Abstract
Introduction
Loss of mitochondrial competency is associated with several chronic illnesses. Therefore, strategies that maintain or increase mitochondrial function will likely be of benefit in a number of clinical settings. Endurance exercise has long been known to increase mitochondrial function in skeletal muscle. Comparatively little is known regarding the impact of resistance exercise training on skeletal muscle mitochondrial respiratory function.
Purpose
The purpose of the current study was to determine the impact of chronic resistance training on skeletal muscle mitochondrial respiratory capacity and function.
Methods
Here, we studied the impact of a 12-week resistance exercise training program on skeletal muscle mitochondrial function in eleven young healthy men. Muscle biopsies were collected before and after the 12-week training program and mitochondrial respiratory capacity determined in permeabilized myofibers by high-resolution respirometry.
Results
Resistance exercise training increased lean body mass and quadriceps muscle strength by 4 and 15%, respectively (P<0.001). Coupled mitochondria respiration supported by complex I, and complex I and II substrates, increased by 2- and 1.4-fold, respectively (P<0.01). The ratio of coupled complex I supported respiration to maximal respiration increased with resistance exercise training (P<0.05), as did complex I protein abundance (P<0.05), while the substrate control ratio for succinate was reduced after resistance exercise training (P<0.001). Transcripts responsible for proteins critical to electron transfer and NAD+ production increased with training (P<0.05), while transcripts involved in mitochondrial biogenesis were unaltered.
Conclusion
Collectively, 12-weeks of resistance exercise training resulted in qualitative and quantitative changes in skeletal muscle mitochondrial respiration. This adaptation occurs with modest changes in mitochondrial proteins and transcript expression. Resistance exercise training appears to be a means to augment the respiratory capacity and intrinsic function of skeletal muscle mitochondria.
Keywords: Resistance training, skeletal muscle, mitochondria, bioenergetic
Introduction
Mitochondria are the cellular organelles responsible for aerobic ATP production; thus mitochondrial “health” is of importance to overall cellular function. The abundance and functional characteristics of mitochondrion within an organ influences its physiological function (17, 23, 24, 27). For example, aging is synonymous with reduced skeletal muscle mitochondrial density and function (29), which is associated with reduced exercise capacity. Conversely, the number and functionality of skeletal muscle mitochondria positively correlate with exercise capacity in healthy humans (15, 25, 38). Further, aerobic exercise is known to be a potent regulator of skeletal muscle oxidative capacity (12, 36), where mitochondrial density and function are elevated in both animals and humans acclimated to endurance exercise (13, 15, 25).
Resistance exercise has emerged as an efficacious intervention which augments muscle mass and function, particularly in populations with diminished lean body mass. However, the impact of resistance exercise on skeletal muscle bioenergetics remains poorly understood. Early reports suggested that skeletal muscle mitochondrial volume (22) and oxidative capacity (5) were reduced by chronic resistance exercise training. More recently, others have shown that resistance exercise increases the activity of oxidative enzymes in tissue homogenates (33) and respiration in skinned muscle fibers (25). However, many other studies have also shown that mitochondrial density (21) and the activity of oxidative enzymes thought to reflect mitochondrial abundance and/or function, such as citrate synthase, β-hydroxyl-acyl-CoA dehydrogenase and succinate dehydrogenase, are largely unaltered by chronic resistance exercise training (1, 10, 11, 26, 34, 35, 37). The discordance in these results are likely attributable to numerous factors, such as the training regime used, participants pre-existing training status, the timing of biopsy collection after exercise (19), and the numerous analytical techniques used to quantify the abundance and activity of mitochondrial enzymes (18).
The majority of studies to date concerning the impact of resistance exercise training on skeletal muscle bioenergetics have determined surrogate measures of oxidative capacity (5, 10, 11, 22, 26, 34, 35, 37), which are more likely to reflect the abundance of mitochondrial proteins rather than mitochondrial function. Surprisingly, only a small number of studies have attempted to determine the impact of resistance exercise training on skeletal muscle mitochondrial function. Salvadego and colleagues measured skeletal muscle mitochondrial respiration in a cross-section of resistance trained athletes and untrained controls (28). These researchersfound that coupled (ATP producing) mitochondrial respiration per mg of muscle was greater in resistance trained athletes, as was the respiratory control ratio for ADP (28), suggesting that mitochondrial ATP production is more efficient in resistance trained individuals.
Recently, Pesta and colleagues showed that endurance training and strength training performed under hypoxia, resulted in similar qualitative and quantitative changes in skeletal muscle mitochondrial respiration (25). Unfortunately, while Pesta and co-workers attempted to study the impact of strength training under normoxic conditions, only 3 participants completed the intervention (25), meaning statistical power was not achieved. As such, a significant knowledge-gap remains concerning the impact of resistance exercise training on skeletal muscle respiratory capacity and function. To address this, we set out to determine the impact of chronic resistance exercise on skeletal muscle mitochondrial respiratory function. To do this, skeletal muscle biopsies were collected from young healthy untrained men before and after a 12-week resistance exercise training program and mitochondria function measurements were performed on fresh muscle biopsy samples. We hypothesized that resistance exercise training would augment mitochondrial respiratory capacity within skeletal muscle.
Methodology
Human subjects and Screening
Eleven young healthy volunteers were recruited for the current study. Participants were young recreationally active males and free from any physical impairment that would prevent participation in a resistanceexercise training program. Participants, while physically active, were not engaged in any structured resistance or endurance training programs at the time or 6 months prior to participation. As such, all participants can be considered as healthy but untrained. The participants were recruited through locally posted flyers, newspaper advertisements, and by word of mouth. Screening of participants was performed in the morning after an overnight fast at the Institute for Translational Sciences-Clinical Research Center (ITS-CRC) at the University of Texas Medical Branch. The screening day included familiarization to strength testing on a Dynamometer, a clinical history and physical exam, and laboratory tests (complete blood count with differential, liver and kidney function tests, coagulation profile, fasting blood glucose, hepatitis B and C screening, HIV test, TSH, lipid profile, urinalysis, and drug screening). All participants provided written informed consent before enrollment in the study. The study was approved by the Institutional Review Board of the University of Texas Medical Branch, and was performed in compliance with the Declaration of Helsinki as revised in 1983.
Experimental Design
Following enrollment, participants completed a 10-14 day pre-training, run-in period that consisted of the pre-training study day at the ITS-CRC and then 3 non-consecutive days of exercise familiarization and baseline 1-repetition max (1-RM) strength testing at the University of Texas Medical Branch Alumni Fieldhouse. The pre-training study day included assessment of body composition, muscle biopsy collection and isokinetic and isometric strength testing. 2-3 days later the participants reported to the University of Texas Medical Branch Alumni Field House for familiarization/testing before beginning 12-weeks of training. After 12-weeks of training, participants were re-tested exactly 3 days following the final exercise session of the training program (see below). Participants reported to the ITS-CRC at the same time in the morning as the pre-training study dayto repeat the same laboratory tests and sample collection.
