Abstract
Research has increasingly suggested a consistent relationship between peripheral hearing and selected measures of cognition in older adults. However, other studies yield conflicting findings.
Objectives
The primary purpose of the present study was to further elucidate the relationship between peripheral hearing and three domains of cognition and one measure of global cognitive status. It was hypothesized that peripheral hearing loss would be significantly associated with poorer performance across measures of cognition, even after adjusting for documented risk factors. No study to date has examined the relationship between peripheral hearing and such an extensive array of cognitive measures.
Design
894 older adult participants from the Staying Keen in Later Life study cohort were eligible, agreed to participate, and completed the baseline evaluation. Inclusion criteria were minimal to include a sample of older adults with a wide range of sensory and cognitive abilities. Multiple linear regression analyses were conducted to evaluate the extent to which peripheral hearing predicted performance on a global measure of cognitive status, as well as multiple cognitive measures in the domains of speed of processing (Digit Symbol Substitution and Copy, Trail-Making Test Part A, Letter and Pattern Comparison, and Useful Field of View), executive function (Trail-Making Test Part B and Stroop Color-Word Interference Task), and memory (Digit Span, Spatial Span, and Hopkins Verbal Learning Test).
Results
Peripheral hearing, measured as the three-frequency PTA in the better ear, accounted for a significant, but minimal, amount of the variance in measures of speed of processing, executive function, and memory, as well as global cognitive status. Alternative measures of hearing (i.e., three-frequency PTAs in the right and left ears and a bilateral, six-frequency PTA [three frequencies per ear]) yielded similar findings across measures of cognition and did not alter the study outcomes in any meaningful way.
Conclusions
Consistent with literature suggesting a significant relationship between peripheral hearing and cognition, and in agreement with our hypothesis, peripheral hearing was significantly related to ten out of eleven measures of cognition that assessed processing speed, executive function, or memory, as well as global cognitive status. Although evidence, including the present results, suggests a relationship between peripheral hearing and cognition, little is known about the underlying mechanisms. Examination of these mechanisms is a critical need in order to direct appropriate treatment.
INTRODUCTION
The prevalence of hearing loss increases substantially with advancing age and hearing loss is often cited by older adults as one of the most frustrating sensory changes (Arlinger 2003; Chien et al. 2012). In a recent study, Chien and Lin (2012) reported that approximately 25-30 million adults aged 50 and older in the United States are affected by clinically significant hearing loss. Peripheral hearing loss significantly affects both the health and quality of life of older adults (Arlinger 2003; Crews et al. 2004; Gates et al. 2005).
Whereas hearing loss has been considered by older adults to be one of the most frustrating and challenging sensory changes associated with aging, second only to vision loss (Crews and Campbell 2004), cognitive impairment is often cited as the most devastating and feared condition that older adults face (Corner et al. 2004; Morris et al. 2001). Cognitive status has been conceptualized on a continuum between normal cognitive function, age-related cognitive decline, mild cognitive impairment, and clinically ascertained dementia such as Alzheimer’s disease. The onset of severe cognitive impairment is insidious and is often preceded by an asymptomatic, preclinical period followed by a transitional, prodromal state termed mild cognitive impairment (Petersen et al. 1999; Smith et al. 1996). The prevalence of mild cognitive impairment in the United States has been reported at 22.6% (Lopez et al. 2003), and individuals diagnosed with MCI are at a substantially elevated risk of developing Alzheimer’s disease or a related dementia (Morris et al. 2001; Petersen 2004). In the United States, 5.2 million adults aged 65 years and older have Alzheimer’s disease or a related dementia; by 2025, this number is expected to increase by 40% to 7.1 million (Thies and Bleier 2013). Given these statistics, there is a critical need to better understand the hearing-cognition relationship and its implications for theory and practice. It is particularly important to understand this relationship among older adults without severe cognitive impairment, when interventions can most effectively be implemented.
A recent surge of research has documented a weak, but significant, relationship between peripheral hearing and cognition in older adults both cross-sectionally (Jupiter 2012; F. Lin 2011; F. Lin, Ferrucci, et al. 2011; Pearman et al. 2000) and longitudinally (Kiely et al. 2012; F. Lin et al. 2013; Valentijn et al. 2005). This work builds upon prior research documenting a connection between sensory and cognitive decline among older adults (e.g., Baltes et al. 1997; Schneider et al. 2000). However, other studies yield conflicting findings (Gates et al. 2002; Gates et al. 1996; Idrizbegovic et al. 2011; M. Y. Lin et al. 2004; Strouse et al. 1995).
There are many known risk factors for peripheral hearing loss. In addition to age, sex and race are known risk factors for peripheral hearing loss (Cruickshanks et al. 1998; Helzner et al. 2005; F. Lin, Thorpe, et al. 2011). In the U.S. National Health and Nutrition Examination Survey, Agrawal and colleagues (2008) reported the odds of hearing loss in males as 5.5 times higher than in females, particularly in the higher frequencies, and as much as 70% lower in Black individuals versus White individuals. Additional risk factors for hearing loss include smoking, noise exposure, and cardiovascular risks (Helzner et al. 2005; Kiely et al. 2012). In the U.S. Health, Aging, and Body Composition study, cardiovascular disease, diabetes mellitus, ear surgery, and poorer cognitive status were also reported as significant risk factors for peripheral hearing loss (Helzner et al. 2005). Similarly, risk factors for cognitive decline include older age, smoking, lower education, depressive symptoms, and poor health (K.J. Anstey et al. 2007; Marquis et al. 2002; Tilvis et al. 2004; Wilson et al. 2009).
Hearing loss and cognitive impairment in older adults have been studied independently for decades. Potentially more interesting, however, is the relationship between hearing and cognition, and the possible mechanisms underlying this relationship (J. Gallacher 2004). Recently, in a secondary analysis using data from the U.S. Aging, Demographics, and Memory Study, Gure and colleagues (2013) found that approximately 42% of individuals diagnosed with dementia and approximately 44% of individuals with cognitive impairment without dementia had comorbid, significant hearing problems, compared to approximately 26% of the healthy older adult group. Further, in one cross-sectional study (F. Lin, Ferrucci, et al. 2011) and two longitudinal studies (F. Lin, Metter, et al. 2011; F. Lin et al. 2013), Lin and colleagues found that peripheral hearing loss was significantly associated with accelerated cognitive decline, incident cognitive impairment, and incident all-cause dementia. Specifically, both cross-sectional and longitudinal studies report significant associations between peripheral hearing and scores on global cognitive assessments (Kiely et al. 2012; F. Lin, Ferrucci, et al. 2011; F. Lin et al. 2013), measures of memory (F. Lin, Ferrucci, et al. 2011; Pearman et al. 2000; van Boxtel et al. 2000), and measures of executive functioning (F. Lin 2011; F. Lin, Ferrucci, et al. 2011; F. Lin et al. 2013). However, a review by Gallacher (2004) demonstrated the mixed nature of the literature in this area; measures, methods, vocabulary, expertise, quality, and findings vary widely across studies.
The primary purpose of the present study was to further elucidate the relationship between peripheral hearing and cognitive function. The current study expands the existing literature by examining the hearing and cognitive function relationship using secondary analyses of existing data from the Staying Keen in Later Life (SKILL) study cohort, which included a large sample of older adults from the Southeast region of the United States (Clay et al. 2009; Edwards, Wadley, et al. 2005; Wood et al. 2005) and a more comprehensive cognitive battery than has been reported previously (e.g., F. Lin et al. 2013). We hypothesized that peripheral hearing would be significantly associated with performance across multiple measures of cognition tapping three cognitive domains (i.e., speed of processing, executive function, and memory) and on a measure of global cognitive status, even after adjusting for documented risk factors, including depression and health. Exploring a broader range of specific cognitive measures potentially associated with peripheral hearing indices in a large population will aid in the understanding of this relationship.
MATERIALS AND METHODS
Study Participants
1052 participants were screened for inclusion in the SKILL study (Clay et al. 2009; Wood et al. 2005). The SKILL study sought to examine the relationships among cognitive, sensory and functional abilities in older adults. Participants were recruited from Bowling Green, Kentucky; Birmingham, Alabama, and surrounding areas. Inclusion criteria were very minimal to include a sample of older adults with a wide range of sensory and cognitive abilities. To participate in baseline, participants were required to complete a screening visit and to demonstrate a visual acuity of 20/80 or better with corrective lenses, if applicable.
One hundred and thirty-four of the 1052 participants refused further participation in the study. Additionally, 24 participants were coded as ineligible. Reasons for ineligibility included (a) did not meet vision inclusion criteria (n=3), (b) did not complete the screening visit (n=6), (c) hearing data were missing (n=8), (d) was unavailable for further testing (n=1). For six of the participants coded as ineligible, the reason was unspecified and could not be determined (n=6). The 168 participants who did not complete baseline had a mean age of 75.8 (SD=2.63) years, completed an average of 10.4 (SD=7.59) years of education, and included 54% Blacks, 42% Whites, and 4% Asians.
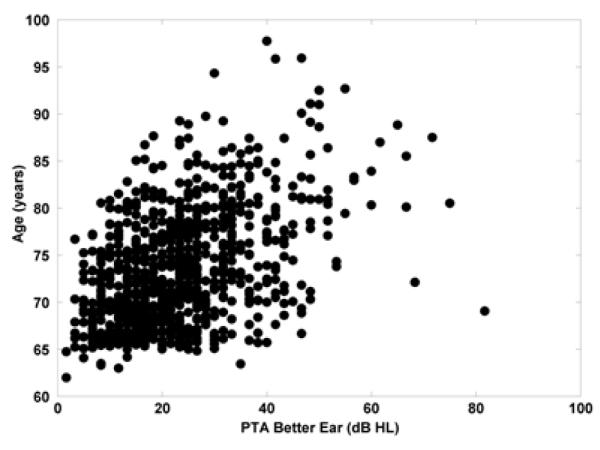
894 participants were eligible, agreed to participate, and completed the baseline evaluation. Demographic information is reported in Table 1. Study participants had a mean age of 73.4 (SD=6.00) years. The participants were mostly women (57.8% female) and White (88.7%); 10.5% of the sample was Black. Participants’ reported levels of education ranged from 6 to 20 years with a mean of 14.0 (SD=2.69) years, which is the equivalent of “some college or vocational training after high school.” Participants were categorized by degree of hearing loss using a three frequency (0.5 kHz, 1 kHz, and 2 kHz) pure tone average in the better hearing ear (see Table 2). The SKILL study did not include measures of hearing at other frequencies. As indicated by Table 2 and Figure 1, the majority of subjects had normal hearing (66.6%; ≤25 dB HL), a finding consistent with other large studies of hearing and cognition (e.g., F. Lin 2011; F. Lin, Metter, et al. 2011).
