Abstract
Background and Aims An endogenous rhythm synchronized to dawn cannot time photosynthesis-linked genes to peak consistently at noon since the interval between sunrise and noon changes seasonally. In this study, a solar clock model that circumvents this limitation is proposed using two daily timing references synchronized to noon and midnight. Other rhythmic genes that are not directly linked to photosynthesis, and which peak at other times, also find an adaptive advantage in entrainment to the solar rhythm.
Methods Fourteen datasets extracted from three published papers were used in a meta-analysis to examine the cyclic behaviour of the Arabidopsis thaliana photosynthesis-related gene CAB2 and the clock oscillator genes TOC1 and LHY in T cycles and N–H cycles.
Key Results Changes in the rhythms of CAB2, TOC1 and LHY in plants subjected to non-24-h light:dark cycles matched the hypothesized changes in their behaviour as predicted by the solar clock model, thus validating it. The analysis further showed that TOC1 expression peaked ∼5·5 h after mid-day, CAB2 peaked close to noon, while LHY peaked ∼7·5 h after midnight, regardless of the cycle period, the photoperiod or the light:dark period ratio. The solar clock model correctly predicted the zeitgeber timing of these genes under 11 different lighting regimes comprising combinations of seven light periods, nine dark periods, four cycle periods and four light:dark period ratios. In short cycles that terminated before LHY could be expressed, the solar clock correctly predicted zeitgeber timing of its expression in the following cycle.
Conclusions Regulation of gene phases by the solar clock enables the plant to tell the time, by which means a large number of genes are regulated. This facilitates the initiation of gene expression even before the arrival of sunrise, sunset or noon, thus allowing the plant to ‘anticipate’ dawn, dusk or mid-day respectively, independently of the photoperiod.
Keywords: Arabidopsis thaliana, CAB2, TOC1, LHY, circadian rhythm, clock genes, photoperiod, solar clock, solar rhythm, zeitgeber
INTRODUCTION
Circadian rhythms in plants and animals refer broadly to various self-sustaining endogenous diurnal periodicities that may differ in key characteristics. In common with all circadian cycles, precision of the rhythm is maintained by daily entrainment to specific environmental references (‘zeitgebers’). Plants are equipped with sensitive light sensors, and so light is commonly the signal that plants use to synchronize their internal clocks to the environment. A well-known example of such a timing cue is the transition from darkness to light at sunrise. The time measured from sunrise (or ‘lights-on’ in the growth chamber) is often referred to as ‘zeitgeber time’, notwithstanding the fact that sunrise is by no means the only zeitgeber operational in circadian rhythms. The rhythms of many arabidopsis cyclic genes, e.g. COLD AND CIRCADIAN-RHYTHM 2 (CCR2) (Alabadí et al., 2002; Roden et al., 2002; McWatters et al., 2007) and CATALASE 3 (CAT3) (Michael et al., 2003), are set by sunrise, with their activities activated at specific intervals timed from the dawn, regardless of the photoperiod. However, timing by sunrise is disadvantageous where genes have functions related to light harvesting in photosynthesis, the efficiency of which is crucial to the competitiveness and ultimate survival of the plant. Since the time lapse between sunrise and noon (the mid-point of the light period) varies seasonally, a cyclic gene with an active phase inflexibly timed from sunrise could peak too early or too late to receive sunlight when it is most intense. The expression of photosynthesis-related genes consistently close to noon is especially crucial at higher latitudes, where the window of sunshine in winter can be very brief, and the opportunity for photosynthesis could otherwise be missed altogether. Indeed, activity of the arabidopsis gene CHLOROPHYLL A-B BINDING PROTEIN 2 (CAB2) peaks consistently close to noon (Millar and Kay, 1996; Tóth et al., 2001; Kim et al., 2003; Michael et al., 2003; Perales and Más, 2007) (Fig. 1). From this, it is apparent that the CAB2 rhythm does not take its timing cue from sunrise, or at least not from sunrise alone. How then is this rhythm maintained?
Fig. 1.
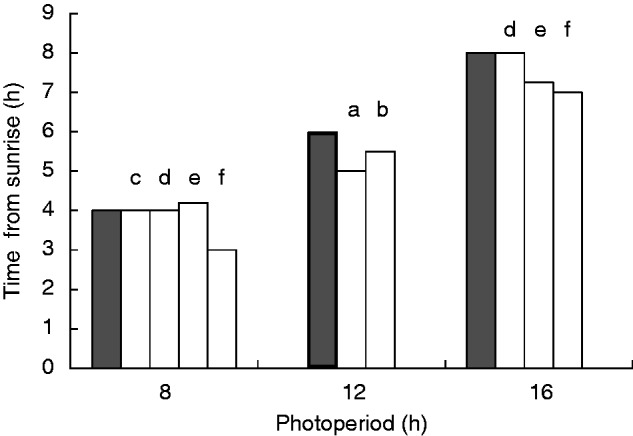
Timing of peak CAB2 activity measured from sunrise for 8-, 12- and 16-h photoperiods in 24-h cycles. Dark shaded columns denote the timing of noon (mid-point of the light period) measured from sunrise. Light shaded columns represent the timing of peak CAB2 activity, based on (a) Fig. 4 of Millar and Kay (1996), (b) Fig. 2 of Tóth et al. (2001), (c) Fig. 1 of Kim et al. (2003), (d) Fig. 2 of Michael et al. (2003), (e) Fig. 4 of McWatters et al. (2007) and (f) Fig. 5 of Perales and Más (2007). Readings were taken at intervals of 2 h or shorter and averaged from two or three cycles, with the exception of the data from Michael et al. (2003), where readings were from the first period after transferring from a light/dark cycle to continuous light.