Pre and Post-Testing Study Day
Participants reported to the ITS-CRC at the University of Texas Medical Branch in the morning following an overnight fast. After voiding, participants lay supine for 30 min prior to assessment of body composition by DEXA scan (dual-energy X-ray absorptiometry) (Hologic ADR 4500W, Bedford, MA).
To maintain a supine position, participants were transported to and from the CRC bed in a stretcher. After the DEXA scan, Ultrasound (Phillips HDI 5000) of the vastus lateralis (VL) was conducted while the participant lay in bed. Ultrasound assessment of VL muscle thickness was assessed as others have previously described (32) with some minor modifications. Briefly, several B-Mode real time images of the VL were taken in the mid-sagittal position at 50 and 75% of the femur length (from the ASIS). The ultrasound head position, pre and post-training, was placed relative to specific measured landmarks. The image that offered the sharpest contrast with the femur was chosen to ensure perpendicular placement of the scan head. VL muscle thickness was assessed as the average distance from the superficial aponeurosis to the deep aponeurosis at these two locations. Preliminary testing, on the same individuals, revealed that the coefficient for variation for measurements taken the day of or several weeks apartwas 1.42±0.20 and 1.84±0.40%, respectively.
A percutaneous biopsy sample of the VL muscle was collected from a randomly selected leg under local lidocaine anesthesia using a suction-adapted 6mm Bergstrom Needle (3). One portion of muscle (~20-30mg) was immediately submerged in an ice-cold, pH adjusted (7.1) relaxation solution (BIOPS buffer) containing 10mM CaK2-EGTA; 7.23mM K2-EGTA; 20mM imidazole; 20mM taurine 50mM K-MES; 0.5mM dithithreitol; 6.56 MgCl2; 5.77mM ATP and 15mM creatine phosphate. The remainder of the sample was snap-frozen in liquid nitrogen and stored at −80°C for future enzyme activity, protein content analysis, lysate separation and RNA isolation.
Peak torque of the quadriceps and biceps femoris muscles of the non-biopsied leg were subsequently determined by dynamometry (Biodex, Shirley, NY). Participants were previously familiarized to the test at the screening. Briefly, subjects were restrained in the dynamometer, with the anatomical access of the knee joint of their dominant leg aligned with the mechanical axis of the dynamometer. After demonstration of proper technique and an explanation of the strength test protocol, subjects performed practice contractions to re-familiarize themselves with the dynamometer and to warm-up. Thereafter, isometric peak torque (extension and flexion) was determined at a 60° angle. Then, isometric peak torque (extension and flexion) was determined at an angular velocity of 120°/sec.
Following the strength test, participants were fed a meal before leaving the unit.All testing was repeated on the post-testing day in the same order.
Resistance exercise training
Following familiarization and 1-RM strength testing, participants began a 12-week whole-body, progressive resistance exercise training (RET) program. All exercise-training sessions were performed on the same equipment at the Alumni Field house at the University of Texas Medical Branch. Exercise sessions were performed on non-consecutive days, 3 times weekly, with 4 rest days per week under supervision of highly qualified personal trainers.RET was performed at an intensity of 60-80% of 1-RM and consisted of 3-4 sets of 8-10 repetitions performed to failure for each exercise. In weeks 1-8, 2 sessions per week were performed at an intensity of 70% 1-RM, where 3 sets of 10 repetitions were performed to momentary muscular failure. Each session consisted of whole body resistance exercise that lasted ~60-70 min. The remaining session was performed at an intensity of 60% 1-RM with the goal of not reaching momentary muscular failure on this day. In weeks 9-12, 2 sessions per week were performed an intensity of 80% 1-RM, where 4 sets of 8 repetitions were performed to momentary muscular failure. The 3rd session was performed at an intensity of 60% 1-RM as before. Each session consisted of whole body resistance exercise that lasted ~70-90 min. Resistance exercises included flat and incline chest press; leg press, curl and extension; seated pull-downs and rows; calf raises; and abdominal exercises. Participants rested for 1-2 minutes between exercises and individuals sets. Strength was re-tested at 3, 6 and 9 weeks so as participants strength increased, absolute training loads could be adjusted to maintain a relative training intensity between 60-80% 1-RM. 1-RM strength tested was performed again at the completion of the training program as the final exercise session. To allow for unforeseen life events, participants were given 13 weeks following the familiarization period to complete 36 exercise sessions. This allowed for 100% attendance. Throughout the RET intervention, subjects were asked to maintain their normal physical activity and dietary habits.
Preparation of permeabilized muscle fibers
Following collection from participants, muscle samples preserved in BIOPS buffer were immediately transferred to the laboratory to be prepared for high-resolution respirometry (HRR). Briefly, is a six well plate, myofiber bundles were manually separated using sharp forceps in ice-cold BIOPS buffer where all visible connective tissue was removed. Chemical permeabilization of the sacrolemmal membrane was achieved by transferring myofiber bundles to 2mls of pH adjusted (7.1) MIR05 buffer (0.5mM EGTA; 3mM MgCl2; 60mM K-lactobionate; 20mM taurine; 10mM KH2PO4; 20mM HEPES; 110mM sucrose: and 1mg/ml essential fatty acid free bovine serum albumin) containing 50μg/ml saponin. Samples were agitated in MIR05 buffer containing saponinfor 30 mins at 4°C. Myofiber bundles were then transferred to 2mls of MIR05 buffer alone and agitated for 15 mins to ensure that any residual saponin was rinsed away. Approximately 1-3 mgs of permeabilizedmyofiber bundles were then weighed on a precision microbalance (Mettler-Toledo, Zaventem, Belgium) and immediately transferred to an Oxygraph-2k (O2K) respirometer (Oroboros Instruments, Innsbruck, Austria).
High-resolution respirometry
Prior to each experiment, the polygraphic oxygen sensors of the O2K were calibrated in MIR05 buffer at air saturation. HRR measurements were performed in 2mls of MIR05 buffer. Temperature was maintained at 37±0.01°C throughout each experiment by an electronic Peltier. Oxygen concentration within the MIR05 was determined at 2 sec intervals and used to compute oxygen flux per mg of tissue by DatLab software (Oroboros Instruments, Innsbruck, Austria). Once myofiber bundles had been introduced into the O2K chamber, an air phase was established by slightly opening the chamber. ~2-3mls of 99% Oxygen was injected into each camber in order to increase the oxygen concentration of the MIR05 buffer. Typically, oxygen concentration was increased to ~450μM prior to each experiment. All experiments were performed with in a range of 200-450μM of oxygen to ensure that oxygen diffusion would not be limiting to respiration.
