Abstract
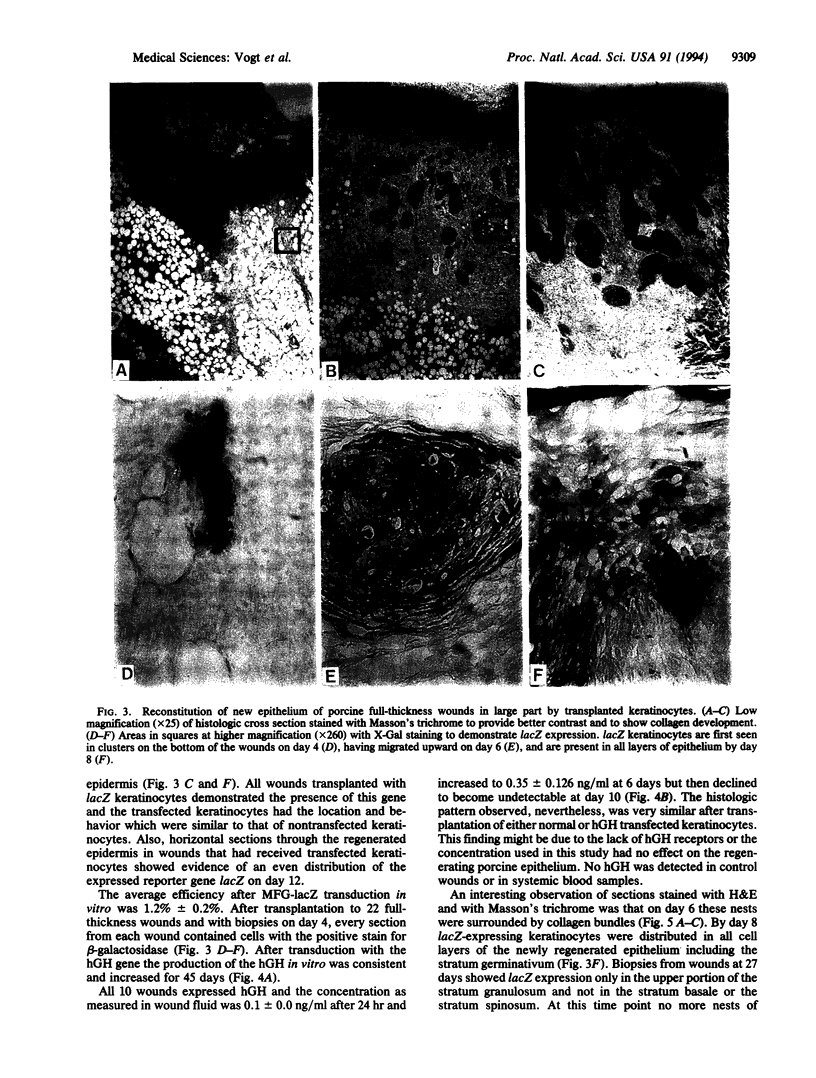
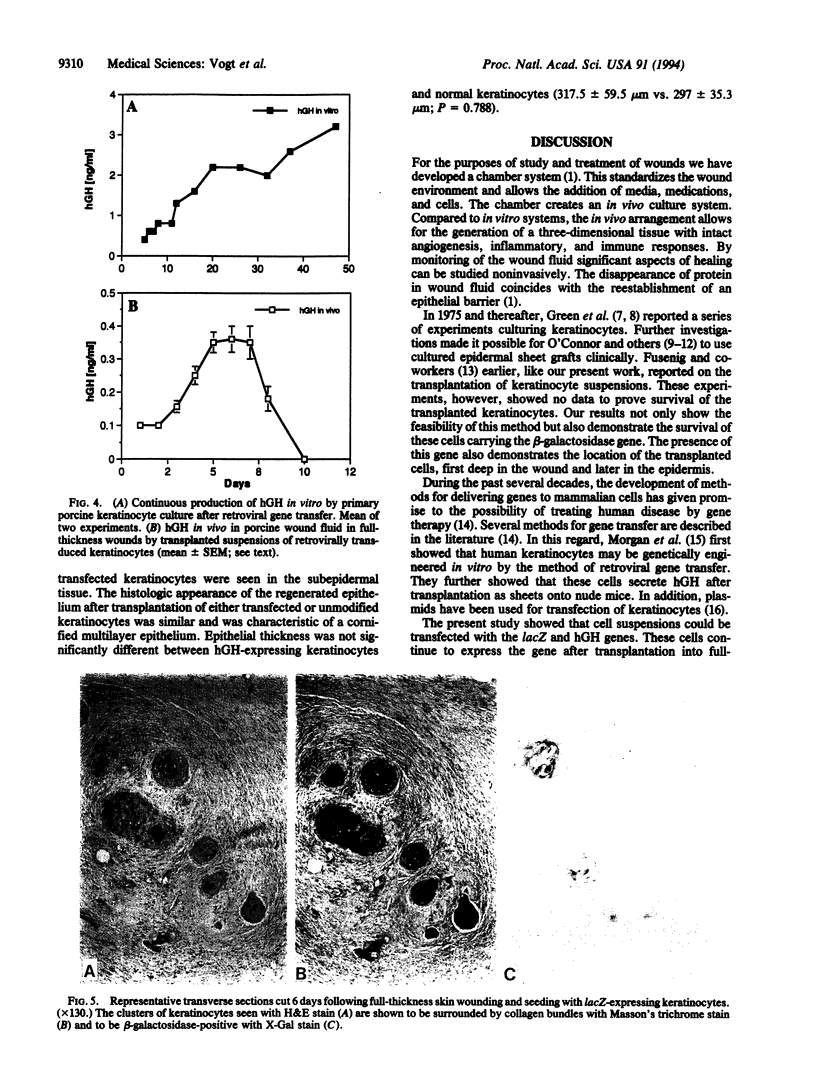
Normal and retrovirally transfected keratinocyte suspensions expressing either the beta-galactosidase gene or the human growth hormone (hGH) gene were transplanted into chamber-enclosed skin full-thickness wounds of Yorkshire pigs. Immunostaining of sequential skin biopsies obtained for 4 weeks after transplantation showed survival of the transplanted keratinocytes as well as expression of beta-galactosidase. Transfected keratinocytes were first seen in the neodermal portions of the wounds, then in the regenerating basal epidermal layer, and finally in the terminally differentiating cells of the stratum spinosum. When keratinocytes transfected with the hGH gene were transplanted into similar wounds, hGH was detected for 10 days in wound fluid. In contrast, hGH was detected in vitro for 47 days. Wounds transplanted with either transfected or normal keratinocytes restored the epithelial barrier function significantly faster than nontransplanted controls (P < 0.05). The study confirms the successful transplantation of keratinocyte suspensions, their reconstitution of the epidermis, and their acceleration of repair. Further, this apparently normal incorporation of genetically engineered transplanted keratinocytes expressing either beta-galactosidase or hGH suggests the possibility of introducing other genes expressing therapeutic proteins into wounds to favorably affect healing. Wound fluid detection of the expressed peptide provided early demonstration of successful transfer of the hGH gene.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Breuing K., Eriksson E., Liu P., Miller D. R. Healing of partial thickness porcine skin wounds in a liquid environment. J Surg Res. 1992 Jan;52(1):50–58. doi: 10.1016/0022-4804(92)90278-8. [DOI] [PubMed] [Google Scholar]
- De Luca M., Albanese E., Bondanza S., Megna M., Ugozzoli L., Molina F., Cancedda R., Santi P. L., Bormioli M., Stella M. Multicentre experience in the treatment of burns with autologous and allogenic cultured epithelium, fresh or preserved in a frozen state. Burns. 1989 Oct;15(5):303–309. doi: 10.1016/0305-4179(89)90007-7. [DOI] [PubMed] [Google Scholar]
- Grafting of burns with cultured epithelium prepared from autologous epidermal cells. Lancet. 1981 Jan 10;1(8211):75–78. [PubMed] [Google Scholar]
- Green H., Kehinde O., Thomas J. Growth of cultured human epidermal cells into multiple epithelia suitable for grafting. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5665–5668. doi: 10.1073/pnas.76.11.5665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumagai N., Nishina H., Tanabe H., Hosaka T., Ishida H., Ogino Y. Clinical application of autologous cultured epithelia for the treatment of burn wounds and burn scars. Plast Reconstr Surg. 1988 Jul;82(1):99–110. [PubMed] [Google Scholar]
