Abstract
We examined the role of innate cells in acquired resistance to the natural murine parasitic nematode, Nippostrongylus brasiliensis. Macrophages obtained as late as 45 days after N. brasiliensis inoculation were able to transfer accelerated parasite clearance to naive recipients. Primed macrophages adhered to larvae in vitro and triggered increased mortality of parasites. Neutrophil depletion in primed mice abrogated the protective effects of transferred macrophages and inhibited their in vitro binding to larvae. Neutrophils in parasite-infected mice showed a distinct transcriptional profile and promoted alternatively activated M2 macrophage polarization through secretory factors including IL-13. Differentially activated neutrophils in the context of a type 2 immune response therefore prime a long-lived effector macrophage phenotype that directly mediates rapid nematode damage and clearance.
Keywords: Parasite, helminth, macrophage, neutrophil, type 2 response, TH2 cytokines
Helminth infection can result in chronic malnutrition, significant morbidity and increased susceptibility to lethal pathogens 1,2. A type 2 immune response is upregulated in humans and mice following helminth infection and components of this response can contribute to both host tolerance and resistance3-5. At present, effective vaccines against helminth parasites have proved elusive perhaps in part due to an incomplete understanding of how components of type 2 immunity mediate host protection and acquired resistance. Infective larval stages of many intestinal nematode parasites typically enter through the skin, migrate to the lung, and then to the enteric region. Studies have suggested that the lung may be an important organ for early parasite clearance in tissues and thus a potential target for vaccine-induced host resistance6.
Immune-mediated resistance to nematodes can include both innate and adaptive components of the type 2 immune response. The cytokines IL-4, IL-5, IL-9, and IL-13, produced by both TH2 cells and innate immune cell populations including eosinophils, basophils, mast cells, and type 2 innate lymphoid cells (ILC2s), promote specific effector mechanisms that contribute to anti-parasite resistance with adaptive immune responses generally thought to drive acquired resistance4,7. Immune components mediating anti-parasite resistance may include increased luminal fluid flow and intestinal muscle contractility8, antibody-dependent mechanisms9, mast cell production of mediators10, secretion of mucous and resistin-like molecule β (RELM-β)11, and effector mechanisms triggered by eosinophils12. Macrophages may also contribute to anti-parasite resistance13,14, although whether they mediate direct effects on helminths is unclear. Neutrophils, essential in resistance to microbial pathogens, have also been associated with helminth infection, in some cases surrounding the parasite in situ13,15. Other studies have suggested that neutrophils may cause damage following contact with helminths in vitro or in diffusion chambers implanted in vivo16. During infectious disease, neutrophils are thought to mediate end-stage effector functions with relatively little evidence as yet suggesting that they can promote the development of other immune effector cell populations. It should also be noted that these various anti-parasite resistance mechanisms have been primarily described in the context of intestinal resistance with as yet few studies identifying effector populations or mechanisms mediating protective immunity in the lung.
Nippostrongylus brasiliensis, an intestinal parasitic nematode of rodents, is a widely used experimental model where infective third-stage larvae (L3) migrate from the skin to the lungs before entering the small intestine.
The lung is an important site where both innate and adaptive components of the type 2 immune response interact with the migrating parasitic larval stages. Resistance mechanisms following primary inoculation lead to parasite expulsion from the intestine; in contrast parasite migration to the intestine following secondary inoculation is impaired with the lung being an important site of parasite clearance6,17. The immune mechanism of parasite damage in the lungs is not well understood and little studied. Although CD4+ T cells are likely required for priming, as the recall response is blocked in major histocompatibility class II (MHC-II) deficient mice6, neither CD4+ T cells18 nor B cells19 are required for the protective immunity leading to an accelerated worm clearance after secondary inoculation. This raises the intriguing possibility that the heightened immune protection compared to the primary response is not mediated through adaptive immunity.
Here we examined the immune mechanism leading to accelerated worm clearance after secondary inoculation with N. brasiliensis. Our findings suggest that neutrophils acquire an alternatively activated (N2) phenotype that is essential in providing helper functions that drive the development of a long-lived persistent macrophage phenotype that subsequently mediates parasitic larval damage during recall responses in the lung.
Results
Larvae induce persistent changes in lung immune cells
To examine whether accelerated parasitic larval clearance following a secondary inoculation was associated with changes in lung innate immune cell populations after primary inoculation, we performed a kinetics analysis of lung immune cell populations extending to three months after primary inoculation. Parasitic N. brasiliensis L3 typically enter the lung between 19 and 32 hours after subcutaneous inoculation, transiently reside in the lung for up to 50 hours, and subsequently migrate to the small intestine, where they are expulsed as early as day eight20. The parasitic larvae cause lung damage resulting in extensive neutrophil inflammation and hemorrhaging by day two, which is largely resolved by day seven after inoculation. Macrophages and eosinophils infiltrate the lung through lung blood vessels by day four21 (Supplementary Fig. 1a). To extend these observations, lung cells were isolated and identified by flow cytometry. Macrophages, defined phenotypically as F4/80hi, CD11cvar (CD11c variable), MHC-IIint-hi)22, were increased by day four and dramatically increased by day seven after inoculation. These elevated numbers were still observed in the lung even as long as three months after N. brasiliensis inoculation (Supplementary Fig. 1b). Similarly, eosinophils (F4/80int, MHCIIneg-lo, CD11clo, SiglecFhi) started to increase at day four, were markedly increased at day seven, but, in contrast to macrophages, decreased to baseline by three months after N. brasiliensis inoculation. Neutrophils also increased transiently, peaking at day two and returning to baseline by day seven. CD4+ T cells slightly increased at day seven and decreased at three months, while CD8+ T cells were unchanged from day two until three months after L3 inoculation (Supplementary Fig. 1b). After secondary inoculation, macrophages and eosinophils rapidly increased by day two after inoculation while neutrophils were decreased compared to primary inoculation, consistent with a more rapidly developing type 2 innate response (Supplementary Fig. 1c). Thus, macrophages persist in the lung for prolonged periods following primary inoculations after other innate immune cells have returned to near baseline.
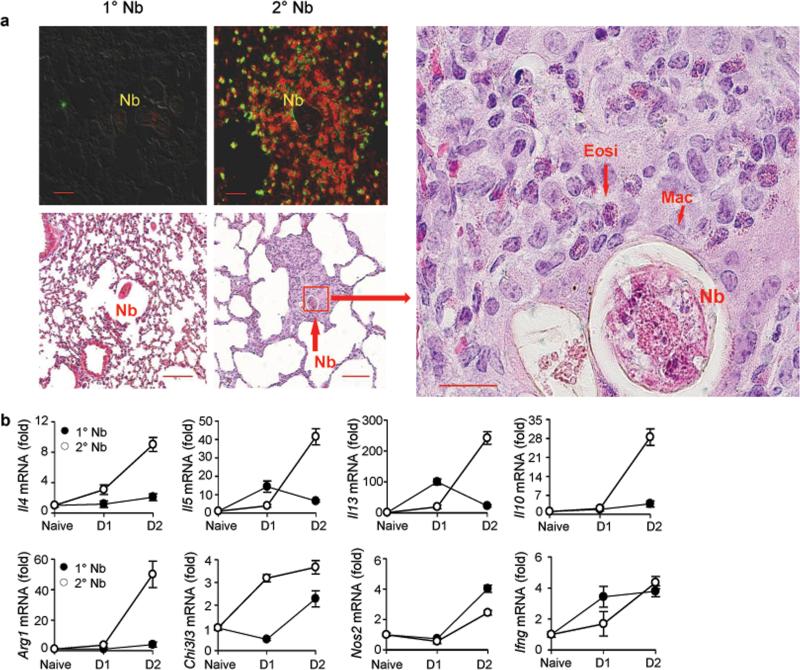
Immune cells surround larvae after secondary inoculation
The persistently elevated macrophages in lung tissue after primary N. brasiliensis inoculation raised the possibility that these innate immune cells may contribute to the accelerated parasite clearance following a secondary inoculation. To directly examine possible interactions between macrophages and invading parasitic larvae, lung tissue cryo-sections were examined at day two and three after N. brasiliensis inoculation. Parasitic larvae in the lung were not surrounded by immune cell populations after primary N. brasiliensis inoculation; however, macrophages and eosinophils were observed immediately surrounding the parasitic larvae after secondary inoculation, as observed using immunofluorescent staining with antibodies against eosinophil cell major basic protein (MBP-Alexa Fluor 488) and F4/80 (R-PE) at day two (Fig. 1a). Macrophages stained red (F4/80+, MBP-) while eosinophils stained yellow-green (MBP+, F4/80var). Histological H&E staining also showed accumulation of macrophages and eosinophils after secondary but not primary inoculation, corroborating the above observations (Fig. 1a). Fewer macrophages were observed in specific sections of the lung with both H&E and immunofluorescent staining after primary inoculation, and these cells were not clustered around the worm (Fig. 1a), To examine changes in gene expression after secondary inoculation, lung tissue was analyzed using quantitative fluorogenic real-time RT-PCR (qPCR). Marked increases in Il4, Il5, Il13, Il10, Arg1, and Chi3l3 (which encodes Ym-1) mRNA were observed as early as two days after secondary inoculation, a time point when relatively little change in these markers of type 2 immunity were observed after primary inoculation. Furthermore, Nos2 and Ifng (characteristic of type 1 responses) mRNA showed only modest changes after either primary or secondary inoculation (Fig. 1b), indicative of a highly polarized type 2 response. Thus, migrating larvae more rapidly encounter macrophages in the lung after secondary inoculation.
