Abstract
The gut immune system and its modification by diet have been implicated in the pathogenesis of type 1 diabetes (T1D). Therefore, we investigated gut immune status in non-diabetes-prone LEW.1AR1 and diabetes-prone LEW.1AR1-iddm rats and evaluated the effect of a low antigen, hydrolysed casein (HC)-based diet on gut immunity and T1D. Rats were weaned onto a cereal-based or HC-based diet and monitored for T1D. Strain and dietary effects on immune homeostasis were assessed in non-diabetic rats (50–60 days old) and rats with recent-onset diabetes using flow cytometry and immunohistochemistry. Immune gene expression was analysed in mesenteric lymph nodes (MLN) and jejunum using quantitative RT-PCR and PCR arrays. T1D was prevented in LEW.1AR1-iddm rats by feeding an HC diet. Diabetic LEW.1AR1-iddm rats had fewer lymphoid tissue T cells compared with LEW.1AR1 rats. The percentage of CD4+ Foxp3+ regulatory T (Treg) cells was decreased in pancreatic lymph nodes (PLN) of diabetic rats. The jejunum of 50-day LEW.1AR1-iddm rats contained fewer CD3+ T cells, CD163+ M2 macrophages and Foxp3+ Treg cells. Ifng expression was increased in MLN and Foxp3 expression was decreased in the jejunum of LEW.1AR1-iddm rats; Ifng/Il4 was decreased in jejunum of LEW.1AR1-iddm rats fed HC. PCR arrays revealed decreased expression of M2-associated macrophage factors in 50-day LEW.1AR1-iddm rats. Wheat peptides stimulated T-cell proliferation and activation in MLN and PLN cells from diabetic LEW.1AR1-iddm rats. LEW.1AR1-iddm rats displayed gut immune cell deficits and decreased immunoregulatory capacity, which were partially corrected in animals fed a low antigen, protective HC diet consistent with other models of T1D.
Keywords: diet, gut immunity, M2 macrophage, regulatory T cell, type 1 diabetes
Introduction
Type 1 diabetes (T1D) results from a heterogeneous disease process caused by poorly understood interactions among several risk genes and environmental factors.1 Onset occurs between 6 months and > 40 years in humans, reflecting the likelihood that the disease develops via many different pathways. There are several animal models of spontaneous T1D, including the diabetes-prone BioBreeding (BBdp) rat, Komeda (KDP) rat, LEW.1AR1-iddm rat and the most studied model, the NOD mouse. Because these animals are highly inbred with a limited number of pathways to T1D,2,3 the relevance to human T1D has been questioned. Translational potential would be increased if outcomes could be demonstrated in more than one animal model.4,5
The LEW.1AR1-iddm rat arose from a spontaneous mutation in LEW.1AR1 rats in Hannover, Germany.6 These animals are distinct from the well-characterized NOD model of T1D in that diabetes develops with equal frequency in both male and female animals and unlike the BBdp rat, LEW.1AR1-iddm rats are not severely lymphopenic, although recent reports have demonstrated a mild T-cell deficit associated with diabetes onset.7,8 Similarities and differences among the various models were recently compared.5 Diabetes develops between 60 and 90 days and is characterized by a rapidly progressing insulitis, which leads to extensive β-cell destruction.9 Diabetes can be induced by transferring autoreactive T cells10 and genetic analyses have identified multiple susceptibility loci.11 In addition to the diabetes-related MHC class II (Iddm1) susceptibility locus, Iddm8 was identified recently as the site of mutation(s) contributing to disease susceptibility in the LEW.1AR1 strain.12
Many studies have linked agents first encountered in the gastrointestinal tract to diabetes development, including enteroviruses and wheat proteins.1 Additional studies have demonstrated increased intestinal permeability in humans and rodents with T1D,13–19 suggesting that the gut barrier is impaired. These data point to the gastrointestinal tract as a critical and perhaps global factor in T1D development. Indeed, there is presently a strong emphasis on the role of gut microbiota in T1D and this has re-focused attention on the gut immune system. However, because diet is the main determinant of gut microbiota composition,20 it is important to examine not just gut microbes but also to understand the role of diet as a primary link between gut microbiota, gut immunity and development of T1D.1,21,22
It has been demonstrated that BBdp rats and NOD mice fed low antigen, hydrolysed casein (HC)-based diets (or other non-diabetogenic amino acid sources23) are protected from T1D compared with animals fed cereal-based, mainly wheat-containing diets.1,21,22,24 We have also demonstrated that mesenteric lymph node (MLN) T cells (but not splenic T cells) from BBdp rats proliferate in response to wheat peptides and secrete pro-inflammatory cytokines such as interferon-γ whereas cells from control animals do not,25 demonstrating impaired oral tolerance. Similarly, peripheral blood mononuclear cells from T1D patients have increased proliferation and secretion of T helper type 1 and T helper type 17 cytokines in response to wheat peptides.26 In the current study, we investigated differences in gut immune homeostasis and oral tolerance between the LEW.1AR1 parental strain and the diabetes-prone LEW.1AR1-iddm strain. We asked whether feeding a low-antigen HC diet affected T1D development in the LEW.1AR1-iddm model. We provide evidence that LEW.1AR1-iddm rats have defects in oral tolerance and immunoregulation in the gut and associated immune tissues compared with the parental strain. Furthermore, the HC diet suppressed diabetes development and modified immune cell distribution in LEW.1AR1-iddm rats.
Materials and methods
Animals
The LEW.1AR1-iddm rat model arose following a spontaneous mutation associated with the telomeric region of rat chromosome 1 (Iddm8) in a colony of LEW.1AR1 rats at the Hannover Medical School.12 They originated from a single breeding cage and have been maintained as a separate strain since the identification of the mutation.6 LEW.1AR1-iddm rats (abbreviated as ‘iddm’ in the figures) and the LEW.1AR1 non-diabetic control strain (abbreviated as ‘1AR1’ in the figures) were obtained from Drs S. Lenzen and D. Wedekind (Hannover Medical School, Hannover, Germany), and were maintained at the Ottawa Hospital Research Institute under specific pathogen-free conditions. The colony was screened quarterly using the Prevalent PCR Rodent Infectious Agent (PRIA) Panel (Charles River, Montreal, QC, Canada) and annually using the Surveillance PRIA Panel (Charles River) and tested negative for the duration of the study. Animals were given free access to water and a standard, cereal-based rodent diet (Teklad Global 18% Protein Rodent diet; Harlan, Montreal, QC, Canada) or an iso-nitrogenous, semi-purified HC diet.27 Some animals were removed at 50 days or 60 days of age before development of T1D and are designated as non-diabetic (nd). Rats were monitored biweekly for glucosuria and diabetes onset was confirmed when fasting blood glucose exceeded 11·1 mmol/mM. All diabetic animals (abbreviated as ‘d’) were killed within 48 hr of diagnosis. Experiments were performed in accordance with the guidelines of the Canadian Council on Animal Care. Studies were approved by the Ottawa Hospital Research Institute Animal Care Committee.
Flow cytometry
Immune cell phenotyping was performed in diabetic rats within 48 hr of onset, as well as 50-day-old normoglycaemic diabetes-prone rats or age-matched controls. Splenocyte, MLN and pancreatic lymph node (PLN) cell suspensions were washed in flow cytometry buffer (IsoFlow™ sheath fluid; Beckman Coulter, Mississauga, ON, Canada) supplemented with 1% BSA. Aliquots of 5 × 105 cells were then incubated with combinations of the following rat-specific antibodies for 30 min at 4° in the dark: FITC-conjugated (-FITC) or phycoerythrin-conjugated (-PE) αβTCR, CD8α-PE (Cedarlane Laboratories, Burlington, ON, Canada), anti-κ chain-FITC, CD4-PE-cyanine 5 (-PC5), CD86-PE (BD Biosciences, Mississauga, ON, Canada), CD11c-FITC, CD103-biotin, CD68-FITC and CD163-PE (AbD Serotec, Raleigh, NC). Analysis of regulatory T (Treg) cells was performed using the PE-conjugated anti-mouse/rat/human Foxp3 Flow Kit (Biolegend, San Diego, CA) and anti-CD4-PC5 antibodies. Phenotyping and cell proliferation analyses were performed using a Beckman Coulter FC500 flow cytometer equipped with CXP software.
