Abstract
Stratified sulfurous lakes are appropriate environments for studying the links between composition and functionality in microbial communities and are potentially modern analogs of anoxic conditions prevailing in the ancient ocean. We explored these aspects in the Lake Banyoles karstic area (NE Spain) through metagenomics and in silico reconstruction of carbon, nitrogen and sulfur metabolic pathways that were tightly coupled through a few bacterial groups. The potential for nitrogen fixation and denitrification was detected in both autotrophs and heterotrophs, with a major role for nitrogen and carbon fixations in Chlorobiaceae. Campylobacterales accounted for a large percentage of denitrification genes, while Gallionellales were putatively involved in denitrification, iron oxidation and carbon fixation and may have a major role in the biogeochemistry of the iron cycle. Bacteroidales were also abundant and showed potential for dissimilatory nitrate reduction to ammonium. The very low abundance of genes for nitrification, the minor presence of anammox genes, the high potential for nitrogen fixation and mineralization and the potential for chemotrophic CO2 fixation and CO oxidation all provide potential clues on the anoxic zones functioning. We observed higher gene abundance of ammonia-oxidizing bacteria than ammonia-oxidizing archaea that may have a geochemical and evolutionary link related to the dominance of Fe in these environments. Overall, these results offer a more detailed perspective on the microbial ecology of anoxic environments and may help to develop new geochemical proxies to infer biology and chemistry interactions in ancient ecosystems.
Introduction
Linking microbial community composition and ecological processes such as carbon (CO2 fixation and respiration), nitrogen (nitrification, denitrification and N2 fixation) and sulfur cycling (sulfur assimilation, anaerobic sulfate respiration and sulfide oxidation) is a primary goal for microbial ecologists. This information is needed to improve our understanding on the structure and functioning of microbial communities, to properly guide experimental research efforts, to promote our ability to understand fundamental mechanisms controlling microbial processes and interactions in situ (Prosser, 2012) and to approach the study of earlier interactions of biosphere–hydrosphere–geosphere (Severmann and Anbar, 2009). However, a detailed comprehension of biological interactions in highly complex systems is however difficult (Bascompte and Sole, 1995).
Stratified lakes with euxinic (anoxic and sulfurous) bottom waters are simplified study systems to explore current biodiversity–biogeochemistry interactions because of their high activity, large biomass and low microbial diversity (Guerrero et al., 1985). Usually, oxic–anoxic interfaces contain conspicuous blooms of photosynthetic bacteria that are often macroscopically visible because of the high intracellular content of pigments, and additional microbial populations also tend to accumulate (Pedrós-Alió and Guerrero, 1993). These blooms are, in fact, natural enrichment cultures that facilitate physiological studies in situ (Van Gemerden et al., 1985). At such interfaces fine gradients of physicochemical conditions are present and tight coupling between different biogeochemical cycles (mainly carbon, nitrogen and sulfur) are established. Microbes adapted to such gradients are difficult to culture because in situ conditions are very difficult to mimic in the laboratory, and their study has improved perceptibly by culture-independent methods (Casamayor et al., 2000).
Stratified euxinic lake systems may also provide potential modern day analogue ecosystems for the oceans during long periods of Earth history. The planet was essentially anoxic until 2.7–2.4 billion years ago, with a ferruginous ocean (Anbar, 2008; Reinhard et al., 2013). With the advent of oxygenic photosynthesis, atmospheric oxygen began to rise, as did the oxygen content in the surface oceans. The deep oceans remained anoxic, but entered a period of temporal and spatial heterogeneity. Strong euxinic conditions might be expected in ancient coastal areas, with merely anoxic conditions in the open ocean, although high Fe deep ocean conditions would have been maintained (Reinhard et al., 2013). In contrast, Fe is low in the deep waters of the modern ocean and, therefore, it is difficult to find appropriate ancient ocean analogue in the current marine realm. With this in mind, stratified aquatic systems with high Fe concentrations in deep waters could be more appropriate modern day analogues of the Proterozoic ocean. Karstic lacustrine systems with a gradient of organic carbon delivery and sulfide concentrations generated by sulfate reduction, and usually rich in iron, would provide reasonable biogeochemical analogues for ancient coastal to open ocean gradients.
In this study, we explored the oxic–anoxic interface (metalimnion) and bottom waters (hypolimnion) from two sulfurous lakes in the Banyoles karstic area (NE Spain) through shotgun metagenomics and in silico analysis of several metabolic pathways. In the framework of paleoreconstruction of anoxic conditions in ancient marine systems, one lake would be representative of strong euxinic conditions (Lake Cisó) and the other of low euxinia and an active iron cycle (basin III of Lake Banyoles). We explore the links between microbial composition and functionality for the carbon, nitrogen and sulfur cycling after phylogenetic and functional identification. The taxonomic identity assigned to each functional step was determined by the closest match in databases, and the relative abundance and distribution of marker genes was comparatively analyzed among samples as a proxy of the potential in situ relevance of these pathways under the specific environmental conditions studied. Because of the lack of oxygen, large microbial biomass and high contribution of deep dark fixation processes to overall CO2 incorporation (Casamayor et al., 2008, 2012; Casamayor, 2010), we hypothesized a high genetic potential for chemotrophic CO2 fixation and a tight redox coupling between carbon, nitrogen and sulfur biogeochemical cycling. In addition, because of its euxinic nature we also expected a low contribution of both methanogens and ammonia oxidizers in the biogeochemical cycles prevailing in these environments.
Materials and methods
Environment and samples collection
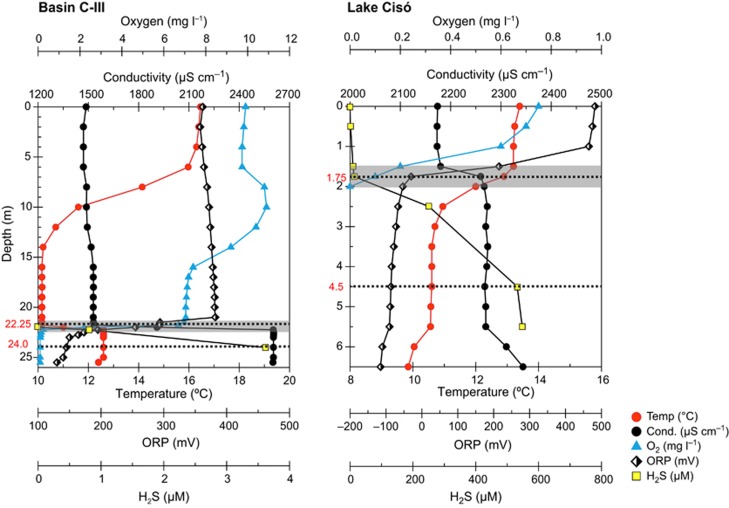
Lake Cisó and basin III of Lake Banyoles (Banyoles C-III) are in the Banyoles karstic area, northeastern Spain (42°8'N, 2°45'E), and the microbial communities inhabiting these water bodies have been extensively studied by limnologists and microbial ecologists (see, for example, Guerrero et al., 1980; Garcia-Gil and Abellà, 1992; Pedrós-Alió and Guerrero, 1993). The lakes were sampled on 8–9 May 2010. Vertical profiles of temperature, conductivity, oxygen and redox potential were measured in situ with a multiparametric probe OTT-Hydrolab MS5 (Hatch Hydromet, Loveland, CO, USA). The different water compartments (oxic epilimnion, metalimnion with the oxic–anoxic interface and anoxic hypolimnion) were determined for each lake according to the physicochemical profiles recorded in situ (Figure 1). For sulfide analyses, 10 ml of subsamples were collected in screw-capped glass tubes and immediately alkalinized by adding 0.1 ml of 1 M NaOH and fixed by adding 0.1 ml of 1 M zinc acetate. Sulfide was analyzed in the laboratory according to Trüper and Schlegel (1964). For pigments, water samples were processed as described by Guerrero et al. (1985) and analyzed by HPLC as previously reported (Borrego et al., 1999). Iron (Fe+2) concentrations were obtained from Garcia-Gil (1990).
Figure 1.
Vertical profiles of physicochemical data for Lake Banyoles basin C-III and Lake Cisó. Metagenomic analyses were carried out at two selected depths (dotted lines): the oxic–anoxic interface (shadowed areas indicate the redoxcline zone) and the anoxic and sulfurous (euxinic) hypolimnia.