Figure 1. Eosinophils and macrophages surround parasitic larvae in the lung and type 2 related cytokines are upregulated shortly after secondary N. brasiliensis (Nb) inoculation.
(a) Top left panels: DIC and Immunofluorescence staining of lung cryosections stained for macrophages (F4/80, PE, red) and eosinophils (MBP, Alexa Fluor488, F4/80var, yellow-green) at day 2 after primary (1°) or secondary (2°) N. brasiliensis inoculation. Scale bars 20 μm. Image is representative of five independent staining preparations of lung tissue from 3 mice infected with N. brasiliensis. Bottom panels; Representative haematoxylin and eosin (H&E) staining of formalin-fixed lung sections. Scale bars, 20 μm. H&E images are representative of five sectional layers for each mouse with each layer 20 layers removed from the other. There were 5 mice for each treatment group for two independent experiments; Nb=larvae; Mac=macrophages; Eosi=eosinophil. (b) At one or two days after primary or secondary Nb inoculation, lung tissue gene expression was determined by qPCR. Gene expression is shown as the fold increase over naive wild type (WT) controls after normalization to 18s RNA. Data shown are the mean and SEM from five individual mice per group and are representative of two independent experiments.
Primed macrophages directly damage N. brasiliensis larvae
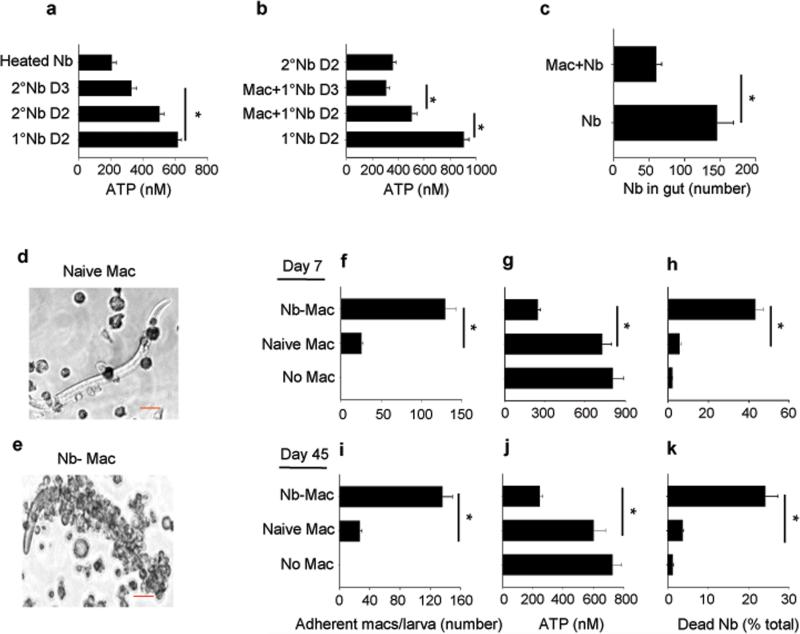
The presence of macrophages immediately surrounding the invading parasite in the lung shortly after secondary, but not primary, inoculation raised the possibility that macrophages were damaging invading larval parasites in the lung. To test this possibility, parasitic larvae were isolated at day two or three after N. brasiliensis L3 primary and secondary inoculation. The larval metabolic activity from secondarily infected mice, as determined by parasite ATP levels11 and as a measure of morbidity, was significantly decreased (Fig. 2a). To further test whether macrophages play a key role in damaging parasitic larvae after secondary inoculation, lung macrophages (F4/80+, CD11cvar, MHC-IIint-hi) obtained at day seven after N. brasiliensis-inoculation, were electronically sorted (>95% purity,-Supplementary Fig. 2a) and transferred to recipients, which were inoculated with N. brasiliensis two days later. At days two and three after N. brasiliensis inoculation of the recipient mice, larval ATP from lung tissue was decreased to concentrations similar to that from secondary inoculated mice (Fig. 2b). At day four after N. brasiliensis inoculation of the recipient mice, the parasite numbers in the gut were decreased compared to N. brasiliensisinoculated mice that did not receive lung macrophages, indicating protective mechanisms in the lung were preventing larval migration to the enteric region (Fig. 2c). Transfer of CFSE-labeled macrophages confirmed that donor macrophages are recruited to the lung (Supplementary Fig. 2b). Lungs of recipient mice showed increased eosinophils and decreased neutrophils as early as day 2 after inoculation (Supplementary Fig. 2c). Il4, Il5, Il13, and Chi3l3 mRNA were elevated, while no changes were detected in Il10, Arg1 and Nos2 mRNA (Supplementary Fig. 2d, 2e). Recent studies have suggested that macrophages can inhibit parasite migration from the site of inoculation in the skin after N. brasiliensis secondary inoculation23. To test whether the transferred primed macrophages could also mediate this effect in naive mice, at day 7 after N. brasiliensis primary inoculation, lung macrophages were electronically sorted, transferred to naive recipient wild-type (WT) mice, and two days later mice were inoculated with N. brasiliensis. At day two after inoculation of recipient mice, parasite numbers in the skin were significantly increased in recipient mice with macrophages from N. brasiliensis-primed WT mice compared to recipient mice with lung macrophages from untreated donor mice (Supplementary Figure 2f). Thus, transferred primed lung macrophages could mediate resistance at the site of subcutaneous inoculation, as well as in the lung.
Figure 2. Macrophages from Nb-primed mice directly damage Nb larvae.
(a) Helminth ATP concentrations were determined for larvae from lung tissue of mice 2 or 3 days after primary (1°) or secondary (2°) Nb inoculation. Heat killed Nb larvae were included as control (Heated Nb). (b) At day 7 after Nb inoculation, donor macrophages were electronically sorted (F4/80hiMHCIIint-hiCD11cvar) and transferred to recipient mice. Larvae were assessed for ATP concentrations at day 2 and 3 after inoculation; controls included larvae ATP concentrations after primary and secondary inoculation. (c) Experiment was performed as in (b) except that recipient mice were examined for intestinal worms at day 4 after inoculation and controls included mice not receiving macrophages. Mean and SEM from 5 mice per treatment group, and experiments were performed two times with similar results. (d-k) Lung macrophages were sorted from naive or from Nb-primed mice at day 7 (d-h) or day 45 (i-k) after inoculation, seeded to 24-well plates (2 × 106 cell/well) and co-cultured with 200 exsheathed third stage larvae (L3); control group included culture with L3 only. At day 5 after culture, macrophages were microscopically imaged (scale bars, 20 μm) (d and e) and the numbers of adherent macrophages (f, i), the worm ATP concentration (g, j), and percent mortality (h, k) were determined. Mean and SEM of triplicate samples obtained from 20 larva from each well for adherent macrophages and 10 larva from each well for determination of ATP concentration; data were adjusted to per worm values. This experiment was repeated three times with similar results. *p<0.01
To directly examine whether macrophages were capable of causing parasite damage in vitro, macrophages were isolated by electronic cell sorting from lungs of naive mice or of mice at day seven after primary inoculation and cultured in vitro with exsheathed L3 larvae. While macrophages from naive mice did not adhere to exsheathed larvae, macrophages from N. brasiliensis-inoculated mice directly adhered in significant numbers to exsheathed larvae (Fig. 2d - f). The worm ATP concentration were significantly decreased (Fig 2g) and the number of nonmotile straightened larvae, with non-refractive internal structures, indicative of inactive dead larvae, were increased following 5 days of culture of larvae with macrophages from primed mice compared to cultures of larvae with macrophages from naive mice, or compared to cultures of larvae without macrophages (Fig. 2h). The larval cultures with naive macrophages were no different with respect to ATP concentration or mortality than the cultures with larva alone (Fig. 2j-h). To examine whether this anti-helminth effector macrophage phenotype persisted for longer time points after primary inoculation, macrophages were isolated at day 45 after primary inoculation. Even at this much later time point macrophages still effectively adhered to parasitic larvae and mediated worm damage and killing to similar extents as macrophages isolated at day 7 after primary inoculation (Fig. 2i-k). These findings thus indicate that macrophages from N. brasiliensis-primed mice preferentially adhere to N. brasiliensis larvae and cause decreased metabolism and increased mortality. Taken together, these studies indicate that macrophages from primed donor mice can directly mediate worm killing, accelerate type 2 immune responses in the lung, and ultimately enhance worm clearance in naive recipient mice after primary inoculation similar to that observed in mice following a secondary inoculation.