Immunohistochemistry
Morphometric analysis was performed on Bouin's-fixed tissues as described previously.22 Briefly, paraffin-embedded jejunum sections from 50-day-old LEW.1AR1-iddm and LEW.1AR1 rats were incubated with anti-CD3 (Abcam, Toronto, ON, Canada), anti-CD8α (BD Biosciences, Mississauga, ON, Canada), anti-Foxp3 (eBioscience, San Diego, CA) or anti-CD163 (Santa Cruz Biotechnology, Santa Cruz, CA) antibodies before the addition of appropriate biotinylated secondary antibodies. Antibody labelling was detected using VECTASTAIN® ABC reagent (Vector Laboratories Canada Inc.), diaminobenzidine and H2O2. Sections were counterstained with haematoxylin. Analysis of coded sections was performed using a Zeiss Axioplan2 microscope and northern eclipse morphometry software (Empix Imaging, Mississauga, ON, Canada).28 Small intestine immune cell populations were quantified as the number of lamina propria lymphocytes per mm2 mucosal area (CD163, CD3 and Foxp3) or as the number of intraepithelial lymphocytes per 100 epithelial cells (CD8α).
Insulitis was evaluated using coded haematoxylin & eosin-stained sections from 50-day-old cereal-fed and HC-fed LEW.1AR1-iddm rats by assessing immune cell infiltration per islet and islet number per cm2 section area as described previously.29
Gene expression assays
Jejunum and MLN from 50-day-old rats were snap-frozen in liquid N2 and stored at −80°. RNA was extracted using TRIzol (Life Technologies, Burlington, ON, Canada). Synthesis of cDNA was performed using M-MLV Reverse Transcriptase (Life Technologies). The reaction was incubated at 42° for 90 min and then inactivated for 5 min at 90° in a thermocycler. Quantitative RT-PCR analysis was performed using an ABI Prism® 7000 Sequence Detection System (Life Technologies) and TaqMan® Gene Expression Assays (Life Technologies) for the following genes: Foxp3, Ctla4, Ifng, Il4, Il23, Il10, Il17a, Tgfb1 and Tnf.
PCR array analysis
Total RNA was isolated from jejunum of 50-day-old cereal-fed LEW.1AR1 (CER, n = 3), normoglycaemic cereal-fed LEW.1AR1-iddm (nd-CER, n = 4) and normoglycaemic HC-fed LEW.1AR1-iddm (nd-HC, n = 4) rats using the NucleoSpin®RNA II Kit (Macherey-Nagel, Bethlehem, PA). RNA quantity and integrity were evaluated on a 6000 Nano LabChip® using an Agilent 2100 Bioanalyzer (OHRI Stemcore Laboratories, Ottawa, ON, Canada). RNA was transcribed to cDNA using the RT2 First Strand cDNA synthesis kit (Qiagen, Toronto, ON, Canada). Expression of a focused panel of immune-related genes was measured using the Innate and Adaptive Immune Responses RT2 Profiler™ PCR Array System (Qiagen).
T-cell proliferation assays
Single-cell suspensions were prepared from spleen and MLN of 60- to 90-day-old diabetic LEW.1AR1-iddm (d-CER) rats or age-matched controls. Cells were cultured in RPMI-1640 medium supplemented with 10% fetal bovine serum (Sigma, Oakville, ON, Canada), 25 mm HEPES, 2·0 mm/l l-glutamine, 50 μm β-mercaptoethanol, 100 U/ml penicillin and 100 μg/ml streptomycin in the presence of medium alone, 1 μm ovalbumin, 2·7 Lf/ml tetanus toxoid, 1·5 μg/ml concanavalin A (ConA; Sigma-Aldrich, Oakville, ON, Canada) or chymotrypsin-treated wheat gluten proteins at 37° and 5% CO2 for 7 days. Cell cultures with ConA were divided as required and supplemented with 10 U/ml interleukin-2 (IL-2; Peprotech, Dollard des Ormeaux, QC, Canada). In some cases, cells were labelled with 5 μm carboxyfluoresceinsuccinimidyl ester (CFSE; Life Technologies) for 20 min at 37° in PBS before antigen stimulation. T-cell proliferation was measured by determining the Cell Division Index (CDI).26 Briefly, the number of CFSEdim events was normalized to 5000 CFSEbright events for each condition, and the CDI was calculated according to the following formula for each antigen tested:
PLN cells were not labelled with CFSE because of the scarcity of this population. Instead, a Cell Activation Index (CAI) was calculated by measuring increased expression of CD25 in αβTCR+ CD4+ cells incubated with antigen compared with cells incubated without antigen from 60-day-old LEW.1AR1 (CER) and LEW.1AR1-iddm (nd-CER) rats according to the formula (number of CD25– events was normalized to 5000 CD25+ events):
Statistics
The Kaplan–Meier survival curve was analysed using the log-rank test in prism statistical software (GraphPad, La Jolla, CA). Age of diabetes onset in LEW.1AR1-iddm (CER) and LEW.1AR1-iddm (HC) rats was compared using t-test (statistica; StatSoft, Tulsa, OK). PCR Array data were analysed using the RT2 profiler™ PCR Array Data Analysis software (http://pcrdataanalysis.sabiosciences.com/pcr/arrayanalysis.php). CDI and CAI data were analysed by factorial analysis of variance with Scheffé post-hoc analysis. Other data were analysed by analysis of variance using statistica with Scheffé post-hoc analysis. Data are presented as mean ± standard deviation (SD) unless noted; P < 0·05 was considered to be statistically significant.
Results
Fewer diabetes cases in HC-fed LEW.1AR1-iddm rats
Fewer animals fed the HC diet developed diabetes compared with those fed the cereal-based diet [incidence 38·5% (10/26) versus 62% (44/71); P = 0·017] and disease onset (defined as the initial detection of fasting hyperglycaemia) was delayed [mean age of 66 ± 7 days (n = 10) versus mean age of 59 ± 7 days (n = 44), P = 0·009, Fig.1]. No differences in insulitis were observed (Table1).
Figure 1.
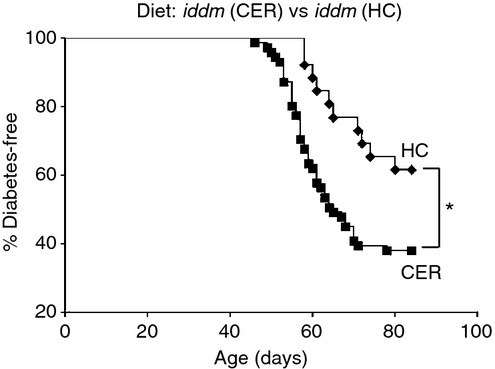
Diabetes incidence in LEW.1AR1-iddm rats modified by diet. Fewer hydrolysed casein (HC)-fed LEW.1AR1-iddm rats developed diabetes (10/26, diamonds) compared with cereal-fed animals (44/71, squares; 38·5% versus 62%, P = 0·017). Kaplan–Meier survival curve was analysed by log-rank test.
Table 1.
Insulitis was evaluated by assessing immune cell infiltration per islet and islet number per cm2 section area
| Diet | Number | Age/Status | Insulitis (±SD) |
|---|---|---|---|
| Cereal | 9 | 50 days (non-diabetic) | 1·28 (±0·36) |
| Cereal | 24 | 60–90 days (diabetic) | 4·48 (±0·62) |
| Cereal | 10 | 90 days (asymptomatic) | 2·00 (±1·33) |
| HC | 8 | 50 (non-diabetic) | 1·06 (±0·18) |
| HC | 10 | 60–90 days (diabetic) | 4·40 (±0·70) |
| HC | 15 | 90 days (asymptomatic) | 1·23 (±0·53) |
No significant difference in insulitis scores was observed among age-matched cereal-fed and hydrolysed casein (HC)-fed LEW.1AR1-iddm rats.
