Abstract
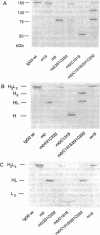
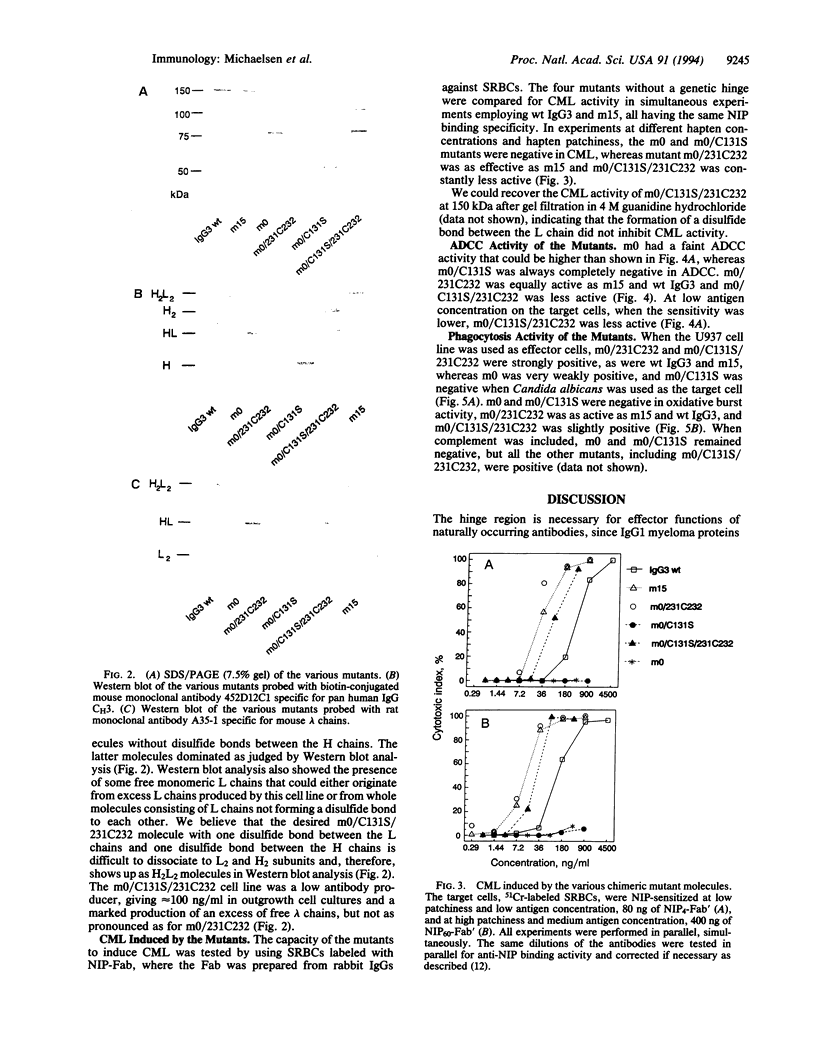
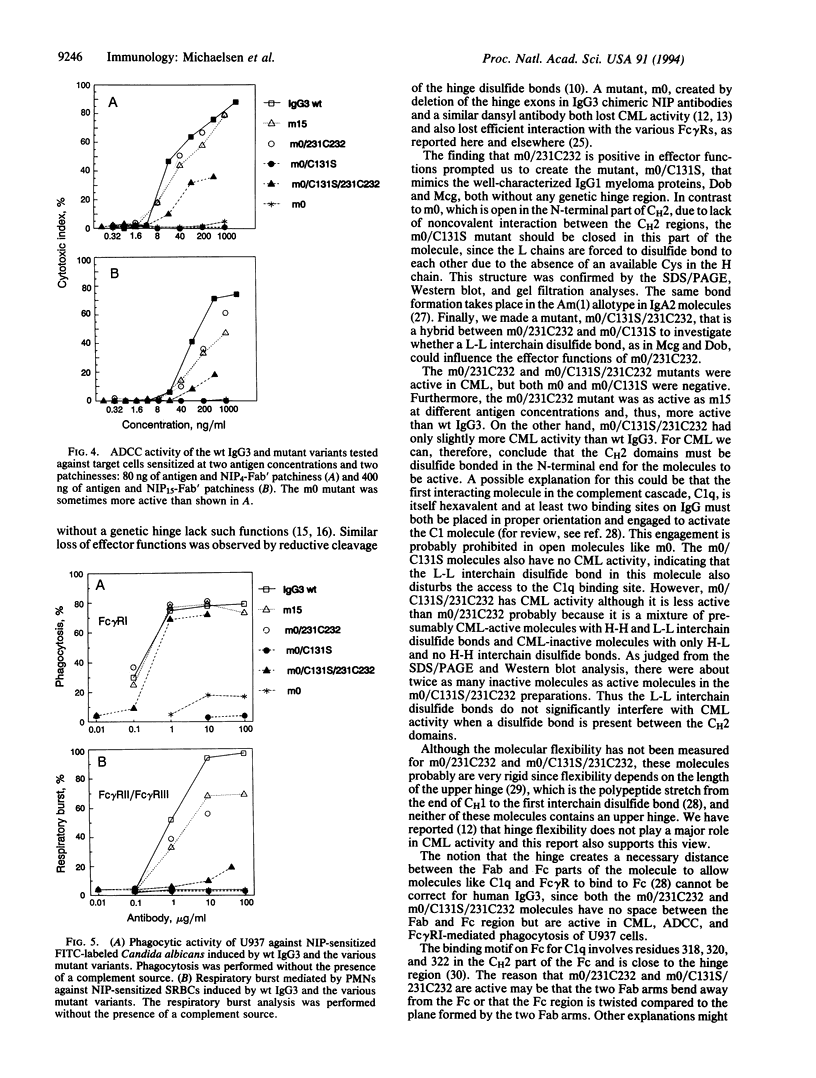
We have created four IgG3 mutants without a normal hinge region: (i) m0 without a genetic hinge; (ii) m0/C131S, where Cys-131 in m0 was mutated to Ser; (iii) m0/231C232 (formerly HM-1), where a Cys residue was inserted in m0 between Ala-231 and Pro-232; (iv) m0/C131S/231C232, which is a hybrid of m0/231C232 and m0/C131S. The wild-type IgG3 and all mutants bind 5-iodo-4-hydroxy-3-nitrophenacetyl groups. The wild type and mutants, m15 (with 15 aa in the hinge), m0/231C232, and m0/C131S/231C232, were all positive for complement-mediated lysis, antibody-dependent cellular cytotoxicity mediated by peripheral blood leukocytes, and phagocytosis by U937. m0/C131S/231C232 was only weakly positive and sometimes negative for respiratory burst activity mediated by peripheral blood neutrophils (polymorphonuclear leukocytes), whereas m15, m0/231C232, and wild-type IgG3 were strongly positive. The m0 and m0/C131S mutants were mainly negative for complement-mediated lysis, antibody-dependent cell-mediated cytotoxicity, and phagocytosis by U937 and polymorphonuclear leukocytes. The results indicate that a hinge spacer region is not necessary, but the correct alignment of the two second heavy chain constant regions in the IgG3 molecule by a minimum of one disulfide bond is necessary and sufficient for effector functions.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aase A., Michaelsen T. E. The use of a hapten-Fab conjugate to sensitize target cells for antibody-dependent complement-mediated lysis and antibody-dependent cell-mediated cytotoxicity. J Immunol Methods. 1991 Feb 15;136(2):185–191. doi: 10.1016/0022-1759(91)90005-z. [DOI] [PubMed] [Google Scholar]
- Aase A., Sandlie I., Norderhaug L., Brekke O. H., Michaelsen T. E. The extended hinge region of IgG3 is not required for high phagocytic capacity mediated by Fc gamma receptors, but the heavy chains must be disulfide bonded. Eur J Immunol. 1993 Jul;23(7):1546–1551. doi: 10.1002/eji.1830230723. [DOI] [PubMed] [Google Scholar]