These lakes are stratified and have incoming sulfate-rich water seeping in through bottom springs, resulting in deep waters rich in reduced sulfur compounds. An oxic–anoxic interface, or redoxcline, is established in the water column where light and sulfide usually coexist. Lake Banyoles is a gypsum karst spring area consisting of six main basins covering a surface area of 1.1 km2. The basin III (C-III) is meromictic with a maximal depth of 32 m, and a redoxcline between 18 and 21 m, depending on the season. Blooms of brown-colored photosynthetic green sulfur bacteria (Chlorobiaceae) and purple sulfur bacteria (Chromatiaceae) have been periodically reported (Garcia-Gil and Abellà, 1992). Lake Cisó is a small monomictic lake (650 m2), located 1 km away from Lake Banyoles with a maximum depth of 6.5 m. The thermocline is at 1.5 m, where different bacterial populations accumulate (Casamayor et al., 2000). The presence of aerobic chemoautotrophic sulfur-oxidizing bacteria and substantial fixation of CO2 in the dark have been previously reported in sulfurous lakes (Casamayor, 2010). Lake Cisó is a small eutrophic water body fully surrounded by trees that strongly limit the incident irradiance on the lake and provide continuous allochthonous organic matter inputs both by leaching from the littoral zone and submerged vegetal debris. The system is therefore prone to a dominance of aerobic respiration and mineralization in surface layers overlying sulfate respiration, fermentation and anaplerotic pathways at depth. In addition, the conspicuous presence of photosynthetic organisms and dissolved and particulate organic matter causes a strong light extinction and quality filtering in the first 2 m that severely limits the development of oxygenic and anoxygenic green phototrophs (Vila and Abellà, 1994). Conversely, the open-basin Banyoles C-III is oligotrophic, with lower influence of the littoral zone. The basin maintains a stable, sharp chemocline that oscillates in depth between 19 and 21 m depending on the season, being shallower during summer. Active sulfate reduction occurring at the permanent anoxic monimolimnion causes sulfide accumulation below the chemocline, usually reaching concentrations of up to 1 mM during summer and late fall. Lower sulfide concentrations are common however during spring. Light intensities reaching the O2/H2S interface are generally low (between 1% and 0.1% of surface incident light in winter and summer, respectively) despite the transparency of the epilimnetic waters of Lake Banyoles. The brown-pigmented green sulfur bacteria are better adapted to low irradiances than the green-pigmented ones (Garcia-Gil and Abellà, 1992) and massively bloom in C-III.
The oxic–anoxic metalimnion interface and the euxinic (anoxic and sulfurous) hypolimnion samples for metagenomic analyses were determined in situ according to the vertical physicochemical profiles. Samples were prefiltered in the field through a 200 μm nylon mesh and kept in the dark in 25 l polycarbonate carboys until further processing in the lab 2–4 h later. The plankton was collected using serial filtration onto 3.0, 0.8 and 0.1 μm Supor 293 mm membrane disc filters (Pall Life Sciences, Port Washington, NY, USA) and stored in liquid nitrogen or −80 °C until DNA extraction. DNA extraction and pyrosequencing was carried out at the J Craig Venter Institute in Rockville, MD, USA as recently reported (Zeigler Allen et al., 2012).
DNA sequences analyses
A shotgun metagenomics approach was applied on all three size fractions of four samples from Lakes Cisó and Banyoles C-III. Identical reads were removed using CD-HIT (Li and Godzik, 2006). Annotation of metagenomic reads was conducted through the JCVI prokaryotic annotation pipeline (Tanenbaum et al., 2010) using Uniref100, PFAM, TIGRfam and KEGG (Kyoto Encyclopedia of Genes and Genomes) Orthologs (KO) databases for taxonomic and functional annotation. JCVI Metagenomics reports (http://jcvi.org/metarep) were used for analysis and comparative metagenomics (Goll et al., 2010). KO annotation was used for functional analysis and KO counts were normalized according to the length of the read and the length of the target gene (Sharon et al., 2009). The communities and functional profiles found in each size fraction were highly similar (Supplementary Figure S1) and, therefore, we pooled all reads after normalizing for sequencing depth for subsequent analyses, which allows for a better comparison of metagenomes.
The functional analyses focused on the three main biogeochemical cycles for this type of lakes, that is, carbon (C), nitrogen (N) and sulfur (S) cycling. The genetic potential of the microbial community was analyzed following the C, N, and S marker genes (KOs) as reported by Lauro et al. (2011) with a few modifications. We amended this previous rubric by adding the anaerobic carbon fixation carried out through the Calvin cycle by Chromatiaceae, and additional genes for polysulfide reduction, nitrate reduction and nitrite oxidation. In addition, the genes pyruvate:ferredoxin oxidoreductase (porA/B) were not considered as marker genes for fermentation as in Lauro et al. (2011), because they are key genes in the reverse tricarboxylic acid cycle used for carbon fixation by Epsilonproteobacteria abundant in our study lakes (Campbell and Cary, 2004; Takai et al., 2005). Because both sulfide oxidation and dissimilatory sulfate reduction pathways are mediated by the same set of genes (aprA, aprB and dsrA) but are found in different families of bacteria, we assigned metagenomic reads to each pathway according to phylogeny, that is, sulfate reduction for Firmicutes and Deltaproteobacteria reads, and sulfide oxidation for Alphaproteobacteria, Betaproteobacteria, Chlorobiaceae and Chromatiaceae. Finally, for the sulfur-oxidizing Epsilonproteobacteria of the order Campylobacterales we specifically searched for sox genes (coding for thiosulfate oxidation) not currently available in the KEGG database. Marker genes used in the present work are shown in Supplementary Table S1. Hierarchical clustering and heatmap plots were generated with R (R Development Core Team, 2012) using the library ‘seriation'. Metagenomic data have been deposited at CAMERA (Sun et al., 2011) under accession number CAM_P_0001174.
Results
Environmental parameters
At the time of sampling (spring 2010), the water column was thermally stratified with thermoclines spanning from 1.5 to 3 m in Lake Cisó, and 7–14 m in basin C-III (Figure 1). Chemical stratification was disconnected from thermal stratification in basin C-III, where a sharp chemocline was detected at 21 m depth based on the higher conductivity of incoming sulfate-rich waters. The epilimnion of C-III showed oxygen concentrations of >6 mg l–1, with rapid drawdowns in the hypolimnion, and the sharp oxic–anoxic interface caused an abrupt decrease in the redox potential and generation of a pronounced redoxcline (Figure 1, shaded area). In Lake Cisó, the epilimnion (0–1.5 m depth) was oxygen deficient (0.2 −1 mg l–1) and the water column became completely anoxic below 2 m depth. In this case, the redoxcline and the oxic–anoxic interface were located in a narrow water layer of 0.5 m width (1.5–2 m depth). The concentration of nitrogen and sulfur species changed according to these physicochemical gradients with high concentrations of ammonia mainly in the hypolimnia (up to 60 μM) and sulfide concentrations ranging between 532 μM in Lake Cisó and <1 μM in C-III in agreement with redox potential (Eh) measurements (Table 1, and Supplementary Figure S2). The concentration of Chl a measured in the lakes agreed with their traditional trophic status (oligotrophic for C-III and mesotrophic for Lake Cisó). Biomarker pigments for green sulfur (BChl c, d and e) and purple sulfur bacteria (BChl a) were detected in the metalimnion and hypolimnion of Lake Cisó and basin C-III. Particularly, conspicuous concentrations of BChl e, the characteristic pigment of brown-colored species of Chlorobium, were measured between 22 and 24 m depth in basin C-III (Table 1). An active Fe2+ cycle has been previously reported in Lake Banyoles with concentrations of 8–10 μM in both the resurgence of groundwater (bottom spring) and water column of basin C-III, inflow velocity of 0.8 mmol total Fe per h, and concentrations of up to 8 mg total Fe per g of sediment (dw) (Garcia-Gil, 1990). Interestingly, we also observed substantial concentrations of nitrate in the bottom of the basin, coming from the groundwater, and high concentration in surface waters originated from the surrounding crop fields and farms (Supplementary Figure S2).