Macrophage-mediated resistance requires IL-4R signaling
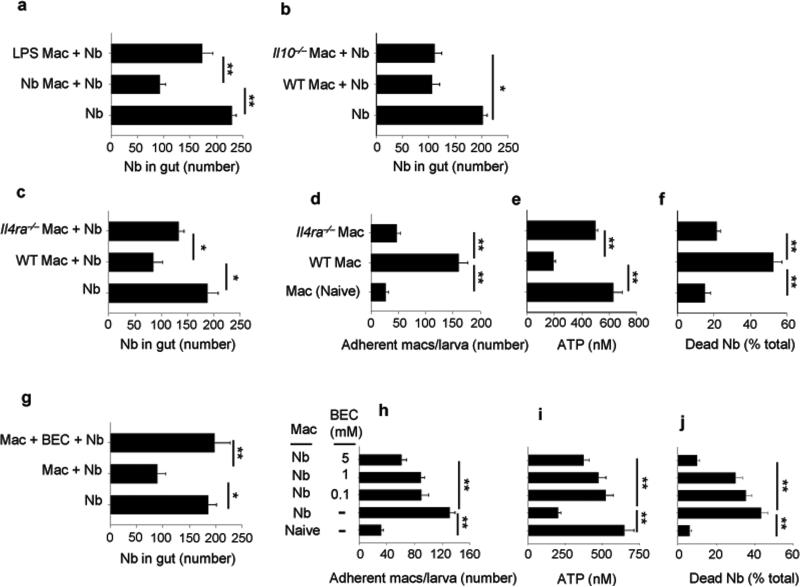
Macrophages can polarize to classically activated macrophages (M1) by bacterial stimulation or alternatively activated macrophages (M2) by helminth infection24. We next examined whether the type 2 immune environment typically associated with helminth infection was also an essential component for the development of the effector macrophages that could mediate enhanced worm expulsion. WT mice were inoculated with N. brasiliensis or administered lipopolysaccharide (LPS), the latter bacterial product promotes M1 macrophage differentiation25,26. Seven days later, macrophages were electronically sorted, transferred to naive recipient WT mice, and two days later mice were inoculated with N. brasiliensis. At day five after inoculation, recipient mice with donor macrophages from N. brasiliensis-inoculated mice, but not from mice administered LPS, showed significantly reduced worm numbers in the gut (Fig. 3a). Transfer of macrophages from naive donor mice to recipient mice had no effect on worm burden, excluding the possibility that transfer of undifferentiated macrophages may be sufficient to promote immunity (Supplementary Figure 2g). We next investigated signaling pathways that may potentially drive the differentiation of the effector anti-helminth macrophages after N. brasiliensis inoculation. Previous studies have shown that after N. brasiliensis inoculation M2 macrophage polarization in the lung is impaired by blocking IL-4Rα signaling but not IL-10 signaling21. Donor WT, Il4ra–/–, and Il10–/– mice were inoculated with N. brasiliensis, macrophages from lungs electronically sorted at day seven and transferred to naive recipient mice, which were then inoculated with N. brasiliensis two days later. At day five after inoculation of recipient mice, parasite numbers in the gut were decreased in recipient mice with macrophages from N. brasiliensis-primed WT and Il10–/– donor mice, but not from N. brasiliensis-primed Il4ra–/– donor mice (Fig. 3b and c). To directly examine whether macrophages from N. brasiliensis-inoculated Il4ra–/– mice showed impaired anti-parasite effects, macrophages from N. brasiliensis-inoculated WT and Il4ra–/– mice were isolated by electronic cell sorting on day 7 after inoculation and cultured with exsheathed-L3 larvae, as already described. Five days later, macrophages from Il4ra–/– mice showed marked reductions in effector function, including pronounced decreases in macrophage-parasite binding (Fig. 3d), markedly reduced capacity to impair worm metabolism, as measured by ATP concentration (Fig. 3e), and larval mortality (Fig. 3f). Previous studies have shown that blockade of Arg1 (arginase) can impair effective worm expulsion13. To examine this possibility in this experimental model, the Arg1 inhibitor, S-(2-bromoeethyl)-L-cysteine (BEC), a specific competitive inhibitor of Arg1 with limited off target effects27-29 , was administered to recipient mice after transfer of macrophages from N. brasiliensis-primed WT donor mice. Arg1 blockade inhibited reduction in parasite numbers in the gut after primary N. brasiliensis inoculation of recipient mice (Fig. 3g) and addition of BEC in cultures of larva with macrophages (cell viability, 90%) from primed mice, resulted in decreased macrophage adherence (Fig. 3h), increased worm ATP concentration (Fig. 3i), and decreased mortality (Fig. 3j). Thus, the development of activated anti-helminth effector macrophages requires IL4R signaling in the context of N. brasiliensis infection.
Figure 3. IL-4R signaling was required for Nb-primed macrophage-mediated resistance to Nb.
WT donor mice were administered LPS or Nb infective larvae (a) or WT, Il4ra–/–, and Il10–/– donor mice were inoculated with Nb (b-c). At day 7 after inoculation electronically sorted donor macrophages were transferred to naive recipient mice, which were inoculated with Nb 2 days later. Five days post-infection gut parasites were enumerated. At day 7 after inoculation electronically sorted macrophages from WT or Il4ra–/– mice were seeded to 24-well plates (2 x 106 cells/well) and co-cultured with 200 exsheathed L3 larvae. At day 5 after culture, larval adherence was determined (d), and larval ATP concentration (e) and percent mortality (f) assessed. (g) WT donor mice were inoculated with Nb and, at day 7 after inoculation, electronically sorted macrophages were transferred to naive recipient mice, with or without administered Arg1 inhibitor BEC, which were then infected with Nb 2 days later. A control group was similarly treated but did not receive donor macrophages. Five days later gut parasites were enumerated. Mean and SEM are shown for five mice/treatment group (a, b, c, g), and these experiments were repeated two times with similar results. (h-j) At day 7 after inoculation electronically sorted macrophages from WT were seeded to 24-well plates (2 × 106 cells/well) and co-cultured with 200 exsheathed L3 larvae, with or without the presence of BEC at different concentrations (0.1-5 mM). At day 5 after culture, larval adherence was determined (h), and larval ATP concentration (i) and percent mortality (j) were assessed. Mean and SEM of triplicate samples (d-f, h-j) are shown for pools of 5 mice/treatment group. This experiment was repeated three times with similar results. *p<0.05, **p<0.01.
Protection in secondary infections requires neutrophils
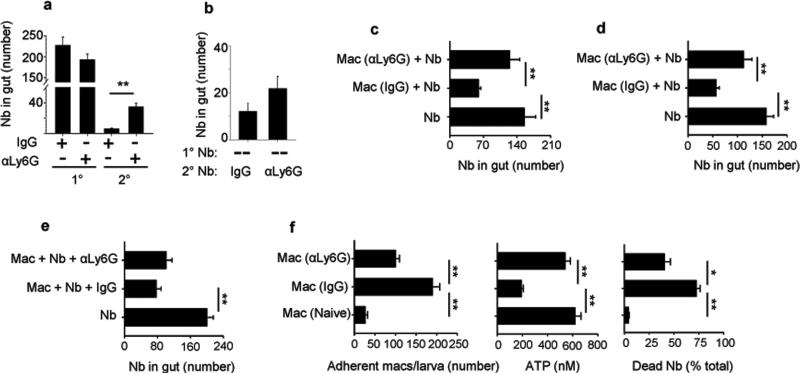
Neutrophils, are key players in type 1 innate immune responses, they provide a first line of defense against invading microorganisms, but few studies have examined their role in resistance against metazoan parasites such as helminths16,30. Neutrophils infiltrate the lung at day two after primary N. brasiliensis inoculation21. To investigate whether neutrophils contribute to host protection against parasitic larvae, neutrophils were depleted in N. brasiliensis-inoculated mice by in vivo administration of antibodies specific to lymphocyte antigen 6 complex, locus G (Ly6G). Administration of anti-Ly6G antibody at day one prior to parasite primary inoculation, and day three and day seven after and day one prior to secondary inoculation and day three after resulted in significantly impaired worm expulsion at day five after secondary inoculation (Fig 4a). Administration of anti-Ly6G antibody at day one prior to and day three after secondary inoculation did not affect the ability of the host to rapidly expel the parasite from the intestine (Fig. 4b). Neutrophil depletion was confirmed by flow cytometry of blood and whole lung (Supplementary Fig. 3a,b). To determine whether the effects of neutrophil depletion altered TH2 cytokine production after primary inoculation, lung tissue was collected and TH2-related cytokine gene expression was determined by qPCR at day 7 after inoculation. Il4, Il13, and Il10 mRNA were up-regulated and not affected after neutrophil depletion during both primary and secondary infection (Supplementary Fig. 3c). However, at day 1 after inoculation, elevations in IL-13 were significantly inhibited after neutrophil depletion (Supplementary Fig. 3d). These observations indicate that neutrophil depletion during priming impairs helminth expulsion after secondary inoculation, and results in a reduction in IL-13 elevations shortly after N. brasiliensis inoculation, but by day 7 TH2 cytokine mRNA levels are largely restored.
Figure 4. Neutrophils are required for differentiation of effector M2 macrophages after Nb primary inoculation.
Parasite numbers were determined for mice administered neutrophil-depleting anti-Ly6G antibody or isotype control at days −1 and 3 after secondary inoculation (2°) (a), or at days -1, 3, and 7 after primary (1°) and days -1 and 3 after secondary (2°) inoculation (b). Donor mice were treated with anti-Ly6G antibody or isotype control on days −1 and 3 (c), or days -1, 3, and 7 (d), after inoculation. At day 7 (c), or day 41(d) after inoculation, macrophages were collected from the lung and transferred to naive recipient mice, which were inoculated 2 days later and assessed for worm expulsion from the intestine on day 5 (c) or day 4 (d) after inoculation. (e) Donor mice were sacrificed at day 7 after Nb inoculation and macrophages were isolated from the lung and transferred to naive recipient mice, which were then treated with anti-Ly6G antibody or isotype control on days −1 and 3 after Nb inoculation. Parasite numbers were assessed in gut at day 5. Data are shown as mean and SEM of 5 mice/group. (f) Nb-inoculated mice administered anti-Ly6G antibody (αLy6G) or isotype control (IgG) were sacrificed at day 7, lung macrophages were electronically sorted and co-cultured with exsheathed L3 larvae. At day 5 after culture, the number of adherent macrophages/L3 was determined and larval damage assessed by parasite ATP concentrations and percent mortality. Mean and SEM of triplicate samples obtained from pools of 5 mice/treatment group. This experiment was repeated three times with similar results. *p<0.05, **p<0.01.
