Abstract
PURPOSE
Examine treatment decisions by ophthalmologists versus Reading Center (RC) fluid identification from OCT in Comparisons of Age-Related Macular Degeneration Treatments Trials (CATT).
METHODS
Fluid in 6210 OCT scans (598 patients) in "as needed treatment" arm of CATT year one was compared to ophthalmologist's treatment: positive fluid agreement (PFA, fluid+, treatment+) and discrepancy (PFD, fluid+, treatment−), negative fluid agreement (NFA, fluid−, treatment−) and discrepancy (NFD, fluid−, treatment+). For PFDs, fluid location and visual acuity (VA) were characterized.
RESULTS
Treatment and RC fluid determination agreed in 72.1% (53.0% PFA, 19.1% NFA) and disagreed in 27.9% (25.7% PFD, 2.2 % NFD) of visits, with no discrepancies for 20.9% of patients. Compared to PFA, PFD occurred more commonly with lower total foveal thickness (mean ±SD: 265 ± 103 PFD, 366 ± 151 microns PFA), presence of intraretinal fluid only, smaller fluid areas (PFA areas > twice those of PFD, p<0.001), and greater decrease in retinal and lesion thickness. Mean acuities before, at and after PFD were 65.8, 66.9 and 66.3 letters.
CONCLUSIONS
Treatment decisions by ophthalmologists matched RC fluid determination in majority of visits. More pronounced response to treatment and smaller foci of fluid likely contributed to PFD. PFD did not have substantial impact on subsequent VA.
INTRODUCTION
Non-invasive cross sectional imaging of the retina and choroid by optical coherence tomography (OCT) enables visualization of anatomic changes common to neovascular age related macular degeneration (NVAMD) such as retinal or retinal pigment epithelium (RPE) elevation over blood or choroidal neovascularization (CNV), accumulation of intraretinal, subretinal and sub-RPE fluid, and deformation, thickening, thinning or loss of retinal layers and choroidal thickness1–3. The ability of OCT to detect fluid indicative of active CNV leakage holds great promise to help rationally direct pharmacologic therapy for NVAMD4–9.
For physicians implementing as needed anti-VEGF therapy, the goal is to maximize visual function while minimizing treatment burden. Pivotal early trials were designed with once monthly intravitreal anti-VEGF treatment10, 11, but frequent dosing is highly resource intensive. Since then, multiple studies have investigated the efficacy of less frequent, as needed treatment dosing based on various criteria5–7, 9. The rewards of using the least injections to obtain optimal outcomes are manifold including increased patient convenience, reduced treatment cost, and decreasing the low, but non-zero rate of injection related complications10–13.
Within the Comparisons of Age-Related Macular Degeneration Treatments Trials (CATT) approximately half of the study patients were randomized to an as needed (pro re nata, PRN), dosing schedule14. For this group, after initial therapy, the treating ophthalmologists evaluated patients every 4 weeks with time-domain TD-OCT (Stratus, Carl Zeiss Meditec, Dublin, California) and treatment was mandated with few exceptions if the ophthalmologist observed any macular fluid on OCT. During the first year of the CATT, the differences in mean change in acuity between monthly versus as needed treatment was equivalent (+1.7 letters) for ranibizumab and inconclusive (+2.1 letters) for bevacizumab15. Prior studies suggest that less frequent injection is associated with less visual gain5 and that as needed dosing can result in decreased visual gain compared to monthly dosing9.
Because macular fluid on OCT has been the predominant reason for treatment decisions for PRN dosing during the CATT and other studies and is commonly used in PRN and treat-and-extend clinical treatment strategies, accurate identification of this fluid is important. It would be helpful to compare the clinicians’ decisions to RC determinations of macular fluid status. In the first year report of the CATT, most discrepancies between OCT findings and treatment decisions in the PRN groups were due to detection of fluid by the RC on OCT scans of patients who were not treated, accounting for 93% of discrepancies in the ranibizumab group and 91% in the bevacizumab group14. In a study of the link between morphology and acuity in the first year of CATT, eyes with residual intraretinal fluid in the fovea had worse mean VA (9 letters) than those without IRF16. We therefore sought to characterize the frequency of discrepancies per eye and the OCT features, associated clinical factors and subsequent visual acuity in these eyes in CATT, currently the largest study to investigate the efficacy of an as needed intravitreal NVAMD pharmacotherapy protocol based on monthly serial assessment of macular fluid.
MATERIALS AND METHODS
The institutional review board for each center approved the study protocol and written consent was obtained from each participant. At specified study visits, certified technicians captured two Stratus OCT scan sets in the study eye following the Macular Thickness Map (MTM) and Fast Macular Thickness Map (FMTM) protocols and submitted these to the RC. Protocol visual acuity was gathered by certified vision examiners at each study visit and submitted to coordinating center15.
CATT treating ophthalmologists had to identify macular fluid on OCT in order for an eye to be enrolled in the study; the RC evaluated the OCT to confirm eligibility after enrollment. To facilitate consistent identification of macular fluid, treating ophthalmologists were provided standardized images of the minimal threshold of IRF, SRF, and sub-RPE fluid on OCT that required treatment. The RC also provided training in standardized OCT interpretation at study startup, investigator meetings and on line. Ophthalmologists were required to review all 12 images from the two OCT scan sets under CATT investigator training. Their certification included review of the treatment protocol and a knowledge assessment test involving interpretation of OCTs.
Within the CATT, patients were divided into four treatment subgroups – monthly or PRN dosing, with bevacizumab or ranibizumab. For PRN dosing patients, after the first mandatory intravitreal injection, the protocol required treating ophthalmologists to examine the eye, review the study visit OCT images and administer the designated treatment at 4-week intervals for predetermined indications. The protocol mandated treatment for macular fluid found on OCT, and macular fluid was the principal indication of for PRN dosing (98.3%) during the first year of follow up. Other non-OCT based criteria mandating treatment included new hemorrhage, persistent hemorrhage, or decreased visual acuity since prior study visit. Fluorescein angiography criteria requiring treatment included increased lesion size or leakage. Treating ophthalmologists could withhold treatment for either definite or possible contraindications. Definite contraindications included intraocular inflammation (greater than 2+ cell), intraocular pressure (IOP) greater than 30 mm Hg, vitreous hemorrhage producing a more than 30 letter decrease in visual acuity, ocular infection, or any anti-VEGF treatment in the study eye within 23 days. Possible contraindications included recent stroke, recent myocardial infarction, new retinal break, new retinal detachment, new macular hole, RPE tear involving the macula, or patient refusal. At the treating ophthalmologist’s discretion treatment could be withheld in any eye not responding to three or more serial injections due to presumed treatment futility.
Comparison of RC and Treating Ophthalmologist Assessments of Fluid
Two certified readers independently analyzed all 12 OCT images from the two scan sets in a systematic fashion for morphological characteristics including intraretinal fluid (IRF), subretinal fluid (SRF), and sub-RPE fluid. Measurements included total thickness at the foveal center (from the internal limiting membrane to Bruch’s membrane). A Senior Reader reconciled disagreements between the initial reader pair. A reader pair and Senior Reader constituted a RC team, and all OCT scans from the CATT were evaluated using this team based approach.
