Abstract
Mps one binder (MOB) proteins are integral components of signaling pathways that control important cellular processes, such as mitotic exit, centrosome duplication, apoptosis, and cell proliferation. However, the biochemical and cellular functions of the human MOB (hMOB) protein family remain largely unknown. The present study investigated the association between hMOB3B expression and clinicopathological characteristics of prostate cancer (PCa).Study subjects included 137 PCa patients and 137 age-matched benign prostatic hyperplasia (BPH) patients. hMOB3B expression was estimated using real-time PCR and compared with clinicopathological parameters of PCa. hMOB3B mRNA expression was significantly lower in PCa tissues than in BPH control tissues (P<0.001). According to receiver operating characteristics curve analysis, the sensitivity of hMOB3B expression for PCa diagnosis was 84.7%, with a specificity of 86% (AUC=0.910; 95% CI=0.869-0.941; P<0.001). hMOB3B expression was significantly lower in patients with elevated prostate specific antigen (PSA) levels (≥10 ng/mL), a Gleason score≥8, and metastatic disease (any T, N+/M+) than in those with low PSA levels, a low Gleason score, and non-metastatic disease (each P<0.05). In conclusion, low levels of hMOB3B are closely associated with aggressive clinicopathologic features in patients with PCa. Our results suggest that hMOB3B may act as a tumor suppressor in human PCa.
Graphical Abstract
Keywords: Prostatic Neoplasms; Gene Expression; Genes, Tumor Suppressor; hMOB3B
INTRODUCTION
Prostate cancer (PCa) is the second most frequently diagnosed cancer and the sixth leading cause of cancer death in men worldwide with an approximately 14% (903,500) of total new cancer cases and 6% (258,400) of the total cancer deaths in males in 2008 (1). In Korea, PCa is the fifth most common cancer in men, and its incidence is the most rapidly increasing of all cancers (2). PCa shows an extremely heterogeneous clinical course, ranging from indolent to aggressive, metastatic lethal disease (3). Consequently, there is a great need to accurately estimate the tumor characteristics of PCa so that appropriate treatment options can be considered. Currently, histopathological analysis (including tumor stage and grade) and serum prostate specific antigen (PSA) levels are key determinants of therapeutic decision-making. However, none of the histological criteria or bio-markers reported to date show sufficient sensitivity and specificity for detecting, monitoring, or determining the prognosis of PCa (4, 5). Thus, there is a critical need for methods capable of predicting oncologic outcomes and responses to therapy in PCa patients. The abnormal expression of certain genes in cancer cells is closely related to distinct aspects of tumor progression, including tumor growth, invasion, and metastasis. Proper cell division requires the precise coordination and execution of several events in the cell cycle, including centrosome duplication, DNA replication, mitotic spindle assembly, chromosome segregation, and cytokinesis (6). A failure in the execution or proper timing of any of these events could lead to chromosome segregation defects, resulting in aneuploidy or polyploidy (7, 8, 9).
Mps one binder (MOB) was originally identified in yeast as a regulator of mitotic exit and cytokinesis, and was later identified as a tumor suppressor (10). hMOB1 can bind to and activate mammalian large tumor suppressor (LATS) and nuclear Dbf2-related (NDR) kinases (LATS1, LATS2, NDR1, and 2) by targeting and activating these kinases at the plasma membrane (11, 12). Increased expression of LATS1 or LATS2 inhibits tumor cell growth by inducing cell cycle arrest or apoptosis (10, 11, 13, 14, 15). Recent studies show that hMOB1 is downregulated in colorectal and non-small cell lung cancers (9, 12). hMOB2 binds to the same domain on NDR1/2, but does not bind to LATS1/2; binding of hMOB2 to NDR1/2 kinases inhibits the phosphorylation of NDR and thereby blocks kinase activation (16). hMOB2 is classified as an inhibitor of NDR signaling, whereas hMOB1 is classified as a co-activator of the NDR/LATS signaling cascades (17, 18). The human MOB protein family consists of six members: hMOB1A, 1B, 2, 3A, 3B, and 3C (18). Unfortunately, although hMOB1 and 2 have been extensively studied, the biological roles and binding partners of hMOB3A/B/C are unknown (18). Recent studies revealed that all six hMOB3B are abundantly expressed in human prostate tissue (10), and that methylation-induced gene silencing of hMOB3B occurs in PCa (19). Based on previous research, we hypothesized that hMOB3B acts as a tumor suppressor, and that its loss of function contributes to the carcinogenesis and aggressiveness of human PCa. Here, we compared the expression levels of hMOB3B in normal and prostate cancer tissues to examine the contribution of this gene to prostate carcinogenesis. Also, and more importantly, we investigated the effect of hMOB3B on the clinicopathological characteristics of PCa in Korean men.
