Abstract
The association between sleep apnea and atrial fibrillation (AF) has not been examined in a multiethnic adult population in prospective community-based studies. We prospectively (2000–2011) investigated the associations of physician-diagnosed sleep apnea (PDSA), which is considered more severe sleep apnea, and self-reported habitual snoring without PDSA (HS), a surrogate for mild sleep apnea, with incident AF in white, black, and Hispanic participants in the Multi-Ethnic Study of Atherosclerosis (MESA) who were free of clinical cardiovascular disease at baseline (2000–2002). Cox proportional hazards models were used to assess the associations, with adjustment for socioeconomic status, traditional vascular disease risk factors, race/ethnicity, body mass index, diabetes, chronic kidney disease, alcohol intake, and lipid-lowering therapy. Out of 4,395 respondents to a sleep questionnaire administered in MESA, 181 reported PDSA, 1,086 reported HS, and 3,128 reported neither HS nor PDSA (unaffected). Over an average 8.5-year follow-up period, 212 AF events were identified. As compared with unaffected participants, PDSA was associated with incident AF in the multivariable analysis, but HS was not (PDSA: hazard ratio = 1.76, 95% confidence interval: 1.03, 3.02; HS: hazard ratio = 1.02, 95% confidence interval: 0.72, 1.44). PDSA, a marker of more severe sleep apnea, was associated with higher risk of incident AF in this analysis of MESA data.
Keywords: atrial fibrillation, longitudinal studies, sleep apnea, snoring
Atrial fibrillation (AF) is prevalent in the elderly population (1), and the occurrence of AF is related to many comorbid clinical conditions such as cigarette smoking, excessive alcohol consumption, hypertension, diabetes mellitus, obesity, chronic kidney disease, structural heart disease, heart failure (HF), coronary heart disease (CHD), inflammation, and sleep apnea (2–7).
Most of the current evidence for the association between sleep apnea and AF comes from patients with multiple comorbidities and established cardiovascular disease (CVD) in cross-sectional, retrospective, or hospital-based studies (8–12). Central sleep apnea is the most common sleep apnea subtype in persons with HF, and it is reported to be associated with an increased risk of AF, especially in elderly populations (13, 14). Across the population, obstructive sleep apnea (OSA) is the major form of sleep apnea, affecting about 15 million US adults (15). A previous observational study found that half of patients with OSA and AF had recurrent AF after electrical cardioversion (7). In addition, treatment of OSA reduces recurrent AF after pulmonary vein isolation (12). In a clinic-based study, some risk factors for incident AF were identified retrospectively in patients with OSA, such as obesity and nocturnal oxygen desaturation, in addition to CHD and HF (11).
To the best of our knowledge, there have been no prospective longitudinal community-based studies to date that have compared the incidence of AF in persons with and without sleep apnea and none which have had the ability to evaluate confounding by an extensive array of CVD risk factors and cardiovascular function. Furthermore, given the known racial/ethnic differences in AF burden (16), it is unclear whether sleep apnea may have similar associations with incident AF in diverse racial/ethnic groups. To further address the longitudinal associations between sleep apnea and incident AF in community-based samples, we prospectively assessed the associations of physician-diagnosed sleep apnea (PDSA), which is considered a marker of more severe sleep apnea, and self-reported habitual snoring without PDSA (HS), a surrogate for mild sleep apnea, with incident AF in a multiethnic adult population (17, 18).
In addition, we explicitly compared models using PDSA and HS to explore the consistency of the associations across racial/ethnic groups. Given prior research, we also explored differences by age and sex as well as by obesity.
METHODS
Study population and data collection
We used data from the Multi-Ethnic Study of Atherosclerosis (MESA), a longitudinal cohort study designed to investigate the prevalence, correlates, and progression of subclinical CVD in persons without clinical CVD at baseline. The study design for MESA has been described in detail elsewhere (17). The cohort includes 6,814 women and men aged 45–84 years recruited from 6 US communities (Baltimore, Maryland; Chicago, Illinois; Forsyth County, North Carolina; Los Angeles County, California; northern Manhattan, New York City; and St. Paul-Minneapolis, Minnesota). At baseline, the participants were 38% white, 28% black, 22% Hispanic, and 12% Chinese. This study was approved by the institutional review board at each study site, and written informed consent was obtained from all participants.
Demographic, medical history, anthropometric, and laboratory data for the present analysis were obtained from the first examination of the MESA cohort (July 2000–August 2002). Body mass index was calculated as weight (kg)/height (m)2. Smoking status was defined as current, former, or never smoking. Alcohol consumption was classified into current and noncurrent (never and former) drinking. Blood pressure was ascertained as the mean of the last 2 of 3 seated measurements. Diabetes mellitus was defined as fasting glucose concentration ≥126 mg/dL or the use of hypoglycemic medication. Chronic kidney disease was defined as an estimated glomerular filtration rate less than 60 mL/minute/1.73 m2 based on the Chronic Kidney Disease Epidemiology Collaboration equation (19). Total cholesterol and high-density lipoprotein cholesterol were measured from blood samples obtained after a 12-hour fast. Medication use was assessed by reviewing participants’ medication containers. Serum levels of interleukin-6 were measured as a marker of systemic inflammation. Levels of interleukin-6 were determined by ultrasensitive enzyme-linked immunosorbent assay (Quantikine HS Human Interleukin-6 Immunoassay; R&D Systems, Minneapolis, Minnesota).
