Abstract
Salmonella enterica serovar Pullorum (S. Pullorum) is a highly adapted pathogen that causes pullorum disease (PD), an important systemic disease of poultry that causes severe economic losses in developing countries. In the interests of developing a safe and immunogenic oral vaccine, the efficacy of a Salmonella pathogenicity island 2 (SPI2)-deleted mutant of S. Pullorum (S06004ΔSPI2) was evaluated in chickens. S06004ΔSPI2 was severely less virulent than the parental wild-type strain S06004 as determined by the 50% lethal dose (LD50) for 3-day-old chickens when injected intramuscularly. Two-day-old chickens immunized with a single oral dose of S06004ΔSPI2 showed no differences in body weight or clinical symptoms compared with those in the negative-control group. S06004ΔSPI2 bacteria were not isolated from livers or spleens of immunized chickens after a short period of time, and specific humoral and cellular immune responses were significantly induced. Immunized chickens were challenged with S. Pullorum strain S06004 and Salmonella enterica serovar Gallinarum (S. Gallinarum) strain SG9 at 10 days postimmunization (dpi), and efficient protection against the challenges was observed. None of the immunized chickens died, the clinical symptoms were slight and temporary following challenge in immunized chickens compared with those in the control group, and these chickens recovered by 3 to 5 dpi. Overall, these results demonstrate that S06004ΔSPI2 can be used as a live attenuated oral vaccine.
INTRODUCTION
S almonella enterica serovar Pullorum (S. Pullorum) is a highly adapted pathogen that causes pullorum disease (PD) in poultry. PD is an acute systemic disease with high morbidity and mortality in young chicks, and causes weight loss, decreased fertility and hatchability, lesions (peritonitis, perihepatitis, and so on), diarrhea, and reproductive tract abnormalities in infected adults. In addition, S. Pullorum can be transmitted to chicks through eggs (1, 2). PD is rare in most developed countries because of modern poultry-rearing facilities and well-established disease control programs, but in recent years, its incidence has been frequently reported in developing countries (3). Salmonella enterica serovar Gallinarum (S. Gallinarum) causes the severe systemic disease fowl typhoid (FT) in young and adult chickens, and multilocus enzyme electrophoresis and comparative sequence analyses show it is closely related to S. Pullorum (4–6). Although largely eradicated in several countries, FT still causes significant morbidity and mortality worldwide, which results in substantial economic losses (7).
Several strategies have been used to prevent and control these diseases, such as antimicrobial therapy, biosecurity practices, and effective vaccination programs. Antimicrobial therapy can result in multidrug-resistant bacteria in poultry. In addition, these multidrug-resistant pathogens can be transmitted to humans via the food chain (8, 9). Vaccination is one of the most effective methods of preventing Salmonella infections (10). Killed vaccines are the most commonly used commercially available vaccines, as only a few Salmonella live vaccines are registered. These live vaccines offer more effective protection than killed vaccines because they can induce more comprehensive cellular immune responses (10).
Salmonella pathogenicity island 2 (SPI2) can encode type III secretion system 2 (T3SS2) and is a major virulence determinant of Salmonella species. T3SS2 is very important for the ability of Salmonella to survive and replicate inside host cells and plays an important role in systemic disease development (11). There are many papers describing the function of SPI2 and the vaccine potential of live SPI2 mutant strains for preventing Salmonella infections (12, 13), but there is no report on the vaccine potential of an SPI2 mutant strain of S. Pullorum.
In this study, we preliminarily evaluated the feasibility of an SPI2-deleted S. Pullorum mutant strain (S06004ΔSPI2) for use as a live attenuated oral vaccine based on the virulence, changes in body weight and clinical symptoms, bacterial persistence, immune responses, and protective effects in susceptible HY-Line white chickens.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
S. Pullorum S06004 (GenBank accession no. CP006575.1) is a virulent nalidixic acid-resistant (Nalr) clinical isolate obtained in 2006 from chickens with pullorum disease in the Jiangsu Province of China and stored in our laboratory (8). The virulent S. Gallinarum SG9 (Nalr) strain was supplied by P. A. Barrow (14). S06004ΔSPI2 (Nalr), the whole SPI2-deleted (∼40 kb) mutant of S. Pullorum S06004, was constructed using the one-step inactivation method described by Datsenko and Wanner and stored in our laboratory (15, 16). Luria-Bertani (LB) broth, LB agar medium (containing 1.5% [wt/vol] agar), and XLT4 agar (Difco) were used for culturing bacteria at 37°C. When needed, nalidixic acid was added to the medium at a final concentration of 40 μg/ml.