Cereal-fed diabetic LEW.1AR1-iddm rats display deficits in T-cell numbers compared with cereal-fed LEW.1AR1 controls
In spleen, MLN and PLN cells from cereal-fed diabetic LEW.1AR1-iddm rats, the proportion of αβTCR+ T cells was smaller than LEW.1AR1 controls (Fig.2a). T-cell deficiencies were particularly striking in the αβTCR+ CD4+ cell population (Fig.2b) whereas αβTCR+ CD8+ cells were deficient in spleen cells, but not MLN and PLN cells (Fig.2c). No significant differences were observed for markers of dendritic cells (CD86, CD11c, CD103) and macrophages (CD68, CD163) in spleen, MLN and PLN, but significantly higher numbers of κ-chain+ cells were observed in the spleen of cereal-fed diabetic LEW.1AR1-iddm rats (data not shown).
Figure 2.
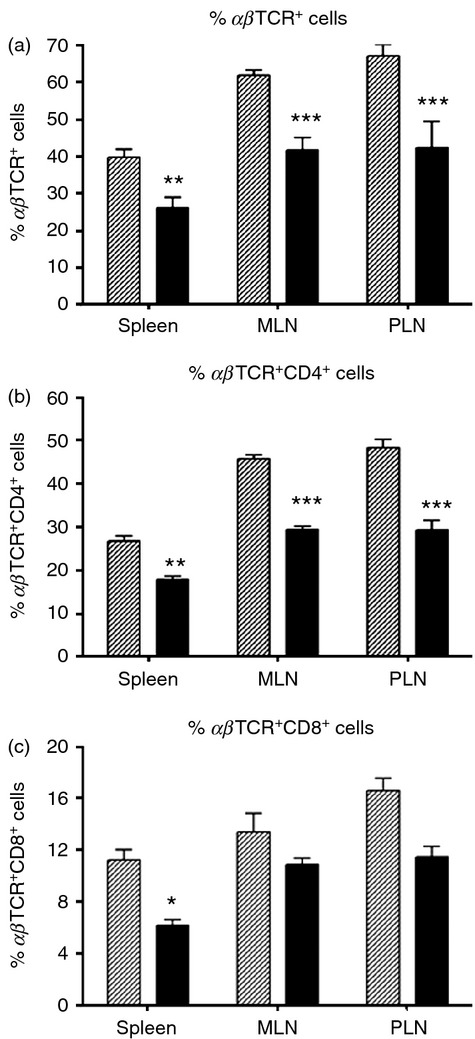
T-cell deficits in diabetic LEW.1AR1-iddm rat spleen, mesenteric lymph node (MLN) and pancreatic lymph node (PLN). (a) Diabetic LEW.1AR1-iddm rats (solid bars, n = 5–6) had fewer αβTCR+ cells compared with control LEW.1AR1 rats (hatched bars, n = 7 or 8) in all three tissues; (b) diabetic rats were also deficient in αβTCR+ CD4+ cells and (c) a significant decrease in αβTCR+ CD8+ cells was observed in the spleen. Data are mean ± SD, analysis of variance followed by Scheffé post-hoc test. *P < 0·05, **P < 0·01, ***P < 0·001.
Because Foxp3+ Treg cells could be deficient and/or defective in T1D,30–33 we quantified Treg cells in spleen, MLN and PLN of cereal-fed diabetic LEW.1AR1-iddm rats. CD4+ Foxp3+ cells were significantly decreased in PLN (Fig.3a,b). Because rats fed an HC diet were protected from diabetes (Fig.1), we investigated potential diet-induced differences in the CD4+ Foxp3+ cells in normoglycaemic 50-day LEW.1AR1-iddm rats fed cereal (nd-CER) and LEW.1AR1-iddm rats fed HC (nd-HC). HC-fed 50-day rats had significantly more CD4+ Foxp3+ MLN cells compared with cereal-fed LEW.1AR1-iddm rats. Though not significantly different from cereal-fed LEW.1AR1-iddm rats (P = 0·07), the number of CD4+ Foxp3+ cells in PLN of HC-fed LEW.1AR1-iddm rats was increased to levels similar to control LEW.1AR1 rats, suggesting a rationale for the protective effects of the HC diet (Fig.3c,d).
Figure 3.
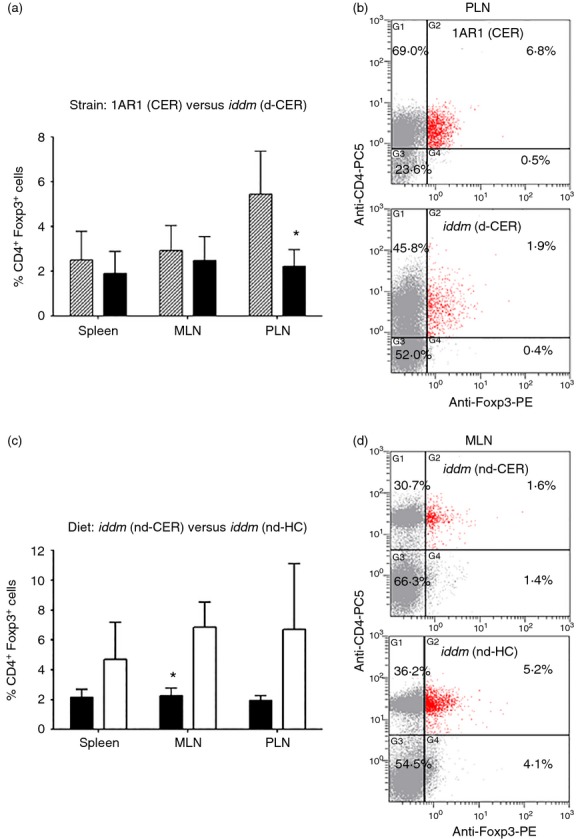
Cereal-fed LEW.1AR1-iddm rats have fewer Foxp3+ CD4+ regulatory T (Treg) cells in pancreatic lymph nodes (PLN) – hydrolysed casein (HC) diet increases mesenteric lymph node (MLN) Treg cells. (a) Diabetic LEW.1AR1-iddm (d-CER) rats (solid bars, n = 4 or 5) had fewer Treg cells in PLN compared with control LEW.1AR1 (CER) animals (hatched bars, n = 7 or 8); (b) representative scatterplot from PLN cells; (c) 50-day LEW.1AR1-iddm (nd-HC) rats (open bars, n = 7 or 8) had increased Treg cells in MLN compared with age-matched LEW.1AR1-iddm (nd-CER) rats (filled bars, n = 5 or 7); (d) representative scatterplot from MLN. Data are mean ± SD, analysis of variance followed by Scheffé post-hoc test. *P < 0·05.
Fewer Foxp3+ cells and M2 macrophages in jejunum of LEW.1AR1-iddm rats
Consistent with results from MLN, analysis of the pan-T-cell marker CD3 showed that 50-day non-diabetic LEW.1AR1-iddm rats had fewer T cells in the lamina propria compartment than controls (Fig.4a); this was also reflected in the intraepithelial CD8α+ T-cell subset (Fig.4b). Regardless of diet, 50-day non-diabetic LEW.1AR1-iddm rats had fewer Foxp3+ cells in the lamina propria (Fig.4c). HC-fed LEW.1AR1-iddm rats had fewer CD3+ and CD8α+ cells than cereal-fed LEW.1AR1-iddm rats (Fig.4a,b) and the Foxp3+/CD3+ ratio was higher (Fig.4d). These data suggest that the HC diet decreased effector T cells and increased the proportion of Treg cells in the lamina propria, consistent with flow cytometry analyses of MLN.
Figure 4.