RC grading of macular fluid was compared to the treating ophthalmologist‘s treatment decision based on OCT guided macular fluid identification. We excluded evaluations with no treatment due to contraindications or futility, and some OCT scans when images were not sufficient quality to determine fluid status by the OCT RC. Definitions of corresponding visits versus RC grading events included: 1) positive fluid agreement (PFA): RC identified macular fluid on OCT and the ophthalmologist administered treatment at the corresponding visit; 2) positive fluid discrepancy (PFD): RC identified macular fluid on OCT and the ophthalmologist did not administer treatment at the corresponding visit when there were no contraindications to treatment; 3) negative fluid agreement (NFA): RC did not identify macular fluid on OCT and the ophthalmologist did not administer treatment at the corresponding visit; 4) negative fluid discrepancy (NFD): RC did not identify macular fluid on OCT and the ophthalmologist administered treatment at the corresponding visit. Cases where an ophthalmologist treated for a reason other than fluid observed on OCT, such as decreased acuity, were specifically excluded from the NFD designation and considered as NFA.
A random sample of 400 PFD (~25%), 100 PFA (100 (~3%)) and 48 NFD (~34%) were selected from week 4 to week 48 visits for measurement of the largest area of fluid (PFD and PFA) or for re-grading for presence of fluid (NFD). The random sample groups contained comparable numbers of patients treated with bevacizumab versus ranibizumab, with visits chosen to be representative across study visits and calendar time. For PFD and PFA, scans were intermixed, and the RC was masked to the designation of the scans. For NFD a senior reader re-graded scans for the presence of fluid with scans intermixed with other OCT scans for grading. For inconclusive or uncertain grades, scans were evaluated in a masked fashion by the RC Director of Grading or Director or at a reader meeting for consensus vote regarding the grade.
For measurement of largest cross-sectional fluid area, Stratus software-based calipers were used to quantify the maximal horizontal and vertical dimensions of the single largest cross sectional area of IRF, SRF, and/or sub-RPE fluid respectively for a specific scan. All 12 OCT images were reviewed to determine the largest dimensions from a single radial line image. Cross sectional area of single largest IRF was approximated as an ellipse [area = π (horizontal dimension/2) × (vertical dimension/2)] and of single largest SRF or sub-RPE fluid, as a hemi-ellipse [area = ½ π (horizontal dimension/2) × (vertical dimension/2)]. If macular fluid was present in more than one location, the single largest area of fluid was calculated for each fluid type, and these were not required to originate from the same radial line scan. In rare cases of macular fluid extending beyond the OCT margin, the largest fluid area visible on OCT was measured.
Statistical Analysis
Descriptive statistics were used to describe the types of agreement and disagreement, the presence of fluid, location of fluid, and VA in the visit subsequent to PFD. Comparison of mean fluid area, total retinal thickness, and change from baseline between eyes with PFD and PFA were performed using generalized linear model with correlations among scans from the same eye accounted for using generalized estimating equations. Comparison of medians was performed using Wilcoxon rank sum test of difference in medians modified to account for correlations among scans from the same eye17. Statistical analyses were performed using SAS (v9.2, SAS Institute, Cary, NC), and two-sided p<0.05 was considered to be statistically significant. To assess the effect of PFD on subsequent VA, we performed a case-control analysis by 1:1 matching of PRN patients with PFD (case) with monthly treated patients (control). The first instance of PFD for a patient was selected for analysis. Cases and controls were matched on treatment drug, OCT fluid status (IRF, SRF, sub-RPE fluid), visit (weeks 4, 8, 12, 24), VA (± 2 letters), and intra-retinal thickness (± 50 microns). We identified 138 cases-control pairs, comparing VA and VA change at 4 weeks after the PFD using a paired t-test and Wilcoxon signed rank test and the mean VA change from baseline at 1 year among groups defined by the total number of PFDs within 1 year (1–2, 3–4, 5+) by using one-way analysis of variance.
RESULTS
Treating ophthalmologist and RC Agreement
A total of 6401 OCT scans were obtained from 598 patients in the PRN groups in Year 1 (week 4 to week 48). After excluding 62 scans (25 ranibizumab and 37 bevacizumab) with treatment contraindications, 12 scans (3 ranibizumab and 9 bevacizumab) with treatment futility and 117 scans (69 ranibizumab and 48 bevacizumab) with image quality insufficient to determine OCT fluid status, 6210 (97%, 3171 ranibizumab and 3039 bevacizumab) OCT scans from 594 patients were used to compare the RC grading of macular fluid to the ophthalmologist’s treatment decision based on identification of macular fluid on OCT. The treatment decision and RC determination of macular fluid status agreed in 4473 (72.1%) visits during the first year of CATT follow up (Table 1). Agreement was comprised of PFA in 3290 (53.0%) and NFA in 1183 (19.1%) of visits, and ranibizumab had a lower rate (48.3%) of PFA than did bevacizumab (57.9%). Discrepancies occurred in 1737 (27.9%) visits with PFD in 1598 (25.7%) and NFD in 139 (2.2%) of visits. Among the 594 patients, 124 (20.9%) had no PFD, 93 (15.7%) patients had 1 PFD, and 255 (42.9%) had 2 to 4 PFD (Table 2).
Table 1.
Agreement between Reading Center (RC) identification of macular fluid on optical coherence tomography (OCT) and ophthalmologist’s treatment decision in the as needed dosing groups during the first year of CATT.
| As needed dosing treatment group |
Number of scans evaluated by both ophthalmologist and RC |
Positive Fluid Agreement n (%) |
Negative Fluid Agreement n (%) |
Positive Fluid Discrepancy n (%) |
Negative Fluid Discrepancy n (%) |
|---|---|---|---|---|---|
| Ranibizumab | 3171 | 1531 (48.3) | 708 (22.3) | 865 (27.3) | 67 (2.1) |
| Bevacizumab | 3039 | 1759 (57.9) | 475 (15.6) | 733 (24.1) | 72 (2.4) |
| All Treatment | 6210 | 3290 (53.0) | 1183 (19.1) | 1598 (25.7) | 139 (2.2) |
Macular fluid: presence of one or more of the following on OCT: intraretinal fluid, subretinal fluid, or sub-retinal pigment epithelium fluid. Positive Fluid Agreement: RC identified macular fluid on OCT and the ophthalmologist administered treatment at the corresponding visit. Negative Fluid Agreement: RC did not identify macular fluid on OCT and the ophthalmologist did not administer treatment at the corresponding visit. Positive Fluid Discrepancy: RC identified macular fluid on OCT and the ophthalmologist did not administer treatment at the corresponding visit when there were no contraindications to treatment. Negative Fluid Discrepancy: RC did not identify macular fluid on OCT and the ophthalmologist administered treatment at the corresponding visit.
Table 2.