- Brekke O. H., Bremnes B., Sandin R., Aase A., Michaelsen T. E., Sandlie I. Human IgG3 can adopt the disulfide bond pattern characteristic for IgG1 without resembling it in complement mediated cell lysis. Mol Immunol. 1993 Nov;30(16):1419–1425. doi: 10.1016/0161-5890(93)90103-i. [DOI] [PubMed] [Google Scholar]
- Brekke O. H., Michaelsen T. E., Sandin R., Sandlie I. Activation of complement by an IgG molecule without a genetic hinge. Nature. 1993 Jun 17;363(6430):628–630. doi: 10.1038/363628a0. [DOI] [PubMed] [Google Scholar]
- Burton D. R., Woof J. M. Human antibody effector function. Adv Immunol. 1992;51:1–84. doi: 10.1016/s0065-2776(08)60486-1. [DOI] [PubMed] [Google Scholar]
- Dangl J. L., Wensel T. G., Morrison S. L., Stryer L., Herzenberg L. A., Oi V. T. Segmental flexibility and complement fixation of genetically engineered chimeric human, rabbit and mouse antibodies. EMBO J. 1988 Jul;7(7):1989–1994. doi: 10.1002/j.1460-2075.1988.tb03037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan A. R., Winter G. The binding site for C1q on IgG. Nature. 1988 Apr 21;332(6166):738–740. doi: 10.1038/332738a0. [DOI] [PubMed] [Google Scholar]
- Duncan A. R., Woof J. M., Partridge L. J., Burton D. R., Winter G. Localization of the binding site for the human high-affinity Fc receptor on IgG. Nature. 1988 Apr 7;332(6164):563–564. doi: 10.1038/332563a0. [DOI] [PubMed] [Google Scholar]
- Guddat L. W., Herron J. N., Edmundson A. B. Three-dimensional structure of a human immunoglobulin with a hinge deletion. Proc Natl Acad Sci U S A. 1993 May 1;90(9):4271–4275. doi: 10.1073/pnas.90.9.4271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huck S., Fort P., Crawford D. H., Lefranc M. P., Lefranc G. Sequence of a human immunoglobulin gamma 3 heavy chain constant region gene: comparison with the other human C gamma genes. Nucleic Acids Res. 1986 Feb 25;14(4):1779–1789. doi: 10.1093/nar/14.4.1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferis R., Reimer C. B., Skvaril F., de Lange G., Ling N. R., Lowe J., Walker M. R., Phillips D. J., Aloisio C. H., Wells T. W. Evaluation of monoclonal antibodies having specificity for human IgG sub-classes: results of an IUIS/WHO collaborative study. Immunol Lett. 1985;10(3-4):223–252. doi: 10.1016/0165-2478(85)90082-3. [DOI] [PubMed] [Google Scholar]
