAR-treated animals, despite a shorter plasma half-life.
Previous reports on DET and the BBB have been contradictory, with one report suggesting that DET is unable to cross the BBB (22) and another reporting that transport is not inhibited (21). Banks et al. (22) reported that DET does not enter the brain from the blood in mice. However, rather than measuring insulin directly, their group administered labeled DET systemically and measured radioactive counts in trichloroacetic acid–precipitable protein in brain extracts, where they found none. While we have no explanation for the difference between our results and those of Banks, it is important to note that our assay measures immunoreactive insulin (as opposed to a radioactive label), and we sampled CSF insulin, which comes from brain interstitial fluid (8). Further, our results are consistent with those of Hennige et al. (21), who found preferential uptake of DET into the brain of mice as assessed by disproportionately elevated insulin in brain tissue extracts and selectively increased phosphorylation of the insulin receptor in mouse hypothalamus. Likewise, overweight humans administered DET intravenously had increased cerebrocortical activity, whereas those administered regular insulin did not (31), and Hallschmid et al. (32) reported that, compared with regular insulin and with plasma glucose comparably clamped, DET elicited a significant change in electroencephalogram activity in the frontal cortex of men and also caused a significant reduction of food intake. Endogenous insulin is believed to cross the BBB via a saturable, IR-mediated process in capillary endothelial cells, and it is probable that DET is transported in the same manner. Thus, our data are consistent with DET being actively transported into the brain.
After peripheral administration in our rats, insulin in the CSF peaked sooner after intraperitoneal DET and remained higher longer than treatment with NPH insulin. In fact, insulin remained significantly elevated in the CSF of DET-treated animals for the duration of the study. The prolonged increase of CSF insulin after DET is likely due to its long peripheral half-life (10) and consequent continued transport into brain after its systemic administration, possibly coupled with slower CNS breakdown (21). The important point is that DET is transported into the CNS and has an earlier peak and prolonged elevated concentration when assessed in CSF, and these phenomena may be responsible for the reduced food intake and weight loss that can occur with DET treatment clinically.
Because DET is used clinically to augment basal insulin, we compared it with another insulin formulation, GLAR, since it is also used for the same purpose but lacks the attached fatty acid side chain of DET. Further, whereas chronic treatment of diabetic patients with GLAR leads to weight gain, treatment with DET at doses that cause the same degree of glucose lowering minimizes weight gain (15,16,33). Similar to previous work in rats (34), the present results demonstrate that whereas 6-week treatment with GLAR caused weight gain relative to controls, treatment with DET caused weight loss in mice. When GLAR and DET were administered more acutely, GLAR had an apparent longer half-life in plasma but shorter half-life in CSF in both rats and mice. The prolonged half-life in the brain likely contributes to the weight-lowering action of chronic DET.
Insulin in the CNS promotes a catabolic state (18,19,35), and administration of insulin directly into the CNS reduces food intake and body weight across diverse species from rodents to humans (36–38). Because we have found that male rats are more sensitive to insulin’s hypophagic action than females (20,39) and because men are analogously more sensitive to the ability of intranasal insulin to reduce body fat than are women (36,40), we utilized male rats in the present experiments. It will be important in future experiments to determine whether there is a sex difference in the transport of insulin into the brain. The current experiment is the first to demonstrate that centrally administered DET is as effective as NPH insulin at acutely reducing food intake. In fact, the effects of a single i3vt bolus of DET on food intake are longer lasting than occur with regular insulin. Return of food intake to normal 48 h after NPH insulin is consistent with previous reports demonstrating rapid return of food intake after cessation of regular insulin infusion (18), and the longer duration of action of DET when it is administered directly into the CNS is presumably the result of a longer functional half-life in the brain.
One limitation of the current study is that we assessed central insulin sensitivity by administering pharmacological doses of insulin into the CSF. These large doses are typically used in such experiments because in order to reach insulin-sensitive sites in the mediobasal hypothalamus, the administered insulin must move against the bulk flow of interstitial fluid out of the brain parenchyma. Baskin et al. (41) found that similar doses of insulin administered directly into the CSF penetrate into the hypothalamic parenchyma sufficiently far to reach the arcuate nucleus, where insulin’s catabolic action is thought to occur (42).