- Lee J. I., Taichman L. B. Transient expression of a transfected gene in cultured epidermal keratinocytes: implications for future studies. J Invest Dermatol. 1989 Feb;92(2):267–271. doi: 10.1111/1523-1747.ep12276837. [DOI] [PubMed] [Google Scholar]
- Marikovsky M., Breuing K., Liu P. Y., Eriksson E., Higashiyama S., Farber P., Abraham J., Klagsbrun M. Appearance of heparin-binding EGF-like growth factor in wound fluid as a response to injury. Proc Natl Acad Sci U S A. 1993 May 1;90(9):3889–3893. doi: 10.1073/pnas.90.9.3889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan J. R., Barrandon Y., Green H., Mulligan R. C. Expression of an exogenous growth hormone gene by transplantable human epidermal cells. Science. 1987 Sep 18;237(4821):1476–1479. doi: 10.1126/science.3629250. [DOI] [PubMed] [Google Scholar]
- Mulligan R. C. The basic science of gene therapy. Science. 1993 May 14;260(5110):926–932. doi: 10.1126/science.8493530. [DOI] [PubMed] [Google Scholar]
- Pittelkow M. R., Scott R. E. New techniques for the in vitro culture of human skin keratinocytes and perspectives on their use for grafting of patients with extensive burns. Mayo Clin Proc. 1986 Oct;61(10):771–777. doi: 10.1016/s0025-6196(12)64815-0. [DOI] [PubMed] [Google Scholar]
- Price J., Turner D., Cepko C. Lineage analysis in the vertebrate nervous system by retrovirus-mediated gene transfer. Proc Natl Acad Sci U S A. 1987 Jan;84(1):156–160. doi: 10.1073/pnas.84.1.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rheinwald J. G., Green H. Serial cultivation of strains of human epidermal keratinocytes: the formation of keratinizing colonies from single cells. Cell. 1975 Nov;6(3):331–343. doi: 10.1016/s0092-8674(75)80001-8. [DOI] [PubMed] [Google Scholar]
- Teumer J., Lindahl A., Green H. Human growth hormone in the blood of athymic mice grafted with cultures of hormone-secreting human keratinocytes. FASEB J. 1990 Nov;4(14):3245–3250. doi: 10.1096/fasebj.4.14.2227214. [DOI] [PubMed] [Google Scholar]
- WEINSTEIN G. D. AUTORADIOGRAPHIC STUDIES ON TURNOVER TIME AND PROTEIN SYNTHESIS IN PIG EPIDERMIS. J Invest Dermatol. 1965 Jun;44:413–419. [PubMed] [Google Scholar]
- Worst P. K., Mackenzie I. C., Fusenig N. E. Reformation of organized epidermal structure by transplantation of suspensions and cultures of epidermal and dermal cells. Cell Tissue Res. 1982;225(1):65–77. doi: 10.1007/BF00216219. [DOI] [PubMed] [Google Scholar]





