Abstract
Topographical cell guidance is utilized to engineer highly organized and aligned cellular constructs for numerous tissue engineering applications. Recently, electrospun scaffolds fabricated using poly(glycerol sebacate) (PGS) and poly(ε-caprolactone) (PCL) have shown a great promise to support valvular interstitial cell functions for the development of tissue engineered heart valves. However, one of the major drawbacks of PGS-PCL scaffolds is the lack of control over cellular alignment. In this work we investigate the role of scaffold architecture on the endothelial cell alignment, proliferation and formation of organized cellular structures. In particular, PGS-PCL scaffolds with randomly oriented and highly aligned fibers with tunable mechanical properties were fabricated using electrospinning technique. After one week of culture, endothelial cells on the aligned scaffolds exhibit higher proliferation compared to those cultures on randomly oriented fibrous scaffolds. Furthermore, the endothelial cells reorganize in response to the topographical features of anisotropic scaffolds forming highly organize cellular constructs. Thus, the topographical contact guidance, provided by aligned PGS-PCL scaffolds, is envisioned to be useful in developing aligned cellular structures for vascular tissue engineering.
Keywords: Electrospun Scaffolds, Aligned Fibers, Tissue Engineering, Poly (glycerol sebacate), Endothelial Cells
1. Introduction
Polyglycerol sebacate (PGS), a biodegradable elastomer, has been extensively evaluated for a broad range of applications in regenerative medicine including cardiovascular patches (Chen et al., 2008), vascular tissue grafts (Motlagh et al., 2006), engineered heart valves (Masoumi et al., 2013), cartilage tissues (Kemppainen and Hollister, 2010), nerve conduits (Sundback et al., 2005), retinal implants (Pritchard et al., 2010), and surgical sealants (Chen et al., 2011). Such diverse applications are primarily attributed to the controlled degradation profile, non-toxic byproducts and highly elastomeric nature of PGS (Rai et al., 2012; Wang et al., 2003a; Wang et al., 2002). In addition, the surface erodible characteristic of PGS makes it desirable to develop scaffolds for tissue engineering applications (Wang et al., 2002; Jaafar et al., 2010; Sun et al., 2011; Sun et al., 2009; Chen et al., 2008). In the body, the cellular microenvironment, exhibits a complex milieu of biophysical and biochemical signals, which play a crucial role in directing cellular functions. For example, extracellular matrix (ECM) proteins as well as the basement membrane are comprised of heterogeneous mixture of pores, ridges and fibers at micro and nano-scale levels, which act as a cellular scaffold to guide various cell functions (Sant et al., 2012; Dolatshahi-Pirouz et al., 2014; Gaharwar et al., 2014b). Such topographical features continuously interact with the cells and influence their physiological function via cell-matrix signaling pathways (Chen et al., 2004; Stevens and George, 2005; Kulangara and Leong, 2009; Nikkhah et al., 2012a; Gaharwar et al., 2014c).
To date, micro- and nanofabrication techniques have been utilized to develop scaffolds with well-defined topographical features to mimic native tissue architecture at different length scales (Gaharwar et al., 2014c; Khademhosseini et al., 2006; Zorlutuna et al., 2012; Mihailaet al., 2013). For example, in a recent study micromolding technique was used to develop PGS scaffolds with diamond-shaped pores that resulted in anisotropic mechanical properties of the scaffolds (Engelmayr et al., 2008; Masoumi et al., 2013). In particular, the importance of designing scaffolds with anisotropic structure was underlined by growing evidence that scaffolds with structural anisotropy play a major role in guiding cellular behaviors. In another work, PGS with accordion-like honeycombs structure was fabricated to promote the formation of aligned cellular structures (Engelmayr et al., 2008). Specifically, the anisotropic structure and directionally dependent mechanical properties were utilized as guidance cues to regulate cell adhesion, shape, spreading, and migration.
In the past few years, electrospinning has become a popular approach to fabricate highly porous tissue engineering scaffolds to mimic the natural ECM microenvironment (Bhardwaj and Kundu, 2010; Gaharwar et al., 2014a; Fleischer and Dvir, 2012; Gaharwar et al., 2014d). For instance, in a recent study, injectable nanofibers of PGS were fabricated to engineer cardiac tissues (Ravichandran et al., 2012). In the proposed approach, a co-axial electrospinning setup was utilized to fabricate scaffolds from PGS and poly-l-lactic acid (PLLA). PLLA was later removed to obtain injectable PGS fibers for minimally invasive therapies. Interestingly, cardiac markers including connexin 43, actinin, troponin, and myosin heavy chain were highly expressed in PGS nanofibers compared to PLLA (Ravichandran et al., 2012). To further enhance physical, chemical and biological functionality, PGS has been blended or copolymerized with a range of natural and synthetic polymers (Ravichandran et al., 2011; Ifkovits et al., 2009; Sant et al., 2011; Tong et al., 2011; Kharaziha et al., 2013). For example, PGS-PEG block copolymers were synthesized with different mechanical properties ranging from soft elastomers to mechanically stiff in order to mimic properties of soft tissues such as cartilage, cardiac tissues, vocal cords (Zhang et al., 2013). In another study, a wide range of mechanical properties of electrospun PGS-gelatin scaffolds was obtained through changing the ratio of PGS and gelatin (Ifkovits et al., 2009; Kharaziha et al., 2013). Modulating the acrylation degree of PGS was also used to alter the degradation profile and mechanical properties of PGS scaffolds for myocardial infarction therapy (Ifkovits et al., 2009). Recently, blend of PGS with poly(ε-caprolactone) (PCL), a semi-crystalline polyester, was used to develop scaffolds that mimic the native mechanical properties of heart valves. (Sant et al., 2011; Sant et al., 2013). The fibrous PGS-PCL scaffolds supported spreading and proliferation of human umbilical vein endothelial cells (HUVECs) and fibroblastic differentiation of mesenchymal stem cells (MSCs) (Sant et al., 2011; Tong et al., 2011). In particular, by controlling the amount of PGS and PCL, it was possible to tailor the degradation rate of the scaffolds to match that of ECM secretion by valvular interstitial cells (VICs). (Sant et al., 2011; Sant et al., 2013) However, one of the limitations of the previously developed PGS-PCL electrospun scaffolds was the lack of control over the structural anisotropy of the construct and subsequent cellular alignment. (Sant et al., 2011; Sant et al., 2013) Moreover in a recent study, it was observed that anisotropic structures are promising candidates for valve tissue engineering, as VICs are proliferate and form aligned structure in anisotropic scaffolds.(Sohier et al., 2014) Therefore, we hypothesized that by controlling the fiber orientation, anisotropy can be incorporated within the fibrous scaffolds to provide topological cues for enhanced cellular signaling.
In this study, we investigated the effect of PGS-PCL fiber organization on endothelial cells behavior. Electrospinning was used to fabricate random and aligned scaffolds from blends of PGS and PCL polymers with tunable mechanical properties. The effect of fiber orientation was evaluated on endothelial cell attachment, proliferation and organization. The specific goal of this project is to investigate the potential applicability of PGS-PCL fibrous scaffolds for engineering vascularized tissue substitutes. We hypothesize that the anisotropic PGS-PCL scaffolds will assist in formation of aligned structure, mimicking native tissue architecture, to develop small and medium-sized tissue engineered vascular grafts.