Neutrophils mediate development of effector macrophages
Unlike macrophages, neutrophils return to baseline numbers in the lung within several days after N. brasiliensis inoculation (Supplementary Fig. 1), making it unlikely that they directly mediate worm clearance, but raising the possibility that they may indirectly contribute to worm clearance by providing signals for effector macrophage development during the primary response. To test this possibility, neutrophils were depleted in donor mice by administration of anti-Ly6G antibody at day one prior to and day three after N. brasiliensis primary inoculation. At day seven after inoculation, electronically sorted macrophages were transferred from donor mice to naive recipient mice, which were inoculated with N. brasiliensis two days later. At day five after primary inoculation, the parasite numbers in the gut were decreased in recipient mice that had received a macrophage transfer from donor mice with intact neutrophil populations, when compared to mock-transferred control mice receiving vehicle only. However, macrophage transfer from donor mice with depleted neutrophil populations did not result in enhanced worm expulsion in the intestine (Fig 4c). These studies thus indicated that neutrophils were required for priming of effector macrophages. To examine how persistent this effector macrophage phenotype was after priming, macrophages, isolated from donor mice at day 41 after primary N. brasiliensis inoculation, were transferred to naive recipient mice. Transferred macrophages still mediated effective worm expulsion in recipient mice at day four after inoculation. However, if donor mice were depleted of neutrophils by administration of anti-Ly6G antibody at day one prior to and day three and seven after inoculation, then transferred macrophages, at day 41 after inoculation, did not mediate accelerated worm expulsion (Fig. 4d). It was possible that this persistent macrophage phenotype was dependent on B cells either secreting factors that contribute to maintaining a type 2 microenvironment in the lung or possibly secreting Abs that would then arm macrophages through Fc receptor binding, thereby facilitating macrophage adhesion to larval parasites. To test this possibility, B cell deficient Jh–/– donor mice were inoculated with N. brasiliensis and 41 days later sorted macrophages were transferred to naive recipient WT mice. Transferred lung macrophages from N. brasiliensis inoculated Jh–/– mice enhanced worm expulsion similar to primed lung macrophages from WT mice, indicating that B cells are not required for the persistent anti-helminth macrophage phenotype (Supplementary Fig. 2h).
To examine whether neutrophils also played a direct role in mediating worm expulsion after secondary inoculation, macrophages were transferred from N. brasiliensis-infected mice at day seven after inoculation to naive recipients, which were then administered anti-Ly6G antibody or the isotype control at day one prior to and day three after inoculation. Transfer of macrophages previously exposed to parasites effectively accelerated worm expulsion even in recipient mice depleted of neutrophils (Fig. 4e). The requirement of neutrophils for the development of effector macrophages that could mediate accelerated worm expulsion in vivo suggested that neutrophils may directly affect the capacity of these effector macrophages to cause parasite damage. To investigate this possibility, macrophages from N. brasiliensis primed mice administered anti-Ly6G antibody or isotype control were cultured with exsheathed L3 larvae for five days. Macrophages from primed mice administered anti-Ly6G antibody showed marked reductions in larval adherent cells, significant increases in worm ATP, and decreased larval mortality compared to macrophages from primed mice given the isotype control antibody (Fig. 4f).
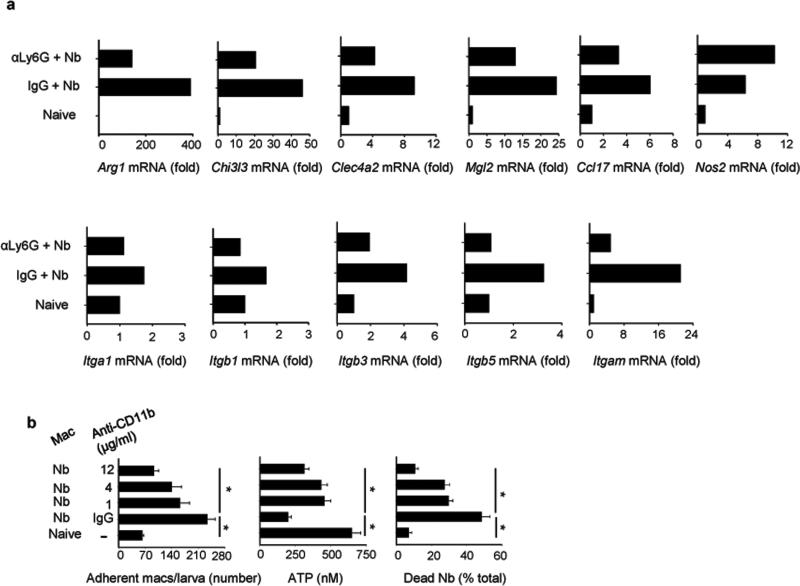
To investigate whether depletion of neutrophils altered the accumulation of immune cell populations, total lung macrophages and eosinophils were assessed in N. brasiliensis-inoculated mice administered either anti-Ly6G or the isotype control. Although macrophages were not affected, consistent decreases in lung eosinophils were observed at day two after inoculation in primed mice administered anti-Ly6G (Supplementary Figure 3a-b). Neutrophil depletion significantly decreased Chi3l3 expression at day seven after primary inoculation (Supplementary Figure 3c). Since the chitinase gene Chi3l3 is characteristic of M2 macrophages, sorted macrophages from N. brasiliensis-infected mice depleted of neutrophils or treated with an isotype control antibody were examined by qPCR for upregulation of relevant genes associated with macrophage activation. Elevations in M2 signature genes, Arg1 and Chi3l3, in highly purified macrophages were partially neutrophil dependent. Furthermore, the lectins Mgl2 and Clec4a2, also associated with M2 polarized macrophages31,32, were downregulated in macrophages from neutrophil-depleted N. brasiliensis inoculated mice. Ccl17, a chemokine that is elevated in M2 polarized macrophages33, was also decreased. In contrast, no decrease was observed in Nos2 mRNA, an M1 macrophage marker, after neutrophil depletion (Fig. 5a). Integrin expression (Itga1, Itgb1, Itgb3, Itgb5) was also examined and elevations in Itgam (the gene encoding integrin alpha M also known as CD11b), associated with leukocyte adhesion and anchoring34, were markedly decreased after neutrophil depletion (Fig. 5a).
Figure 5. Depletion of neutrophils impaired effector lung macrophage polarization after primary Nb inoculation.
(a) Macrophages were electronically sorted from lung of naive mice or mice at day 7 after Nb inoculation treatment with αLy6G or isotype control (day -1, 3). Gene expression for characteristic M1 and M2 macrophage markers and integrins were analyzed by qPCR. Data shown are the mean from a pool of five mice per group (expressed as fold increase over mRNA from lungs of naive mice) and are representative of two independent experiments. (b) Flow sorted macrophages from WT mice at day 7 post infection were seeded to 24-well plates and co-cultured with exsheathed L3 larvae, with or without the presence of anti-CD11b antibody at different concentration. At day 5, larval adherence, larval ATP concentration, and percent mortality were assessed. Mean and SEM of triplicate samples obtained from pools of 5 mice per treatment group. This experiment was repeated three times with similar results. *p<0.01
The marked dependence of macrophage Itgam mRNA expression on the presence of neutrophils raised the possibility that CD11b may be an important mediator of neutrophil-dependent anti-helminth macrophage effector function. To examine a potential role for CD11b, macrophages from N. brasiliensis-primed mice were cultured with N. brasiliensis larvae in the presence of CD11b blocking antibodies (anti-CD11b Ab). Significant decreases in macrophages binding to parasites were observed with increasing concentrations of anti-CD11b blocking antibody. Furthermore, direct effects of macrophages on worm metabolism and mortality were impaired following CD11b blockade (Fig 5b). To examine whether CD4 T cells were required for neutrophil recruitment, mice were administered depleting anti-CD4 (GK1.5) antibody two days before primary N. brasiliensis inoculation. Therefore neutrophil recruitment was not affected by CD4 T cell depletion (Supplementary Figure 4c).
