Abstract
Objective:
The objective of this study was to demonstrate the feasibility of timely multimodal MRI screening before thrombolysis in acute stroke patients.
Methods:
Quality improvement processes were initiated in 2013 to reduce door-to-needle (DTN) time at the 2 hospitals where the NIH stroke team provides clinical care. Acute ischemic stroke (AIS) patients who received IV tissue plasminogen activator (tPA) ≤4.5 hours from last known normal were identified. Demographic and clinical characteristics and timing metrics were analyzed comparing the time periods before, during, and after the quality improvement processes.
Results:
There were 157 patients treated with IV tPA for AIS during 2012–2013, of whom 135 (86%) were screened with MRI. DTN time was significantly reduced by 40% during this period from a median of 93 minutes in the first half of 2012 to 55 minutes in the last half of 2013 (p < 0.0001) with a significant 4-fold increase in the proportion of treated patients with DTN time ≤60 minutes from 13.0% to 61.5%, respectively (p < 0.00001). Improvement in DTN time was associated with reduced door-to-MRI time, and there were no differences in demographic or clinical characteristics (p = 0.21–0.76).
Conclusions:
It is feasible and practical to consistently and rapidly deliver IV tPA to AIS patients within national benchmark times using MRI as the routine screening modality. The processes used in the SMART (Screening with MRI for Accurate and Rapid Stroke Treatment) Study to reduce DTN time have the potential to be widely applicable to other hospitals.
Early stroke treatment correlates with improved clinical outcomes.1 The American Heart Association/American Stroke Association's (AHA/ASA) quality improvement (QI) program Target: Stroke, launched in 2011, promotes reducing door-to-needle (DTN) time for IV tissue plasminogen activator (tPA) therapy to ≤60 minutes.2 CT is typically used to screen acute ischemic stroke (AIS) patients for IV tPA eligibility, and application of lean manufacturing principles have been used to expedite this process.3 Past studies have demonstrated that obtaining an MRI before IV tPA treatment is feasible.4–6 However, operational requirements for around-the-clock immediate access to MRI and concerns about delaying IV tPA has limited use of MRI as the routine first-line method of neuroimaging to a very few centers around the world.7 To date, it has not been reported that MRI-based screening protocols can consistently achieve the DTN time benchmark of ≤60 minutes. This study sought to demonstrate that with a focused QI process, it is feasible to routinely screen AIS patients with multimodal MRI before IV tPA treatment and consistently meet the national benchmark DTN time of ≤60 minutes.
METHODS
NIH stroke team infrastructure.
The NIH stroke team provides stroke care at MedStar Washington Hospital Center (MWHC), a 900-bed tertiary care hospital in Washington, DC, with 1,000 stroke admissions annually and Suburban Hospital (SH), a 230-bed community hospital in Bethesda, MD, with 400 stroke admissions annually. Both are certified by the Joint Commission, MWHC as a Comprehensive Stroke Center and SH as a Primary Stroke Center, and both participate in AHA/ASA Get With the Guidelines–Stroke. Coverage is provided at these hospitals during “on hours” (Monday–Friday, 7 am to 5 pm) by an on-site acute stroke team consisting of a vascular neurology attending and fellow, a stroke nurse responder, a transporter, and a research assistant, and during “off hours” (Monday–Thursday, 5 pm to 7 am, and Friday 5 pm to Monday 7 am) by an on-site stroke nurse responder and transporter, with the on-call vascular neurology fellow and attending located off-site. An in-house MRI technologist is present 24/7 at MWHC; at SH, an in-house MRI technologist is present from 7 am to 11 pm and is on-call but not in-house between 11 pm and 7 am. At both hospitals, the MRI suite is located on the same floor as the emergency department (ED) within approximately 150 feet. MRI technologists receive pager notification from the stroke physician about the acute stroke patient coming for MRI and are instructed to interrupt any ongoing scan and take that patient off the table within 15 minutes of the code initiation to accommodate the acute stroke patient when there is another scan in progress.
MRI protocol.