2. Experimental Section
2.1 Materials
PGS (Molecular weight~5,012 Da and PDI~2.64) was synthesized according to previously published polycondensation methods (Wang et al., 2003a; Zhang et al., 2013). Briefly, equimolar glycerol (Sigma-Aldrich, USA) and sebacic acid (Sigma-Aldrich) were heated to 130°C for 2 h under Argon gas in a round bottom reactor. The pressure was gradually decreased to 50 mTorr and the reaction was continued under vacuum condition for 48 h. PCL (C6H10O2)n (Molecular weight 70-90 kDa) was obtained from Sigma-Aldrich (USA).
2.2 Scaffold Fabrication
The electrospun scaffolds were fabricated by first dissolving PCL (10 % w/v) and PGS (5 % w/v) in 9:1 ratio of anhydrous chloroform and ethanol according to previously published methods (Sant et al., 2011; Sant et al., 2013). Electrospinning of the PGS-PCL mixture was carried out using a blunt needle (21G), 2 mL/h flow rate, 12.5 kV (Glassman High Voltage, INC., USA) and 18 cm distance between the collector and needle. Random scaffolds were obtained by using a circular aluminum collector plate (8 inches in diameter) while aligned scaffolds were fabricated by using a parallel collector plate (5 inches in length with a gap of 1 inch). The voltage and distance were kept constant to obtain random and aligned scaffolds. The fabricated electrospun scaffolds were dried in vacuum overnight to remove the residual solvent.
2.3 Microstructure Evaluation
Electrospun fiber structure and morphology was evaluated by scanning electron microscopy (SEM) (JSM 5600LV, JEOL, USA). The fabricated scaffolds were coated with Au/Pd for 2 min using a Hummer 6.2 sputter coater (Ladd Research, USA). SEM images were acquired at 5KV accelerating voltage, 5mm working distance and a spot size of 20. The images were analyzed using Image J (NIH, USA) software and fiber diameter was calculated from at least 100 fibers. The alignment of fibers was characterized using directional plugin of ImageJ to obtain Fast Fourier transformation (FFT) of SEM images.
2.4 Chemical characterization
Fourier Transform Infrared (FTIR) spectra of the fabricated electrospun scaffolds were obtained using Alpha FTIR spectrometer (Bruker, USA). For each sample, at least 24 scans with 4 cm−1 resolution were collected. Thermal properties of PGS-PCL scaffolds were determined by Differential Scanning Calorimeter (DSC) 8500 (Perkin-Elmer, USA). Pre-weighted scaffolds were sealed in aluminum pans and were heated at 10 °C/min. The samples were then subjected to two heating and cooling cycles between −70 °C to 150 °C under nitrogen gas. The second heating cycle was used to determine the melting temperature (Tm) and the melting enthalpy (ΔHm).
2.5 Mechanical Testing
The mechanical properties of the blended PGS-PCL scaffolds were characterized using Instron 5943 Materials Testing System (Instron, USA) at 10 mm/min strain rate with 50 N load cell. The scaffolds were cut in rectangular shapes (10×5 mm2) with approximate thickness of 100-140 μm. For the tensile test, samples were griped from two end using rubber griped to avoid slippage. A preload of 0.01N was applied on the scaffolds before the start of the experiment. The elastic modulus was calculated from the linear section of stress-strain curve within 5-15% strain range. The morphology of electrospun scaffolds was evaluated after the mechanical deformation using SEM imaging. The fractured samples were coated with Au/Pd before the imaging to determine the effect of mechanical deformation on topography.
2.6 Cell seeding and proliferation
GFP-expressing human umbilical vein endothelial cells (HUVECs) (passage ~ 10-15) were used in this work. The cells were cultured in endothelial specific growth medium (EGM-2, Lonza, USA) containing 2% fetal bovine serum (FBS), human fibroblast growth factor (hFGF), vascular endothelial growth factor (VEGF), human epidermal growth factor (hEGF), insulin growth factor (R3-IGF-I), ascorbic acid, hydrocortisone, heparin, and 1 % gentamicin and amphotericin B. The fibrous scaffolds (5×5 mm2) were sterilized using 70% ethanol and the cells were seeded on each scaffold with a density of 2×105 cells in ultra-low adhesion plates. The effect of fiber orientation on the metabolic activity of the cells was evaluated using Alamar Blue assay (Invitrogen, USA) over a period of 7 days.
2.7 Histology
Cell-seeded scaffolds were fixed using 4% paraformaldehyde (PF) solution and then dehydrated using an alcohol gradient and paraffin-embedding. The fixed samples were then sectioned (10 μm) and mounted on glass slides and washed in xylene. The sections were finally rehydrated before staining with hemotoxylin and eosin (H&E).
2.8 Cellular alignment analysis
After 7 days of culture, the cell-seeded scaffolds were fixed in 4% PF solution and cell nuclei were stained with 4’,6-diamidino-2-phenylindole (DAPI). The confocal images were obtained using inverted laser scanning confocal microscope (SP5 X MP, Leica). The 3D projected image was obtained using Leica application suite (LAS) software by stacking the acquired confocal images using Imaris software (Bitplane, Zurich, Swiss). The nuclei alignment was determined according to our previously published protocols (Nikkhah et al., 2012b).
2.9 Immunofluorescence
Actin cytoskeleton organization and endothelial cell specific intercellular junction marker (CD31) expression were evaluated using confocal microscopy on the random and aligned scaffolds. Cell-seeded scaffolds were fixed in 4% PF solution and the cell membrane was permeabilized using 0.1% Triton X-100 for 30 minutes. Subsequently, the samples were blocked in 1% bovine serum albumin (BSA) for actin cytoskeleton and in 10% horse serum for CD-31 staining, respectively. Actin filaments were stained using 1:40 dilution of Alexa Fluor-594 phalloidin (Abcam) in 0.1% BSA, while CD-31 staining was performed using 1:100 dilution of monoclonal mouse anti-CD31 primary antibody (Abcam) and 1:200 dilution of Alexa Fluor-594 conjugated goat anti-mouse secondary antibody in 10% horse serum.
2.10 Statistical Analysis
All experimental data were presented is mean ± standard deviation (SD). Student's t-test was used to analyze the differences between the groups for structural analysis and mechanical testing (*p<0.05).
3. Results and Discussion
Both, PGS and PCL are considered as aliphatic polyesters. PGS is an amorphous polymer at room temperature and has a faster in vivo degradation rate compared to PCL (Wang et al., 2003b; Sundback et al., 2005). Alternatively, PCL is a semi-crystalline polymer which slowly degrades over a period of 2-4 years depending on its specific properties (i.e molecular weight and crystallinity)(Lam et al., 2008). Through blending PGS and PCL, it is possible to develop hybrid biomaterials resembling the natural ECM architecture with a broad spectrum of mechanical properties and degradation profile to modulate various cell functions (Sant et al., 2011; Sant et al., 2013). Due to their unique property combinations, PGS-PCL based electrospun scaffolds can be extensively used for cardiovascular tissue engineering applications (i.e. heart valves, vascular grafts).