Table 1.
Participant Demographics
| Variable | M (n) | SD or (%) | Minimum | Maximum |
|---|---|---|---|---|
| Age (years) | 73.47 | 6 | 62 | 97.73 |
| Education (years) | 13.98 | 2.69 | 6 | 20 |
| Female | (517) | (57.8) | ||
| White | (793) | (88.7) | ||
| Black | (94) | (10.5) | ||
| MMSE | 28.17 | 1.9 | 14 | 30 |
| Better ear PTA | 23.09 | 11.80 | 1.67 | 81.67 |
Note. MMSE = Mini-Mental State Exam
Table 2.
Peripheral Hearing Status
| Peripheral Hearing Status |
% Left Ear |
% Right Ear |
% Better Ear |
% Poorer Ear |
|---|---|---|---|---|
| Normal Hearing (PTA ≤25 dB HL) |
57.3 | 57.8 | 66.6 | 48.5 |
| Mild Hearing Loss (PTA = 26-40 dB HL) |
28.5 | 29.5 | 24.6 | 33.4 |
| Moderate Hearing Loss (PTA = 41-70 dB HL) |
12.9 | 11.2 | 8.4 | 15.8 |
| Severe Hearing Loss (PTA = 71-90 dB HL) |
1.2 | 1.0 | 0.34 | 1.9 |
Figure 1.
The relationship between age and pure tone average in the better hearing ear.
Procedure
Participants were screened for eligibility in the SKILL study. Visual acuity, contrast sensitivity, and hearing were assessed at screening. Participants also completed a fifth grade literacy assessment, the Mini-Mental State Exam (MMSE; Folstein et al. 1975), and the Useful Field of View test (UFOV; Edwards, Vance, et al. 2005). The screening visit was approximately 1.5 hours in duration. Within three weeks following the screening, eligible participants completed a baseline assessment lasting approximately 2.5 hours that consisted of a battery of cognitive, sensory, and functional measures, as well as a health questionnaire and a measure of depression (CES-D). Measures of global cognitive status (i.e., MMSE), speed of processing (i.e., Digit Symbol Substitution and Copy, Trail Making Test Part A, Letter and Pattern Comparison, UFOV®), executive function (i.e., Trail Making Test Part B, Stroop Color-Word Interference Task), and memory (i.e., Wechsler Memory Scale-III Digit and Spatial Span, Hopkins Verbal Learning Test) were included at either the screening visit or the baseline assessment.
Measures
A thorough description of the SKILL measures has been reported previously (Clay et al. 2009; Wood et al. 2005). Please refer to Table 4 for an overview of the cognitive measures.
Table 4.
Descriptive Statistics for Cognitive Variables
| Variable | N | M | SD | Minimum | Maximum |
|---|---|---|---|---|---|
| Speed of Processing | |||||
| Digit Symbol Substitution (number) | 894 | 40.04 | 11.66 | 0 | 74 |
| Digit Symbol Copy (seconds) | 891 | 106.48 | 43.14 | 51.07 | 613.25 |
| Trails A (seconds) | 893 | 45.07 | 26.87 | 14.61 | 480 |
| Letter Comparison (number) | 891 | 39.40 | 9.37 | 12 | 69 |
| Pattern Comparison (number) | 893 | 26.94 | 6.53 | 4 | 45 |
| UFOV® (milliseconds) | 890 | 877.53 | 290.15 | 302 | 2000 |
| Executive Function | |||||
| Trails B (seconds) | 893 | 139.86 | 105.78 | 36.6 | 480 |
| Stroop (seconds) | 845 | 32.28 | 17.07 | 3.12 | 147.56 |
| Memory | |||||
| Digit Span (number) | 894 | 9.55 | 2.13 | 4 | 16 |
| Spatial Span (number) | 894 | 7.39 | 1.77 | 0 | 13 |
| HVLT (number) | 894 | 23.35 | 5.58 | 0 | 36 |
Note. UFOV® = Useful Field of View test; HVLT = Hopkins Verbal Learning Test; variable units are listed in parentheses
Audiometry
Pure-tone air conduction hearing thresholds were measured using a calibrated GSI-17 (Grason-Stadler Instruments) portable audiometer and TDH-39 (Telephonics Corp.) earphones at 0.5 kHz, 1 kHz, and 2 kHz in each ear. The protocol of the SKILL study did not include measurement of hearing thresholds at other audiometric frequencies; however, for the frequencies included, the ASHA 1978 method was used to determine threshold (American Speech-Language-Hearing Association Comittee on Audiometric Evaluation 1978). The pure-tone thresholds were averaged to give a three-frequency pure-tone average (PTA) for each ear. The PTA in the better hearing ear was used for all analyses due to the fact that this ear would likely be the primary determinant of participants’ everyday hearing perceptual abilities (World Health Organization Prevention of Blindness and Deafness Program 2011). Additionally, in order to determine whether other measures of peripheral hearing would yield unique relationships with cognitive variables, the individual ear PTAs and the bilateral six-frequency PTA (three frequencies per ear) were also examined.
Global Cognitive Status
MMSE
The MMSE (Folstein et al. 1975) is a screening tool used to briefly assess global cognitive function by tapping domains such as orientation, memory, attention, language, and spatial skills. Instructions are given verbally; two of the items involve visual stimuli. Scores on the MMSE ranged from 14 to 30 points out of a maximum 30 points.
Speed of Processing
Digit Symbol Substitution and Copy
The Wechsler Adult Intelligence Scale-Revised Digit Symbol Substitution is a measure of psychomotor speed (Lezak et al. 2012). Instructions are given verbally, but the stimuli are visual. The task requires participants to quickly fill in a grid of empty squares with geometric symbols by associating the number appearing above each square with the geometric symbol paired with that number in a key at the top of the page (Wechsler 1981). Participants must complete as many substitutions as possible in 90 seconds. The number of correctly completed substitutions in 90 seconds was recorded.
Digit Symbol Copy is a measure of motor speed (Tun et al. 1997). Instructions are given verbally but the stimuli are visual. The task requires participants to quickly fill in a grid of empty squares by copying the corresponding geometric shape located directly above the empty square. Participants are instructed to work as quickly and as accurately as possible. The time it took for participants to complete the task was recorded.
Trail Making Test Part A
The Trail Making Test Part A (Trails A) is a measure of processing speed (Reitan et al. 1985, 1993). Instructions are given verbally but the stimuli are visual. The task requires participants to navigate a series of numbers and connect them in sequential order. The time required to complete the task was the recorded score.
Letter and Pattern Comparison
The Letter and Pattern Comparison Tasks have been used as indices of processing speed (Salthouse et al. 1991). Instructions are given verbally but the stimuli are visual. The Letter Comparison Test was adapted from Salthouse and Babcock (1991). Participants compared paired sets of letters (three, six, or nine letters per set) and determined whether the sets were the same or different. Similarly, in the Pattern Comparison Task, participants compared paired line patterns (three, six, or nine letters per set) to determine as quickly as possible whether the sets were the same or different. For each task, participants were given 20 seconds per section to complete as many items as possible. The number of correct determinations for each task was summed across tests.
UFOV®
The UFOV® is a measure of processing speed that is designed to determine the minimum display duration at which participants can process visual information for four increasingly difficult subtests (Edwards, Vance, et al. 2005). Instructions are given verbally but also are provided visually as a written text; all exercises are visual. In subtest 1, a silhouette of either a car or a truck presented inside of a fixation box must be identified. Subtest 2 measures processing speed while the participant identifies a car or truck presented in the fixation box and localizes a car presented in the periphery. Subtest 3 requires the same two responses, but includes distractors surrounding the peripheral car in the display. Subtest 4 requires participants to indicate whether two items presented simultaneously in the fixation box are the same or different. The briefest duration (in ms) at which participants perform accurately on 75% of trials in each subtest is recorded. Performance across the four subtests is summed and recorded.
Executive Function
Trail Making Test Part B
The Trail Making Test Part B (Trails B) is commonly used to assess executive functioning (Reitan and Wolfson 1985, 1993). Instructions are given verbally but the stimuli are visual. The task requires participants to navigate a series of letters and numbers and connect them in alternating sequential order. The time required to complete the task was the recorded score. In the present study, the time limit for completion of Trails B was set at 480 seconds.
Stroop Color-Word Interference Task
A computerized adaptation of the original Stroop task was used in the present study (Trenerry et al. 1989). Instructions are given verbally but the exercises are visual. The modified Stroop task measures the time it takes to complete each of the following three task conditions: (1) Text/Word Naming – reading words that name colors; (2) Color Naming - naming the color of color blocks; and (3) Text Color Naming - naming the text color in which the words in the series appear, as opposed to the text/word. A composite score was created by first subtracting the reaction time in condition 2 (i.e., color naming) from the reaction time in condition 3 (i.e., text color naming), adjusted by adding a time penalty for the number of uncorrected mistakes during color naming1 (i.e., condition 3). See Wood et al. (2005) for further detail.
Memory
Wechsler Memory Scale-III Digit Span
Digit Span is measure of immediate memory (Wechsler 1987). Instructions and test items are given verbally. Progressively longer strings of numbers were provided as stimuli, and participants were required to recall the number strings until they correctly recalled the most difficult series or repeatedly failed to recall the series. The number of the most difficult series correctly repeated was the recorded score.
Wechsler Memory Scale-III Spatial Span
The WMS-III Spatial Span subtest was administered to assess spatial memory (Wechsler 1987). Instructions are given verbally; the task is visual. Participants viewed a white board that contained 10 blue pegs while the tester touched the pegs (2 to 9 pegs), one peg per second, in a particular order. The participant was then instructed to repeat the peg sequence demonstrated by the tester. The number of pegs included in a sequence increased over trials, thus the task became increasingly difficult due to higher demands on working memory. Task completion occurred when participants either failed trials repeatedly, resulting in a cut-off point, or when participants correctly reproduced the most demanding peg sequence. The number of peg sequences correctly replicated was the score used in the analyses.