MATERIALS AND METHODS
Study population
This case-control study was included 137 cases of newly diagnosed PCa and 137 age-matched benign prostatic hyperplasia (BPH) controls. The study cases were recruited from patients with histologically confirmed primary adenocarcinoma of the prostate at our hospital. Controls were selected from a database of BPH patients who underwent transurethral resection of the prostate (TURP), and were one-to-one matched (as far as possible) according to age and date of blood sampling. Controls with high PSA (serum PSA levels >2.5 ng/mL) underwent transrectal prostate biopsy before TURP to rule out the presence of cancer, and those with PSA levels >10 ng/mL were excluded from the study. Subjects with a suspicious history of previous management for PCa or incomplete medical records were also excluded. Gleason grade and 2002 TNM stage were used as prognostic factors. Gleason grade was measured in 12-core transrectal biopsy, TURP, or radical prostatectomy specimens. Tumor stage was estimated from the radical prostatectomy specimens, or from computed tomography (CT), magnetic resonance imaging (MRI), or bone scan results.
RNA extraction and construction of cDNA
TRIzol (1 mL; Invitrogen, Carlsbad, USA) was added to BPH control and PCa tissues and homogenized in a 5 mL glass tube. The homogenate was transferred to a 1.5 mL tube and mixed with 200 µL chloroform. After incubating for 5 min at 4℃, the homogenate was centrifuged for 13 min at 13,000×g at 4℃. The upper aqueous phase was transferred to a clean tube and 500 µL isopropanol was added, followed by incubation for 60 min at 4℃. The tube was then centrifuged for 8 min at 13,000×g and 4℃. Next, the upper aqueous phase was removed, mixed with 500 µL of 75% ethanol, and centrifuged for 5 min at 13,000×g and 4℃. After the upper aqueous layer was discarded, the pellet was dried at room temperature, dissolved with diethylpyrocarbonate (DEPC)-treated water, and stored at -80℃. The quality and integrity of the RNA were confirmed by agarose gel electrophoresis and ethidium bromide staining, followed by visual examination under ultraviolet light. cDNA was then prepared from 1 µg f RNA by random priming with a First-Strand cDNA Synthesis Kit (Amersham Biosciences Europe GmbH, Freiburg, Germany) according to the manufacturer's protocol.
Real-time PCR
To quantify the expression of hMOB3B, real-time PCR was performed using a Rotor Gene 6000 instrument (Corbett Research, Mortlake, Australia). Real-time PCR assays using SYBR Premix EX Taq (TAKARA BIO INC., Otsu, Japan) were carried out in micro-reaction tubes (Corbett Research, Mortlake, Australia) using hMOB3B (6,528 bp) sense (5'-GTG GCA GGA TGA TCT CAA-3') and antisense (5'-CGG CAC AGG ATC TTC TTG-3') primers. The PCR reaction was performed in a final volume of 10 µL, comprising 5 µL of 2×SYBR Premix EX Taq buffer, 0.5 µL of each 5' and 3' primer (10 pM/µL), and 1 µL of sample cDNA. The products were purified with a QIAquick Extraction kit (QIAGEN, Hilden, Germany), quantified in a spectrometer (Perkin Elmer MBA2000, Fremont, USA), and sequenced using an automated laser fluorescence sequencer (ABI PRISM 3100 Genetic Analyzer, Foster City, USA). A known concentration of the PCR product was then 10-fold serially diluted from 100 pg/µL to 0.1 pg/µL and used to establish a standard curve. The real-time PCR conditions were as follows: 1 cycle at 96℃ for 20 sec, followed by 40 cycles of 3 sec at 96℃ for denaturation, 15 sec at 60℃ for annealing, and 15 sec at 72℃ for extension. The melting program was performed at 72-95℃, with a heating rate of 1℃ per 45 sec. Spectral data were PCatured and analyzed using Rotor Gene Real-Time Analysis Software 6.0 Build 14 (Corbett Research, Mortlake, Australia). All samples were run in triplicate. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as an endogenous RNA reference gene. Gene expression was normalized to the expression of GAPDH.