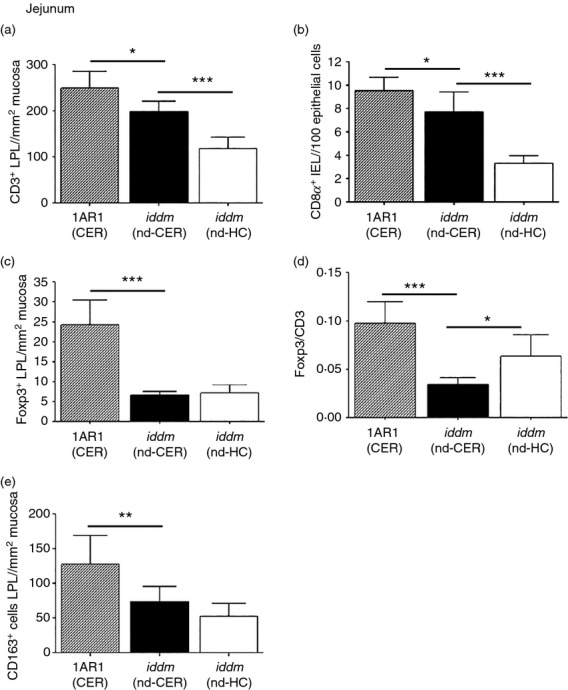
Lamina propria lymphocytes (LPL) and macrophages in jejunum of 50-day LEW.1AR1 and LEW.1AR1-iddm rats fed a cereal or hydrolysed casein (HC) diet. Immunohistochemical analysis of LPL populations in 50-day LEW.1AR1 (CER) (hatched bars, n = 7), LEW.1AR1-iddm (nd-CER) (solid bars, n = 7) and LEW.1AR1-iddm (nd-HC) rats (open bars, n = 8). (a) The number of CD3+ LPL was larger in cereal-fed LEW.1AR1(CER) rats compared with LEW.1AR1-iddm (nd-CER) rats, which had more CD3+ cells than HC-fed LEW.1AR1-iddm (nd-HC) rats as also seen in (b) the number of CD8α+ intraepithelial lymphocytes; (c) LEW.1AR1 (CER) had more Foxp3+ LPL than LEW.1AR1-iddm (nd-CER) rats; (d) LEW.1AR1-iddm (nd-CER) rats had the lowest Foxp3/CD3+ ratio compared with LEW.1AR1 (CER) rats and LEW.1AR1-iddm (nd-HC) fed rats; (e) LEW.1AR1 (CER) rats had more CD163+ M2 macrophages than LEW.1AR1-iddm (nd-CER) rats. Data are mean ± SD, analysis of variance followed by Scheffé post-hoc test. *P < 0·05, **P < 0·01, ***P < 0·001.
We also analysed the number of CD163+ cells in the lamina propria (Fig.4e). CD163 is a scavenger receptor expressed by tissue-resident macrophages, which have anti-inflammatory activity and are associated with the M2 phenotype.34 Regardless of diet, 50-day diabetes-prone rats had fewer CD163+ cells compared with the control strain, suggesting that M2 macrophages are deficient in LEW.1AR1-iddm rats similar to the BBdp rat.22
Increased pro-inflammatory cytokines in jejunum and MLN of 50-day LEW.1AR1-iddm rats
Previous studies in the BBdp rat have demonstrated a pro-inflammatory cytokine bias in the gut and associated lymphoid tissues.25 We hypothesized that a similar situation prevails in the gut-associated lymphoid tissues of the LEW.1AR1-iddm rat. We investigated cytokine mRNA expression in MLN and jejunum of 50-day non-diabetic rats using quantitative RT-PCR.
Expression of Il10, Il4, Il17a, Il23, Tgfb1, Tnf and Ifng in MLN was compared among cereal-fed (control) LEW.1AR1, LEW.1AR1-iddm and HC-fed LEW.1AR1-iddm rats. Expression of Ifng was significantly increased in 50-day cereal-fed LEW.1AR1-iddm rats compared with controls (Fig.5a). No differences were observed for other cytokines (Tnf, Il23, Il17a, Tgfb1, Il10) or Il4 expression (Fig.5b). Cereal-fed LEW.1AR1-iddm rats had an increased Ifng/Il4 ratio25,35 in MLN compared with the parental cereal-fed LEW.1AR1 strain (Fig.5c), similar to the T helper type 1 bias we observed in BBdp rats.25 Foxp3 (Fig.5d) and Ctla4 (Fig.5e) gene expression was significantly decreased in the jejunum of 50-day cereal-fed LEW.1AR1-iddm rats compared with the control strain. The decreased ratio of Ifng/Il4 in jejunum of young 50-day HC-fed diabetes-prone rats (Fig.4f) suggested that the protective HC diet decreased inflammatory responses in the lamina propria.
Figure 5.
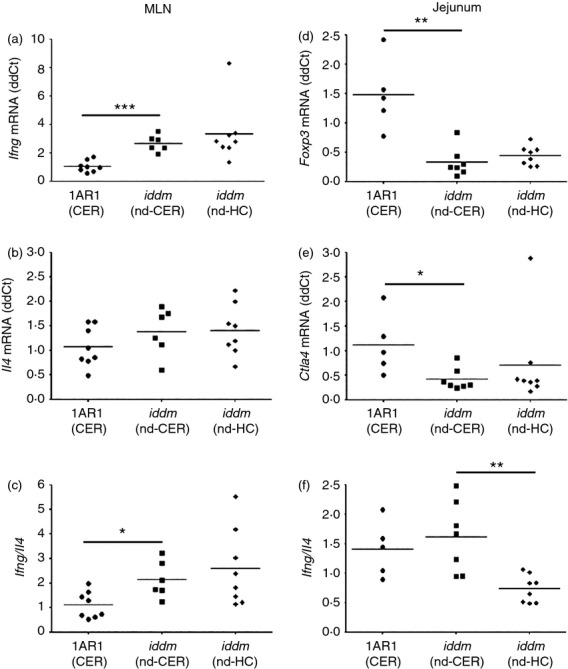
Cereal-fed 50-day LEW.1AR1-iddm rats have a pro-inflammatory bias in the mesenteric lymph nodes (MLN) and jejunum. Quantitative RT-PCR analyses of 50-day rat MLN (a–c) and jejunum (d–f) in LEW.1AR1 (CER) (filled circles, n = 5–8), LEW.1AR1-iddm (nd-CER) (filled squares, n = 6–7) and LEW.1AR1-iddm (nd-HC) (filled diamonds, n = 8) rats: (a) increased expression of Ifng in LEW.1AR1-iddm (nd-CER) versus LEW.1AR1 (CER) MLN; (b) no difference in Il4 expression in MLN; (c) increased Ifng/Il4 ratio in the LEW.1AR1-iddm (nd-CER) MLN compared with controls; (d) high levels of Foxp3 and (e) Ctla4 in LEW.1AR1 (CER) rat jejunum compared with LEW.1AR1-iddm (nd-CER) rats; (f) ratio of Ifng/Il4 increased in LEW.1AR1-iddm (nd-CER) compared with LEW.1AR1-iddm (nd-HC) rats. Mean ± SD, analysis of variance followed by Scheffé post-hoc test. *P < 0·05, **P < 0·01, ***P < 0·001.
Next, we used RT2 Profiler™ PCR Arrays to investigate the expression of a panel of genes associated with innate and adaptive immune responses in the jejunum of cereal-fed control LEW.1AR1 rats (n = 3), cereal-fed diabetes-prone LEW.1AR1-iddm rats (n = 4), as well as HC-fed LEW.1AR1-iddm rats (n = 4/group). Cereal-fed LEW.1AR1-iddm rats had significantly decreased expression of Fn1 and Stab1 (Fig.6a), both of which are associated with the M2 macrophage phenotype. This is consistent with the immunohistochemistry data showing fewer CD163+ cells in the lamina propria (Fig.4e). Cxcr4 and Casp1 were also down-regulated in diabetes-prone rats (Fig.6a). In contrast, comparison of gene expression in the jejunum of cereal-fed and HC-fed LEW.1AR1-iddm rats demonstrated that the cereal diet decreased expression of the anti-inflammatory genes Nfkbia and Il1f10 (Fig.6b).
Figure 6.