- Johnson P. M., Michaelsen T. E., Scopes P. M. Conformation of the hinge region and various fragments of human IgG3. Scand J Immunol. 1975;4(2):113–119. doi: 10.1111/j.1365-3083.1975.tb02607.x. [DOI] [PubMed] [Google Scholar]
- Krawinkel U., Rabbitts T. H. Comparison of the hinge-coding segments in human immunoglobulin gamma heavy chain genes and the linkage of the gamma 2 and gamma 4 subclass genes. EMBO J. 1982;1(4):403–407. doi: 10.1002/j.1460-2075.1982.tb01182.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel T. A., Roberts J. D., Zakour R. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Methods Enzymol. 1987;154:367–382. doi: 10.1016/0076-6879(87)54085-x. [DOI] [PubMed] [Google Scholar]
- Michaelsen T. E., Aase A., Norderhaug L., Sandlie I. Antibody dependent cell-mediated cytotoxicity induced by chimeric mouse-human IgG subclasses and IgG3 antibodies with altered hinge region. Mol Immunol. 1992 Mar;29(3):319–326. doi: 10.1016/0161-5890(92)90018-s. [DOI] [PubMed] [Google Scholar]
- Michaelsen T. E., Aase A., Westby C., Sandlie I. Enhancement of complement activation and cytolysis of human IgG3 by deletion of hinge exons. Scand J Immunol. 1990 Nov;32(5):517–528. doi: 10.1111/j.1365-3083.1990.tb03192.x. [DOI] [PubMed] [Google Scholar]
- Michaelsen T. E. Alteration of the conformation of human IgG subclasses by reduction of the hinge S-S bonds. Mol Immunol. 1988 Jul;25(7):639–646. doi: 10.1016/0161-5890(88)90099-5. [DOI] [PubMed] [Google Scholar]
- Michaelsen T. E., Frangione B., Franklin E. C. Primary structure of the "hinge" region of human IgG3. Probable quadruplication of a 15-amino acid residue basic unit. J Biol Chem. 1977 Feb 10;252(3):883–889. [PubMed] [Google Scholar]
- Michaelsen T. E., Natvig J. B. Unusual molecular properties of human IgG3 proteins due to an extended hinge region. J Biol Chem. 1974 May 10;249(9):2778–2785. [PubMed] [Google Scholar]