Statistical analysis
Clinical variables such as age, PSA, prostate size, and BMI were compared using the Mann-Whitney U-test. To evaluate tumor characteristics, Gleason scores were classified as ≤7 and ≥8, and the clinical stage was categorized as ≤T4 or metastatic disease (any nodal or distant metastasis). Statistical analysis was performed using SPSS 20.0 software (SPSS Inc., Chicago, IL, USA), and a P value <0.05 was considered statistically significant.
Ethics statement
The study protocol of collection and analyses of all samples was reviewed and approved by the institutional review board of Chungbuk National University (IRB approved number 2006-01-001). Informed consent was obtained from each subject.
RESULTS
Baseline characteristics
Table 1 lists the baseline clinical and pathologic characteristics of the 137 BPH controls and the 137 PCa patients. The mean age of the PCa patients and controls was 69.2 yr. The serum PSA level was higher in PCa patients than in the BPH controls (124.9±380.6 vs. 4.0±5.2; P<0.001). There was no significant difference between cases and controls regarding prostate size (P=0.037). Among the 137 PCa patients, 72 (52.6%) underwent radical prostatectomy and the other 65 (47.4%) underwent palliative TURP. Stage and Gleason grade at diagnosis were as follows: 91 cases had localized-to-advanced disease (T2-4N0M0) and 46 (33.6%) had metastatic disease (any T, N+/M+); the Gleason score was ≤7 and ≥8 for 64 (46.7%) and 73 (53.3%) patients, respectively.
Table 1. Clinicopathologic characteristics of prostate cancer patients and BPH controls.
| Characteristics | BPH | PCa | P value |
|---|---|---|---|
| No. | 137 | 137 | |
| Age (yr; range) | 69.2 (46-85) | 69.2 (48-86) | 0.994 |
| PSA±SD (ng/mL) | 4.0±5.2 | 124.9±380.6 | <0.001 |
| Prostate size (gram) | 38.8±23.0 | 41.3±22.2 | 0.387 |
| Operation (%) | |||
| TURP | 137 (100) | 65 (47.4) | |
| Radical prostatectomy | 72 (52.6) | ||
| Gleason score, No. (%) | |||
| ≤7 | 64 (46.7) | ||
| ≥8 | 73 (53.3) | ||
| Stage (%) | |||
| T1-4N0M0 | 91 (66.4) | ||
| Metastatic (any T N+/M+) | 46 (33.6) | ||
| hMOB3B expression, median (IQR; × 104 copies per µg) | 1,108.5 (797.0-1,428.0) | 426.2 (197.6-637.0) | <0.001 |
P values were obtained from the Mann-Whitney U-test. BPH, benign prostatic hyperplasia; PCa, prostate cancer; IQR, interquartile range; SD, standard deviation; TURP, transurethral resection of the prostate.
