Abstract
Gabapentin reduces behavioral signs of stimulus-evoked allodynia and hyperalgesia in preclinical studies of traumatic nerve injury, but its effects on more clinically-relevant measures of stimulus-independent pain are unclear. To address this gap, we determined whether gabapentin would relieve affective pain after spared nerve injury (SNI). Twelve days after sham or SNI surgery, we administered gabapentin over three consecutive conditioning days and then evaluated conditioned place preference (CPP). Gabapentin produced CPP and reversed mechanical hypersensitivity in SNI but not sham rats at a dose (100 mg/kg) that did not change open field activity. These results show for the first time that gabapentin provides relief from affective pain without producing locomotor sedation, and adds to a limited clinical literature suggesting that its use can be extended to treat pain arising from traumatic nerve injury.
Keywords: Conditioned place preference, neuropathic, pain, gabapentin, SNI, affective
Introduction
Preclinical research targeting discovery of novel treatments for neuropathic pain primarily rely on mechanical or thermal stimulus-evoked behavioral outcomes. However, this approach fails to mimic the affective and spontaneous aspects of chronic pain that are most relevant to pharmacotherapy in humans, as indicated by the high failure rate of analgesic drug candidates in clinical trials. The use of conditioned place preference (CPP) to assess non-evoked pain, originally described two decades ago [20], has re-emerged as a leading measure of affective neuropathic pain [13] and has the potential to address the disconnect between preclinical and clinical efficacy [12]. The use of CPP to measure preclinical pain relief is advantageous because the test is performed in the absence of an exogenous stimulus [21], incorporates the motivation to seek reward [3], and evaluates the affective [13] pain relieving effects of analgesic drug administration [12].
Gabapentin (Neurontin®) is a primary treatment for neuropathic pain [1] in patients with trigeminal neuralgia [14], post-herpetic neuralgia [19], painful chemoneuropathy [5], and painful diabetic neuropathy [2;22]. Reverse translation studies in rodents indicate that gabapentin attenuates affective pain produced by cisplatin [18] or streptozotocin [23], as well as the evoked hypersensitivity associated with traumatic nerve injury [25]. However, no study has evaluated whether gabapentin reduces affective pain after traumatic nerve injury. To address this question, we performed gabapentin CPP in rats with spared nerve injury (SNI), a widely used preclinical model of traumatic nerve injury [8].
Materials and Methods
Animals
Male Sprague-Dawley rats (Charles River Laboratories, Wilmington, MA) weighing 200–250 g at the time of surgery and 300–350 g at the time of behavioral procedures were housed 2 per cage on a 12-hour light/dark cycle (7am lights on / 7pm lights off) in a temperature (68–72° F) and humidity controlled room with food and water provided ad libitum. All efforts were made to minimize animal suffering, to reduce the number of animals used, and to utilize alternatives to in vivo techniques, in accordance with the International Association for the Study of Pain and the National Institutes of Health Office of Laboratory Animal Welfare Guide for the Care and Use of Laboratory Animals. All behavioral procedures were carried out between 8am–6pm and approved by the Institutional Animal Care and Use Committee (IACUC) at University of Kentucky. Behavioral measurements were performed by an observer blinded to experimental treatments.
Spared Nerve Injury (SNI) surgery
Sham and SNI surgeries were performed as previously described [8]. To generate surgical sham control subjects, all steps were performed except ligation and transection of the common peroneal and tibial nerves. The day of sham or SNI surgery is referred to as day 0.
Measurement of pain-like behavior and open field activity
Animals were acclimated in individual Plexiglas boxes (4″ × 8″ × 4″) on top of a raised stainless steel mesh grid for 1 h. Mechanical hypersensitivity was assessed using von Frey filaments (Stoelting, Inc., Wood Dale, IL) using a modified up-down method [6;9] as previously described [15]. The calculated 50% withdraw threshold is reported.
A photobeam activity system (PAS; 16 × 16 array; San Diego Instruments, San Diego, CA) was used to measure exploratory locomotion in a clear, square box surrounded by the photobeam array. Saline or gabapentin (100 mg/kg) was administered i.p. prior to placing the rat into the open field chamber. The total number of photobeam breaks was automatically quantified by the PAS software for 30 min in 5 min bins in the absence of any observer.