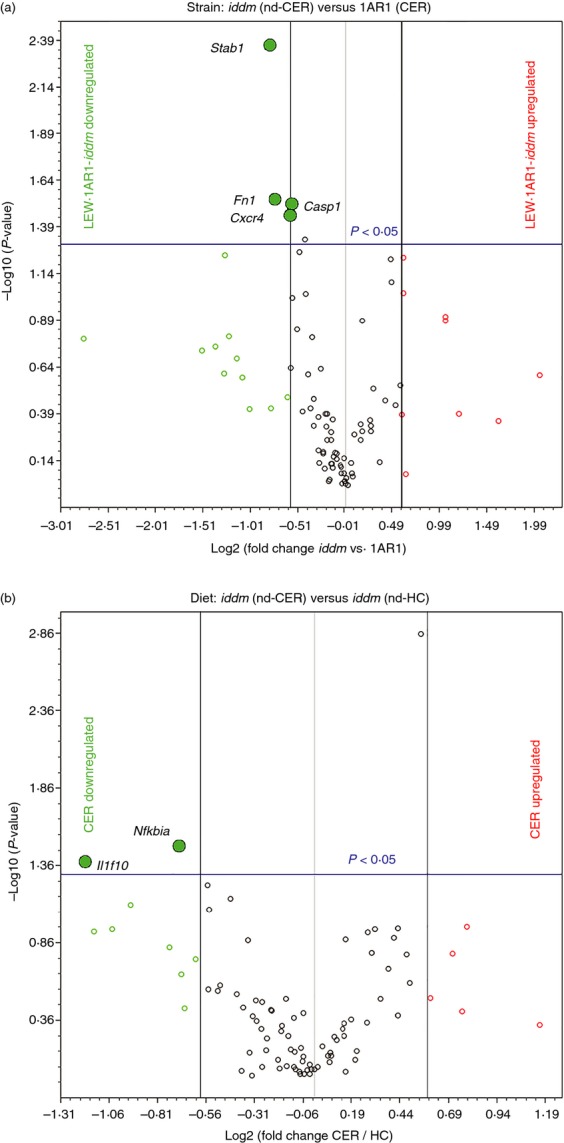
Strain- and diet-related changes in pro-inflammatory and regulatory gene expression in LEW.1AR1-iddm jejunum. Young 50-day cereal-fed LEW.1AR1 rats, LEW.1AR1-iddm (nd-CER) rats, and LEW.1AR1-iddm (nd-HC) rats were profiled by PCR array for intestinal expression of a panel of innate and adaptive immune genes. (a) Strain comparison: LEW.1AR1-iddm (nd-CER) (n = 4) versus LEW.1AR1 (CER) (n = 3); results presented as a volcano plot of gene expression in LEW.1AR1-iddm rats relative to LEW.1AR1 rats. (b) Diet comparison: volcano plot shows gene expression in LEW.1AR1-iddm (nd-CER) versus LEW.1AR1-iddm (nd-HC) (n = 4) rats, vertical (black line) boundaries were set at ± 1·5-fold change and horizontal (blue line) denotes P = 0·05 significance cut-off.
Cereal-fed LEW.1AR1-iddm rats have increased T-cell proliferation in MLN and increased CD25 expression in PLN in response to wheat peptides
Wheat peptides induce T-cell proliferation and pro-inflammatory cytokine production in subjects with T1D.25,26 Therefore, we incubated CFSE-labelled splenocytes and MLN cells with hydrolysed wheat gluten or control antigens for 7 days and measured αβTCR+ CD4+ T-cell proliferation by flow cytometry in cereal-fed diabetic LEW.1AR1-iddm (d-CER, n = 6) and age-matched cereal-fed LEW.1AR1 rats (n = 4). MLN cells from diabetic rats displayed increased proliferation to wheat peptides compared with control animals and control antigens (Fig.7a). Splenocytes did not respond to wheat peptide stimulation in either strain.
Figure 7.
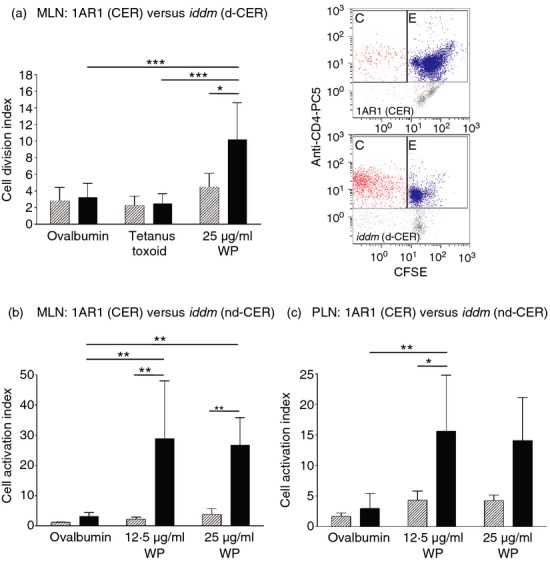
Mesenteric lymph node (MLN) and pancreatic lymph node (PLN) T-cell reactivity to wheat peptides. (a) CFSE-labelled, αβTCR+ CD4+ MLN cells from cereal-fed diabetic LEW.1AR1-iddm rats (n = 6, solid bars) incubated with wheat peptides (WP) displayed increased proliferation compared with cereal-fed LEW.1AR1 rats (n = 4, hatched bars) and with cells incubated with control antigens (ovalbumin and tetanus toxoid); representative dot plots are shown; (b) increased numbers of CD25+ CD4+ αβTCR+ MLN cells were observed in cereal-fed normoglycaemic 60-day LEW.1AR1-iddm rats (n = 6, solid bars) versus cereal-fed LEW.1AR1 control rats (n = 6, hatched bars) when stimulated with 12·5 μg/ml WP and 25 μg/ml WP and compared with ovalbumin; (c) number of CD25+ CD4+ αβTCR+ PLN cells was increased in response to 12·5 μg/ml WP in diabetes-prone rats compared with controls and ovalbumin. Data are mean ± SD, factorial analysis of variance followed by Scheffé post-hoc test. Representative dot plots are shown. *P < 0·05 (except in (a) where P = 0·050), **P < 0·01, ***P < 0·001.
We also measured T-cell activation in response to wheat peptides in MLN and PLN cells from normoglycaemic 60-day cereal-fed LEW.1AR1-iddm and LEW.1AR1 rats (n = 6/group). After 7 days in culture, the cells were labelled with CD25-FITC, αβTCR+-PE and CD4-PC5 antibodies and analysed by flow cytometry. CD25 expression was increased in LEW.1AR1-iddm MLN cells in response to wheat peptides but not to control ovalbumin (Fig.7b) and tetanus toxoid (data not shown) antigens as observed in the cell proliferation assays (Fig.7a); splenocytes did not show increased expression of CD25. Interestingly, PLN also showed increased CD25 expression in response to wheat peptides, suggesting that wheat-specific T cells traffic to the PLN36 (Fig.7c).
Discussion
Current evidence suggests that both genetic predisposition and environmental factors act in concert to break self-tolerance and induce T1D. We recently demonstrated22 that antigen-rich cereal diets provoke pro-inflammatory immune responses in diabetes-prone rats with underlying defects in gut immune regulation, which can be corrected by feeding a low antigen hydrolysed casein diet. Studies in humans have shown that T1D is associated with abnormal responses to dietary antigens.37,38 In particular, we demonstrated that peripheral blood mononuclear cells from ∽ 50% of human patients displayed pro-inflammatory responses to wheat peptides and other dietary proteins in vitro.26 In the current study, cereal-fed LEW.1AR1-iddm rats had fewer Foxp3+ Treg cells and CD163+ M2 cells in the jejunum associated with an increased Ifng/Il4 ratio in the MLN and associated immune tissues. Additionally, we demonstrated increased T-cell proliferation and activation in response to wheat peptides, characteristic of impaired oral tolerance. We also observed increased T-cell activation in the PLN where β-cell-specific effector T cells are activated, suggesting these PLN-resident, wheat-specific cells participate in the autoimmune attack. Furthermore, the low antigen HC diet protected a subset of animals from diabetes development in association with increased CD3+ Foxp3+ cells in the lamina propria and MLN and a decreased Ifng/Il4 ratio in the jejunum, indicating that a substantial portion of diabetes in the LEW.1AR1-iddm rat is gut-associated and linked with exposure to dietary antigens. Diabetes development in human patients39 and other rodent models13,15,16,40 can be associated with enteropathy and increased gut permeability, and the HC diet ameliorated the permeability defects in the BBdp rat.18 Whereas histological evidence of overt enteropathy was not observed in this study, it would be beneficial to investigate gut permeability in this model in future studies.
Gene expression analysis of jejunum from cereal-fed and HC-fed LEW.1AR1-iddm rats demonstrated that cereal-fed rats had decreased expression of Nfkbia and Il1f10. Nfkbia encodes nuclear factor of κ light polypeptide gene enhancer in B-cells inhibitor α protein, which inhibits nuclear factor-κB activity, and has been linked to inflammation and autoimmunity.41 Interleukin-1f10 (also known as IL-38) negatively regulates IL-17 and IL-22 production through IL-36 receptor.42 This suggests that the anti-inflammatory effects of the low antigen HC diet are mediated through suppression of the nuclear factor-κB and Th17/Th22 pathways, combined with an increase in the proportion of Treg cells.