Hopkins Verbal Learning Test
The Hopkins Verbal Learning Test (HVLT) is used to assess verbal learning and memory (Brandt 1991). Instructions and test items are given verbally. The test consists of a list of 12 words used to assess verbal learning, recall, and recognition. Participants were given three memorization trials. After each memorization trial, participants were asked to recall all of the words from the 12-item list. The sum of words recalled across the three memorization trials was recorded.
Risk Factors
Center for Epidemiological Studies Depression Scale
The Center for Epidemiological Studies Depression Scale (CES-D) is a brief screening measure of depressive symptomology in the general population (Kohout et al. 1993; Radloff 1977). The CES-D consists of 20 items; participants are asked to indicate the frequency of occurrence (as indicated by the corresponding statement) over the past week. CES-D scores range from 0 to 60, with higher scores indicating increasing presence of depressive symptomology. Depressive symptoms were adjusted for in analyses as a covariate given that such symptoms can negatively affect cognitive performance (Arlinger 2003; Crews and Campbell 2004; Heine et al. 2002; Mohr et al. 2000; Rabinowitz 2000; Weinstein et al. 1982).
Health Questionnaire
Participants were asked to self-report health conditions with a previously validated questionnaire (Jobe et al. 2001) by prompting, “Has a doctor or nurse ever told you that you had…” for fourteen different conditions. Health conditions known to be associated with hearing loss, including diabetes, hypertension, stroke, and heart disease (Agrawal et al. 2008; Cruickshanks et al. 1998; Helzner et al. 2005; Kiely et al. 2012; F. Lin, Thorpe, et al. 2011) were included as covariates in analyses. These variables were coded dichotomously.
Analyses
Locally weighted scatterplot smoothing (LOESS; Cleveland et al. 1988) was used to graphically explore the association of peripheral hearing and age with scores on cognitive measures and to identify nonlinear data trends. The linearity of relationship between covariates and outcome variables was checked with component-residual plots. We did not observe nonlinear relationships. Linear regression using untransformed data was used to model the association between measures of cognition and peripheral hearing while adjusting for age and other covariates. Log transformations were made to variables with non-normal distributions (i.e., PTA, Stroop, and Digit Symbol Substitution). Regression analyses were conducted with and without log transformations for non-normal distributions. Results from log transformed data and untransformed data did not vary significantly. Thus, the untransformed data analyses from linear regressions are reported below.
Multiple linear regression analyses were conducted to evaluate the extent to which peripheral hearing (defined in four ways: three-frequency PTA in the better hearing ear, three-frequency PTA in the right ear, three-frequency PTA in the left ear, or a bilateral six-frequency PTA [across both ears]) predicted performance on a measure of global cognitive status and cognitive measures of executive function, memory, and speed of processing. Known risk factors of hearing loss and cognitive decline including age, race, gender, education, diabetes, heart disease, hypertension, stroke were examined as covariates. Depressive symptomology (CES-D), which can detrimentally affect cognitive performance, was also included as a covariate (Arlinger 2003; Crews and Campbell 2004; Heine and Browning 2002; Mohr et al. 2000; Rabinowitz 2000; Weinstein and Ventry 1982). Covariates were entered in Step 1 (termed “Model 1” below), and each of the hearing measures were entered individually in Step 2 (Model 2). An alpha level of < 0.05 was considered significant, and SPSS version 21 (SPSS IBM, New York, USA) was used to conduct all analyses.
RESULTS
The purpose of the present study was to further elucidate the relationship between peripheral hearing and cognitive function. Descriptive statistics for the cognitive measures and MMSE are reported in Table 4. Data were missing for the Digit Symbol Substitution task (n = 3), Trails A test (n = 1), Letter Comparison (n = 3), Pattern Comparison (n = 1), UFOV® (n = 4), Trails B (n = 1), and Stroop (n = 49). One additional participant was excluded from analyses due to an improperly recorded hearing threshold. Cases with missing data were excluded from analyses.
Three-frequency PTAs in the better hearing ear ranged from 2 to 82 dB HL (Mean=26.21, SD=12.76), with a range of 2 to 88 dB HL in the left ear and a range of 2 to 83 dB HL in the right ear. Thus, consistent with other large database studies in this area (e.g., F. Lin 2011; F. Lin, Metter, et al. 2011), the majority (i.e., 66.6%) of participants had normal hearing. The other three PTAs (i.e., right, left, and bilateral six-frequency PTA [three frequencies per ear]) were strongly correlated (rs = .734-.953) within and between ears (ps < .001).
For all of the outcomes, results for the three-frequency PTA in the better hearing ear are reported in Table 5 Model 2 and are described below. This was chosen: (1) in order to prevent regression dilution—that is, to minimize noise in the measurement of hearing loss produced by a unilateral insult that might attenuate the hearing and cognition relationship, (2) to remain consistent with previous studies (e.g., F. Lin 2011; F. Lin, Ferrucci, et al. 2011), and (3) due to the fact that the better hearing ear would likely be the primary determinant of participants’ everyday hearing perceptual abilities (F. Lin, Ferrucci, et al. 2011; World Health Organization Prevention of Blindness and Deafness Program 2011).
Table 5.
Regression Models for Cognitive Function and Global Cognitive Status Outcomes
| Model 1 |
Model 2 |
|||||
|---|---|---|---|---|---|---|
| Variable | β | 95% CI | t | β | 95% CI | t |
| Digit Symbol Substitution | ||||||
| PTA Better Hearing Ear | - | - | - | −.094 | −.15 – −.03 | −2.99 ** |
| Age | −.302 | −.70 – .48 | −10.67*** | −.260 | −.63 – −.39 | −8.28*** |
| Race | .267 | 7.79 – 12.0 | 9.22*** | .267 | 7.81 – 12.0 | 9.27*** |
| Sex | −.210 | −6.28 – −3.64 | −7.36*** | −.201 | −6.07 – −3.42 | −7.03*** |
| Education | .248 | .830 – 1.31 | 8.70*** | .241 | .80 – 1.28 | 8.45*** |
| Diabetes | −.100 | −5.14 – −1.44 | −3.49*** | −.098 | −5.06 – −1.37 | −3.42*** |
| Hypertension | −.088 | −3.39 – −.73 | −3.04** | −.085 | −3.30 – −.66 | −2.93** |
| Stroke | −.032 | −3.40 – .91 | −1.13 | −.032 | −3.39 – .90 | −1.14 |
| Heart Disease | −.054 | −3.49 – .08 | −1.88 | −.060 | −3.65 – −.09 | −2.06* |
| CES-D | −.123 | −.30 – −.11 | −4.31*** | −.117 | −.29 – −.10 | −4.11*** |
| R2 | .319 | .326 | ||||
| Δ R 2 | .007 | |||||
|
| ||||||
| Digit Symbol Copy | ||||||
| PTA Better Hearing Ear | - | - | - | .084 | .07 – .54 | 2.56 * |
| Age | .303 | 1.77 – 2.61 | 10.21*** | .266 | 1.45 – 2.39 | 8.06*** |
| Race | −.266 | −44.39 – −28.12 | −8.75*** | −.266 | −44.41 – −28.19 | −8.79*** |
| Sex | .112 | 4.58 – 14.80 | 3.72*** | .103 | 3.85 – 14.10 | 3.44*** |
| Education | −.212 | −4.31 – −2.44 | −7.09*** | −.205 | −4.20 – −2.33 | −6.86*** |
| Diabetes | .049 | −1.22 – 13.06 | 1.63 | .047 | −1.46 – 12.78 | 1.56 |
| Hypertension | .074 | 1.22 – 11.49 | 2.43* | .071 | .97 – 11.21 | 2.33* |
| Stroke | .041 | −2.55 – 14.17 | 1.37 | .041 | −2.50 – 14.16 | 1.37 |
| Heart Disease | .022 | −4.36 – 9.43 | .72 | .027 | −3.81 – 9.95 | .88 |
| CES-D | .123 | .40 – 1.11 | 4.13*** | .118 | .36 – 1.08 | 3.95*** |
| R2 | .253 | .259 | ||||
| Δ R 2 | .006 | |||||
|
| ||||||
| Trail Making Test Part A | ||||||
| PTA Better Hearing Ear | - | - | - | .062 | −.01 – .29 | 1.85 |
| Age | .312 | 1.14 – 1.66 | 10.40*** | .285 | .98 – 1.57 | 8.50*** |
| Race | −.313 | −31.95 – −21.64 | −10.20*** | −.313 | −31.96 – −21.66 | −10.22*** |
| Sex | .112 | 2.85 – 9.32 | 3.69*** | .106 | 2.50 – 9.00 | 3.47*** |
| Education | −.159 | −2.18 – −1.00 | −5.26*** | −.154 | −2.13 – −.95 | −5.09*** |
| Diabetes | .046 | −1.01 – 8.04 | 1.52 | .045 | −1.13 – 7.91 | 1.47 |
| Hypertension | .037 | −1.26 – 5.25 | 1.21 | .035 | −1.38 – 5.12 | 1.13 |
| Stroke | −.015 | −6.56 – 3.98 | −.48 | −.015 | −6.56 – 3.97 | −.48 |
| Heart Disease | −.029 | −6.43 – 2.30 | −.93 | −.025 | −6.19 – 2.55 | −.82 |
| CES-D | .085 | .10 – .55 | 2.82** | .081 | .08 – .54 | 2.68** |