Table 1. Biogeochemical data for Lake Cisó and Banyoles basin C-III.
| Cisó ML | Cisó HL | C-III ML | C-III HL | |
|---|---|---|---|---|
| Depth (m) | 1.75 | 4.5 | 22.25 | 24 |
| Temperature (°C) | 12.9 | 10.6 | 12.6 | 12.6 |
| Conductivity (μS cm-1) | 2260 | 2268 | 2603 | 2604 |
| Eh (mV) | −30 | −86 | 195 | 145 |
| Oxygen (mg l−1) | 0.10 | 0 | 0.25 | 0 |
| H2S (μM) | 12.8 | 531.9 | 0.8 | 3.6 |
| Light (% incident) | 1% | <0.1% | 1% | <0.1% |
| TOC (mg l−1) | 5 | 3 | 1.5 | 3 |
| pH | 7.40 | 7.23 | 7.14 | 7.15 |
| TDP (μM) | 1.05 | 2.83 | 0.33 | 0.37 |
| NH4 (μM) | 44.39 | 50.99 | 25.04 | 37.52 |
| NO2 (μM) | 0.75 | b.d.l. | 0.21 | 0.00 |
| NO3 (μM) | 2.20 | 1.44 | 6.20 | 0.54 |
| Urea (μM) | 4.84 | 0.17 | 1.91 | 1.08 |
| Si (μM) | 185.0 | 168.1 | 144.8 | 114.5 |
| Chl a (μg l−1) | 1.7 | 22.5 | 1.1 | 0.8 |
| BChl a (μg l−1) | 2.4 | 123.7 | 1.1 | 1.6 |
| BChl c and d (μg l−1) | 5.4 | 39.3 | 0 | 0 |
| BChl e (μg l−1) | 0.8 | 13.6 | 25.8 | 40.6 |
Abbreviations: BChl, bacteriochlorophyll; b.d.l., below detection limits; Chla, chlorophyll a; HL, hypolimnion; Eh, redox potential; ML, metalimnion; TOC, total organic carbon; TDP, total dissolved phosphorus.
Taxonomic structure of the microbial communities
The overall taxonomic breakdown of the communities was assessed using the phylogenetic annotation of the metagenomic reads. The domain Bacteria numerically dominated the genetic composition of the microbial communities, both at the oxic–anoxic interfaces and at the anoxic hypolimnia (Table 2). More than 95% of all taxonomically assigned metagenomic reads matched bacteria, with a few representatives of archaea (range 0.7–3.5%), phages (0.8–4.0%) and eukaryotes (0.7–2.8%). Archaeal metagenomic reads were more abundant in the hypolimnion (2.67±1.21% of total reads) than in the metalimnion (1.09±0.47%). Most of the archaeal metagenomics reads matched methanogens within Euryarchaeota (c. 88%), with a few additional representatives within Thermococci, Thermoplasmata, Archaeoglobi and Haloarchaea (Supplementary Figure S3). The 16S rRNA gene in the metagenomics data set agreed with the broad taxonomic picture provided by the functional genes (Table 2), that is, 98–100% of the 16S rRNA gene affiliated to Bacteria, whereas Archaea were a minor component more abundant in the hypolimnion (1.4±0.7%) than in the metalimnion (0.2±0.2%).
Table 2. Total number of metagenomic reads (averaged c. 1 million per sample) for Lake Cisó and Banyoles basin C-III.
| Cisó ML | Cisó HL | C-III ML | C-III HL | |
|---|---|---|---|---|
| Total number of reads | 869 947 | 991 056 | 1 071 206 | 1 077 431 |
| Taxonomically assigned reads (%) | 46.7 | 53.5 | 54.2 | 62.4 |
| Bacteria (%) | 92.5 | 94.6 | 91.9 | 93.7 |
| Archaea (%) | 0.7 | 3.5 | 1.4 | 1.7 |
| Eukarya (%) | 2.8 | 1.1 | 2.7 | 0.7 |
| Viruses (%) | 4.0 | 0.8 | 4.0 | 3.9 |
| 16S rRNA genes in the metagenomic pool | 465 | 578 | 690 | 787 |
| Bacteria (%) | 99.6 | 97.9 | 100 | 99.4 |
| Archaea (%) | 0.4 | 2.1 | 0 | 0.6 |
| Functionally assigned metagenomic reads (%) | 25.8 | 27.5 | 30.5 | 32.6 |
| Reads of key genes in C, N and S cycles | 2392 | 3574 | 4773 | 5162 |
Abbreviations: HL, hypolimnion; ML, metalimnion.
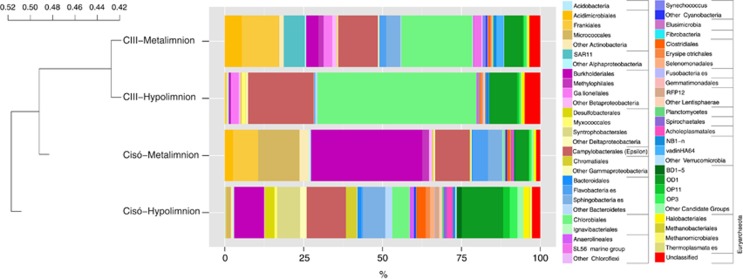
Interestingly, we observed higher proportion of functional reads affiliated to Crenarchaeota–Thaumarchaeota at the oxic–anoxic interface (12.3±0.4% of total archaeal reads) than at the anoxic and sulfurous bottom of the lakes (8.6±0.5%). Thaumarchaeota metagenomic reads putatively assigned to ammonia oxidizers were 0.03% of total reads but were not detected in the 16S rRNA pool. Conversely, ammonia-oxidizing bacteria (AOB, Nitrosomonadales- and Nitrosococcus-like) and nitrite-oxidizing bacteria (Nitrospirae-like) metagenomic reads were detected at 10 times higher concentration (0.3% of total reads). AOB were also detected in the 16S rRNA pool at similar concentrations (0.1% of total 16S rRNA gene). Overall, the most abundantly recovered 16S rRNA gene from the metagenomic data set matched Chlorobiales (green sulfur bacteria; 20%, range 5–50%), Campylobacterales (Epsilonproteobacteria; 14%, range 11–21%), Burkholderiales (Betaproteobacteria; 12%, range 0.5–35%), OD1 (8%, range 4–13%) and Frankiales (Actinobacteria; 5%, range 0.3–12%), among others (Figure 2, and Supplementary Table S2). These populations were differentially distributed between layers and lakes (Figure 2, and see details in Supplementary Table S2) and yielded a taxonomic clustering according to the redox potential, with samples with higher redox (>−30 mV) and lower sulfide concentrations (sulfide <13 μM) closer to each other than to the most euxinic sample (Lake Cisó hypolimnion, sulfide >500 μM, redox −86 mV; Table 1).
Figure 2.
Prokaryotic community structure (relative abundances at the Order level) of Lake Banyoles basin C-III and Lake Cisó obtained from the 16S rRNA gene present in the metagenomic pool. See detailed information in Supplementary Table S2. Hierarchical clustering based on Bray–Curtis dissimilarity matrices.
Functional structure of the microbial communities
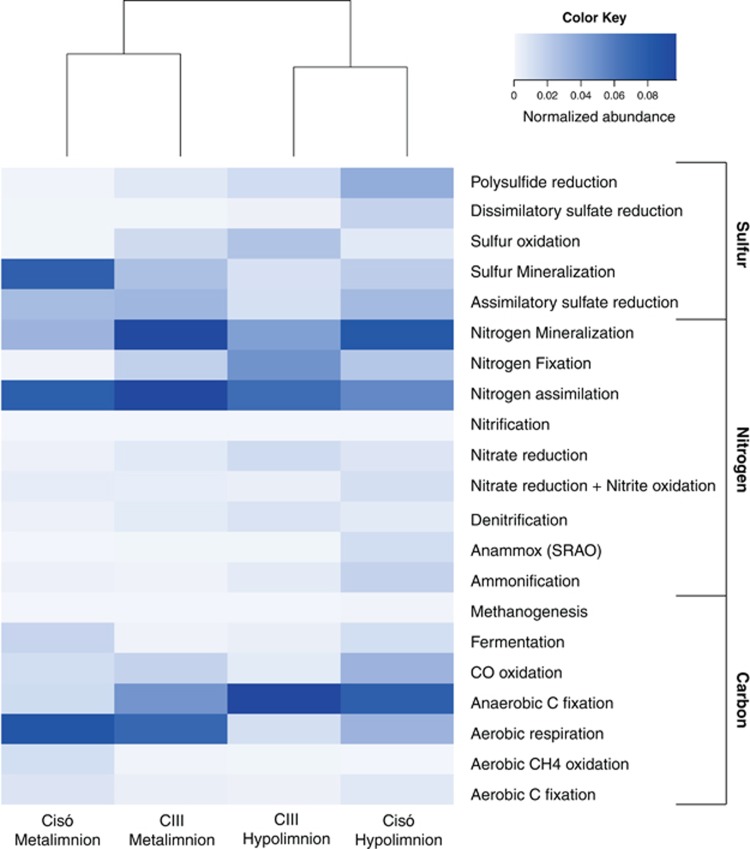
The metagenomic data set comprised four million reads of average length 377 bp and 54% of the metagenomic reads were taxonomically assigned based on the APIS or BLAST, whereas 22% could be assigned KO numbers and thus putative functions (e-value 10−5). From the identified KOs, we selected marker genes related to C, N and S cycling (Supplementary Table S1). Anaerobic C fixation, nitrogen fixation and assimilatory sulfate reduction genes accounted for a substantial percentage of annotated reads in the hypolimnia, whereas genes for aerobic respiration, nitrogen assimilation and sulfur mineralization were more abundant at the oxic–anoxic interfaces (Supplementary Table S3). Other less abundant metabolic pathways such as ammonification, anammox–SRAO (sulfate-reducing anaerobic ammonia oxidation; Rikmann et al., 2012) and dissimilatory sulfate reduction were detected, mostly in the hypolimnion of Lake Cisó. Such differences were globally captured by a functional-level (C, N and S pathways examined) hierarchical analysis that grouped the samples according to presence/absence of oxygen (Figure 3). This clustering analysis produced the same result using multiple other functional annotations, including KEGG (EC), Gene Ontology terms and MetaCyc. Similarly, repeating this analysis with all size fractions as separate libraries (data not shown) and housekeeping genes (Supplementary Figure S4) gave similar results, with redox being a more structuring factor than geographical distribution.