We observed decreased T-cell numbers in both the MLN and lamina propria immune compartments, in agreement with recent reports of decreased CD3+ and CD4+ cells in the periphery of (cereal-fed) LEW.1AR1-iddm rats.7,8 Arndt et al. demonstrated that the variability in T-cell frequency was associated with T1D development and linked to the Iddm8 susceptibility locus.7,8 This is similar to the mild T-cell deficits observed in the NOD mouse model,43 and reminiscent of the more severe lymphopenia observed in the BBdp rat.44 T-cell homeostatic expansion under lymphopenic conditions promotes the development of autoreactive T cells45–47 particularly in the absence of a robust regulatory immune system, possibly because expansion of effector cells outpaces Treg cell proliferation in a lymphopenic environment.48 Imbalances between regulatory and effector T cells in diabetes have been reported,22,46,49 and Treg deficiencies have effects on other regulatory populations, further biasing the immune response to a pro-inflammatory phenotype.50,51 Additionally, we observed decreased expression of the chemokine receptor CXCR4, which participates in T-cell recruitment in partnership with CXCL12, in diabetes-prone rats. Blockade of the CXCR4/CXCL12 pathway causes T1D in the NOD mouse,52 and may be particularly relevant for the maintenance of the PLN Treg population.53
We recently reported that CD163+ macrophages are deficient in cereal-fed BBdp rats22 and observed putative autoantibodies to anti-inflammatory CD163+ intestinal macrophages in a patient with T1D and coeliac disease.54 We observed decreased numbers of CD163+ M2 macrophages in the lamina propria of LEW.1AR1-iddm rats and analysis of gene expression by PCR array demonstrated that the CD163+ cell deficit was associated with decreased expression of the M2-macrophage-associated genes Stab1 and Fn1. Fibronectin (Fn1) is an extracellular matrix protein associated with the wound-healing process. Stabilin-1 (Stab1) is a phagocytic receptor on M2 macrophages55,56 that has been reported to mediate remodelling of the extracellular-matrix via endocytosis of SPARC (secreted protein acidic and rich in cysteine).57 In addition, endothelial expression of stabilin-1 supports leucocyte extravasation, and has been shown to support preferential migration of Treg cells into sites of inflammation.58 While the HC diet did not correct the CD163+ cell deficit, it did significantly decrease the Ifng/Il4 ratio, suggesting that the HC diet impacted M1/M2-associated cytokine balance.
In summary, combined defects in adaptive and innate immune regulation result in a persistent inflammatory bias in the gut of LEW.1AR1-iddm rats fed a cereal-based diet, leading to a loss of tolerance towards dietary antigens. These deficits were partially corrected by feeding a low antigen, hydrolysed casein diet, in association with protection from diabetes. A defect in M2 macrophage function would impair anti-inflammatory responses such as the clearance of apoptotic cellular debris,59 predisposing individuals to chronic inflammation, particularly in a lymphopenic environment that favours the development of autoimmunity.45–47 These combined risk factors could be critical for T1D development, as suggested by our recent studies, which demonstrate innate immunoregulatory defects in the BBdp rat,22 and a study in which T1D was prevented in NOD mice by adoptive transfer of M2 macrophages.60
Acknowledgments
This work was supported by the Canadian Institutes of Health Research and the Canadian Diabetes Association. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
C. Patrick was supported by the Queen Elizabeth II Graduate Scholarship in Science and Technology, Ontario Graduate Scholarship program and Ontario Graduate Scholarships in Science and Technology; J.A. Noel was supported by the Natural Sciences and Engineering Research Council and by the Ontario Graduate Scholarship program.
JAC, CP and GSW performed the experiments, analysed the data and wrote the manuscript. JAN performed the experiments and analysed the data. FWS conceived and designed the study, analysed the data, and wrote the manuscript. FWS is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Parts of this work were presented at the 70th Scientific Sessions of the American Diabetes Association (Orlando, USA, Diabetes 2010;59 (Suppl. 1):OR-333), and at the 2nd International Conference on Immune Tolerance (Amsterdam, the Netherlands, 2011).
We are grateful to Dr S. Lenzen and Dr D. Wedekind (Hannover Medical School) for the kind gift of LEW.1AR1 and LEW.1AR1-iddm rats. We thank Dr C. Kennedy, A. Côté, A. Hendin, G. Rodger and P. Bradley (OHRI) for technical assistance.
Glossary
- 1AR1
control, non-diabetes-prone LEW.1AR1 rat, cereal-fed
- BBdp
diabetes-prone BioBreeding rat
- CAI
Cell Activation Index
- CDI
Cell Division Index
- d
recent onset diabetes
- HC
hydrolysed casein
- iddm (d-CER)
LEW.1AR1-iddm diabetic rat, cereal-fed
- iddm (nd-CER)
LEW.1AR1-iddm non-diabetic (50 days old), cereal-fed rat
- iddm (nd-HC)
LEW.1AR1-iddm non-diabetic (50 days old), HC-fed rat
- IEL
intraepithelial lymphocytes
- LPL
lamina propria lymphocytes
- MLN
mesenteric lymph node
- nd
non-diabetic
- NOD
non-obese diabetic mouse
- PLN
pancreatic lymph node
- Treg
regulatory T cells
Disclosures
No potential conflicts of interest relevant to this article were reported.
References
- Lefebvre DE, Powell KL, Strom A, Scott FW. Dietary proteins as environmental modifiers of type 1 diabetes mellitus. Annu Rev Nutr. 2006;26:175–202. doi: 10.1146/annurev.nutr.26.061505.111206. [DOI] [PubMed] [Google Scholar]
- Leslie RD. Toward secondary prevention of type 1 diabetes. Diabet Med. 2011;28:1140. doi: 10.1111/j.1464-5491.2011.03418.x. [DOI] [PubMed] [Google Scholar]
- Phillips B, Trucco M, Giannoukakis N. Current state of type 1 diabetes immunotherapy: incremental advances, huge leaps, or more of the same? Clin Dev Immunol. 2011;2011:432016. doi: 10.1155/2011/432016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greiner DL, Rossini AA, Mordes JP. Translating data from animal models into methods for preventing human autoimmune diabetes mellitus: caveat emptor and primum non nocere. Clin Immunol. 2001;100:134–43. doi: 10.1006/clim.2001.5075. [DOI] [PubMed] [Google Scholar]
- Jorns A, Arndt T, Meyer zu Vilsendorf A, et al. Islet infiltration, cytokine expression and beta cell death in the NOD mouse, BB rat, Komeda rat, LEW.1AR1-iddm rat and humans with type 1 diabetes. Diabetologia. 2014;57:512–21. doi: 10.1007/s00125-013-3125-4. [DOI] [PubMed] [Google Scholar]
- Lenzen S, Tiedge M, Elsner M, et al. The LEW.1AR1/Ztm-iddm rat: a new model of spontaneous insulin-dependent diabetes mellitus. Diabetologia. 2001;44:1189–96. doi: 10.1007/s001250100625. [DOI] [PubMed] [Google Scholar]