| R2 | .234 | .237 | ||||
| Δ R 2 | .003 | |||||
|
| ||||||
| Letter Comparison | ||||||
| PTA Better Hearing Ear | - | - | - | −.092 | −.13 – −.02 | −2.76 ** |
| Age | −.313 | −.58 – −.40 | −10.38*** | −.272 | −.53 – −.32 | −8.11*** |
| Race | .166 | 3.14 – 6.75 | 5.39*** | .167 | 3.16 – 6.75 | 5.42*** |
| Sex | −.170 | −4.36 – −2.10 | −5.60*** | −.162 | −4.20 – −1.93 | −5.30*** |
| Education | .216 | .54 – .96 | 7.11*** | .209 | .52 – .93 | 6.88*** |
| Diabetes | −.093 | −4.03 – −.87 | −3.04** | −.091 | −3.97 – −.81 | −2.97** |
| Hypertension | −.058 | −2.22 – .06 | −1.86 | −.054 | −2.15 – .12 | −1.75 |
| Stroke | −.039 | −3.07 – .64 | −1.29 | −.039 | −3.07 – .63 | −1.30 |
| Heart Disease | −.044 | −2.63 – .43 | −1.41 | −.049 | −2.75 – .29 | −1.58 |
| CES-D | −.077 | −.18 – −.02 | −2.53* | −.071 | −.17 – −.02 | −2.34* |
| R2 | .228 | .235 | ||||
| Δ R 2 | .007 | |||||
|
| ||||||
| Pattern Comparison | ||||||
| PTA Better Hearing Ear | - | - | - | −.110 | −.10 – −.03 | −3.48 *** |
| Age | −.380 | −.47 – −.35 | −13.22*** | −.330 | −.43 – −.29 | −10.37*** |
| Race | .195 | 2.86 – 5.25 | 6.65*** | .196 | 2.87 – 5.25 | 6.70*** |
| Sex | −.047 | −1.37 – .13 | −1.62 | −.036 | −1.23 – .28 | −1.24 |
| Education | .239 | .44 – .72 | 8.26*** | .230 | .42 – .69 | 7.96*** |
| Diabetes | −.119 | −3.23 – −1.13 | −4.08*** | −.116 | −3.17 – −1.09 | −4.01*** |
| Hypertension | −.054 | −1.46 – .05 | −1.84 | −.05 | −1.40 – .10 | −1.71 |
| Stroke | −.042 | −2.13 – .31 | −1.46 | −.042 | −2.12 – .31 | −1.47 |
| Heart Disease | −.053 | −1.94 – .09 | −1.80 | −.059 | −2.04 – −.03 | −2.01* |
| CES-D | −.130 | −.17 – −.07 | −4.49*** | −.123 | −.17 – −.06 | −4.28*** |
| R2 | .300 | .310 | ||||
| Δ R 2 | .010 | |||||
|
| ||||||
| UFOV® | ||||||
| PTA Better Hearing Ear | - | - | - | .090 | .68 – 3.73 | 2.84 ** |
| Age | .447 | 18.84 – 24.27 | 15.59*** | .406 | 16.60 – 22.64 | 12.76*** |
| Race | −.261 | −293.96 – −187.74 | −8.90*** | −.261 | −293.90 – −188.10 | −8.94*** |
| Sex | .050 | −3.96 – 62.45 | 1.73 | .041 | −9.25 – 57.29 | 1.42 |
| Education | −.123 | −19.37 – −7.21 | −4.29*** | −.117 | −18.62 – −6.46 | −4.05*** |
| Diabetes | .096 | 31.71 – 124.56 | 3.30*** | .094 | 29.98 – 122.50 | 3.24*** |
| Hypertension | .019 | −22.12 – 44.66 | .66 | .016 | −5.12 – 84.27 | .56 |
| Stroke | −.019 | −72.97 – 35.64 | −.68 | −.019 | −72.88 – 35.29 | −.68 |
| Heart Disease | .046 | −9.01 – 80.58 | 1.57 | .051 | −5.12 – 84.27 | 1.74 |
| CES-D | .142 | 3.51 – 8.16 | 4.92*** | .136 | 3.28 – 7.92 | 4.73*** |
| R2 | .307 | .313 | ||||
| Δ R 2 | .006 | |||||
|
| ||||||
| Trail-Making Test Part B | ||||||
| PTA Better Hearing Ear | - | - | - | .150 | .77 – 1.91 | 4.59 *** |
| Age | .272 | 3.76 – 5.81 | 9.15*** | .205 | 2.47 – 4.74 | 6.25*** |
| Race | −.304 | −121.90 – −81.87 | −9.99*** | −.304 | −121.82 – −82.24 | −10.12*** |
| Sex | .046 | −2.76 – 22.36 | 1.53 | .031 | −5.86 – 19.13 | 1.04 |
| Education | −.203 | −10.24 – −5.65 | −6.79*** | −.191 | −9.77 – −5.21 | −6.45*** |
| Diabetes | .095 | 10.51 – 45.64 | 3.14** | .091 | 9.56 – 44.31 | 3.04** |
| Hypertension | .067 | 1.51 – 26.75 | 2.20* | .061 | .45 – 25.43 | 2.03* |
| Stroke | −.018 | −26.76 – 14.16 | −.60 | −.018 | −26.55 – 13.91 | −.61 |
| Heart Disease | .028 | −8.85 – 25.04 | .94 | .037 | −6.35 – 27.22 | 1.22 |
| CES-D | .094 | .53 – 2.30 | 3.15** | .085 | .40 – 2.14 | 2.85** |
| R2 | .251 | .269 | ||||
| Δ R 2 | .017 | |||||
|
| ||||||
| Stroop | ||||||
| PTA Better Hearing Ear | - | - | - | .076 | .01 – .18 | 2.16 * |
| Age | .271 | .60 – .97 | 8.36*** | .238 | .51 – .91 | 6.66*** |
| Race | −.191 | −14.56 – −7.22 | −5.82*** | −.193 | −14.71 – −7.38 | −5.89*** |
| Sex | .116 | 1.80 – 6.23 | 3.56*** | .109 | 1.44 – 5.90 | 3.33*** |
| Education | −.198 | −1.66 – −.86 | −6.13*** | −.192 | −1.63 – −.82 | −5.95*** |
| Diabetes | .079 | .75 – 6.93 | 2.44* | .078 | .73 – 6.90 | 2.41* |
| Hypertension | .056 | −.31 – 4.10 | 1.69 | .052 | −.53 – 3.89 | 1.57 |
| Stroke | .022 | −2.32 – 4.87 | .70 | .023 | −2.30 – 4.87 | .42 |
| Heart Disease | −.001 | −3.08 – 2.99 | −.03 | .003 | −2.89 – 3.18 | .09 |
| CES-D | .091 | .07 – .38 | 2.83** | .086 | .06 – .38 | 2.65** |
| R2 | .172 | .177 | ||||
| Δ R 2 | .005 | |||||
|
| ||||||
| Digit Span | ||||||
| PTA Better Hearing Ear | - | - | - | −.095 | −.03 – −.00 | −2.64 ** |
| Age | −.124 | −.07 – −.02 | −3.84*** | −.082 | −.05 – −.00 | −2.28* |
| Race | .156 | .62 – 1.50 | 4.71*** | .156 | .62 – 1.50 | 4.74*** |
| Sex | −.069 | −.57 – −.02 | −2.10* | −.059 | −.53 – .02 | −1.81 |
| Education | .183 | .09 – .20 | 5.63*** | .176 | .09 – .19 | 5.39*** |
| Diabetes | −.082 | −.87 – −.10 | −2.48* | −.079 | −.86 – −.09 | −2.42* |
| Hypertension | −.072 | −.58 – −.03 | −2.17* | −.069 | −.57 – −.02 | −2.07* |
| Stroke | −.033 | −.68 – .22 | −1.01 | −.033 | −.68 – .22 | −1.02 |
| Heart Disease | −.022 | −.50 – .25 | −.65 | −.027 | −.53 – .22 | −.81 |
| CES-D | −.045 | −.03 – .06 | −1.37 | −.039 | −.03 – .01 | −1.19 |
| R2 | .108 | .115 | ||||
| Δ R 2 | .007 | |||||
|
| ||||||
| Spatial Span | ||||||
| PTA Better Hearing Ear | - | - | - | −.074 | −.02 – −.00 | −2.06 * |
| Age | −.150 | −.06 – −.03 | −4.63*** | −.117 | −.06 – −.01 | −3.24*** |
| Race | .185 | .68 – 1.41 | 5.57*** | .185 | .68 – 1.41 | 5.59*** |
| Sex | .000 | −.23 – .23 | −.00 | .007 | −.21 – .26 | .22 |
| Education | .131 | .04 – .13 | 4.00*** | .125 | .04 – .12 | 3.81*** |
| Diabetes | −.042 | −.53 – .11 | −1.28 | −.040 | −.53 – .12 | −1.23 |
| Hypertension | −.022 | −.31 .15 | −.67 | −.020 | −.30 – .16 | −.59 |
| Stroke | .062 | −.01 – .74 | 1.89 | .062 | −.01 – .74 | 1.90 |
| Heart Disease | .043 | −.11 – .52 | 1.30 | .039 | −.13 – .50 | 1.18 |
| CES-D | −.121 | −.05 – −.01 | −3.70*** | −.116 | −.05 – −.01 | −3.56*** |
| R2 | .104 | .108 | ||||
| Δ R 2 | .004 | |||||
|
| ||||||
| Hopkins Verbal Learning Test | ||||||
| PTA Better Hearing Ear | - | - | - | −.168 | −.11 – −.05 | −5.15 *** |
| Age | −.245 | −.28 – −.17 | −8.22*** | −.170 | −.22 – −.10 | −5.19*** |
| Race | .249 | 3.37 – 5.50 | 8.18*** | .250 | 3.40 – 5.49 | 8.32*** |
| Sex | −.298 | −4.03 – −2.70 | −9.90*** | −.281 | −3.84 – −2.52 | −9.44*** |
| Education | .173 | .24 – .48 | 5.77*** | .160 | .21 – .45 | 5.38*** |
| Diabetes | −.074 | −2.10 – −.23 | −2.45* | −.070 | −2.02 – −.17 | −2.34* |
| Hypertension | .015 | −.50 – .84 | .50 | .021 | −.43 – .90 | .70 |
| Stroke | .014 | −.83 – 1.35 | .47 | .014 | −.81 – 1.33 | .47 |
| Heart Disease | −.034 | −1.42 – .38 | −1.13 | −.044 | −1.55 – .23 | −1.45 |
| CES-D | −.144 | −.16 – −.07 | −4.81*** | −.134 | −.15 – −.06 | −4.52*** |
| R2 | .245 | .267 | ||||
| Δ R 2 | .022 | |||||
|
| ||||||
| Mini Mental State Exam | ||||||
| PTA Better Hearing Ear | - | - | - | −.106 | −.03 – −.01 | −3.16 ** |
| Age | −.260 | −.10 – −.06 | −8.60*** | −.213 | −.88 – −.05 | −6.34*** |
| Race | .271 | 1.30 – 2.00 | 8.76*** | .271 | 1.27 – 2.00 | 8.82*** |
| Sex | −.199 | −1.00 – −.54 | −6.53*** | −.189 | −.96 – −.50 | −6.19*** |
| Education | .207 | .10 – .19 | 6.79*** | .198 | .10 – .18 | 6.52*** |
| Diabetes | −.050 | −.59 – .05 | −1.65 | −.048 | −.58 – .07 | −1.57 |
| Hypertension | −.027 | −.33 – .13 | −.87 | −.023 | −.32 – .14 | −.75 |
| Stroke | .035 | −.16 – −59 | 1.15 | .035 | −.15 – .59 | 1.15 |
| Heart Disease | .029 | −.16 – .46 | .951 | .024 | −.19 – .43 | .77 |
| CES-D | −.092 | −.04 – −.01 | −3.04** | −.086 | −.04 – −.01 | −2.83** |
| R2 | .222 | .231 | ||||
| Δ R 2 | .009 | |||||
p ≤ .05,
p ≤ .01,
p ≤ .001
Note. PTA = pure-tone average. Model 1(columns 2-4) included known risk factors of hearing loss. Model 2 (columns 5-7) added three-frequency PTA in the better hearing ear after all covariates were accounted for. The change in R2 represents the amount of variance that can be contributed to PTA in the better hearing ear after all other variables are considered. The β weights (column 2 for model 1 and column 5 for model 2) indicate the standard deviation change in the outcome associated with a 12 dB change in hearing. The 95% CI (column 3 for model 1 and column 6 for model 2) indicate the confidence interval or range of values across which β would be expected to occur 95% of the time. The t statistic (column 4 for model 1 and column 7 for model 2) is the β coefficient divided by its standard error. The asterisks beside the t values indicates whether or not the variable contributed significant variance, and if so, at what level.