That the CAB2 rhythm is linked to that of TIMING OF CAB EXPRESSION 1 (TOC1) in some way (Millar et al., 1995) is self-evident from the appellation of the latter. In fact, TOC1 plays an important role as a key component of the circadian clock’s central oscillator, which generates a self-perpetuating rhythm in arabidopsis. The clock oscillator function that is achieved in concert with another principal oscillator gene, LATE ELONGATED HYPOCOTYL (LHY) and its homologue CIRCADIAN CLOCK ASSOCIATED 1 (CCA1) maintains an endogenous rhythm through a transcriptional feedback mechanism that also involves a host of other regulatory genes (Alabadí et al., 2001; Huang et al., 2012; Pokhilko et al., 2012; Hsu and Harmer, 2014). Nevertheless, a sustained cycle is only one characteristic of the circadian rhythm. A further provision requires the rhythm to be entrained to a consistent 24-h period. While the oscillator feedback mechanism is capable of perpetuating an endogenous rhythm, sustaining its periodicity with a degree of precision would require calibration against an environmental timing reference captured by the plant’s photoreceptors (Salomé and McClung, 2005). For genes like CAB2, as noted above, the light transition at sunrise on its own does not fulfil this role. What other light signals from the environment might therefore be better suited to this task?
Past studies on plant clock models have elucidated in some detail the various clock components, the paths they take and how they interact with one another. But left largely unsaid is how and where, amidst these paths and loops, timing references (zeitgebers) act to entrain the rhythms of many genes. It is one thing to get a clock running, but quite another to keep it running on time. What zeitgeber positions the CAB2 peak at the mid-point of the light period? A similar question applies to many other plant genes. For example, what zeitgeber entrains the cycles of the ‘morning genes’ CCA1 and LHY? While both genes peak in the early morning, their activities are initiated in darkness, well before sunrise. Past researchers have often been reticent and vague when drilling down to specific zeitgebers, alluding only to ‘the input of light signals’ (Zhou et al., 2007), ‘the resetting light signals’ (Tóth et al., 2001), ‘light signals [that] are transduced to entrain a circadian oscillator’ (Webb, 2003) or ‘light signals [that] reset or entrain the oscillator’ (Murtas and Millar, 2000). The nature of these ‘light signals’ is therefore the focus of the present paper. The solar clock model presented here seeks to complement rather than supplant the well-researched existing clock models. Whereas current models explain how the plant clock runs, the model examined here specifically addresses how the clock is set.
In this meta-analysis, evidence is presented to validate a model for an endogenous plant rhythm that circumvents the limitations of a rhythm entrained to the zeitgeber of sunrise. This is the solar rhythm that is controlled by a clock synchronized to noon and midnight, much the same way we set our clocks and watches to chronometer time. The solar rhythm allows the plant clock to be set, rendering it functional. A clock, no matter how well it runs, does not tell the time until it is set. The solar clock model also allows the timing of genes cycling on solar time to be predicted in plants subjected to different photoperiods, cycle periods or dark/light ratios.
METHODS
Fourteen datasets extracted from three published papers were used in a meta-analysis to examine the cyclic behaviour of the Arabidopsis thaliana genes CAB2, TOC1 and LHY in T cycles and Nanda–Hamner (N–H) cycles. In T cycles, the ratio of the dark to light periods is fixed while the cycle period varies, whereas in N–H cycles (Nanda and Hamner, 1958) the light period is fixed while the dark period (and hence the cycle period) is variable. Details of the data used in the analysis are shown in Table 1.
Table 1.