Figure 3.
Heatmap plot and functional clustering of the selected KEGG Orthologs for the predicted open reading frames (ORFs) from the metagenomic reads for Lake Cisó and Banyoles basin C-III.
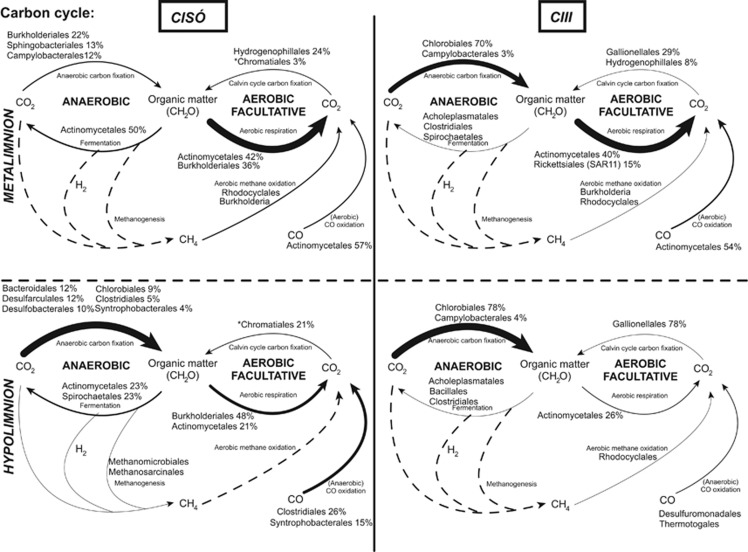
As Bacteria and Archaea accounted for most of total metagenomic reads, we focused on the prokaryotes for a comparative study of the geochemistry of carbon (Figure 4), nitrogen (Figure 5) and sulfur (Figure 6) along the redoxcline. We used the relative abundance of the detected functional genes as a proxy of the potential relevance of each pathway in situ without considering the role of microscopic algae. For the C cycling, the main pathway detected in the oxic–anoxic interface was aerobic respiration by heterotrophic Actinomycetales and Burkholderiales in Lake Cisó, and by Actinomycetales and Pelagibacterales (SAR11-like) in Lake Banyoles C-III. In the hypolimnion, the abundant pathways were various forms of anaerobic carbon fixation: by Chromatiales (anoxygenic phototrophy by the Calvin cycle), Bacteroidales (probably anaplerotic) and sulfate-reducing bacteria (SRB) (probably the reductive citric acid cycle/Arnon pathway; Fuchs, 2011) in Lake Cisó, and Chlorobiales (anoxygenic phototrophy by the Arnon cycle) in Banyoles C-III (Table 3). Chemolithotrophic aerobic carbon fixation via the Calvin cycle, which was rare, was mostly related to Betaproteobacteria of the genus Hydrogenophilales (Thiobacillus-like) and Gallionellales (Syderoxydans-like). Chemolithotrophic Epsilonproteobacteria with genes for the Arnon cycle (Campylobacterales on Figure 4) were found related to the genera Arcobacter, Sulfuricurvum and Sulfurimonas (Table 3). Carbon monoxide (CO) oxidation marker genes were also present (3–14% of those targeted marker genes selected for the carbon cycle, Supplementary Table S3) and related to heterotrophic bacteria. The potential for fermentation was mostly observed in Lake Cisó. Both methanogenesis and methane oxidation-specific marker genes had low abundances in all four environments, and even in those samples where such genes were not specifically detected (Figure 4, dotted lines) we found additional metagenomic reads taxonomically matching methanogens and methane oxidizers clades.
Figure 4.
Genetic potential for several steps of the carbon cycle in Lake Cisó and Banyoles basin C-III using a combination of normalized marker genes. Arrow size proportional to the potential flux of the carbon pathways (100% value, see Supplementary Table S3). Dotted lines: not detected marked genes but putative presence of the pathway (see main text). Relative abundances for the main microbes potentially driving each conversion step are shown (only for those that contributed >1% of the marker genes mixture). *Chromatiales: anoxygenic phototrophy through the Calvin cycle.
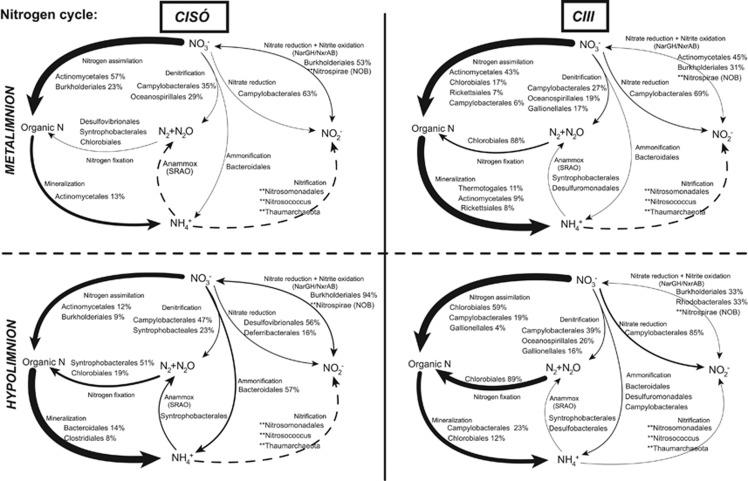
Figure 5.
Genetic potential for several steps of the nitrogen cycle in Lake Cisó and Banyoles basin C-III using a combination of normalized marker genes. Arrow size proportional to the potential flux of the nitrogen pathways (100% value, see Supplementary Table S3). Dotted lines: not detected marked genes but putative presence of the pathway (see main text). Relative abundances for the main microbes potentially driving each conversion step are shown (only for those that contributed >1% of the marker genes mixture). **Presence of AOA, AOB and nitrite-oxidizing bacteria (NOB) reads in the metagenomic pool.
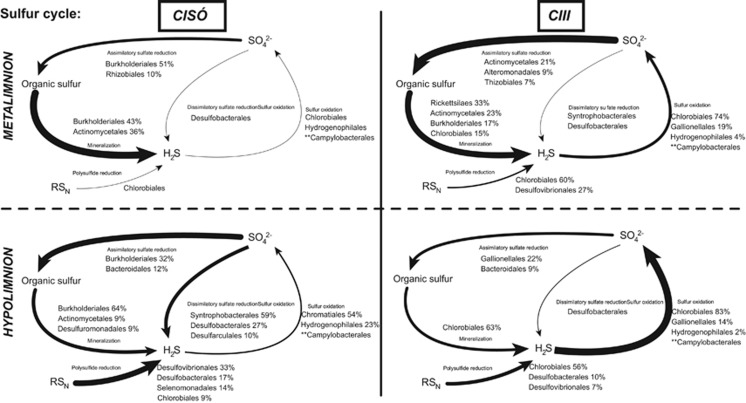
Figure 6.