Details of the MRI screening protocol have been previously published.4 Image sequences include diffusion-weighted imaging (DWI), gradient echo, fluid-attenuated inversion recovery, intracranial time-of-flight magnetic resonance angiography (MRA), and perfusion-weighted imaging (PWI). Imaging was performed using a 3T (Achieva; Philips, Andover, MA) MRI machine at MWHC and a 1.5T (TwinSpeed; General Electric, Waukesha, WI) MRI machine at SH. DWI was an isotropically weighted series with b = 0 and b = 1,000 s/mm2, repetition time/echo time (TR/TE) = 6,000–9,000/72–90 milliseconds (ms), and acquisition matrix of 128 × 128 or 256 × 256. Gradient echo used T2* imaging with TR/TE = 800/20 ms, acquisition matrix of 256 × 192, 20-7-mm-thick contiguous axial oblique slices, and 24 cm field of view. Fluid-attenuated inversion recovery had TR/TE = 9,000/92–146 ms, TI inversion time = 2,200 ms, acquisition matrix of 256 × 128 or 256 × 256, either 66-2-mm-thick or 20-7-mm-thick contiguous axial oblique slices, and 24 cm field of view. The 3-dimensional time-of-flight MRA consisted of a single slab, approximately 7 cm thick, centered in the region of the circle of Willis, coplanar to the other slice prescriptions. MRA parameters were TR/TE = 39/6.9 ms, 25° flip angle, field of view of 24 × 18 cm with a matrix of 224 × 160 for an in-plane resolution of approximately 1 mm, reconstructed to 92 axial images, 1.6 mm thick with a 0.8-mm overlap, for a total acquisition time of 3 minutes and 11 seconds. PWIs were obtained using the standard bolus passage of contrast method by injecting gadolinium (0.1 mmol/kg dose via power injector) with gradient-echo echo planar imaging. PWI parameters were TR/TE = 1,500–2,200/45 ms; 25 to 40 phases, 2 seconds per phase; and a matrix of 64 × 64, 128 × 128, or 256 × 256. Maps of normalized mean transit time were calculated using concentration-time curves obtained from the PWI time series. The total scan time of the screening MRI is 15 minutes. Only patients with a contraindication to MRI (e.g., pacemaker, severe claustrophobia, medically unstable) or who are within 30 minutes of the 4.5-hour IV tPA time window are screened with CT at these 2 hospitals.
QI process.
Beginning with the second quarter of 2013, independent QI processes at both hospitals were implemented, which included interventions to expedite IV tPA treatment with participation from ED physicians, nurses, and technicians, radiology and laboratory staff, and acute stroke team physicians and nurses. These interventions to amend the time-consuming bottlenecks were identified by formal lean process initiatives utilizing 6 sigma principles at both of the hospitals, which involved creating value stream maps delineating all the parallel and sequential steps in the process of delivering IV tPA to acute stroke patients arriving in ED. Further analyses were done to identify steps that did not add value and contributed to key time delays. Targeted areas for improvement were identified at each hospital and specific interventions were implemented to minimize DTN times.
Data collection.
A review was performed of consecutive patients evaluated for possible AIS by the NIH stroke team from January 2012 to December 2013. Patients were included in the Screening with MRI for Accurate and Rapid Stroke Treatment (SMART) Study if they received IV tPA at either of the 2 hospitals; patients transferred from outside hospitals after IV tPA administration or those treated with IV tPA in a clinical trial were excluded. Data were collected prospectively for QI purposes in compliance with Human Subjects Protection policies and included the following: patient demographics, stroke risk factors, pretreatment NIH Stroke Scale (NIHSS) score, modified Rankin Scale score, onset-to-door time, door-to-stroke team paging time, door-to-MRI start time, MRI-to-needle time, last seen normal-to-needle time, and DTN time.
Statistical analysis.
Data were grouped in consecutive 6-month intervals for comparison of demographic and clinical characteristics and timing metrics. Kruskal-Wallis analysis of variance and χ2 tests were used to detect significance in continuous and binary data, respectively (p < 0.005 defined significance). To assess which clinical or time variable independently correlated with reduced DTN time, a linear regression model was created with predictors selected using a forward stepwise procedure (p ≤ 0.20 required for entry; p ≤ 0.05 required to be retained). Predictors considered for the model included variables from timing metrics and all characteristics. Statistical analysis was performed using SPSS version 21.0 (IBM Corp., Armonk, NY).