Conditioned Place Preference
The use of conditioned place preference (CPP) as a tool to measure the ongoing aversiveness (i.e. affective pain) after injury or preference for rewards has been well established [3;13;20;24]. Eight rat CPP boxes (Med Associates, St Albans, VT) were used to assess chamber preference before and after the drug conditioning phase. The experimental timeline and details of the CPP apparatus are illustrated in Figure 1. Rats were able to discriminate the drug- versus vehicle-paired chamber using visual (wall color), tactile (flooring), and olfactory (Lipsmackers Chapstick, Bonne Bell, Westlake, OH) cues. Preliminary experiments indicated no preference for vanilla (white chamber) or kiwi (black chamber) chapstick olfactory cues in sham or SNI rats. To reduce time spent in the gray chamber lighting in the white and black chambers was adjusted to 25% of that in the grey chamber. Manual guillotine doors were used to isolate the white and black pairing chambers from the grey chamber during conditioning. Each individual CPP box was fully contained in a sound and light attenuating enclosure. Time of testing, animal handling method, and cleaning of the CPP boxes were held constant.
Fig 1. Experimental timeline and diagram of the conditioning place preference (CPP) apparatus.
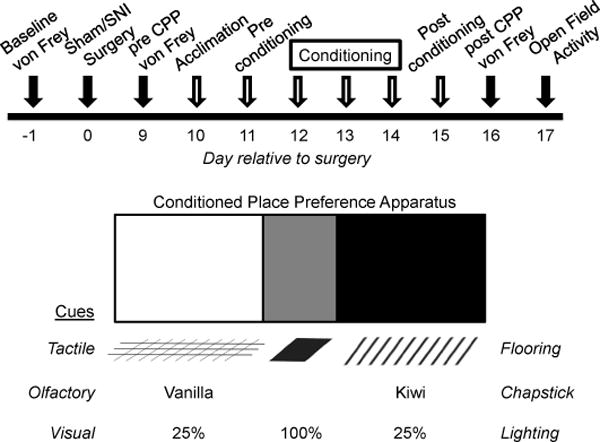
(Top) Baseline von Frey thresholds were measured on d -1 prior to Sham or Spared Nerve Injury (SNI) surgery on d 0. Mechanical hypersensitivity (von Frey) was measured on d 9 (pre CPP) and d 16 (post CPP). The Conditioned Place Preference (CPP) assay was performed on d 10 – 15 (open arrows) and consisted of acclimation, preconditioning, conditioning, and postconditioning. Open field activity was determined on d 17. (Bottom) The CPP apparatus consisted of: (left) a white chamber with grid flooring, vanilla chapstick, and 25% light intensity; (middle) a grey chamber with solid flooring, no olfactory cue, and 100% light intensity; (right) a black chamber with bar flooring, kiwi chapstick, and 25% light intensity.
Preconditioning
The CPP procedure spanned six consecutive days. On Day 1, subjects were acclimated to the CPP boxes for 30 min, with open access to each of the three chambers. On Day 2 (preconditioning), animals were placed in the grey middle chamber, and then we determined time spent in the white or black pairing chambers for 15 min. Animals that spent <20% or >80% time in the black and/or white chamber (i.e. showing an apparatus bias or initial, unconditioned preference) during preconditioning were removed from the experiment [13]. By these criteria, ten animals were removed from von Frey and CPP analyses.
Conditioning
On Days 3, 4 and 5 (conditioning), we used a biased assignment approach to drug pairing: saline was paired with the preferred chamber in the morning, and gabapentin was paired with the non-preferred chamber in the afternoon. Our biased approach was chosen for five reasons: 1) increases assay sensitivity; 2) allows for a within subjects design and statistical analysis [7;21]; 3) of all CPP studies in 2001, 30% used a biased approach and 42% analyzed results using a difference score (postconditioning minus preconditioning) [7]; 4) a biased approach was very recently used to assess gabapentin CPP in the streptozotocin model of painful diabetic neuropathy [23]; 5) Cunningham et al demonstrated that if the CPP apparatus is not biased (time spent in the white chamber = time spend in black chamber when average across all subjects, as in the current study), then the use of either a biased or unbiased chamber-assignment approach does not affect the ability to produce CPP [7].