Frequency of Positive Fluid Discrepancies per eye during the first year of CATT
| Number of Positive Fluid Discrepancies |
Ranibizumab n (%) eyes |
Bevacizumab n (%) eyes |
All Treatment n (%) eyes |
|||
|---|---|---|---|---|---|---|
| 0 | 42 (14.1) | 82 (27.7) | 124 (20.9) | |||
| 1 | 49 (16.4) | 44 (14.9) | 93 (15.7) | |||
| 2 | 50 (16.8) | 48 (16.2) | 98 (16.5) | |||
| 3 | 47 (15.8) | 34 (11.5) | 81 (13.6) | |||
| 4 | 47 (15.8) | 29 (9.8) | 76 (12.8) | |||
| 5 | 26 (8.7) | 24 (8.1) | 50 (8.4) | |||
| 6 | 18 (6.0) | 13 (4.4) | 31 (5.2) | |||
| 7 | 9 (3.0) | 8 (2.7) | 17 (2.9) | |||
| 8 | 6 (2.0) | 8 (2.7) | 14 (2.4) | |||
| 9 | 2 (0.7) | 3 (1.0) | 5 (0.8) | |||
| 10 | 2 (0.7) | 3 (1.0) | 5 (0.8) | |||
| Any | 298 (100.0) | 296 (100.0) | 594 (100.0) | |||
Positive Fluid Discrepancy: RC identified macular fluid on OCT and the ophthalmologist did not administer treatment at the corresponding visit when there were no contraindications to treatment.
Localization, Distribution and Persistence of Fluid for Positive Fluid Discrepancies
Types of macular fluid, combinations of fluid present, and treatment status at each visit are detailed in Table 3A and in Supplemental Tables 3B (ranibizumab) and 3C (bevacizumab). The most common pattern of fluid identified by the RC was IRF alone, present at 1548 (31.7%) of the 4888 visits when some type of fluid was present. SRF alone was more likely to be treated (71.1%) than either IRF alone (49.0%) or Sub-RPE fluid (46.4%) alone. When SRF was present in any combination, treatment was more likely than at a visit without SRF. The proportion treated with IRF alone or sub RPE fluid alone was comparable (49.0% versus 46.4%). When two or three types of fluid were present, the proportion treated was higher than when only one type of fluid was present. These relationships generally held in subgroup analysis by assigned drug (supplemental Tables 3B and 3C).
Table 3A.
Frequency of treatment compared to Reading Center determined macular fluid location on optical coherence tomography in year 1 of CATT
| Reading Center Determined Fluid Location |
No. of Scans with Fluid Subtype (%) |
No. of PFA: Treatment at Corresponding Study Visit (%) |
No. of PFD: No Treatment at Corresponding Study Visit (%) |
|---|---|---|---|
| IRF only | 1548 (31.7) | 759 (49.0) | 789 (51.0) |
| SRF only | 519 (10.6) | 369 (71.1) | 150 (28.9) |
| Sub-RPE fluid only | 401 (8.20) | 186 (46.4) | 215 (53.6) |
| IRF and SRF | 704 (14.4) | 565 (80.3) | 139 (19.7) |
| IRF and Sub-RPE fluid | 509 (10.4) | 345 (67.8) | 164 (32.2) |
| SRF and Sub-RPE fluid | 434 (8.88) | 380 (87.6) | 54 (12.4) |
| IRF and SRF and Sub-RPE fluid | 773 (15.8) | 686 (88.8) | 87 (11.3) |
| Any IRF | 3534 (72.3) | 2355 (66.6) | 1179 (33.4) |
| Any SRF | 2430 (49.7) | 2000 (82.3) | 430 (17.7) |
| Any Sub-RPE fluid | 2117 (43.3) | 1597 (75.4) | 520 (24.6) |
| Total | 4888 | 3290 (67.3) | 1598 (32.7) |
IRF = intraretinal fluid, SRF = subretinal fluid, RPE = retinal pigment epithelium. RC performed grading of optical coherence tomography scans from as needed dosing patients evaluated during CATT year-one visits (week 4 till week 48). PFA= Positive Fluid Agreement: RC identified macular fluid on OCT and the ophthalmologist administered treatment at the corresponding visit. PFD= Positive Fluid Discrepancy: RC identified macular fluid on OCT and the ophthalmologist did not administer treatment at the corresponding visit when there were no contraindications to treatment. Macular fluid = presence of any one or more of IRF, SRF, or sub-RPE fluid on OCT.
At the visit following a PFD, fluid persisted in 1409/1443 visits (97.6%). Fluid at the subsequent visit to PFD was still most likely to be IRF alone (405/1443, 28.1%) or in any combination (897/1443, 62.2%) (Table 4A). At the subsequent visit to a PFD event, ophthalmologists often did not administer treatment (779/1443, 54.0% of visits) (Table 5A). Fluid and subsequent treatment status at the visits following a PFD event is detailed by assigned drug in Supplemental Tables 4B and 5B.
Table 4A.
Macular fluid status at visit subsequent to Positive Fluid Discrepancy (PFD) event.
| Macular Fluid Status at Subsequent Visit to PFD Event |
All Treatment PFD Events n (%) |
|---|---|
| No fluid | 189 (13.1) |
| IRF only | 405 (28.1) |
| SRF only | 135 (9.4) |
| Sub-RPE fluid only | 105 (7.3) |
| IRF and SRF | 191 (13.2) |
| IRF and Sub-RPE Fluid | 98 (6.8) |
| SRF and Sub-RPE fluid | 89 (6.2) |
| IRF and SRF and Sub-RPE fluid | 203 (14.1) |
| Unknown | 28 (1.9) |
| Any IRF | 897 (62.2) |
| Any SRF | 618 (42.8) |
| Any Sub-RPE fluid | 495 (34.3) |
| Total | 1443* (100.0) |
Legend: PFD= Positive Fluid Discrepancy: RC identified macular fluid on OCT and the ophthalmologist did not administer treatment at the corresponding visit when there were no contraindications to treatment. Macular fluid = presence of one or more of the following on OCT: intraretinal fluid, subretinal fluid, and sub-retinal pigment epithelium (RPE) fluid.
155 eyes that did not have subsequent visit after positive fluid discrepancy were excluded.
Table 5A.
Treatment status after Positive Fluid Discrepancy (PFD) event.
| Treatment Status at Subsequent Visit after PFD Event | All Treatment PFD Events n (%) |
|---|---|
| Not treated | 779 (54.0) |
| Treated for macular fluid on OCT only | 520 (36.0) |
| Treated for macular fluid on OCT and another reason | 99 (6.9) |
| Treated for persistent subretinal hemorrhage or new hemorrhage | 14 (1.0) |
| Treated for leakage on fluorescein angiography | 3 (0.2) |
| Treated for decreased visual acuity only | 17 (1.2) |
| Multiple non-OCT reasons | 5 (0.3) |
| Other | 6 (0.4) |
| Total | 1443* (100.0) |
PFD= Positive Fluid Discrepancy: RC identified macular fluid on OCT and the ophthalmologist did not administer treatment at the corresponding visit when there were no contraindications to treatment. Macular fluid = presence of one or more of the following on OCT: intraretinal fluid, subretinal fluid, and sub-retinal pigment epithelium (RPE) fluid.
155 eyes that did not have subsequent visit after positive fluid discrepancy were excluded.