Characteristics of T cycles and N–H cycles in arabidopsis experiments. Readings were taken at 2-h intervals
| Cycle period (h) | Light period (h) | Dark period (h) | Ratio Light:dark periods | Cycle characteristics | Data source | |
|---|---|---|---|---|---|---|
| TOC1 T cycles | 28 | 14 | 14 | 1:1 | Light:dark period ratio fixed at 1:1; cycle period, light and dark periods variable | Fig. 2 of Dodd et al. (2014) |
| 24 | 12 | 12 | 1:1 | |||
| 20 | 10 | 10 | 1:1 | |||
| CAB2 T cycles | 28 | 9·3 | 18·7 | 1: 2 | Light:dark period ratio fixed at 1:2; cycle period, light and dark periods variable | Fig. 1 of Kim et al. (2003), averaged over three cycles; |
| 24 | 8·0 | 16·0 | 1: 2 | |||
| 20 | 6·7 | 13·3 | 1: 2 | |||
| LHY T cycles | 28 | 9·3 | 18·7 | 1: 2 | Light:dark period ratio fixed at 1:2; cycle period, light and dark periods variable | Figs 2 and 3 of Roden et al. (2002), averaged over three cycles |
| 24 | 8·0 | 16·0 | 1: 2 | |||
| 20 | 6·7 | 13·3 | 1: 2 | |||
| 16 | 5·3 | 10·7 | 1: 2 | |||
| LHY N–H cycles | 28 | 8 | 20 | 1: 2·5 | Light period fixed at 8 h; dark period, cycle period and light:dark period ratio variable | Figs 2 and 3 of Roden et al. (2002), averaged over two cycles |
| 24 | 8 | 16 | 1: 2·0 | |||
| 20 | 8 | 12 | 1: 1·5 | |||
| 16 | 8 | 8 | 1: 1·0 |
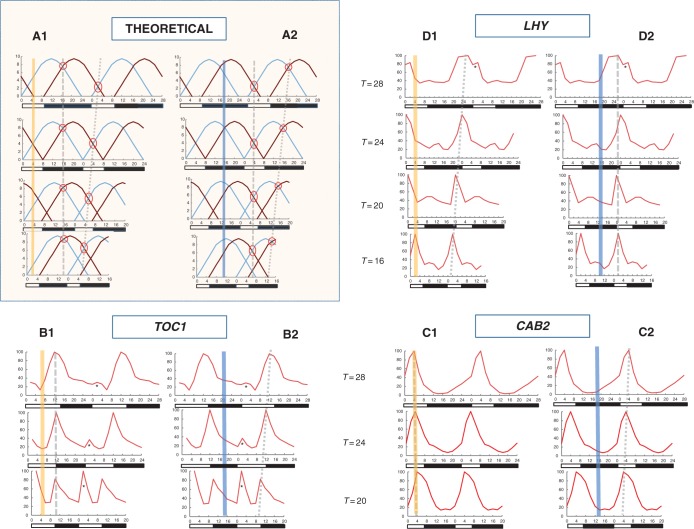
In the originally published graphs of TOC1, CAB2 and LHY rhythms (Roden et al., 2002; Kim et al., 2003; Dodd et al., 2014) that are re-constructed in Fig. 3 and Supplementary Data Fig. S1, the scales of the x-axes were normalized, resulting in all T or N–H cycles on the x-axis being equal in length even though the cycle periods varied from 16 to 28 h; the x-axes were therefore scaled differently for the different cycle periods. In the present analysis, the x-axis scales are identical for all the cycle periods. Uniform scaling of the x-axis is essential to observing the alignment of gene peaks across the different cycle periods under comparison in Fig. 3 and Supplementary Data Fig. S1.
Fig. 3.
Predicted (theoretical) timing of the day calibrator and night calibrator compared with the observed timing of TOC1, CAB2 and LHY phases in T cycles. In the theoretical model (A), the dawn signal (blue line) and the dusk signal (brown line) are re-timed when sunrise and sunset are shifted in various T cycles; the signal arcs are otherwise unaltered. In the process, the day calibrator (marked by an oval) maintains a constant time interval from midnight (panel A2), but not from noon, whereas the night calibrator (marked by a circle) maintains a constant time interval from noon (panel A1), but not from midnight. Observations of gene expression in T cycles (B to D) show that TOC1 is expressed at a constant time interval from noon (panel B1), CAB2 is expressed at noon (panel C1), while LHY is expressed at a constant time interval from midnight (panel D2) across all T cycles. [Besides their regulation by the solar clock, TOC1 and LHY also show activity spikes induced by the dark-to-light transition at sunrise (asterisks). The TOC1 spikes become more marked as the cycle period shortens.] The x-axes show zeitgeber time. Vertical bands indicate noon or midnight. Black and white bars below the x-axes denote dark and light periods respectively. A constant time interval across all T cycles between a calibration marker or gene expression peak and either noon or midnight is denoted by a dashed line, while a dotted line denotes dissimilar time intervals. Readings for each curve were taken at intervals of 2 h and are normalized by assigning a value of 100 for the highest reading. Details of photoperiods and cycle periods are given in Table 1.
ANALYSIS AND DISCUSSION
The plant solar rhythm is ubiquitous
To accommodate rhythmic genes that peak close to noon year-round, a 24-h solar clock has been proposed that is referenced not to sunrise, but to noon and midnight (Yeang, 2009, 2013). Taking the broad definition of a circadian rhythm as any endogenous rhythm that maintains an ∼24-h diurnal cycle, the solar rhythm is itself a class of circadian rhythms. But unlike the better known circadian rhythm that is entrained to sunrise, the solar rhythm is set to noon and midnight. The significant characteristic of the solar rhythm is that, unlike sunrise, its reference markers, noon and midnight, do not shift with the seasons.
Genes with photosynthesis functions are not the only ones that benefit from tethering to the solar rhythm. Other rhythmic genes that peak at other times may also find an adaptive advantage in entrainment to the solar clock. For instance, plants may schedule the expression of UV-sensitive cellular processes in the night to avoid light-induced damage (Nikaido and Johnson, 2000; Michael et al., 2008).