Standard protocol approvals, registrations, and patient consents.
Data were collected prospectively as a QI initiative. Office of Human Subjects Research exemptions and local institutional review board approvals were obtained for use of clinical and research data. Because of its focus on QI, no individual patient consent was required.
RESULTS
Improved acute stroke assessment protocol.
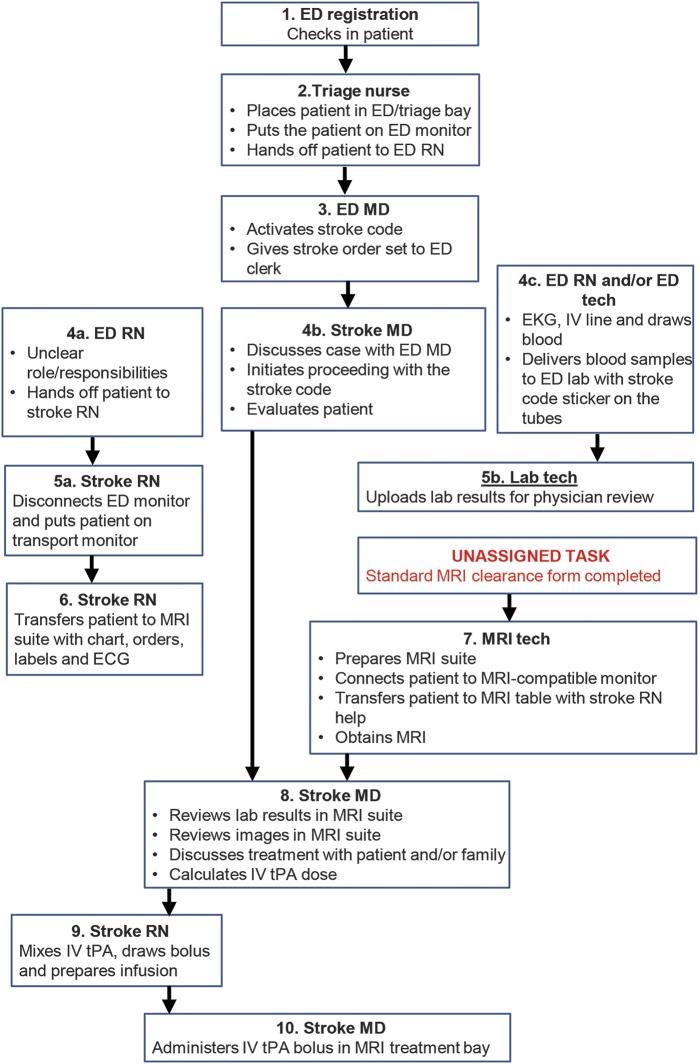
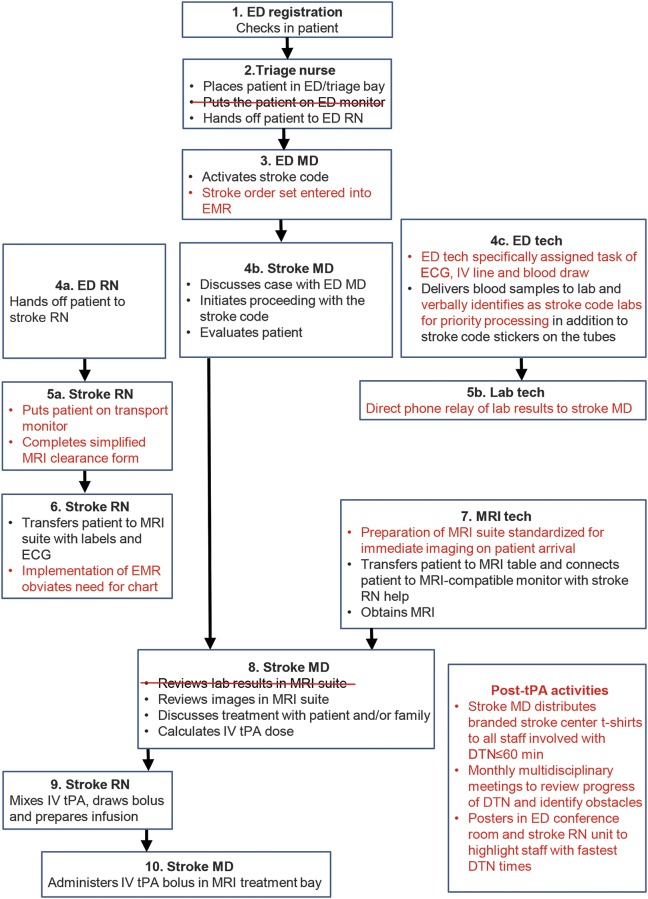
Independent multidisciplinary QI processes were undertaken at MWHC and SH in the first quarter of 2013 to identify specific opportunities to reduce DTN times, and interventions identified as a result of these processes were implemented during the second quarter of 2013. While there were some targets for improvement identified that were similar between the 2 hospitals, others were unique given their different infrastructures. At MWHC, the stroke code page to MRI start time was identified as the interval with the most opportunity to improve efficiency. Specific interventions included the following: (1) defining specific and separate roles and responsibilities for the ED nurse, ED technologist, and stroke nurse, (2) eliminating use of the ED monitor and immediately placing the patient on the transport monitor, (3) simplifying the MRI clearance form (figure e-1 on the Neurology® Web site at Neurology.org), (4) requiring the MRI technologist to consistently have the MRI suite prepared to receive the patient and begin immediate scanning, and (5) distribution of branded stroke center t-shirts by stroke director to all staff involved with DTN time ≤60 minutes to reinforce emphasis on reducing DTN time. Figure 1 illustrates the DTN time workflow before the QI