Overall, the current studies demonstrate that DET crosses the BBB and reduces food intake. This provides support for the hypothesis that DET improves weight management by an enhanced and prolonged centrally mediated reduction of energy intake. Insulin receptors are found in many areas of the brain in addition to the hypothalamus, including the hippocampus and several limbic areas (42), and because insulin entering the brain via the blood-brain barrier presumably accesses all brain insulin receptors as opposed to mainly those in the hypothalamus as occurs after its i3vt administration, the catabolic action of insulin from the brain likely results from both homeostatic and nonhomeostatic circuits (43–45). Greater understanding of insulin penetration into the brain will allow studies to incorporate this process as a novel strategy for the prevention and treatment of obesity and type 2 diabetes (46–48).
Article Information
Funding. This work was supported by National Institutes of Health grants DK017844 and DK095440 to S.C.W., DK092779 to M.L. and S.C.W., DK57900 to D.A.D., and DK54080 to R.J.S. D.P.B. was supported by National Health and Medical Research Council of Australia Early Career Fellowship 1013264.
Duality of Interest. D.A.D. consults for Interarcia, Lilly, and Novo Nordisk; receives research support from Johnson & Johnson and Mannkind; and participates in clinical trials sponsored by Sanofi. R.J.S. has consultancies and research support or is a paid speaker with the following companies: Ethicon Endo-Surgery/Johnson & Johnson, Novo Nordisk, Merck, Novartis, Angiochem, Zafgen, Takeda, Ablaris, Pfizer, Eli Lilly, and Zealand Pharma. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. D.P.B., D.A.D., R.J.S., and S.C.W. designed the experiments. D.P.B., A.A.M., J.D.M., and M.L. collected and analyzed data. D.P.B., D.A.D., and S.C.W. wrote the manuscript. A.A.M., J.D.M., M.L., and R.J.S. reviewed and edited the manuscript. D.P.B. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
References
- 1.Begg DP, Woods SC. Interactions between the central nervous system and pancreatic islet secretions: a historical perspective. Adv Physiol Educ 2013;37:53–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baskin DG, Woods SC, West DB, et al. Immunocytochemical detection of insulin in rat hypothalamus and its possible uptake from cerebrospinal fluid. Endocrinology 1983;113:1818–1825 [DOI] [PubMed] [Google Scholar]
- 3.van Houten M, Posner BI. Insulin binds to brain blood vessels in vivo. Nature 1979;282:623–625 [DOI] [PubMed] [Google Scholar]
- 4.Banks WA. The source of cerebral insulin. Eur J Pharmacol 2004;490:5–12 [DOI] [PubMed] [Google Scholar]
- 5.Ward KR, Bardgett JF, Wolfgang L, Stocker SD. Sympathetic response to insulin is mediated by melanocortin 3/4 receptors in the hypothalamic paraventricular nucleus. Hypertension 2011;57:435–441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jaillard T, Roger M, Galinier A, et al. Hypothalamic reactive oxygen species are required for insulin-induced food intake inhibition: an NADPH oxidase-dependent mechanism. Diabetes 2009;58:1544–1549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Babri S, Badie HG, Khamenei S, Seyedlar MO. Intrahippocampal insulin improves memory in a passive-avoidance task in male wistar rats. Brain Cogn 2007;64:86–91 [DOI] [PubMed] [Google Scholar]
- 8.Baura GD, Foster DM, Porte D Jr, et al. Saturable transport of insulin from plasma into the central nervous system of dogs in vivo. A mechanism for regulated insulin delivery to the brain. J Clin Invest 1993;92:1824–1830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Banks WA, Jaspan JB, Huang W, Kastin AJ. Transport of insulin across the blood-brain barrier: saturability at euglycemic doses of insulin. Peptides 1997;18:1423–1429 [DOI] [PubMed] [Google Scholar]
- 10.Danne T, Lüpke K, Walte K, Von Schuetz W, Gall MA. Insulin detemir is characterized by a consistent pharmacokinetic profile across age-groups in children, adolescents, and adults with type 1 diabetes. Diabetes Care 2003;26:3087–3092 [DOI] [PubMed] [Google Scholar]
- 11.Saltiel AR, Kahn CR. Insulin signalling and the regulation of glucose and lipid metabolism. Nature 2001;414:799–806 [DOI] [PubMed] [Google Scholar]