The three-frequency PTA in the better hearing ear accounted for a significant amount of variance in the global status measure and all cognitive outcomes except for Trails A (Table 5 Model 2). For all of the cognitive outcomes (speed of processing: Digit Symbol Substitution and Copy, Trails A, Letter and Pattern Comparison, UFOV®; executive function: Trails B, Stroop; memory: Digit and Spatial Span, HVLT) and global cognitive status (MMSE), age, race, and education were significant covariates. Sex was a significant covariate for all outcomes except for Pattern Comparison, UFOV®, Trails B, and Spatial Span. Diabetes was a significant covariate for Digit Symbol Substitution, Letter and Pattern Comparison, UFOV®, Trails B, Stroop, Digit Span, and HVLT. Hypertension was a significant covariate for Digit Symbol Substitution and Copy, Trails B, and Digit Span. Neither stroke nor heart disease was a significant covariate in any of the models. Depressive symptomology was a significant covariate for all outcomes except for Digit Span.
Alternative measures of hearing (i.e., three-frequency PTAs in the right and left ears and bilateral six-frequency PTA [three frequencies per ear]) yielded similar findings across ten measures of cognitive function and the global screening measure. However, results across the four PTAs varied for Trails A. Results of regression models for the other three hearing indices for Trails A are reported in Table 6.
Table 6.
Regression Models with Alternative Hearing Indices for Trail Making Test Part A
| Model 1 |
Model 2 |
|||||
|---|---|---|---|---|---|---|
| Hearing Index | β | 95% CI | t | β | 95% CI | t |
| Three Frequency PTA: Left Ear | ||||||
| PTA Left | - | - | - | .109 | .09 – .34 | 3.391*** |
| Age | .312 | 1.14 – 1.66 | 10.399*** | .271 | .93 – 1.50 | 8.424*** |
| Race | −.313 | −31.95 – −21.64 | −10.201*** | −.315 | −32.06 – −21.81 | −10.313*** |
| Sex | .112 | 2.85 – 9.32 | 3.688*** | .096 | 1.95 – 8.47 | 3.139** |
| Education | −.159 | 2.18 – −1.00 | −5.261*** | −.151 | −2.09 – −.91 | −4.999*** |
| Diabetes | .046 | −1.01 – −8.04 | 1.524 | .045 | −1.10 – 7.90 | 1.484 |
| Hypertension | .037 | −1.26 – 5.25 | 1.205 | .029 | −1.67 – 4.81 | .952 |
| Stroke | −.015 | −6.56 – 3.98 | −.482 | −.015 | −6.56 – 3.92 | −.495 |
| Heart Disease | −.029 | −6.43 – 2.30 | −.928 | −.022 | −5.95 – 2.75 | −.720 |
| CES-D | .085 | .10 – .55 | 2.816** | .082 | .09 – .54 | 2.721** |
| R2 | .234 | .244 | ||||
| Δ R 2 | .234 | .010 | ||||
|
| ||||||
| Three Frequency PTA: Right Ear | ||||||
| PTA Right | - | - | - | .037 | −.05 – .20 | 1.158 |
| Age | .313 | 1.14 – 1.67 | 10.411*** | .298 | 1.05 – 1.62 | 9.141*** |
| Race | −.313 | −31.93 – −21.62 | −10.190*** | −.313 | −31.96 – −21.65 | −10.202*** |
| Sex | .113 | 2.89 – 9.37 | 3.713*** | .110 | 2.72 – 9.22 | 3.605*** |
| Education | −.160 | −2.18 – −1.00 | −5.272*** | −.157 | −2.16 – −.97 | −5.172*** |
| Diabetes | .046 | −1.03 – 8.02 | 1.514 | .044 | −1.17 – 7.89 | 1.454 |
| Hypertension | .038 | −1.22 – 5.29 | 1.230 | .037 | −1.28 – 5.23 | 1.191 |
| Stroke | −.015 | −6.59 – 3.96 | −.490 | −.015 | −6.61 – 3.93 | −.499 |
| Heart Disease | −.029 | −6.47 – 2.27 | −.945 | −.027 | −6.36 – 2.39 | −.892 |
| CES-D | .085 | .10 – .55 | 2.81** | .083 | .09 – .55 | 2.743** |
| R2 | .235 | .236 | ||||
| Δ R 2 | .235 | .001 | ||||
|
| ||||||
|
PTA Across Ears
| ||||||
| PTA Across Ears | - | - | - | .081 | .03 - .31 | 2.457* |
| Age | .312 | 1.14 – 1.66 | 10.399*** | .279 | .96 – 1.54 | 8.494*** |
| Race | −.313 | −31.95 – −21.64 | −10.201*** | −.314 | −32.03 – −21.74 | −10.263*** |
| Sex | .112 | 2.85 – 9.32 | 3.688*** | .102 | 2.29 – 8.80 | 3.344*** |
| Education | −.159 | −2.18 – −1.00 | −5.261*** | −.153 | −2.12 – −.931 | −5.047*** |
| Diabetes | .046 | −1.01 – 8.04 | 1.524 | .044 | −1.20 – 7.83 | 1.442 |
| Hypertension | .037 | −1.26 – 5.25 | 1.205 | .033 | −1.50 – 5.00 | 1.057 |
| Stroke | −.015 | −6.56 – 3.98 | −.482 | −.015 | −6.59 – 3.92 | −.498 |
| Heart Disease | −.029 | −6.43 – 2.30 | −.928 | −.024 | −6.10 –2.62 | −.783 |
| CES-D | .085 | .10 – .55 | 2.816** | .082 | .09 – .54 | 2.709** |
| R2 | .234 | .240 | ||||
| Δ R 2 | .234 | .005 | ||||
p ≤ .05,
p ≤ .01,
p ≤ .001
Note. PTA = pure-tone average. Model 1 included known risk factors of hearing loss. Model 2 added three-frequency PTA in the left ear, three-frequency PTA in the right ear, and three-frequency PTA across both ears, respectively, after all covariates were accounted for. The β weights indicate the standard deviation change in the outcome associated with a 12 dB change in hearing.
DISCUSSION
Several studies have documented a significant, yet weak, relationship between peripheral hearing and global measures of cognitive status (e.g., F. Lin, Ferrucci, et al. 2011; Gallacher et al. 2012; Jupiter 2012; Kiely et al. 2012; F. Lin et al. 2013), and limited measures of speed of processing (e.g., F. Lin 2011; F. Lin, Ferrucci, et al. 2011; F. Lin et al. 2013), executive function (e.g., F. Lin, Ferrucci, et al. 2011), and memory (e.g., Pearman et al. 2000; F. Lin, Ferrucci, et al. 2011; Gallacher et al. 2012). Consistent with these findings and in agreement with our hypothesis, the present results indicate that peripheral hearing, indexed by the three-frequency PTA in the better hearing ear, was significantly related to global cognitive status (i.e., MMSE) as well as a broader range of cognitive measures of processing speed (i.e., Digit Symbol Substitution and Copy, Letter and Pattern Comparison, and UFOV®), executive function (i.e., Trails B and Stroop), and memory (i.e., Digit Span, Spatial Span, and HVLT) than has been reported previously. Digit Symbol Copy, Letter and Pattern Comparison, UFOV®, Digit Span, Spatial Span, and HVLT performance related to peripheral hearing has not previously been reported in the literature. It is of interest to note, however, that after accounting for known risk factors of hearing loss and cognitive decline, peripheral hearing accounted for only a minimal, albeit significant, amount of variance across measures of speed of processing (0.6% to 1%), executive function (0.5% and 1.7%), memory (0.4% to 2.2%), and global cognitive status (0.9%).
It is possible that these minimal effect sizes are due to the fact that the majority (66.6%) of study participants had normal peripheral hearing. To determine the extent to which the inclusion of a large number of participants with normal hearing (between 0.5 and 2 kHz) diffused the effects of peripheral hearing loss on measures of cognition, we repeated our regression analyses within two groups: (1) Normal Hearing (NH), 595 participants with better ear PTA ≤ 25 dB HL, and (2) Impaired Hearing (IH), 299 participants with better ear PTA > 25 dB HL. For the NH participants, peripheral hearing was significantly related to one measure of executive function (Trails B; ΔR2 = .007, p = .022) but not to global cognitive status (MMSE; ΔR2 < .001, p = .916) or any of the other cognitive measures (ΔR2 = 0 - .005, ps = .065 - .544). Among the IH participants, peripheral hearing was significantly related to global cognitive status (MMSE; ΔR2 = .014, p = .018 ), one measure of executive function (Trails B; ΔR2 = .013, p = .020), and all three measures of memory (ΔR2 = .011 - .021, ps = .009 - .048) but was not significantly related to any of the five measures of processing speed (ΔR2 = .002 - .007, ps = .080 - .421) or the other measure of executive function (Stroop; ΔR2 = .008, p = 090). This suggests that the significant relationship between hearing and cognition is driven by the older adults with impaired hearing. However, the effect sizes were still minimal, as they were for the full group of NH and IH participants combined.