Genetic potential for several steps of the sulfur cycle in Lake Cisó and Banyoles basin C-III using a combination of normalized marker genes. Arrow size proportional to the potential flux of the sulfur pathways (100% value, see Supplementary Table S3). Relative abundances for the main microbes potentially driving each conversion step are shown (only for those that contributed >1% of the marker genes mixture). **Campylobacteraceae contributed through sox genes not reported in KEGG database.
Table 3. Carbon fixation cycles and main metabolic traits of the C-fixing microorganisms found in this study.
| Taxa/phylogeny | C-fixation pathway | Traits | Main genera identified in Lakes Cisó and C-III from the 16S rRNA gene present in the metagenomic pool |
|---|---|---|---|
| Gallionellales Betaproteobacteria | Calvin | Facultative Chemolithoautotroph Energy sources: Fe(II)/sulfide Denitrification | Sideroxydans |
| Hydrogenophilales Betaproteobacteria | Calvin | Chemolithoautotroph Sulfide oxidation | |
| Campylobacterales Epsilonproteobacteria | Arnona | Chemolithoautotroph Denitrification Sulfide oxidation | Arcobacter, Sulfuricurvum, Sulfurimonas |
| Chromatiales Gammaproteobacteria | Calvin | Photolithoautotroph Anaerobic Tolerates oxygen | Lamprocystis |
| Chlorobiales Chlorobi | Arnona | Photolithoautotroph Anaerobic (strict) N fixation | Chlorobium luteolum |
| Desulfobacterales Deltaproteobacteria | Arnona Reductive acetyl-CoAb | Heterotroph Sulfate reducers | Desulfatiferula, Desulfobulbus, Desulfocapsa, Desulfosalsimonas |
| Syntrophobacterales Deltaproteobacteria | Arnona Reductive acetyl-CoA(?)b | Heterotroph Sulfate reducer/ sulfide oxidation SRAO | Desulfomonile |
| Desulfuromonadales Deltaproteobacteria | Reductive acetyl-CoAb | Nitrate dependent Fe(II) oxidation with production of ammonium (Weber et al., 2006) |
Abbreviation: SRAO, sulfate-reducing ammonium oxidation.
Also known as reverse Krebs cycle, reverse tricarboxylic acid cycle (rTCA) and reverse citric acid cycle.
Also known as Wood–Ljungdahl pathway.
For the nitrogen cycle, most of the detected marker genes catalyzed N assimilation and mineralization (Figure 5 and Supplementary Table S3). Denitrification was observed in low abundance in all the cases (c. 3% of the nitrogen functional reads selected), and the main taxa involved were Campylobacterales (autotrophic Sulfurimonas and Arcobacter), Oceanospirillales (heterotrophs) and Gallionellales (autotrophic Sideroxydans). Conversely, the potential for nitrogen fixation (nif genes) was observed in all the cases, although in higher abundance under euxinia (18±11%) than in the oxic–anoxic interfaces (4±4%). The nif genes were most related to Chlorobium in Lake Banyoles, whereas in Lake Cisó they were most similar to Syntrophobacterales. Under the most euxinic conditions, c. 6% of the total nitrogen marker genes examined were the anammox catalyzing enzyme hydrazine oxidoreductase, although these were associated with Syntrophobacterales instead of the planctomycetales found in oceanic anoxic zones. Both aerobic ammonia oxidation and nitrification marker genes had very low abundance, and were only properly detected in Lake Banyoles C-III hypolimnion (amoC gene 97% identical to Nitrosospira multiformis). However, metagenomic reads matching Thaumarchaeota (ammonia-oxidizing archaea (AOA)), Nitrosomonadales and Nitrosococcus (AOB) and Nitrospirae nitrite-oxidizing bacteria were detected in all lakes and water layers (Figure 5, dotted lines), pointing out that the genetic potential to close the nitrogen cycle was there, but at very low abundance as compared with other pathways in the cycle.
Finally, in the S cycle (Figure 6 and Supplementary Table S3) the highest percentage of the reads matched assimilatory sulfate reduction (28±9% of those targeted sulfur marker genes) and sulfur mineralization (35±25%), mostly driven by the predominant heterotrophic organisms found in each water layer (Actinomycetales and Burkholderiales). Most sulfide oxidation genes likely originated from Chlorobiales in Lake Banyoles C-III, and Chromatiales in Lake Cisó, with further contributions from chemolithotrophs Gallionellales, Hydrogenophilales and Campylobacterales. The potential for planktonic sulfate reduction was only observed in strong euxinia (Lake Cisó hypolimnion, 16% of targeted sulfur reads as compared with 1.4±1% in the remaining samples), with reads likely originating from Desulfobacterales and most probably Syntrophobacterales, although members of this group may carry out both reductive and oxidative parts of the sulfur cycle. Interestingly, we observed a high richness of sulfate-reducing bacteria genera (Table 3) with the potential to degrade a wide variety of carbon compounds to help to maintain the high sulfide concentrations found in Lake Cisó.
Although the metagenomic data set does not contain transcriptome or proteome data, and thus only indicates potential function, we observed a direct linear relationship between relative abundance of dissimilatory sulfate reduction genes and in situ sulfide concentrations (r=0.998, P=0.002). Although this comparison should be carefully interpreted because of the low number of samples compared, it suggests a close link between both the abundance of planktonic SRB and sulfide production. We also observed significant direct linear relationships between the relative abundance of anaerobic carbon fixation genes from bacterial chemotrophs and denitrification (r=0.958, P=0.042), suggesting a close link between chemoautotrophy and the nitrogen cycle.
Discussion
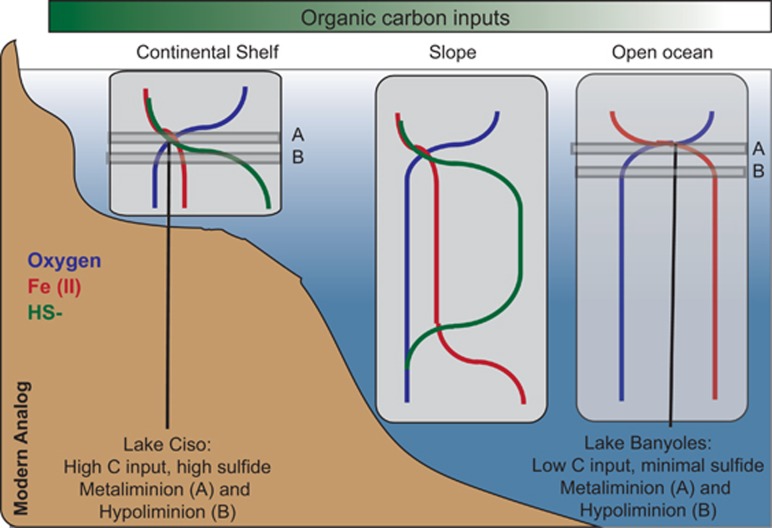
Stratified planktonic environments with sharp chemical gradients and sulfide-rich bottom waters are valuable current windows on past Earth conditions. Anoxic and euxinic conditions were common but spatially and temporally heterogeneous in ancient oceans during Proterozoic (Reinhard et al., 2013; Lyons et al., 2014), and may have played an important role in mass extinctions during Phanerozoic (Meyer and Kump, 2008). The presence of marker pigments for photosynthetic sulfur bacteria (that is, isorenieratene and okenone) have been often reported as evidence of euxinic conditions in ancient oceans (Damsté and Köster, 1998; Brocks et al., 2005). These conditions are not common nowadays, although persistent euxinia can be found in deep silled basins such as the Black Sea, Baltic Sea and Cariaco Basin (Millero, 1991; Stewart et al., 2007). Future climate change scenarios predict, however, an increasing of euxinia phenomena, mainly in coastal marine ecosystems (Diaz and Rosenberg, 2008). The study of stratified sulfurous lakes has, therefore, an additional interest to predict biogeochemical functioning and microbial interactions in such future scenarios. In the present study, we explored the community composition and functional gene content along a gradient of redox conditions in a karstic sulfurous area. Continental systems are cheaper and easier to sample than marine basins, and a large variety of photo- and chemolithotrophs organisms, sulfide-oxidizing and sulfate-reducing bacteria, fermenters, denitrifying microbes, methanogens and methane oxidizers are expected to be found, among others, according to previous studies (see, for example, Casamayor et al., 2000; Barberán and Casamayor, 2011). The metabolisms harbored by these microorganisms have the potential to provide insights into the ecosystems operating in euxinic early stages of Earth. The strong euxinic conditions found in Lake Cisó may match biogeochemistry in ancient coastal areas, whereas basin C-III in Lake Banyoles may represent the transition from euxinic coastal areas to merely anoxic and rich Fe conditions in the ancient open ocean (Figure 7).