Cyclic genes that are regulated by the solar rhythm are in fact very common. Timed from sunrise (zeitgeber time), the phase of a gene cycling on a solar rhythm is delayed as the photoperiod increases; this delay is half of the difference between the longer and shorter photoperiods (Yeang, 2009). On the other hand, the phase of a gene timed by the solar clock is the same for any photoperiod when expressed in chronometer time because both the solar clock and the chronometer are referenced to noon and midnight, independently of the photoperiod. (Chronometer time in civilian use is average solar time, which accommodates slight variation in the time taken for the earth to complete its rotation at different times of the year.) Thus, for example, an examination of Fig. 2 of Roden et al. (2002) shows that initiation of LHY gene expression occurred at 0200 h (i.e. 2 h after midnight) regardless of whether the photoperiod was short (8 h) or long (16 h). This behaviour is confirmed in Fig. S1 of Hemmes et al. (2012), which also reveals a similar trend in the phase of its homologue CCA1. Also in the same figure, the initial rise in TOC1 expression occurred close to 1400 h under both the 8-h and the 16-h photoperiod. It is hence noteworthy that LHY/CCAI and TOC1, commonly described as morning- and evening-phased genes, respectively, observe solar time (or chronometer time), and that they are triggered 12 h apart at around 0200 h (2 a.m.) and 1400 h (2 p.m.) respectively, independently of the photoperiod. Does an activator kick-start the LHY/CCA1 cycle at 0200 h and does another trigger the TOC1 cycle at 1400 h? While no direct connection has been established, it is nevertheless noteworthy that 0200 h and 1400 h are the two points on the solar clock to which arabidopsis rhythmic genes are most frequently phased (Supplementary Data Fig. S2).
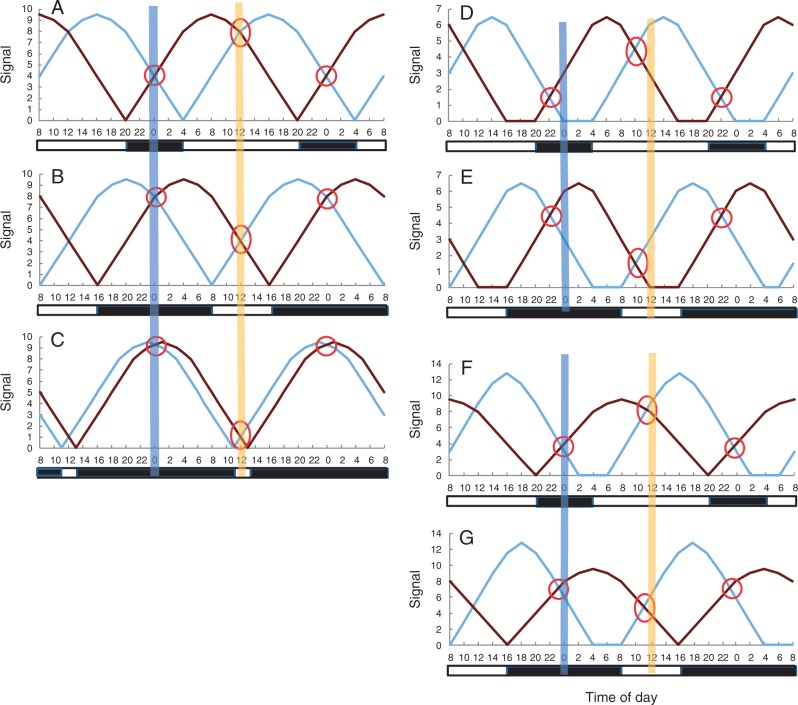
Fig. 2.
The solar clock model is robust. A dawn signal (light blue line) is initiated at the light transition of sunrise and a complementary dusk signal (dark brown line) is initiated at the light transition of sunset. The portion of the signal curve before it reaches its peak is the ascending signal; the curve after the peak is the descending signal. The day calibrator (marked by an oval) is the intersect between the dawn ascending signal and the dusk descending signal. The night calibrator (marked by a circle) is the intersect between the dawn descending and dusk ascending signals. A plant rhythm that is entrained to either the day or night calibrator cycles on solar time/chronometer time. Such a rhythm is unaffected by changes in the photoperiod. The actual duration of the signals is unknown, but it can be seen that where the duration of the signal (from initiation to termination) is exactly 24 h, the day calibrator and night calibrator are set at noon and midnight, respectively, in a 24-h cycle, regardless of the photoperiod, e.g. whether it is 16 h (A), 8 h (B) or 2 h (C). Where the duration of the signal is less than 24 h, the day and night calibrators would be at a constant interval (offset) from noon and midnight (D, E). Rhythmic genes timed by these off-noon/midnight calibrators would still be observing solar timing. The solar clock model holds even where the dawn and dusk signals differ in magnitude and duration (F, G). Vertical bands indicate noon or midnight. The x-axes show chronometer time (Crt), with Crt = 0 at midnight. Black and white bars below the x-axes denote dark and light periods respectively.
While empirical evidence in support of the solar rhythm is abundant in the literature (look for identical chronometer timing of gene phases under different photoperiods), the mechanism underlying the solar clock remains contentious. Light sensors on the plant can readily differentiate between the presence and absence of light, and so detecting the light:dark transitions that take place at sunrise and sunset poses no difficulty. But noon and midnight, which are the two mid-points between sunrise and sunset, are bereft of obvious timing cues. One opportunity for a mid-day zeitgeber in the natural environment lies in the increase in sunshine intensity from dawn to a maximum at mid-day before declining towards dusk. However, the fact that solar rhythms are maintained in growth chambers where artificial lighting is uniform from lights-on to lights-off argues against maximal light intensity at noon or changes in spectral properties being the reference marker.
It has long been a contention of researchers (Lam and Chua, 1989; Millar and Kay, 1996; McClung, 2006) that the daily light transitions, both at dawn and at dusk, are crucial to the timing of the CAB genes, even though how the zeitgeber operates has remained obscure. Such conjecture is reasonable and logical. The mid-point of any measurement can be determined only if both its beginning and its end are known. To position peak CAB2 expression consistently at the mid-point of the light period (a moving target in the natural environment as the seasons change), the plant has to be able to detect and utilize the timing of the light period’s beginning (sunrise) and its end (sunset).