Chickens.
HY-Line white chicken eggs were hatched, and neither clinical symptoms of enteric disease nor Salmonella was detected in the chickens. All chickens were maintained in wire cages and reared with commercial feed and drinking water. Experiments were undertaken with the permission of the Animal Care and Ethics Committee of Yangzhou University.
Virulence assessment.
To investigate the virulence of mutant S06004ΔSPI2 in 3-day-old chickens, 60 chickens were randomly assigned into six groups (n = 10). Each group was inoculated intramuscularly with 10-fold dilutions of the mutant strain, from 1 × 1010 to 1 × 105 CFU, in 100 μl of phosphate-buffered saline (PBS). Another 60 chickens were injected with the same doses of the parental strain S06004. Ten control chickens received 200 μl of PBS via the same route. Chickens that died or were killed humanely were recorded over the 3-week experimental period. The 50% lethal dose (LD50) was calculated using the Karber and Behrens method (17).
Changes in body weight and clinical symptoms.
Sixty 2-day-old chickens were randomly divided into 3 groups of 20. The infected group received 2 × 108 CFU of S06004 in 100 μl of PBS orally, the vaccinated group received an equal dose of S06004ΔSPI2 (2 × 108 CFU), and the PBS group received 100 μl of PBS as a negative control. The body weights of these chickens were recorded at 5, 12, and 19 days postinoculation (dpi), and clinical symptoms were observed daily from 1 to 19 dpi.
Colonization and persistence assay.
To evaluate bacterial persistence in the internal organs of chickens following oral immunization, four chickens from each group (infected group, vaccinated group, and PBS group) were euthanized at 5, 7, 10, 14, and 21 dpi, and liver and spleen samples were aseptically collected. Samples were weighed, suspended in 1 ml of PBS, and homogenized individually. One hundred microliters of serial 10-fold dilutions of the homogenates was plated onto XLT4 agar containing 40 μg/ml nalidixic acid and then incubated at 37°C for 20 h. Bacteria were counted and expressed as log10 CFU/g. Negative samples were indicated as 0 CFU/g.
Enzyme-linked immunosorbent assay (ELISA) for serum IgG.
Serum samples were collected from four chickens of each group at 3, 7, 14, and 21 dpi. Specific antibody levels were assessed by ELISA, using heat-killed whole S. Pullorum bacteria as the coating antigen, as previously described (18). Briefly, 96-well plates were coated with 50 μl of antigen (1 × 108 CFU/ml) in PBS overnight at 4°C. Plates were washed three times with PBS containing 0.02% Tween 20 and blocked for 2 h with 100 μl of 10% fetal calf serum in PBS at 37°C. To determine specific serum IgG, serum samples (diluted 1:50) were used as the primary antibody. Horseradish peroxidase (HRP)-conjugated rabbit anti-chicken IgG (diluted 1:10,000) was used as the secondary antibody. The bound HRP activity was determined using o-phenylenediamine dihydrochloride (Sigma), and the absorbance was measured at 492 nm using an ELISA reader after the reactions were stopped by the addition of 2 M H2SO4.
Lymphocyte proliferation assay.
Peripheral lymphocytes were separated from the blood of four chickens per group using Histopaque-1077 (Sigma) at 7, 14, and 21 dpi. Cell viability was determined using trypan blue dye exclusion. The lymphocyte proliferation assay was performed with specific antigens prepared from the wild-type S. Pullorum S06004 strain as described previously (19, 20). Briefly, a viable mononuclear cell suspension (100 μl) at 1 × 105 cells/ml in RPMI 1640 medium (containing 10% fetal calf serum, 2 mM l-glutamine, 50 U/ml of penicillin, and 50 μg/ml of streptomycin) was incubated in triplicate in 96-well tissue culture plates with 50 μl of medium alone or with medium containing 4 μg/ml of soluble antigen at 41°C in a humidified 5% CO2 atmosphere for 72 h. Lymphocyte proliferation activity was measured using ATP bioluminescence with a ViaLight Plus kit (Lonza, Rockland, ME, USA). The blastogenic response for the assay was expressed as the mean stimulation index (SI), as previously described (19).
Protection assessment.