- 12.Haak T, Tiengo A, Draeger E, Suntum M, Waldhäusl W. Lower within-subject variability of fasting blood glucose and reduced weight gain with insulin detemir compared to NPH insulin in patients with type 2 diabetes. Diabetes Obes Metab 2005;7:56–64 [DOI] [PubMed] [Google Scholar]
- 13.Hermansen K, Davies M, Derezinski T, Martinez Ravn G, Clauson P, Home P. A 26-week, randomized, parallel, treat-to-target trial comparing insulin detemir with NPH insulin as add-on therapy to oral glucose-lowering drugs in insulin-naive people with type 2 diabetes. Diabetes Care 2006;29:1269–1274 [DOI] [PubMed] [Google Scholar]
- 14.Vague P, Selam J-L, Skeie S, et al. Insulin detemir is associated with more predictable glycemic control and reduced risk of hypoglycemia than NPH insulin in patients with type 1 diabetes on a basal-bolus regimen with premeal insulin aspart. Diabetes Care 2003;26:590–596 [DOI] [PubMed] [Google Scholar]
- 15.Dornhorst A, Lüddeke HJ, Koenen C, et al.; PREDICTIVE Study Group . Transferring to insulin detemir from NPH insulin or insulin glargine in type 2 diabetes patients on basal-only therapy with oral antidiabetic drugs improves glycaemic control and reduces weight gain and risk of hypoglycaemia: 14-week follow-up data from PREDICTIVE. Diabetes Obes Metab 2008;10:75–81 [DOI] [PubMed] [Google Scholar]
- 16.Zachariah S, Sheldon B, Shojaee-Moradie F, et al. Insulin detemir reduces weight gain as a result of reduced food intake in patients with type 1 diabetes. Diabetes Care 2011;34:1487–1491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hermansen K, Davies M. Does insulin detemir have a role in reducing risk of insulin-associated weight gain? Diabetes Obes Metab 2007;9:209–217 [DOI] [PubMed] [Google Scholar]
- 18.Woods SC, Lotter EC, McKay LD, Porte D Jr. Chronic intracerebroventricular infusion of insulin reduces food intake and body weight of baboons. Nature 1979;282:503–505 [DOI] [PubMed] [Google Scholar]
- 19.Benoit SC, Air EL, Coolen LM, et al. The catabolic action of insulin in the brain is mediated by melanocortins. J Neurosci 2002;22:9048–9052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Clegg DJ, Brown LM, Woods SC, Benoit SC. Gonadal hormones determine sensitivity to central leptin and insulin. Diabetes 2006;55:978–987 [DOI] [PubMed] [Google Scholar]
- 21.Hennige AM, Sartorius T, Tschritter O, et al. Tissue selectivity of insulin detemir action in vivo. Diabetologia 2006;49:1274–1282 [DOI] [PubMed] [Google Scholar]
- 22.Banks WA, Morley JE, Lynch JL, Lynch KM, Mooradian AD. Insulin detemir is not transported across the blood-brain barrier. Peptides 2010;31:2284–2288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu M, Shen L, Begg DP, D’alessio DA, Woods SC. Insulin increases central apolipoprotein E levels as revealed by an improved technique for collection of cerebrospinal fluid from rats. J Neurosci Methods 2012;209:106–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu M, Kuhel DG, Shen L, Hui DY, Woods SC. Apolipoprotein E does not cross the blood-cerebrospinal fluid barrier, as revealed by an improved technique for sampling CSF from mice. Am J Physiol Regul Integr Comp Physiol 2012;303:R903–R908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mul JD, Begg DP, Barrera JG, et al. High-fat diet changes the temporal profile of GLP-1 receptor-mediated hypophagia in rats. Am J Physiol Regul Integr Comp Physiol 2013;305:R68–R77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Begg DP, Mul JD, Liu M, et al. Reversal of diet-induced obesity increases insulin transport into cerebrospinal fluid and restores sensitivity to the anorexic action of central insulin in male rats. Endocrinology 2013;154:1047–1054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mul JD, Seeley RJ, Woods SC, Begg DP. Angiotensin-converting enzyme inhibition reduces food intake and weight gain and improves glucose tolerance in melanocortin-4 receptor deficient female rats. Physiol Behav 2013;121:43–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Heise T, Nosek L, Rønn BB, et al. Lower within-subject variability of insulin detemir in comparison to NPH insulin and insulin glargine in people with type 1 diabetes. Diabetes 2004;53:1614–1620 [DOI] [PubMed] [Google Scholar]