3.1. Structural and chemical characterization of electrospun scaffolds
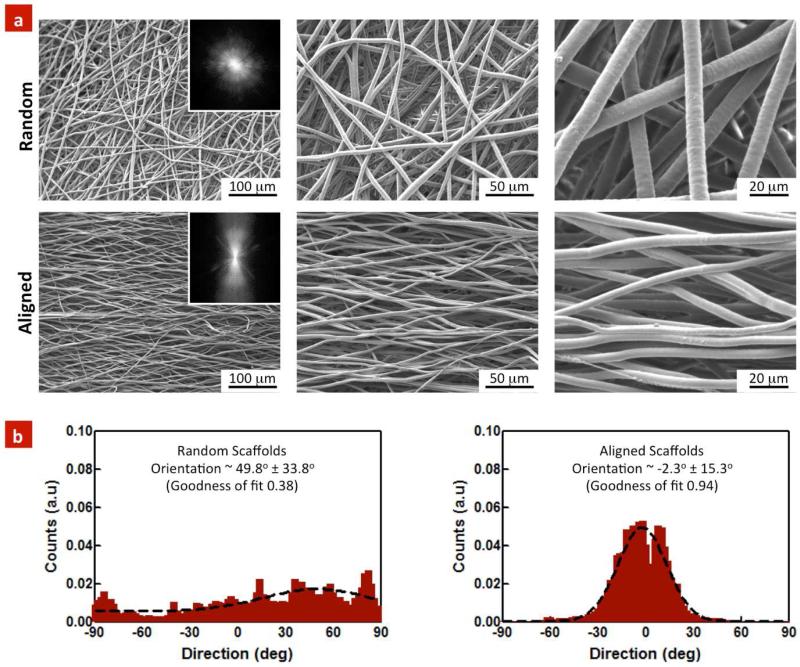
The fiber morphology of random and aligned scaffolds was determined using SEM imaging (Figure 1a). Both random and aligned scaffold exhibited fibers with uniform and smooth surface morphology with diameter in the range of 3.4±0.78 μm and 4.7±0.6 μm respectively. The microscopic images indicated enhanced fiber orientation within the aligned scaffolds compared to the random ones. To further analyze the degree of alignment, we performed two-dimensional (2D) Fast Fourier transform (FFT) of the SEM images. We first converted the spatial organization of fibers into a mathematically defined frequency domain. This process maps the rate of change of intensities across the original images and the output image depicts the orientation of the object in the original image. The 2D FFT images (Figure 1a) indicated that the random scaffolds comprised of fibers with a small degree of orientation. Alternatively, aligned scaffold exhibited highly oriented structure as indicated by an intense peak perpendicular to the fiber orientation.
Figure 1. Topographical evaluation of random and aligned PGS-PCL scaffolds.
(a) SEMs images indicate arbitrary orientation of individual fibers within the random scaffolds, whereas highly organized and aligned fibers were observed in the aligned scaffolds. Inset: 2D FFT of SEM images further verified highly oriented structure within the aligned scaffolds. (b) The random scaffolds exhibited only a small degree of preferred orientation (49.8±33.8°), as compared to the aligned scaffolds (2.3±15.3°). The data represents fiber diameter calculated from at least 100 fibers (n=3).
The preferred orientation of fibers within aligned and random scaffolds was also investigated using ImageJ software (Directional plugin). The results indicated relatively flat and skewed histogram for random and normal distribution comprised of a sharp peak within the aligned scaffolds. The fitting data indicated a small degree of preferred orientation in the random scaffolds at 49.8±33.8° (goodness of fit = 0.38). On the other hand, preferred orientation was −2.3±15.3° within the aligned scaffolds (goodness of fit = 0.94) (Fig 1b). The goodness of the fit indicated that the aligned scaffolds had almost 94% of the fibers in the range of −2.3±15.3°. In the current study, we selected only random and highly aligned scaffold, however there are range of parameters in electrospinning processes that can be tuned to obtain variable degree of fiber alignment.(Courtney et al., 2006)
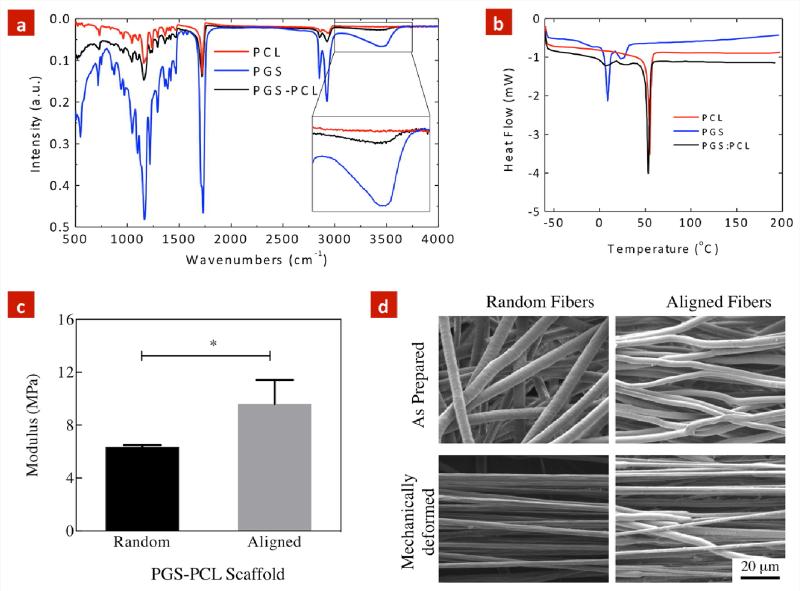
FTIR spectroscopy was performed to determine the presence of PGS and PCL within electrospun scaffolds. Both PCL and PGS showed very similar IR bands at 2923 cm−1 (CH2 stretching-asymmetric), 2857 cm−1 (CH2 stretching-symmetric), 1293 cm−1 (C–O and C–C stretching in the crystalline phase), 1720 cm−1 (carbonyl stretching) and 1240 cm−1 (C-O-C stretching-asymmetric) (Figure 2a). Additionally, PGS showed an addition hydroxyl peak at 3500 cm−1, which was not observed in pure PCL. Overall, electrospun PGS-PCL scaffolds showed both PGS and PCL peaks, indicating composite structure of the fibers.
Figure 2. Chemical and Physical Characterization of PGS-PCL scaffolds.
(a) FTIR spectra of pure PCL, pure PGS and random PGS-PCL electrospun scaffolds. (b) DSC thermogram (heating cycle) of pure PCL, pure PGS and random PGS-PCL electrospun scaffolds. (c) Uniaxial tensile test of electrospun scaffold demonstrating higher modulus of the aligned scaffolds compared to the random scaffolds (*p<0.05, student's t-test). (d) The effect of tensile mechanical stress on the fiber rearrangement and deformation evaluated using SEM images. Significant reduction in fiber diameter and increase in fiber orientation was observed in response to mechanical stress.
We further used DSC to determine the effect of addition of PGS on the thermal properties of the electrospun PGS-PCL scaffolds. Figure 2b shows the DSC thermograms (heating cycles) of PGS, PCL and PGS-PCL scaffolds. The melting temperature (Tm) of pure PCL and pure PGS was calculated to be 57.8 °C and 12.4 °C while the melting enthalpy (ΔH) was found to be 47.96 J/g and 1.72 J/g, respectively. Notably, electrospun scaffolds, exhibited two distinct Tm (56.1 and 11.9 °C) indicating presences of both PGS and PCL within the blended scaffolds. The presence of two distinct peaks indicates that PGS and PCL are not fully miscible and phase separation might occur after the electrospinning process. These results were in agreement with the previously published reports (Bolgen et al., 2005; Cai and Liu, 2008; Liu et al., 2007).