To examine the protective efficacy of mutant S06004ΔSPI2, 50 two-day-old chickens were randomly divided into 5 groups of 10 chickens (groups A to E). Groups A and C were immunized orally with 2 × 108 CFU of S06004ΔSPI2 in 100 μl of PBS, while groups B, D, and E received 100 μl of PBS. After 10 days, groups A and B were challenged intramuscularly with 1 × 109 CFU of strain S06004 in 100 μl of PBS. Groups C and D received equal amounts of SG9. Group E received 100 μl PBS as a negative control. The surviving chickens were counted at 21 days postchallenge, and clinical symptoms (including anorexia, diarrhea, depression, high morbidity, and mortality) were recorded daily from 1 to 35 dpi.
Statistical analysis.
All data are expressed as mean ± standard error of the mean (SEM) unless otherwise specified. All statistical analyses were performed using GraphPad Prism. P values of <0.05 were considered significant when using one-way analysis of variance.
RESULTS
Virulence of S. Pullorum mutant S06004ΔSPI2.
The virulence of parental and mutant strains was evaluated in 3-day-old HY-Line white chickens. The LD50 of the S. Pullorum mutant S06004ΔSPI2 (2.08 × 109 CFU) was 208-fold higher than that of the wild-type parental strain S06004 (1.00 × 107 CFU), indicating that the virulence of the S. Pullorum mutant S06004ΔSPI2 was attenuated.
Changes in body weight and clinical symptoms following immunization.
The mean body weights of chickens in the three treatment groups (infected group, vaccinated group, and PBS group) at 5, 12, and 19 dpi are shown in Table 1. No statistically significant differences were observed between the three groups, although the body weights in the vaccinated group and PBS group were slightly higher than those in the infected group. Clinical signs of disease, including slight and temporary lethargy, anorexia, and diarrhea, were observed in chickens from the infected group, but these clinical symptoms were absent in chickens of the vaccinated and PBS groups.
TABLE 1.
Mean body weights of chickens following immunization
| Group | Immunization |
Mean body wt (g) per chicken at: |
||||
|---|---|---|---|---|---|---|
| Strain or control | Route | Dose (CFU) | 5 dpi | 12 dpi | 19 dpi | |
| Infected | S06004 | Oral | 2 × 108 | 62.79 ± 0.36 | 110.22 ± 0.51 | 181.35 ± 0.55 |
| Vaccinated | S06004ΔSPI2 | Oral | 2 × 108 | 66.43 ± 0.29 | 114.55 ± 0.49 | 186.05 ± 0.12 |
| PBS | PBS | 66.80 ± 0.11 | 115.12 ± 0.23 | 185.17 ± 0.26 | ||
Bacterial colonization and persistence in internal organs.
All liver and spleen samples from the negative-control group (PBS group) were negative for bacterial recovery. As shown in Fig. 1, the viable counts in organs from chickens inoculated with the wild-type parent strain S06004 (infected group) were higher than those from chickens inoculated with the S. Pullorum mutant S06004ΔSPI2 (vaccinated group). Of the four examined livers of the vaccinated group, one and two samples were negative at 7 and 10 dpi, respectively, and no colonies were recovered at 14 dpi. In contrast, three and two liver samples were positive in the infected group at 14 and 21 dpi, respectively. From the four examined spleens of the vaccinated group, one and two samples were negative at 10 and 14 dpi, respectively, and no colonies were recovered from any animal at 21 dpi. However, all four spleen samples from the infected group were positive at 21 dpi.
FIG 1.
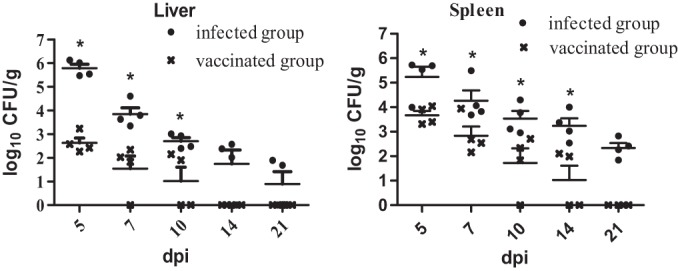
Bacterial colonization and persistence in liver and spleen of chickens following immunization. The infected group received 2 × 108 CFU of strain S06004 orally; the vaccinated group received 2 × 108 CFU of mutant S06004ΔSPI2. Values represent the mean ± SEM log10 CFU/g. *, P < 0.05.
Determination of serum IgG by ELISA.