Figure 7.
Lake Cisó and basin C-III of Lake Banyoles as modern analogs of anoxic conditions prevailing in the ancient ocean. The illustration shows the geochemical distributions of Fe, S, C and O2 in depth profiles and along different oceanic regimes (shelf, slope, open ocean) during Proterozoic (Lyons et al., 2014). Lake Cisó would be closer to coastal and continental shelf areas, whereas Banyoles C-III would be a more open ocean analogue.
The very low abundance of genes for nitrification, the minor presence of anammox genes, the high potential for nitrogen fixation and mineralization and the potential for chemotrophic CO2 fixation and CO oxidation all provide potential clues on the ancient oceanic anoxic zones functioning. The low abundance of ammonia oxidizers (AOA and AOB) agrees with the high ammonia accumulation in the anoxic bottom of the lakes, the lack of oxygen and presence of potentially toxic sulfide. We observed, however, a higher gene abundance of AOB relative to AOA in the metagenomic pool that may have a geochemical link related to the abundance of Fe in these environments. AOA have a highly copper-dependent system for ammonia oxidation and electron transport (Walker et al., 2010), completely different from the iron-dependent system present in AOB. The tradeoff in Fe- vs Cu-rich ammonia oxidation enzymatic systems would suggest that AOA evolved relatively recently (<550 million years ago) and that the Proterozoic oceans, which would have been Fe rich, would have been AOB dominated. Interestingly, the evolutionary dynamics of the amoA gene cladogenesis events visualized using lineage through time plots displays a different scenario for AOA and AOB, with AOB showing a more constant cladogenesis through the evolutionary time, whereas AOA experienced two fast diversification events separated by a long steady-state period (Fernàndez-Guerra and Casamayor, 2012).
The potential for nitrogen fixation and denitrification was detected in both autotroph and heterotroph microbial lineages, suggesting a diverse range of potential overlaps between carbon and nitrogen cycling in the ancient ocean, and an active nitrogen cycle in anoxic systems. Our results show a potential major contribution to nitrogen fixation by Chlorobiaceae under euxinic conditions. Chlorobiaceae were also the major contributors to carbon fixation in Banyoles C-III coupled to sulfide oxidation through the Arnon cycle. Therefore, the reported presence of Chlorobiaceae in the ancient ocean (Damsté and Köster, 1998; Brocks et al., 2005) would have been of major relevance not only for the carbon but also for the nitrogen cycling. Campylobacterales (Epsilonproteobacteria) accounted for a large percentage of the denitrification genes in the anaerobic layers of both lakes, but were taxonomically segregated (Arcobacter dominated in Cisó, Sulfurimonas was present in C-III). Both genera respire nitrate coupled to C fixation in the dark through the reverse tricarboxylic acid cycle (Labrenz et al., 2005; Burgin and Hamilton, 2007; Grote et al., 2012), being potentially able to couple denitrification to sulfur oxidation (Ghosh and Dam, 2009). The other important group involved in denitrification was the chemolithoautotrophic Gallionellales oxidizing sulfide or Fe2+ while respiring nitrate and producing NH4+ or N2. The presence of Gallionellales exclusively in C-III is probably because of their close relation with the iron cycle (Weber et al., 2006), and by the fact that an active Fe2+ cycle has been previously detected in Lake Banyoles (Garcia-Gil et al., 1990). The potential role of Gallionellales in ancient oceans with an active iron cycle is therefore of major interest.
The case of Bacteroidales also deserves to be mentioned. Bacteroidales have been typically considered aerobic or microaerophilic chemoorganoheterotrophic bacteria (Reichenbach, 2006), and have been recurrently detected in the Banyoles area (Casamayor et al., 2000; Casamayor et al., 2002; Casamayor et al., 2012) and in the marine realm (Fernández-Gómez et al., 2013). However, their role in anaerobic, sulfide-rich layers was not elucidated. Here, we assigned Bacteroidales as potentially catalyzing DNRA (dissimilatory nitrate reduction to ammonium), coupling the electron flow from organic matter to the reduction of nitrate. Thus, we would expect a potential gradient of distribution for anaerobic Bacteroidales in the ancient ocean being more abundant in the organic carbon- and sulfide-rich coastal zones (Figure 7) than in the anoxic and more oligotrophic open ocean. We also noticed the low abundance of key processes in the anaerobic carbon cycle such as CH4 cycling, probably because in the presence of high levels of sulfate, methanogens are generally poor competitors with sulfate reducers in stratified natural environments (Raskin et al., 1996). Sulfate reduction normally occurs in fully anoxic sediments by SRB (Holmer and Storkholm, 2001). However, as shown here, a water column with euxinic conditions and a high availability of organic carbon is also suitable for the growth of an important community of planktonic SRB.
Previous studies in Banyoles area measured unexpected high rates of dark carbon fixation at the oxic–anoxic interface and the hypolimnetic waters, accounting for 58% of total annual fixed carbon in Lake Cisó (Garcia-Cantizano et al., 2005). It was proposed that photosynthetic bacteria could be partly carrying out dark carbon incorporation in situ (Casamayor et al., 2008), and Thiobacilli may actively fix CO2 at certain depths (Casamayor, 2010). However, the ecological factors modulating the process and the microbial populations performing dark carbon fixation are still not well understood (Casamayor et al., 2012). In the present investigation, we detected the potential for chemotrophic CO2 fixation mainly through the reverse tricarboxylic acid cycle (K00174, K00175 and K00244 from KEGG Orthology) in Bacteroidales, Campylobacterales and Desulfarculales. In addition, other SRB such as Desulfobacterales may also participate through the anaerobic C1-pathway (Wood–Ljungdahl pathway, K00194 and K00197) yielding formate assimilation and CO2 fixation (Hugler et al., 2003; Sun et al., 2010; Fuchs, 2011). Interestingly, the diversity of taxa potentially participating in carbon fixation in the dark was larger in Lake Cisó than in C-III, in agreement with in situ measurements carried out in former investigations (Garcia-Cantizano et al., 2005; Casamayor, 2010). These findings would suggest an active carbon-fixing activity in ancient euxinic oceans beyond the euphotic zone that certainly deserves further investigations.
In addition, the oxidation of CO generates adenosine triphosphate and CO2 that may be further processed through one of the reductive CO2 fixation pathways to be used as C source (Ragsdale, 2004; King and Weber, 2007). Some studies indicate that organisms using CO as both energy and C source can be viewed as the extant survivors of early metabolic processes (Huber and Wächtershäuser, 1997). In the hypoxic layers we found that the heterotrophic group of Actinomycetales accounted for most of monooxygenase CO genes in agreement with their mixotrophic lifestyle (Schmidt and Conrad, 1993). More interestingly, in the anoxic depths of Lake Cisó we found that CO oxidation genes were mainly related to SRB from Deltaproteobacteria group (Geobacter and deltaproteobacterium NaphS2) and to Firmicutes (Carboxidothermus hydrogenoformans, Moorella thermoacetica and Clostridium spp.). This finding suggests that the fate of the reducing equivalents from CO oxidation in anaerobic conditions could be coupled to sulfate reduction (carried out by SRB) to produce sulfide, or to CO2 reduction to produce acetate (SRB and Firmicutes) (Roberts et al., 2004; King and Weber, 2007). To check whether CO oxidation could be coupled to CO2 reduction to yield acetate (Ragsdale, 2004; Roberts et al., 2004), we identified the phylogenetic affiliation of acetyl-CoA synthase genes (ACS, K14138), and found that Desulfobacterales and Firmicutes had the potential to use the Wood–Ljungdahl pathway to obtain energy and fix carbon from CO in the hypolimnion of Lake Cisó. However, although the CO-oxidizing genes were detected, we cannot assess their relevance in the lake or the ancient oceans because CO-oxidizing bacteria carry out a facultative mixotrophic metabolism (Gadkari et al., 1990).