To explain the solar clock, therefore, it is worthwhile directing particular attention to the timing of both sunrise and sunset, and to the possible interplay between these two light transitions, which could regulate CAB2 expression to peak at mid-day whatever the photoperiod.
The proposed solar clock mechanism is robust
To explain the solar clock, a mechanism has been proposed whereby sunrise activates a dawn light transition signal (possibly a gene expression) that rises to a peak before declining to base level, while a complementary dusk light transition signal is activated at sunset. According to this model (Yeang, 2009), which is elaborated on in Fig. 2, zeitgebers are established for the solar rhythm when the dawn and dusk light transition signals intersect. This occurs twice within a 24-h period, giving rise to the day calibrator and the night calibrator. As shown in Fig. 2, the day calibrator and night calibrator would be set at noon and midnight, respectively (Fig. 2A–C) or at a constant time offset from both noon and midnight (Fig. 2D, E), regardless of the photoperiod. Any gene rhythm entrained to either of these two calibrators would cycle on solar time. The proposed solar clock model is robust and remains operational even under extreme photoperiods, such as 2 h light:22 h dark (Fig. 2C), or even if the morning and evening signals are dissimilar in magnitude or duration (Fig. 2F, G). Nevertheless, while the model can account for the observed characteristics of the solar rhythm, actual experimental results that verify its proposed mechanism are lacking. Validation of the solar clock model is hence a main objective of the present report.
The approach taken to validate the solar clock is to create a situation that alters the timing of the hypothesized day and night calibrators. The timing of the gene phases that they control should then change accordingly in a predictable manner. Validation is achieved if readings from actual experiments match the predicted changes in the model. The problem here is that the exact timings of the day and night calibrators in the normal 24-h cycle are not even known. As shown in Fig. 2, they can be set at noon or midnight or at any constant time offset from noon or midnight. Therefore, even if the calibrators were changed in their timings, how might it be shown that the gene phases in actual experiments are following suit? As described below, this is achieved through an indirect approach that analyses the phases of the TOC1, CAB2 and LHY endogenous rhythms under varying cycle periods ranging from 16 to 28 h in T cycles and N–H cycles.
Predicted changes to the solar clock calibrators in T and N–H cycles
The characteristics of the dawn and dusk light transition signals of the plant could change over evolutionary time if appropriate selection pressure were brought to bear over multiple generations. In the short term, however, the predetermined paths of the timing signals adapted for the 24-h cycle should remain unchanged over a non-24-h period adopted in an experiment. An analogy can be made of the flight path of a shell fired from a gun. If the gun is scheduled to be fired at sunrise or sunset, the time when it is fired changes when the timing of sunrise or sunset is altered. But once the shell leaves the gun, its trajectory is fixed regardless of when it is fired. The shell follows the path specified for the design of the gun and shell.
In non-24-h T cycles, dawn and dusk light transition signals are triggered as usual at sunrise and sunset. The two intersects between the dawn signal and the dusk signal that constitute the zeitgebers still occur, but their timing would be altered as shown in Fig. 3A. The important feature here is that, irrespective of the cycle period, the day calibrator remains at a constant time interval from midnight (Fig. 3A, panel 2) while the night calibrator remains at a constant interval from noon (Fig. 3A, panel 1). The ratio of light to dark periods is fixed at 1:2 for all T cycles in the theoretical model presented, but similar results (not shown) are obtained for dark and light periods of 1:1. It should be emphasized that while noon and midnight may be the actual day and night calibrators (Fig. 2A–C), this model also allows for the possibility that the calibrators are not set exactly at noon and midnight. In the latter situation, noon and midnight nevertheless serve as convenient surrogates for the actual day and night calibrators cycling on solar time (Fig. 2D, E). To determine whether the timings of gene phases change in tandem with the shift in the day and night calibrators, it is not necessary to know precisely where the calibrators are located in the solar cycle. What is looked for is the constant time interval being maintained between gene activity and noon or midnight across different cycle periods. Thus, when a gene is expressed at the same interval timed from noon irrespective of photoperiod or cycle period, it is an indication that the gene is being regulated by the night calibrator; it is the day calibrator that enables a gene to maintain a constant phase from midnight. The same behaviour of the clock calibrators described for T cycles above also applies to N–H cycles, in which the duration of the light period is fixed and that of the dark period varies (Supplementary Data Fig. S1).
Non-24-h cycles can yield further information on how gene expression is regulated. Two solar time calibrators operating in a day as postulated by the model suggest two separate sets of genes tethered to the solar rhythm, viz. those synchronized to the day calibrator and those to the night calibrator. However, it is not known which calibrator a particular gene uses as its zeitgeber since a gene phase is synchronized to both noon and midnight in a 24-h cycle; any phase measured from noon is concurrently that phase plus 12 h when measured from the preceding midnight. (For example, it would be equally correct to say that TOC1 peaks 5–6 h after the preceding noon or that it peaks 17–18 h after the preceding midnight.) In non-24-h cycles, however, a gene phase would no longer be synchronized to both noon and midnight; the phase would be at a constant interval measured from either noon or midnight (Fig. 3A, panels 1 and 2). Therefore, non-24-h cycles essentially break the ‘concurrently referenced to noon and midnight’ mould. This provides the opportunity to determine whether a gene is entrained to the day calibrator or the night calibrator by observing whether it is noon or midnight to which the gene maintains a constant phase.