To evaluate the humoral immune response following immunization, serum IgG levels were examined using indirect ELISA. Chickens inoculated with the wild-type parent strain S06004 (infected group) and the S. Pullorum mutant S06004ΔSPI2 (vaccinated group) showed significantly elevated levels of serum IgG at 3, 7, 14, and 21 dpi (Fig. 2). The considerably elevated serum IgG levels of the infected and vaccinated groups were continuously observed through 21 dpi.
FIG 2.
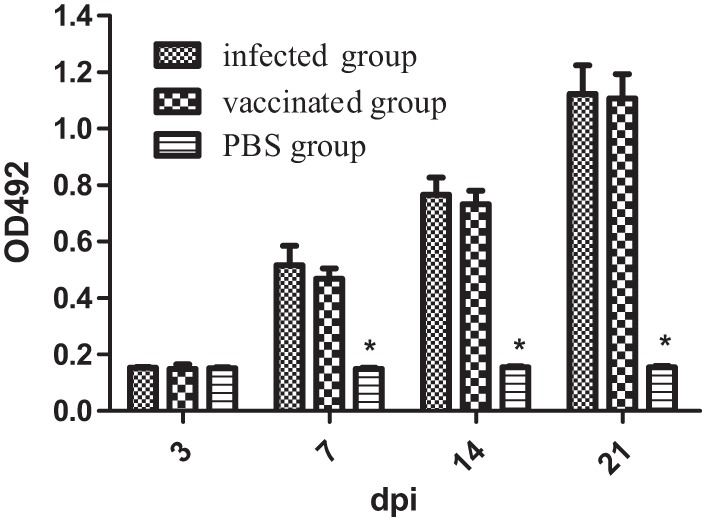
Determination of serum IgG levels. The infected group received 2 × 108 CFU of strain S06004 orally, the vaccinated group received 2 × 108 CFU of mutant S06004ΔSPI2, and the PBS group received 100 μl of PBS. Values represent the mean ± SEM. OD492, optical density at 492 nm. *, P < 0.05.
Lymphocyte proliferation assay.
To evaluate cellular immune responses following immunization, a peripheral lymphocyte proliferation assay was performed using soluble antigen. As shown in Fig. 3, the SI values of chickens inoculated with mutant S06004ΔSPI2 (vaccinated group) were 2.711 ± 0.152, 2.959 ± 0.156, and 1.778 ± 0.203 at 7, 14, and 21 dpi, respectively, and the values of chickens inoculated with strain S06004 (infected group) were 2.755 ± 0.102, 3.131 ± 0.257, and 2.861 ± 0.184 at the same respective dpi. The SI values of the infected and vaccinated groups were significantly increased at 7, 14, and 21 dpi, and the SI value of the infected group was also significantly higher than that of the vaccinated group at 21 dpi. Sequential monitoring of lymphocyte responses revealed considerably elevated SI values in the infected and vaccinated groups; significantly elevated SI values in the infected and vaccinated groups were observed at 14 dpi but were reduced at 21 dpi.
FIG 3.
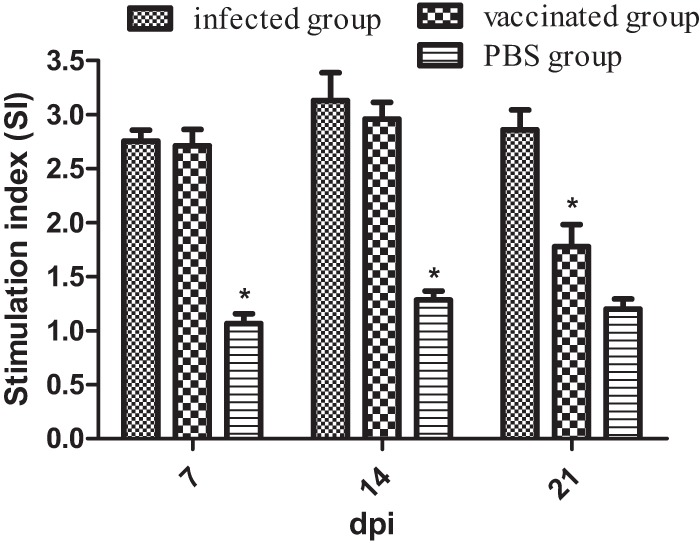
Stimulation index of chicken lymphocyte samples determined by peripheral lymphocyte proliferation assay using soluble antigen. The infected group received 2 × 108 CFU of strain S06004 orally, the vaccinated group received 2 × 108 CFU of mutant S06004ΔSPI2, and the PBS group received 100 μl of PBS. Values represent the mean ± SEM. *, P < 0.05.