Participants were evaluated at baseline with 12-lead electrocardiography and magnetic resonance imaging (MRI). Novacode 6.1 was used to define left ventricular hypertrophy, Novacode 7.1 for left atrial enlargement (LAE), Novacode 8.1 for right ventricular hypertrophy, and Novacode 9.1 for right atrial enlargement on 12-lead electrocardiography (20). In addition, left and right ventricular mass were measured in participants who agreed to undergo cardiac MRI at study entry. The MRI protocol and analysis methods have been previously described (21).
Collection of data on PDSA and self-reported snoring
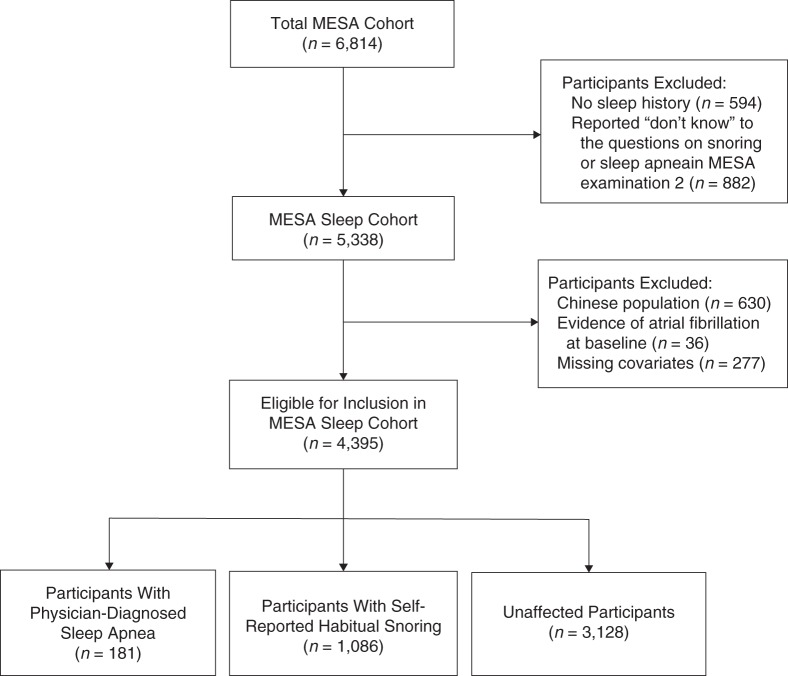
During the second MESA examination (September 2002–February 2004), a self-administered sleep history questionnaire was completed by participants. Among the questions included were 1) “Have you ever snored (now or at any time in the past)?” and (if yes) “How frequently and loudly do you snore?”; and 2) “Have you ever been told by a doctor that you had sleep apnea (a condition in which breathing stops briefly during sleep)?” Participants were provided with 3 potential responses to each question: “yes,” “no,” and “don't know.” Participants with HS were defined as those who did not report PDSA and reported snoring at least 3–5 days/week. Participants who did not report sleep apnea or habitual snoring were classified as “unaffected.” Among the 6,814 MESA participants, 594 did not participate in the sleep history study, and 882 responded “don't know” to either the sleep apnea question or the snoring questions and were excluded from this analysis, yielding a sample size of 5,338 (17). Of this sample, there were only 9 Chinese participants with PDSA (18). Therefore, the Chinese participants were excluded (n = 630). Non-Chinese participants found by the Centers for Medicare and Medicaid Services (through events ascertainment) to have AF prior to entry into MESA or evidence of AF at baseline electrocardiography (n = 36) and those with missing data on covariates (n = 277) were also excluded, resulting in 4,395 participants in the following analysis. Figure 1 shows the process used for selection of the eligible MESA sleep cohort.
Figure 1.
Selection of the overall cohort for the sleep study, Multi-Ethnic Study of Atherosclerosis (MESA), 2000–2011.
Ascertainment of incident AF events
Current self-reported AF was an exclusion criterion for recruitment in MESA, and participants underwent electrocardiography at baseline. Incident AF was identified from hospital discharge diagnosis codes for AF or atrial flutter (International Classification of Diseases, Ninth Revision, diagnosis codes 427.31 and 427.32) as ascertained by the MESA events detection protocol or from inpatient Medicare claims data. A validation substudy reviewing a random sample of 45 of 185 MESA participants with hospital discharge diagnosis codes for AF showed that AF was confirmed in 93% of the hospitalizations, implying a high positive predictive value for the diagnosis (22).
Statistical analysis
Demographic characteristics of participants in each of the 3 groups—PDSA, HS, and unaffected (neither sleep apnea nor snoring)—were compared using either analysis of variance or χ2 analysis. Results are reported as mean values with standard deviations for continuous variables and percentages for categorical variables.
Since PDSA and HS referred to conditions or symptoms that preceded MESA examination 2, follow-up started at MESA examination 1 and continued until the first occurrence of incident AF, death, loss to follow-up, or the end of follow-up (December 31, 2011). Kaplan-Meier analysis was used to assess the associations between the 3 groups and incident AF. Cox proportional hazards regression analyses were used to assess the multivariable association of each group with incident AF, adjusting for potential confounders.
In model 1, covariates included age, sex, race/ethnicity, study site, health insurance status, educational attainment, body mass index, diabetes, systolic blood pressure, total cholesterol, high-density lipoprotein cholesterol, chronic kidney disease, smoking status, alcohol intake status, and use of antihypertensive and lipid-lowering agents, which we defined as conventional risk factors. In model 2, covariates included log interleukin-6 and conventional risk factors. In model 3, covariates included electrocardiographic LAE and the model 2 covariates. In model 4, covariates included interim HF or CHD, which was defined as myocardial infarction or definite or probable angina pectoris (if followed by coronary artery bypass grafting or percutaneous coronary intervention) (17), and the covariates in model 3. These potential confounders or mediators were chosen on the basis of prior published associations with AF or sleep disorders.