Expression levels of hMOB3B mRNA in normal and cancer tissues
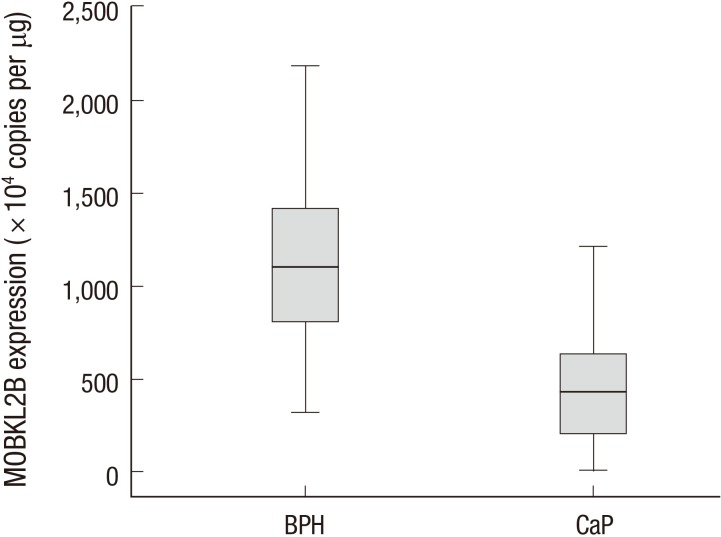
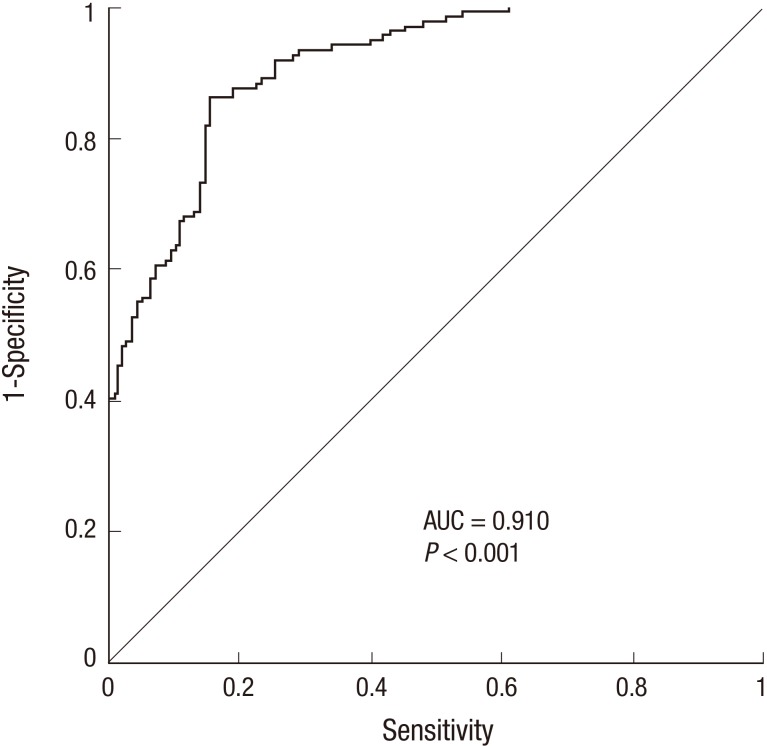
To identify whether hMOB3B is involved in prostate cancer susceptibility, we compared mRNA expression levels in BPH and PCa tissues. The median hMOB3B mRNA expression level in PCa tissues was significantly lower (median 426.2×104 copies per µg; IQR, 197.6-637.0) than that in BPH tissues (median 1,108.5×104 copies per µg; IQR, 797.0-1,428.0) (P<0.001, Table 1 and Fig. 1). According to receiver operating curve (ROC) statistical analysis, the sensitivity of hMOB3B expression for diagnosing prostate cancer was 84.7%, with a specificity of 86% at a reference value of 737.5×104 copies per µg (AUC, 0.910; 95% CI, 0.869-0.941; P<0.001) (Fig. 2).
Fig. 1. hMOB3B mRNA expression levels in normal and cancer tissues. BPH, benign prostatic hyperplasia; CaP, prostate cancer.
Fig. 2. Receiver operating characteristic (ROC) curve generated to calculate the sensitivity and specificity of hMOB3B for detecting prostate cancer. AUC, area under the curve.