process, and figure 2 after the QI process at MWHC. At SH, key interventions included (1) bypassing the ED bay and bringing the patient directly to MRI on the emergency medical services stretcher, and (2) creating a treatment bay within the MRI suite so that IV tPA could be administered in the MRI suite rather than transferring the patient back to the ED before treatment (already in place at MWHC). Figure e-2 illustrates the pre- and post-QI process DTN time workflow at SH.
Figure 1. Pre-SMART door-to-needle process at MWHC.
ED = emergency department; MWHC = MedStar Washington Hospital Center; SMART = Screening with MRI for Accurate and Rapid Stroke Treatment; tPA = tissue plasminogen activator.
Figure 2. Post-SMART DTN process at MWHC.
Post-SMART DTN process at MWHC. Key differences between DTN processes at MWHC (figures 1 and 2) are highlighted in red. DTN = door-to-needle; ED = emergency department; EMR = electronic medical record; MWHC = MedStar Washington Hospital Center; SMART = Screening with MRI for Accurate and Rapid Stroke Treatment; tPA = tissue plasminogen activator.
Patient characteristics.
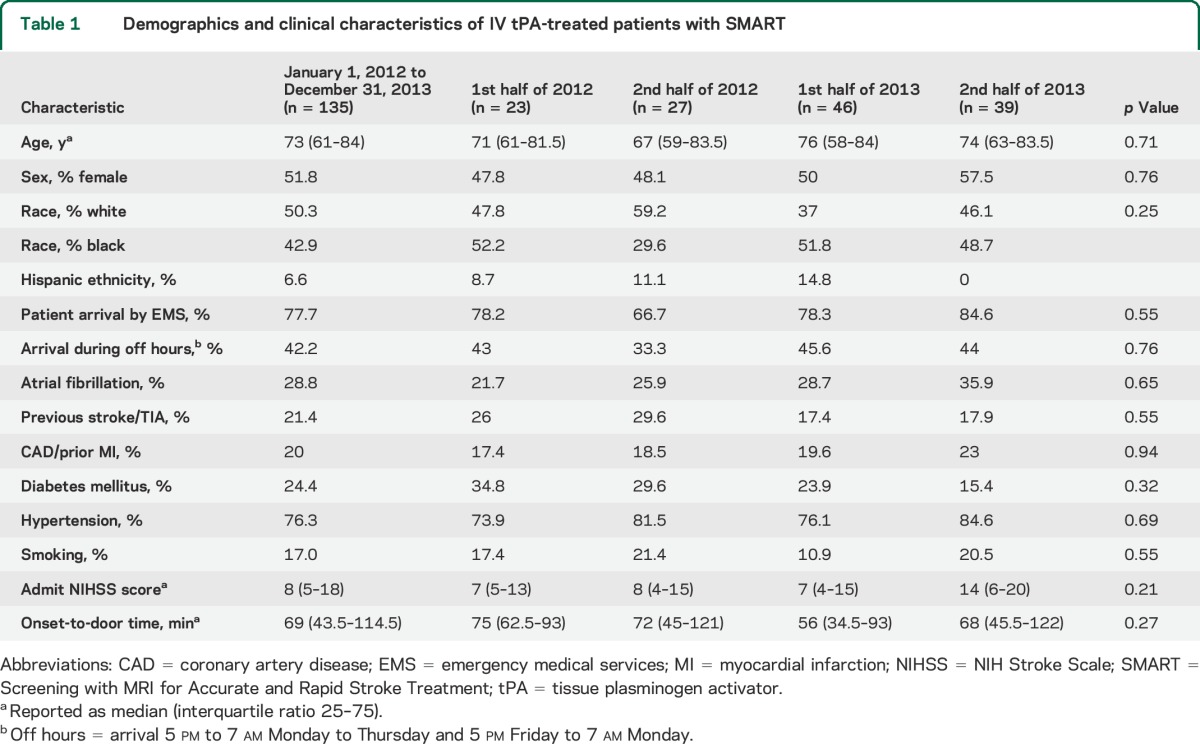
Demographic and clinical characteristics of the patients treated with IV tPA are shown in table 1. There were no differences in these characteristics during the time period studied including age, male sex, race, admit NIHSS score, onset-to-door time, and arrival during off hours.
Table 1.
Demographics and clinical characteristics of IV tPA-treated patients with SMART
Improvement in DTN times with SMART interventions.
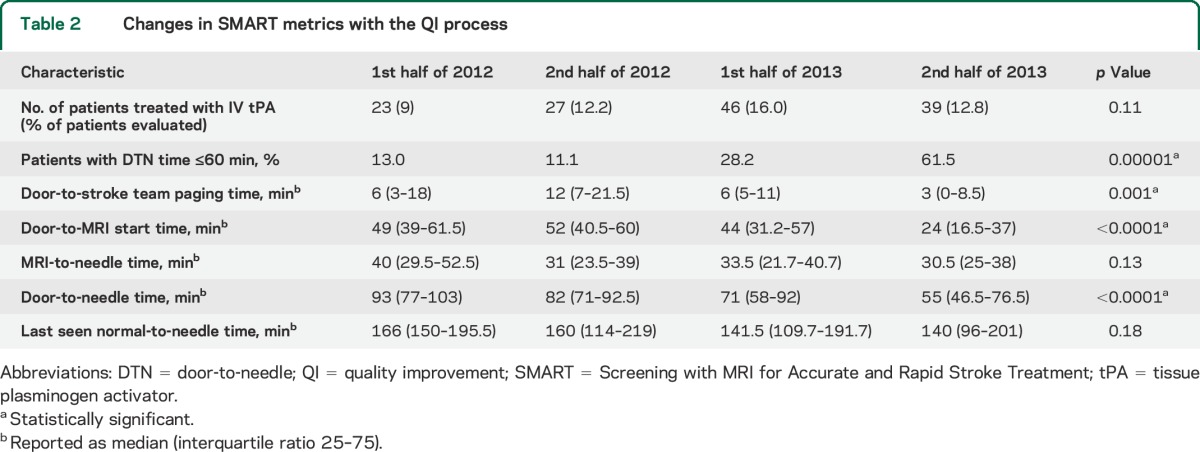