We conducted another stepwise multivariable analysis in participants who had available data on MRI-measured left and right ventricular mass, as a sensitivity analysis for the overall cohort. Stratified analyses exploring the association between PDSA and AF within racial/ethnic, age, sex, and body mass index groups, as well as formal testing for multiplicative interactions, were performed. In addition, mediation analyses were conducted to evaluate the potential mediating effects of log interleukin-6 and electrocardiographic LAE on the associations in the overall cohort (23). A 2-tailed P value less than 0.05 was considered significant. All statistical analyses were performed using SAS, version 9.4 (SAS Institute, Inc., Cary, North Carolina).
RESULTS
In the overall cohort, 181 participants (4.1%) reported PDSA, 1,086 (24.7%) reported HS, and 3,128 (71.2%) reported neither PDSA nor HS. As compared with unaffected participants and those with HS, participants with PDSA were more obese, were more likely to be male, and had higher proportions of chronic kidney disease and use of medication for hypertension and dyslipidemia. In addition, those with PDSA had higher levels of interleukin-6, higher proportions of electrocardiographic left ventricular hypertrophy and LAE, and greater left and right ventricular mass as measured by cardiac MRI at baseline (Table 1).
Table 1.
Demographic Characteristics of Participants With Physician-Diagnosed Sleep Apnea, Participants With Self-Reported Habitual Snoring, and Unaffected Participants in the Multi-Ethnic Study of Atherosclerosis, 2000–2011
| Variable | Sleep Apnea (n = 181) |
Habitual Snoring (n = 1,086) |
Unaffected (n = 3,128) |
P Value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. | % | Mean (SD) | No. | % | Mean (SD) | No. | % | Mean (SD) | ||
| Age, years | 59.9 (9.4) | 59.3 (9.3) | 62.1 (10.2) | <0.0001 | ||||||
| Female sex | 61 | 33.7 | 454 | 41.8 | 1,712 | 54.7 | <0.0001 | |||
| Race/ethnicity | ||||||||||
| White | 87 | 48.1 | 435 | 40.1 | 1,471 | 47.0 | <0.0001 | |||
| Black | 61 | 33.7 | 282 | 26.0 | 948 | 30.3 | ||||
| Hispanic | 33 | 18.2 | 369 | 34.0 | 709 | 22.7 | ||||
| Body mass indexa | 32.4 (6.3) | 30.3 (5.5) | 28.4 (5.0) | <0.0001 | ||||||
| Diabetes mellitus | 22 | 12.2 | 144 | 13.3 | 339 | 10.8 | 0.09 | |||
| Systolic blood pressure, mm Hg | 126 (19) | 126 (21) | 126 (21) | 0.96 | ||||||
| Diastolic blood pressure, mm Hg | 73 (10) | 73 (10) | 71 (10) | <0.0001 | ||||||
| Total cholesterol, mg/dL | 186.6 (31.5) | 195.5 (35.7) | 194.4 (35.9) | 0.0082 | ||||||
| HDL cholesterol, mg/dL | 47.4 (12.5) | 48.3 (12.9) | 51.9 (15.3) | <0.0001 | ||||||
| Triglycerides, mg/dL | 132.0 (75.1) | 140.1 (93.9) | 126.0 (80.0) | <0.0001 | ||||||
| Chronic renal disease | 18 | 9.9 | 75 | 6.9 | 292 | 9.3 | 0.043 | |||
| Cigarette smoking status | ||||||||||
| Never smoker | 71 | 39.2 | 462 | 42.5 | 1,511 | 48.3 | 0.001 | |||
| Former smoker | 77 | 42.5 | 459 | 42.3 | 1,233 | 39.4 | ||||
| Current smoker | 33 | 18.2 | 165 | 15.2 | 384 | 12.3 | ||||
| Current alcohol drinking | 65.2 | 61.8 | 60.3 | 0.33 | ||||||
| Use of antihypertensive agents | 91 | 50.3 | 385 | 35.5 | 1,124 | 35.9 | 0.0004 | |||
| Use of lipid-lowering agents | 43 | 23.8 | 166 | 15.3 | 502 | 16.1 | 0.015 | |||
| Interleukin-6, pg/mL | 1.8 (1.2) | 1.6 (1.1) | 1.5 (1.2) | 0.013 | ||||||
| Electrocardiography | ||||||||||
| LVH by Novacode 6.1 | 15 | 8.3 | 65 | 6.0 | 153 | 4.9 | 0.072 | |||
| LAE by Novacode 7.1 | 41 | 22.7 | 146 | 13.4 | 441 | 14.1 | 0.0040 | |||
| RVH by Novacode 8.1 | 1 | 0.55 | 6 | 0.55 | 17 | 0.54 | >0.99 | |||
| RAE by Novacode 9.1 | 0 | 0 | 3 | 0.28 | 3 | 0.10 | 0.34 | |||
| MRI findingsb | ||||||||||
| Left ventricular mass, g | 170.9 (43.5) | 157.0 (39.1) | 146.3 (38.4) | <0.0001 | ||||||
| Right ventricular mass, g | 23.3 (4.5) | 22.4 (4.5) | 21.3 (4.4) | <0.0001 | ||||||
Abbreviations: HDL, high-density lipoprotein; LAE, left atrial enlargement; LVH, left ventricular hypertrophy; MRI, magnetic resonance imaging; RAE, right atrial enlargement; RVH, right ventricular hypertrophy; SD, standard deviation.
a Weight (kg)/height (m)2.
b In the MRI subcohort (n = 2,770).