In addition to further examining the relationship between hearing and cognition using a broader variety of cognitive measures, we set out to explore whether a single PTA for the better hearing ear is the optimal audiometric measure for studying this relationship. Results across PTAs for the left ear, right ear, and both ears combined were largely consistent and differed significantly only for Trails A. Although peripheral hearing measured as the three-frequency PTA in the better hearing ear was not significantly related to Trails A performance, remaining measures of peripheral hearing (i.e., 3-frequency PTA in the right ear only or the left ear only, and 6-frequency PTA across right and left ears) yielded significant relationships.
Although somewhat varying relationships emerged across measures of peripheral hearing for Trails A performance, the discrepancies were minimal. Thus, given that the better hearing ear would likely be the primary determinant of participants’ everyday hearing perceptual abilities (World Health Organization Prevention of Blindness and Deafness Program 2011) , the need for consistency across studies (e.g., J. Gallacher 2004; F. Lin 2011), and the fact that the present results indicate that the relationships between alternative measures of peripheral hearing were largely consistent across variables, the PTA in the better hearing ear appears to be a useful and ecologically valid metric of peripheral hearing when examining the relationship between peripheral hearing and cognition.
In spite of a growing body of literature documenting significant relationships between peripheral hearing and cognition (F. Lin 2011; F. Lin, Ferrucci, et al. 2011; F. Lin et al. 2013), a few studies have failed to yield similar results (Gates et al. 2002; Gates et al. 1996; Idrizbegovic et al. 2011; M. Y. Lin et al. 2004; Strouse et al. 1995). For example, M. Y. Lin and colleagues (2004) found no relationship between peripheral hearing and changes in global mental status over four years when adjusting for covariates (e.g., age, education, smoking, baseline mental status). However, a relatively imprecise measure of peripheral hearing (i.e., categorized as greater or less than 40 dB at 2 kHz) was examined, the outcome was change in an insensitive measure of global cognitive status (i.e., Modified MMSE), and 80% of the sample had normal hearing. Further, several studies that have examined the relationship between peripheral hearing and dementia status have failed to yield significant findings. For example, Gates and colleagues (1996; 1995) found no relationship between peripheral hearing (PTA at 0.5- 1- and 2- kHz) and incident dementia either cross-sectionally (determined by the Clinical Dementia Rating Scale) or six years later (determined by changes in MMSE). Strouse et al. (1995) compared a small sample (N=20) of older adults with and without dementia and found no group differences in peripheral hearing measured by high frequency PTA (average threshold at 2, 4, and 8 kHz). However, the sample was not adequately powered to detect significant group differences given the minimal effect sizes subsequently observed in the present study and other studies (Baltes and Lindenberger 1997; F. Lin 2011; F. Lin, Ferrucci, et al. 2011).
The effect sizes observed in the present study between peripheral hearing and cognition among older adults without dementia are similar to those observed by Baltes and Lindenberger (1997) who found that hearing accounted for 1.1% of the variance in a cognitive composite. Although statistical techniques and outcomes have varied across studies, the reported effect sizes of the relationship between peripheral hearing and cognition are, at best, small. Effect sizes for memory outcomes (which typically involve only auditory stimuli) in our study as well as others (J. Gallacher et al. 2012; F. Lin, Ferrucci, et al. 2011; Pearman et al. 2000; van Boxtel et al. 2000) have tended to be slightly larger than other cognitive outcomes, but the magnitude is still minimal to small. Among older adults without dementia, correlations between hearing and cognitive performance have ranged from r = .04 - .36, depending on adjustments for covariates, both cross-sectionally and longitudinally (Baltes and Lindenberger 1997; Uhlmann et al. 1989; Valentijn et al. 2005).
In addition to small effect sizes, some null findings may be attributed to the inclusion of a sample of older adult participants with primarily normal hearing. For example, Idrizbegovic et al. (2011) found no significant group differences in peripheral hearing when comparing individuals with a clinical diagnosis of mild cognitive impairment, Alzheimer’s disease, or subjective memory complaints with intact cognitive status. However, the participants’ hearing thresholds did not exceed 20 dB at any frequency between 0.125 and 2 kHz (Idrizbegovic et al. 2011).
In summary, the present study and prior research indicate that, among older adults without dementia, in both cross-sectional and longitudinal analyses, there is a consistent, significant relationship between peripheral hearing and multiple domains of cognitive performance and global cognitive status, but the effect sizes are minimal. Thus, our results indicate that discrepant findings regarding whether or not there is a relationship between peripheral hearing and cognition are not due to using varied indices of cognitive performance, and neither can discrepant findings be attributed to the use of the better ear PTA instead of individual ear PTAs or a bilateral PTA. Given the minimal effect sizes of the peripheral hearing/cognition relationship, discrepant findings are most likely due to small sample sizes, analyses of group differences (rather than examining continuous relationships), and particularly the inclusion of samples of participants with dementia and/or primarily participants with normal hearing. Although the present findings and literature reviewed support the relationship between peripheral hearing and cognition among older adults without dementia, we have yet to explain why this relationship exists.
There are several limitations to the present study. First, pure-tone hearing was only measured at three frequencies in each ear (0.5, 1, and 2 kHz) and it is likely that many participants would have had high frequency sensorineural hearing loss that was not captured in the current study due to the limited range of frequencies tested. Second, the SKILL study (Clay et al. 2009; Edwards, Wadley, et al. 2005; Wood et al. 2005) used a community-based sample, thus the majority of the participants had normal hearing within this range of frequencies. Another limitation is that although theories such as the “common cause” and sensory degradation have been proposed indicating that visual and auditory sensory functioning are both related to cognitive decline (Baltes and Lindenberger 1997; Pichora-Fuller et al. 1995; Schneider and Pichora-Fuller 2000; Valentijn et al. 2005), the present study focused on only hearing. Previous publications have described the relationship between vision and cognition in the SKILL study (Clay et al. 2009). Similarly, a review of speech recognition in noise and cognition indicated a significant relationship between the two (Akeroyd 2008); however, no speech recognition measures were administered in SKILL.
Given that approximately 25-30 million Americans have peripheral hearing loss, and it is the third most prevalent chronic health condition facing older adults (Collins 1997; National Institute on Deafness and Other Communication Disorders 2010), it is imperative to better understand the mechanisms underlying the hearing and cognition relationship. Although the hearing and cognition relationship has been explored for decades and ample evidence, including the results presented in this study, suggests a relationship between the two conditions, little is known about the underlying mechanisms responsible for the relationship. Several potential mechanisms are possible: (1) cognitive impairment may be overdiagnosed in those with hearing loss or vice versa (Arlinger et al. 2009; Herbst et al. 1980; Jorgensen 2012; Valentijn et al. 2005); (2) widespread neural degeneration may cause declines in both hearing and cognition (“common cause”; K.J. Anstey et al. 2001; Baltes and Lindenberger 1997; Lindenberger et al. 1994); (3) sensory deprivation/degradation may negatively impact cognitive processing (Oster 1976; Pichora-Fuller et al. 1995; Schneider and Pichora-Fuller 2000); (4) differential patterns of cognitive resource allocation and depletion may occur in those with hearing loss due to neural reorganization and/or compensation (K. Z. Li et al. 2002; K. Z. H. Li et al. 2001; Schneider and Pichora-Fuller 2000; Tun et al. 2009); (5) social isolation/depression experienced by individuals with hearing loss may ultimately cause cognitive decline (Gopinath, Hickson, et al. 2012; Gopinath, Schneider, et al. 2012; Kramer et al. 2002; Mick et al. 2014); or (6) the combination of any or all of the above (e.g., multi-level model; K. Z. Li and Lindenberger 2002). Furthermore, the relationship between hearing and cognition may be bi-directional in nature (Kiely et al. 2012). Examination of these mechanisms is critical in order to direct appropriate treatment. At present, it is unknown whether existing treatments for peripheral hearing loss (e.g., hearing aids, cochlear implants, assistive listening devices, auditory-cognitive training) have the potential to remediate cognitive declines in the domains of speed of processing, executive function, and/or memory.
To date, predominately behavioral measures (both audiometric and cognitive) have been utilized to explore the proposed mechanisms underlying the hearing and cognition relationship. In some cases, this approach might be appropriate. However, to fully address the proposed mechanisms, it is more likely that multiple, interdisciplinary approaches are required. Absent from this literature and critical for advancing the field are longitudinal studies of multiple domains of both hearing and cognition using behavioral and neurophysiological methods.
Supplementary Material
SHORT SUMMARY.
Research has suggested a significant relationship between peripheral hearing and limited measures of cognition in older adults. However, it is unclear whether this relationship extends to multiple domains and measures of cognitive performance. Using a sample of 894 participants from the Staying Keen in Later Life (SKILL) study, significant relationships were observed between peripheral hearing and eleven cognitive measures tapping global cognitive status, speed of processing, executive function, and memory. Results provide comprehensive evidence of a minimal, but significant, relationship between peripheral hearing and multiple domains of cognition in older adults. Future research should address potential mechanisms underlying this relationship.
Table 3.