Evaluation of immune protection.
The percentage of survival in chickens orally vaccinated with the S. Pullorum mutant S06004ΔSPI2, followed by intramuscular challenge with the parent S. Pullorum strain S06004 or S. Gallinarum strain SG9, at 10 dpi is shown in Table 2. None of the immunized chickens died, whereas four chickens died in control group B following challenge with S06004, and seven chickens died in control group D following challenge with SG9. The clinical symptoms in the immunized chickens were slight and temporary following challenge, compared with those of the blank control group (group E), and the chickens had recovered by 3 to 5 days postchallenge, indicating the protection was effective. The classical clinical symptoms, such as anorexia, diarrhea, depression, high morbidity, and mortality, were observed in groups B and D.
TABLE 2.
Protective efficacy of mutant S06004ΔSPI2 in chickens
| Group | Vaccination |
Challenge |
No. of survivors/total no. of chickens | ||||
|---|---|---|---|---|---|---|---|
| Strain or control | Route | Dose (CFU) | Strain | Route | Dose (CFU) | ||
| A | S06004ΔSPI2 | Oral | 2 × 108 | S06004 | Intramuscular | 1 × 109 | 10a/10 |
| B | PBS | S06004 | Intramuscular | 1 × 109 | 6/10 | ||
| C | S06004ΔSPI2 | Oral | 2 × 108 | SG9 | Intramuscular | 1 × 109 | 10a/10 |
| D | PBS | SG9 | Intramuscular | 1 × 109 | 3/10 | ||
| E | PBS | 10/10 | |||||
P < 0.05 for comparison of group A with group B and group C with group D.
DISCUSSION
PD and FT are two important systemic diseases in poultry that are caused by S. Pullorum and S. Gallinarum, respectively. They remain common and can result in substantial economic losses in many countries (2). Thus, effective measures are needed to prevent and control the diseases, and vaccination may be a viable choice. Many live attenuated Salmonella vaccines have been described and are generally more efficacious than killed vaccines (21, 22).
SPI1 and SPI2 mutants were identified as having vaccine potential in Salmonella enterica serovar Enteritidis (S. Enteritidis), Salmonella enterica serovar Typhimurium (S. Typhimurium), and Salmonella enterica serovar Typhi (S. Typhi) (23, 24). In this study, we evaluated the efficacy of a candidate live-attenuated oral vaccine, S06004ΔSPI2, for PD and FT based on the virulence, changes in body weight and clinical symptoms, colonization, serum IgG response, peripheral lymphocyte proliferation response, and protective efficiency in susceptible HY-Line white chickens.
The ideal vaccine should be avirulent, and this is especially important for live vaccines. SG9R has been used for nearly 50 years as an S. Gallinarum vaccine for FT, but it is not an optimal vaccine strain because of its residual virulence and controversial protection (25). S. Enteritidis and S. Typhimurium mutants with deletion of SPI2 or other key genes located within the pathogenicity island display decreased virulence in poultry, pigs, cattle, mice, and humans (13, 24, 26–29). Compared with that of the parental wild-type strain S06004, the virulence of S. Pullorum mutant S06004ΔSPI2 was attenuated, as demonstrated by the LD50 of 3-day-old chickens inoculated by intramuscular injection. We next evaluated the effects of S06004ΔSPI2 on chicken growth by recording body weight and observing clinical symptoms following oral immunization, which showed that S06004ΔSPI2 has almost no side effects in terms of growth performance in chickens.
T3SS2 is necessary for maintenance of infection and, hence, persistence. Salmonella lacking a functional T3SS2 is cleared from the host more rapidly than the parental wild-type strain, and some studies have failed to detect SPI2 mutant strains in the liver and spleen following oral inoculation (7, 14, 30). Our results showed that mutant S06004ΔSPI2 can persist for a short time in chicken liver and spleen, but it was more rapidly cleared from these organs than parent strain S06004 after oral inoculation. This may be related to the inoculation dose and the breed of chicken.