Cross Sectional Area of Fluid and Total Foveal Thickness in Positive Fluid Agreement versus Disagreement
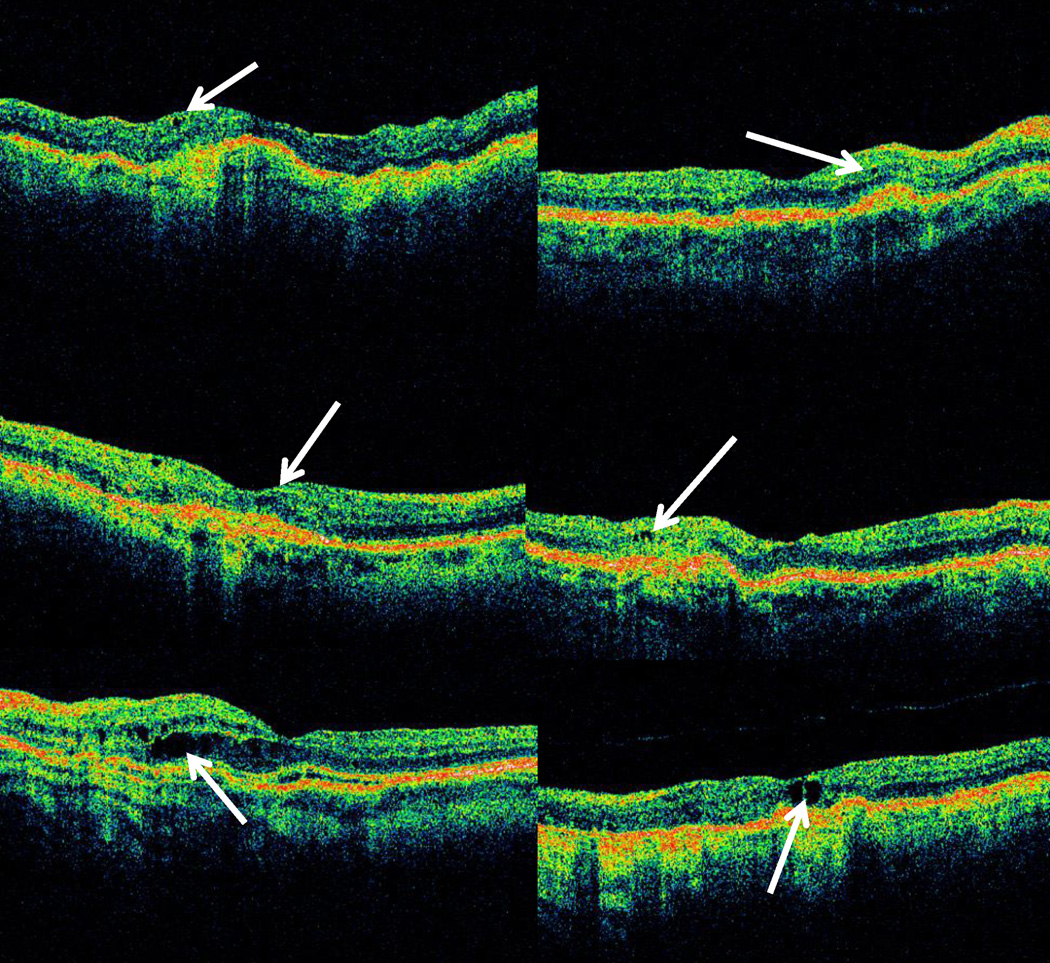
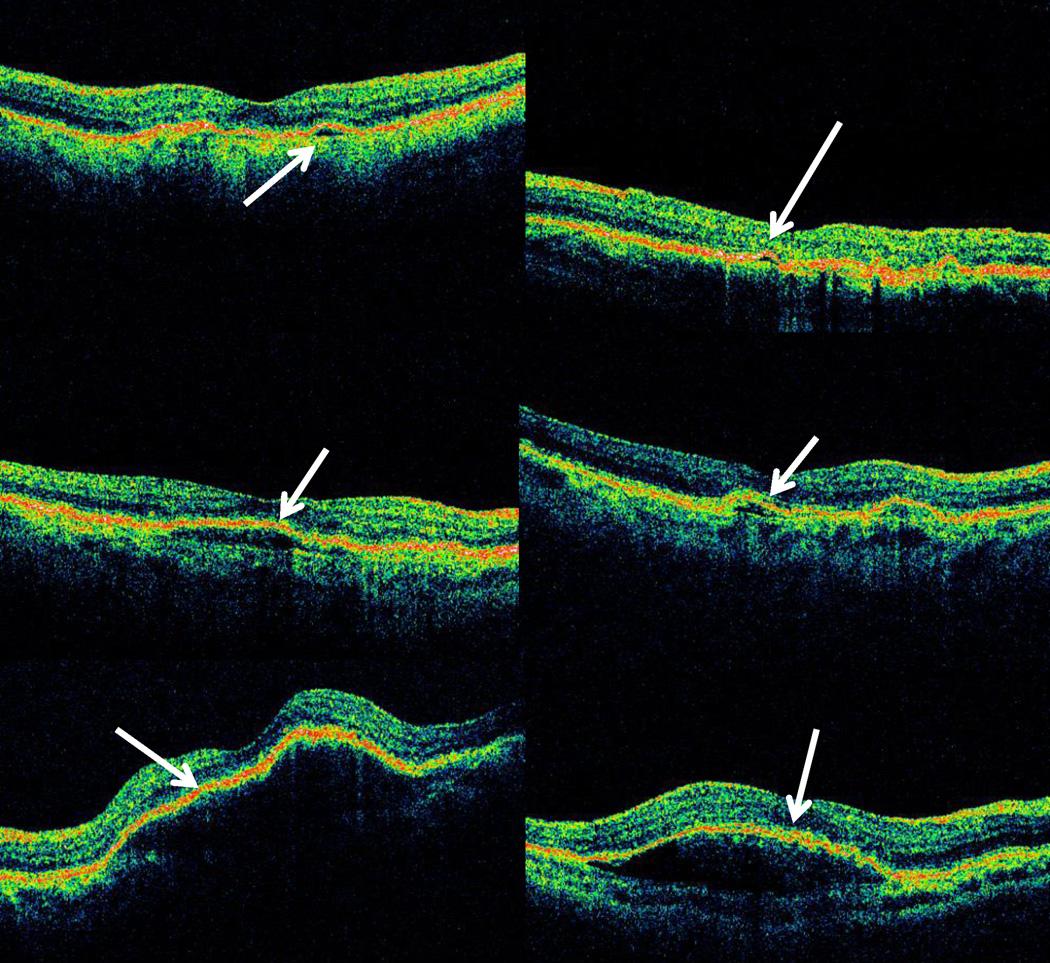
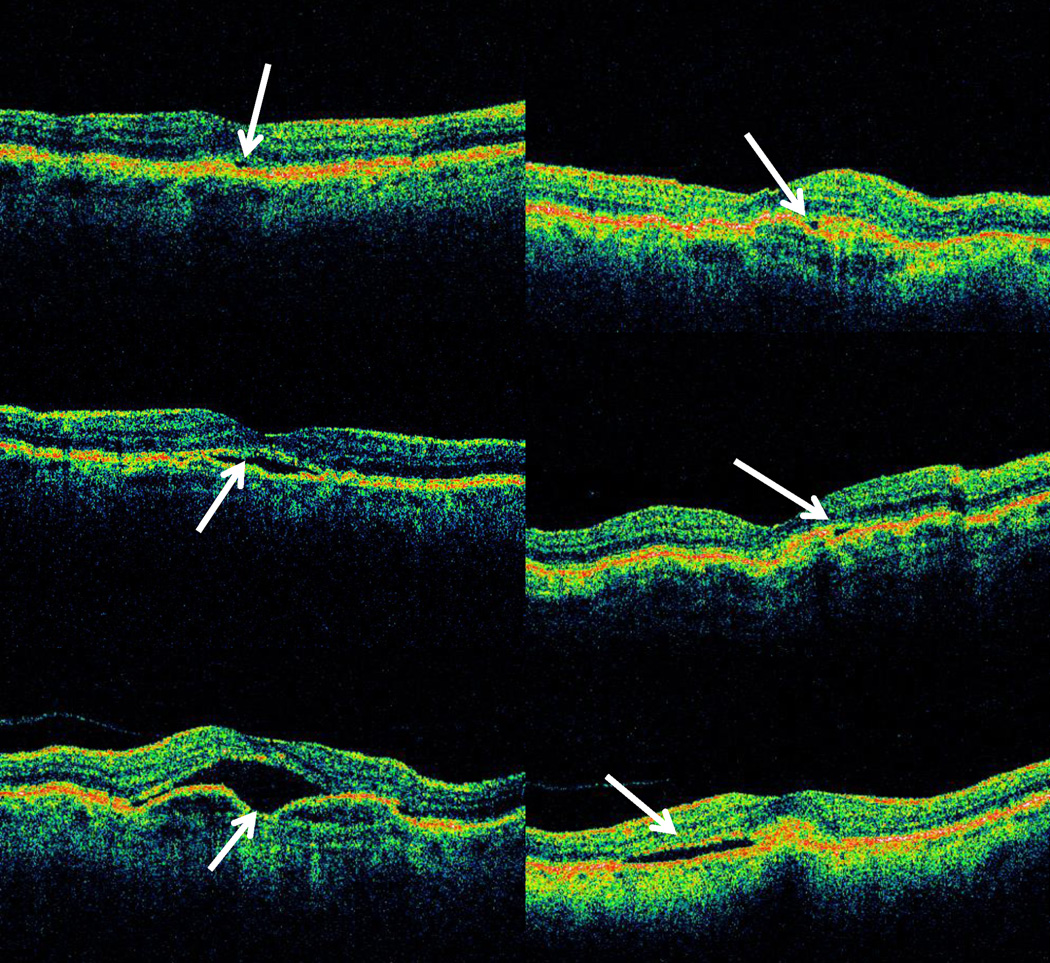
The median of the single largest cross-sectional area of fluid of each type was greater for cases of PFA than for cases of PFD (Table 6; Figures 1– 3). For both PFD and PFA groups, the largest median cross sectional area of fluid on OCT was for sub-RPE fluid, while the smallest median area was for IRF. The median cross-sectional area for PFA scans relative to PFD scans was twice as large for IRF (p < 0.001; Figure 1c–d) and over four times as large for SRF (p < 0.001; Figure 2c–d), and for sub-RPE fluid (p<0.001; Figure 3c–d).
Table 6.
Comparison of single largest cross sectional fluid area found on OCT scans from random samples of Positive Fluid Agreements versus Positive Fluid Discrepancies in year 1 of CATT
| Positive Fluid Agreement | Positive Fluid Discrepancy | p-value* | |||||||
|---|---|---|---|---|---|---|---|---|---|
| n | Median (Q1, Q3) | Min | Max | n | Median (Q1, Q3) | Min | Max | ||
| Intraretinal Fluid Area (×10−3 mm2) | 74 | 12.8 (8.67, 21.8) | 2.59 | 129 | 290 | 6.28 (3.78, 10.1) | 0.60 | 60.7 | < 0.0001 |
| Subretinal Fluid Area (×10−3 mm2) | 44 | 24.9 (10.7, 45.2) | 1.26 | 354 | 102 | 5.43 (2.71, 10.9) | 1.06 | 213 | < 0.0001 |
| Sub-RPE Fluid Area (×10−3 mm2) | 36 | 45.0 (14.9, 121) | 1.87 | 806 | 126 | 10.0 (4.15, 33.6) | 0.66 | 1370 | < 0.0004 |
P-value is based on modified version of the Wilcoxon rank sum test to compare median areas of fluid between groups17. Positive Fluid Agreement: RC identified macular fluid on OCT and the ophthalmologist administered treatment at the corresponding visit. Positive Fluid Discrepancy: RC identified macular fluid on OCT and the ophthalmologist did not administer treatment at the corresponding visit when there were no contraindications to treatment. Single largest cross sectional area of intraretinal fluid was approximated as an ellipse using the following formula: area = π × [(Horizontal Dimension/2) × (Vertical Dimension/2)]. Single largest cross sectional area of subretinal fluid and sub-RPE fluid was approximated as a hemi-ellipse using the following formula: area = π/2 × [(Horizontal Dimension/2) × (Vertical Dimension/2)].