Over an average follow-up period of 8.5 years, 212 AF events were identified in the overall cohort, including 151 in unaffected participants, 45 in participants with HS, and 16 in participants with PDSA. The observation periods were 25,102, 8,778, and 1,405 person-years for unaffected participants, participants with HS, and participants with PDSA, respectively. Figure 2 displays results of the Kaplan-Meier analysis, showing that participants with PDSA had a lower event-free survival rate (without incident AF events) than either unaffected participants or participants with HS (log-rank P = 0.02). AF event rates were 6.02, 5.13, and 11.39 per 1,000 person-years for unaffected participants, participants with HS, and participants with PDSA, respectively. There were no significant differences between the event-free survival rates of participants with HS and unaffected participants in MESA (log-rank P = 0.53).
Figure 2.

Kaplan-Meier curves for event-free survival (no incident atrial fibrillation events) among all participants with physician-diagnosed sleep apnea (short-dashed line), self-identified habitual snorers (long-dashed line), and unaffected participants (solid line), Multi-Ethnic Study of Atherosclerosis, 2000–2011.
Comparison of the risks of incident AF between participants with HS and unaffected participants in the overall cohort
As shown in Table 2, participants with HS did not have a significantly higher risk of incident AF as compared with unaffected participants in the multivariable analysis adjusting for the covariates in model 1 (hazard ratio (HR) = 1.02). The association was not significant in other models either.
Table 2.
Associations of Physician-Diagnosed Sleep Apnea, Self-Reported Habitual Snoring, and Other Potential Confounders or Mediators With Incident Atrial Fibrillation in the Overall Sleep Cohort (Multivariate Models), Multi-Ethnic Study of Atherosclerosis, 2000–2011
| Variable | Model 1a |
Model 2b |
Model 3c |
Model 4d |
||||
|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | HR | 95% CI | HR | 95% CI | HR | 95% CI | |
| Age, years | 1.09e | 1.07, 1.11 | 1.08e | 1.06, 1.10 | 1.08e | 1.06, 1.10 | 1.08e | 1.06, 1.10 |
| Male sex | 2.03e | 1.44, 2.93 | 2.11e | 1.50, 2.98 | 2.12e | 1.50, 2.98 | 2.02e | 1.43, 2.86 |
| Black race/ethnicity | 0.47e | 0.32, 0.69 | 0.47e | 0.32, 0.68 | 0.46e | 0.32, 0.67 | 0.46e | 0.32, 0.68 |
| Hispanic race/ethnicity | 0.55e | 0.34, 0.88 | 0.53e | 0.33, 0.86 | 0.53e | 0.33, 0.86 | 0.55e | 0.34, 0.89 |
| Body mass indexf | 1.03 | 1.00, 1.06 | 1.02 | 0.99, 1.05 | 1.01 | 0.98, 1.05 | 1.01 | 0.98, 1.04 |
| Systolic blood pressure, mm Hg | 1.01 | 1.00, 1.01 | 1.01 | 1.00, 1.01 | 1.01 | 1.00, 1.01 | 1.01 | 1.00, 1.01 |
| Use of antihypertensive agents | 1.60e | 1.20, 2.16 | 1.59e | 1.18, 2.13 | 1.58e | 1.18, 2.12 | 1.55e | 1.15, 2.08 |
| Use of lipid-lowering agents | 0.63e | 0.44, 0.93 | 0.65e | 0.45, 0.95 | 0.65e | 0.45, 0.96 | 0.65e | 0.44, 0.95 |
| Chronic kidney disease | 1.47e | 1.03, 2.11 | 1.46e | 1.02, 2.09 | 1.48e | 1.03, 2.12 | 1.43e | 1.00, 2.05 |
| Log interleukin-6, pg/mL | 1.44e | 1.14, 1.82 | 1.44e | 1.14, 1.82 | 1.41e | 1.12, 1.79 | ||
| Electrocardiographic LAE | 1.32 | 0.94, 1.85 | 1.33 | 0.95, 1.86 | ||||
| Interim CHD or heart failure | 3.04e | 2.04, 4.53 | ||||||
| Habitual snoring | 1.02 | 0.72, 1.44 | 1.02 | 0.72, 1.44 | 1.02 | 0.72, 1.45 | 1.08 | 0.76, 1.54 |
| Sleep apnea | 1.76e | 1.03, 3.02 | 1.74e | 1.01, 2.99 | 1.67 | 0.97, 2.88 | 1.52 | 0.88, 2.63 |
Abbreviations: CHD, coronary heart disease; CI, confidence interval; HR, hazard ratio; LAE, left atrial enlargement.
a Adjusted for age, sex, race/ethnicity, study site, health insurance status, educational level, body mass index, diabetes, systolic blood pressure, total and high-density lipoprotein cholesterol, chronic kidney disease, smoking status, alcohol intake status, use of antihypertensive agents, and use of lipid-lowering agents.
b Additionally adjusted for log interleukin-6.
c Additionally adjusted for electrocardiographic LAE.
d Additionally adjusted for interim CHD or heart failure events.
e P < 0.05.
f Weight (kg)/height (m)2.
Comparison of the risks of incident AF between participants with PDSA and unaffected participants in the overall cohort
Participants with PDSA had a higher risk of incident AF independently of conventional CVD risk factors as compared with unaffected participants in the multivariable analysis (model 1) (HR = 1.76). The association was similar in the multivariable analysis sequentially adjusting for log interleukin-6 (model 2) (HR = 1.74). After additional adjustment for electrocardiographic LAE (model 3) and interim CHD or HF (model 4), the association between PDSA and incident AF was further attenuated and became nonsignificant (HR = 1.67 and HR = 1.52, respectively) (Table 2).