Parasite-primed neutrophils express a distinct transcriptome
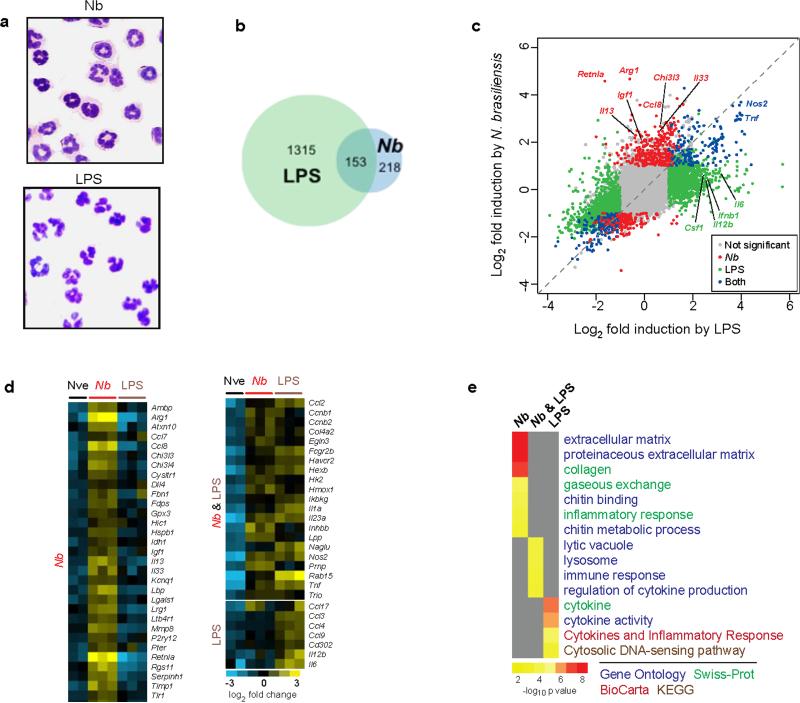
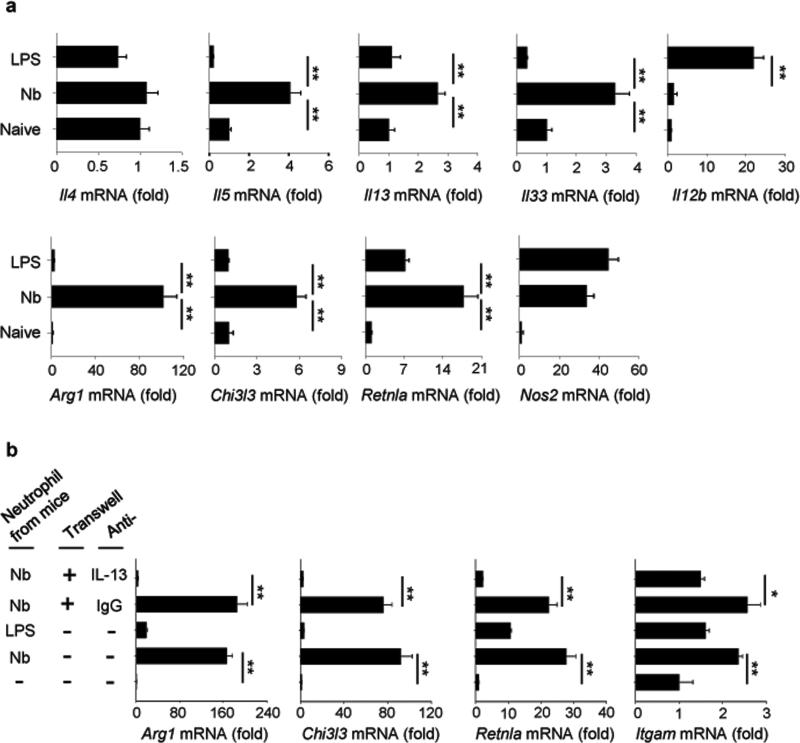
To explore the mechanism of helminth-induced neutrophil interactions with macrophages, mice were inoculated with L3 larvae or were intratracheally inoculated with LPS to drive a predominantly type 1 response. Two days later, lung neutrophils were electronically sorted by selecting Ly6Ghi and CD11bhi. There were no detectable basophils (FcεR1+, DX5+) in the neutrophil sorted population (Supplementary Figure 4). Sorted cells were cytospun, and stained with Wright-Giemsa. Purity of the neutrophil cell population was confirmed by homogeneity of stained cells on cytospin preparations, with macrophage or eosinophil phenotypes not detected. Neutrophils from N. brasiliensis-inoculated mice exhibited a characteristic phenotype with a ring-form nucleus. In contrast, neutrophils from LPS-treated mice exhibited a more typical multilobular nucleus, similar to sorted lung neutrophils from naive mice (Fig. 6a). To further characterize lung neutrophil populations in the context of a helminth-induced type 2 immune response, mRNA was isolated from sort-purified lung neutrophils from untreated, LPS-inoculated, and N. brasiliensis inoculated mice and analyzed on whole genome gene expression microarrays. Relative to untreated controls, neutrophils showed a distinct cytokine gene expression pattern when activated with LPS versus N. brasiliensis (Fig. 6b). Although 153 genes were similarly upregulated in neutrophils following N. brasiliensis or LPS inoculation, 218 genes were uniquely upregulated after N. brasiliensis inoculation while 1315 genes were only upregulated after LPS inoculation. Neutrophils from N. brasiliensis-inoculated mice uniquely upregulated Il13, Il33, Igf1, Retnla, and Chi3l3, while LPS-inoculated mice showed distinct elevations in Il6 and Il12b (Fig. 6c). Tnf and Nos2 were similarly upregulated in both treatments. A heat map was generated using genes characteristically upregulated by immune cells during type 1 and type 2 immune responses. Neutrophils showed a distinctive gene expression pattern, with numerous genes associated with type 2 immune responses elevated after N. brasiliensis but not LPS inoculation (Fig. 6d). Additionally, functional enrichment analysis using DAVID35 further confirmed a distinctive transcriptional signature in neutrophils from N. brasiliensis-inoculated mice, which was significantly enriched in genes that are part of known type 2-associated pathways such as collagen, extracellular matrix, and chitin metabolism. In contrast, neutrophils from LPS-inoculated mice showed enrichment in immune response genes characteristic of the type 1 response (Fig. 6e). QPCR of select genes associated with type 1 and 2 immune responses were used to corroborate findings with global transcriptome analyses. Highly purified neutrophils from N. brasiliensis-inoculated mice, but not LPS-inoculated mice, showed elevations in Il5, Il13, and Il33 but not Il4 and also increases in M2 markers, while lung neutrophils from LPS inoculated mice showed elevations in Il12b (Fig. 7a). Neutrophils, but neither macrophages nor CD4+ T cells from N. brasiliensis-inoculated mice producing IL-13 and IL-33 were further detected by stimulating lung cells with PMA-ionomycin following antibody staining and flow cytometry analysis at day 2 after primary inoculation (Supplementary Fig. 5).
Figure 6. Nb-primed neutrophils show a distinct gene expression profile.
Mice were inoculated with Nb L3 or intratracheally administered LPS. Two days later neutrophils were purified from lung tissue of untreated controls and treatment groups using electronic cell sorting. (a) Wright-Giemsa staining of sorted CD11b+Ly6G+ cells from the lung showed characteristic homogeneous neutrophil populations in each treatment group. (b) Global intersectional overview of upregulated transcripts relative to untreated controls by microarray analysis from sorted neutrophils obtained from lungs of naive mice (Nve) or mice infected with Nb or LPS. Genes were determined to be significantly differentially regulated by Significance Analysis of Microarrays (3% FDR, 1.5-fold change) (c) Scatterplot of gene induction by LPS and/or Nb relative to untreated controls. (d) Heat map presentation of selected representative genes characteristic of polarized type 1 and type 2 immune responses. (e) Functional analysis of gene expression showing characteristic categories according to classifications from the Gene Ontology (GO) project, SwissProt, BioCarta, and KEGG and aggregated in DAVID.
Figure 7. Nb-primed neutrophils polarize to an alternatively activated neutrophil phenotype that promotes M2 macrophage differentiation in vitro.
(a) Gene expression of characteristic immune response markers, identified by microarray, from sorted neutrophils (CD11b+Ly-6G+) analyzed by qPCR. Data shown are the mean of triplicate samples from a pool of five mice per group (expressed as fold increase over mRNA from neutrophils of naive mice) and are representative of two independent experiments. (b) Bone marrow derived macrophages (BMDM) were cultured with neutrophils from the lung of mice infected with Nb or treated with LPS. The neutrophil culture was separated from macrophages by transwell in some group, and anti-IL-13 antibody (10 μg /ml) was also added to the culture in some groups. At day 3, Arg1, Chi3l3, Retnla, and Itgam gene expression was determined by qPCR. Gene expression is presented as the ratio of treated/BMDM group without neutrophils added. Data shown are the mean and SEM of triplicate samples and repeated three times with similar results. *p<0.05, **p<0.01.
The known effect of IL-13 on M2 cell differentiation raised the possibility that neutrophils may enhance M2 cell differentiation by their secretion of IL-13. To test this possibility, bone marrow derived macrophages were cultured with neutrophils from lungs of N. brasiliensis- or LPS-inoculated mice at day 2 after treatment. Marked increases in macrophage Arg1, Chi3l3, Retnla, and Itgam were observed only after coculture with neutrophils from N. brasiliensis-inoculated mice (Fig. 7b). To investigate whether neutrophil-derived IL-13 might be contributing to M2 cell differentiation, N. brasiliensis activated neutrophils were cultured with bone marrow derived macrophages in separate chambers in transwell plates. Although separated by a 0.4 μm semi-permeable membrane N. brasiliensis-activated neutrophils still stimulated differentiation of M2 markers, when cultured with an isotype control antibody. However, administration of blocking anti-IL-13 Ab effectively inhibited mRNA elevations in M2 markers and in Itgam (Fig. 7b). These results indicate that soluble factors, including IL-13, provide essential neutrophil-derived signals for M2 cell polarization. Taken together, our findings suggest that shortly after priming, neutrophils polarize to a distinctive phenotype and provide essential signals to macrophages causing them to differentiate to effector macrophages with anti-helminth functions (Supplementary Figure 6).