Figure 1.
Representative optical coherence tomography (OCT) images comparing areas of single largest intraretinal fluid (IRF) between cases where RC identified OCT macular fluid and treatment was administered by the ophthalmologist (Positive Fluid Agreement or PFA) compared to cases where RC identified OCT macular fluid and treatment was not administered by the ophthalmologist at corresponding visit (Positive Fluid Discrepancy or PFD): PFA IRF 5th percentile area (upper left), PFD IRF 5th percentile area (upper right), PFA IRF median area (center left), PFD IRF median area (center right), PFA IRF 95th percentile area (lower left), and PFD IRF 95th percentile area (lower right).
Figure 3.
Representative optical coherence tomography (OCT) images comparing areas of single largest sub-retinal pigment epithelium (RPE) fluid between cases where RC identified OCT macular fluid and treatment was administered by an treating ophthalmologist (Positive Fluid Agreement or PFA) compared to cases where RC identified OCT macular fluid and treatment not administered by treating ophthalmologist at corresponding visit (Positive Fluid Discrepancy or PFD): PFA sub-RPE fluid 5th percentile area (upper left), PFD sub-RPE fluid 5th percentile area (upper right), PFA sub-RPE fluid median area (center left), PFD sub-RPE fluid median area (center right), PFA sub-RPE fluid 95th percentile area (lower left), and PFD sub-RPE fluid 95th percentile area (lower right).
Figure 2.
Representative optical coherence tomography (OCT) images comparing areas of single largest subretinal fluid (SRF) between cases where RC identified OCT macular fluid and treatment was administered by an treating ophthalmologist (Positive Fluid Agreement or PFA) compared to cases where RC identified OCT macular fluid and treatment not administered by treating ophthalmologist at corresponding visit (Positive Fluid Discrepancy or PFD): PFA SRF 5th percentile area (upper left), PFD SRF 5th percentile area (upper right), PFA SRF median area (center left), PFD SRF median area (center right), PFA SRF 95th percentile area (lower left), and PFD SRF 95th percentile area (lower right).