It is crucial that live attenuated Salmonella vaccines stimulate the humoral and the cellular immune responses in the host (10). Determination of the specific serum IgG antibody level by indirect ELISA showed that a strong humoral antibody response was induced, and antibodies were detectable at 7 dpi. Chickens inoculated with S06004ΔSPI2 showed significantly higher levels of serum IgG and levels similar to those of chickens inoculated with S06004. A previous report showed that an S. Enteritidis SPI2 mutant also leads to a significant antibody increase in chickens (13). Salmonella is a facultative intracellular pathogen, meaning that cellular immune responses are required for adequate host defense, which can only be induced by live Salmonella (31). As avian lymphocytes are mostly composed of T cells, we can evaluate cellular immune responses using a peripheral lymphocyte proliferation assay. In the present study, cellular immune responses were clearly observed in the vaccinated chickens. The SI value of the vaccinated group was significantly lower than that of the infected group at 21 dpi; this may be related to bacterial colonization and persistence in internal organs (32, 33).
Live Salmonella enterica vaccines can confer cross-protection immunity to different pathogenic Salmonella serotypes (34). To evaluate the protective efficacy of the candidate vaccine, we determined protection rates and observed clinical symptoms following intramuscular challenge with strains S06004 and SG9. None of the immunized chickens died, which demonstrated that the candidate vaccine S06004ΔSPI2 provides high levels of protection in HY-Line white chickens. Clinical symptoms in immunized chickens following challenge were slight, temporary, and resolved quickly, but classical clinical symptoms were observed in control groups (groups B and D). These results showed that S06004ΔSPI2 can offer efficient protection against acute systemic PD and FT infection.
In conclusion, the present study demonstrated that the live vaccine candidate S06004ΔSPI2 can induce acquired immunity, including humoral and cellular immune responses, and can provide efficient protection against systemic PD and FT infection. Furthermore, vaccinated chickens did not display adverse side effects. Taken together, these data showed that S06004ΔSPI2 can be used as a novel live attenuated oral vaccine, although further trials are needed.
ACKNOWLEDGMENTS
This work was supported by the Key Program of the National Natural Science Foundation of China (grant 31230070), the Colleges and Universities of Jiangsu Province Plans to Graduate Research and Innovation (grant CXZZ13_0917), the National Science & Technology Pillar Program (grant 2014BAD13B02), the National Natural Science Foundation of China (grant 31201905), the 333 Project in Jiangsu Province (grant BRA2011141), and the Priority Academic Program Development (PAPD) of Jiangsu Higher Education Institutions.
REFERENCES
- 1.Rettger LF. 1909. Further studies on fatal septicemia in young chickens, or “white diarrhea.” J Med Res 21:115–123. [PMC free article] [PubMed] [Google Scholar]
- 2.Li Q, Hu Y, Chen J, Liu Z, Han J, Sun L, Jiao X. 2013. Identification of Salmonella enterica serovar Pullorum antigenic determinants expressed in vivo. Infect Immun 81:3119–3127. doi: 10.1128/IAI.00145-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barrow PA, Freitas Neto OC. 2011. Pullorum disease and fowl typhoid–new thoughts on old diseases: a review. Avian Pathol 40:1–13. doi: 10.1080/03079457.2010.542575. [DOI] [PubMed] [Google Scholar]
- 4.Barrow PA, Simpson JM, Lovell MA, Binns MM. 1987. Contribution of Salmonella Gallinarum large plasmid toward virulence in fowl typhoid. Infect Immun 55:388–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baumler AJ, Tsolis RM, Ficht TA, Adams LG. 1998. Evolution of host adaptation in Salmonella enterica. Infect Immun 66:4579–4487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li J, Smith NH, Nelson K, Crichton PB, Old DC, Whittam TS, Selander RK. 1993. Evolutionary origin and radiation of the avian-adapted non-motile salmonellae. J Med Microbiol 38:129–139. doi: 10.1099/00222615-38-2-129. [DOI] [PubMed] [Google Scholar]