3.2 Effect of Electrospun Fiber Orientation on Mechanical Properties of Scaffolds
The mechanical properties of biomaterials are among crucial parameters, which significantly influence cell functions. In particular, previous studies have demonstrated that scaffolds with low stiffness result in cells with round morphology, while scaffolds with high stiffness will lead to stretched and spindle like cellular morphology.(Engler et al., 2006) On the other hand, organs and tissues within the body exhibit different mechanical properties depending on their functionality. For instance, vascular system undergoes continuous cyclic mechanical deformation, exhibits elastic modulus in the range of 2.25-130 MPa depending on the physiological anatomy, location and functionality of the blood vessel.(Hasan et al., 2014; Sacks et al., 2009; Wagenseil and Mecham, 2009) Therefore, we investigated the mechanical properties of fibrous scaffolds using uniaxial tensile test (performed horizontal to the direction of the aligned fibers) (Figure 2c). The results indicated that random and aligned PGS-PCL scaffolds exhibited elastic modulus of 6.3±0.1 MPa and 9.6±1.1 MPa, respectively (*p<0.05, Student's t-test). It is anticipated that the increase in the mechanical strength of the aligned scaffolds is attributed to the oriented architecture of the scaffold, which is capable of bearing a higher load compared to the random scaffolds. Under mechanical stress, during initial loading process, fibrous scaffolds undergo fiber rearrangement before the mechanical deformation of individual fibers. In case of aligned scaffolds, this process shortens as most of the fibers are aligned and significant fiber rearrangement is not required. Due to such behavior, the modulus of aligned scaffolds was significantly higher compared to the random scaffolds.
Our mechanical testing results are in agreement with previously published results.(Sant et al., 2011; Sant et al., 2013) Both PCL and PGS-PCL scaffolds have shown to have modulus around 7-8 MPa.(Sant et al., 2011; Sant et al., 2013) The addition of PGS to PCL does not result in any significant change in mechanical properties. However, alignment of fibers has shown to have significant effect on the mechanical stiffness of PCL scaffolds.(Baker et al., 2008) For example, aligned PCL scaffolds have two-fold higher modulus compared to random scaffolds.(Baker et al., 2008) In PGS-PCL scaffolds, a dramatic increase in modulus was not observed. Such behavior might be attributed to the presence of PGS that result in phase separation from PCL upon electrospinning. This is further supported by the DSC data where, two distinct peak of PGS and PCL was observed.
The effect of mechanical deformation on microstructure was evaluated further using SEM imaging. Figure 2d compares the microstructure of random and aligned scaffolds before and after mechanical deformation. In random scaffolds, the mechanical deformation resulted in a decrease in fiber diameter from 4.7±0.6 μm to 2.6±0.2 μm, and significant fiber alignment. The deformed fibers showed uniform and smooth surface morphology. Alternatively, within aligned scaffolds, the mechanical deformation resulted in a decrease in fiber diameter from 3.2±0.8 μm to 1.5±0.5 μm. The deformed fibers showed necking behavior in the aligned scaffolds.
3.3 Effect of electrospun fiber orientation on cell spreading and proliferation
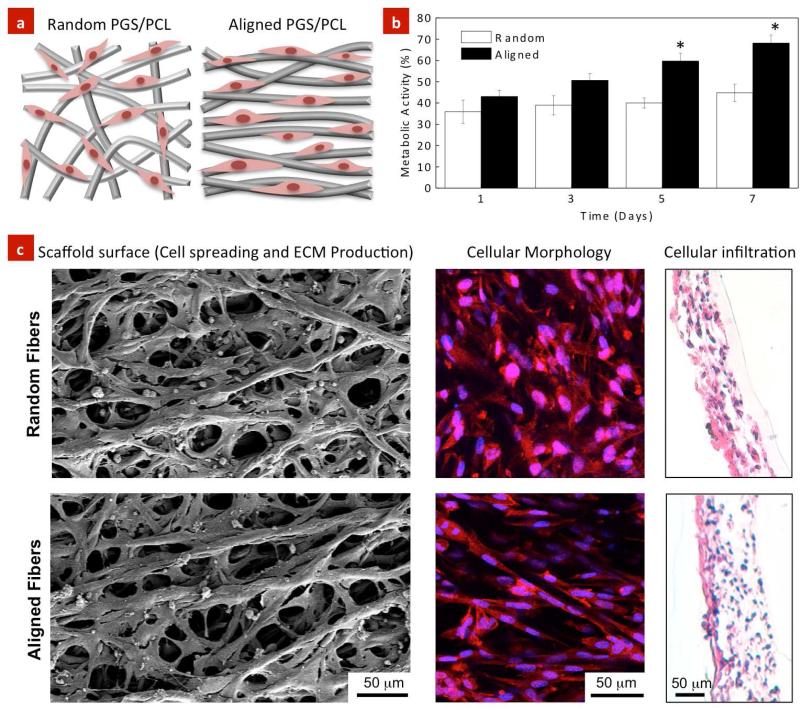
Earlier studies have reported that electrospun PGS-PCL scaffolds promote cell adhesion and proliferation compared to PCL scaffolds (Sant et al., 2011), mainly due to their enhanced hydrophilicity compared to hydrophobic PCL scaffolds (Sant et al., 2011). Herein, the effect of random and aligned scaffolds on endothelial cells adhesion and spreading was investigated (Figure 3a). The effect of fiber orientation on the metabolic activity of HUVECs was investigated using Alamar Blue assay. The metabolic activity of HUVECs seeded on the aligned scaffolds was higher compared to the random scaffolds (Figure 3b). In particular, aligned scaffolds induced higher metabolic activity toward the formation of a confluent monolayer over a longer period of culture time (i.e. day 7).
Figure 3. Effect of topography on cellular adhesion, proliferation and infiltration within the PGS-PCL scaffolds.
(a) Schematic showing cellular morphology with respect to the fiber orientation. (b) Effect of scaffolds architecture on cellular proliferation after 7 days of culture. Endothelial cells exhibited higher proliferation on the aligned scaffolds than the random scaffolds. (c) HUVECs readily attached on both random and aligned scaffolds and spread along the fiber axis as observed by SEM and fluorescence images (red-actin filament, blue nucleus (dapi)). Histological evaluation indicated that cells infiltrated within the aligned and random scaffolds due to highly porous architecture of the scaffolds. The data represents mean ± standard deviation (n=5, Student's t-test *p<0.05).
SEM images clearly demonstrated that HUVECs adhered and spread on both, random and aligned scaffolds suggesting the dominance of material properties over structural (fiber organization) cues on cellular attachment (Figure 3c). On the aligned scaffolds, the cells exhibited spindle-like morphology and were highly organized and elongated along the fiber direction. Alternatively, the cells demonstrated stellate-shape morphology and were distributed in different directions as well as in between the neighboring fibers on the random scaffolds. Fluorescence images shown in Figure 3c were in agreement with SEM images confirming that the cells extended their cytoskeletal structure in a direction parallel to the electrospun fibers on the aligned scaffolds. Furthermore, the histological sections of the scaffolds confirmed homogenous penetration and uniform migration of the cells within the interior of regions of the electrospun scaffolds due to highly porous architecture of the scaffolds (Figure 3c) irrespective of their alignment.