There were 1,066 patients screened and diagnosed on admission with an AIS by the NIH stroke team at both hospitals combined between January 2012 and December 2013, of whom 157 (15%) received IV tPA. Eighty-six percent (135/157) were screened with multimodal MRI before IV tPA treatment. The rate of IV tPA utilization during the study period was unchanged. There was a statistically significant 40% reduction in median DTN time from 93 to 55 minutes (p < 0.0001) and a 4-fold increase in the proportion of patients treated with IV tPA in ≤60 minutes from 13.0% in the first half of 2012 to 61.5% in the last half of 2013 (table 2). There was no difference in the baseline patient characteristics, mode of arrival, or onset-to-arrival during this time period. The improvement in DTN time and proportion of patients treated in ≤60 minutes was seen at both hospitals (tables e-1 and e-2). These improvements were largely attributable to the faster door-to-MRI start time as shown by linear regression for prediction of DTN time (regression coefficient, β [SE] = 0.81 [0.08]; p < 0.0001). Thus, shorter MRI start times were associated with shorter DTN times. While there was a trend showing decrease in overall last seen normal-to-needle time, it was not statistically significant because of low sample size. In-hospital mortality, discharge to home or inpatient rehabilitation rates, and modified Rankin Scale score were not statistically different across the study period (table e-3) and stroke mimic rate remained consistently 0%, showing that with improvement in efficiency, there was no detrimental effect on safety. Although the rate of patients discharged to home or inpatient rehabilitation was lower in the second half of 2013 compared with the earlier time periods, this was likely attributable to the more severe admit NIHSS scores of those patients.