Conditioning consisted of the following sequential steps: i.p. injection, return of the animal to its home cage for 5 min, and then placement within the white or black chamber for 30 min (injections were never paired with the grey, middle chamber). We used a 30 min conditioning time based on reports that gabapentin maximally reduced mechanical hypersensitivity at 30–60 min after injection [18;23]. We chose 3 d of gabapentin conditioning because 1 d was not sufficient to produce CPP in a mouse model of chemoneuropathy [18].
Postconditioning and analysis
On Day 6 (postconditioning), animals were placed into the grey chamber and we evaluated time spent in either the white or black chamber. The difference score for each subject was calculated by subtracting the time spent in the saline- or gabapentin paired chamber before pairing (during preconditioning) from the time spent in each chamber after pairing (postconditioning), and then averaged within each group.
Experimental Design
Evoked mechanical sensitivity was measured prior to sham or SNI surgery (day 0), 9 d after surgery (pre CPP), and after completion of the CPP procedure on d 15 (post CPP). The CPP assay was performed on days 10 – 15. Following post CPP measurement of von Frey withdraw thresholds to confirm the sustained presence of mechanical hypersensitivity, saline or gabapentin (100 mg/kg) was injected i.p. and von Frey thresholds were recorded 15, 30, and 60 min later. A 24 h timepoint was taken to determine whether the anti-hypersensitivity effects of gabapentin endured from one conditioning day to the next. Open field activity after i.p. saline or gabapentin administration was performed at the conclusion of von Frey and CPP experiments on d 17.
Drugs
Gabapentin (Spectrum Chemical, Gardena, CA) was dissolved in 0.9% saline immediately prior to injections and administered i.p. in a volume of 0.5–1.0 ml (final dose = 100 mg / kg body weight).
Statistical Analysis
A paired t-test was used to compare the effect of sham or SNI surgery on mechanical sensitivity prior to CPP, preconditioning versus postconditioning time spent in CPP chambers, and CPP difference scores. Gabapentin effect on behavior in the von Frey assay was compared for significant differences over time using repeated measures two-way ANOVA followed by Holm-Sidak multiple comparison correction. An alpha value of α = 0.05 was used to determine statistical significance. All data were analyzed and graphed using Prism 6.0 (GraphPad, La Jolla, CA) and are presented as mean ± SEM.
Results
SNI produces evoked mechanical hypersensitivity
Spared nerve injury (SNI) evokes mechanical hypersensitivity that begins a few days after surgery and lasts for at least 6 months [8]. To compare evoked mechanical hypersensitivity and affective pain, we performed von Frey testing prior to surgery (baseline), before CPP (pre), after CPP (post), and for 60 min following i.p. gabapentin administration in sham and SNI rats. CPP conditioning occurred at day 12–14 during established mechanical hypersensitivity. As illustrated in Fig 2A, SNI produced hypersensitivity to von Frey mechanical stimulation at the pre CPP timepoint [p < 0.0001]. There was no change in mechanical thresholds in sham animals [p > 0.05].
Fig 2. Gabapentin reverses evoked mechanical hypersensitivity associated with nerve injury.
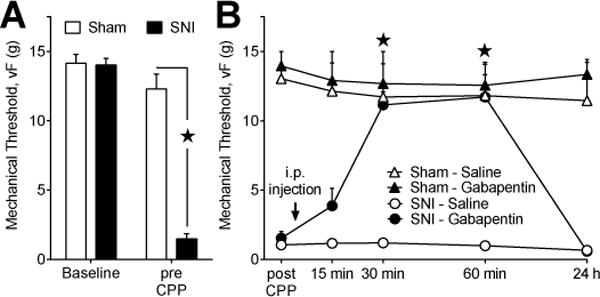
(A) Mechanical thresholds are shown at baseline (before surgery) and d 9 (pre CPP) after sham or spared nerve injury (SNI) surgery prior to conditioned place preference testing. SNI (n=17) decreased mechanical thresholds relative to sham (n=8) controls. (B) On d 16 (post CPP), mechanical thresholds remain decreased in SNI but not sham animals. Gabapentin (100 mg/kg; i.p.) increased mechanical thresholds in SNI (n=11) but not sham (n=5) animals. Saline did not change mechanical thresholds in sham (n=3) or SNI (6) animals. (A), ★ “SNI” at the pre CPP timepoint significantly different from all other groups. (B), ★ “SNI – Gabapentin” significantly different from “SNI – Saline”.