Total foveal center point thickness (ILM to Bruch’s membrane) reflected the presence and amount fluid and neovascular complex at the foveal center. For the 3290 study visits with PFA, mean total foveal center point thickness (366 ± 151 microns) was greater than for the 1598 study visits with PFD (265 ± 103; p<0.0001). The mean decrease in total foveal thickness from baseline to the study visit was less for visits with PFA (−120 ± 172 microns compared to those with PFD (−170 ± 164 microns), p<0.0001). At the subsequent visit, the mean thickness decreased (−27±83) for PFA (treatment administered) while the mean thickness increased (+34±76) for PFD (treatment not given; p<0.001).
Positive Fluid Discrepancy Visual Outcomes
The visual acuities at the visit prior to, at visit of and at next visit after the PFD were similar (mean VA 66.4, 67.6 and 66.9 letters [≈20/50], respectively) and this pattern was consistent in ranibizumab and bevacizumab PRN treated patients (Table 7). The case-control analysis showed that visual acuity at 4 weeks after the 1st PFD was similar to the matched monthly treated patients (mean VA 68.1 vs. 69.4 letters, p=0.16). The VA change at 4 weeks after the 1st PFD visits was also similar to matched controls (−1.0 letters vs. 0.22 letters, p=0.15) (Table 8).
Table 7.
Visual acuity prior to, at and after the Positive Fluid Discrepancy (PFD) events
| All Treatments PFD Events |
Ranibizumab PFD Events |
Bevacizumab PFD Events |
||||
|---|---|---|---|---|---|---|
| n | Mean (SD) | n | Mean (SD) | n | Mean (SD) | |
| VA prior to PFD | 1597 | 66.4 (16.3) | 865 | 67.0 (15.3) | 733 | 65.8 (17.3) |
| At PFD | 1597 | 67.6 (16.1) | 865 | 68.1 (15.0) | 733 | 66.9 (17.3) |
| After PFD | 1597 | 66.9 (16.7) | 865 | 67.4 (15.6) | 733 | 66.3 (17.9) |
PFD= Positive Fluid Discrepancy: RC identified macular fluid on OCT and the ophthalmologist did not administer treatment at the corresponding visit when there were no contraindications to treatment.
Table 8.
Comparison of visual acuity at 4 weeks after the first PFD in PRN groups and their matched controls in monthly treated group
| PFD Case | Monthly treated Control |
P Value | |
|---|---|---|---|
| VA at 4 weeks after the 1st PFD | |||
| N | 138 | 138 | |
| Mean (SE) | 68.1 (1.00) | 69.4 (0.95) | 0.16* |
| Median (Min, Max) | 70 (33, 88) | 72 (27, 88) | 0.08† |
| VA change at 4 weeks after the 1st PFD | |||
| Mean (SE) | −1.0 (0.70) | 0.22 (0.59) | 0.15* |
| Median (Min, Max) | −1.0 (−43, 22) | 1 (−25, 32) | 0.08† |
P value is from paired t test
P value is from signed rank test
PFD= Positive Fluid Discrepancy: RC identified OCT macular fluid and treatment not administered by treating ophthalmologist at corresponding visit.
When change in visual acuity at year one was stratified by frequency of PFD, the 191 (41%) eyes with 1–2 PFD had mean VA gain of 7.7 letters versus mean gain of 7.2 letters for 157 (33%) eyes with 3–4 PFD and mean gain of 6.1 letters in 122 (26%) eyes with ≥ 5 PFD. These small differences were not statistically significant (p=0.58) (Table 9) and remained non-significant when analyzed by drug group.
Table 9.
Change in visual acuity from baseline at Year 1 stratified by number of discrepant treatments in CATT as needed dosing group patients.
| Overall | Ranibizumab | Bevacizumab | ||||
|---|---|---|---|---|---|---|
| Number of missed treatment in Year 1 |
n | Mean VA change from baseline at 1 Year (SD) |
n | Mean VA change from baseline at 1 Year (SD) |
n | Mean VA change from baseline at 1 Year (SD) |
| 1–2 | 191 | 7.7 (12.5) | 99 | 8.0 (12.8) | 92 | 7.3 (12.2) |
| 3–4 | 157 | 7.2 (13.4) | 94 | 6.6 (12.7) | 63 | 8.0 (14.4) |
| 5+ | 122 | 6.1 (13.4) | 63 | 5.8 (12.7) | 59 | 6.3 (14.3) |
| P-value | 0.58 | 0.57 | 0.78 |
DISCUSSION
In this study of OCT image review for anti-VEGF treatment decisions in the PRN groups at year one of the CATT, the treatment decisions of ophthalmologists matched RC determination of macular fluid status in the majority (72.1%) of the 6210 examinations. Disagreement on macular fluid status was most commonly PFD in which the ophthalmologist did not treat and the RC detected macular fluid. This occurred more commonly for intraretinal than for any other fluid (SRF and sub-RPE fluid). Relative to eyes with PFA, PFD occurred in visits with thinner retinas, smaller cross-sectional fluid areas and greater decrease in total retinal thickness at the foveal center, suggesting that eyes with smaller sites of fluid and greater improvement from baseline were less likely to have fluid identified for treatment. At visits after a PFD event, the fluid often persisted, and often was not treated. The PFD did not have substantial impact on the early subsequent VA (4 weeks later). At one year, eyes with repeated PFD had no significant difference in VA change compared to eyes with rare PFD. Repeat evaluation of NFD scans rarely revealed fluid not reported during original grading.
Every OCT was independently graded by at least 2 readers at the RC using a standardized protocol, so it is not surprising that macular fluid, subtle or otherwise was more often noted by the RC than by individual treating ophthalmologists. Supporting this assertion, of the 6210 OCT scans evaluated by both treating ophthalmologists and the RC, 1598 (25.7%) discrepancies were noted where the RC graded macular fluid present on OCT and treating ophthalmologists did not administer treatment presumably due to not observing the same fluid on OCT, while treating ophthalmologists administered treatment in only 139 (2.2%) events when the RC graded macular fluid as absent. Diffuse thickening without cystoid spaces may have influenced the observing ophthalmologist in some of the 139 cases, as IRF was positive at the RC only if cystoid spaces were observed. Repeat RC evaluation of a randomized selection 48 of these NFD scans revealed only one (2.1%) scan with macular fluid.