Relationship between hMOB3B mRNA expression levels and clinicopathologic features
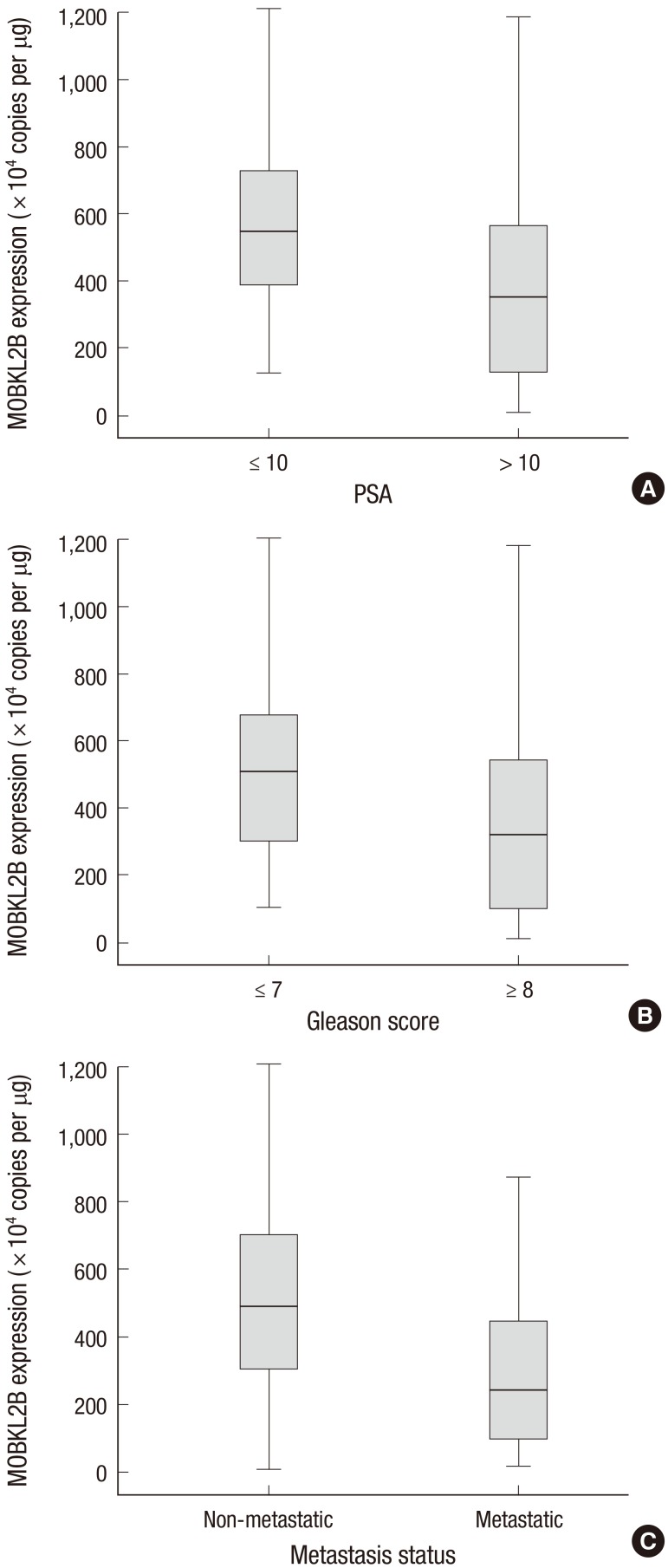
hMOB3B mRNA expression was significantly lower in patients with elevated PSA levels (≥10 ng/mL) than in those with low PSA levels (P=0.001). In addition, hMOB3B expression was significantly lower in cancer tissue specimens from patients with a higher Gleason score (≥8) and metastatic disease (any T, N+/M+) than in samples from those with a lower Gleason score (≤7) and non-metastatic disease (T2-4N0M0) (each P<0.001) (Table 2 and Fig. 3A-C).
Table 2. hMOB3B mRNA expression levels according to PSA, Gleason score, and metastasis status in prostate cancer patients.
| Characteristics | No | hMOB3B expression, median (IQR; × 104 copies/µg) | P value |
|---|---|---|---|
| PSA | 0.001 | ||
| ≤10 | 38 | 545.2 (382.9-730.3) | |
| >10 | 99 | 349.6 (123.6-569.9) | |
| Gleason score | <0.001 | ||
| ≤7 | 64 | 509.8 (300.6-687.3) | |
| ≥8 | 73 | 320.9 (96.9-545.2) | |
| Stage | <0.001 | ||
| T1-4N0M0 | 91 | 491.1 (299.2-716.4) | |
| any T N+/M+ | 46 | 243.8 (96.4-448.4) |
P values were obtained from the Mann-Whitney U-test. IQR, interquartile range; PSA, prostate specific antigen; SD, standard deviation.
Fig. 3. Relationship between hMOB3B mRNA expression levels and clinicopathologic features in prostate cancer. (A) PSA, (B) Gleason score, and (C) metastatic status.