Gabapentin reverses SNI-induced evoked mechanical hypersensitivity
After CPP testing, we assessed inhibition of SNI-induced mechanical hypersensitivity by measuring von Frey withdraw thresholds after systemic administration of gabapentin at the same dose used during CPP conditioning (100 mg/kg). Fig 2B illustrates that gabapentin significantly attenuated evoked mechanical hypersensitivity in rats with SNI at 30 [p < 0.05] and 60 [p < 0.05] min after i.p. injection [drug × time; F (3, 36) = 17.5; P < 0.0001]. Mechanical withdraw thresholds were slightly, but insignificantly, increased at 15 min. Sham animals did not exhibit evoked mechanical hypersensitivity (compared to baseline) [p > 0.05] and von Frey withdraw thresholds were unaltered by gabapentin [drug × time; F (3, 18) = 0.015; P > 0.05].
Gabapentin produces CPP in rats with SNI but not sham surgery
To confirm that animals do not prefer one chamber over another, we assessed time spent in each. This was done prior to drug-pairing during conditioning. In sham rats, time spent in the white [355.1 ± 16.0 s] and black [363.2 ± 18.8 s] chambers was similar [p = 0.37]. In SNI rats, time spent in the white [336.2 ± 15.6 s] and black [346.1 ± 18.6 s] chambers was also similar [p = 0.34]. These data indicate that there is no initial bias for the CPP apparatus and that injury did not alter preconditioning preferences.
To determine whether gabapentin alleviates affective pain after traumatic nerve injury, we assessed CPP in sham and SNI rats. Fig 3A illustrates our biased conditioning approach: preconditioning time spent in the saline-paired chamber was greater than time spent in the gabapentin-paired chamber in both sham [p < 0.05] and SNI [p < 0.0001] rats. Biased drug pairing remained counterbalanced, where half the animals received gabapentin in the white chamber and half in the black chamber. Conditioning to gabapentin produced an increase in time spent in the gabapentin-paired chamber in SNI [p = 0.0043] but not sham rats [p = 0.2]. When compared to saline difference scores, Fig 3B illustrates a significantly higher gabapentin difference score in SNI [p < 0.0001] but not sham [p = 0.70] rats. These results indicate that gabapentin produces CPP in SNI but not sham rats at 2 wks after injury. A previous study indicates that the antihyperalgesic effect of gabapentin varies over time after nerve injury [11]. Future studies could investigate the ability of gabapentin to produce CPP at later timepoints.
Fig 3. Gabapentin attenuates affective pain associated with traumatic nerve injury.
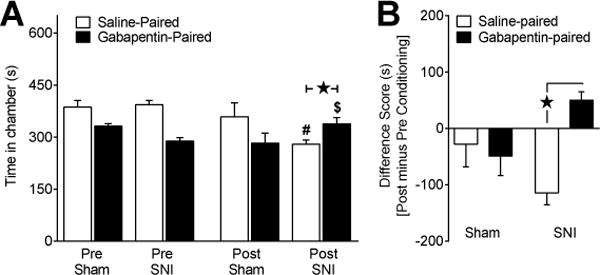
To determine affective pain relief we performed CPP with three days of conditioning (saline or gabapentin; 100 mg/kg; i.p.). (A) Time spent in the saline-paired chamber during preconditioning was greater than time spent in the gabapentin-paired chamber as a result of our biased drug-pairing approach. In sham rats (n=8), there was no change in preference during postconditioning (“Post”) when compared to preconditioning baselines (“Pre”). In SNI rats (n=17), gabapentin produced an increase in time spent in the gabapentin-paired chamber when compared to preconditioning baseline. (B) Saline and gabapentin difference scores were significantly different in SNI but not sham rats. These results taken together indicate gabapentin induces CPP thereby relieving affective pain in rats with traumatic nerve injury. # Significantly different from preconditioning saline-paired in SNI. $ Significantly different from preconditioning gabapentin-paired in SNI. ★ Significant difference between indicated groups.