Discussion
Both plants36 and invertebrates37 exhibit acquired resistance and furthermore studies indicate that vertebrate innate immune compartments exhibit recall responses (so-called ‘trained immunity’)38. Our studies indicate that macrophages primed during a type 2 immune response and under the influence of neutrophils maintain a long-lived phenotype that mediates accelerated helminth expulsion in the absence of T or B cells. This is consistent with a study suggesting that lung macrophages can maintain a long-lived desensitized state after lung influenza infection39. The long-lived lung macrophage phenotype may be an important target for vaccine development against helminths as primed effector macrophages alone were sufficient to trigger accelerated worm expulsion in naive recipient mice. Identification of this persistent effector macrophage phenotype may explain previous studies where CD4+ T cell depletion after secondary N. brasiliensis inoculation did not impair resistance18. The persistence of M2 macrophages is associated with the development of fibrosis and emphysema-like impaired lung function40,41. It may be that the persistence of the M2 macrophages requires a type 2 immune microenvironment. Alternatively, a more fixed phenotype, not as dependent on extrinsic signals, may develop in M2 macrophages perhaps associated with stimulus-responsive regulatory molecules or epigenetic changes as recently described in innate immune cells42,43. Studies suggest that basophil-primed macrophages in the skin inhibit migration of N. brasiliensis L3 from the subcutaneous site of inoculation23. We now show that transfer of primed lung macrophages to naive recipients inhibits parasite migration from the skin after primary inoculation, indicating that these primed anti-helminth macrophages also affect migrating larvae in skin, as well as the lung, shortly after inoculation.
Neutrophils are often studied during microbial infections in the context of type 1 immune responses44. However, neutrophils are also recruited to sites of helminth infection15,21 and when combined with macrophages in vitro can damage helminths16,45. Different subsets of neutrophils were previously identified in a Staphylococcus aureus infection model with one subset promoting M2 macrophage differentiation and also exhibiting a ring-form nucleus46. Our studies now demonstrate that helminth infection triggers the development of an alternatively activated neutrophil (‘N2’) population that shows a characteristic global transcriptional profile distinct from LPS-activated neutrophils (‘N1’). Although there was overlap between the two differentially activated neutrophil subsets, a number of genes were uniquely upregulated, including genes characteristic of type 2 responses. Our studies further showed that N2 neutrophils specifically activated in the context of helminth infection and IL-4R signaling mediated development of effector anti-helminth macrophages. Furthermore, neutrophil-mediated M2 macrophage differentiation was dependent on IL-13 production by neutrophils, although other soluble factors may also be involved. Adherence of in vivo N. brasiliensis-primed macrophages to parasitic larvae was blocked if neutrophils were depleted during priming. Indeed, neutrophil granule proteins can enhance monocyte adhesion mediated by beta-2 integrins47. Elevations in M2 markers and integrins were accordingly reduced in macrophages from neutrophil-depleted N. brasiliensis-primed mice. Our findings thus suggest that during helminth infection a specific N2 population develops that can interact with macrophages to upregulate both M2 markers and adhesion molecules.
Close interactions of macrophages with parasitic larvae were also observed in vivo after secondary but not primary inoculation, consistent with primed macrophages surrounding and adhering to invasive larvae. Large numbers of macrophages adhering to migrating larvae may impair chemosensory function and access to nutrients, thereby contributing to parasite damage. Our findings that upregulated Itgam expression on macrophages during N. brasiliensis infection was dependent on neutrophils and that blocking CD11b interactions inhibited macrophage adhesion to parasites indicates one mechanism through which neutrophils may promote anti-helminth macrophage effector function. Indeed, complement-opsonized adhesion of Leishmania promastigotes to macrophages is CD11b-dependent.48 It will be of interest to examine whether complement plays a role in opsonizing N. brasiliensis larval parasites for macrophage adhesion. Furthermore our studies indicated an important role for arginase 1 in parasite damage and killing which is consistent with studies suggesting that arginase 1 blockade can impair host resistance to tissue-dwelling metazoan parasites13,23.
Eosinophils also contribute to protective immunity to helminth infection because transgenic mice with increased blood eosinophils show increased helminth resistance49. Our immunofluorescence analyses of infiltrates surrounding invading larvae in the lung indicate rapid accumulation of both macrophages and eosinophils, suggesting that eosinophils might also contribute to larval killing in vivo. It should be noted, however, that highly purified macrophages from helminth-infected mice were sufficient to kill N. brasiliensis larvae in vitro. Basophils have also been reported to mediate accelerated protective responses after secondary N. brasiliensis inoculation, although other studies have suggested they may not be essential50. Further studies are needed to examine the role of basophils, eosinophils and now neutrophils interacting with macrophages to mediate acquired resistance.
Materials and Methods
Mice
Female five–eight week old BALB/c mice were purchased from NCI. Il4ra−/− BALB/c mice and Il10–/– BALB/c mice were purchased from The Jackson Laboratory (Bar Harbor, ME). Jh–/– BALB/c mice were from TACONIC Laboratory (Hudson, NY). Colonies of genetically deficient mice were maintained and used with WT controls at ages 6-20 weeks. All mice were maintained in a specific pathogen-free, virus Ab-free facility during the experiments. Healthy mice were selected for treatment groups from purchased or bred colonies, without using specific randomization methods or specific blinding methods. No samples or animals were excluded from the analyses. The studies have been reviewed and approved by the Institutional Animal Care and Use Committee at Rutgers-the State University of New Jersey. The experiments herein were conducted according to the principles set forth in the Guide for the Care and Use of Laboratory Animals, Institute of Animal Resources, National Research Council, Department of Health, Education and Welfare (US National Institutes of Health).
Parasite inoculation, antibody, LPS, and S-(2-bromoeethyl)-L-cysteine (BEC) administration
Nippostrongylus brasiliensis L3 were incubated with 400U penicillin, 400 μg ml−1 streptomycin plus 400 μg ml−1 Neomycin (GIBCO, Rockville, MD) for 2 h at room temperature, and then washed with sterile PBS, as described previously51. For neutrophil depletion, Ly6G-specific-antibody (BioXcell, West Lebanon, NH) or isotype control IgG was administered to mice both intraperitoneally (i.p.) (0.5 mg in 0.2 ml) and intratracheally (i.t.) (0.2 mg in 0.05 ml) one day before and 3 or 7 day after parasite inoculation, as described previously21. To deplete the CD4 T cell function in vivo, 800 ug of anti-CD4 mAb (GK1.5) was given by i.p. administration 2 days before N. brasiliensis infection. LPS (Lipopolysaccharides from Escherichia coli, Sigma, St. Louis, MO) was administered to mice i.t (50 μg in 50 μl of PBS). BEC was delivered orally to mice 10 mg / mice per day from day −1 to +4 after Nb inoculation, at doses previously shown to effectively block arginase activity in the lung21.
Flow cytometry and cell sorting
Lung tissue was minced and incubated with stirring at 37°C for 30 min in Hank's balanced salt solution (HBSS) with 1.3 mM EDTA (GIBCO), followed by treatment at 37°C for 1 hour with collagenase (1 mg ml−1; Sigma, St. Louis, MO) in RPMI1640 (Mediatech, Manassas, VA) with 5% fetal calf serum (Biowest, Ocala, FL) and with 100 μg ml−1 of DNase (Sigma) for 10 min. Cells were lysed with ACK Lysing Buffer (Lonza, Walkersville, MD) to remove erythrocytes, blocked with Fc Block (BD Biosciences, San Jose, CA), directly stained with fluorochromeconjugated antibodies against CD3, CD4, CD8, siglecF, Ly6G (1A8), MHCII, CD11c, CD11b, or F4/80 (BD Biosciences), and analyzed by flow cytometry uing an LSRII flow cytometry (BD Biosciences Immunocytometry Systems, San Jose, CA). For intracellular staining, lung cells were stimulated with phorbol myristate acetate-ionomycin (PMA/Ion) for 5h in the presence of Brefeldin A (SIGMA, St. Louis, MO). Cells were fixed and permeabilized with Saponin buffers followed by staining with anti-mouse IL-13 (clone: eBio13A; eBioscience) or IL-33 (clone: 396118, R&D Systems, Minneapolis, MN). In some experiments, macrophages or neutrophils were sorted for adoptive transfer or cell culture using a BD FACSAria II (BD Biosciences). The sorted macrophages were confirmed by Giemsa-Wright (CAMCO, Fort Lauderdale, FL) stained cytospin slides with more than 95% purity. To track the position of transferred cells, sorted macrophages were incubated for 30 min at 37°C in RPMI containing 5 μM carboxyfluorescein diacetate succinimidyl ester (CFSE). Following incubation, cells were washed before IV instillation into naive mice. Mice were killed 3d later, and the presence of fluorescently labeled cells in the lung was assessed by flow cytometry.
Adoptive transfer of macrophages
Cell suspensions were prepared from whole lungs of donor mice as described above and macrophages were electronically sorted with antibodies against F4/80, MHCII, and CD11c (F4/80high MHCIIint-highCD11cvar), as previously described22. 2.5–5×106 cells (in 100 μl of PBS) were transferred into WT naive recipient mice through i.v. injection. After 2 days, mice were inoculated with infective N. brasiliensis L3.parasites. In some experiments, donor cells were collected from mice administered neutrophil-anti-Ly6G antibody or isotype control IgG. In another experiments, donor cells were collected from mice administered LPS, as described above.