- 7.Wigley P, Berchieri A Jr, Page KL, Smith AL, Barrow PA. 2001. Salmonella enterica serovar Pullorum persists in splenic macrophages and in the reproductive tract during persistent, disease-free carriage in chickens. Infect Immun 69:7873–7879. doi: 10.1128/IAI.69.12.7873-7879.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Geng S, Jiao X, Barrow PA, Pan Z, Chen X. 2014. Virulence determinants of Salmonella Gallinarum biovar Pullorum identified by PCR signature-tagged mutagenesis and the spiC mutant as a candidate live attenuated vaccine. Vet Microbiol 168:388–394. doi: 10.1016/j.vetmic.2013.11.024. [DOI] [PubMed] [Google Scholar]
- 9.Tollefson L, Miller MA. 2000. Antibiotic use in food animals: controlling the human health impact. J AOAC Int 83:245–254. [PubMed] [Google Scholar]
- 10.Mastroeni P, Chabalgoity JA, Dunstan SJ, Maskell DJ, Dougan G. 2001. Salmonella: immune responses and vaccines. Vet J 161:132–164. doi: 10.1053/tvjl.2000.0502. [DOI] [PubMed] [Google Scholar]
- 11.Galan JE. 2001. Salmonella interactions with host cells: type III secretion at work. Annu Rev Cell Dev Biol 17:53–86. doi: 10.1146/annurev.cellbio.17.1.53. [DOI] [PubMed] [Google Scholar]
- 12.Hindle Z, Chatfield SN, Phillimore J, Bentley M, Johnson J, Cosgrove CA, Ghaem-Maghami M, Sexton A, Khan M, Brennan FR, Everest P, Wu T, Pickard D, Holden DW, Dougan G, Griffin GE, House D, Santangelo JD, Khan SA, Shea JE, Feldman RG, Lewis DJ. 2002. Characterization of Salmonella enterica derivatives harboring defined aroC and Salmonella pathogenicity island 2 type III secretion system (ssaV) mutations by immunization of healthy volunteers. Infect Immun 70:3457–3467. doi: 10.1128/IAI.70.7.3457-3467.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Matulova M, Havlickova H, Sisak F, Rychlik I. 2012. Vaccination of chickens with Salmonella pathogenicity island (SPI) 1 and SPI2 defective mutants of Salmonella enterica serovar Enteritidis. Vaccine 30:2090–2097. doi: 10.1016/j.vaccine.2012.01.050. [DOI] [PubMed] [Google Scholar]
- 14.Jones MA, Wigley P, Page KL, Hulme SD, Barrow PA. 2001. Salmonella enterica serovar Gallinarum requires the Salmonella pathogenicity island 2 type III secretion system but not the Salmonella pathogenicity island 1 type III secretion system for virulence in chickens. Infect Immun 69:5471–5476. doi: 10.1128/IAI.69.9.5471-5476.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Datsenko KA, Wanner BL. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci U S A 97:6640–6645. doi: 10.1073/pnas.120163297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yin J, Wu Y, Lin Z, Wang X, Hu Y, Li Q, Geng S, Jiao X. 13 February 2015 Construction and characterization of SPI-2 deletion mutant of Salmonella Pullorum S06004. Wei Sheng Wu Xue Bao (In Chinese.) 10.13343/j.cnki.wsxb.20140621. [DOI] [PubMed] [Google Scholar]
- 17.Gilles HJ. 1974. Calculation of the index of acute toxicity by the method of linear regression. Comparison with the method of “Karber and Behrens.” Eur J Toxicol Environ Hyg 7:77–84. (In French.) [PubMed] [Google Scholar]
- 18.Haneda T, Okada N, Kikuchi Y, Takagi M, Kurotaki T, Miki T, Arai S, Danbara H. 2011. Evaluation of Salmonella enterica serovar Typhimurium and Choleraesuis slyA mutant strains for use in live attenuated oral vaccines. Comp Immunol Microbiol 34:399–409. doi: 10.1016/j.cimid.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 19.Rana N, Kulshreshtha RC. 2006. Cell-mediated and humoral immune responses to a virulent plasmid-cured mutant strain of Salmonella enterica serotype Gallinarum in broiler chickens. Vet Microbiol 115:156–162. doi: 10.1016/j.vetmic.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 20.Song H, Yan R, Xu L, Song X, Shah MA, Zhu H, Li X. 2010. Efficacy of DNA vaccines carrying Eimeria acervulina lactate dehydrogenase antigen gene against coccidiosis. Exp Parasitol 126:224–231. doi: 10.1016/j.exppara.2010.05.015. [DOI] [PubMed] [Google Scholar]