- 29.Carver C. Insulin treatment and the problem of weight gain in type 2 diabetes. Diabetes Educ 2006;32:910–917 [DOI] [PubMed] [Google Scholar]
- 30.Banks WA, Jaspan JB, Kastin AJ. Selective, physiological transport of insulin across the blood-brain barrier: novel demonstration by species-specific radioimmunoassays. Peptides 1997;18:1257–1262 [DOI] [PubMed] [Google Scholar]
- 31.Tschritter O, Hennige AM, Preissl H, et al. Cerebrocortical beta activity in overweight humans responds to insulin detemir. PLoS ONE 2007;2:e1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hallschmid M, Jauch-Chara K, Korn O, et al. Euglycemic infusion of insulin detemir compared with human insulin appears to increase direct current brain potential response and reduces food intake while inducing similar systemic effects. Diabetes 2010;59:1101–1107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pontiroli AE, Miele L, Morabito A. Increase of body weight during the first year of intensive insulin treatment in type 2 diabetes: systematic review and meta-analysis. Diabetes Obes Metab 2011;13:1008–1019 [DOI] [PubMed] [Google Scholar]
- 34.Rojas JM, Printz RL, Niswender KD. Insulin detemir attenuates food intake, body weight gain and fat mass gain in diet-induced obese Sprague-Dawley rats. Nutr Diabetes 2011;1:e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Begg DP, Woods SC. The endocrinology of food intake. Nat Rev Endocrinol 2013;9:584–597 [DOI] [PubMed] [Google Scholar]
- 36.Hallschmid M, Benedict C, Schultes B, Fehm HL, Born J, Kern W. Intranasal insulin reduces body fat in men but not in women. Diabetes 2004;53:3024–3029 [DOI] [PubMed] [Google Scholar]
- 37.Hallschmid M, Higgs S, Thienel M, Ott V, Lehnert H. Postprandial administration of intranasal insulin intensifies satiety and reduces intake of palatable snacks in women. Diabetes 2012;61:782–789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Air EL, Benoit SC, Blake Smith KA, Clegg DJ, Woods SC. Acute third ventricular administration of insulin decreases food intake in two paradigms. Pharmacol Biochem Behav 2002;72:423–429 [DOI] [PubMed] [Google Scholar]
- 39.Clegg DJ, Benoit SC, Barrera JG, Woods SC. Estrogen mediates body fat distribution and brain sensitivity to adiposity signals. Diabetes 2003;52 [Google Scholar]
- 40.Benedict C, Kern W, Schultes B, Born J, Hallschmid M. Differential sensitivity of men and women to anorexigenic and memory-improving effects of intranasal insulin. J Clin Endocrinol Metab 2008;93:1339–1344 [DOI] [PubMed] [Google Scholar]
- 41.Baskin DG, Brewitt B, Davidson DA, et al. Quantitative autoradiographic evidence for insulin receptors in the choroid plexus of the rat brain. Diabetes 1986;35:246–249 [DOI] [PubMed] [Google Scholar]
- 42.Begg DP, Woods SC. The central insulin system and energy balance. Handb Exp Pharmacol 2012;209:111–129 [DOI] [PubMed] [Google Scholar]
- 43.Figlewicz DP, Sipols AJ. Energy regulatory signals and food reward. Pharmacol Biochem Behav 2010;97:15–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stice E, Figlewicz DP, Gosnell BA, Levine AS, Pratt WE. The contribution of brain reward circuits to the obesity epidemic. Neurosci Biobehav Rev 2013;37:2047–2058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schilling TM, Ferreira de Sá DS, Westerhausen R, et al. Intranasal insulin increases regional cerebral blood flow in the insular cortex in men independently of cortisol manipulation. Hum Brain Mapp 2014;35:1944–1956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hallschmid M, Benedict C, Schultes B, Born J, Kern W. Obese men respond to cognitive but not to catabolic brain insulin signaling. Int J Obes (Lond) 2008;32:275–282 [DOI] [PubMed] [Google Scholar]
- 47.Kern W, Benedict C, Schultes B, et al. Low cerebrospinal fluid insulin levels in obese humans. Diabetologia 2006;49:2790–2792 [DOI] [PubMed] [Google Scholar]
- 48.Gray SM, Meijer RI, Barrett EJ. Insulin regulates brain function, but how does it get there? Diabetes 2014;63:3992–3997 [DOI] [PMC free article] [PubMed] [Google Scholar]