Macrophage co-cultures and larval ATP assay
Macrophages were electronically sorted and placed on 24-well plates (2 × 106) in 2 ml of RPMI1640 medium with 10% of FBS, 400U penicillin, 400 μg /ml streptomycin, and 100 μg/ ml gentamycin. 100 μl of serum from the donor mice was also added to medium. Two hundred exsheathed L3 larvae were added to each well. In some experiments, anti-CD11b antibody (eBioscience) (1-12 μg /ml) was also added to the macrophage –larvae co-culture. In other experiments, BEC was added, using previously published doses (0.1, 1, 5 mM)28, to each well of the in vitro macrophage - larva co-culture medium. Cells and worms were co-cultured for 5 days at 37°C. The procedure to ex-sheath larvae included washing L3 six times with sterile PBS containing 400U penicillin and 400 μg ml−1 streptomycin. The parasitic L3 were then incubated with 6.7 mM sodium hypochlorite in PBS for 15 min. at room temperature and exsheathed parasites removed for subsequent culture. For the macrophage-larvae adherence assay, 20 larval parasites were removed from cultures using a p1000 pipetman, washed in PBS, incubated in PBS with 0.5 mM EDTA for 1hr to remove adherent macrophages, and then counted using a hemacytometer. The ATP levels for the L3 were performed according to the manufacturer's instructions. Briefly, 10 N. brasiliensis L3 were collected with 100 μl of RPMI1640 medium and 100μl of the Celltiter-Glo Luminescent reagent (Promega, Madison, WI) was then added. The larvae were homogenized and incubated for five minutes at room temperature to stabilize the luminescence signal. After the homogenate was centrifuged at 1000g for 2 minutes, 100 μl of supernatant was applied to the luminometer to measure luminescence. As a negative control, larvae in RPMI1640 medium were treated in boiling water for five minutes and after cooling, the worms were homogenized with reagent. Dead larvae after incubation with macrophages were defined as non-motile, outstretched bodies, with non-refractive internal structures. In other studies, bone marrow derived macrophages (BMDM) (1x 106) were cultured in 24 well plates with sorted lung neutrophils (1: 1 ratio) obtained from mice at 2 days after inoculation with N. brasiliensis or after intracheal administration of LPS. Neutrophils from each treatment were cultured with macrophages in separate chambers of Costar transwell (0.4 μm) plates (Corning Incorporated, Corning, NY). In some cases, anti-IL-13 antibody or its isotype control (R&D Systems, Minneapolis, MN) was added to the co-cultures at a final concentration of 10 ug /ml. At day 3, the wells were washed with cold PBS and BMDM were harvested and RNA analyzed for qene expression by qPCR.
Gene expression by microarray and qPCR
For microarray, lung macrophages (10,000 cells) were directly sorted into RNA lysis buffer (Ambion RNAqueous Micro for RNA isolation) and frozen on dry ice. RNA was isolated, amplified using the AminoAllyl MessageAmp II kit (Life Technologies, Grand Island, NY), coupled to Cy3, and hybridized to SurePrint Mouse 8x60k gene expression microarrays (Agilent Technologies, Santa Clara CA) as per manufacturer recommendations. Microarrays were scanned on an Agilent scanner, and spot intensities extracted with Feature Extraction software (Agilent Technologies). Data were quantile normalized, and statistical analysis was performed using Significance Analysis of Microarrays52. All microarray data are available from the NCBI Gene Expression Omnibus under accession number GSE46437. For qPCR, RNA was extracted from lung tissue or sorted macrophages, and then reverse transcribed to cDNA, as previously described53,54. qPCR was performed using Taqman® (Applied Biosystems, Foster City, CA) kits and the Applied Biosystems 7500 Real-Time PCR System. All data were normalized to 18S ribosomal values and the quantification of differences between treatment groups was calculated according to the manufacturer's instructions. Gene expression is presented as the fold increase over naive WT controls or other groups indicated in detail in legends.
Histology
Lungs were formalin-fixed and embedded in paraffin and 5μm sections were cut and stained with hematoxylin and eosin (H&E) to assess the inflammatory infiltrate, as previously described21.
Immunohistological staining
Lungs were perfused with 200μl of PBS and Tissue-Tek O.C.T. compound (Sakura, Torrance, CA), excised and frozen in chilled acetone. 5 μm tissue sections were obtained using a HM505E cryostat (Microm International GmbH, Waldorf, Germany) and stored at −80°C. The tissue sections were allowed to dry at room temperature for one hour, fixed in cold acetone for 10 min, and then stained with antibodies specific to F4/80 and MBP (supplied by Dr. James J. Lee, Mayo Clinic, Scottsdale, AZ). Coverslips were applied to the slides using Fluoromount-G mounting medium (Southern Biotechnology Associates, Inc., Birmingham, AL). Images were taken using a Leica DM6000B and were tiled together using Image-Pro Plus 7.0 software (Media Cybernetics, Rockville, MD). Fluorescent channels were photographed separately, merged together and overlaid with corresponding Nomarski images. Exposure times and fluorescence intensities were normalized to appropriate control images.
Statistical analysis
Data were analyzed using SigmaPlot 12 (Systat Software, San Jose, CA, USA) and are reported as means ± s.e. Differences between multiple groups were assessed by one way ANOVA and individual comparisons were analyzed using Holm-Sidak test. Tests were only reported where data met assumptions of tests. Differences of p < 0.05 were considered statistically significant. Based on preliminary experimental data, a power analysis of 0.8 with p<0.05 indicates a minimum number 3-4 inbred mice per group but in most cases 5 mice/group were used.
Supplementary Material
Acknowledgements
This work was in part supported by National Institutes of health grants 1R01AI107588 (W.C.G. And C.C.K.), 5R01AI031678 (W.C.G.), R00AI085035 (C.C.K.), and USDA/ARS 1235-51000-058 (J.F.U). Mention of trade names or commercial products in this publication is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the U.S. Department of Agriculture. The USDA is an equal opportunity provider and employer.
Footnotes
Author contributions
W.C.G., F. C., C.C.K., and J.F.U. designed the experiments; F. C., W.W., A.M. J.F.C., E.C., and N.P. did the experiments; W.C.G., F.C., W.W., and C.C.K. wrote the manuscript.
COMPETING FINANCIAL INTERESTS
The authors declare no competing financial interests.
Accession codes:
Microarray data are available in GEO under accession GSE46437.
References
- 1.King CH. Health metrics for helminthic infections. Advances in parasitology. 2010;73:51–69. doi: 10.1016/S0065-308X(10)73003-7. [DOI] [PubMed] [Google Scholar]
- 2.Salgame P, Yap GS, Gause WC. Effect of helminth-induced immunity on infections with microbial pathogens. Nat Immunol. 2013;14:1118–1126. doi: 10.1038/ni.2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gaze S, et al. Characterising the mucosal and systemic immune responses to experimental human hookworm infection. PLoS pathogens. 2012;8:e1002520. doi: 10.1371/journal.ppat.1002520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anthony RM, Rutitzky LI, Urban JF, Jr., Stadecker MJ, Gause WC. Protective immune mechanisms in helminth infection. Nat Rev Immunol. 2007;7:975–987. doi: 10.1038/nri2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gause WC, Wynn TA, Allen JE. Type 2 immunity and wound healing: evolutionary refinement of adaptive immunity by helminths. Nat Rev Immunol. 2013 doi: 10.1038/nri3476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harvie M, et al. The lung is an important site for priming CD4 T-cell-mediated protective immunity against gastrointestinal helminth parasites. Infect Immun. 2010;78:3753–3762. doi: 10.1128/IAI.00502-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maizels RM, et al. Immune modulation and modulators in Heligmosomoides polygyrus infection. Exp Parasitol. 2012;132:76–89. doi: 10.1016/j.exppara.2011.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhao A, et al. Dependence of IL-4, IL-13, and nematode-induced alterations in murine small intestinal smooth muscle contractility on Stat6 and enteric nerves. J Immunol. 2003;171:948–954. doi: 10.4049/jimmunol.171.2.948. [DOI] [PubMed] [Google Scholar]
- 9.Harris N, Gause WC. To B or not to B: B cells and the Th2-type immune response to helminths. Trends Immunol. 2011;32:80–88. doi: 10.1016/j.it.2010.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Finkelman FD, et al. Interleukin-4- and interleukin-13-mediated host protection against intestinal nematode parasites. Immunological reviews. 2004;201:139–155. doi: 10.1111/j.0105-2896.2004.00192.x. [DOI] [PubMed] [Google Scholar]
- 11.Herbert DR, et al. Intestinal epithelial cell secretion of RELM-beta protects against gastrointestinal worm infection. J Exp Med. 2009;206:2947–2957. doi: 10.1084/jem.20091268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Knott ML, et al. Impaired resistance in early secondary Nippostrongylus brasiliensis infections in mice with defective eosinophilopoeisis. Int J Parasitol. 2007;37:1367–1378. doi: 10.1016/j.ijpara.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 13.Anthony RM, et al. Memory T(H)2 cells induce alternatively activated macrophages to mediate protection against nematode parasites. Nat Med. 2006;12:955–960. doi: 10.1038/nm1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang Z, et al. Macrophages as IL-25/IL-33-responsive cells play an important role in the induction of type 2 immunity. PloS one. 2013;8:e59441. doi: 10.1371/journal.pone.0059441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morimoto M, et al. Peripheral CD4 T cells rapidly accumulate at the host: parasite interface during an inflammatory Th2 memory response. J Immunol. 2004;172:2424–2430. doi: 10.4049/jimmunol.172.4.2424. [DOI] [PubMed] [Google Scholar]