Among the covariates, older age, male sex, antihypertensive therapy, chronic kidney disease, higher log interleukin-6 levels at baseline, and incident CHD or HF were associated with higher risk of AF, whereas black and Hispanic race/ethnicity and lipid-lowering therapy were associated with lower risk of AF.
Comparison of the risks of incident AF between participants with PDSA and unaffected participants in subgroup analyses by race/ethnicity, age, sex, and body mass index in the overall cohort
Table 3 shows the results of a priori subgroup analyses adjusting for the covariates in model 1. Among whites and blacks, the point estimate for risk of incident AF was approximately 2-fold higher in persons with PDSA than in unaffected participants. In contrast, among Hispanics, the risk of incident AF was similar in participants with PDSA and unaffected participants. Irrespective of the obvious difference between racial/ethnic groups in the association of PDSA with incident AF, a significant interaction between racial/ethnic groups was not observed (P = 0.97).
Table 3.
Association of Physician-Diagnosed Sleep Apnea With Incident Atrial Fibrillation in the Overall Sleep Cohort, According to Model 1 Covariates (Stratified Multivariable Cox Proportional Hazards Regression Model), Multi-Ethnic Study of Atherosclerosis, 2000–2011
| Variable | Hazard Ratioa |
95% Confidence Interval |
No. of AF Events |
P Value |
P for Interaction |
|---|---|---|---|---|---|
| Race/ethnicity | |||||
| White | 1.85 | 0.97, 3.51 | 132 | 0.06 | 0.97 |
| Black | 2.25 | 0.65, 7.98 | 43 | 0.20 | |
| Hispanic | 0.71 | 0.09, 5.91 | 37 | 0.76 | |
| Age, years | |||||
| ≥65 | 1.27 | 0.63, 2.57 | 159 | 0.51 | 0.11 |
| <65 | 3.20 | 1.32, 7.74 | 53 | 0.01 | |
| Sex | |||||
| Female | 3.97 | 1.48, 10.65 | 71 | 0.006 | 0.08 |
| Male | 1.48 | 0.77, 2.85 | 141 | 0.24 | |
| Body mass indexb | |||||
| ≥30 | 2.73 | 1.33, 5.60 | 73 | 0.006 | 0.15 |
| <30 | 1.04 | 0.41, 2.59 | 139 | 0.94 |
Abbreviation: AF, atrial fibrillation.
a All subgroup analyses adjusted for age, sex, race/ethnicity, study site, health insurance status, educational level, smoking status, alcohol intake status, body mass index, diabetes, systolic blood pressure, total and high-density lipoprotein cholesterol, chronic kidney disease, use of antihypertensive agents, and use of lipid-lowering agents. Unaffected participants were the reference group.
b Weight (kg)/height (m)2.
There was a tendency for the association of PDSA with incident AF to vary somewhat by age, sex, and body mass index, but the interaction P values were not significant. PDSA was significantly associated with a higher risk of AF in participants under age 65 years but not in elderly participants; in women but not in men; and in obese participants (body mass index ≥30) but not in nonobese participants. The results of subgroup analyses were similar in the multivariable analysis adjusting for all covariates in model 4 (data not shown).
Comparison of the risks of incident AF between participants with PDSA and unaffected participants in multivariable models and subgroup analyses in the MRI subcohort
There were 2,770 participants and 122 incident AF events identified in the subcohort that underwent cardiac MRI examination. Web Table 1 (available at http://aje.oxfordjournals.org/) shows that PDSA was significantly associated with incident AF in the MRI subcohort in the multivariable analysis adjusting for the covariates in model 1. The association remained significant in models 2–4. In addition, Web Table 2 shows that the results in the subgroup analyses adjusting for the covariates in model 1 in the MRI subcohort were similar to those in the overall cohort.
Evaluation of the potential mediating effects of interleukin-6 and electrocardiographic LAE on the associations of HS and PDSA with incident AF in the overall cohort
Using the “%MEDIATE” macro (23) in model 2, log interleukin-6 was not intermediate to either HS, as it did not attenuate the estimated association for HS, or PDSA (percentage of treatment effect: −34.29% (P = 0.92) for HS and 2.38% (P = 0.29) for PDSA) with regard to incident AF. In model 3, electrocardiographic LAE was intermediate to PDSA but, again, not to HS (percentage of treatment effect: 7.47% (P = 0.0085) and −12.46% (P = 0.90) for HS and PDSA, respectively) with regard to incident AF (data not shown).
DISCUSSION
We assessed the associations of PDSA and HS with incident AF among white, black, and Hispanic participants in MESA, a prospective community-based study. In the overall cohort and in multivariable models adjusting for conventional vascular risk factors and a marker of systemic inflammation (interleukin-6) during an average 8.5-year follow-up period, we found that participants with PDSA had a 1.74-fold higher risk of incident AF than unaffected persons. Notably, the magnitude of association was attenuated somewhat and became nonsignificant after additional adjustment for electrocardiographic LAE and interim CHD or HF. This means that left atrial remodeling and interim CHD or HF may be potential mediators between more severe sleep apnea and AF.
Habitual snoring without sleep apnea has been reported to be unassociated with increased risk of incident CVD in some studies, including MESA (17, 24). Although Sands et al. (25) observed a significant association in postmenopausal women, the risk of CVD was relatively small (approximately 1.12). In the present study, once we distinguished persons likely to have more severe sleep apnea—that is, those with PDSA—from the larger group reporting habitual snoring, we found that habitual snoring was not associated with higher risk of incident AF.