DISCUSSION
In this study, we identified a relationship between hMOB3B expression and an increased risk of PCa. In addition, hMOB3B expression was closely associated with clinicopathologic features in patients with PCa. MOB proteins are crucial regulators of NDR family kinases and are conserved from yeast to humans. Members of the MOB protein family regulate mitosis, cell proliferation, apoptosis, centrosome biology, and morphological changes (12, 20). Signal transduction cascades control essential biological processes such as cell division, morphogenesis, cell growth, and controlled cell death/apoptosis (21). The vast majority of signaling cascades transmit extra- and intracellular inputs via protein kinases (11, 22). A conserved property of MOB proteins is its association with and activation of NDR (nuclear-Dbf2-related) kinases belonging to the AGC family, which includes PKA, PKG, and PKC (18, 23). Human cells express four related NDR kinases: NDR1 (also known as serine/threonine kinase 38 or STK38), NDR2 (or STK38L), LATS1 (large tumor suppressor-1), and LATS2 (11, 24, 25, 26). Originally, the biological roles of MOB proteins were investigated mainly using budding and fission yeast. In budding and fission yeast, MOB proteins are expressed from two independent genes. Mob1p regulates both the mitotic exit network in budding yeast and the septation initiation network in fission yeast by binding to and activating Dbf2p and Sid2p kinases, respectively (11, 12, 25, 27). In addition, Mob1p also plays a major role in modulating cytokinesis, the last stage of the cell cycle (28). MOB2 regulates cell polarity and daughter-specific gene expression programs during the yeast cell cycle by modulating Cbk1p and Orb6p kinases (29). The human MOB protein family consists of six distinct members (hMOB1A, 1B, 2, 3A, 3B, and 3C), with hMOB1A/B being the best studied due to their putative tumor suppressive functions, which are mediated through regulation of the NDR/LATS kinases (10, 11, 18, 24). The biological features of the other hMOBs are largely unknown. hMOB1A binds to and activates human NDR1/2 kinases by stimulating autophosphorylation of the activation segment. Similarly, hMOB1A binds to and activates LATS1 and 2 (11, 18, 24). By contrast, hMOB2 binds to NDR1 and NDR2, but not to LATS1 (16). hMOB3A/B/C do not associate with any NDR/LATS kinases, and the binding partners of hMOB3A/B/C are currently unknown. Recently, a study by Haldrup et al. (16) reported that hypermethylation of six novel genes, AOX1, C1orf114, GAS6, HAPLN3, KLF8, and MOB3B, was highly cancer specific. Although we did not measure the correlation between hMOB3B methylation and hMOB3B expression levels, our data support methylation-induced gene silencing of hMOB3B.
A possible limitation of our study is that we did not determine the levels of hMOB3B protein, for example, by Western blotting or immunohistochemical staining. Second, we could not evaluate the prognostic value of hMOB3 due to relatively small sample size and inconsistent treatment modalities. Most importantly, the physiological binding partners and functions of the hMOB3 proteins remain undefined. Thus, further research is needed to identify physiological binding partners and to confirm the prognostic value of hMOB3 in PCa.
In conclusion, low levels of hMOB3B are closely associated with aggressive clinicopathologic features of PCa. Our results suggest a functional role for hMOB3B as a tumor suppressor in human PCa.
ACKNOWLEDGEMENT
The biospecimens for this study were provided by the Chungbuk National University Hospital, a member of the National Biobank of Korea, which is supported by the Ministry of Health, Welfare and Family Affairs. All samples derived from the National Biobank of Korea were obtained with informed consent under institutional review board-approved protocols.
Footnotes
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIP) (No. NRF-2014R1A2A1A09006983, 2014R1A2A2A04007036).
DISCLOSURE: The authors have no potential financial conflicts on this subject.