- Neuberger M. S., Williams G. T., Mitchell E. B., Jouhal S. S., Flanagan J. G., Rabbitts T. H. A hapten-specific chimaeric IgE antibody with human physiological effector function. Nature. 1985 Mar 21;314(6008):268–270. doi: 10.1038/314268a0. [DOI] [PubMed] [Google Scholar]
- Norderhaug L., Brekke O. H., Bremnes B., Sandin R., Aase A., Michaelsen T. E., Sandlie I. Chimeric mouse human IgG3 antibodies with an IgG4-like hinge region induce complement-mediated lysis more efficiently than IgG3 with normal hinge. Eur J Immunol. 1991 Oct;21(10):2379–2384. doi: 10.1002/eji.1830211013. [DOI] [PubMed] [Google Scholar]
- Rajan S. S., Ely K. R., Abola E. E., Wood M. K., Colman P. M., Athay R. J., Edmundson A. B. Three-dimensional structure of the Mcg IgG1 immunoglobulin. Mol Immunol. 1983 Jul;20(7):787–799. doi: 10.1016/0161-5890(83)90057-3. [DOI] [PubMed] [Google Scholar]
- Rothe G., Oser A., Valet G. Dihydrorhodamine 123: a new flow cytometric indicator for respiratory burst activity in neutrophil granulocytes. Naturwissenschaften. 1988 Jul;75(7):354–355. doi: 10.1007/BF00368326. [DOI] [PubMed] [Google Scholar]
- Sandlie I., Aase A., Westby C., Michaelsen T. E. C1q binding to chimeric monoclonal IgG3 antibodies consisting of mouse variable regions and human constant regions with shortened hinge containing 15 to 47 amino acids. Eur J Immunol. 1989 Sep;19(9):1599–1603. doi: 10.1002/eji.1830190912. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjöberg B., Rosenqvist E., Michaelsen T., Pap S., Osterberg R. The solution shapes of IgG3 immunoglobulin and its Fch and Fc fragments. A small-angle X-ray scattering study. Biochim Biophys Acta. 1980 Sep 23;625(1):10–17. doi: 10.1016/0005-2795(80)90103-8. [DOI] [PubMed] [Google Scholar]
- Tan L. K., Shopes R. J., Oi V. T., Morrison S. L. Influence of the hinge region on complement activation, C1q binding, and segmental flexibility in chimeric human immunoglobulins. Proc Natl Acad Sci U S A. 1990 Jan;87(1):162–166. doi: 10.1073/pnas.87.1.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfenstein-Todel C., Frangione B., Franklin E. C. Partial amino acid sequence of an IgA2 human immunoglobulin heavy chain. Biochim Biophys Acta. 1975 Feb 27;379(2):627–637. doi: 10.1016/0005-2795(75)90169-5. [DOI] [PubMed] [Google Scholar]