Mitochondrial respiratory function was determined by the sequential addition of substrates and uncouplers. State 2 leak respiration supported primarily by electron flow through complex I of the respiratory chain (LI) was achieved by the titration of 1.5mM octanoyl-l-carnitine, 5mM pyruvate, 2mM malate and 10mM glutamate into the O2K chamber. Electron transfer was then coupled to phosphorylation by the addition of 5mM ADP, accessing coupled state 3 respiration with electron transfer supported by Complex I (PI). 10mM succinate was added to the O2K chamber to induce maximal state 3 (oxphos) respiration with parallel electron input from Complex I and II (PI+II). 10μM cytochrome C was added to access the competence of the outer mitochondrial membrane. The absence of a significant increase in respiratory flux following the addition of cytochrome C indicates that the outer mitochondrial membranes are intact. Finally, oxidative phosphorylation was uncoupled by the titration of carbonyl cyanide m-chlorophenylhydrazone (CCCP) to a final concentration of 5μM to access maximal electron transfer capacity (E).
Citrate Synthase Activity
Approximately 20mg of wet muscle tissue was lyophilized for 48hr. Freeze-dried muscle tissue was dissected free of visible blood and connective tissue. 1-3 mg of freeze dried tissue was homogenized in a glass tissue grinder in an ice-cold 175mM KCl buffer containing 2mM EDTA and 1% Triton. Muscle homogenates were then centrifuged at 2000rpm at 4°C. Supernatants were stored at −80C until analysis for citrate synthase (CS) activity and protein content.
CS activity was determined spectrophotometrically by a modified version of the method originally devised by Srere (30). Briefly, muscle lysates were diluted 1 in 10 in a 100mM TRIS-HCl buffer (pH 8.1) containing 300 μM acetyl-CoA and 100 μM of (5,5'-dithiobis-(2-nitrobenzoic acid) (DTNB). Thereafter, oxaloacetate was added to each well at a final concentration of 500 μM to initiate the CS reaction. Light absorbance at 412nM was then recorded every 30 sec for a total of 10 mins at 37°C (BioTek Eon™, Winooski, VT). The change in light absorbance is proportional to the reaction of DTNB with free thiol groups (Coenzyme A production following the condensation of oxaloacetate and acetyl-CoA). CS activity (μmol/g/min) was calculated from the linear change in absorbance over time.
CS activity was normalized to the protein content of the muscle lysate. Lysate protein concentration was determined using a modified Bradford assay. Samples and bovine serum albumin standards were incubated at room temperature in protein quantification dye (BIO-RAD, Hercules, CA) for 30 mins. Light absorbance was read in a 96-well plate at 595nM (BioTek®Eon™, Winooski, VT) in a 200μl aliquot each sample mixed with protein quantification dye. Lysate protein concentrations were calculated from the extinction coefficient bovine the 1 mg/ml serum albumin standard.
Protein Expression
Homogenates were prepared from 40-50 mg of muscle tissue for Western blotting analysis as previously conducted (7) with a minor change. Mitochondrial respiratory complexes I-V protein abundance was quantified using a Mitosciences®Oxphos human antibody cocktail ab110411 (Abcam, Eugene, Oregon). The β-mercaptothenaol was omitted from the sample buffer to ensure non-reducing, denaturing conditions for SDS-PAGE, per the manufacture instructions. Immunoblot data were normalized the manufacturer's internal loading control, isolated human heart mitochondria, which was loaded on all gels for comparison across blots. Data were further normalized to Ponceau S to account for differences in loading.
RNA extraction and Semiquantitative real-time PCR
RNA isolation, cDNA synthesis, and real-time qPCR were performed as we have previously described(8). Total RNA was isolated by homogenizing 10-20 mg tissue with a hand-held homogenizing dispenser (T10 Basic Ultra Turrax, IKA, Wilmington, NC) in 1 ml of Tri reagent. The RNA was separated into an aqueous phase using 0.2 ml of chloroform and subsequently precipitated from the aqueous phase using 0.5 ml of isopropanol. RNA was washed twice with 1 ml of 75% ethanol, air-dried, and suspended in a known amount of nuclease-free water. RNA concentration was determined using a NanoDrop 2000 spectrophotometer (Thermo Fisher Scientific, Wilmington, DE) and RNA was DNase-treated using a commercially available kit (DNA-free, Ambion, Austin, TX). A total of 2 μg of RNA was reverse transcribed into cDNA according to the directions provided by the manufacturer (iScript, BioRad, Hercules, CA). Real-time qPCR was carried out using the PrimePCR system with a CFX Connect PCR cycler (BioRad). cDNA was analyzed with SYBR green fluorescence (iQ SYBR green supermix; BioRad). Validated gene targets and unique Bio-Rad assay ID”s are as follows (COX4I1, qHsaCID0006289; COX18, qHsaCID0017694; MFN1, qHsaCED0046771; NAMPT, qHsaCED0043104; NDRG2, qHsaCED0056853; PPARGC1A, qHsaCID0006418; TFAM, qHsaCED0037846). The geometric mean of 3 targets (ACTB, qHsaCED0036269; B2M, qHsaCID0015347; RPL13A, qHsaCED0020417) were selected as a normalization/housekeeping genes. B2M was stable pre to post training for all but one subject, however ACTB and RLP13A were stable for all subjects. Thus the geometric mean of 2 targets (ACTB& RPL13A) wereutilized as a normalization/housekeeping genes. The target stability values for these normalization genes were exceptional as demonstrated by a CV of 0.027 and M value of 0.078. Relative fold changes were determined from the Ct values using the 2−ΔΔCt method (20)calculated via Bio-Rad”s CFX Manager software.
Statistical analysis
Data are present as group means ± the standard error (S.E.) unless stated otherwise. Group means were analyzed for significance using a two-tailed Paired t-test using GraphPad Prism version 6 (GraphPad software, La Jolla, CA). Significance was accepted when P≤0.05.
Results
Subject characteristics
Eleven young (26±5 years) healthy men participated in the current study. Basic subject characteristics before and after RET are presented in Table 1. Total body mass increased by 1.9±2 kg following 12 weeks of RET (P<0.01). Body mass index (BMI) also increased significantly with RET (P<0.01). Subject gained a significant amount (2.5±1.4 kg) of fat free mass with RET (P<0.001), while absolute fat mass was not significantly different pre and post RET. Subsequently, there was a significant increase in the relative proportion of fat free mass (P<0.05), and a significant decrease in the relative proportion of fat mass (P<0.05) following RET. Muscle thickness data before and after RET is presented in Table 1. RET significantly increased muscle thickness of the VL by 12% (P<0.001). While habitual physical activity and dietary habits were not determined in the current study, it is likely that participants increased their dietary caloric and nitrogen intake, as indicated by the increase in body mass and fat free mass following RET.
Table 1.