- 16.Bonne-Annee S, et al. Human and mouse macrophages collaborate with neutrophils to kill larval Strongyloides stercoralis. Infect Immun. 2013;81:3346–3355. doi: 10.1128/IAI.00625-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harvie M, Camberis M, Le Gros G. Development of CD4 T Cell Dependent Immunity Against N. brasiliensis Infection. Frontiers in immunology. 2013;4:74. doi: 10.3389/fimmu.2013.00074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Katona IM, Urban JF, Jr., Finkelman FD. The role of L3T4+ and Lyt-2+ T cells in the IgE response and immunity to Nippostrongylus brasiliensis. J Immunol. 1988;140:3206–3211. [PubMed] [Google Scholar]
- 19.Liu Q, et al. B cells have distinct roles in host protection against different nematode parasites. Journal of immunology. 2010;184:5213–5223. doi: 10.4049/jimmunol.0902879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Patel N, Kreider T, Urban JF, Jr., Gause WC. Characterisation of effector mechanisms at the host:parasite interface during the immune response to tissue-dwelling intestinal nematode parasites. Int J Parasitol. 2009;39:13–21. doi: 10.1016/j.ijpara.2008.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen F, et al. An essential role for T(H)2-type responses in limiting acute tissue damage during experimental helminth infection. Nat Med. 2012;18:260–266. doi: 10.1038/nm.2628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Smith KA, et al. Type 2 Innate Immunity in Helminth Infection Is Induced Redundantly and Acts Autonomously following CD11c+ Cell Depletion. Infect Immun. 2012;80:3481–3489. doi: 10.1128/IAI.00436-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Obata-Ninomiya K, et al. The skin is an important bulwark of acquired immunity against intestinal helminths. J Exp Med. 2013 doi: 10.1084/jem.20130761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kreider T, Anthony RM, Urban JF, Jr., Gause WC. Alternatively activated macrophages in helminth infections. Curr Opin Immunol. 2007;19:448–453. doi: 10.1016/j.coi.2007.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dobrovolskaia MA, Vogel SN. Toll receptors, CD14, and macrophage activation and deactivation by LPS. Microbes Infect. 2002;4:903–914. doi: 10.1016/s1286-4579(02)01613-1. [DOI] [PubMed] [Google Scholar]
- 26.Murray PJ, Wynn TA. Protective and pathogenic functions of macrophage subsets. Nature reviews. Immunology. 2011;11:723–737. doi: 10.1038/nri3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morris SM., Jr. Recent advances in arginine metabolism: roles and regulation of the arginases. British journal of pharmacology. 2009;157:922–930. doi: 10.1111/j.1476-5381.2009.00278.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ckless K, et al. Inhibition of arginase activity enhances inflammation in mice with allergic airway disease, in association with increases in protein S-nitrosylation and tyrosine nitration. J Immunol. 2008;181:4255–4264. doi: 10.4049/jimmunol.181.6.4255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Herbert DR, et al. Arginase I suppresses IL-12/IL-23p40-driven intestinal inflammation during acute schistosomiasis. J Immunol. 2010;184:6438–6446. doi: 10.4049/jimmunol.0902009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bonne-Annee S, Hess JA, Abraham D. Innate and adaptive immunity to the nematode Strongyloides stercoralis in a mouse model. Immunologic research. 2011;51:205–214. doi: 10.1007/s12026-011-8258-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gong D, et al. TGFbeta signaling plays a critical role in promoting alternative macrophage activation. BMC Immunol. 2012;13:31. doi: 10.1186/1471-2172-13-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oo-Puthinan S, et al. The amino acids involved in the distinct carbohydrate specificities between macrophage galactose-type C-type lectins 1 and 2 (CD301a and b) of mice. Biochim Biophys Acta. 2008;1780:89–100. doi: 10.1016/j.bbagen.2007.10.017. [DOI] [PubMed] [Google Scholar]
- 33.Staples KJ, et al. Phenotypic characterization of lung macrophages in asthmatic patients: overexpression of CCL17. J Allergy Clin Immunol. 2012;130:1404–1412. e1407. doi: 10.1016/j.jaci.2012.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Becker HM, et al. alpha1beta1 Integrin-Mediated Adhesion Inhibits Macrophage Exit from a Peripheral Inflammatory Lesion. J Immunol. 2013;190:4305–4314. doi: 10.4049/jimmunol.1202097. [DOI] [PubMed] [Google Scholar]
- 35.Huang da W, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nature protocols. 2009;4:44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- 36.Durrant WE, Dong X. Systemic acquired resistance. Annual review of phytopathology. 2004;42:185–209. doi: 10.1146/annurev.phyto.42.040803.140421. [DOI] [PubMed] [Google Scholar]
- 37.Kurtz J. Specific memory within innate immune systems. Trends Immunol. 2005;26:186–192. doi: 10.1016/j.it.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 38.Netea MG, Quintin J, van der Meer JW. Trained immunity: a memory for innate host defense. Cell host & microbe. 2011;9:355–361. doi: 10.1016/j.chom.2011.04.006. [DOI] [PubMed] [Google Scholar]
- 39.Didierlaurent A, et al. Sustained desensitization to bacterial Toll-like receptor ligands after resolution of respiratory influenza infection. The Journal of experimental medicine. 2008;205:323–329. doi: 10.1084/jem.20070891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Reece JJ, Siracusa MC, Scott AL. Innate immune responses to lung-stage helminth infection induce alternatively activated alveolar macrophages. Infect Immun. 2006;74:4970–4981. doi: 10.1128/IAI.00687-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Marsland BJ, Kurrer M, Reissmann R, Harris NL, Kopf M. Nippostrongylus brasiliensis infection leads to the development of emphysema associated with the induction of alternatively activated macrophages. Eur J Immunol. 2008;38:479–488. doi: 10.1002/eji.200737827. [DOI] [PubMed] [Google Scholar]
- 42.Satoh T, et al. The Jmjd3-Irf4 axis regulates M2 macrophage polarization and host responses against helminth infection. Nat Immunol. 2010;11:936–944. doi: 10.1038/ni.1920. [DOI] [PubMed] [Google Scholar]
- 43.Monticelli S, Natoli G. Short-term memory of danger signals and environmental stimuli in immune cells. Nat Immunol. 2013;14:777–784. doi: 10.1038/ni.2636. [DOI] [PubMed] [Google Scholar]
- 44.Mantovani A, Cassatella MA, Costantini C, Jaillon S. Neutrophils in the activation and regulation of innate and adaptive immunity. Nat Rev Immunol. 2011;11:519–531. doi: 10.1038/nri3024. [DOI] [PubMed] [Google Scholar]
- 45.Galioto AM, et al. Role of eosinophils and neutrophils in innate and adaptive protective immunity to larval strongyloides stercoralis in mice. Infect Immun. 2006;74:5730–5738. doi: 10.1128/IAI.01958-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tsuda Y, et al. Three different neutrophil subsets exhibited in mice with different susceptibilities to infection by methicillin-resistant Staphylococcus aureus. Immunity. 2004;21:215–226. doi: 10.1016/j.immuni.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 47.Soehnlein O, Weber C, Lindbom L. Neutrophil granule proteins tune monocytic cell function. Trends Immunol. 2009;30:538–546. doi: 10.1016/j.it.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 48.Rosenthal LA, Sutterwala FS, Kehrli ME, Mosser DM. Leishmania major-human macrophage interactions: cooperation between Mac-1 (CD11b/CD18) and complement receptor type 1 (CD35) in promastigote adhesion. Infect Immun. 1996;64:2206–2215. doi: 10.1128/iai.64.6.2206-2215.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dent LA, et al. Interleukin-5 transgenic mice show enhanced resistance to primary infections with Nippostrongylus brasiliensis but not primary infections with Toxocara canis. Infect Immun. 1999;67:989–993. doi: 10.1128/iai.67.2.989-993.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Voehringer D. Protective and pathological roles of mast cells and basophils. Nat Rev Immunol. 2013;13:362–375. doi: 10.1038/nri3427. [DOI] [PubMed] [Google Scholar]
- 51.Pesce JT, et al. Neutrophils clear bacteria associated with parasitic nematodes augmenting the development of an effective Th2-type response. J Immunol. 2008;180:464–474. doi: 10.4049/jimmunol.180.1.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tusher VG, Tibshirani R, Chu G. Significance analysis of microarrays applied to the ionizing radiation response. Proc Natl Acad Sci U S A. 2001;98:5116–5121. doi: 10.1073/pnas.091062498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liu Z, et al. IL-2 and autocrine IL-4 drive the in vivo development of antigen-specific Th2 T cells elicited by nematode parasites. J Immunol. 2005;174:2242–2249. doi: 10.4049/jimmunol.174.4.2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gause WC, Adamovicz J. The use of the PCR to quantitate gene expression. PCR methods and applications. 1994;3:S123–135. doi: 10.1101/gr.3.6.s123. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.