Several additional reasons may account for the treating ophthalmologist – RC discrepancies such as: varied interpretation of fluid across ophthalmologists in CATT, impact of other clinical data on treating ophthalmologist interpretation of OCT or interpretation of lesion activity and other circumstances such as patient reticence. Readers were provided OCT scans in a batch format via a standard computer interface and followed a standardized grading protocol reviewing all 12 radial line images (6 MTM and 6 FMTM). Treating ophthalmologists were trained in OCT review, provided with reference OCT images and instructed to review all 12 radial line OCT images. Although the treating ophthalmologists were able to use analysis tools such as the retinal mapping function, they also reviewed OCT images on a variety of sources, ranging from printed images to digital images on an assortment of monitors. These may have increased variability of their evaluation and may have presented obstacles to reviewing all of the OCT scans. For all fluid types, in some cases very large areas of IRF, SRF and sub-RPE fluid were present at a PFD (Fig 2e–f, 3e–f, and 4e–f). Many of these scans with larger areas of fluid were not on the horizontal or vertical axis and thus some perhaps were missed if select scans, rather than the entire set, were reviewed at a particular visit. Although treatment in the PRN groups, with exceptions noted in methods, per protocol were based on OCT finding of fluid, the clinician had access to interval change information, visual acuity, angiography and symptoms that all may impact the decision-making process. A more pronounced improvement in these multiple factors may have made subsequent OCT based treatment administration more challenging. RC OCT interpretation was not provided to the treating ophthalmologist as feedback, in part so as not to bias treatment decisions that would then not reflect clinical practice. Treating ophthalmologists most commonly agreed with RC determination of fluid in CATT and the small size of most PFD suggest that review of the full OCT scan set was performed. One must recognize the careful review of the OCT scan set by the treating ophthalmologist at monthly visits when generalizing from the outcomes of the PRN treatment in CATT.
There were differences in PFD by location of fluid. Intraretinal fluid only was the most common (789 of 1598 total discrepancies, 49.3%) cause of treatment discrepancy. Treating ophthalmologists may have less frequently identified IRF on OCT for several reasons. IRF though frequently present on OCT images, was small in cross sectional area and thus more difficult to detect or distinguish from normal pixel variation (Figure 2A–D). Hyporeflectivity of the OCT scan at the foveal center can add to difficulty in delineating subtle IRF from pixel void artifact, and discrepancies in identifying small areas of IRF may reflect the resolution limits of TD-OCT. In some cases, treating ophthalmologists may have been more prone to interpret subtle IRF as noise or artifact and not administer treatment, while conversely, RC teams may have graded artifact as subtle IRF, again leading to discrepancy. In contrast to the other fluid types, when SRF was present, whether alone or in combination, the treating ophthalmologist was most likely to agree with RC regarding treatment of fluid. Both SRF and sub-RPE fluid appeared to match RC findings except when the area of fluid was relatively small. The median largest cross-sectional area for PFD were <¼ that for PDA for both SRF and sub-RPE fluid. For all three types of fluid, the cross sectional fluid area was consistently smaller, total thickness at the foveal center point was smaller and foveal center point decreased more from baseline for PFD compared to PFA.
Discrepant fluid from PFD events frequently persisted in study eyes which often had PFD at the visit following a PFD event. This prevalence of macular fluid was high compared to the prevalence of persistent fluid in monthly treatment patients. At one year of follow up, 53.2% and 70.9% of patients dosed monthly with ranibizumab and bevacizumab respectively demonstrated persistent fluid on RC OCT review. In comparison, macular fluid was observed by the RC in 97.6% of eyes during the subsequent visit after a PFD event occurred although these were throughout rather than at the end of one year of treatment. Minimizing subsequent discrepant fluid after PFD would diminish overall rates of macular fluid during PRN treatment.
Secondary analysis did not reveal pronounced disparities between patients in the ranibizumab and bevacizumab PRN dosing groups. For both drug groups, the frequency and distribution of discrepant treatment were comparable. After PFD events, fluid persisted at comparable rates for both groups, though rate of treatment was slightly higher at subsequent visit to PFD events for eyes in the ranibizumab group. Cases in the PRN ranibizumab group demonstrated a larger reduction in total thickness at the foveal center point from baseline to PFD than cases in the bevacizumab group, however total foveal thicknesses at one year were comparable (294 ± 139 for bevacizumab PRN and 308 ± 127 for ranibizumab PRN)14.
Though we have shown that generally smaller areas of discrepant fluid are widespread and persistent, the ultimate impact of discrepant macular fluid on visual outcomes is currently not well understood. Multiple studies have investigated variable dosing regimens based in part on OCT assessment of fluid as an alternative protocol to maximize visual gain while minimizing treatment burden6, 7. Across these studies, criteria for treatment differed across the variable-dosing regimens6, 7, and assessment of fluid through detailed morphological analysis involved as few as two cross hair scans6. None of the studies compared decisions to treat to RC interpretations of fluid on OCT.
For CATT study patients at one year, the differences in mean change in acuity were +1.7 letters between ranibizumab monthly and PRN groups and +2.1 letters between the bevacizumab monthly and PRN groups. These outcomes were obtained with a mean 6.9 ± 3.0 ranibizumab and 7.7 ± 3.5 bevacizumab injections respectively14. The overall visual and anatomical impact of treatment discrepancies was not notable at the 4-week visit after PFD. Moreover, when we compared the one year visual acuity between eyes with >5 PFD and eyes with minimal PFD, there was no significant difference in acuity. Thus from this study, we could not identify a difference in acuity in the PRN groups that could be based on PFD.
This work must be interpreted in the context of several limitations. Since treating ophthalmologists did not report OCT grading like RC teams, agreement was approximated by comparing treating ophthalmologist treatment decisions to RC team OCT grading. If the rationale for treatment was not reported correctly, then agreement rates may be inaccurate. Agreement may have been overestimated if eyes were treated for other factors, but macular fluid was reported as the indication for treatment. Conversely, though the protocol mandated treatment for any macular fluid on OCT, a treating ophthalmologist may have withheld treatment despite residual fluid due to excellent visual acuity, marked treatment response to the previous injection or patient reticence. Another limitation is that the total cross sectional areas of all IRF, SRF, and sub-RPE fluid on a radial line image were not calculated. Rather the single largest area for each type of macular fluid present on an OCT scan was approximated. Since this method was consistently applied, relative comparisons could be made with reasonable certainty across groups. This work is the first to characterize treatment discrepancies from a large cohort of patients prospectively undergoing OCT guided PRN treatment of NVAMD. By examining the treatment decision, this study is instructive regarding the nuances in implementing an OCT guided PRN treatment protocol in real world practice.
Spectral domain OCT technology (SD-OCT) offers many promises for future clinical trials, some of which may facilitate OCT based PRN dosing. Compared to conventional time domain OCT (TD-OCT), SD-OCT offers increased image resolution and more rapid data acquisition leading to subsequent decreased motion artifact18, 19. Thus SD-OCT may facilitate recognition of macular fluid, although, as with TD-OCT, a review of the full scan set would likely be important. Sayanagi et al reported that various SD-OCT platforms compared to TD-OCT were superior in detection of IRF, SRF, and sub-RPE fluid in eyes with NVAMD20. Folgar et al, in a review of over 1200 pairs of SD-OCT and TD-OCT scans from study visits in year two of the CATT, found 6% greater frequency of IRF detected on TD-OCT possibly due to lower resolution with interpretation of dark pixels as cystoid edema, although fluid overall was detected with 5% greater frequency with SD-OCT21. Improved fluid detection may result in increased treatment frequency, improved outcomes, and decreased inter-treating ophthalmologist variability.