In the Sleep Disorders in Older Men Study, comprising 2,911 elderly (age ≥65 years) men (14), AF was identified cross-sectionally using overnight electrocardiography from a sleep study. Odds ratios for AF were 1.58 and 2.15 times higher in participants with respiratory disturbance indices of 12–23.8 and ≥23.9, respectively, than in those with an index less than 5.9. In addition, Gami et al. (11) retrospectively analyzed a cohort of 3,542 sleep-disordered patients in a clinic-based study, 74% of whom were diagnosed with OSA. They showed that patients with OSA had a 2.18 times' higher risk of incident AF than those without OSA after an average follow-up period of 4.7 years. A case-crossover analysis of overnight sleep studies showed that individual apneas and hypopneas served as triggers of paroxysmal arrhythmias (26). The results of these cohort studies, although limited by either a cross-sectional design or a limited ability to adjust for multiple CVD markers, accord with our findings in a group free of CVD at baseline.
In MESA, we observed an AF burden that was 2-fold higher in whites than in blacks and Hispanics, which is consistent with previous epidemiologic studies of the general US population (27). As compared with unaffected participants, the risk of incident AF was 1.9 times higher in white participants with PDSA. In the Sleep Disorders in Older Men Study and the Sleep Heart Health Study, most of the participants were white, and thus the findings of those studies are consistent with ours for whites (15, 28). In addition, the risk of incident AF was 2.3 times higher in black participants with PDSA. However, the risk of incident AF was not increased in Hispanics with PDSA. The results of some studies have implied that the roles of stress and medications in triggering AF may differ among racial/ethnic groups (16, 29). Since the sample size of our participants with PDSA was small, further research is needed to understand ethnic and racial differences in sleep apnea–related AF susceptibility.
Sleep apnea has been associated with intermittent hypoxemia or hypercapnia, metabolic abnormalities, inflammation, poor control of hypertension, and left atrial and ventricular remodeling, which may lead to an increased risk of CVD and AF (30–32). Recent evidence has suggested that the severity of OSA is correlated with left atrium size and dysfunction and that continuous positive airway pressure therapy could improve left atrial remodeling, which may reduce the occurrence of AF (33, 34). This evidence is consistent with our findings that left atrial abnormality may be a mediator of the association of more severe sleep apnea (PDSA) with incident AF. In addition, our findings suggest that interim CHD or HF may be a mediator between PDSA and AF.
In subgroup analyses, there was a tendency for the association of PDSA with incident AF to be stronger in middle-aged or obese persons and in women. Prior research in the Sleep Heart Health Study also showed stronger associations in middle-aged persons compared with younger persons (28). Furthermore, in the Sleep Disorders in Older Men Study cohort, a cohort of older men, indices of sleep apnea severity were associated with AF, albeit less strongly than in the Sleep Heart Health Study cohort. Gami et al. have postulated that the presence of multiple cardiovascular risk factors and cardiac dysfunction in older people may attenuate the association of sleep apnea with the occurrence of AF (11). However, it is also possible that differences in autonomic nervous system responses to sleep apnea in older persons as compared with younger persons also may influence sleep apnea–related arrhythmia. This finding is also consistent with overall stronger associations of sleep apnea with mortality and CHD (35, 36).
With regard to the influence of obesity, it is possible that underlying obesity, which is associated with insulin resistance and inflammation, may be related to sleep apnea–associated stresses that contribute to AF (37). Iwasaki et al. (38) found the mechanism of forced inspiration-induced left atrial distension in an animal model to be a critical component of the occurrence of AF during OSA episodes in obese patients.
With regard to the sex difference, the prevalence of clinically relevant OSA in the general population is only 6% in women as compared with 14% in men (39). Differences in craniofacial morphology and function, body-fat distribution, and sex-hormonal influences may play a role in the sex-specific pathogenesis of PDSA (40, 41). Women are usually diagnosed with sleep apnea after menopause, at later ages, and with higher prevalences of obesity than men. The stronger associations between PDSA and AF observed in women may reflect either increased susceptibility of women to sleep apnea–related stresses, which is consistent with some data indicating more endothelial dysfunction among women with sleep apnea compared with men (42), or a greater severity of sleep apnea in women who are clinically diagnosed with the disorder.
The strengths of our study included the ethnic diversity of the cohort; the availability of data on conventional risk factors, inflammatory markers, and measures of cardiac structure at baseline; and adjudicated information on subsequent CHD or HF. In addition, to our knowledge, MESA is the first prospective study to have demonstrated long-term associations of PDSA and HS with incident AF in a broad community cohort.
Our study had several limitations, however—notably the lack of objective data on the severity and forms of sleep apnea and the use of a self-reported measure of sleep. Despite these limitations, our participants with PDSA had characteristics similar to those of persons with more severe sleep apnea diagnosed by polysomnography (17, 27). In addition, basing the diagnosis of AF on hospital admission records may have produced underestimation of many paroxysmal or persistent AF events among participants who were treated in outpatient clinics. Although the use of a sleep history questionnaire may have allowed us to follow up on AF events occurring after MESA examination 1, the associations became nonsignificant in a sample excluding persons with evidence of AF prior to MESA examination 2 in model 1 (HR = 1.59, P = 0.12) and in other models. We also had limited statistical power to test for subgroup differences. Finally, the model did not adjust for sleep apnea therapy (such as continuous positive airway pressure) that would reduce the risk of incident AF in the PDSA group, since this would not have affected the final result.
In conclusion, our findings suggest that PDSA, a marker of more severe sleep apnea, but not HS was associated with an increased risk of incident AF after adjustment for multiple potential confounders or mediators in MESA. In addition, there was a tendency for the association of PDSA with incident AF to be stronger in middle-aged or obese persons and in women.