Previous studies by Zhang et al. also confirmed similar growth behavior of human aortic endothelial cells on random silk nanofibrous scaffolds for 7 days of culture (Zhang et al., 2008). Their studies demonstrated that the cell proliferation was increased two folds on day 14 as compared to day 7. On the other hand, Heath et al. reported a opposite finding showing that HUVECs seeded on randomly distributed hexyl methacrylate (HMA)/methyl methacrylate (MMA)/methacrylic acid (MAA) scaffolds with rubber-like material properties (Elastic Modulus ~ 3±2 MPa) and small pore area (~270±190 μm2) exhibited a significantly higher proliferation compared to the aligned scaffolds (Heath et al., 2010). They concluded that that the pore size is a major factor in regulating proliferation and enzymatic activity of the cells. Therefore, we expect that the metabolic activity of HUVECs seeded on the aligned PGS-PCL scaffolds is governed by the material properties and structural characteristics of the scaffolds, by providing a coherent and unidirectional signaling in between the neighboring cells.
3.4 Cellular orientation on random and aligned scaffolds
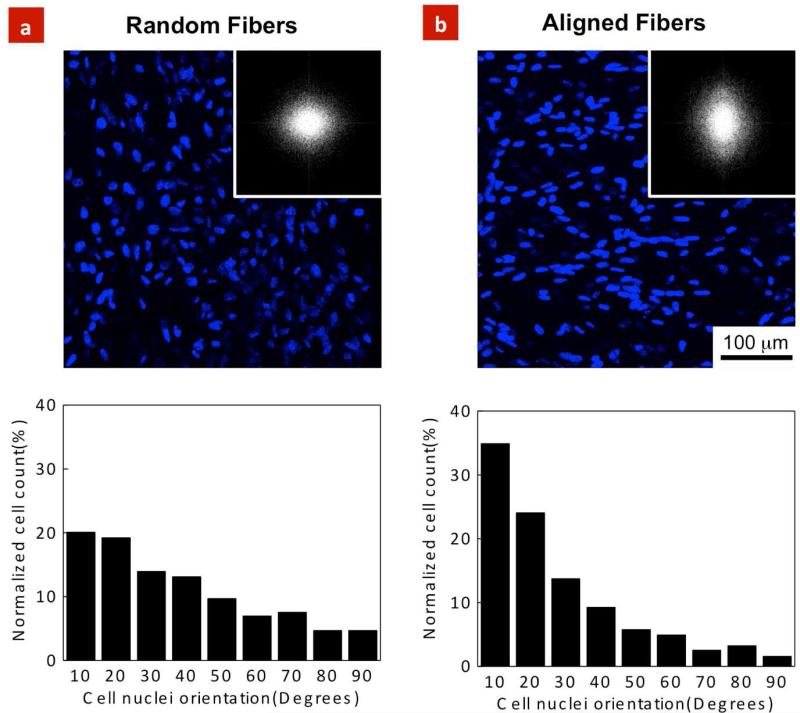
To investigate the role of fiber orientation on cellular organization, nuclear alignment of HUVECs was quantified on the random and aligned scaffolds after 7 days of culture. As demonstrated in Figures 4a, b, fiber organization had a pronounced effect on the cell nuclear alignment. Specifically, on the aligned and random scaffolds, 35% of the cells (n=836) and 20% of the cells (n=713) were aligned within 10° preferred angle respectively. FFT analysis further confirmed nuclear alignment obtained through fluorescence images. To date, a large number of studies have demonstrated cellular alignment along the major axis of micro and nano-scale ridges, grooves, and micropatterns; a phenomenon known as “contact guidance” (Teixeira et al., 2003; Curtis and Wilkinson, 2001; Nikkhah et al., 2012a). Contact guidance plays a significant role in numerous biological processes such as cell migration (Clark et al., 1990). In addition, cytoskeletal components including actin filaments as well as focal adhesion complexes are organized along with the direction of the features (Nikkhah et al., 2012a; Gaharwar et al., 2011). Therefore, similar process is expected to take place when endothelial cells are cultured on the PGS-PCL scaffolds. As the cells adhere on the aligned scaffolds, they probe and sense their local microenvironment through their integrin receptors and focal adhesion complexes. This ultimately results in reorganization of the cytoskeletal structure (i.e. actin filaments) of the cells and alters their morphology toward alignment and elongation along the fibers.
Figure 4. HUVEC alignment on the PGS-PCL scaffolds.
HUVEC nuclei alignment within the random and aligned scaffolds after 7 days of culture; Microscopic images indicated significant role of fiber architecture on cellular alignment. Fluorescence images along with FFT analysis confirmed significantly higher cell nuclei alignment on the (b) aligned scaffolds compare to the (a) random scaffolds. The data represents normalized cell counts calculated from at least 100 cells /images.
3.5 Effect of topography on cytoskeletal organizations
One of the major themes in vascularization is the development of biomaterials, which enable rapid formation of cord-like structures. To date, several techniques have been developed to integrate vascularized networks within engineered tissue scaffolds (Kannan et al., 2005; Lovett et al., 2009; Khademhosseini and Langer, 2007; Chen et al., 2012). A number of these strategies are cell-based approaches where endothelial cells are incorporated within hydrogels and porous scaffolds to facilitate formation of capillary networks and cord- like structures (Nikkhah et al., 2012b; Du et al., 2011). In this regard, selection of a proper biomaterial plays a dominant role in the formation of functional vascularized networks. Herein, we investigated the role of fiber architecture on the formation of organized cellular structures.
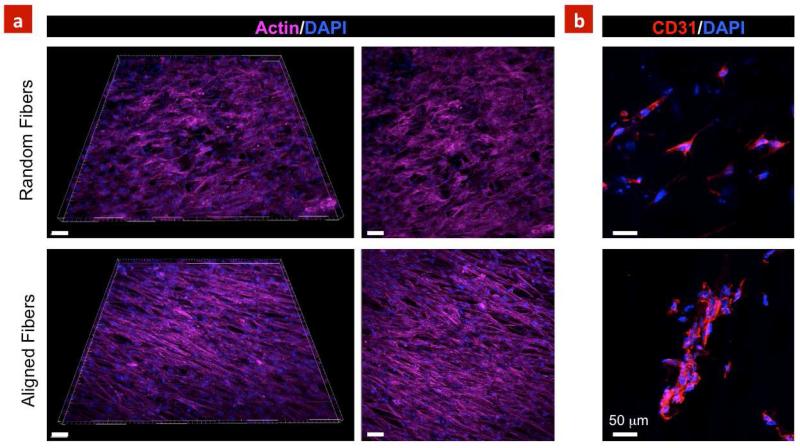
Immunostaining demonstrated that the cells reorganized and formed a complex and highly aligned networks comprised of highly oriented actin fibers on the aligned scaffolds (Figure 5a). The expressions of CD31, as a major determinant in angiogenesis (Newman et al., 1990), was also investigated on the developed scaffolds. Figure 5b indicates higher expression of CD-31 within the aligned scaffolds as compared to the random scaffolds. Although it is not clear if the fiber alignment result in enhanced CD-31 expression or the 3D microenvironment provided by electrospun scaffold play a major role. Specifically, CD-31 expression within clusters of the cells was along the fiber directions demonstrating the importance of the aligned scaffolds for tissue engineering applications. Overall, our findings are in agreement with previous studies showing that electrospun scaffolds significantly affect morphology, proliferation and alignment of endothelial cells and can serve as suitable material to develop vascularized tissue constructs (Heath et al., 2010; Zhang et al., 2008). Furthermore, the developed scaffolds can be used a suitable biomaterials to enhance endothelial cells infiltration for potential applications in designing vascular graft.
Figure 5. Cytoskeleton organization and expression of CD31 marker.