Subject characteristic pre and post resistance exercise training. Values are presented as group means ± standard deviation.
| Subject Characteristics | ||||
|---|---|---|---|---|
| Pre RET | Post RET | Δ | P | |
| Age | 26±5 | 26±5 | ||
| Height (cm) | 180±9 | 180±9 | ||
| Weight (kg) | 86.4±14.4 | 88.2±13.5 | 1.87±2.0 | 0.010 |
| BMI (kg/m2) | 26.5±3.1 | 27.1±2.7 | 0.57±0.58 | 0.009 |
| Fat mass (kg) | 23.1±8.2 | 22.7±7.8 | 0.45±1.45 | 0.332 |
| Fat-free mass (kg) | 62.7±8.1 | 65.2±7.9 | 2.54±1.41 | 0.000 |
| BMC (kg)† | 3.3±0.5 | 3.3±0.4 | 0.01±0.03 | 0.459 |
| % Fat mass | 27.4±6.4 | 26.3±6.2 | 1.1±1.4 | 0.023 |
| % Fat-free mass | 72.6±6.4 | 73.7±6.2 | 1.1±1.4 | 0.023 |
| VL Thickness (cm)†† | 2.5±0.4 | 2.8±0.3 | 0.3±0.2 | 0.000 |
Bone mineral content.
Vastus lateralis muscle thickness.
Muscle strength
Muscle strength data before and after RET is presented in Table 2. RET significantly increased the isometric peak torque of both the quadriceps (P<0.001) and biceps femoris (P<0.001) muscle groups of the leg. Isokinetic peak torque of the quadriceps muscles also increased with RET (P<0.001). The increase in isokinetic peak torque of the biceps femoris muscle group following RET did not reach statistical significance.
Table 2.
Muscle strength before and after 12 weeks of resistance exercise training. Values are presented as group means ± standard error.
| Pre | Post | % Δ | P | ||
|---|---|---|---|---|---|
| Leg Isometric Peak Torque (N/m) | Extension | 282±18 | 324±19 | 15 | 0.0001 |
| Flexion | 153±10 | 170±11 | 11 | 0.0008 | |
| Leg Isokinetic Peak Torque (N/m) | Extension | 195±9 | 212±11 | 9 | 0.0008 |
| Flexion | 121±8 | 126±8 | 5 | 0.2104 |
Skeletal muscle mitochondrial respiration
Mitochondrial respiratory capacity per mg-wet weight of muscle tissue was increased by RET (Figure 1). State 2 leak respiration (LI) was increased 2-fold by RET (10.7±1.5 vs. 21.8±3.2 pmols/sec/mg; P<0.05). Similarly, coupled state 3 respiration with electron input from complex I (PI) was 2-fold increased after RET (30.2±2.8 vs. 58.9±3.5 pmols/sec/mg; P<0.001). Maximal coupled (oxphos) respiration with electron input from both complex I and II of the electron transport chain (PI+II) was 1.4-fold increased after RET (54.5±6.5 vs. 75.6±5.8 pmols/sec/mg; P<0.05). The addition of cytochrome C had little effect on respiratory flux, suggesting that the muscle biopsy procedure and the preparation of permeabilized myofiber bundles did no damage the outer mitochondrial membrane. Addition of 10μM cytochrome C increased respiration by 2.1±1.5% on average pre RET and 1.6±1.0% on average post RET. Maximal uncoupled mitochondrial respiration, a marker of mitochondrial respiratory capacity, was achieved by titration of the ionophore CCCP. RET significantly increased electron transfer system capacity (E) by 65% (64.0±5.1 vs. 104.4±9.8 pmols/sec/mg; P<0.001).
Figure 1. Skeletal muscle mitochondrial respiration.
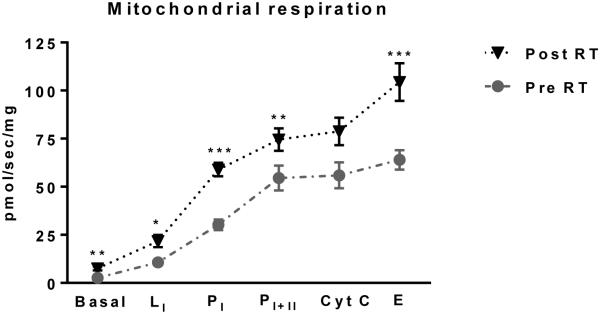
Skeletal muscle mitochondrial respiration in MIR05 buffer alone (basal), following the addition of pyruvate, octanoyl-l-carnitine, glutamate and malate (LI), ADP (PI), succinate (PI+II), cytochrome C (Cyt C), and CCCP (E), before (circles) and after (triangles) 12 weeks of resistance exercise training. L and P denote leak and phosphorylating respiratory states, respectively. I and II indicate electron transfer through complex I and complex II of the electron transport chain, respectively. The absence in a respiratory response to cytochrome C indicates that mitochondria within myofiber bundles were viable. *P<0.05, **P<0.01, ***P<0.001.
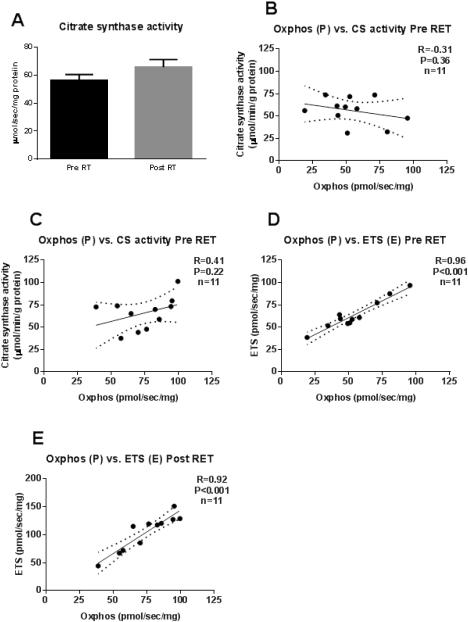
Skeletal muscle oxidative capacity
Skeletal muscle CS activity, a marker of oxidative capacity, is presented in Figure 2A. RET tended to increase CS activity in skeletal muscle (56.1±4.5 vs. 65.8±5.4 (μmol/g/min)/(mg protein)). However, this 17% increase was not statistically significant. CS activity was not correlated with maximal coupled mitochondrial respiration (P) before (Figure 2B) or after (Figure 2C) RET. Maximal respiratory capacity (E) correlated with maximal coupled respiration (P) both before (R=0.96, P<0.001; Figure 2D) and after (R=0.92, P<0.001; Figure 2E) RET.
Figure 2. Skeletal muscle respiratory capacity.
Skeletal muscle citrate synthase activity (Panel 2A) before (black bars) and after (grey bars) resistance exercise training. There was no correlation between citrate synthase (CS) and PI+II before (Panel 2B) or after (Panel 2C) RET. There was a significant correlation between PI+IIand E before (Panel 2D) and after (Panel 2E) RET.