Table 2.
Changes in SMART metrics with the QI process
DISCUSSION
This study demonstrates that consistently achieving DTN time of ≤60 minutes is feasible using multimodal MRI as the first-line imaging modality to screen patients. The DTN time achieved and the proportion of patients receiving IV tPA in ≤60 minutes with screening MRI in this study are comparable to the results reported by other large centers using CT as the primary screening modality.3,8–10 In fact, the reduction in DTN time and the increase in proportion of patients treated ≤60 minutes were greater than that recently reported from AHA/ASA Get With the Guidelines–Stroke participating hospitals in aggregate.8 While a recent study suggests that prolonged imaging-to-needle time is the primary contributor to delay in DTN time, the lean process analyses at our hospitals found that the door-to-imaging time had the greatest opportunity for interventions to reduce DTN time.11 Strengths of this study include achieving this target in 2 very different health care settings independent of stroke severity and time of stroke presentation. We are planning continued monitoring of DTN time closely to ensure that these improvements are sustainable and not attributable to specific attention to the QI process at the time of implementation.
Although no study to date has demonstrated improved outcomes using a multimodal MRI-based approach compared with traditional noncontrast CT for the screening of acute stroke patients, multimodal MRI does provide comprehensive information including location and size of cerebral ischemia, its relative duration, extent of the penumbra, presence of acute and chronic hemorrhage, vessel occlusion, and insight into underlying etiology, which can be useful for clinical decision-making and prognostication purposes.12 Presence of persistent perfusion deficits on multimodal MRI can influence treatment decisions, particularly in mild stroke, which has both clinical and cost implications.13 Furthermore, multimodal MRI can be used to exclude stroke mimics14 and indeed our prospective collection of detailed clinical data including final discharge diagnosis indicated that none of the patients treated with IV tPA at the admitting hospitals had stroke mimics. Previous reports using multimodal MRI before IV tPA treatment had much longer DTN time, prompting concerns about delaying time-sensitive treatment.6,15 In this SMART Study, through the QI processes at the 2 hospitals involved, we identified several inefficiencies in the DTN time workflow before and after the acquisition of MRI, and implemented interventions without any cost-intensive upgrades of existing infrastructure to yield a systemic reduction in the total DTN time using an MRI-based screening approach.
Several elements of the SMART processes described here are generalizable, including creating process maps to identify roadblocks causing delays, reorganizing the work flow to reduce hand-offs, and assigning specific roles to each member of the team, and can serve as a guide to assist other stroke centers and EDs to expedite delivery of time-sensitive care. A 2005 survey showed that 66% of EDs in the United States have access to on-site MRI,16 and with the help of the strategies described here, it should be possible to create the infrastructure necessary to use MRI for rapid and accurate decision-making in acute stroke thrombolysis.
The limitations of this study include the inability to achieve ultrafast DTN time reported by selective centers using CT.17,18 Although DTN time of ≤20 minutes was not achievable in this post-SMART process, continued advances in MRI technology allowing for faster image acquisition times along with improvement in other aspects of the workflow, e.g., hospital prenotification, will likely lead to further reduction of DTN time in the future.19,20 The MRI protocol describing image acquisition time comparable to multimodal CT was recently published using echo-planar imaging and parallel acquisition technique with newer 3.0T multicoil magnetic resonance scanners. This protocol has yet to be tested for thrombolysis screening and its adaptation would require cost-intensive hardware upgrades. Other recent publications have described use of DWI-only MRI as a very sensitive and specific tool for ruling out intracerebral hemorrhage and stroke mimics and confirming the diagnosis of AIS.21,22 While this latter approach can be useful to shorten the scanning time, it does not provide the comprehensive information obtainable through multimodal MRI. Our work here shows that it is possible to reduce DTN time without modifying the MRI acquisition protocol and sacrificing any radiologic information provided by multimodal MRI.