- 21.Smith HW. 1956. The use of live vaccines in experimental Salmonella Gallinarum infection in chickens with observations on their interference effect. J Hyg (Lond) 54:419–432. doi: 10.1017/S0022172400044685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Van Immerseel F, Methner U, Rychlik I, Nagy B, Velge P, Martin G, Foster N, Ducatelle R, Barrow PA. 2005. Vaccination and early protection against non-host-specific Salmonella serotypes in poultry: exploitation of innate immunity and microbial activity. Epidemiol Infect 133:959–978. doi: 10.1017/S0950268805004711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bohez L, Ducatelle R, Pasmans F, Haesebrouck F, Van Immerseel F. 2007. Long-term colonisation-inhibition studies to protect broilers against colonisation with Salmonella Enteritidis, using Salmonella pathogenicity island 1 and 2 mutants. Vaccine 25:4235–4243. doi: 10.1016/j.vaccine.2007.02.082. [DOI] [PubMed] [Google Scholar]
- 24.Khan SA, Stratford R, Wu T, McKelvie N, Bellaby T, Hindle Z, Sinha KA, Eltze S, Mastroeni P, Pickard D, Dougan G, Chatfield SN, Brennan FR. 2003. Salmonella Typhi and S. Typhimurium derivatives harbouring deletions in aromatic biosynthesis and Salmonella pathogenicity island-2 (SPI-2) genes as vaccines and vectors. Vaccine 21:538–548. doi: 10.1016/S0264-410X(02)00410-3. [DOI] [PubMed] [Google Scholar]
- 25.Bouzoubaa K, Nagaraja KV, Kabbaj FZ, Newman JA, Pomeroy BS. 1989. Feasibility of using proteins from Salmonella Gallinarum vs. 9R live vaccine for the prevention of fowl typhoid in chickens. Avian Dis 33:385–391. doi: 10.2307/1591094. [DOI] [PubMed] [Google Scholar]
- 26.Boyen F, Pasmans F, Van Immerseel F, Morgan E, Botteldoorn N, Heyndrickx M, Volf J, Favoreel H, Hernalsteens JP, Ducatelle R, Haesebrouck F. 2008. A limited role for SsrA/B in persistent Salmonella Typhimurium infections in pigs. Vet Microbiol 128:364–373. doi: 10.1016/j.vetmic.2007.10.031. [DOI] [PubMed] [Google Scholar]
- 27.Coombes BK, Coburn BA, Potter AA, Gomis S, Mirakhur K, Li Y, Finlay BB. 2005. Analysis of the contribution of Salmonella pathogenicity islands 1 and 2 to enteric disease progression using a novel bovine ileal loop model and a murine model of infectious enterocolitis. Infect Immun 73:7161–7169. doi: 10.1128/IAI.73.11.7161-7169.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dieye Y, Ameiss K, Mellata M, Curtiss R III. 2009. The Salmonella pathogenicity island (SPI) 1 contributes more than SPI2 to the colonization of the chicken by Salmonella enterica serovar Typhimurium. BMC Microbiol 9:3. doi: 10.1186/1471-2180-9-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Karasova D, Sebkova A, Havlickova H, Sisak F, Volf J, Faldyna M, Ondrackova P, Kummer V, Rychlik I. 2010. Influence of 5 major Salmonella pathogenicity islands on NK cell depletion in mice infected with Salmonella enterica serovar Enteritidis. BMC Microbiol 10:75. doi: 10.1186/1471-2180-10-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cirillo DM, Valdivia RH, Monack DM, Falkow S. 1998. Macrophage-dependent induction of the Salmonella pathogenicity island 2 type III secretion system and its role in intracellular survival. Mol Microbiol 30:175–188. doi: 10.1046/j.1365-2958.1998.01048.x. [DOI] [PubMed] [Google Scholar]
- 31.Collins FM, Scott MT. 1974. Effect of Corynebacterium parvum treatment on the growth of Salmonella enteritidis in mice. Infect Immun 9:863–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Matsuda K, Chaudhari AA, Lee JH. 2011. Evaluation of safety and protection efficacy on cpxR and lon deleted mutant of Salmonella Gallinarum as a live vaccine candidate for fowl typhoid. Vaccine 29:668–674. doi: 10.1016/j.vaccine.2010.11.039. [DOI] [PubMed] [Google Scholar]
- 33.Wigley P, Jones MA, Barrow PA. 2002. Salmonella enterica serovar Pullorum requires the Salmonella pathogenicity island 2 type III secretion system for virulence and carriage in the chicken. Avian Pathol 31:501–506. doi: 10.1080/0307945021000005879. [DOI] [PubMed] [Google Scholar]
- 34.Heithoff DM, House JK, Thomson PC, Mahan MJ. 2015. Development of a Salmonella cross-protective vaccine for food animal production systems. Vaccine 33:100–107. doi: 10.1016/j.vaccine.2014.11.012. [DOI] [PubMed] [Google Scholar]