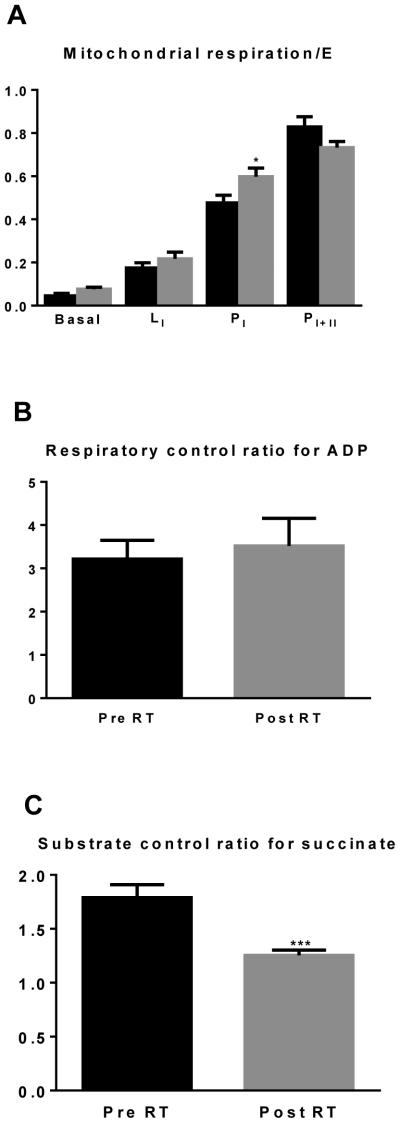
Skeletal muscle mitochondrial function
Skeletal muscle mitochondrial respiration values were normalized to maximal uncoupled respiration (E) in order to control for mitochondrial respiratory capacity (Figure 3A). Leak respiration with complex I substrates (LI) normalized to E was not altered by RET (0.17±0.02 vs. 0.22±0.03). Coupled respiration supported by complex I (PI) was significantly increased by RET when normalized to E (0.48±0.04 vs. 0.60±0.04, P<0.05). Coupled respiration supported by both complex I and II was not altered by RET when normalized to E (0.83±0.05 vs. 0.73±0.03).The respiratory control ratio (RCR) for ADP was not different after RET (3.2±0.4 vs. 3.5±0.6; Figure 3B). The substrate control ratio (SCR) for succinate was significantly reduced after RET (1.8±0.1 vs. 1.3±0.1; Figure 3C; P<0.001).
Figure 3. Specific mitochondrial respiration and coupling control.
Skeletal muscle mitochondrial respiration normalized to respiratory capacity (E) before and after RET (panel 3A). The respiratory control ratio for ADP calculated as PI/LI and the substrate control ratio for succinate calculated as PI+II/PI are presented in Panels 3B and 3C, respectively. *P<0.05, ***P<0.001.
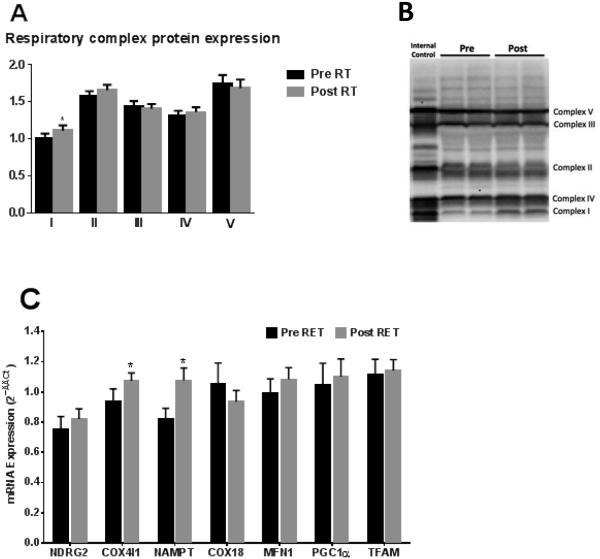
Electron transport chain protein expression
The protein abundances of complex I to IV of the electron transport chain and ATP synthase (complex V) are presented in Figure 4. Complex II, III and IV protein expression was not different following RET. Similarly, complex V protein expression was unaltered by RET. The protein expression of complex I of the electron transport chain (NADH oxidase), increased by 11% with RET (P=0.054).
Figure 4. Altered mitochondrial gene expression and protein abundance following RET.
Mitochondrial respiratory chain protein levels determined in cytoplasmic skeletal muscle lysates before (black bars) and after (grey bars) 12 weeks of RET. I, II, III, IV and V represent complex I (NADH oxidase), complex II (succinate dehydrogenase), complex III (cytochrome bcoxidoreductase), complex IV (cytochrome C oxidase) and complex V (ATP synthase) of the respiratory chain are shown in Panel 4A. Representative blot images of the human heart mitochondria isolate internal control and a pre and post sample (loaded in duplicate) are shown in Panel 4B. Changes in RNA levels of COX18, COX4I1, MFN1, NAMPT, NDRG2, PGC1α, and TFAM pre and post RET are presented in Panel4C. *P<0.05.
Select mitochondrial mRNA expression
The mRNA expression of COX18 (cytochrome C oxidase assembly factor), MFN1 (mitofusion 1), NDRG2 (N-myc downstream-regulated gene 2), PGC1α (peroxisome proliferator-activated receptor gamma co-activator), andTFAM (mitochondrial transfer factor A) were unaltered by RET, whereas the mRNA expression of COX4I1 (cytochrome C oxidase subunit 4) and NAMPT (nicotinamidephosphoribosyltransferase)were significantly increased following RET (P<0.05, Figure 4C).
Discussion
The number and intrinsic function of mitochondria within skeletal muscle influences substrate metabolism and exercise capacity. Skeletal muscle mitochondria exhibit marked plasticity in response to numerous physiological stimuli and pathophysiological states (4, 17, 23, 24, 27), making them a target for interventions aimed at improving metabolic health and physical function. Aerobic exercise has long been known to be a potent regulator of skeletal muscle oxidative capacity (12, 13, 36), as chronic endurance exercise increases mitochondrial number/density and intrinsic mitochondrial function (15). In contrast, surprisingly little is known regarding the impact of resistance exercise on skeletal muscle mitochondrial function. We determined the effects of chronic RET on skeletal muscle mitochondrial function. We show that RET elicits both quantitative and qualitative adaptations in skeletal muscle mitochondrial respiration. This data suggest that RET is a means of augmenting mitochondrial respiratory capacity and function in skeletal muscle.
RET has received significant interest recently as a relatively straightforward strategy for increasing muscle mass and strength. In the current study, a 12-week progressive RET program resulted in a 1.8 kg increase in body mass. This was attributable to increased fat-free mass. Indeed, the relative contribution of fat and fat-free mass to whole body mass significantly changed over the study period, with participants become leaner. Thus, RET was effective in increasing body mass, and in particular, fat free mass (i.e., skeletal muscle), as evidenced by a 12% increase in VL thickness. Twelve weeks of RET also significantly increased strength. Specifically, quadriceps (knee extensors) isometric and isokinetic peak torque increased after RET. Therefore, increased lean body mass was accompanied by increased muscle strength, highlighting the efficacy of the RET program with regards to augmenting muscle mass and function.