Gabapentin did not change locomotor activity in sham or SNI rats
Gabapentin produces adverse effects in humans including somnolence, dizziness, peripheral edema, infection, and ataxia [19]. To address the potential effect of gabapentin on exploratory or somatomotor activity, we assessed open field activity. Fig 4A indicates that gabapentin did not change locomotor activity in sham [drug; F (1, 6) = 0.18; P > 0.05] or SNI [drug; F (1, 8) = 0.005; P > 0.05] rats. Furthermore, there was no difference in activity between sham and SNI animals treated with saline [injury; F (1, 8) = 0.02; P = 0.89] indicating that SNI did not change locomotor function. Additional studies are needed to evaluate alternative adverse effects of gabapentin to rule out confounding effects on mechanical thresholds or CPP.
Fig 4. Gabapentin does not alter locomotor activity.
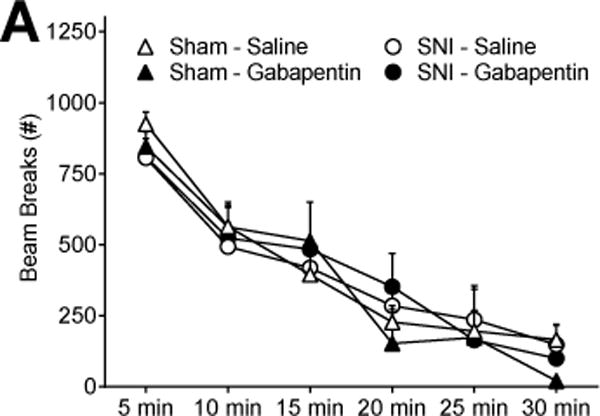
(A) The number of beam breaks in sham or SNI animals treated with saline or gabapentin (100 mg/kg; i.p.) was not different in an open field photobeam activity assay (n=5–6).
Discussion
Here we present the first data indicating that gabapentin relieves affective pain (i.e. produces CPP) associated with traumatic nerve injury in a preclinical model. Our current results are consistent with recent findings in other rodent models of neuropathic pain. For example, gabapentin produces CPP in mice following chronic cisplatin treatment [18] or in the streptozotocin model of type I painful diabetic neuropathy [23]. Xie et al reported that gabapentin reversed mechanical hyperalgesia associated with spinal nerve ligation (SNL), without measuring its effect on affective pain using CPP [25]. Confirmatory CPP studies in other models such as SNL are needed to generalize our findings to multiple types of neuropathic pain induced by nerve injury.
The current study shares important experimental design characteristics with Park et al and Wagner et al [18;23], but in the setting of traumatic nerve injury. First, gabapentin produced CPP rapidly, within 30 min of administration. Second, gabapentin did not produce CPP in control animals, ruling out the possibility that gabapentin is intrinsically rewarding. This is in contrast to rewarding analgesic drugs such as morphine, which produce CPP in naïve or uninjured subjects, thus complicating interpretation of effects on affective pain [20;21]. Third, gabapentin reduced both evoked and affective measures of pain. This is striking in light of recent reports indicating that other analgesic drugs such as TRP antagonists [4] inhibit evoked but not affective pain in preclinical models of inflammation [16], osteoarthritis [17], type I diabetes [24], and SNI [24]. Fourth, a single systemic dose of 100 mg/kg was administered over three conditioning days [18;23]. To determine whether CPP might reveal enhanced potency of gabapentin as compared to evoked measures of pain, further dose-response studies are needed.
Conclusion
We conclude that gabapentin alleviates affective pain after SNI in rodents. Further studies to determine the clinical efficacy of gabapentin for the treatment of chronic pain associated with traumatic nerve injury are warranted. Indeed, a randomized, double-blind, placebo-controlled, cross-over, multicenter clinical trial involving patients with peripheral nerve injury due to trauma or surgery reported that, compared to placebo, gabapentin provided better pain relief and increased the number of subjects with a pain reduction of at least 30% [10]. Our results highlight the importance of measuring the affective component of pain in preclinical studies to better predict clinical efficacy of pain-relieving drugs.