Observed changes to the timing of TOC1, CAB2 and LHY activity in T cycles and N–H cycles
The present study reinterprets data from the literature on TOC1, CAB2 and LHY rhythmic behaviour to arrive at new conclusions not offered in the original reports.
Figure 3B–D show how TOC1, CAB2 and LHY rhythms match the predictions in Fig. 3A for solar timing over the different cycle periods. TOC1 peaks consistently ∼5·5 h from the preceding noon in T cycles of 20, 24 or 28 h (Fig. 3B). On the other hand, there is no consistent interval between midnight and the TOC1 peak phase. This indicates that TOC1 is a gene timed by the night calibrator according to the solar clock model in Fig. 3A, panel 1, as explained above. In the case of CAB2, the gene peaks consistently at noon, indicating again that this gene is regulated by the night calibrator (Fig. 3C). As is true for TOC1, there is no consistent time interval between CAB2 expression peak and midnight. Unlike TOC1 and CAB2, however, LHY does not have peak activity at a constant phase when measured from noon. Instead, the gene peaks ∼7·5 h from midnight in all T cycles of periods ranging from 16 to 28 h (Fig. 3D). Hence, the LHY rhythm is timed by the day calibrator following the prediction in Fig. 3A, panel 2. There is further opportunity to observe the rhythmic behaviour of LHY in non-24-h cycles when plants are subjected to N–H cycles (Supplementary Data Fig. S1). Here again, LHY activity does not show a constant phase to noon, but peaks ∼7·5 h after midnight in all N–H cycles.
In these observations, the photoperiod and cycle period change over the different T cycles, while the cycle period and light/dark period ratio are altered over the different N–H cycles. Yet the phases of TOC1 and CAB2 in T cycles, and LHY in both T cycles and N–H cycles, are all predictable. Such predictions are possible because each gene phase maintains a constant time interval from either noon or midnight (and by extension, from either the night or day calibrator respectively), regardless of the cycle period, the photoperiod or the light/dark period ratio, as proposed for the solar rhythm in Fig. 3A. The solar clock model is thus validated.
It is noted that the difference in timing of the CAB2 peak between the shortest cycle (20 h) and longest cycle (28 h) from the available data is only 1·4 h, which is below the resolution of the readings taken at 2 h intervals. Hence, any inference on the model drawn from CAB2 alone is not sufficiently conclusive. However, this caveat does not arise in the case of TOC1, where the timings of the corresponding peaks differ by 2 h. The results are unequivocal in the case of LHY, where there is a difference of 8 h for T cycles and 6 h for N–H cycles between the emergence of the peaks in the shortest cycle (16 h) and the longest cycle (28 h). The phases mentioned above refer to the principal phases of gene transcription. Gene activity is frequently influenced by the action of other genes or by environmental events, such as the light transition at dawn that induces a reaction in LHY (Locke et al., 2006; Pokhilko et al., 2012). Activity spikes triggered by sunrise for both LHY and TOC1 are seen in Fig. 3 and Supplementary Data Fig. S1. There have also been reports of CAB2 being induced by the light at lights-on (Anderson and Kay, 1995), although this is not clearly seen in the present data.
Prediction of gene phases on zeitgeber time
With any gene cycling on a solar rhythm maintaining a fixed phase from either noon or midnight, the peak phases of TOC1, CAB2 and LHY on zeitgeber time (i.e. timed from sunrise) can be predicted for any photoperiod and any cycle period by the following equations.
To estimate the zeitgeber time (Zn) of the peak phase of cyclic genes referenced to noon (e.g. TOC1 or CAB2),
| (1) |
and to estimate the zeitgeber time (Zm) of the peak phase of genes referenced to midnight (e.g. LHY),
| (2) |
where P is the photoperiod (duration of the light period), C is the cycle period, P is the time interval from sunrise to noon, (C + P) is the time interval from sunrise to midnight, N is the time of gene peak phase measured from noon (e.g. 5·5 h for TOC1, 0 h for CAB2) and M is the time of gene peak phase measured from midnight (e.g. 7·5 h for LHY).
As shown in Fig. 4, the phases of all the TOC and CAB2 peaks predicted by eqn (1) match their observed timings in actual experiments.
Fig. 4.
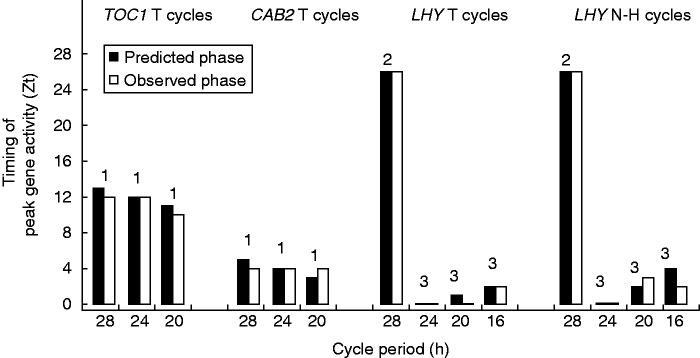
Observed and predicted zeitgeber timings of the peak activities of TOC1 and CAB2 in T cycles and of LHY in T and N–H cycles. Observed timings are based on experimental data for TOC1 from Fig. 2 of Dodd et al. (2014), CAB2 from Fig. 1 of Kim et al. (2003) and LHY from Figs 2 and 3 of Roden et al. (2002). Readings (averaged over two or three cycles) were taken at intervals of 2 h. Predicted timings are from eqn (1), (2) or (3) as indicated above the respective columns. Observed and predicted readings are rounded to the nearest hour. Details of photoperiods and cycle periods are given in Table 1.