CATT treating ophthalmologist decisions suggest that ophthalmologists less frequently identified OCT macular fluid than the RC, however, clinical decisions were also likely to be impacted by other factors (acuity, presence of blood, patient reticence to treatment) that would not be considered by a reading center. The areas of discrepant fluid were most commonly located within the retina and were smaller than corresponding areas in eyes undergoing protocol treatment. A more pronounced anatomical response to treatment, including larger decrease in total thickness at the foveal center and smaller macular fluid may have contributed to increased discrepancy rates. Fluid tended to persist after a PFD, and often it was not treated. The visual impact for missed fluid was minimal at the subsequent examination. Although repeated PFD might affect visual acuity, this was not evident in the one-year study. There were infrequent large areas of fluid in OCTs at PFD which is an alert to the importance of review of all scans for a clinical visit in which a treatment decision is made for NVAMD.
Supplementary Material
REFERENCES
- 1.Hee MR, Baumal CR, Puliafito CA, et al. Optical coherence tomography of age-related macular degeneration and choroidal neovascularization. Ophthalmology. 1996;103(8):1260–1270. doi: 10.1016/s0161-6420(96)30512-5. [DOI] [PubMed] [Google Scholar]
- 2.Ting TD, Oh M, Cox TA, et al. Decreased visual acuity associated with cystoid macular edema in neovascular age-related macular degeneration. Arch Ophthalmol. 2002;120(6):731–737. doi: 10.1001/archopht.120.6.731. [DOI] [PubMed] [Google Scholar]
- 3.Rahman W, Chen FK, Yeoh J, da Cruz L. Enhanced depth imaging of the choroid in patients with neovascular age-related macular degeneration treated with anti-VEGF therapy versus untreated patients. Graefes Arch Clin Exp Ophthalmol. 2012 doi: 10.1007/s00417-012-2199-x. [DOI] [PubMed] [Google Scholar]
- 4.Gupta OP, Shienbaum G, Patel AH, et al. A Treat and Extend Regimen Using Ranibizumab for Neovascular Age-Related Macular Degeneration Clinical and Economic Impact. Ophthalmology. doi: 10.1016/j.ophtha.2010.02.032. [DOI] [PubMed] [Google Scholar]
- 5.Dadgostar H, Ventura AA, Chung JY, et al. Evaluation of injection frequency and visual acuity outcomes for ranibizumab monotherapy in exudative age-related macular degeneration. Ophthalmology. 2009;116(9):1740–1747. doi: 10.1016/j.ophtha.2009.05.033. [DOI] [PubMed] [Google Scholar]
- 6.Rothenbuehler SP, Waeber D, Brinkmann CK, et al. Effects of ranibizumab in patients with subfoveal choroidal neovascularization attributable to age-related macular degeneration. Am J Ophthalmol. 2009;147(5):831–837. doi: 10.1016/j.ajo.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 7.Fung AE, Lalwani GA, Rosenfeld PJ, et al. An optical coherence tomography-guided, variable dosing regimen with intravitreal ranibizumab (Lucentis) for neovascular age-related macular degeneration. Am J Ophthalmol. 2007;143(4):566–583. doi: 10.1016/j.ajo.2007.01.028. [DOI] [PubMed] [Google Scholar]
- 8.Lalwani GA, Rosenfeld PJ, Fung AE, et al. A variable-dosing regimen with intravitreal ranibizumab for neovascular age-related macular degeneration: year 2 of the PrONTO Study. Am J Ophthalmol. 2009;148(1):43–58. e1. doi: 10.1016/j.ajo.2009.01.024. [DOI] [PubMed] [Google Scholar]
- 9.Holz FG, Amoaku W, Donate J, et al. Safety and Efficacy of a Flexible Dosing Regimen of Ranibizumab in Neovascular Age-Related Macular Degeneration: The SUSTAIN Study. Ophthalmology. 118(4):663–671. doi: 10.1016/j.ophtha.2010.12.019. [DOI] [PubMed] [Google Scholar]
- 10.Rosenfeld PJ, Brown DM, Heier JS, et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355(14):1419–1431. doi: 10.1056/NEJMoa054481. [DOI] [PubMed] [Google Scholar]
- 11.Brown DM, Kaiser PK, Michels M, et al. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med. 2006;355(14):1432–1444. doi: 10.1056/NEJMoa062655. [DOI] [PubMed] [Google Scholar]
- 12.Wu L, Martinez-Castellanos MA, Quiroz-Mercado H, et al. Twelve-month safety of intravitreal injections of bevacizumab (Avastin): results of the Pan-American Collaborative Retina Study Group (PACORES) Graefes Arch Clin Exp Ophthalmol. 2008;246(1):81–87. doi: 10.1007/s00417-007-0660-z. [DOI] [PubMed] [Google Scholar]
- 13.Fung AE, Rosenfeld PJ, Reichel E. The International Intravitreal Bevacizumab Safety Survey: using the internet to assess drug safety worldwide. Br J Ophthalmol. 2006;90(11):1344–1349. doi: 10.1136/bjo.2006.099598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martin DF, Maguire MG, Ying GS, et al. Ranibizumab and bevacizumab for neovascular age-related macular degeneration. N Engl J Med. 2011;364(20):1897–1908. doi: 10.1056/NEJMoa1102673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.CATT. Comparison of Age-Related Macular Degeneration Treatments Trials Manual of Procedures. http://www.med.upenn.edu/cpob/studies/documents/CATTManualofProceduresJan2011.pdf. [Google Scholar]
- 16.Ying GS, Huang J, Maguire MG, et al. Baseline predictors for one-year visual outcomes with ranibizumab or bevacizumab for neovascular age-related macular degeneration. Ophthalmology. 2013;120(1):122–129. doi: 10.1016/j.ophtha.2012.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rosner B, Glynn RJ, Lee ML. Incorporation of clustering effects for the Wilcoxon rank sum test: a large-sample approach. Biometrics. 2003;59(4):1089–1098. doi: 10.1111/j.0006-341x.2003.00125.x. [DOI] [PubMed] [Google Scholar]
- 18.Srinivasan VJ, Wojtkowski M, Witkin AJ, et al. High-definition and 3-dimensional imaging of macular pathologies with high-speed ultrahigh-resolution optical coherence tomography. Ophthalmology. 2006;113(11):2054 e1–2054 e14. doi: 10.1016/j.ophtha.2006.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wojtkowski M, Bajraszewski T, Gorczynska I, et al. Ophthalmic imaging by spectral optical coherence tomography. Am J Ophthalmol. 2004;138(3):412–419. doi: 10.1016/j.ajo.2004.04.049. [DOI] [PubMed] [Google Scholar]
- 20.Sayanagi K, Sharma S, Yamamoto T, Kaiser PK. Comparison of spectral-domain versus time-domain optical coherence tomography in management of age-related macular degeneration with ranibizumab. Ophthalmology. 2009;116(5):947–955. doi: 10.1016/j.ophtha.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 21.Folgar FA, Jaffe GJ, Ying GS, et al. Comparison of Optical Coherence Tomography Assessments in the Comparison of Age-Related Macular Degeneration Treatments Trials. Ophthalmology. 2014 May 15; doi: 10.1016/j.ophtha.2014.04.020. [epub]. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.