Acknowledgments
We thank Emily Denehy for her support in housing the animals and scheduling use of the CPP equipment, Justin Yates for his guidance using the CPP equipment, Renee Donahue for her assistance with scheduling and protocol writing, and Tracy Butler for critical reading of the manuscript.
Funding Sources: R01NS062306 and R01NS045954 to BKT; T32NS077889 and F31NS083292 to RBG
Footnotes
Conflict of Interest Statement
The authors declare that there are no conflicts of interest.
References
- 1.Attal N, Cruccu G, Baron R, Haanpaa M, Hansson P, Jensen TS, Nurmikko T. EFNS guidelines on the pharmacological treatment of neuropathic pain: 2010 revision. European journal of neurology : the official journal of the European Federation of Neurological Societies. 2010;17(9):1113–e1188. doi: 10.1111/j.1468-1331.2010.02999.x. [DOI] [PubMed] [Google Scholar]
- 2.Backonja M, Beydoun A, Edwards KR, et al. Gabapentin for the symptomatic treatment of painful neuropathy in patients with diabetes mellitus: A randomized controlled trial. JAMA : the journal of the American Medical Association. 1998;280(21):1831–1836. doi: 10.1001/jama.280.21.1831. [DOI] [PubMed] [Google Scholar]
- 3.Bardo MT, Bevins RA. Conditioned place preference: what does it add to our preclinical understanding of drug reward? Psychopharmacology. 2000;153(1):31–43. doi: 10.1007/s002130000569. [DOI] [PubMed] [Google Scholar]
- 4.Brederson JD, Kym PR, Szallasi A. Targeting TRP channels for pain relief. Eur J Pharmacol. 2013;716(1–3):61–76. doi: 10.1016/j.ejphar.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 5.Caraceni A, Zecca E, Bonezzi C, Arcuri E, Yaya Tur R, Maltoni M, Visentin M, Gorni G, Martini C, Tirelli W, Barbieri M, De Conno F. Gabapentin for neuropathic cancer pain: a randomized controlled trial from the Gabapentin Cancer Pain Study Group. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2004;22(14):2909–2917. doi: 10.1200/JCO.2004.08.141. [DOI] [PubMed] [Google Scholar]
- 6.Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. Journal of Neuroscience Methods. 1994;53(1):55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- 7.Cunningham CL, Ferree NK, Howard MA. Apparatus bias and place conditioning with ethanol in mice. Psychopharmacology (Berl) 2003;170(4):409–422. doi: 10.1007/s00213-003-1559-y. [DOI] [PubMed] [Google Scholar]
- 8.Decosterd I, Woolf CJ. Spared nerve injury: an animal model of persistent peripheral neuropathic pain. Pain. 2000;87(2):149–158. doi: 10.1016/S0304-3959(00)00276-1. [DOI] [PubMed] [Google Scholar]
- 9.Dixon WJ. Efficient analysis of experimental observations. Annual review of pharmacology and toxicology. 1980;20:441–462. doi: 10.1146/annurev.pa.20.040180.002301. [DOI] [PubMed] [Google Scholar]
- 10.Gordh TE, Stubhaug A, Jensen TS, Arner S, Biber B, Boivie J, Mannheimer C, Kalliomaki J, Kalso E. Gabapentin in traumatic nerve injury pain: a randomized, double-blind, placebo-controlled, cross-over, multi-center study. Pain. 2008;138(2):255–266. doi: 10.1016/j.pain.2007.12.011. [DOI] [PubMed] [Google Scholar]
- 11.Hama AT, Borsook D. The effect of antinociceptive drugs tested at different times after nerve injury in rats. Anesth Analg. 2005;101(1):175–179. doi: 10.1213/01.ANE.0000155247.93604.62. table of contents. [DOI] [PubMed] [Google Scholar]
- 12.King T, Porreca F. Preclinical Assessment of Pain: Improving Models in Discovery Research. Springer Berlin Heidelberg; 2014. pp. 1–20. [DOI] [PubMed] [Google Scholar]