(a) Aligned scaffolds resulted in formation of highly organized cord-like structures, whereas cells were randomly distributed on the random scaffolds. (b) Random scaffolds indicated less expression of CD31 compared to align scaffolds. Moreover, aligned scaffolds promoted clustering of HUVECs demonstrating the suitability of the aligned architecture to form vascularized networks within 7 days of culture. Scale bars represent 50 μm.
Earlier reports have also highlighted the use of aligned scaffolds for tissue engineering (Yang et al., 2005; Meng et al., 2010; Xie et al., 2010; Nguyen et al., 2012; Tseng et al., 2014). For example, our recent study on PGS-gelatin fibrous scaffold indicates that cardiomyocytes seeded on aligned scaffolds showed higher expression of sarcomeric α-actinin, Cx-43 and cardiac troponin I (Kharaziha et al., 2013). In another study, mesenchymal progenitor cell were seeded on aligned and random scaffolds of poly(ester urethane) urea elastomer (Bashur et al., 2009). They demonstrated that the fiber morphology and alignment play an important role on the expression of ligament specific markers such as collagen 1α1, decorin, and tenomodulin. Thus aligned PGS-PCL scaffold can be used to engineer aligned tissue structures.
4. Conclusions
In this work, we investigated the effect of anisotropy of PGS-PCL scaffolds with variable mechanical properties on endothelial cell behavior. SEM images confirmed successful fabrication of aligned and random fibrous scaffolds. Uniaxial tensile test revealed higher elastic modulus of aligned scaffold as compared to the random scaffolds. Notably, aligned PGS-PCL scaffold enhanced cellular proliferation and alignment and led to the formation of organized endothelial constructs as a necessary step for the development of vascularized tissue structures.
Acknowledgements
This research was funded by the US Army Engineer Research and Development Center, the Institute for Soldier Nanotechnology, the NIH (EB009196; DE019024; EB007249; HL099073; AR057837), and the National Science Foundation CAREER award (AK).
REFERENCES
- Baker BM, Gee AO, Metter RB, Nathan AS, Marklein RA, Burdick JA, Mauck RL. The potential to improve cell infiltration in composite fiber-aligned electrospun scaffolds by the selective removal of sacrificial fibers. Biomaterials. 2008;29:2348–58. doi: 10.1016/j.biomaterials.2008.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bashur CA, Shaffer RD, Dahlgren LA, Guelcher SA, Goldstein AS. Effect of fiber diameter and alignment of electrospun polyurethane meshes on mesenchymal progenitor cells. Tissue Engineering Part A. 2009;15:2435–45. doi: 10.1089/ten.tea.2008.0295. [DOI] [PubMed] [Google Scholar]
- Bhardwaj N, Kundu SC. Electrospinning: a fascinating fiber fabrication technique. Biotechnology Advances. 2010;28:325–47. doi: 10.1016/j.biotechadv.2010.01.004. [DOI] [PubMed] [Google Scholar]
- Bolgen N, Menceloglu YZ, Acatay K, Vargel I, Piskin E. In vitro and in vivo degradation of non-woven materials made of poly(epsilon-caprolactone) nanofibers prepared by electrospinning under different conditions. J. Biomater. Sci.-Polym. Ed. 2005;16:1537–55. doi: 10.1163/156856205774576655. [DOI] [PubMed] [Google Scholar]
- Cai W, Liu L. Shape-memory effect of poly (glycerol–sebacate) elastomer. Materials Letters. 2008;62:2171–3. [Google Scholar]
- Chen CS, Tan J, Tien J. Mechanotransduction at cell-matrix and cell-cell contacts. Annual Review of Biomedical Engineering. 2004;6:275–302. doi: 10.1146/annurev.bioeng.6.040803.140040. [DOI] [PubMed] [Google Scholar]
- Chen Q, Liang S, Thouas GA. Synthesis and characterisation of poly (glycerol sebacate)-co-lactic acid as surgical sealants. Soft Matter. 2011;7:6484–92. [Google Scholar]
- Chen QZ, Bismarck A, Hansen U, Junaid S, Tran MQ, Harding SE, Ali NN, Boccaccini AR. Characterisation of a soft elastomer poly (glycerol sebacate) designed to match the mechanical properties of myocardial tissue. Biomaterials. 2008;29:47–57. doi: 10.1016/j.biomaterials.2007.09.010. [DOI] [PubMed] [Google Scholar]
- Chen Y-C, Lin R-Z, Qi H, Yang Y, Bae H, Melero-Martin JM, Khademhosseini A. Functional human vascular network generated in photocrosslinkable gelatin methacrylate hydrogels. Advanced Functional Materials. 2012;22:2027–39. doi: 10.1002/adfm.201101662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark P, Connolly P, Curtis A, Dow J, Wilkinson C. Topographical control of cell behaviour: II. Multiple grooved substrata. Development. 1990;108:635–44. doi: 10.1242/dev.108.4.635. [DOI] [PubMed] [Google Scholar]
- Courtney T, Sacks MS, Stankus J, Guan J, Wagner WR. Design and analysis of tissue engineering scaffolds that mimic soft tissue mechanical anisotropy. Biomaterials. 2006;27:3631–8. doi: 10.1016/j.biomaterials.2006.02.024. [DOI] [PubMed] [Google Scholar]
- Curtis A, Wilkinson C. Nantotechniques and approaches in biotechnology. TRENDS in Biotechnology. 2001;19:97–101. doi: 10.1016/s0167-7799(00)01536-5. [DOI] [PubMed] [Google Scholar]
- Dolatshahi-Pirouz A, Nikkhah M, Gaharwar AK, Hashmi B, Guermani E, Aliabadi H, Camci-Unal G, Ferrante T, Foss M, Ingber DE. A combinatorial cell-laden gel microarray for inducing osteogenic differentiation of human mesenchymal stem cells. Scientific reports. 2014;4 doi: 10.1038/srep03896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Y, Ghodousi M, Qi H, Haas N, Xiao W, Khademhosseini A. Sequential assembly of cell, Äêladen hydrogel constructs to engineer vascular, Äêlike microchannels. Biotechnology and bioengineering. 2011;108:1693–703. doi: 10.1002/bit.23102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelmayr GC, Cheng M, Bettinger CJ, Borenstein JT, Langer R, Freed LE. Accordion-like honeycombs for tissue engineering of cardiac anisotropy. Nature Materials. 2008;7:1003–10. doi: 10.1038/nmat2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–89. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- Fleischer S, Dvir T. Tissue engineering on the nanoscale: lessons from the heart. Current Opinion in Biotechnology. 2012 doi: 10.1016/j.copbio.2012.10.016. [DOI] [PubMed] [Google Scholar]