Descriptions of Outcome Measures
| Measure Name | Cognitive Domain | Data |
|---|---|---|
| Digit Symbol Substitution (Tun et al. 1997; Wechsler 1981) |
Speed of processing | Number correct |
| Digit Symbol Copy (Wechsler 1981) | Speed of processing | Total time to complete task in s |
| Trail Making Test Part A (Reitan and Wolfson 1985, 1993) |
Speed of processing | Total time to complete task in s |
| Letter and Pattern Comparison (Salthouse and Babcock 1991) | Speed of processing | Sum of correctly identified pairs |
| Useful Field of View® (UFOV; Edwards, Vance, et al. 2005) | Speed of processing | Threshold display speed in ms across four subtests |
| Trail Making Test Part B (Reitan and Wolfson 1985, 1993) |
Executive function; inhibition; set- shifting |
Total time to complete task in s (480 s max.) |
| Stroop Color Word Test (Trenerry et al. 1989) | Executive function; inhibition |
Completion reaction time difference in s between ink color naming and color block naming tasks, adjusted for number of uncorrected mistakes during color naming task |
| Digit Span (Wechsler 1987) | Memory (verbal memory span) |
Number of series correctly repeated |
| Spatial Span (Wechsler 1987) | Memory (visual- spatial memory span) |
Number of series correctly replicated |
| Hopkins Verbal Learning Test (HVLT; Brandt 1991) | Memory (verbal episodic) |
Total number of words recalled across three free recall trials |
| Mini Mental State Exam (MMSE; Cockrell et al. 1987; Folstein et al. 1975) |
Global screening measure of mental status |
Total points out of 30 possible. Items screen orientation, registration, recall, calculation, attention, naming, repetition, comprehension, reading, writing, and drawing abstraction |
Acknowledgments
Dr. Lin’s work was supported in by part by NIH K23DC011279, the Eleanor Schwartz Charitable Foundation, and a Triological Society/American College of Surgeons Clinician Scientist Award.
The SKILL study was supported in part by the National Institutes of Health/National Institute on Aging grant 5 R37 AG05739-16, Improvement of Visual Processing in Older Adults, Karlene K. Ball, principal investigator. Jerri D. Edwards was supported as a Co-Investigator of this study. The authors wish to acknowledge Dr. Karlene K. Ball, who was awarded the grant to conduct the SKILL study, and the other investigators of SKILL, Drs. Daniel Roenker, Lesley Ross, David Roth, Virginia Wadley, and David Vance. We also thank the staff and students of the University of Alabama at Birmingham Center for Translational Research on Aging and Mobility.
Footnotes
The time penalty resulted in longer reaction times.
Financial Disclosures/Conflicts of Interest: Disclosures: Dr. Lin reports being on the scientific advisory board of Pfizer and Autifony Therapeutics, a consultant to Cochlear Ltd, and a speaker for Med El and Amplifon.
REFERENCES
- Agrawal Y, Platz EA, Niparko JK. Prevalence of hearing loss and differences by demographic characteristics among US adults - Data from the National Health and Nutrition Examination Survey, 1999-2004. Archives of Internal Medicine. 2008;168:1522–1530. doi: 10.1001/archinte.168.14.1522. [DOI] [PubMed] [Google Scholar]
- Akeroyd MA. Are individual differences in speech reception related to individual differences in cognitive ability? A survey of twenty experimental studies with normal and hearing-impaired adults. International Journal of Audiology. 2008;47:S53–S71. doi: 10.1080/14992020802301142. [DOI] [PubMed] [Google Scholar]
- American Speech-Language-Hearing Association Comittee on Audiometric Evaluation Guidelines for manual pure-tone threshold audiometry. ASHA. 1978;20:297–301. [PubMed] [Google Scholar]
- Anstey KJ, Luszcz MA, Sanchez L. Two-year decline in vision but not hearing is associated with memory decline in very old adults in a population-based sample. Gerontology. 2001;47:289–293. doi: 10.1159/000052814. [DOI] [PubMed] [Google Scholar]
- Anstey KJ, von Sanden C, Salim A, et al. Smoking as a risk factor for dementia and cognitive decline: A meta-analysis of prospective studies. American Journal of Epidemiology. 2007;166:367–378. doi: 10.1093/aje/kwm116. [DOI] [PubMed] [Google Scholar]
- Arlinger S. Negative consequences of uncorrected hearing loss-a review. International Journal of Audiology. 2003;42:S17–S20. [PubMed] [Google Scholar]
- Arlinger S, Lunner T, Lyxell B, et al. The emergence of cognitive hearing science. Scandinavian Journal of Psychology. 2009;50:371–384. doi: 10.1111/j.1467-9450.2009.00753.x. [DOI] [PubMed] [Google Scholar]
- Baltes P, Lindenberger U. Emergence of a powerful connection between sensory and cognitive functions across the adult life span: a new window to the study of cognitive aging? Psychology and Aging. 1997;12:12–21. doi: 10.1037//0882-7974.12.1.12. [DOI] [PubMed] [Google Scholar]
- Brandt J. The Hopkins Verbal Learning Test: Development of a new memory test with six equivalent forms. The Clinical Neuropsychologist. 1991;5:125–142. [Google Scholar]
- Chien W, Lin F. Prevalence of hearing aid use among older adults in the United States. Archives of Internal Medicine. 2012;172:292–293. doi: 10.1001/archinternmed.2011.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clay OJ, Edwards JD, Ross LA, et al. Visual function and cognitive speed of processing mediate age-related decline in memory span and fluid intelligence. Journal of Aging and Health. 2009;21:547–566. doi: 10.1177/0898264309333326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleveland WS, Devlin SJ. Locally weighted regression: an approach to regression analysis by local fitting. Journal of the American Statistical Association. 1988;83:596–610. [Google Scholar]
- Cockrell JR, Folstein MF. Mini-Mental State Examination (MMSE) Psychopharmacology Bulletin. 1987;24:689–692. [PubMed] [Google Scholar]
- Collins JG. Vital and Health Statistics. National Center for Health Statistics; Hyattsville, MD: 1997. Prevalence of selected chronic conditions: United States 1990–1992. [PubMed] [Google Scholar]
- Corner L, Bond J. Being at risk of dementia: Fears and anxieties of older adults. Journal of Aging Studies. 2004;18:143–155. [Google Scholar]
- Crews JE, Campbell VA. Vision impairment and hearing loss among community-dwelling Americans: Implications for health and functioning. American Journal of Public Health. 2004;94:823–829. doi: 10.2105/ajph.94.5.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruickshanks K, Wiley T, Tweed T, et al. Prevalence of hearing loss in older adults in Beaver Dam, Wisconsin. The Epidemiology of Hearing Loss Study. American Journal of Epidemiology. 1998;148:879–886. doi: 10.1093/oxfordjournals.aje.a009713. [DOI] [PubMed] [Google Scholar]
- Edwards JD, Vance DE, Wadley VG, et al. The reliability and validity of the Useful Field of View Test as administered by personal computer. Journal of Clinical and Experimental Neuropsychology. 2005;27:529–543. doi: 10.1080/13803390490515432. [DOI] [PubMed] [Google Scholar]
- Edwards JD, Wadley VG, Vance DE, et al. The impact of speed of processing training on cognitive and everyday performance. Aging & Mental Health. 2005;9:262–271. doi: 10.1080/13607860412331336788. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”: A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Gallacher J. Hearing, cognitive impairment and aging: A critical review. Reviews in Clinical Gerontology. 2004;14:199–209. [Google Scholar]
- Gallacher J, Ilubaera V, Ben-Shlomo Y, et al. Auditory threshold, phonologic demand, and incident dementia. Neurology. 2012;79:1583–1590. doi: 10.1212/WNL.0b013e31826e263d. [DOI] [PubMed] [Google Scholar]
- Gates G, Beiser A, Rees T, et al. Central auditory dysfunction may precede the onset of clinical dementia in people with probable Alzheimer’s disease. Journal of the American Geriatrics Society. 2002;50:482–488. doi: 10.1046/j.1532-5415.2002.50114.x. [DOI] [PubMed] [Google Scholar]
- Gates G, Cobb J, Linn R, et al. Central auditory dysfunction, cognitive dysfunction, and dementia in older people. Archives of Otolaryngology -- Head and Neck Surgery. 1996;122:161–167. doi: 10.1001/archotol.1996.01890140047010. [DOI] [PubMed] [Google Scholar]
- Gates G, Karzon R, Garcia P, et al. Auditory dysfunction in aging and senile dementia of the Alzheimer’s type. Archives of Neurology. 1995;52:626–634. doi: 10.1001/archneur.1995.00540300108020. [DOI] [PubMed] [Google Scholar]
- Gates G, Mills JH. Presbycusis. The Lancet. 2005;366:1111–1120. doi: 10.1016/S0140-6736(05)67423-5. [DOI] [PubMed] [Google Scholar]
- Gopinath B, Hickson L, Schneider J, et al. Hearing-impaired adults are at increased risk of experiencing emotional distress and social engagement restrictions five years later. Age and Ageing. 2012;41:618–623. doi: 10.1093/ageing/afs058. [DOI] [PubMed] [Google Scholar]
- Gopinath B, Schneider J, McMahon CM, et al. Severity of age-related hearing loss is associated with impaired activities of daily living. Age and Ageing. 2012;41:195–200. doi: 10.1093/ageing/afr155. [DOI] [PubMed] [Google Scholar]
- Gure TR, Langa KM, Fisher GG, et al. Functional limitations in older adults who have cognitive impairment without dementia. Journal of Geriatric Psychiatry and Neurology. 2013;26:78–85. doi: 10.1177/0891988713481264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heine C, Browning CJ. Communication and psychosocial consequences of sensory loss in older adults: overview and rehabilitation directions. Disability & Rehabilitation. 2002;24:763–773. doi: 10.1080/09638280210129162. [DOI] [PubMed] [Google Scholar]
- Helzner EP, Cauley JA, Pratt SR. Race and sex differences in age-related hearing loss: The Health, Aging and Body Composition Study. Journal of the American Geriatrics Society. 2005;53:2119–2127. doi: 10.1111/j.1532-5415.2005.00525.x. [DOI] [PubMed] [Google Scholar]