AUTHOR CONTRIBUTION: Conception and design of the study: Kim EA, Kang HW, Yun SJ. Acquisition of data: Kim EA, Kim YH, Yoon HY. Statistical analysis: Kim WT, Kim YJ, Yun SJ. First draft of manuscript: Kim EA, Kang HW. Revision and critical review of the manuscript: Moon SK, Choi YH, Kim IY, Lee SC, Kim WJ. Manuscript approval: all authors.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Lee HW, Jeon HG, Jeong BC, Seo SI, Jeon SS, Choi HY, Lee HM. Comparison of pathological and biochemical outcomes after radical prostatectomy in Korean patients with serum PSA ranges. J Korean Med Sci. 2015;30:317–322. doi: 10.3346/jkms.2015.30.3.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Seo WI, Kang PM, Chung JI. Predictive value of the cancer of the prostate risk assessment score for recurrence-free survival after radical prostatectomy in Korea: a single-surgeon series. Korean J Urol. 2014;55:321–326. doi: 10.4111/kju.2014.55.5.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kattan MW, Eastham JA, Stapleton AM, Wheeler TM, Scardino PT. A preoperative nomogram for disease recurrence following radical prostatectomy for prostate cancer. J Natl Cancer Inst. 1998;90:766–771. doi: 10.1093/jnci/90.10.766. [DOI] [PubMed] [Google Scholar]
- 5.Partin AW, Kattan MW, Subong EN, Walsh PC, Wojno KJ, Oesterling JE, Scardino PT, Pearson JD. Combination of prostate-specific antigen, clinical stage, and Gleason score to predict pathological stage of localized prostate cancer. a multi-institutional update. JAMA. 1997;277:1445–1451. [PubMed] [Google Scholar]
- 6.Negrini S, Gorgoulis VG, Halazonetis TD. Genomic instability: an evolving hallmark of cancer. Nat Rev Mol Cell Biol. 2010;11:220–228. doi: 10.1038/nrm2858. [DOI] [PubMed] [Google Scholar]
- 7.Charames GS, Bapat B. Genomic instability and cancer. Curr Mol Med. 2003;3:589–596. doi: 10.2174/1566524033479456. [DOI] [PubMed] [Google Scholar]
- 8.Romanowski P, Madine MA. Mechanisms restricting DNA replication to once per cell cycle: MCMS, pre-replicative complexes and kinases. Trends Cell Biol. 1996;6:184–188. doi: 10.1016/0962-8924(96)10015-5. [DOI] [PubMed] [Google Scholar]
- 9.Sasaki H, Kawano O, Endo K, Suzuki E, Yukiue H, Kobayashi Y, Yano M, Fujii Y. Human MOB1 expression in non-small-cell lung cancer. Clin Lung Cancer. 2007;8:273–276. doi: 10.3816/CLC.2007.n.006. [DOI] [PubMed] [Google Scholar]
- 10.Chow A, Hao Y, Yang X. Molecular characterization of human homologs of yeast MOB1. Int J Cancer. 2010;126:2079–2089. doi: 10.1002/ijc.24878. [DOI] [PubMed] [Google Scholar]
- 11.Hergovich A. MOB control: reviewing a conserved family of kinase regulators. Cell Signal. 2011;23:1433–1440. doi: 10.1016/j.cellsig.2011.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lai ZC, Wei X, Shimizu T, Ramos E, Rohrbaugh M, Nikolaidis N, Ho LL, Li Y. Control of cell proliferation and apoptosis by mob as tumor suppressor, mats. Cell. 2005;120:675–685. doi: 10.1016/j.cell.2004.12.036. [DOI] [PubMed] [Google Scholar]
- 13.Yang X, Li DM, Chen W, Xu T. Human homologue of Drosophila lats, LATS1, negatively regulate growth by inducing G(2)/M arrest or apoptosis. Oncogene. 2001;20:6516–6523. doi: 10.1038/sj.onc.1204817. [DOI] [PubMed] [Google Scholar]
- 14.Zhang J, Smolen GA, Haber DA. Negative regulation of YAP by LATS1 underscores evolutionary conservation of the Drosophila Hippo pathway. Cancer Res. 2008;68:2789–2794. doi: 10.1158/0008-5472.CAN-07-6205. [DOI] [PubMed] [Google Scholar]