A novel finding of the current study was that RET significantly increased skeletal muscle mitochondrial respiration. To our knowledge, this is the first prospective study to show that chronic RET augments skeletal muscle mitochondrial respiration. Specifically, we show a marked increase in mitochondrial respiration coupled to ATP production (P) following RET, where the increase in P when complex I substrates were provided (PI) was particularly striking. These data show that the respiratory capacity and perhaps more importantly, the ATP producing capacity of skeletal muscle mitochondria significantly increase followingRET. These results are in good agreement with those of Salvadego et al., (28), who showed that coupled skeletal muscle mitochondrial respiration was significantly greater in a cross-section of resistance trained athletes when compared to untrained individuals. Further, the current data are similar to those showing that aerobic training increases skeletal muscle mitochondrial function (15, 25).
CS activity is often reported as a biochemical read-out of skeletal muscle oxidative capacity (18). In the current study, CS activity increased following RET, however, this increase was not statistically significant (Figure 2A). This is in agreement with most(1, 28, 34, 35, 37)but not all (33) reports of the impact of chronic RET on skeletal muscle oxidative capacity. In the cohort of participants studied here, CS activity was not correlated with maximal coupled mitochondrial respiration (P) before (Figure 2B) or after (Figure 2C) RET, suggesting that changes in mitochondrial respiratory capacity with RET are not accompanied by concurrent alterations in markers of skeletal muscle oxidative capacity.
Mitochondrial respiratory capacity (E) was assessed in uncoupled organelles using the proton ionophore CCCP. CCCP dissipates mitochondrial membrane potential and increases respiration above that of maximal coupled respiration, since the inhibitory effect of proton accumulation on electron transfer is removed (9). RET significantly increased mitochondrial respiratory capacity by (Figure 2D). These findings are similar to those showing mitochondrial respiratory capacity is greater in endurance-trained individuals compared to untrained individuals (15). Further, mitochondrial respiratory capacity (E) was correlated with maximal coupled respiration (P) before (R=0.96; Figure 3E) and after (R=0.92; Figure 3F) RET.Collectively, this suggests that like aerobic exercise training (8, 13),RET increases coupled mitochondrial respiration as well as mitochondrial respiratory capacity.
A benefit of determining mitochondrial function by HRR is that numerous respiratory states can be measured sequentially within the same sample. In the current study, leak (L), phosphorylating (P) and maximal (E) mitochondrial respiration were determined in each myofiber preparation within a single experiment lasting. Critically, this means that respiratory fluxes were determined within the same pool of mitochondria. An advantage of this approach is that sensitivity of a sample to a particular substrate or uncoupler reflects the intrinsic functional characteristics of the mitochondrial pool within that sample, and is independent of mitochondria density. In the current study, normalizing leak (L) and phosphorylating (P) respiratory states to respiratory capacity (E)provides a means of normalizing absolute respiratory rates to an internal control, and thus accounts for any change in respiratory capacity/mitochondrial density following RET.
Our present data show that the ratio of leak mitochondrial respiration to mitochondrial respiratory capacity (L/E) was unchanged by 12-weeks of RET (Figure 3A). Interestingly, the ratio of coupled respiration supported by complex I substrates to respiratory capacity (PI/E) was significantly increased by RET (Figure 3A), suggesting that in addition to quantitative alterations in respiratory capacity, chronic RET brings about qualitative changes in skeletal muscle mitochondria. However, when coupled respiration was supported by both complex I and II substrates, the ratio of P/E was not different, again suggesting that increases in maximal coupled respiration following RET (PI+II) is largely supported by increased respiratory capacity.
The respiratory control ratio for ADP, an indicator of how coupled mitochondria are, was calculated as the ratio of PI to LI respiration. The RCR for ADP was not altered by chronic RET (Figure 3B). These current data are in contrast to those of Salvadego and co-workers(28), who demonstrated that skeletal muscle mitochondrial RCR for ADP was greater in a cohort of resistance trained individuals when compared to untrained individuals. The discrepancy between our current findings and those previously reported (28)may be attributable to experimental design. Here, participants were studied prospectively, whereas Salvadego and colleagues (28) studied a cohort of resistance trained and untrained individuals. Further, Salvadego and colleagues used a slightly different substrate combination than the one employed in the current study. With that said, our finding that chronic resistance exercise does not alter the RCR for ADP (Figure 3B) is supported by our flux control ratio data (Figure 3A), where L/E and PI+II/E were not different post RET, suggesting that the proportion of respiratory capacity accounted for by leak and coupled respiration were similar before and after RET.
In the current study, PI/E increased following RET. This alteration in intrinsic mitochondrial function is indicative of skeletal muscle mitochondria becoming more sensitive to the complex I substrate NADH.In support of this, the SCR for succinate (PI+II/PI) was significantly reduced following RET. The conversion of succinate to fumarate in the Krebs cycle produces FADH2, from which electrons are transferred to ubiquinone via the inner mitochondrial membrane bound enzyme succinate dehydrogenase (complex II of the electron transport chain). This suggests that the contribution of complex I and II to total electron transfer and thus mitochondrial respiration is altered after RET, where complex I electron transfer to ubiquinone increases, while complex II electron transfer to ubiquinone decreases following RET.
Blunted mitochondrial sensitivity to succinate post RET suggests reduced electron flow from complex II. However, since electron flow from complex I to ubiquinone increased after RET, as did both coupled (P) and uncoupled (E)respiration, it would appear that there is a shift in the relative contribution of complex I and II to maximal electron transfer, as opposed to diminished complex II function. This is an important point, as previous studies have reported reduced Complex II activity following chronic RET (2, 5), concluding that RET may reduce skeletal muscle oxidative capacity. Further, while we show the contribution of complex II to total respiratory capacity is diminished, mitochondrial respiratory capacity increases with RET. Such a finding underscores the fact that caution should be exercised when attempting to infer deficits in mitochondrial function from single measurements of mitochondrial enzyme activity.
In accordance with functional data showing a significant increase in coupled respiration supported by electron input from complex I of the electron transport chain, we found the complex I protein abundance was also significantly elevated after 12 weeks of RET (Figure 4). This adds credence to our supposition that RET results in qualitative changes in mitochondrial respiratory function, where complex I function is augmented. Interestingly, while the protein abundance of the other four components of the electron transport change were unaltered by chronic RET, the fact that complex II protein levels did not change (Figure 4), further suggests that reduced succinate sensitivity following RET is likely due to increased complex I function rather than deficit in complex II abundance or function.