Supplementary Material
ACKNOWLEDGMENTS
Author affiliations: Department of Preventive Medicine, Feinberg School of Medicine, Northwestern University, Chicago, Illinois (Gen-Min Lin, Laura A. Colangelo, Donald M. Lloyd-Jones, Kiang Liu); Department of Medicine, Hualien Armed Forces General Hospital, Hualien, Taiwan (Gen-Min Lin); Department of Medicine, Harvard Medical School, Brigham and Women's Hospital, and Beth Israel Deaconess Medical School, Boston, Massachusetts (Susan Redline); Department of Internal Medicine/Cardiology, School of Medicine, Wake Forest University, Winston-Salem, North Carolina (Joseph Yeboah); Department of Epidemiology, School of Public Health, University of Washington, Seattle, Washington (Susan R. Heckbert); Section for Cardiac Electrophysiology, Division of Cardiology, School of Medicine, Johns Hopkins University, Baltimore, Maryland (Saman Nazarian); Division of Epidemiology and Community Health, School of Public Health, University of Minnesota, Minneapolis, Minnesota (Alvaro Alonso); National Institute for Biomedical Imaging and Bioengineering, National Institutes of Health, Bethesda, Maryland (David A. Bluemke); Department of Radiology, School of Medicine, Johns Hopkins University, Baltimore, Maryland (David A. Bluemke); Division of Pulmonary and Critical Care Medicine, School of Medicine, Johns Hopkins University, Baltimore, Maryland (Naresh M. Punjabi); and Department of Epidemiology, Bloomberg School of Public Health, Johns Hopkins University, Baltimore, Maryland (Moyses Szklo).
This research was supported by National Institutes of Health contracts N01-HC-95159 through N01-HC-95167 and grant R21HL121348 from the National Heart, Lung, and Blood Institute. G.-M.L. was supported by a scholarship grant from the Ministry of Defense, Taiwan.
Conflict of interest: none declared.
REFERENCES
- 1.Benjamin EJ, Chen PS, Bild DE, et al. Prevention of atrial fibrillation: report from a National Heart, Lung, and Blood Institute workshop. Circulation. 2009;1194:606–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kannel WB, Wolf PA, Benjamin EJ, et al. Prevalence, incidence, prognosis, and predisposing conditions for atrial fibrillation: population-based estimates. Am J Cardiol. 1998;82(8A):2N–9N. [DOI] [PubMed] [Google Scholar]
- 3.Chamberlain AM, Agarwal SK, Folsom AR, et al. Smoking and incidence of atrial fibrillation: results from the Atherosclerosis Risk in Communities (ARIC) Study. Heart Rhythm. 2011;88:1160–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Conen D, Tedrow UB, Cook NR, et al. Alcohol consumption and risk of incident atrial fibrillation in women. JAMA. 2008;30021:2489–2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nelson SE, Shroff GR, Li S, et al. Impact of chronic kidney disease on risk of incident atrial fibrillation and subsequent survival in Medicare patients. J Am Heart Assoc. 2012;14:e002097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rienstra M, Sun JX, Magnani JW, et al. White blood cell count and risk of incident atrial fibrillation (from the Framingham Heart Study). Am J Cardiol. 2012;1094:533–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gami AS, Pressman G, Caples SM, et al. Association of atrial fibrillation and obstructive sleep apnea. Circulation. 2004;1104:364–367. [DOI] [PubMed] [Google Scholar]
- 8.Mooe T, Gullsby S, Rabben T, et al. Sleep-disordered breathing: a novel predictor of atrial fibrillation after coronary artery bypass surgery. Coron Artery Dis. 1996;76:475–478. [PubMed] [Google Scholar]
- 9.Tanigawa T, Yamagishi K, Sakurai S, et al. Arterial oxygen desaturation during sleep and atrial fibrillation. Heart. 2006;9212:1854–1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pedrosa RP, Drager LF, Genta PR, et al. Obstructive sleep apnea is common and independently associated with atrial fibrillation in patients with hypertrophic cardiomyopathy. Chest. 2010;1375:1078–1084. [DOI] [PubMed] [Google Scholar]
- 11.Gami AS, Hodge DO, Herges RM, et al. Obstructive sleep apnea, obesity, and the risk of incident atrial fibrillation. J Am Coll Cardiol. 2007;495:565–571. [DOI] [PubMed] [Google Scholar]
- 12.Fein AS, Shvilkin A, Shah D, et al. Treatment of obstructive sleep apnea reduces the risk of atrial fibrillation recurrence after catheter ablation. J Am Coll Cardiol. 2013;624:300–305. [DOI] [PubMed] [Google Scholar]
- 13.Leung RS, Huber MA, Rogge T, et al. Association between atrial fibrillation and central sleep apnea. Sleep. 2005;2812:1543–1546. [DOI] [PubMed] [Google Scholar]
- 14.Mehra R, Stone KL, Varosy PD, et al. Nocturnal arrhythmias across a spectrum of obstructive and central sleep-disordered breathing in older men: Outcomes of Sleep Disorders in Older Men (MrOS Sleep) Study. Arch Intern Med. 2009;16912:1147–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med. 2002;1659:1217–1239. [DOI] [PubMed] [Google Scholar]
- 16.Gbadebo TD, Okafor H, Darbar D. Differential impact of race and risk factors on incidence of atrial fibrillation. Am Heart J. 2011;1621:31–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yeboah J, Redline S, Johnson C, et al. Association between sleep apnea, snoring, incident cardiovascular events and all-cause mortality in an adult population: MESA. Atherosclerosis. 2011;2192:963–968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nieto FJ, Young TB, Lind BK, et al. Association of sleep-disordered breathing, sleep apnea, and hypertension in a large community-based study. Sleep Heart Health Study. JAMA. 2000;28314:1829–1836. [DOI] [PubMed] [Google Scholar]