- 15.Ke H, Pei J, Ni Z, Xia H, Qi H, Woods T, Kelekar A, Tao W. Putative tumor suppressor Lats2 induces apoptosis through downregulation of Bcl-2 and Bcl-x(L) Exp Cell Res. 2004;298:329–338. doi: 10.1016/j.yexcr.2004.04.031. [DOI] [PubMed] [Google Scholar]
- 16.Hergovich A. Regulation and functions of mammalian LATS/NDR kinases: looking beyond canonical Hippo signalling. Cell Biosci. 2013;3:32. doi: 10.1186/2045-3701-3-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hergovich A. Mammalian Hippo signalling: a kinase network regulated by protein-protein interactions. Biochem Soc Trans. 2012;40:124–128. doi: 10.1042/BST20110619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kohler RS, Schmitz D, Cornils H, Hemmings BA, Hergovich A. Differential NDR/LATS interactions with the human MOB family reveal a negative role for human MOB2 in the regulation of human NDR kinases. Mol Cell Biol. 2010;30:4507–4520. doi: 10.1128/MCB.00150-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haldrup C, Mundbjerg K, Vestergaard EM, Lamy P, Wild P, Schulz WA, Arsov C, Visakorpi T, Borre M, Høyer S, et al. DNA methylation signatures for prediction of biochemical recurrence after radical prostatectomy of clinically localized prostate cancer. J Clin Oncol. 2013;31:3250–3258. doi: 10.1200/JCO.2012.47.1847. [DOI] [PubMed] [Google Scholar]
- 20.Hergovich A, Cornils H, Hemmings BA. Mammalian NDR protein kinases: from regulation to a role in centrosome duplication. Biochim Biophys Acta. 2008;1784:3–15. doi: 10.1016/j.bbapap.2007.07.017. [DOI] [PubMed] [Google Scholar]
- 21.Manning G, Whyte DB, Martinez R, Hunter T, Sudarsanam S. The protein kinase complement of the human genome. Science. 2002;298:1912–1934. doi: 10.1126/science.1075762. [DOI] [PubMed] [Google Scholar]
- 22.Hergovich A, Stegert MR, Schmitz D, Hemmings BA. NDR kinases regulate essential cell processes from yeast to humans. Nat Rev Mol Cell Biol. 2006;7:253–264. doi: 10.1038/nrm1891. [DOI] [PubMed] [Google Scholar]
- 23.Tamaskovic R, Bichsel SJ, Hemmings BA. NDR family of AGC kinases--essential regulators of the cell cycle and morphogenesis. FEBS Lett. 2003;546:73–80. doi: 10.1016/s0014-5793(03)00474-5. [DOI] [PubMed] [Google Scholar]
- 24.Praskova M, Xia F, Avruch J. MOBKL1A/MOBKL1B phosphorylation by MST1 and MST2 inhibits cell proliferation. Curr Biol. 2008;18:311–321. doi: 10.1016/j.cub.2008.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bothos J, Tuttle RL, Ottey M, Luca FC, Halazonetis TD. Human LATS1 is a mitotic exit network kinase. Cancer Res. 2005;65:6568–6575. doi: 10.1158/0008-5472.CAN-05-0862. [DOI] [PubMed] [Google Scholar]
- 26.Yabuta N, Okada N, Ito A, Hosomi T, Nishihara S, Sasayama Y, Fujimori A, Okuzaki D, Zhao H, Ikawa M, et al. Lats2 is an essential mitotic regulator required for the coordination of cell division. J Biol Chem. 2007;282:19259–19271. doi: 10.1074/jbc.M608562200. [DOI] [PubMed] [Google Scholar]
- 27.Hou MC, Salek J, McCollum D. Mob1p interacts with the Sid2p kinase and is required for cytokinesis in fission yeast. Curr Biol. 2000;10:619–622. doi: 10.1016/s0960-9822(00)00492-9. [DOI] [PubMed] [Google Scholar]
- 28.Luca FC, Mody M, Kurischko C, Roof DM, Giddings TH, Winey M. Saccharomyces cerevisiae Mob1p is required for cytokinesis and mitotic exit. Mol Cell Biol. 2001;21:6972–6983. doi: 10.1128/MCB.21.20.6972-6983.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hou MC, Wiley DJ, Verde F, McCollum D. Mob2p interacts with the protein kinase Orb6p to promote coordination of cell polarity with cell cycle progression. J Cell Sci. 2003;116:125–135. doi: 10.1242/jcs.00206. [DOI] [PubMed] [Google Scholar]