- Arndt T, Jorns A, Hedrich HJ, Lenzen S, Wedekind D. Variable immune cell frequencies in peripheral blood of LEW.1AR1-iddm rats over time compared to other congenic LEW strains. Clin Exp Immunol. 2014;177:168–78. doi: 10.1111/cei.12323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arndt T, Jorns A, Weiss H, Tiedge M, Hedrich HJ, Lenzen S, Wedekind D. A variable CD3+ T-cell frequency in peripheral blood lymphocytes associated with type 1 diabetes mellitus development in the LEW.1AR1-iddm rat. PLoS ONE. 2013;8:e64305. doi: 10.1371/journal.pone.0064305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorns A, Gunther A, Hedrich HJ, Wedekind D, Tiedge M, Lenzen S. Immune cell infiltration, cytokine expression, and β-cell apoptosis during the development of type 1 diabetes in the spontaneously diabetic LEW.1AR1/Ztm-iddm rat. Diabetes. 2005;54:2041–52. doi: 10.2337/diabetes.54.7.2041. [DOI] [PubMed] [Google Scholar]
- Wedekind D, Weiss H, Jorns A, Lenzen S, Tiedge M, Hedrich HJ. Effects of polyinosinic-polycytidylic acid and adoptive transfer of immune cells in the Lew. 1AR1-iddm rat and in its coisogenic LEW.1AR1 background strain. Autoimmunity. 2005;38:265–75. doi: 10.1080/08916930500114321. [DOI] [PubMed] [Google Scholar]
- Weiss H, Bleich A, Hedrich HJ, et al. Genetic analysis of the LEW.1AR1-iddm rat: an animal model for spontaneous diabetes mellitus. Mamm Genome. 2005;16:432–41. doi: 10.1007/s00335-004-3022-8. [DOI] [PubMed] [Google Scholar]
- Weiss H, Arndt T, Jorns A, Lenzen S, Cuppen E, Hedrich HJ, Tiedge M, Wedekind D. The mutation of the LEW.1AR1-iddm rat maps to the telomeric end of rat chromosome 1. Mamm Genome. 2008;19:292–7. doi: 10.1007/s00335-008-9102-4. [DOI] [PubMed] [Google Scholar]
- Meddings JB, Jarand J, Urbanski SJ, Hardin J, Gall DG. Increased gastrointestinal permeability is an early lesion in the spontaneously diabetic BB rat. Am J Physiol. 1999;276:G951–7. doi: 10.1152/ajpgi.1999.276.4.G951. [DOI] [PubMed] [Google Scholar]
- Malaisse WJ, Courtois P, Scott FW. Insulin-dependent diabetes and gut dysfunction: the BB rat model. Horm Metab Res. 2004;36:585–94. doi: 10.1055/s-2004-825920. [DOI] [PubMed] [Google Scholar]
- Watts T, Berti I, Sapone A, Gerarduzzi T, Not T, Zielke R, Fasano A. Role of the intestinal tight junction modulator zonulin in the pathogenesis of type I diabetes in BB diabetic-prone rats. Proc Natl Acad Sci U S A. 2005;102:2916–21. doi: 10.1073/pnas.0500178102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham S, Courtois P, Malaisse WJ, Rozing J, Scott FW, Mowat AM. Enteropathy precedes type 1 diabetes in the BB rat. Gut. 2004;53:1437–44. doi: 10.1136/gut.2004.042481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee AS, Gibson DL, Zhang Y, Sham HP, Vallance BA, Dutz JP. Gut barrier disruption by an enteric bacterial pathogen accelerates insulitis in NOD mice. Diabetologia. 2010;53:741–8. doi: 10.1007/s00125-009-1626-y. [DOI] [PubMed] [Google Scholar]
- Visser JT, Lammers K, Hoogendijk A, et al. Restoration of impaired intestinal barrier function by the hydrolysed casein diet contributes to the prevention of type 1 diabetes in the diabetes-prone BioBreeding rat. Diabetologia. 2010;53:2621–8. doi: 10.1007/s00125-010-1903-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosi E, Molteni L, Radaelli MG, et al. Increased intestinal permeability precedes clinical onset of type 1 diabetes. Diabetologia. 2006;49:2824–7. doi: 10.1007/s00125-006-0465-3. [DOI] [PubMed] [Google Scholar]
- Muegge BD, Kuczynski J, Knights D, et al. Diet drives convergence in gut microbiome functions across mammalian phylogeny and within humans. Science. 2011;332:970–4. doi: 10.1126/science.1198719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen DS, Krych L, Buschard K, Hansen CH, Hansen AK. Beyond genetics. Influence of dietary factors and gut microbiota on type 1 diabetes. FEBS Lett. 2014;588:4234–43. doi: 10.1016/j.febslet.2014.04.010. [DOI] [PubMed] [Google Scholar]
- Patrick C, Wang GS, Lefebvre DE, et al. Promotion of autoimmune diabetes by cereal diet in the presence or absence of microbes associated with gut immune activation, regulatory imbalance and altered cathelicidin antimicrobial peptide. Diabetes. 2013;62:2036–47. doi: 10.2337/db12-1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott FW. Food-induced type 1 diabetes in the BB rat. Diabetes Metab Rev. 1996;12:341–59. doi: 10.1002/(SICI)1099-0895(199612)12:4<341::AID-DMR173>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Beales PE, Elliott RB, Flohe S, et al. A multi-centre, blinded international trial of the effect of A(1) and A(2) β-casein variants on diabetes incidence in two rodent models of spontaneous Type I diabetes. Diabetologia. 2002;45:1240–6. doi: 10.1007/s00125-002-0898-2. [DOI] [PubMed] [Google Scholar]
- Chakir H, Lefebvre DE, Wang H, Caraher E, Scott FW. Wheat protein-induced proinflammatory T helper 1 bias in mesenteric lymph nodes of young diabetes-prone rats. Diabetologia. 2005;48:1576–84. doi: 10.1007/s00125-005-1842-z. [DOI] [PubMed] [Google Scholar]
- Mojibian M, Chakir H, Lefebvre DE, Crookshank JA, Sonier B, Keely E, Scott FW. A diabetes-specific HLA-DR restricted pro-inflammatory T cell response to wheat polypeptides in tissue transglutaminase antibody negative patients with type 1 diabetes. Diabetes. 2009;58:1789–96. doi: 10.2337/db08-1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacFarlane AJ, Burghardt KM, Kelly J, Simell T, Simell O, Altosaar I, Scott FW. A type 1 diabetes- related protein from wheat (Triticum aestivum). cDNA clone of a wheat storage globulin, Glb1, linked to islet damage. J Biol Chem. 2003;278:54–63. doi: 10.1074/jbc.M210636200. [DOI] [PubMed] [Google Scholar]
- Wang GS, Kauri LM, Patrick C, Bareggi M, Rosenberg L, Scott FW. Enhanced islet expansion by beta-cell proliferation in young diabetes-prone rats fed a protective diet. J Cell Physiol. 2011;224:501–8. doi: 10.1002/jcp.22151. [DOI] [PubMed] [Google Scholar]
- Scott FW, Rowsell P, Wang GS, Burghardt K, Kolb H, Flohe S. Oral exposure to diabetes-promoting food or immunomodulators in neonates alters gut cytokines and diabetes. Diabetes. 2002;51:73–8. doi: 10.2337/diabetes.51.1.73. [DOI] [PubMed] [Google Scholar]
- D'Alise AM, Ergun A, Hill JA, Mathis D, Benoist C. A cluster of coregulated genes determines TGF-β-induced regulatory T-cell (Treg) dysfunction in NOD mice. Proc Natl Acad Sci USA. 2011;108:8737–42. doi: 10.1073/pnas.1105364108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker CF, Nebane-Ambe DL, Chhabra A, Parnell SA, Zhao Y, Alard P, Kosiewicz MM. Decreased frequencies of CD4+ CD25+ Foxp3+ cells and the potent CD103+ subset in peripheral lymph nodes correlate with autoimmune disease predisposition in some strains of mice. Autoimmunity. 2011;44:453–64. doi: 10.3109/08916934.2011.568553. [DOI] [PubMed] [Google Scholar]
- Badami E, Sorini C, Coccia M, et al. Defective differentiation of regulatory FoxP3+ T cells by small-intestinal dendritic cells in patients with type 1 diabetes. Diabetes. 2011;60:2120–4. doi: 10.2337/db10-1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider A, Rieck M, Sanda S, Pihoker C, Greenbaum C, Buckner JH. The effector T cells of diabetic subjects are resistant to regulation via CD4+ FOXP3+ regulatory T cells. J Immunol. 2008;181:7350–5. doi: 10.4049/jimmunol.181.10.7350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonier B, Patrick C, Ajjikuttira P, Scott FW. Intestinal immune regulation as a potential diet-modifiable feature of gut inflammation and autoimmunity. Int Rev Immunol. 2009;28:414–45. doi: 10.3109/08830180903208329. [DOI] [PubMed] [Google Scholar]