To further examine the mechanisms underpinning improvements in mitochondrial function following RET, we examined the expression of several mRNA transcripts pre and post-training. It has been postulated that increased content of mitochondrial proteins is drivenby increased transcriptional regulation of mitochondrial biogenesis (14). Similar to previous reports(1), we did not see a pronounced increase in the expression of mRNA”s involved in mitochondrial biogenesis (COX18, MFN1, NDRG2, PPARGC1A (PGC1α) and TFAM), assessed 3 days after the last day of RET. It should be noted that marked improvements in aerobic capacity can be see without chronic increases (31), or even a decrease (16) in markers of mitochondrial biogenesis. It appears that PGC1α, due to its short half-life, may be more reflective of a transcriptional regulation of the acute response, and thus the timing of biopsy (3 days post exercise in the case of the current study), may explain the absence of any transcriptional response. However, mRNA expression of COX4I1 and NAMPT significantly increased following RET.IncreasedCOX4I1expression RET has been reported previously (1) and unlike PGC1α, may reflect chronic transcriptional regulation of mitochondria not necessarily to an acute training response(31). Given that complex IV (COX) is the location of electron transfer to molecular oxygen within mitochondria, lends some mechanistic insight into the increased respiratory capacity seen following RET. NAMPT controls conversion of nicotinamide to nicotinamide adenine dinucleotide (NAD+), the principle electron carrier within mitochondrion. Cross-sectional and longitudinal studies demonstrate that NAMPT mRNA and protein are increased with aerobic-like exercise and that the NAMPT protein content is correlated with mitochondrial content(6). This current finding may linked to increasedcomplex I function, since complex I (NADH oxidase) respiratory flux post RET may require greater mitochondrial NAD+.
To the best of our knowledge, this is the first study to comprehensively determine the impact of chronic RET on mitochondrial respiratory capacity within skeletal muscle. Our novel findings demonstrate that a 12-week RET program results in both qualitative and qualitative adaptations in skeletal muscle mitochondria of young healthy adults. These changes appear to occur with modest changes in mitochondrial proteins and mRNAs. Increased capacity for coupled respiration, particularly supported by complex I activity was accompanied by increased mitochondrial respiratory capacity. Collectively, these findings demonstrate that chronic RET improves mitochondrial respiratory function within skeletal muscle.
Acknowledgements
We wish to thank the Clinical Research staff of the Institute for Translational Science Clinical Research Center at UTMB for assisting in screening and consenting patients and participants and for assisting in data collection. We wish to thank Benjamin Brightwell, Camille Brightwell and Jennifer Thedinga for their assistance in supervising the exercise training of research participants.
This work was supported by grants from the National Institutes of Health (RO1 AR049877 and P30 AG024832), and Shriners Hospitals for Children (84090). Study visits were conducted with the support of the Institute for Translational Sciences at the University of Texas Medical Branch, which is supported by a Clinical and Translational Science Award (UL1TR000071) from the National Center for Advancing Translational Sciences, National Institutes of Health. CP is supported by an Interdisciplinary Rehabilitation Research Postdoctoral Training Grant (H133P110012) from the National Institute for Disabilities and Rehabilitation Research and the Department of Education.
The authors wish to state that the results of this study do not constitute endorsement by the American College of Sports Medicine.
Footnotes
Conflict of Interest: None of the authors have any relevant conflicts of interest to disclose.
References
- 1.Alvehus M, Boman N, Söderlund K, Svensson M, Burén J. Metabolic adaptations in skeletal muscle, adipose tissue, and whole-body oxidative capacity in response to resistance training. Eur J Appl Physiol. 2014;114:1463–71. doi: 10.1007/s00421-014-2879-9. [DOI] [PubMed] [Google Scholar]
- 2.Bell G, Syrotuik D, Martin T, Burnham R. Effect of concurrent strength and endurance training on skeletal muscle properties and hormone concentrations in humans. Eur J Appl Physiol. 2000;81:418–27. doi: 10.1007/s004210050063. [DOI] [PubMed] [Google Scholar]
- 3.Bergström J. Percutaneous needle biopsy of skeletal muscle in physiological and clinical research. Scand J Clin Lab Invest. 1975;35:609–16. (Bergström J) [PubMed] [Google Scholar]
- 4.Boushel R, Gnaiger E, Schjerling P, Skovbro M, Kraunsøe R, Dela F. Patients with type 2 diabetes have normal mitochondrial function in skeletal muscle. Diabetologia. 2007;50:790–6. doi: 10.1007/s00125-007-0594-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chilibeck P, Syrotuik D, Bell G. The effect of strength training on estimates of mitochondrial density and distribution throughout muscle fibres. Eur J Appl Physiol Occup Physiol. 1999;80:604–9. doi: 10.1007/s004210050641. [DOI] [PubMed] [Google Scholar]
- 6.Costford S, Bajpeyi S, Pasarica M, et al. Skeletal muscle NAMPT is induced by exercise in humans. Am J Physiol Endocrinol Metab. 2010;298:E117–26. doi: 10.1152/ajpendo.00318.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dreyer H, Fujita S, Cadenas J, Chinkes D, Volpi E, Rasmussen B. Resistance exercise increases AMPK activity and reduces 4E-BP1 phosphorylation and protein synthesis in human skeletal muscle. J Physiol. 2006;576:613–24. doi: 10.1113/jphysiol.2006.113175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Drummond M, Glynn E, Fry C, Timmerman K, Volpi E, Rasmussen B. An increase in essential amino acid availability upregulates amino acid transporter expression in human skeletal muscle. Am J Physiol Endocrinol Metab. 2010;298:E1011–8. doi: 10.1152/ajpendo.00690.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gnaiger E. Capacity of oxidative phosphorylation in human skeletal muscle: new perspectives of mitochondrial physiology. Int J Biochem Cell Biol. 2009;41:1837–45. doi: 10.1016/j.biocel.2009.03.013. [DOI] [PubMed] [Google Scholar]
- 10.Green H, Dahly A, Shoemaker K, Goreham C, Bombardier E, Ball-Burnett M. Serial effects of high-resistance and prolonged endurance training on Na+-K+ pump concentration and enzymatic activities in human vastus lateralis. Acta Physiol Scand. 1999;165:177–84. doi: 10.1046/j.1365-201x.1999.00484.x. [DOI] [PubMed] [Google Scholar]