Overall, the metagenomics approach unveiled the interrelationships between microbes and biogeochemical cycling in a comparative framework of two lakes that are modern analogs of ancient ocean conditions. These results may also help to develop new geochemical proxies to infer ancient ocean biology and chemistry. A major pitfall in our metagenomic approach is the reliance on the assumption that the genes come from a particular bacteria or archaea according to phylogentic annotation; lateral gene transfer would compromise the direct link of phylogeny to a metabolic pathway. In most of the cases we found the 16S rRNA gene counterpart present in the metagenomic data pool, giving additional support to such links. Obvious next steps include an experimental quantification of the energy and matter fluxes involved in each of the metabolic pathways to get a complete picture on the tight coupling between microbes and biogeochemical cycling in anoxic ecosystems.
Acknowledgments
JC Auguet, M Llirós, F Gich, FM Lauro and JM Gasol are acknowledged for field and lab assistance and ancillary data. We sincerely appreciate insightful comments and suggestions from anonymous reviewers and the editor. This research was funded by Grants GOS-LAKES CGL2009-08523-E and DARKNESS CGL2012-32747 to EOC from the Spanish Office of Science (MINECO), from financial support by the Beyster Family Fund of the San Diego Foundation and the Life Technologies Foundation to the J Craig Venter Institute, and the NASA Astrobiology Institute to CLD.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies this paper on The ISME Journal website (http://www.nature.com/ismej)
Supplementary Material
References
- Anbar AD. Oceans elements and evolution. Science. 2008;322:1481–1483. doi: 10.1126/science.1163100. [DOI] [PubMed] [Google Scholar]
- Barberán A, Casamayor EO. Euxinic freshwater hypolimnia promote bacterial endemicity in continental areas. Microb Ecol. 2011;61:465–472. doi: 10.1007/s00248-010-9775-6. [DOI] [PubMed] [Google Scholar]
- Bascompte J, Sole RV. Rethinking complexity - modeling spatiotemporal dynamics in ecology. Trends Ecol Evol. 1995;10:361–366. doi: 10.1016/s0169-5347(00)89134-x. [DOI] [PubMed] [Google Scholar]
- Borrego CM, Baneras L, Garcia-Gil J. Temporal variability of Chlorobium phaeobacteroides antenna pigments in a meromictic karstic lake. Aquat Microb Ecol. 1999;17:121–129. [Google Scholar]
- Brocks JJ, Love GD, Summons RE, Knoll AH, Logan GA, Bowden SA. Biomarker evidence for green and purple sulphur bacteria in a stratified Palaeoproterozoic sea. Nature. 2005;437:866–870. doi: 10.1038/nature04068. [DOI] [PubMed] [Google Scholar]
- Burgin AJ, Hamilton SK. Have we overemphasized the role of denitrification in aquatic ecosystems? A review of nitrate removal pathways. Front Ecol Environ. 2007;5:89–96. [Google Scholar]
- Campbell BJ, Cary SC. Abundance of reverse tricarboxylic acid cycle genes in free-living microorganisms at deep-sea hydrothermal vents. Appl Environ Microbiol. 2004;70:6282–6289. doi: 10.1128/AEM.70.10.6282-6289.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casamayor EO, Schafer H, Baneras L, Pedrós-Alió C, Muyzer G. Identification of and spatio-temporal differences between microbial assemblages from two neighboring sulfurous lakes: comparison by microscopy and denaturing gradient gel electrophoresis. Appl Environ Microbiol. 2000;66:499–508. doi: 10.1128/aem.66.2.499-508.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casamayor EO, Pedrós-Alió C, Muyzer G, Amann R. Microheterogeneity in 16S ribosomal DNA-defined bacterial populations from a stratified planktonic environment is related to temporal changes and to ecological adaptations. Appl Environ Microbiol. 2002;68:1706–1714. doi: 10.1128/AEM.68.4.1706-1714.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casamayor EO, Garcia-Cantizano J, Pedrós-Alió C. Carbon dioxide fixation in the dark by photosynthetic bacteria in sulfide-rich stratified lakes with oxic-anoxic interfaces. Limnol Oceanogr. 2008;53:1193–1203. [Google Scholar]
- Casamayor EO. Vertical distribution of planktonic autotrophic thiobacilli and dark CO2 fixation rates in lakes with oxygen-sulfide interfaces. Aquat Microb Ecol. 2010;59:217–228. [Google Scholar]
- Casamayor EO, Llirós M, Picazo A, Barberán A, Borrego CM, Camacho A. Contribution of deep dark fixation processes to overall CO2 incorporation and large vertical changes of microbial populations in stratified karstic lakes. Aquat Sci. 2012;74:61–75. [Google Scholar]
- Damsté JSS, Köster J. A euxinic southern North Atlantic Ocean during the Cenomanian/Turonian oceanic anoxic event. Earth Planet Sci Lett. 1998;158:165–173. [Google Scholar]
- Diaz RJ, Rosenberg R. Spreading dead zones and consequences for marine ecosystems. Science. 2008;321:926–929. doi: 10.1126/science.1156401. [DOI] [PubMed] [Google Scholar]
- Fernández-Gómez B, Richter M, Schüler M, Pinhassi J, Acinas SG, González JM, et al. Ecology of marine Bacteroidetes: a comparative genomics approach. ISME J. 2013;7:1026–1037. doi: 10.1038/ismej.2012.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernàndez-Guerra A, Casamayor EO. Habitat-associated phylogenetic community patterns of microbial ammonia oxidizers. PLoS One. 2012;7:e47330. doi: 10.1371/journal.pone.0047330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs G.2011Alternative pathways of carbon dioxide fixation: insights into the early evolution of lifeIn: Gottesman S, Harwood CS (eds)Annual Review of Microbiology vol. 65Annual Reviews: Palo Alto; p631. [DOI] [PubMed] [Google Scholar]
- Gadkari D, Schricker K, Acker G, Kroppenstedt RM, Meyer O. Streptomyces thermoautotrophicus sp. nov., a thermophilic CO- and H(2)-oxidizing obligate chemolithoautotroph. Appl Environ Microbiol. 1990;56:3727–3734. doi: 10.1128/aem.56.12.3727-3734.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Cantizano J, Casamayor EO, Gasol JM, Guerrero R, Pedrós-Alió C. Partitioning of CO2 incorporation among planktonic microbial guilds and estimation of in situ specific growth rates. Microb Ecol. 2005;50:230–241. doi: 10.1007/s00248-004-0144-9. [DOI] [PubMed] [Google Scholar]
- Garcia-Gil LJ.1990Phototrophic bacteria and iron cycle in Lake Banyoles. PhD thesis Autonomous University of Barcelona; (in Spanish). [Google Scholar]
- Garcia-Gil LJ, Salagenoher L, Esteva JV, Abellà CA. Distribution of iron in lake Banyoles in relation to the ecology of purple and green sulur bacteria. Hydrobiologia. 1990;192:259–270. [Google Scholar]
- Garcia-Gil LJ, Abellà CA.1992Population dynamics of phototrophic bacteria in three basins of Lake Banyoles (Spain)In: Ilmavirta V, Jones R (eds)The Dynamics and Use of Lacustrine Ecosystems Springer: The Netherlands; 87–94. [Google Scholar]
- Ghosh W, Dam B. Biochemistry and molecular biology of lithotrophic sulfur oxidation by taxonomically and ecologically diverse bacteria and archaea. FEMS Microbiol Rev. 2009;33:999–1043. doi: 10.1111/j.1574-6976.2009.00187.x. [DOI] [PubMed] [Google Scholar]
- Goll J, Rusch DB, Tanenbaum DM, Thiagarajan M, Li K, Methe BA, et al. METAREP: JCVI metagenomics reports—an open source tool for high-performance comparative metagenomics. Bioinformatics. 2010;26:2631–2632. doi: 10.1093/bioinformatics/btq455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grote J, Schott T, Bruckner CG, Glockner FO, Jost G, Teeling H, et al. Genome and physiology of a model Epsilonproteobacterium responsible for sulfide detoxification in marine oxygen depletion zones. Proc Natl Acad Sci USA. 2012;109:506–510. doi: 10.1073/pnas.1111262109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrero R, Montesinos E, Esteve I, Abellà C.1980Physiological adaptation and growth of purple and green sulfur bacteria in a meromictic lake (Vila) as compared to a holomictic lake (Siso)In: Dokulil M, Metz H, Jewson D (eds)Shallow Lakes Contributions to their Limnology Springer: The Netherlands; 161–171. [Google Scholar]