Our study demonstrates that rapid and efficient delivery of IV tPA within national benchmark times is feasible and practical using MRI as the routine screening modality. The processes we have implemented and illustrated are applicable to other centers performing or considering screening MRI for acute stroke treatment evaluation.
Supplementary Material
ACKNOWLEDGMENT
The authors acknowledge and thank the NIH Stroke Team and all members of the multidisciplinary committees who participated in and continue to sustain these successful quality improvement initiatives at MedStar Washington Hospital Center and Suburban Hospital.
GLOSSARY
- AHA/ASA
American Heart Association/American Stroke Association
- AIS
acute ischemic stroke
- DTN
door-to-needle
- DWI
diffusion-weighted imaging
- ED
emergency department
- MRA
magnetic resonance angiography
- MWHC
MedStar Washington Hospital Center
- NIHSS
NIH Stroke Scale
- PWI
perfusion-weighted imaging
- QI
quality improvement
- SH
Suburban Hospital
- SMART
Screening with MRI for Accurate and Rapid Stroke Treatment
- TE
echo time
- tPA
tissue plasminogen activator
- TR
repetition time
Footnotes
Editorial, page 2394
Supplemental data at Neurology.org
AUTHOR CONTRIBUTIONS
Shreyansh Shah: designing, drafting, and revising manuscript, drafting result tables and performing statistical calculations. Marie Luby: designing, drafting, and revising manuscript and drafting and revising illustrations. Karen Poole: devising and implementing quality improvement process at MedStar Washington Hospital Center, revising illustrations. Teresa Morella: devising and implementing quality improvement process at Suburban Hospital, revising illustrations. Elizabeth Keller: devising quality improvement strategy at MedStar Washington Hospital Center, revising manuscript and illustrations. Richard T. Benson: devising and implementing quality improvement strategy at MedStar Washington Hospital Center, revising manuscript. John Lynch: implementing quality improvement process at Suburban Hospital. Zurab Nadareishvili: implementing quality improvement process at Suburban Hospital, revising manuscript. Amie Hsia: devising and implementing quality improvement process at MedStar Hospital Center, designing, drafting, and revising manuscript and drafting and revising illustrations.
STUDY FUNDING
This research was supported by the Division of Intramural Research of the National Institute of Neurological Disorders and Stroke, NIH.
DISCLOSURE
The authors report no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Saver JL, Fonarow GC, Schwamm LH. Acute ischemic stroke and timing of treatment: reply. JAMA 2013;310:1856–1857. [DOI] [PubMed] [Google Scholar]
- 2.Fonarow GC, Smith EE, Saver JL, et al. Improving door-to-needle times in acute ischemic stroke: the design and rationale for the American Heart Association/American Stroke Association's Target: stroke initiative. Stroke 2011;42:2983–2989. [DOI] [PubMed] [Google Scholar]
- 3.Ford AL, Williams JA, Spencer M, et al. Reducing door-to-needle times using Toyota's lean manufacturing principles and value stream analysis. Stroke 2012;43:3395–3398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Luby M, Warach SJ, Nadareishvili Z, Merino JG. Immediate changes in stroke lesion volumes post thrombolysis predict clinical outcome. Stroke 2014;45:3275–3279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schellinger PD, Jansen O, Fiebach JB, et al. Feasibility and practicality of MR imaging of stroke in the management of hyperacute cerebral ischemia. AJNR Am J Neuroradiol 2000;21:1184–1189. [PMC free article] [PubMed] [Google Scholar]