What happens in an experiment where the T or N–H cycle is so shortened that a gene peak is scheduled to emerge only after the termination of the truncated cycle? In this scenario, the next cycle would have begun even before the gene is expressed in the current cycle. Might the gene peak concerned then be annulled? The LHY peaks for the 28-h T cycle (Fig. 3D) and 28-h N–H cycle (Supplementary Data Fig. S1) both occur before sunrise at around zeitgeber time 26, as predicted by eqn (2). However, this equation is no longer applicable to shorter cycle periods (24, 20 and 16 h), where there is an apparent pronounced advance (earlier expression) of the activity peak to the early hours after sunrise, a behaviour not seen with TOC1 or CAB2 rhythms. Examination of Fig. 3D and Supplementary Data Fig. S1 shows that the LHY phase has not, in fact, advanced. In maintaining its activity peak 7·5 h after midnight, the LHY peak has simply transgressed into the succeeding cycle. Thus, where the gene peak phase (Zm) is equal to or exceeds the cycle period (C), C is subtracted from eqn (2) to show Zm on zeitgeber time of the following cycle:
| (3) |
The correct prediction of the zeitgeber timing of the LHY peak in short cycles (Fig. 4) following eqn (3) provides further validation of the solar clock and its proposed mode of action.
The meta-analysis in this study taken as a whole shows the solar clock model correctly predicting 14 zeitgeber timings involving three genes (CAB2, TOCI and LHY) cycling under 11 different lighting regimes that comprised various combinations of seven light periods, nine dark periods, four cycle periods and four light:dark period ratios (Fig. 4). The functional versatility of the model points to a basic, broad-encompassing control mechanism that sets the arabidopsis solar clock and regulates the timing for the expression of genes entrained to the solar rhythm.
CONCLUDING REMARKS
Plants gain in fitness when they are physiologically prepared for the arrival of light or darkness (Somers, 1999; Yerushalmi et al., 2011; Herrero et al., 2012). In this connection, the initiation of gene expression even before the arrival of sunrise and sunset enables plants to ‘anticipate’ the dawn or dusk (Webb, 2003; McClung, 2006). Since it is only when the clock is set that it tells the time, plants must first calibrate their internal clocks by making use of environmental cues. Although various environmental variables, such as temperature or light wavelength and intensity, can influence the plant’s endogenous rhythm, it is the timing of the periods of light and darkness in the cycle that principally regulates the phases of TOC1, CAB2 and LHY. For example, while peak levels of these three genes are altered by changes in ambient temperature, their cycle periods and the timing of their phases are unaffected (Gould et al., 2006; Michael et al., 2008). It is the ability to tell time that allows the plant to anticipate the dawn by synchronizing the cycle of LHY/CCA1 to midnight and to anticipate the dusk by synchronizing the TOC1 cycle to noon. Of no lesser importance, of course, is the plant’s anticipation of mid-day, with CAB2 synchronized to noon and the plant’s light-harvesting apparatus primed and ready.
Over the course of the year, the timing of dawn and dusk varies, arriving early or late depending on the seasonal daylength. But the timing of noon and midnight are constants in the diurnal cycle. The solar rhythm offers a temporal scaffold upon which various plant genes can be advantageously scheduled for expression in light or in darkness year round, regardless of the season or daylength.
SUPPLEMENTARY DATA
Supplementary data are available online at www.aob.oxfordjournals.org and consist of the following. Figure S1: predicted (theoretical) timing of the day calibrator and night calibrator compared with the observed timing of LHY phases in N–H cycles. Figure S2: relative number of arabidopsis rhythmic genes phased to each hour under an 8-h photoperiod and a 16-h photoperiod.