- Guerrero R, Montesinos E, Pedrós-Alió C, Esteve I, Mas J, Van Gemerden H, et al. Phototrophic sulfur bacteria in two Spanish lakes: vertical distribution and limiting factors. Limnol Oceanogr. 1985;30:919–931. [Google Scholar]
- Holmer M, Storkholm P. Sulphate reduction and sulphur cycling in lake sediments: a review. Freshw Biol. 2001;46:431–451. [Google Scholar]
- Huber C, Wächtershäuser G. Activated acetic acid by carbon fixation on (Fe,Ni)S under primordial conditions. Science. 1997;276:245–247. doi: 10.1126/science.276.5310.245. [DOI] [PubMed] [Google Scholar]
- Hugler M, Huber H, Stetter KO, Fuchs G. Autotrophic CO2 fixation pathways in archaea (Crenarchaeota) Arch Microbiol. 2003;179:160–173. doi: 10.1007/s00203-002-0512-5. [DOI] [PubMed] [Google Scholar]
- King GM, Weber CF. Distribution, diversity and ecology of aerobic CO-oxidizing bacteria. Nat Rev Microbiol. 2007;5:107–118. doi: 10.1038/nrmicro1595. [DOI] [PubMed] [Google Scholar]
- Labrenz M, Jost G, Pohl C, Beckmann S, Martens-Habbena W, Jurgens K. Impact of different in vitro electron donor/acceptor conditions on potential chemolithoautotrophic communities from marine pelagic redoxclines. Appl Environ Microbiol. 2005;71:6664–6672. doi: 10.1128/AEM.71.11.6664-6672.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauro FM, DeMaere MZ, Yau S, Brown MV, Ng C, Wilkins D, et al. An integrative study of a meromictic lake ecosystem in Antarctica. ISME J. 2011;5:879–895. doi: 10.1038/ismej.2010.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li WZ, Godzik A. Cd-hit: a fast program for clustering and comparing large sets of protein or nucleotide sequences. Bioinformatics. 2006;22:1658–1659. doi: 10.1093/bioinformatics/btl158. [DOI] [PubMed] [Google Scholar]
- Lyons TW, Reinhard CT, Planavsky NJ. The rise of oxygen in Earth's early ocean and atmosphere. Nature. 2014;506:307–315. doi: 10.1038/nature13068. [DOI] [PubMed] [Google Scholar]
- Meyer KM, Kump LR. Oceanic euxinia in Earth history: causes and consequences. Annu Rev Earth Planet Sci. 2008;36:251–288. [Google Scholar]
- Millero FJ. The oxidation of H2S in Framvaren fjord. Limnol Oceanogr. 1991;36:1006–1014. [Google Scholar]
- Pedrós-Alió C, Guerrero R. Microbial ecology in Lake Cisó. Adv Microb Ecol. 1993;13:155–209. [Google Scholar]
- Prosser JI. Ecosystem processes and interactions in a morass of diversity. FEMS Microbiol Ecol. 2012;81:507–519. doi: 10.1111/j.1574-6941.2012.01435.x. [DOI] [PubMed] [Google Scholar]
- R Develppment Core Team . R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing: Vienna, Austria; 2012. [Google Scholar]
- Ragsdale SW. Life with carbon monoxide. Crit Rev Biochem Mol Biol. 2004;39:165–195. doi: 10.1080/10409230490496577. [DOI] [PubMed] [Google Scholar]
- Raskin L, Rittmann BE, Stahl DA. Competition and coexistence of sulfate-reducing and methanogenic populations in anaerobic biofilms. Appl Environ Microbiol. 1996;62:3847–3857. doi: 10.1128/aem.62.10.3847-3857.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichenbach H.2006The order CytophagalesIn: Dworkin M, Falkow S, Rosenberg E, Schleifer K-H, Stackebrandt E (eds)Prokaryotes Springer: Berlin; 549–590. [Google Scholar]
- Reinhard CT, Planavsky NJ, Robbins LJ, Partin CA, Gill BC, Lalonde SV, et al. Proterozoic ocean redox and biogeochemical stasis. Proc Natl Acad Sci USA. 2013;110:5357–5362. doi: 10.1073/pnas.1208622110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rikmann E, Zekker I, Tomingas M, Tenno T, Menert A, Loorits L, et al. Sulfate-reducing anaerobic ammonium oxidation as a potential treatment method for high nitrogen-content wastewater. Biodegradation. 2012;23:509–524. doi: 10.1007/s10532-011-9529-2. [DOI] [PubMed] [Google Scholar]
- Roberts GP, Youn H, Kerby RL. CO-sensing mechanisms. Microbiol Mol Biol Rev. 2004;68:453–473. doi: 10.1128/MMBR.68.3.453-473.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt U, Conrad R. Hydrogen, carbon-monoxide, and methane dynamics in Lake Constance. Limnol Oceanogr. 1993;38:1214–1226. [Google Scholar]
- Severmann S, Anbar AD. Reconstructing paleoredox conditions through a multitracer approach: the key to the past is the present. Elements. 2009;5:359–364. [Google Scholar]
- Sharon I, Pati A, Markowitz VM, Pinter RY.2009A statistical framework for the functional analysis of metagenomesIn: Batzoglou S (ed)Research in Computational Molecular Biology, Proceedings Springer: Berlin, Heidelberg, Germany; 496–511. [Google Scholar]
- Stewart K, Kassakian S, Krynytzky M, DiJulio D, Murray J.2007Oxic, suboxic, and anoxic conditions in the Black SeaIn: Yanko-Hombach V, Gilbert A, Panin N, Dolukhanov P (eds)The Black Sea Flood Question: Changes in Coastline, Climate, and Human Settlement Springer: The Netherlands; 1–21. [Google Scholar]
- Sun H, Spring S, Lapidus A, Davenport K, Del Rio TG, Tice H, et al. Complete genome sequence of Desulfarculus baarsii type strain (2st14(T)) Stand Genomic Sci. 2010;3:276–284. doi: 10.4056/sigs.1243258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun SL, Chen J, Li WZ, Altintas I, Lin A, Peltier S, et al. Community cyberinfrastructure for Advanced Microbial Ecology Research and Analysis: the CAMERA resource. Nucleic Acids Res. 2011;39:D546–D551. doi: 10.1093/nar/gkq1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takai K, Campbell BJ, Cary SC, Suzuki M, Oida H, Nunoura T, et al. Enzymatic and genetic characterization of carbon and energy metabolisms by deep-sea hydrothermal chemolithoautotrophic isolates of Epsilonproteobacteria. Appl Environ Microbiol. 2005;71:7310–7320. doi: 10.1128/AEM.71.11.7310-7320.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanenbaum DM, Goll J, Murphy S, Kumar P, Zafar N, Thiagarajan M, et al. The JCVI standard operating procedure for annotating prokaryotic metagenomic shotgun sequencing data. Stand Genomic Sci. 2010;2:229–237. doi: 10.4056/sigs.651139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trüper HG, Schlegel HG. Sulphur metabolism in Thiorhodaceae.1. Quantitative measurements on growing cells of Chromatium Okenii. Anton Van Lee J M S. 1964;30:225–22. doi: 10.1007/BF02046728. [DOI] [PubMed] [Google Scholar]
- Van Gemerden H, Montesinos E, Mas J, Guerrero R. Diel cycle of metabolism of phototrophic purple sulfur bacteria in Lake Cisó (Spain) Limnol Oceanogr. 1985;30:932–943. [Google Scholar]
- Vila X, Abellà CA. Effects of light quality on the physiology and the ecology of planktonic green sulfur bacteria in lakes. Photosynthesis Res. 1994;41:53–65. doi: 10.1007/BF02184145. [DOI] [PubMed] [Google Scholar]
- Walker CB, de la Torre JR, Klotz MG, Urakawa H, Pinel N, et al. Nitrosopumilus maritimus genome reveals unique mechanisms for nitrification and autotrophy in globally distributed marine crenarchaea. Proc Natl Acad Sci USA. 2010;107:8818–8823. doi: 10.1073/pnas.0913533107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber KA, Achenbach LA, Coates JD. Microorganisms pumping iron: anaerobic microbial iron oxidation and reduction. Nat Rev Microbiol. 2006;4:752–764. doi: 10.1038/nrmicro1490. [DOI] [PubMed] [Google Scholar]
- Zeigler Allen L, Allen EE, Badger JH, McCrow JP, Paulsen IT, Elbourne LD, et al. Influence of nutrients and currents on the genomic composition of microbes across an upwelling mosaic. ISME J. 2012;6:1403–1414. doi: 10.1038/ismej.2011.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.