- Chakir H, Wang H, Lefebvre DE, Webb J, Scott FW. T-bet/GATA-3 ratio as a measure of the Th1/Th2 cytokine profile in mixed cell populations: predominant role of GATA-3. J Immunol Methods. 2003;278:157–69. doi: 10.1016/s0022-1759(03)00200-x. [DOI] [PubMed] [Google Scholar]
- Turley SJ, Lee JW, Dutton-Swain N, Mathis D, Benoist C. Endocrine self and gut non-self intersect in the pancreatic lymph nodes. Proc Natl Acad Sci USA. 2005;102:17729–33. doi: 10.1073/pnas.0509006102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auricchio R, Paparo F, Maglio M, et al. In vitro-deranged intestinal immune response to gliadin in type 1 diabetes. Diabetes. 2004;53:1680–3. doi: 10.2337/diabetes.53.7.1680. [DOI] [PubMed] [Google Scholar]
- Troncone R, Franzese A, Mazzarella G, Paparo F, Auricchio R, Coto I, Mayer M, Greco L. Gluten sensitivity in a subset of children with insulin dependent diabetes mellitus. Am J Gastroenterol. 2003;98:590–5. doi: 10.1111/j.1572-0241.2003.07301.x. [DOI] [PubMed] [Google Scholar]
- Sapone A, de Magistris L, Pietzak M, et al. Zonulin upregulation is associated with increased gut permeability in subjects with type 1 diabetes and their relatives. Diabetes. 2006;55:1443–9. doi: 10.2337/db05-1593. [DOI] [PubMed] [Google Scholar]
- Courtois P, Nsimba G, Jijakli H, Sener A, Scott FW, Malaisse WJ. Gut permeability and intestinal mucins, invertase, and peroxidase in control and diabetes-prone BB rats fed either a protective or a diabetogenic diet. Dig Dis Sci. 2005;50:266–75. doi: 10.1007/s10620-005-1594-0. [DOI] [PubMed] [Google Scholar]
- Zhang GL, Zou YF, Feng XL, Shi HJ, Du XF, Shao MH, Gu Y, Zhou Q. Association of the NFKBIA gene polymorphisms with susceptibility to autoimmune and inflammatory diseases: a meta-analysis. Inflamm Res. 2011;60:11–8. doi: 10.1007/s00011-010-0216-2. [DOI] [PubMed] [Google Scholar]
- van de Veerdonk FL, Stoeckman AK, Wu G, et al. IL-38 binds to the IL-36 receptor and has biological effects on immune cells similar to IL-36 receptor antagonist. Proc Natl Acad Sci U S A. 2012;109:3001–5. doi: 10.1073/pnas.1121534109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King C, Ilic A, Koelsch K, Sarvetnick N. Homeostatic expansion of T cells during immune insufficiency generates autoimmunity. Cell. 2004;117:265–77. doi: 10.1016/s0092-8674(04)00335-6. [DOI] [PubMed] [Google Scholar]
- Ramanathan S, Poussier P. BB rat lyp mutation and Type 1 diabetes. Immunol Rev. 2001;184:161–71. doi: 10.1034/j.1600-065x.2001.1840115.x. [DOI] [PubMed] [Google Scholar]
- Calzascia T, Pellegrini M, Lin A, Garza KM, Elford AR, Shahinian A, Ohashi PS, Mak TW. CD4 T cells, lymphopenia, and IL-7 in a multistep pathway to autoimmunity. Proc Natl Acad Sci USA. 2008;105:2999–3004. doi: 10.1073/pnas.0712135105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saoudi A, Seddon B, Fowell D, Mason D. The thymus contains a high frequency of cells that prevent autoimmune diabetes on transfer into prediabetic recipients. J Exp Med. 1996;184:2393–8. doi: 10.1084/jem.184.6.2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Saout C, Mennechet S, Taylor N, Hernandez J. Memory-like CD8+ and CD4+ T cells cooperate to break peripheral tolerance under lymphopenic conditions. Proc Natl Acad Sci USA. 2008;105:19414–9. doi: 10.1073/pnas.0807743105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaminitz A, Mizrahi K, Yaniv I, Stein J, Askenasy N. Immunosuppressive therapy exacerbates autoimmunity in NOD mice and diminishes the protective activity of regulatory T cells. J Autoimmun. 2010;35:145–52. doi: 10.1016/j.jaut.2010.06.002. [DOI] [PubMed] [Google Scholar]
- van den Brandt J, Fischer HJ, Walter L, Hunig T, Kloting I, Reichardt HM. Type 1 diabetes in BioBreeding rats is critically linked to an imbalance between Th17 and regulatory T cells and an altered TCR repertoire. J Immunol. 2010;185:2285–94. doi: 10.4049/jimmunol.1000462. [DOI] [PubMed] [Google Scholar]
- Liu G, Ma H, Qiu L, Li L, Cao Y, Ma J, Zhao Y. Phenotypic and functional switch of macrophages induced by regulatory CD4+ CD25+ T cells in mice. Immunol Cell Biol. 2011;89:130–42. doi: 10.1038/icb.2010.70. [DOI] [PubMed] [Google Scholar]
- Tiemessen MM, Jagger AL, Evans HG, van Herwijnen MJ, John S, Taams LS. CD4+ CD25+ Foxp3+ regulatory T cells induce alternative activation of human monocytes/macrophages. Proc Natl Acad Sci USA. 2007;104:19446–51. doi: 10.1073/pnas.0706832104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aboumrad E, Madec AM, Thivolet C. The CXCR4/CXCL12 (SDF-1) signalling pathway protects non-obese diabetic mouse from autoimmune diabetes. Clin Exp Immunol. 2007;148:432–9. doi: 10.1111/j.1365-2249.2007.03370.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nti BK, Markman JL, Bertera S, Styche AJ, Lakomy RJ, Subbotin VM, Trucco M, Zorina TD. Treg cells in pancreatic lymph nodes: the possible role in diabetogenesis and beta cell regeneration in a T1D model. Cell Mol Immunol. 2012;9:455–63. doi: 10.1038/cmi.2012.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonier B, Strom A, Wang G-S, Patrick C, Crookshank JA, Mojibian M, MacFarlane AJ, Scott FW. Antibodies from a patient with type 1 diabetes and celiac disease bind to macrophages that express the scavenger receptor CD163. Can J Gastroenterol. 2011;25:327–9. doi: 10.1155/2011/758579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palani S, Maksimow M, Miiluniemi M, Auvinen K, Jalkanen S, Salmi M. Stabilin-1/CLEVER-1, a type 2 macrophage marker, is an adhesion and scavenging molecule on human placental macrophages. Eur J Immunol. 2011;41:2052–63. doi: 10.1002/eji.201041376. [DOI] [PubMed] [Google Scholar]
- Park SY, Jung MY, Lee SJ, Kang KB, Gratchev A, Riabov V, Kzhyshkowska J, Kim IS. Stabilin-1 mediates phosphatidylserine-dependent clearance of cell corpses in alternatively activated macrophages. J Cell Sci. 2009;122:3365–73. doi: 10.1242/jcs.049569. [DOI] [PubMed] [Google Scholar]
- Kzhyshkowska J, Workman G, Cardo-Vila M, et al. Novel function of alternatively activated macrophages: stabilin-1-mediated clearance of SPARC. J Immunol. 2006;176:5825–32. doi: 10.4049/jimmunol.176.10.5825. [DOI] [PubMed] [Google Scholar]
- Shetty S, Weston CJ, Oo YH, et al. Common lymphatic endothelial and vascular endothelial receptor-1 mediates the transmigration of regulatory T cells across human hepatic sinusoidal endothelium. J Immunol. 2011;186:4147–55. doi: 10.4049/jimmunol.1002961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zizzo G, Hilliard BA, Monestier M, Cohen PL. Efficient clearance of early apoptotic cells by human macrophages requires M2c polarization and MerTK induction. J Immunol. 2012;189:3508–20. doi: 10.4049/jimmunol.1200662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsa R, Andresen P, Gillett A, Mia S, Zhang XM, Mayans S, Holmberg D, Harris RA. Adoptive transfer of immunomodulatory M2 macrophages prevents type 1 diabetes in NOD mice. Diabetes. 2012;61:2881–92. doi: 10.2337/db11-1635. [DOI] [PMC free article] [PubMed] [Google Scholar]