- 6.Sølling C, Ashkanian M, Hjort N, Gyldensted C, Andersen G, Ostergaard L. Feasibility and logistics of MRI before thrombolytic treatment. Acta Neurol Scand 2009;120:143–149. [DOI] [PubMed] [Google Scholar]
- 7.Hjort N, Butcher K, Davis SM, et al. Magnetic resonance imaging criteria for thrombolysis in acute cerebral infarct. Stroke 2005;36:388–397. [DOI] [PubMed] [Google Scholar]
- 8.Fonarow GC, Zhao X, Smith EE, et al. Door-to-needle times for tissue plasminogen activator administration and clinical outcomes in acute ischemic stroke before and after a quality improvement initiative. JAMA 2014;311:1632–1640. [DOI] [PubMed] [Google Scholar]
- 9.Lin CB, Peterson ED, Smith EE, et al. Emergency medical service hospital prenotification is associated with improved evaluation and treatment of acute ischemic stroke. Circ Cardiovasc Qual Outcomes 2012;5:514–522. [DOI] [PubMed] [Google Scholar]
- 10.Xian Y, Smith EE, Zhao X, et al. Strategies used by hospitals to improve speed of tissue-type plasminogen activator treatment in acute ischemic stroke. Stroke 2014;45:1387–1395. [DOI] [PubMed] [Google Scholar]
- 11.Sauser K, Levine DA, Nickles AV, Reeves MJ. Hospital variation in thrombolysis times among patients with acute ischemic stroke: the contributions of door-to-imaging time and imaging-to-needle time. JAMA Neurol 2014;71:1155–1161. [DOI] [PubMed] [Google Scholar]
- 12.Chalela JA, Kidwell CS, Nentwich LM, et al. Magnetic resonance imaging and computed tomography in emergency assessment of patients with suspected acute stroke: a prospective comparison. Lancet 2007;369:293–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kruetzelmann A, Siemonsen S, Gerloff C, et al. Thrombolysis targeting MRI defined tissue at risk in minor stroke. J Neurol Neurosurg Psychiatry 2009;80:1156–1158. [DOI] [PubMed] [Google Scholar]
- 14.Freeman JW, Luby M, Merino JG, et al. Negative diffusion-weighted imaging after intravenous tissue-type plasminogen activator is rare and unlikely to indicate averted infarction. Stroke 2013;44:1629–1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Griebe M, Kern R, Eisele P, et al. Continuous magnetic resonance perfusion imaging acquisition during systemic thrombolysis in acute stroke. Cerebrovasc Dis 2013;35:554–559. [DOI] [PubMed] [Google Scholar]
- 16.Ginde AA, Foianini A, Renner DM, Valley M, Camargo CA., Jr Availability and quality of computed tomography and magnetic resonance imaging equipment in U.S. emergency departments. Acad Emerg Med 2008;15:780–783. [DOI] [PubMed] [Google Scholar]
- 17.Meretoja A, Strbian D, Mustanoja S, Tatlisumak T, Lindsberg PJ, Kaste M. Reducing in-hospital delay to 20 minutes in stroke thrombolysis. Neurology 2012;79:306–313. [DOI] [PubMed] [Google Scholar]
- 18.Meretoja A, Weir L, Ugalde M, et al. Helsinki model cut stroke thrombolysis delays to 25 minutes in Melbourne in only 4 months. Neurology 2013;81:1071–1076. [DOI] [PubMed] [Google Scholar]
- 19.Nael K, Khan R, Choudhary G, et al. Six-minute magnetic resonance imaging protocol for evaluation of acute ischemic stroke: pushing the boundaries. Stroke 2014;45:1985–1991. [DOI] [PubMed] [Google Scholar]
- 20.McKinney JS, Mylavarapu K, Lane J, Roberts V, Ohman-Strickland P, Merlin MA. Hospital prenotification of stroke patients by emergency medical services improves stroke time targets. J Stroke Cerebrovasc Dis 2013;22:113–118. [DOI] [PubMed] [Google Scholar]
- 21.Eichel R, Hur TB, Gomori JM, Cohen JE, Leker RR. Use of DWI-only MR protocol for screening stroke mimics. J Neurol Sci 2013;328:37–40. [DOI] [PubMed] [Google Scholar]
- 22.Leker RR, Keigler G, Eichel R, Ben Hur T, Gomori JM, Cohen JE. Should DWI MRI be the primary screening test for stroke? Int J Stroke 2014;9:696–697. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.