LITERATURE CITED
- Alabadí D, Oyama T, Yanovsky MJ, Harmon FG, Más P, Kay SA. 2001. Reciprocal regulation between TOC1 and LHY/CCA1 within the Arabidopsis circadian clock. Science 293: 880–883. [DOI] [PubMed] [Google Scholar]
- Alabadí D, Yanovsky MJ, Más P, Harmer SL, Kay SA. 2002. Critical role for CCA1 and LHY in maintaining circadian rhythmicity in Arabidopsis . Current Biology 12: 757–761. [DOI] [PubMed] [Google Scholar]
- Anderson SL, Kay SA. 1995. Functional dissection of circadian clock- and phytochrome-regulated transcription of the Arabidopsis CAB2 gene. Proceedings of the National Academy of Sciences of the USA 92: 1500–1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodd AN, Dalchau N, Gardner MJ, Baek S-J, Webb AAR. 2014. The circadian clock has transient plasticity of period and is required for timing of nocturnal processes in Arabidopsis. New Phytologist 201: 168–179. [DOI] [PubMed] [Google Scholar]
- Gould PD, Locke JCW, Larue C, et al. 2006. The molecular basis of temperature compensation in the Arabidopsis circadian clock. Plant Cell 18: 1177–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemmes H, Henriques R, Jang I, -C, Kim S, Chua N-H. 2012. Circadian clock regulates dynamic chromatin modifications associated with Arabidopsis CCA1/LHY and TOC1 transcriptional rhythms. Plant Cell Physiology 53: 2016–2029. Online Supplementary Data Fig. S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrero E, Kolmos E, Bujdoso N, et al. 2012. EARLY FLOWERING4 recruitment of EARLY FLOWERING3 in the nucleus sustains the Arabidopsis circadian clock. Plant Cell 24: 428–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu PY, Harmer SL. 2014. Wheels within wheels: the plant circadian system. Trends in Plant Science 19: 240–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W, Pérez-García P, Pokhilko A, et al. 2012. Mapping the core of the Arabidopsis circadian clock defines the network structure of the oscillator. Science 336: 75–79. [DOI] [PubMed] [Google Scholar]
- Kim J-Y, Song H-R, Taylor BL, Carré IA. 2003. Light-regulated translation mediates gated induction of the Arabidopsis clock protein LHY. EMBO Journal 22: 935–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam E, Chua N-H. 1989. Light to dark transition modulates the phase of antenna chlorophyll protein gene expression. Journal of Biological Chemistry 264: 20175–20176. [PubMed] [Google Scholar]
- Locke JCW, Kozma-Bognár L, Gould PD, et al. 2006. Experimental validation of a predicted feedback loop in the multi-oscillator clock of Arabidopsis thaliana . Molecular Systems Biology 2: 59, doi:10.1038/msb4100102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClung CR. 2006. Plant circadian rhythms. Plant Cell 23: 792–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McWatters HG, Kolmos E, Hall A, et al. 2007. ELF4 is required for oscillatory properties of the circadian clock. Plant Physiology 144: 391–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michael TP, Salomé PA, McClung CR. 2003. Two Arabidopsis circadian oscillators can be distinguished by differential temperature sensitivity. Proceedings of the National Academy of Sciences of the USA 100: 6878–6883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michael TP, Mockler TC, Breton G, et al. 2008. Network discovery pipeline elucidates conserved time-of-day–specific cis-regulatory modules. PLoS Genetics 4: e14 doi:10.1371/journal.pgen.0040014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millar AJ, Kay SA. 1996. Integration of circadian and phototransduction pathways in the network controlling CAB gene transcription in Arabidopsis . Proceedings of the National Academy of Sciences of the USA 93: 15491–15496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millar AJ, Carré IA, Strayer CA, Chua NH, Kay SA. 1995. Circadian clock mutants in Arabidopsis identified by luciferase imaging. Science 267:1161–1163. [DOI] [PubMed] [Google Scholar]
- Murtas G, Millar AJ. 2000. How plants tell the time. Current Opinion in Plant Biology 3: 43–46. [DOI] [PubMed] [Google Scholar]
- Nanda KK, Hamner KC. 1958. Studies on the nature of the endogenous rhythm affecting photoperiodic response of Biloxi soybean. Botanical Gazette 120: 14–25. [Google Scholar]
- Nikaido SS, Johnson CH. 2000. Daily and circadian variation in survival from ultraviolet radiation in Chlamydomonas reinhardtii . Photochemistry and Photobiology 71: 768–765. [DOI] [PubMed] [Google Scholar]
- Perales M, Más P. 2007. A functional link between rhythmic changes in chromatin structure and the Arabidopsis biological clock. Plant Cell 19: 2111–2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pokhilko A, Fernández AP, Edwards KD, Southern MM, Halliday KJ, Millar AJ. 2012. The clock gene circuit in Arabidopsis includes a repressilator with additional feedback loops. Molecular Systems Biology 8: 574, doi:10.1038/msb.2012.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roden LC, Song H-R, Jackson S, Morris K, Carré IA. 2002. Floral responses to photoperiod are correlated with the timing of rhythmic expression relative to dawn and dusk in Arabidopsis . Proceedings of the National Academy of Sciences of the USA 99: 13313–13318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salomé PA, McClung CR. 2005. What makes the Arabidopsis clock tick on time? A review on entrainment. Plant, Cell & Environment 28: 21–38. [Google Scholar]
- Somers DE. 1999. The physiology and molecular bases of the plant circadian clock. Plant Physiology 121: 9–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tóth R, Kevei E, Hall A, Millar AJ, Nagy F, Kozma-Bognár L. 2001. Circadian clock-regulated expression of phytochrome and cryptochrome genes in Arabidopsis. Plant Physiology 127: 1607–1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb AAR. 2003. The physiology of circadian rhythms in plants. New Phytologist 160: 281–303. [DOI] [PubMed] [Google Scholar]
- Yeang HY. 2009. Circadian and solar clocks interact in seasonal flowering. BioEssays 31: 1211–1218. [DOI] [PubMed] [Google Scholar]
- Yeang HY. 2013. Solar rhythm in the regulation of photoperiodic flowering of long-day and short-day plants. Journal of Experimental Botany 64: 2643–2652. [DOI] [PubMed] [Google Scholar]
- Yerushalmi S, Yakir E, Green RM. 2011. Circadian clocks and adaptation in Arabidopsis. Molecular Ecology 20: 1155–1165. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Sun X-D, Ni M. 2007. Timing of photoperiodic flowering: light perception and circadian clock. Journal of Integrative Plant Biology 49: 28–34. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.