- Herbst KG, Humphrey C. Hearing impairment and mental state in the elderly living at home. British Medical Journal. 1980;281:903–905. doi: 10.1136/bmj.281.6245.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Idrizbegovic E, Hederstierna C, Dahlquist M, et al. Central auditory function in early Alzheimer’s disease and in mild cognitive impairment. Age and Ageing. 2011;40:249–254. doi: 10.1093/ageing/afq168. [DOI] [PubMed] [Google Scholar]
- Jobe JB, Smith DM, Ball KK, et al. ACTIVE: A cognitive intervention trial to promote independence in older adults. Controlled Clinical Trials. 2001;22:453–479. doi: 10.1016/s0197-2456(01)00139-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgensen LE. Doctoral dissertation. University of Pittsburgh; 2012. The potential impact of undiagnosed hearing loss on the diagnosis of dementia. [Google Scholar]
- Jupiter T. Cognition and screening for hearing loss in nursing home residents. Journal of the American Medical Directors Association. 2012;13:744–747. doi: 10.1016/j.jamda.2012.07.010. [DOI] [PubMed] [Google Scholar]
- Kiely K, Gopinath B, Mitchell P, et al. Cognitive, health, and sociodemographic predictors of longitudinal decline in hearing acuity among older adults. Journals of Gerontology. Series A, Biological Sciences and Medical Sciences. 2012;67:997–1003. doi: 10.1093/gerona/gls066. [DOI] [PubMed] [Google Scholar]
- Kohout FJ, Berkman LF, Evans DA, et al. Two shorter forms of the CES-D (Center for Epidemiological Studies Depression) depression symptoms index. Journal of Aging and Health. 1993;5:179–193. doi: 10.1177/089826439300500202. [DOI] [PubMed] [Google Scholar]
- Kramer SE, Kapteyn TS, Kuik DJ, et al. The association of hearing impairment and chronic diseases with psychosocial health status in older age. Journal of Aging and Health. 2002;14:122–137. doi: 10.1177/089826430201400107. [DOI] [PubMed] [Google Scholar]
- Lezak MD, Howieson DB, Bigler ED, et al. Neuropsychological Assessment. 5th ed Oxford University Press; New York: 2012. [Google Scholar]
- Li KZ, Lindenberger U. Relations between aging sensory/sensorimotor and cognitive functions. Neuroscience & Biobehavioral Reviews. 2002;26:777–783. doi: 10.1016/s0149-7634(02)00073-8. [DOI] [PubMed] [Google Scholar]
- Li KZH, Lindenberger U, Freund AM, et al. Walking while memorizing: age-related differences in compensatory behavior. Psychological Science. 2001;12:230–237. doi: 10.1111/1467-9280.00341. [DOI] [PubMed] [Google Scholar]
- Lin F. Hearing loss and cognition among older adults in the United States. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 2011;66:1131–1136. doi: 10.1093/gerona/glr115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin F, Ferrucci L, Metter EJ, et al. Hearing loss and cognition in the Baltimore Longitudinal Study of Aging. Neuropsychology. 2011;25:763–770. doi: 10.1037/a0024238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin F, Metter E, O’Brien R, et al. Hearing loss and incident dementia. Archives of Neurology. 2011;68:214–220. doi: 10.1001/archneurol.2010.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin F, Thorpe R, Gordon-Salant S, et al. Hearing loss prevalence and risk factors among older adults in the United States. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 2011;66A:582–590. doi: 10.1093/gerona/glr002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin F, Yaffe K, Xia J, et al. Hearing loss and cognitive decline in older adults. JAMA Internal Medicine. 2013;173:293–299. doi: 10.1001/jamainternmed.2013.1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin MY, Gutierrez PR, Stone KL, et al. Vision impairment and combined vision and hearing impairment predict cognitive and functional decline in older women. Journal of the American Geriatrics Society. 2004;52:1996–2002. doi: 10.1111/j.1532-5415.2004.52554.x. [DOI] [PubMed] [Google Scholar]
- Lindenberger U, Baltes PB. Sensory functioning and intelligence in old age: A strong connection. Psychology and Aging. 1994;9:339–355. doi: 10.1037//0882-7974.9.3.339. [DOI] [PubMed] [Google Scholar]
- Lopez OL, Jagust WJ, DeKosky ST, et al. Prevalence and classification of mild cognitive impairment in the Cardiovascular Health Study Cognition Study: part 1. Archives of Neurology. 2003;60:1385–1389. doi: 10.1001/archneur.60.10.1385. [DOI] [PubMed] [Google Scholar]
- Marquis S, Moore M, Howieson DB, et al. Independent predictors of cognitive decline in healthy elderly persons. Archives of Neurology. 2002;59:601–606. doi: 10.1001/archneur.59.4.601. [DOI] [PubMed] [Google Scholar]
- Mick P, Kawachi I, Lin F. The association between hearing loss and social isolation in older adults. Otolaryngology - Head and Neck Surgery. 2014;150:378–384. doi: 10.1177/0194599813518021. [DOI] [PubMed] [Google Scholar]
- Mohr PE, Feldman JJ, Dunbar JL, et al. The societal costs of severe to profound hearing loss in the United States. International journal of technology assessment in health care. 2000;16:1120–1135. doi: 10.1017/s0266462300103162. [DOI] [PubMed] [Google Scholar]
- Morris JC, Storandt M, Miller P. Mild cognitive impairment represents early-stage Alzheimer’s disease. Archives of Neurology. 2001;58:397–405. doi: 10.1001/archneur.58.3.397. [DOI] [PubMed] [Google Scholar]
- National Institute on Deafness and Other Communication Disorders Quick statistics. 2010 from http://www.nidcd.nih.gov/health/statistics/Pages/quick.aspx.
- Oster C. Sensory deprivation in geriatric patients. Journal of the American Geriatrics Society. 1976;24:461–464. doi: 10.1111/j.1532-5415.1976.tb03261.x. [DOI] [PubMed] [Google Scholar]
- Pearman A, Friedman L, Brooks J, et al. Hearing impairment and serial word recall in older adults. Experimental Aging Research. 2000;26:383–391. doi: 10.1080/036107300750015769. [DOI] [PubMed] [Google Scholar]
- Petersen RC, Smith GE, Waring SC, et al. Mild cognitive impairment: Clinical characterization and outcome. Archives of Neurology. 1999;56:303–308. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- Pichora-Fuller MK, Schneider BA, Daneman M. How young and old adults listen to and remember speech in noise. Journal of the Acoustical Society of America. 1995;97:593–608. doi: 10.1121/1.412282. [DOI] [PubMed] [Google Scholar]
- Rabinowitz PM. Noise-induced hearing loss. American Family Physician. 2000;61:2759–2760. [PubMed] [Google Scholar]
- Radloff L. The CES-D scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- Reitan RM, Wolfson D. The Halstead-Reitan Neuropsychological Test Battery. Neuropsychological Press; Tucson, AZ: 1985. [Google Scholar]
- Reitan RM, Wolfson D. The Halstead-Reitan Neuropsychological Test Battery: Theory and Clinical Interpretation. Neuropsychology Press; South Tucson, AZ: 1993. [Google Scholar]
- Salthouse TA, Babcock RL. Decomposing adult age differences in working memory. Developmental Psychology. 1991;27:763–776. [Google Scholar]
- Schneider BA, Pichora-Fuller MK. Implications of perceptual deterioration for cognitive aging research. In: Craik FIM, Salthouse TA, editors. The Handbook of Aging and Cognition. Lawrence Erlbaum Associates, Inc.; Mahwah, NJ: 2000. pp. 155–220. [Google Scholar]
- Smith GE, Petersen RC, Parisi JE, et al. Definition, course, and outcome of mild cognitive impairment. Aging, Neuropsychology, and Cognition. 1996;3:141–147. [Google Scholar]
- Strouse A, Hall J, Burger C. Central auditory processing in Alzheimer’s Disease. Ear and Hearing. 1995;16:230–238. doi: 10.1097/00003446-199504000-00010. [DOI] [PubMed] [Google Scholar]
- Tilvis RS, Kahonen-Vare MH, Jolkkonen J, et al. Predictors of cognitive decline and mortality of aged people over a 10 year period. Journals of Gerontology. Series A, Biological Sciences and Medical Sciences. 2004;59A:268–274. doi: 10.1093/gerona/59.3.m268. [DOI] [PubMed] [Google Scholar]
- Trenerry MR, Crosson B, DeBoe J, et al. Stroop Neurological Screening Test. Psychological Assessment Resources; Lutz: 1989. [Google Scholar]
- Tun PA, McCoy S, Wingfield A. Aging, hearing acuity, and the attentional costsof effortful listening. Psychology and Aging. 2009;24:761–766. doi: 10.1037/a0014802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tun PA, Wingfield A, Lindfield KC. Motor-speed baseline for the Digit-Symbol Substitution Test. Clin Gerontol. 1997;18:47–51. [Google Scholar]
- Uhlmann RF, Larson EB, Rees TS, et al. Relationship of hearing impairment to dementia and cognitive dysfunction in older adults. JAMA. 1989;261:1916–1919. [PubMed] [Google Scholar]
- Valentijn SAM, van Boxtel MPJ, van Hooren SAH, et al. Change in sensory functioning predicts change in cognitive functioning: Results from a 6-year follow-up in the Maastricht aging study. Journal of the American Geriatrics Society. 2005;53:374–380. doi: 10.1111/j.1532-5415.2005.53152.x. [DOI] [PubMed] [Google Scholar]
- van Boxtel MPJ, van Beijsterveldt CEM, Houx PJ, et al. Mild hearing impairment can reduce verbal memory performance in a healthy adult population. Journal of Clinical and Experimental Neuropsychology. 2000;22:147–154. doi: 10.1076/1380-3395(200002)22:1;1-8;FT147. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale-Revised. Psychological Corporation; San Antonio, TX: 1981. [Google Scholar]
- Wechsler D. Wechsler Memory Scale-Revised Manual. The Psychological Corporation; San Antonio: 1987. [Google Scholar]
- Weinstein B, Ventry I. Hearing impairment and social isolation in the elderly. Journal of Speech and Hearing Research. 1982;25:593–599. doi: 10.1044/jshr.2504.593. [DOI] [PubMed] [Google Scholar]
- Wilson RS, Hebert LE, Scherr PA, et al. Educational attainment and cognitive decline in old age. Neurology. 2009;72:460–465. doi: 10.1212/01.wnl.0000341782.71418.6c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood KM, Edwards JD, Clay OJ, et al. Sensory and cognitive factors influencing functional ability in older adults. Gerontology. 2005;51:131–141. doi: 10.1159/000082199. [DOI] [PubMed] [Google Scholar]
- World Health Organization Prevention of Blindness and Deafness Program [Retrieved July 16, 2014];Prevention of deafness and hearing impaired grades of hearing impairment. 2011 2014 from http://www.who.int/pbd/deafness/hearing_impairment_grades/en/
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.