- 13.King T, Vera-Portocarrero L, Gutierrez T, Vanderah TW, Dussor G, Lai J, Fields HL, Porreca F. Unmasking the tonic-aversive state in neuropathic pain. Nat Neurosci. 2009;12(11):1364–1366. doi: 10.1038/nn.2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lemos L, Alegria C, Oliveira J, Machado A, Oliveira P, Almeida A. Pharmacological versus microvascular decompression approaches for the treatment of trigeminal neuralgia: clinical outcomes and direct costs. Journal of pain research. 2011;4:233–244. doi: 10.2147/JPR.S20555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morgenweck J, Griggs RB, Donahue RR, Zadina JE, Taylor BK. PPARgamma activation blocks development and reduces established neuropathic pain in rats. Neuropharmacology. 2013;70:236–246. doi: 10.1016/j.neuropharm.2013.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Okun A, DeFelice M, Eyde N, Ren J, Mercado R, King T, Porreca F. Transient inflammation-induced ongoing pain is driven by TRPV1 sensitive afferents. Molecular pain. 2011;7:4. doi: 10.1186/1744-8069-7-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Okun A, Liu P, Davis P, Ren J, Remeniuk B, Brion T, Ossipov MH, Xie J, Dussor GO, King T, Porreca F. Afferent drive elicits ongoing pain in a model of advanced osteoarthritis. Pain. 2012;153(4):924–933. doi: 10.1016/j.pain.2012.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Park HJ, Stokes JA, Pirie E, Skahen J, Shtaerman Y, Yaksh TL. Persistent hyperalgesia in the cisplatin-treated mouse as defined by threshold measures, the conditioned place preference paradigm, and changes in dorsal root ganglia activated transcription factor 3: the effects of gabapentin, ketorolac, and etanercept. Anesth Analg. 2013;116(1):224–231. doi: 10.1213/ANE.0b013e31826e1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rowbotham M, Harden N, Stacey B, Bernstein P, Magnus-Miller L. Gabapentin for the treatment of postherpetic neuralgia: a randomized controlled trial. JAMA : the journal of the American Medical Association. 1998;280(21):1837–1842. doi: 10.1001/jama.280.21.1837. [DOI] [PubMed] [Google Scholar]
- 20.Sufka KJ. Conditioned place preference paradigm: a novel approach for analgesic drug assessment against chronic pain. Pain. 1994;58(3):355–366. doi: 10.1016/0304-3959(94)90130-9. [DOI] [PubMed] [Google Scholar]
- 21.Tzschentke TM. Measuring reward with the conditioned place preference (CPP) paradigm: update of the last decade. Addiction biology. 2007;12(3–4):227–462. doi: 10.1111/j.1369-1600.2007.00070.x. [DOI] [PubMed] [Google Scholar]
- 22.Vincent AM, Callaghan BC, Smith AL, Feldman EL. Diabetic neuropathy: cellular mechanisms as therapeutic targets. Nat Rev Neurol. 2011 doi: 10.1038/nrneurol.2011.137. [DOI] [PubMed] [Google Scholar]
- 23.Wagner K, Yang J, Inceoglu B, Hammock BD. Soluble epoxide hydrolase inhibition is antinociceptive in a mouse model of diabetic neuropathy. J Pain. 2014 doi: 10.1016/j.jpain.2014.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wei H, Viisanen H, Amorim D, Koivisto A, Pertovaara A. Dissociated modulation of conditioned place-preference and mechanical hypersensitivity by a TRPA1 channel antagonist in peripheral neuropathy. Pharmacology, biochemistry, and behavior. 2013;104:90–96. doi: 10.1016/j.pbb.2012.12.014. [DOI] [PubMed] [Google Scholar]
- 25.Xie JY, Qu C, Patwardhan A, Ossipov MH, Navratilova E, Becerra L, Borsook D, Porreca F. Activation of mesocorticolimbic reward circuits for assessment of relief of ongoing pain: a potential biomarker of efficacy. Pain. 2014 doi: 10.1016/j.pain.2014.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]