- Gaharwar AK, Mihaila SM, Kulkarni AA, Patel A, Di Luca A, Reis RL, Gomes ME, van Blitterswijk C, Moroni L, Khademhosseini A. Amphiphilic beads as depots for sustained drug release integrated into fibrillar scaffolds. J. Control. Release. 2014a;187:66–73. doi: 10.1016/j.jconrel.2014.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaharwar AK, Mihaila SM, Kulkarni AA, Patel A, Di Luca A, Reis RL, Gomes ME, van Blitterswijk C, Moroni L, Khademhosseini A. Amphiphilic beads as depots for sustained drug release integrated into fibrillar scaffolds. Journal of Controlled Release. 2014b;187:66–73. doi: 10.1016/j.jconrel.2014.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaharwar AK, Mukundan S, Karaca E, Dolatshahi-Pirouz A, Patel A, Rangarajan K, Mihaila SM, Iviglia G, Zhang H, Khademhosseini A. Nanoclay-Enriched Poly (ε-caprolactone) Electrospun Scaffolds for Osteogenic Differentiation of Human Mesenchymal Stem Cells. Tissue Engineering Part A. 2014c doi: 10.1089/ten.tea.2013.0281. Doi:10.1089/ten.tea.2013.0281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaharwar AK, Mukundan S, Karaca E, Dolatshahi-Pirouz A, Patel A, Rangarajan K, Mihaila SM, Iviglia G, Zhang H, Khademhosseini A. Nanoclay-Enriched Poly(varepsilon-caprolactone) Electrospun Scaffolds for Osteogenic Differentiation of Human Mesenchymal Stem Cells. Tissue Eng. Pt. A. 2014d doi: 10.1089/ten.tea.2013.0281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaharwar AK, Schexnailder PJ, Dundigalla A, White JD, Matos-Pérez CR, Cloud JL, Seifert S, Wilker JJ, Schmidt G. Highly Extensible Bio-Nanocomposite Fibers. Macromolecular Rapid Communications. 2011;32:50–7. doi: 10.1002/marc.201000556. [DOI] [PubMed] [Google Scholar]
- Hasan A, Memic A, Annabi N, Hossain M, Paul A, Dokmeci MR, Dehghani F, Khademhosseini A. Electrospun scaffolds for tissue engineering of vascular grafts. Acta biomaterialia. 2014;10:11–25. doi: 10.1016/j.actbio.2013.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath DE, Lannutti JJ, Cooper SL. Electrospun scaffold topography affects endothelial cell proliferation, metabolic activity, and morphology. Journal of Biomedical Materials Research Part A. 2010;94:1195–204. doi: 10.1002/jbm.a.32802. [DOI] [PubMed] [Google Scholar]
- Ifkovits JL, Devlin JJ, Eng G, Martens TP, Vunjak-Novakovic G, Burdick JA. Biodegradable Fibrous Scaffolds with Tunable Properties Formed from Photo-Cross-Linkable Poly(glycerol sebacate) ACS Applied Materials & Interfaces. 2009;1:1878–86. doi: 10.1021/am900403k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaafar IH, Ammar MM, Jedlicka SS, Pearson RA, Coulter JP. Spectroscopic evaluation, thermal, and thermomechanical characterization of poly(glycerol-sebacate) with variations in curing temperatures and durations. J. Mater. Sci. 2010;45:2525–9. [Google Scholar]
- Kannan RY, Salacinski HJ, Sales K, Butler P, Seifalian AM. The roles of tissue engineering and vascularisation in the development of micro-vascular networks: a review. Biomaterials. 2005;26:1857–75. doi: 10.1016/j.biomaterials.2004.07.006. [DOI] [PubMed] [Google Scholar]
- Kemppainen JM, Hollister SJ. Tailoring the mechanical properties of 3D-designed poly (glycerol sebacate) scaffolds for cartilage applications. Journal of Biomedical Materials Research Part A. 2010;94:9–18. doi: 10.1002/jbm.a.32653. [DOI] [PubMed] [Google Scholar]
- Khademhosseini A, Langer R. Microengineered hydrogels for tissue engineering. Biomaterials. 2007;28:5087–92. doi: 10.1016/j.biomaterials.2007.07.021. [DOI] [PubMed] [Google Scholar]
- Khademhosseini A, Langer R, Borenstein J, Vacanti JP. Microscale technologies for tissue engineering and biology. Proceedings of the National Academy of Sciences. 2006;103:2480–7. doi: 10.1073/pnas.0507681102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kharaziha M, Nikkhah M, Shin S-R, Annabi N, Masoumi N, Gaharwar AK, Camci-Unal G, Khademhosseini A. PGS:Gelatin nanofibrous scaffolds with tunable mechanical and structural properties for engineering cardiac tissues. Biomaterials. 2013;34:6355. doi: 10.1016/j.biomaterials.2013.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulangara K, Leong KW. Substrate topography shapes cell function. Soft Matter. 2009;5:4072–6. [Google Scholar]
- Lam CXF, Savalani MM, Teoh S-H, Hutmacher DW. Dynamics of in vitro polymer degradation of polycaprolactone-based scaffolds: accelerated versus simulated physiological conditions. Biomedical Materials. 2008;3:034108. doi: 10.1088/1748-6041/3/3/034108. [DOI] [PubMed] [Google Scholar]
- Liu QY, Tian M, Shi R, Zhang LQ, Chen DF, Tian W. Structure and properties of thermoplastic poly(glycerol sebacate) elastomers originating from prepolymers with different molecular weights. J. Appl. Polym. Sci. 2007;104:1131–7. [Google Scholar]
- Lovett M, Lee K, Edwards A, Kaplan DL. Vascularization strategies for tissue engineering. Tissue Engineering Part B: Reviews. 2009;15:353–70. doi: 10.1089/ten.teb.2009.0085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masoumi N, Johnson KL, Howell MC, Engelmayr GC., Jr Valvular interstitial cell seeded poly(glycerol sebacate) scaffolds: Toward a biomimetic in vitro model for heart valve tissue engineering. Acta Biomaterialia. 2013 doi: 10.1016/j.actbio.2013.01.001. DOI 10.1016/j.actbio.2013.01.001. [DOI] [PubMed] [Google Scholar]
- Meng Z, Wang Y, Ma C, Zheng W, Li L, Zheng Y. Electrospinning of PLGA/gelatin randomly-oriented and aligned nanofibers as potential scaffold in tissue engineering. Materials Science and Engineering: C. 2010;30:1204–10. [Google Scholar]
- Mihaila SM, Gaharwar AK, Reis RL, Marques AP, Gomes ME, Khademhosseini A. Photocrosslinkable Kappa-Carrageenan Hydrogels for Tissue Engineering Applications. Advanced Healthcare Materials. 2013:n/a–n/a. doi: 10.1002/adhm.201200317. [DOI] [PubMed] [Google Scholar]
- Motlagh D, Yang J, Lui KY, Webb AR, Ameer GA. Hemocompatibility evaluation of poly (glycerol-sebacate) in vitro for vascular tissue engineering. Biomaterials. 2006;27:4315–24. doi: 10.1016/j.biomaterials.2006.04.010. [DOI] [PubMed] [Google Scholar]
- Newman PJ, Berndt MC, Gorski J, White GC, Lyman S, Paddock C, Muller WA. PECAM-1 (CD31) cloning and relation to adhesion molecules of the immunoglobulin gene superfamily. Science. 1990;247:1219–22. doi: 10.1126/science.1690453. [DOI] [PubMed] [Google Scholar]
- Nguyen LT, Liao S, Chan CK, Ramakrishna S. Enhanced osteogenic differentiation with 3D electrospun nanofibrous scaffolds. Nanomedicine. 2012;7:1561–75. doi: 10.2217/nnm.12.41. [DOI] [PubMed] [Google Scholar]