- 19.Levey AS, Stevens LA. Estimating GFR using the CKD Epidemiology Collaboration (CKD-EPI) creatinine equation: more accurate GFR estimates, lower CKD prevalence estimates, and better risk predictions. Am J Kidney Dis. 2010;554:622–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rautaharju PM, Calhoun HP, Chaitman BR. NOVACODE serial ECG classification system for clinical trials and epidemiologic studies. J Electrocardiol. 1992;24(suppl):179–187. [DOI] [PubMed] [Google Scholar]
- 21.Natori S, Lai S, Finn JP, et al. Cardiovascular function in Multi-Ethnic Study of Atherosclerosis: normal values by age, sex, and ethnicity. AJR Am J Roentgenol. 2006;186(6 suppl 2):S357–S365. [DOI] [PubMed] [Google Scholar]
- 22.Patton KK, Heckbert SR, Alonso A, et al. N-terminal pro-B-type natriuretic peptide as a predictor of incident atrial fibrillation in the Multi-Ethnic Study of Atherosclerosis: the effects of age, sex and ethnicity. Heart. 2013;9924:1832–1836. [DOI] [PubMed] [Google Scholar]
- 23.Hertzmark E, Pazaris M, Spiegelman D. The SAS MEDIATE Macro. Boston, MA: Harvard School of Public Health; 2009. http://www.hsph.harvard.edu/spiegelman/mediate/mediate.pdf Accessed September 15, 2014. [Google Scholar]
- 24.Marshall NS, Wong KK, Cullen SR, et al. Snoring is not associated with all-cause mortality, incident cardiovascular disease, or stroke in the Busselton Health Study. Sleep. 2012;359:1235–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sands M, Loucks EB, Lu B, et al. Self-reported snoring and risk of cardiovascular disease among postmenopausal women (from the Women's Health Initiative). Am J Cardiol. 2013;1114:540–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Monahan K, Storfer-Isser A, Mehra R, et al. Triggering of nocturnal arrhythmias by sleep-disordered breathing events. J Am Coll Cardiol. 2009;5419:1797–1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alonso A, Agarwal SK, Soliman EZ, et al. Incidence of atrial fibrillation in whites and African-Americans: the Atherosclerosis Risk in Communities (ARIC) Study. Am Heart J. 2009;1581:111–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mehra R, Benjamin EJ, Shahar E, et al. Association of nocturnal arrhythmias with sleep-disordered breathing: the Sleep Heart Health Study. Am J Respir Crit Care Med. 2006;1738:910–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bush D, Martin LW, Leman R, et al. Atrial fibrillation among African Americans, Hispanics and Caucasians: clinical features and outcomes from the AFFIRM trial. J Natl Med Assoc. 2006;983:330–339. [PMC free article] [PubMed] [Google Scholar]
- 30.Mehra R, Redline S. Sleep apnea: a proinflammatory disorder that coaggregates with obesity. J Allergy Clin Immunol. 2008;1215:1096–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Todd K, McIntyre WF, Baranchuk A. Obstructive sleep apnea and atrial fibrillation. Nat Sci Sleep. 2010;2:39–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stoohs R, Guilleminault C. Cardiovascular changes associated with obstructive sleep apnea syndrome. J Appl Physiol. 1992;722:583–589. [DOI] [PubMed] [Google Scholar]
- 33.Kim SM, Cho KI, Kwon JH, et al. Impact of obstructive sleep apnea on left atrial functional and structural remodeling beyond obesity. J Cardiol. 2012;606:475–483. [DOI] [PubMed] [Google Scholar]
- 34.Colish J, Walker JR, Elmayergi N, et al. Obstructive sleep apnea: effects of continuous positive airway pressure on cardiac remodeling as assessed by cardiac biomarkers, echocardiography, and cardiac MRI. Chest. 2012;1413:674–681. [DOI] [PubMed] [Google Scholar]
- 35.Punjabi NM, Caffo BS, Goodwin JL, et al. Sleep-disordered breathing and mortality: a prospective cohort study. PLoS Med. 2009;68:e1000132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gottlieb DJ, Yenokyan G, Newman AB, et al. Prospective study of obstructive sleep apnea and incident coronary heart disease and heart failure: the Sleep Heart Health Study. Circulation. 2010;1224:352–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schäfer H, Pauleit D, Sudhop T, et al. Body fat distribution, serum leptin, and cardiovascular risk factors in men with obstructive sleep apnea. Chest. 2002;1223:829–839. [DOI] [PubMed] [Google Scholar]
- 38.Iwasaki YK, Shi Y, Benito B, et al. Determinants of atrial fibrillation in an animal model of obesity and acute obstructive sleep apnea. Heart Rhythm. 2012;99:1409–1416.e1. [DOI] [PubMed] [Google Scholar]
- 39.Peppard PE, Young T, Barnet JH, et al. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol. 2013;1779:1006–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brooks LJ, Strohl KP. Size and mechanical properties of the pharynx in healthy men and women. Am Rev Respir Dis. 1992;1466:1394–1397. [DOI] [PubMed] [Google Scholar]
- 41.Bixler EO, Vgontzas AN, Lin HM, et al. Prevalence of sleep-disordered breathing in women: effects of gender. Am J Respir Crit Care Med. 2001;1633:608–613. [DOI] [PubMed] [Google Scholar]
- 42.Faulx MD, Larkin EK, Hoit BD, et al. Sex influences endothelial function in sleep-disordered breathing. Sleep. 2004;276:1113–1120. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.