- 11.Green H, Goreham C, Ouyang J, Ball-Burnett M, Ranney D. Regulation of fiber size, oxidative potential, and capillarization in human muscle by resistance exercise. Am J Physiol. 1999;276:591–6. doi: 10.1152/ajpregu.1999.276.2.R591. [DOI] [PubMed] [Google Scholar]
- 12.Holloszy J. Biochemical adaptations in muscle: Effects of exercise on mitochondrial oxygen uptake and respiratory enzyme activity in skeletal muscle. J Biol Chem. 1967;242:2278–82. [PubMed] [Google Scholar]
- 13.Holloszy J, Oscai L, Don I, Molé P. Mitochondrial citric acid cycle and related enzymes: adaptive response to exercise. Biochem Biophys Res Commun. 1970;40:1368–73. doi: 10.1016/0006-291x(70)90017-3. [DOI] [PubMed] [Google Scholar]
- 14.Hoppeler H, Fluck M. Plasticity of skeletal muscle mitochondria: structure and function. Med Sci Sports Exerc. 2003;35:95–104. doi: 10.1249/01.MSS.0000043292.99104.12. [DOI] [PubMed] [Google Scholar]
- 15.Jacobs R, Lundby C. Mitochondria express enhanced quality as well as quantity inassociation with aerobic fitness across recreationally active individuals up to elite athletes. J Appl. Physiol. 2013;26:5192–200. doi: 10.1152/japplphysiol.01081.2012. [DOI] [PubMed] [Google Scholar]
- 16.Konopka A, Douglass M, Kaminsky L, et al. Molecular adaptations to aerobic exercise training in skeletal muscle of older women. J Gerontol A Biol Sci Med Sci. 2010;65:1201–7. doi: 10.1093/gerona/glq109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Larsen S, Ara I, Rabøl R, et al. Are substrate use during exercise and mitochondrial respiratory capacity decreased in arm and leg muscle in type 2 diabetes? Diabetologia. 2009;52:1400–8. doi: 10.1007/s00125-009-1353-4. [DOI] [PubMed] [Google Scholar]
- 18.Larsen S, Nielsen J, Hansen CN, et al. Biomarkers of mitochondrial content in skeletal muscle of healthy young human subjects. J Physiol. 2012;590:3349–60. doi: 10.1113/jphysiol.2012.230185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leek B, Mudaliar S, Henry R, Mathieu-Costello O, Richardson R. Effect of acute exercise on citrate synthase activity in untrained and trained human skeletal muscle. Am J Physiol Regul Integr Comp Physiol. 2011;280:441–7. doi: 10.1152/ajpregu.2001.280.2.R441. [DOI] [PubMed] [Google Scholar]
- 20.Livak K, Schmittgen T. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 21.Lüthi J, Howald H, Claassen H, Rösler K, Vock P, Hoppeler H. Structural changes in skeletal muscle tissue with heavy-resistance exercise. Int J Sports Med. 1986;7:123–7. doi: 10.1055/s-2008-1025748. [DOI] [PubMed] [Google Scholar]
- 22.MacDougall J, Sale D, Moroz J, Elder G, Sutton J, Howald H. Mitochondrial volume density in human skeletal muscle following heavy resistance training. Med Sci Sports Exerc. 1979;11:164–6. [PubMed] [Google Scholar]
- 23.Menshikova EV, Ritov VB, Fairfull L, Ferrell RE, Kelley DE, Goodpaster BH. Effects of exercise on mitochondrial content and function in aging human skeletal muscle. J Gerontol A Biol Sci Med Sci. 2006;61:534–40. doi: 10.1093/gerona/61.6.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Menshikova EV, Ritov VB, Toledo FG, Ferrell RE, Goodpaster BH, Kelley DE. Effects of weight loss and physical activity on skeletal muscle mitochondrial function in obesity. Am J Physiol Endocrinol Metab. 2005;288:818–25. doi: 10.1152/ajpendo.00322.2004. [DOI] [PubMed] [Google Scholar]
- 25.Pesta D, Hoppel F, Macek C, et al. Similar qualitative and quantitative changes of mitochondrial respiration following strength and endurance training in normoxia and hypoxia in sedentary humans. Am J Physiol Regul Integr Comp Physiol. 2011;301:1078–87. doi: 10.1152/ajpregu.00285.2011. [DOI] [PubMed] [Google Scholar]
- 26.Ploutz L, Tesch P, Biro R, Dudley G. Effect of resistance training on muscle use during exercise. J Appl Physiol. 1994;76:1675–81. doi: 10.1152/jappl.1994.76.4.1675. [DOI] [PubMed] [Google Scholar]
- 27.Porter C, Herndon D, Borscheim E, et al. Uncoupled skeletal muscle mitochondria contribute to hypermetabolism in severely burned adults. Am J Physiol Endocrinol Metab. 2014 doi: 10.1152/ajpendo.00206.2014. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Salvadego D, Domenis R, Lazzer S, et al. Skeletal muscle oxidative function in vivo and ex vivo in athletes with marked hypertrophy from resistance training. J Appl Physiol. 2013;114:1527–35. doi: 10.1152/japplphysiol.00883.2012. [DOI] [PubMed] [Google Scholar]
- 29.Short KR, Bigelow ML, Kahl J, et al. Decline in skeletal muscle mitochondrial function with aging in humans. Proc Natl Acad Sci U S A. 2005;102:5618–23. doi: 10.1073/pnas.0501559102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Srere P. Citrate Synthase. Methods Enzymol. 1969;13:3–11. [Google Scholar]
- 31.Stepto N, Benziane B, Wadley G, et al. Short-term intensified cycle training alters acute and chronic responses of PGC1α and Cytochrome C oxidase IV to exercise in human skeletal muscle. PLoS One. 2012;7:e53080. doi: 10.1371/journal.pone.0053080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Suetta C, Andersen J, Dalgas U, et al. Resistance training induces qualitative changes in muscle morphology, muscle architecture, and muscle function in elderly postoperative patients. J Appl Physiol (1985) 2008;105:180–6. doi: 10.1152/japplphysiol.01354.2007. [DOI] [PubMed] [Google Scholar]
- 33.Tang J, Hartman J, Phillips S. Increased muscle oxidative potential following resistance training induced fibre hypertrophy in young men. Appl Physiol Nutr Metab. 2006;5:495–501. doi: 10.1139/h06-026. [DOI] [PubMed] [Google Scholar]
- 34.Tesch P, Komi P, Häkkinen K. Enzymatic adaptations consequent to long-term strength training. Int J Sports Med. 1987;8:66–9. doi: 10.1055/s-2008-1025706. [DOI] [PubMed] [Google Scholar]
- 35.Tesch P, Thorsson A, Colliander E. Effects of eccentric and concentric resistance training on skeletal muscle substrates, enzyme activities and capillary supply. Acta Physiol Scand. 1990;140:575–80. doi: 10.1111/j.1748-1716.1990.tb09035.x. [DOI] [PubMed] [Google Scholar]
- 36.Varnauskas E, Björntorp P, Fahlén M, Prerovský I, Stenberg J. Effects of physical training on exercise blood flow and enzymatic activity in skeletal muscle. Cardiovasc Res. 1970;4:418–22. doi: 10.1093/cvr/4.4.418. [DOI] [PubMed] [Google Scholar]
- 37.Wang N, Hikida R, Staron R, Simoneau J. Muscle fiber types of women after resistance training--quantitative ultrastructure and enzyme activity. Pflugers Arch. 1993;424:494–502. doi: 10.1007/BF00374913. [DOI] [PubMed] [Google Scholar]
- 38.Zoll J, Sanchez H, N'Guessan B, et al. Physical activity changes the regulation of mitochondrial respiration in humans skeletal muscle. J Physiol. 2002;543:191–200. doi: 10.1113/jphysiol.2002.019661. [DOI] [PMC free article] [PubMed] [Google Scholar]