- Nikkhah M, Edalat F, Manoucheri S, Khademhosseini A. Engineering microscale topographies to control the cell–substrate interface. Biomaterials. 2012a;33:5230–46. doi: 10.1016/j.biomaterials.2012.03.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikkhah M, Eshak N, Zorlutuna P, Annabi N, Castello M, Kim K, Dolatshahi-Pirouz A, Edalat F, Bae H, Yang Y, Khademhosseini A. Directed endothelial cell morphogenesis in micropatterned gelatin methacrylate hydrogels. Biomaterials. 2012b;33:9009. doi: 10.1016/j.biomaterials.2012.08.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pritchard CD, Arnér KM, Langer RS, Ghosh FK. Retinal transplantation using surface modified poly (glycerol-co-sebacic acid) membranes. Biomaterials. 2010;31:7978–84. doi: 10.1016/j.biomaterials.2010.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rai R, Tallawi M, Grigore A, Boccaccini AR. Synthesis, properties and biomedical applications of poly(glycerol sebacate) (PGS): A review. Prog. Polym. Sci. 2012;37:1051–78. [Google Scholar]
- Ravichandran R, Venugopal JR, Sundarrajan S, Mukherjee S, Ramakrishna S. Poly (Glycerol sebacate)/gelatin core/shell fibrous structure for regeneration of myocardial infarction. Tissue Engineering Part A. 2011;17:1363–73. doi: 10.1089/ten.TEA.2010.0441. [DOI] [PubMed] [Google Scholar]
- Ravichandran R, Venugopal JR, Sundarrajan S, Mukherjee S, Sridhar R, Ramakrishna S. Minimally invasive injectable short nanofibers of poly (glycerol sebacate) for cardiac tissue engineering. Nanotechnology. 2012;23:385102. doi: 10.1088/0957-4484/23/38/385102. [DOI] [PubMed] [Google Scholar]
- Sacks MS, Schoen FJ, Mayer JE., Jr Bioengineering challenges for heart valve tissue engineering. Annual review of biomedical engineering. 2009;11:289–313. doi: 10.1146/annurev-bioeng-061008-124903. [DOI] [PubMed] [Google Scholar]
- Sant S, Coutinho DF, Sadr N, Reis RL, Khademhosseini A. Biomimetic Approaches for Biomaterials Development. Wiley-VCH Verlag GmbH & Co. KGaA; 2012. pp. 471–93. [Google Scholar]
- Sant S, Hwang CM, Lee S-H, Khademhosseini A. Hybrid PGS–PCL microfibrous scaffolds with improved mechanical and biological properties. Journal of Tissue Engineering and Regenerative Medicine. 2011;5:283–91. doi: 10.1002/term.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sant S, Iyer D, Gaharwar AK, Patel A, Khademhosseini A. Effect of biodegradation and de novo matrix synthesis on the mechanical properties of VIC-seeded PGS-PCL scaffolds. Acta Biomaterialia. 2013;9:5963–73. doi: 10.1016/j.actbio.2012.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohier J, Carubelli I, Sarathchandra P, Latif N, Chester AH, Yacoub MH. The potential of anisotropic matrices as substrate for heart valve engineering. Biomaterials. 2014;35:1833–44. doi: 10.1016/j.biomaterials.2013.10.061. [DOI] [PubMed] [Google Scholar]
- Stevens MM, George JH. Exploring and engineering the cell surface interface. Science. 2005;310:1135–8. doi: 10.1126/science.1106587. [DOI] [PubMed] [Google Scholar]
- Sun ZJ, Chen C, Sun MZ, Ai CH, Lu XL, Zheng YF, Yang BF, Dong DL. The application of poly (glycerol–sebacate) as biodegradable drug carrier. Biomaterials. 2009;30:5209–14. doi: 10.1016/j.biomaterials.2009.06.007. [DOI] [PubMed] [Google Scholar]
- Sun ZJ, Sun CW, Sun B, Lu XL, Dong DL. The Polycondensing Temperature rather than Time Determines the Degradation and Drug Release of Poly(Glycerol-Sebacate) Doped with 5-Fluorouracil. Journal of Biomaterials Sciences Polymer Edition. 2011 doi: 10.1163/092050611X562157. [DOI] [PubMed] [Google Scholar]
- Sundback CA, Shyu JY, Wang YD, Faquin WC, Langer RS, Vacanti JP, Hadlock TA. Biocompatibility analysis of poly(glycerol sebacate) as a nerve guide material. Biomaterials. 2005;26:5454–64. doi: 10.1016/j.biomaterials.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Teixeira AI, Abrams GA, Bertics PJ, Murphy CJ, Nealey PF. Epithelial contact guidance on well-defined micro-and nanostructured substrates. Journal of Cell Science. 2003;116:1881–92. doi: 10.1242/jcs.00383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong Z, Sant S, Khademhosseini A, Jia X. Controlling the Fibroblastic Differentiation of Mesenchymal Stem Cells Via the Combination of Fibrous Scaffolds and Connective Tissue Growth Factor. Tissue Engineering Part A. 2011;17:2773–85. doi: 10.1089/ten.tea.2011.0219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng H, Puperi DS, Kim EJ, Ayoub S, Shah JV, Cuchiara ML, West JL, Grande-Allen KJ. Anisotropic poly (ethylene glycol)/polycaprolactone (PEG/PCL) hydrogel fiber composites for heart valve tissue engineering. Tissue Engineering. 2014 doi: 10.1089/ten.tea.2013.0397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagenseil JE, Mecham RP. Vascular Extracellular Matrix and Arterial Mechanics. 2009;89 doi: 10.1152/physrev.00041.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Kim YM, Langer R. In vivo degradation characteristics of poly(glycerol sebacate) Journal of Biomedical Materials Research Part A. 2003a;66A:192–7. doi: 10.1002/jbm.a.10534. [DOI] [PubMed] [Google Scholar]
- Wang YD, Ameer GA, Sheppard BJ, Langer R. A tough biodegradable elastomer. Nat. Biotechnol. 2002;20:602–6. doi: 10.1038/nbt0602-602. [DOI] [PubMed] [Google Scholar]
- Wang YD, Kim YM, Langer R. Journal of Biomedical Materials Research Part A. 2003b;In vivo degradation characteristics of poly(glycerol sebacate)66A:192–7. doi: 10.1002/jbm.a.10534. [DOI] [PubMed] [Google Scholar]
- Xie J, Li X, Lipner J, Manning CN, Schwartz AG, Thomopoulos S, Xia Y. “Aligned-to-random” nanofiber scaffolds for mimicking the structure of the tendon-to-bone insertion site. Nanoscale. 2010;2:923–6. doi: 10.1039/c0nr00192a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang F, Murugan R, Wang S, Ramakrishna S. Electrospinning of nano/micro scale poly (L-lactic acid) aligned fibers and their potential in neural tissue engineering. Biomaterials. 2005;26:2603–10. doi: 10.1016/j.biomaterials.2004.06.051. [DOI] [PubMed] [Google Scholar]
- Zhang H, Patel A, Gaharwar AK, Mihaila SM, Iviglia G, Mukundan S, Bae H, Yang H, Khademhosseini A. Hyperbranched polyester hydrogels with controlled drug release and cell adhesion properties. Biomacromolecules. 2013;14:1299–310. doi: 10.1021/bm301825q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Baughman CB, Kaplan DL. In vitro evaluation of electrospun silk fibroin scaffolds for vascular cell growth. Biomaterials. 2008;29:2217–27. doi: 10.1016/j.biomaterials.2008.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorlutuna P, Annabi N, Camci-Unal G, Nikkhah M, Cha JM, Nichol JW, Manbachi A, Bae H, Chen S, Khademhosseini A. Microfabricated biomaterials for engineering 3D tissues. Advanced Materials. 2012;24:1782–804. doi: 10.1002/adma.201104631. [DOI] [PMC free article] [PubMed] [Google Scholar]